LED Lights Influenced Phytochemical Contents and Biological Activities in Kale (Brassica oleracea L. var. acephala) Microgreens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Quantitative Real-Time Polymerase Chain Reaction
2.3. Extraction of Carotenoid
2.4. Analysis of Carotenoid
2.5. Extraction of Desulfo-Glucosinolates
2.6. Analysis of Desulfo-Glucosinolates
2.7. Extraction of Phenolics
2.8. Analysis of Phenolics
2.9. Total Phenolic Content
2.10. Measurement of Antioxidant Activity
2.11. Antibacterial Screening of Kale Microgreens
2.12. Statistical Analysis
2.13. Chemicals
3. Results
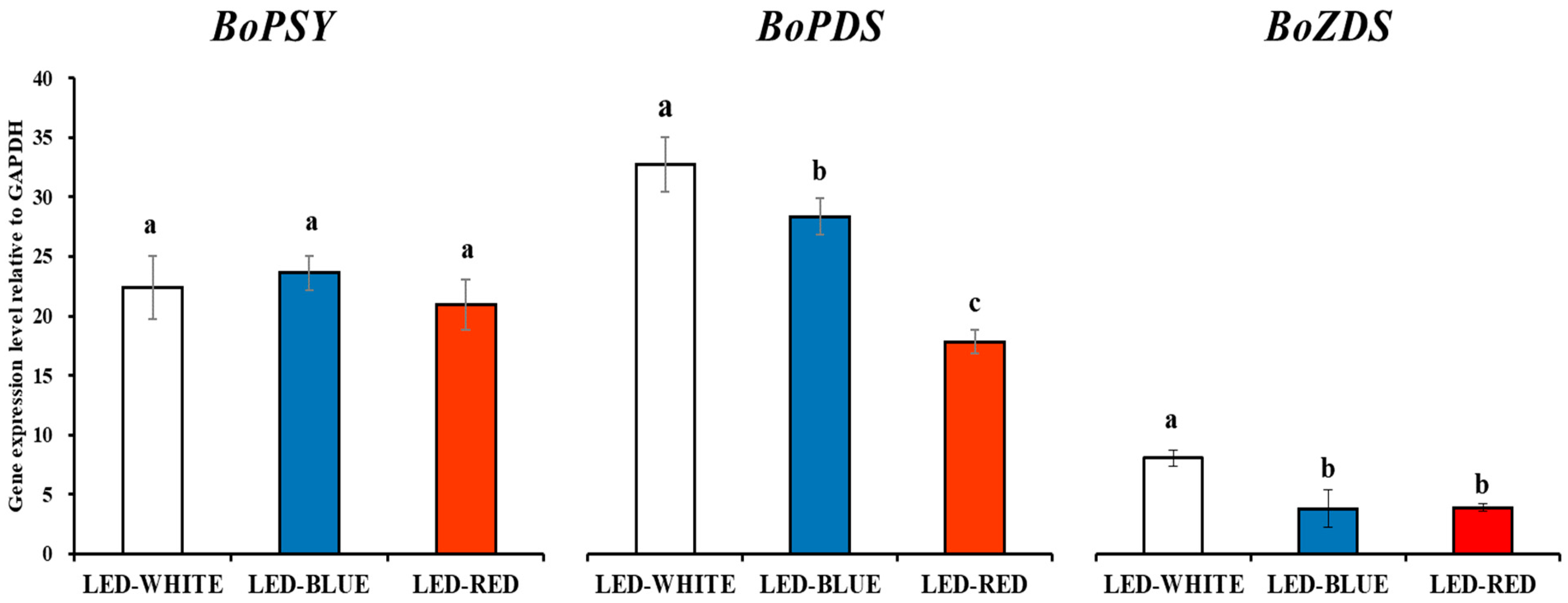
3.1. Carotenoid Biosynthesis Gene Expression Analysis in Kale Microgreens Irradiated with White-, Blue-, and Red-LED Lights
3.2. Carotenoid Contents in Kale Microgreens Irradiated with White-, Blue-, and Red-LED Lights
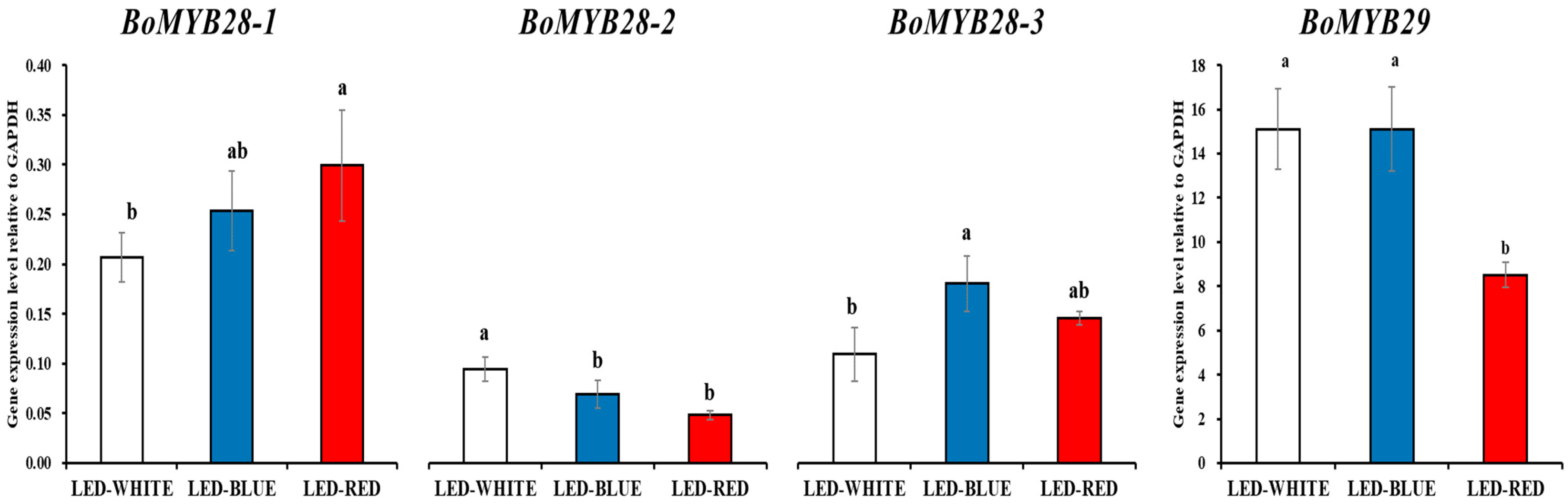
3.3. Alipathic Glucosinolate Biosynthesis Involved Tion Factor Expression Analysis in Kale Microgreens Irradiated with White-, Blue-, and Red-LED Lights
3.4. Alipathic Glucosinolate Contents in Kale Microgreens Irradiated with White-, Blue-, and Red-LED Lights
3.5. Phenolic Contents in Kale Microgreens Irradiated with White-, Blue-, and Red-LED Lights
3.6. Total Phenolic Content and DPPH Assay
3.7. The Antimicrobial Effect of Kale Microgreens Irradiated with White-, Blue-, and Red-LED Lights
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sikora, E.; Bodziarczyk, I. Composition and antioxidant activity of kale (Brassica oleracea L. var. acephala) raw and cooked. Acta Sci. Pol. Technol. Aliment. 2012, 11, 239–248. [Google Scholar] [PubMed]
