Valorization of Defatted Cherry Seed Residues from Liquor Processing by Matrix Solid-Phase Dispersion Extraction: A Sustainable Strategy for Production of Phenolic-Rich Extracts with Antioxidant Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deffated Cherry Seeds (Prunus avium L.) before Fermentation, after Fermentation, and after Both Fermentation and Distillation
2.2. Reagents, Solvents, and Polyphenol Standards
2.3. Extraction of Phenolic Compounds from Residual Cherry Seeds
2.4. MSPD Optimized Extraction Method
2.5. Total Phenolic Content
2.6. Total Flavonoid Content
2.7. Antioxidant Activity
2.8. Chromatographic Analysis of Phenolic Extracts by HPLC-ESI-QTOF-MS
2.9. Statistical Analysis
3. Results
3.1. Moisture Content of the Defatted Sweet Cherry Seeds
3.2. Defatted Seed Yield of Sweet Cherry Seeds
3.3. MSPD Extraction Method Optimization
3.3.1. Initial MSPD Assays
3.3.2. Experimental Design and Optimization
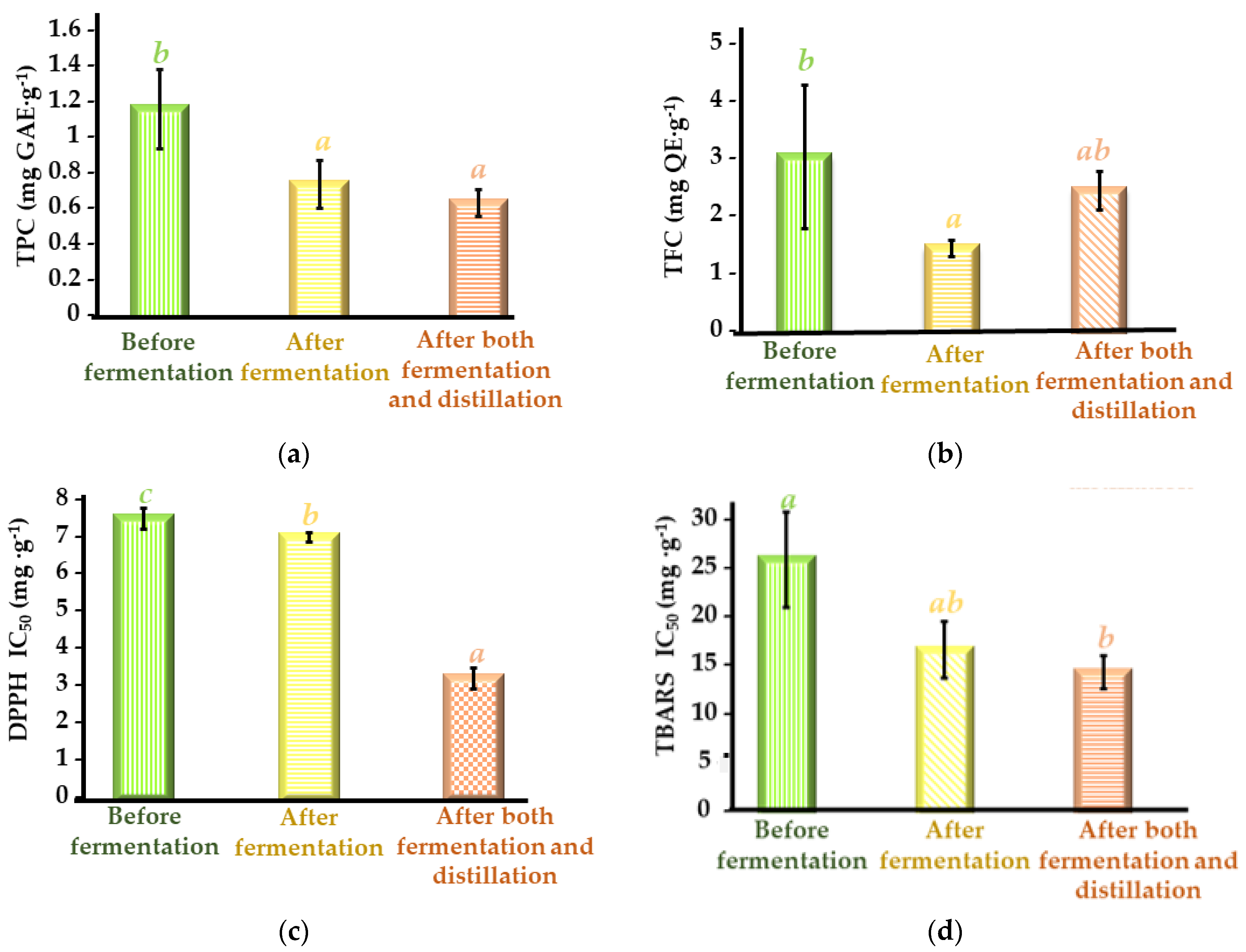
3.4. Total Phenolic Content and Antioxidant Activities of Cherry Seed Extracts
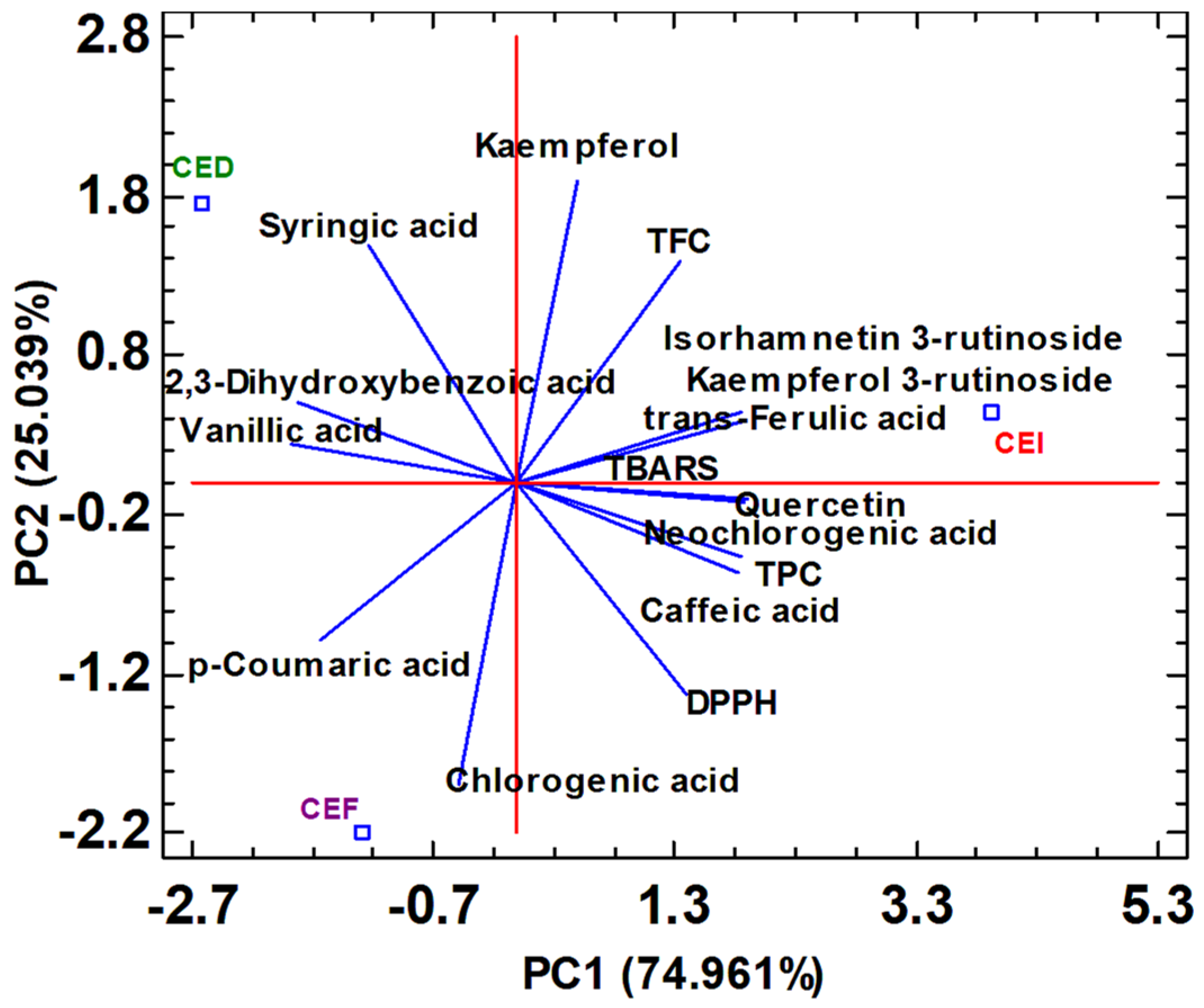
3.5. Phenolic Profile of Cherry Seed Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, Organic Acids, Phenolic Composition and Antioxidant Activity of Sweet Cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Muñoz Mingarro, D. Comparison of Phenolic Compounds Profile and Antioxidant Properties of Different Sweet Cherry (Prunus avium L.) Varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Blázquez, S.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; García-Sánchez, B.; Miranda, R. Valorization of Prunus Seed Oils: Fatty Acids Composition and Oxidative Stability. Molecules 2023, 28, 7045. [Google Scholar] [CrossRef] [PubMed]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet Cherry: Composition, Postharvest Preservation, Processing and Trends for Its Future Use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Ristovski, B.; Jakopic, J.; Slatnar, A.; Stampar, F.; Bocevska, M. Polyphenols in Traditional Sour Cherry Liqueurs-Beverages with Health Benefits. EJSDR 2016, 1, 11–17. [Google Scholar]
- Venegas-Gómez, A.; Gómez-Corzo, M.; MacIás-Garciá, A.; Carrasco-Amador, J.P. Charcoal Obtained from Cherry Stones in Different Carbonization Atmospheres. J. Environ. Chem. Eng. 2020, 8, 103561. [Google Scholar] [CrossRef]
- Balas, F.; López-Corrales, M.; Serradilla, M.J.; Cai, L.; Iezzoni, A.; Wünsch, A. Firmness QTL Mapping Using an “Ambrunés” × “Sweetheart” Sweet Cherry Population. Acta Hortic. 2019, 1235, 41–46. [Google Scholar] [CrossRef]
- Farag, M.A.; Bahaa Eldin, A.; Khalifa, I. Valorization and Extraction Optimization of Prunus Seeds for Food and Functional Food Applications: A Review with Further Perspectives. Food Chem. 2022, 388, 132955. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Mišina, I.; Segliņa, D. Impact of Cultivar on Profile and Concentration of Lipophilic Bioactive Compounds in Kernel Oils Recovered from Sweet Cherry (Prunus avium L.) by-Products. Plant Foods Hum. Nutr. 2016, 71, 158–164. [Google Scholar] [CrossRef]
- Araujo, M.N.; dos Santos, K.C.; do Carmo Diniz, N.; de Carvalho, J.C.; Corazza, M.L. A Biorefinery Approach for Spent Coffee Grounds Valorization Using Pressurized Fluid Extraction to Produce Oil and Bioproducts: A Systematic Review. Bioresour. Technol. Rep. 2022, 18, 101013. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; García, M.C.; Marina, M.L.; Plaza, M. Pressurized Liquid Extraction Combined with Enzymatic-Assisted Extraction to Obtain Bioactive Non-Extractable Polyphenols from Sweet Cherry (Prunus avium L.) Pomace. Nutrients 2021, 13, 3242. [Google Scholar] [CrossRef] [PubMed]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical Characterisation and Bioactive Properties of Prunus avium L.: The Widely Studied Fruits and the Unexplored Stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Selli, S. Evaluation of Chemical Constituents and Antioxidant Activity of Sweet Cherry (Prunus avium L.) Cultivars. Int. J. Food Sci. Technol. 2011, 46, 2530–2537. [Google Scholar] [CrossRef]
- Aires, A.; Dias, C.; Carvalho, R.; Saavedra, M.J. Analysis of Glycosylated Flavonoids Extracted from Sweet-Cherry Stems, as Antibacterial Agents against Pathogenic Escherichia coli Isolates. Acta Biochim. Pol. 2017, 64, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic Compounds Profile and Antioxidant Properties of Six Sweet Cherry (Prunus avium L.) Cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Commisso, M.; Bianconi, M.; Di Carlo, F.; Poletti, S.; Bulgarini, A.; Munari, F.; Negri, S.; Stocchero, M.; Ceoldo, S.; Avesani, L.; et al. Multi-Approach Metabolomics Analysis and Artificial Simplified Phytocomplexes Reveal Cultivar-Dependent Synergy between Polyphenols and Ascorbic Acid in Fruits of the Sweet Cherry (Prunus avium L.). PLoS ONE 2017, 12, 889. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Screening the Extraction Process of Phenolic Compounds from Pressed Grape Seed Residue: Towards an Integrated and Sustainable Management of Viticultural Waste. LWT 2022, 169, 113988. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Mikkelsen, L.H.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. A Combined Approach Based on Matrix Solid-Phase Dispersion Extraction Assisted by Titanium Dioxide Nanoparticles and Liquid Chromatography to Determine Polyphenols from Grape Residues. J. Chromatogr. A 2021, 1644, 462128. [Google Scholar] [CrossRef]