- Ayaz, F.A.; Glew, R.H.; Millson, M.; Huang, H.; Chuang, L.; Sanz, C.; Hayırlıoglu-Ayaz, S. Nutrient contents of kale (Brassica oleraceae L. var. acephala DC.). Food Chem. 2006, 96, 572–579. [Google Scholar] [CrossRef]
- Lučić, D.; Pavlović, I.; Brkljačić, L.; Bogdanović, S.; Farkaš, V.; Cedilak, A.; Nanić, L.; Rubelj, I.; Salopek-Sondi, B. Antioxidant and Antiproliferative Activities of Kale (Brassica oleracea L. var. acephala DC.) and Wild Cabbage (Brassica incana Ten.) Polyphenolic Extracts. Molecules 2023, 28, 1840. [Google Scholar] [CrossRef]
- da Silva, L.C.R.; Azevedo, A.M.; Almeida, A.C.; da Fonseca, F.S.A.; Brandi, I.V.; Ferreira, E.A.; Fernandes, A.C.G.; Valadares, N.R. Antioxidant and antimicrobial capacity of aqueous extract of kale and potential supplementation in fermented dairy beverage. Sci. Plena 2021, 17, 101502. [Google Scholar] [CrossRef]
- Park, C.H.; Park, S.-Y.; Park, Y.J.; Kim, J.K.; Park, S.U. Metabolite profiling and comparative analysis of secondary metabolites in Chinese cabbage, radish, and hybrid xBrassicoraphanus. J. Agric. Food Chem. 2020, 68, 13711–13719. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Orozco, R.; Piskula, M.K.; Zielinski, H.; Kozlowska, H.; Frias, J.; Vidal-Valverde, C. Germination as a process to improve the antioxidant capacity of Lupinus angustifolius L. var. Zapaton. Eur. Food Res. Technol. 2006, 223, 495–502. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts and microgreens—Novel food sources for healthy diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef]
- Rhee, J.-H.; Choi, S.; Lee, J.-E.; Hur, O.-S.; Ro, N.-Y.; Hwang, A.-J.; Ko, H.-C.; Chung, Y.-J.; Noh, J.-J.; Assefa, A.D. Glucosinolate content in Brassica genetic resources and their distribution pattern within and between inner, middle, and outer leaves. Plants 2020, 9, 1421. [Google Scholar] [CrossRef]