- García, M.C.; Endermann, J.; González-García, E.; Marina, M.L. HPLC-Q-TOF-MS Identification of Antioxidant and Antihypertensive Peptides Recovered from Cherry (Prunus cerasus L.) Subproducts. J. Agric. Food Chem. 2015, 63, 1514–1520. [Google Scholar] [CrossRef]
- Antognoni, F.; Potente, G.; Mandrioli, R.; Angeloni, C.; Freschi, M.; Malaguti, M.; Hrelia, S.; Lugli, S.; Gennari, F.; Muzzi, E.; et al. Fruit Quality Characterization of New Sweet Cherry Cultivars as a Good Source of Bioactive Phenolic Compounds with Antioxidant and Neuroprotective Potential. Antioxidants 2020, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Agulló-Chazarra, L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A.; Micol, V.; Herranz-López, M.; Barrajón-Catalán, E. Sweet Cherry Byproducts Processed by Green Extraction Techniques as a Source of Bioactive Compounds with Antiaging Properties. Antioxidants 2020, 9, 418. [Google Scholar] [CrossRef]
- Nazaruddin, R.; Seng, L.K.; Hassan, O.; Said, M. Effect of Pulp Preconditioning on the Content of Polyphenols in Cocoa Beans (Theobroma Cacao) during Fermentation. Ind. Crops Prod. 2006, 24, 87–94. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Rizzi, C.; Mihaylova, D.; Lante, A. Effect of the Distillation Process on Polyphenols Content of Grape Pomace. Eur. Food Res. Technol. 2022, 248, 929–935. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M. New Insights into the Application of MSPD in Various Fields of Analytical Chemistry. Trends Anal. Chem. 2019, 112, 29–51. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). AOAC Method 925.10 (Air Oven Method) for Moisture in Flour, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Method 960.39, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J.L. Valorisation Potential of Cabernet Grape Pomace for the Recovery of Polyphenols: Process Intensification, Optimisation and Study of Kinetics. Food Bioprod. Process. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Determination of Phenolic Compounds in Residual Brewing Yeast Using Matrix Solid-Phase Dispersion Extraction Assisted by Titanium Dioxide Nanoparticles. J. Chromatogr. A 2019, 1601, 255–265. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Sacristán, I.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Effect of Storage and Drying Treatments on Antioxidant Activity and Phenolic Composition of Lemon and Clementine Peel Extracts. Molecules 2023, 28, 1624. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Sacristán, I.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Valorization of Citrus Reticulata Blanco Peels to Produce Enriched Wheat Bread: Phenolic Bioaccessibility and Antioxidant Potential. Antioxidants 2023, 12, 1742. [Google Scholar] [CrossRef]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of Ultrasound-Assisted Extraction with Conventional Extraction Methods of Oil and Polyphenols from Grape (Vitis vinifera L.) Seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef]
- Daneshfar, A.; Hashemi, P.; Delfan, B.; Tavakkoli, M.; Rashno, P.M. The Efficient Extraction of Phenolic Compounds from Oak Gall Using a Miniaturized Matrix Solid-Phase Dispersion Method before Their HPLC Determination. Herb. Med. J. Spring 2017, 2, 71–80. [Google Scholar] [CrossRef]
- Minuti, L.; Pellegrino, R. Determination of Phenolic Compounds in Wines by Novel Matrix Solid-Phase Dispersion Extraction and Gas Chromatography/Mass Spectrometry. J. Chromatogr. A 2008, 1185, 23–30. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Samperi, R.; Stampachiacchiere, S.; Ventura, S.; Laganà, A. Recent Advances and Developments in Matrix Solid-Phase Dispersion. Trends Anal. Chem. 2015, 71, 186–193. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Dimitrov, K. Optimization of Antioxidant Phenolic Compounds Extraction from Quinoa (Chenopodium quinoa) Seeds. J. Food Sci. Technol. 2015, 52, 4396–4404. [Google Scholar] [CrossRef]
- Yüksekkaya, Ş.; Başyiğit, B.; Sağlam, H.; Pekmez, H.; Cansu, Ü.; Karaaslan, A.; Karaaslan, M. Valorization of Fruit Processing By-Products: Free, Esterified, and Insoluble Bound Phytochemical Extraction from Cherry (Prunus avium L.) Tissues and Their Biological Activities. J. Food Meas. Charact. 2021, 15, 1092–1107. [Google Scholar] [CrossRef]
- Hu, T.; Subbiah, V.; Wu, H.; BK, A.; Rauf, A.; Alhumaydhi, F.A.; Suleria, H.A.R. Determination and Characterization of Phenolic Compounds from Australia-Grown Sweet Cherries (Prunus avium L.) and Their Potential Antioxidant Properties. ACS Omega 2021, 6, 34687–34699. [Google Scholar] [CrossRef]
- Hernández, D.; Fernández-Puratich, H.; Cataldo, F.; González, J. Particle Boards Made with Prunus avium L. Fruit Waste. Case Stud. Constr. Mater. 2020, 12, e00336. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Alves, G.; Falcão, A.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability. Foods 2021, 10, 1185. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of Solid-State Fermentation with Two Filamentous Fungi on the Total Phenolic Contents, Flavonoids, Antioxidant Activities and Lipid Fractions of Plum Fruit (Prunus domestica L.) by-Products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Jericó Santos, T.R.; Santos Vasconcelos, A.G.; Lins de Aquino Santana, L.C.; Gualberto, N.C.; Buarque Feitosa, P.R.; Pires de Siqueira, A.C. Solid-State Fermentation as a Tool to Enhance the Polyphenolic Compound Contents of Acidic Tamarindus Indica by-Products. Biocatal. Agric. Biotechnol. 2020, 30, 101851. [Google Scholar] [CrossRef]