- Frerigmann, H.; Gigolashvili, T. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol. Plant 2014, 7, 814–828. [Google Scholar] [CrossRef]
- Li, Y.; Sawada, Y.; Hirai, A.; Sato, M.; Kuwahara, A.; Yan, X.; Hirai, M.Y. Novel insights into the function of Arabidopsis R2R3-MYB transcription factors regulating aliphatic glucosinolate biosynthesis. Plant Cell Physiol. 2013, 54, 1335–1344. [Google Scholar] [CrossRef]
- Vale, A.P.; Santos, J.; Brito, N.V.; Fernandes, D.; Rosa, E.; Oliveira, M.B.P. Evaluating the impact of sprouting conditions on the glucosinolate content of Brassica oleracea sprouts. Phytochemistry 2015, 115, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Abreu, A.C.; Ferreira, C.; Saavedra, M.J.; Simões, L.C.; Simões, M. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J. Food Sci. Technol. 2015, 52, 4737–4748. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, Y.; Matsumoto, H.; Kato, M. Diversity in the carotenoid profiles and the expression of genes related to carotenoid accumulation among citrus genotypes. Breed. Sci. 2016, 66, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light emitting diodes (LEDs) as agricultural lighting: Impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Park, C.H.; Xu, H.; Yeo, H.J.; Park, Y.E.; Hwang, G.-S.; Park, N.I.; Park, S.U. Enhancement of the flavone contents of Scutellaria baicalensis hairy roots via metabolic engineering using maize Lc and Arabidopsis PAP1 transcription factors. Metab. Eng. 2021, 64, 64–73. [Google Scholar] [CrossRef]
- ISO 9167-1:1992; Rapeseed—Determination of glucosinolates content—Part 1: Method using high-performance liquid chromatography. ISO: Geneva, Switzerland, 1992; pp. 1–9.