- Bhanja Dey, T.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant Phenolics and Their Microbial Production by Submerged and Solid State Fermentation Process: A Review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of Heat Processing on Thermal Stability and Antioxidant Activity of Six Flavonoids. J. Food Process. Preserv. 2017, 41, 13203. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Fruit Seeds of the Rosaceae Family: A Waste, New Life, or a Danger to Human Health? J. Agric. Food Chem. 2017, 65, 10621–10629. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, X.; Yuan, S.; Liu, H.; Liu, M.; Zhang, Y.; Zhang, H.; Gao, Y.; Lin, R.; Li, X. Identification and Analysis of Amygdalin, Neoamygdalin and Amygdalin Amide in Different Processed Bitter Almonds by HPLC-ESI-MS/MS and HPLC-DAD. Molecules 2017, 22, 1425. [Google Scholar] [CrossRef] [PubMed]
- Badr, J.; Tawfik, M.K.; Badr, J.M. Analytical and Pharmacological Investigation of Amygdalin in Prunus armeniaca L. Kernels. J. Pharm. Res. 2010, 3, 2134–2137. [Google Scholar]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic Extract of Propolis (EEP) Enhances the Apoptosis- Inducing Potential of TRAIL in Cancer Cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef]
- Blaheta, R.A.; Nelson, K.; Haferkamp, A.; Juengel, E. Amygdalin, Quackery or Cure? Phytomedicine 2016, 23, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Alshimali, S.I.; Abdellatif, A.A.H. Amygdalin: A Review on Its Characteristics, Antioxidant Potential, Gastrointestinal Microbiota Intervention, Anticancer Therapeutic and Mechanisms, Toxicity, and Encapsulation. Biomolecules 2022, 12, 1514. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Anwar, S.; Yunusa, B.M.; Nayik, G.A.; Mousavi Khaneghah, A. The Potential of Apricot Seed and Oil as Functional Food: Composition, Biological Properties, Health Benefits & Safety. Food Biosci. 2023, 51, 102336. [Google Scholar] [CrossRef]
- Kashyap, P.; Singh Riar, C.; Jindal, N. Detoxification of Meghalayan Cherry (Prunus nepalensis) Kernel and Its Effect on Structural and Thermal Properties of Proteins. Food Res. Int. 2023, 164, 112437. [Google Scholar] [CrossRef]
- Jasar, D.; Filipovski, V.; Kubelka -Sabit, K.; Curcic-Trajkovska, B. Potential benefits and controversies related to use of amygdalin (vitamin b17). J. Hyg. Eng. Des. 2008, 23, 57–63. [Google Scholar]
- Tsaur, I.; Thomas, A.; Monecke, M.; Zugelder, M.; Rutz, J.; Grein, T.; Maxeiner, S.; Xie, H.; Chun, F.K.H.; Rothweiler, F.; et al. Amygdalin Exerts Antitumor Activity in Taxane-Resistant Prostate Cancer Cells. Cancers 2022, 14, 3111. [Google Scholar] [CrossRef]
- Song, Z.; Xu, X. Advanced Research on Anti-Tumor Effects of Amygdalin. J. Cancer Res. Ther. 2014, 10, C3–C7. [Google Scholar] [CrossRef]