- Eum, H.L.; Park, Y.; Yi, T.G.; Lee, J.W.; Ha, K.-S.; Choi, I.-Y.; Park, N.I. Effect of germination environment on the biochemical compounds and anti-inflammatory properties of soybean cultivars. PLoS ONE 2020, 15, e0232159. [Google Scholar] [CrossRef]
- Yi, T.G.; Park, Y.; Park, J.-E.; Park, N.I. Enhancement of phenolic compounds and antioxidative activities by the combination of culture medium and methyl jasmonate elicitation in hairy root cultures of Lactuca indica L. Nat. Prod. Commun. 2019, 14, 1934578X19861867. [Google Scholar] [CrossRef]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2019, 59, 2411–2422. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, J.; Wan, J.; Pham, Q.; Zhang, Z.; Sun, J.; Yu, L.; Luo, Y.; Wang, T.T.; Chen, P. Profiling of polyphenols and glucosinolates in kale and broccoli microgreens grown under chamber and windowsill conditions by ultrahigh-performance liquid chromatography high-resolution mass spectrometry. ACS Food Sci. Technol. 2021, 2, 101–113. [Google Scholar] [CrossRef]
- Waterland, N.L.; Moon, Y.; Tou, J.C.; Kopsell, D.A.; Kim, M.J.; Park, S. Differences in leaf color and stage of development at harvest influenced phytochemical content in three cultivars of kale (Brassica oleracea L. and B. napus). J. Agric. Sci. 2019, 11, 14. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Artés-Hernández, F. Periodical UV-B radiation hormesis in biosynthesis of kale sprouts nutraceuticals. Plant Physiol. Biochem. 2021, 165, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Biegańska-Marecik, R.; Radziejewska-Kubzdela, E.; Marecik, R. Characterization of phenolics, glucosinolates and antioxidant activity of beverages based on apple juice with addition of frozen and freeze-dried curly kale leaves (Brassica oleracea L. var. acephala L.). Food Chem. 2017, 230, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, S.U. Accumulation of carotenoids in green and red Chinese cabbage (Brassica rapa ssp. pekinensis) in response to light-emitting diodes. Biosci. Res. 2018, 15, 41–47. [Google Scholar]
- Frede, K.; Schreiner, M.; Baldermann, S. Light quality-induced changes of carotenoid composition in pak choi Brassica rapa ssp. chinensis. J. Photochem. Photobiol. B Biol. 2019, 193, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Frede, K.; Schreiner, M.; Zrenner, R.; Graefe, J.; Baldermann, S. Carotenoid biosynthesis of pak choi (Brassica rapa ssp. chinensis) sprouts grown under different light-emitting diodes during the diurnal course. Photochem. Photobiol. Sci. 2018, 17, 1289–1300. [Google Scholar] [CrossRef]
- Frede, K.; Winkelmann, S.; Busse, L.; Baldermann, S. The effect of LED light quality on the carotenoid metabolism and related gene expression in the genus Brassica. BMC Plant Biol. 2023, 23, 328. [Google Scholar] [CrossRef]
- Gangappa, S.N.; Botto, J.F. The multifaceted roles of HY5 in plant growth and development. Mol. Plant 2016, 9, 1353–1365. [Google Scholar] [CrossRef]
- Park, C.H.; Park, Y.E.; Yeo, H.J.; Kim, J.K.; Park, S.U. Effects of light-emitting diodes on the accumulation of phenolic compounds and glucosinolates in Brassica juncea sprouts. Horticulturae 2020, 6, 77. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.B.; Li, X.; Choi, S.R.; Park, S.; Park, J.S.; Lim, Y.P.; Park, S.U. Accumulation of phenylpropanoids by white, blue, and red light irradiation and their organ-specific distribution in Chinese cabbage (Brassica rapa ssp. pekinensis). J. Agric. Food Chem. 2015, 63, 6772–6778. [Google Scholar] [CrossRef]