- El-Ela, F.I.A.; Gamal, A.; Elbanna, H.A.; ElBanna, A.H.; Salem, H.F.; Tulbah, A.S. In Vitro and In Vivo Evaluation of the Effectiveness and Safety of Amygdalin as a Cancer Therapy. Pharmaceuticals 2022, 15, 1306. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Narendhirakannan, R.T. Evaluation of Antioxidant and Cytotoxicity Properties of Amygdalin Extracted from Prunus Dulcis. Kongunadu Res. J. 2019, 6, 8–12. [Google Scholar] [CrossRef]
- Kitic, D.; Miladinovic, B.; Randjelovic, M.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Seidel, V. Anticancer Potential and Other Pharmacological Properties of Prunus armeniaca L.: An Updated Overview. Plants 2022, 11, 1885. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-Y.; Hong, S.-P.; Hahn, D.-H.; Kim, J.H. Apoptosis Induction of Persicae Semen Extract in Human Promyelocytic Leukemia (HL-60) Cells. Arch. Pharm. Res. 2003, 26, 157–161. [Google Scholar] [PubMed]
- Gago-López, N.; Lagunas Arnal, C.; Perez, J.J.; Wagner, E.F. Topical Application of an Amygdalin Analogue Reduces Inflammation and Keratinocyte Proliferation in a Psoriasis Mouse Model. Exp. Dermatol. 2021, 30, 1662–1674. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.H.; Ramadan, A.E.R.G.; Alsanory, T.A.; Herdan, M.O.; Fathallah, O.M.; Alsanory, A.A. Experimental and Therapeutic Trials of Amygdalin. Int. J. Biochem. Pharmacol. 2019, 1, 21–26. [Google Scholar] [CrossRef]
- De Leo, M.; Iannuzzi, A.M.; Germanò, M.P.; D’Angelo, V.; Camangi, F.; Sevi, F.; Diretto, G.; De Tommasi, N.; Braca, A. Comparative Chemical Analysis of Six Ancient Italian Sweet Cherry (Prunus avium L.) Varieties Showing Antiangiogenic Activity. Food Chem. 2021, 360, 129999. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of Grape (Vitis vinifera L.) Seed Oil Production as a Valuable Source of Phenolic Antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Kim, D.O.; Ho, J.H.; Young, J.K.; Hyun, S.Y.; Lee, C.Y. Sweet and Sour Cherry Phenolics and Their Protective Effects on Neuronal Cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- Liana, L.; Rizal, R.; Widowati, W.; Khainir Akbar, F.; Fachrial, E.; Ehrich, N. Antioxidant and Anti-Hyaluronidase Activities of Dragon Fruit Peel Extract and Kaempferol-3-O-Rutinoside Aktivitas Antioksidan Dan Anti-Hialuronidase Ekstrak Kulit Buah Naga Dan Kaempferol-3-O-Rutinoside. J. Kedokt. Brawijaya 2019, 30, 247–252. [Google Scholar] [CrossRef]
- Yu, M.H.; Hung, T.W.; Wang, C.C.; Wu, S.W.; Yang, T.W.; Yang, C.Y.; Tseng, T.H.; Wang, C.J. Neochlorogenic Acid Attenuates Hepatic Lipid Accumulation and Inflammation via Regulating Mir-34a in Vitro. Int. J. Mol. Sci. 2021, 22, 3163. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, P.; Deepak, D.; Rai, K. Quercetin: A Versatile Flavonoid. Internet J. Med. Update 2007, 2, 22–37. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The Potential Effects of Chlorogenic Acid, the Main Phenolic Components in Coffee, on Health: A Comprehensive Review of the Literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Álvarez-Cilleros, D.; Martín, M.Á.; Ramos, S. (-)-Epicatechin and the Colonic 2,3-Dihydroxybenzoic Acid Metabolite Regulate Glucose Uptake, Glucose Production, and Improve Insulin Signaling in Renal NRK-52E Cells. Mol. Nutr. Food Res. 2018, 62, 470. [Google Scholar] [CrossRef]
- Ingole, A.; Kadam, M.P.; Dalu, A.P.; Kute, S.M.; Mange, P.R.; Theng, V.D.; Lahane, O.R.; Nikas, A.P.; Kawal, Y.V.; Nagrik, S.U.; et al. A Review of the Pharmacological Characteristics of Vanillic Acid. J. Drug Deliv. Ther. 2021, 11, 200–204. [Google Scholar] [CrossRef]
- Czyzowska, A.; Pogorzelski, E. Changes to Polyphenols in the Process of Production of Must and Wines from Blackcurrants and Cherries. Part I. Total Polyphenols and Phenolic Acids. Eur. Food Res. Technol. 2002, 214, 148–154. [Google Scholar] [CrossRef]
- Marinova, E.M.; Yanishlieva, N. V Antioxidant Activity and Mechanism of Action of Some Phenolic Acids at Ambient and High Temperatures. Food Chem. 2003, 81, 189–197. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial Polyphenols as Potential Natural Food Preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.; Kamel, G.; Shokry, A.A.; El-Shiekh, R.A. A Potent Anti-Ageing and Immunomodulatory Activity of Apricot Seed Standardized Extract and Its Major Compound; Amygdalin. Trop. J. Nat. Prod. Res. 2020, 4, 728–733. [Google Scholar] [CrossRef]



| Experiment | Factors | ||
|---|---|---|---|
| Sample–Dispersant Ratio (w/w) | Ethanol–Water Ratio (v/v) | Vortex Stirring Time (min) | |
| 1 | 1/1 (−1) | 20:80 (−1) | 5 (−1) |
| 2 | 1/5 (1) | 20:80 (−1) | 5 (−1) |
| 3 | 1/1 (−1) | 80:20 (1) | 5 (−1) |
| 4 | 1/1 (−1) | 20:80 (−1) | 10 (1) |
| 5 | 1/3 (0) | 50:50 (0) | 7.5 (0) |
| 6 | 1/5 (1) | 20:80 (−1) | 10 (1) |
| 7 | 1/3 (0) | 50:50 (0) | 7.5 (0) |
| 8 | 1/5 (1) | 80:20 (1) | 10 (1) |
| 9 | 1/1 (−1) | 80:20 (1) | 10 (1) |
| 10 | 1/5 (1) | 80:20 (1) | 10 (1) |
| 11 | 1/3 (0) | 50:50 (0) | 7.5 (0) |
| Experiment | Factors | Responses | ||||
|---|---|---|---|---|---|---|
| Sample–Dispersant Ratio (w/w) | Ethanol–Water Ratio (v/v) | Vortex Stirring Time (min) | TPC (mg GAE·g−1) | TFC (mg QE·g−1) | DPPHIC50 (mg·g−1) | |
| 1 | 1/1 (−1) | 20:80 (−1) | 5 (−1) | 0.344 | 0.741 | 5.122 |
| 2 | 1/5 (1) | 20:80 (−1) | 5 (−1) | 0.441 | 0.157 | 4.369 |
| 3 | 1/1 (−1) | 80:20 (1) | 5 (−1) | 1.078 | 1.588 | 10.816 |
| 4 | 1/1 (−1) | 20:80 (−1) | 10 (1) | 0.358 | 0.480 | 8.191 |
| 5 | 1/3 (0) | 50:50 (0) | 7.5 (0) | 0.494 | 1.854 | 4.389 |
| 6 | 1/5 (1) | 20:80 (−1) | 10 (1) | 0.341 | 0.545 | 7.485 |
| 7 | 1/3 (0) | 50:50 (0) | 7.5 (0) | 0.450 | 1.497 | 4.372 |
| 8 | 1/5 (1) | 80:20 (1) | 10 (1) | 1.118 | 1.812 | 7.190 |