- Sathasivam, R.; Park, S.U.; Kim, J.K.; Park, Y.J.; Kim, M.C.; Nguyen, B.V.; Lee, S.Y. Metabolic profiling of primary and secondary metabolites in kohlrabi (Brassica oleracea var. gongylodes) sprouts exposed to different light-emitting diodes. Plants 2023, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Liu, H.; Zhang, Y.; Hao, Y.; Song, S.; Lei, B. Effect of supplemental blue light intensity on the growth and quality of Chinese kale. Hortic. Environ. Biotechnol. 2019, 60, 49–57. [Google Scholar] [CrossRef]
- Chung, I.M.; Paudel, N.; Kim, S.-H.; Yu, C.Y.; Ghimire, B.K. The influence of light wavelength on growth and antioxidant capacity in Pachyrhizus erosus (L.) Urban. J. Plant Growth Regul. 2020, 39, 296–312. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Jeong, B.R. Physiological and proteomic insights into red and blue light-mediated enhancement of in vitro growth in Scrophularia kakudensis—A potential medicinal plant. Front. Plant Sci. 2021, 11, 607007. [Google Scholar] [CrossRef]
- Gupta, R.; Sood, H. Emerging Technologies for the Production of In Vitro Raised Quality Rich Swertia chirayita by Using LED Lights. Sustainability 2023, 15, 1714. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kim, W.W.; Park, C.H.; Cho, D.H. Effect of artificial LED light and far infrared irradiation on phenolic compound, isoflavones and antioxidant capacity in soybean (Glycine max L.) sprout. Foods 2018, 7, 174. [Google Scholar] [CrossRef]
- Yin, L.; Chen, H.; Cao, B.; Lei, J.; Chen, G. Molecular characterization of MYB28 involved in aliphatic glucosinolate biosynthesis in Chinese kale (Brassica oleracea var. alboglabra Bailey). Front. Plant Sci. 2017, 8, 1083. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Engqvist, M.; Yatusevich, R.; Müller, C.; Flügge, U.I. HAG2/MYB76 and HAG3/MYB29 exert a specific and coordinated control on the regulation of aliphatic glucosinolate biosynthesis in Arabidopsis thaliana. New Phytol. 2008, 177, 627–642. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Yatusevich, R.; Berger, B.; Müller, C.; Flügge, U.I. The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 2007, 51, 247–261. [Google Scholar] [CrossRef]
- Yatusevich, R.; Mugford, S.G.; Matthewman, C.; Gigolashvili, T.; Frerigmann, H.; Delaney, S.; Koprivova, A.; Flügge, U.I.; Kopriva, S. Genes of primary sulfate assimilation are part of the glucosinolate biosynthetic network in Arabidopsis thaliana. Plant J. 2010, 62, 1–11. [Google Scholar] [CrossRef]
- Zhuang, L.; Huang, G.; Li, X.; Xiao, J.; Guo, L. Effect of different LED lights on aliphatic glucosinolates metabolism and biochemical characteristics in broccoli sprouts. Food Res. Int. 2022, 154, 111015. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Arasu, M.V.; Park, S.; Byeon, D.H.; Chung, S.-O.; Park, S.U. LED lights enhance metabolites and antioxidants in Chinese cabbage and kale. Braz. Arch. Biol. Technol. 2016, 59, e16150546. [Google Scholar] [CrossRef]
- Shamlan, K.A.; Yahya, H.; Ismail, I.N.A.; Yahya, H.N. Antibacterial Activities of Microgreens and Mature Extract of Kale and Red Spinach Against Selected Pathogenic Bacteria. East Afr. Sch. J. Agric. Life Sci. 2020, 3, 337–342. [Google Scholar] [CrossRef]
- Burgaz, E.; Sezener, M.G.; Dikbas, C.; Ceylan, A.K.; Andac, M.; Ciftci, A. Determination of antibacterial properties of silver nanoparticles with aqueous extracts of Brassica oleracea L. var. acephala dc in cotton textiles. J. Elem. 2021, 26, 447–462. [Google Scholar]
- Ayaz, F.A.; Hayırlıoglu-Ayaz, S.; Alpay-Karaoglu, S.; Grúz, J.; Valentová, K.; Ulrichová, J.; Strnad, M. Phenolic acid contents of kale (Brassica oleraceae L. var. acephala DC.) extracts and their antioxidant and antibacterial activities. Food Chem. 2008, 107, 19–25. [Google Scholar] [CrossRef]
- Hu, S.H.; Wang, J.C.; Kung, H.F.; Wang, J.T.; Lee, W.L.; Yang, Y.H. Antimicrobial effect of extracts of cruciferous vegetables. Kaohsiung J. Med. Sci. 2004, 20, 591–599. [Google Scholar] [CrossRef]

| White | Red | Blue | |
|---|---|---|---|
| Lutein | 1070 ± 10.7 a * | 1080 ± 71.0 a | 965 ± 6.35 b |
| 13-cis-β-Carotene | 153 ± 0.0780 a | 106 ± 3.87 c | 117 ± 7.23 b |
| α-Carotene | 31.5 ± 0.232 a | 25.0 ± 0.516 c | 28.8 ± 1.59 b |
| β-Carotene | 1980 ± 10.2 a | 1680 ± 88.4 c | 1850 ± 52.3 b |
| 9-cis-β-Carotene | 120 ± 3.85 a | 96.7 ± 4.31 b | 105 ± 5.16 b |
| White | Red | Blue | |
|---|---|---|---|
| Glucoiberin | 0.357 ± 0.0431 a * | 0.359 ± 0.00507 a | 0.298 ± 0.0248 b |
| Progoitrin | 27.3 ± 0.119 a | 23.9 ± 0.614 b | 29.1 ± 2.13 a |
| Glucoraphanin | 0.889 ± 0.0146 b | 0.737 ± 0.0304 c | 1.01 ± 0.0662 a |
| Sinigrin | 0.0913 ± 0.00819 a | 0.0644 ± 0.00233 b | 0.0993 ± 0.0123 a |
| Glucobrassicanapin | 0.271 ± 0.00952 a | 0.240 ± 0.00282 b | 0.274 ± 0.0235 a |
| Glucoerucin | 0.700 ± 0.111 ab | 0.816 ± 0.0283 a | 0.615 ± 0.0288 b |
| White | Red | Blue | |
|---|---|---|---|
| Gallic acid | 14.1483 ± 0.3128 c * | 17.8989 ± 1.773 b | 75 ± 0.1472 a |
| Catechin | 72.1758 ± 1.7539 b | 68.4374 ± 1.4468 c | 90.8036 ± 1.9068 a |
| Ferulic acid | 1.8538 ± 0.1143 b | 1.2222 ± 0.1239 c | 1.7846 ± 0.0677 a |
| Sinapic acid | 16.4898 ± 3.6659 b | 7.2225 ± 1.7981 c | 14.9793 ± 0.7898 a |
| Rutin | 134.9817 ± 0.7248 a | 141.8442 ± 6.2921 a | 136.1821 ± 0.5638 a |
| Quercetin | 112.4698 ± 0.0742 b | 110.4018 ± 0.0645 b | 114.8303 ± 2.0245 a |
| White | Red | Blue | |
|---|---|---|---|
| TPC [mg gallic acid equivalent (GAE)/g Dry weight] | 83.32 ± 3.35 b * | 73.06 ± 5.58 c | 93.39 ± 0.84 a |
| DPPH (Inhibition%) | 88.16 ± 1.42 ab | 87.19 ± 1.42 b | 90.50 ± 0.73 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Park, C.H.; Kim, J.K.; Ahn, K.; Kwon, H.; Kim, J.K.; Park, S.U.; Yeo, H.J. LED Lights Influenced Phytochemical Contents and Biological Activities in Kale (Brassica oleracea L. var. acephala) Microgreens. Antioxidants 2023, 12, 1686. https://doi.org/10.3390/antiox12091686
Lee S, Park CH, Kim JK, Ahn K, Kwon H, Kim JK, Park SU, Yeo HJ. LED Lights Influenced Phytochemical Contents and Biological Activities in Kale (Brassica oleracea L. var. acephala) Microgreens. Antioxidants. 2023; 12(9):1686. https://doi.org/10.3390/antiox12091686
Chicago/Turabian StyleLee, Seom, Chang Ha Park, Jin Kyung Kim, Kyungmin Ahn, Haejin Kwon, Jae Kwang Kim, Sang Un Park, and Hyeon Ji Yeo. 2023. "LED Lights Influenced Phytochemical Contents and Biological Activities in Kale (Brassica oleracea L. var. acephala) Microgreens" Antioxidants 12, no. 9: 1686. https://doi.org/10.3390/antiox12091686
APA StyleLee, S., Park, C. H., Kim, J. K., Ahn, K., Kwon, H., Kim, J. K., Park, S. U., & Yeo, H. J. (2023). LED Lights Influenced Phytochemical Contents and Biological Activities in Kale (Brassica oleracea L. var. acephala) Microgreens. Antioxidants, 12(9), 1686. https://doi.org/10.3390/antiox12091686