| 9 | 1/1 (−1) | 80:20 (1) | 10 (1) | 1.272 | 4.451 | 7.734 |
| 10 | 1/5 (1) | 80:20 (1) | 10 (1) | 1.171 | 3.013 | 8.184 |
| 11 | 1/3 (0) | 50:50 (0) | 7.5 (0) | 0.445 | 1.357 | 4.331 |
| Responses | Coefficients | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | SEE | ||||||||
| TPC (mg GAE·g−1) | 0.7654 | 0.0024 | 0.3944 | 0.0201 | −0.0176 | −0.0319 | 0.0416 | 0.999 | 0.009 |
| TFC (mg QE·g−1) | 1.5984 | −0.2166 | 1.1176 | 0.5239 | −0.0869 | −0.1266 | 0.4921 | 0.956 | 0.817 |
| DPPH IC50 (mg·g−1) | 7.3865 | −0.5793 | 1.0946 | 0.5123 | −0.2146 | 0.5155 | −1.0341 | 0.926 | 1.424 |
| Experimental Responses | Experimental Factors | ||
|---|---|---|---|
| Sample–Dispersant Ratio (w/w) | Ethanol–Water Ratio (v/v) | Vortex Stirring Time (min) | |
| RSA | |||
| TPC (mg GAE·g−1) | 1/1 | 80:20 | 10 |
| TFC (mg QE·g−1) | 1/1 | 80:20 | 10 |
| DPPH IC50 (mg·g−1) | 1/5 | 20:80 | 5 |
| MRA | 1/1 | 80:20 | 10 |
| Experimental Responses | Experimental Factors | Responses | Comparison | |||
|---|---|---|---|---|---|---|
| Sample–Dispersant Ratio (w/w) | Ethanol–Water Ratio (v/v) | Vortex Stirring Time (min) | Predicted | Experimental (Mean ± SD) | tcalc | |
| TPC (mg GAE·g−1) | 1/1 | 80:20 | 10 | 1.268 | 1.37 ± 0.08 | 2.08 |
| TFC (mg QE·g−1) | 4.162 | 3 ± 1 | 2.44 | |||
| DPPH IC50 (mg·g−1) | 8.237 | 7.4 ± 0.3 | 5.22 | |||
| Peak Number | Compound | RT (min) | Molecular Formula | [M-H]− (m/z) | MS/MS Fragments (m/z) |
|---|---|---|---|---|---|
| 1 | Gallic acid a | 2.7 | C7H6O5 | 169.1128 | 125.0306; 97.0302; 79.0243; 51.0305 |
| 2 | 2,3-Dihydroxybenzoic acid a | 4.9 | C7H6O4 | 153.1138 | 125.5103; 109.0303; 91.0201; 65.006 |
| 3 | Neochlorogenic acid b | 4.9 | C16H18O9 | 353.0879 | 191.0433; 179.0335; 161.1005; 135.0433; 107.0429 |
| 4 | Chlorogenic acid a | 7.8 | C16H18O9 | 353.3015 | 319.6037; 257.5812, 191.0613; 161.0299; 85.0372 |
| 5 | Vanillic acid a | 9.2 | C8H8O4 | 167.0351 | 151.0007; 108.0227; 91.0128; 80.5217 |
| 6 | Caffeic acid a | 9.6 | C9H8O4 | 179.1502 | 135.0469; 117.0299, 89.0406; 65.0374 |
| 7 | Syringic acid a | 9.7 | C9H10O5 | 197,0456 | 181.0194; 167.0176; 151.9915; 138.0294; 123.0072; 95.0121 |
| 8 | p-Coumaric acid a | 13.8 | C9H8O3 | 163.1508 | 119.0516; 93.0364; 65.0359 |
| 9 | trans-Ferulic acid a | 15.2 | C10H10O4 | 193.1768 | 150.0655; 134.0400; 106.0488; 89.0389 |
| 10 | Kaempferol 3-rutinoside b | 17.7 | C27H30O15 | 593.1513 | 423.0438; 367.7453; 285.0372; 195.1208; 61.9900 |
| 11 | Isorhamnetin 3-rutinoside c | 17.9 | C28H32O16 | 623.1618 | 577.2840; 315.0504; 300.0258; 271.6939; 243.0098 |
| 12 | Quercetin a | 27.0 | C15H10O7 | 301.2285 | 273.0425; 229.0516; 179.0033; 151.0081; 121.0317 |
| 13 | Kaempferol a | 32.6 | C15H10O6 | 285.2291 | 255.0218; 229.0400; 185.0529; 151.0335; 93.0308 |
| Compound | Initial Seed (µg·g−1) | Seed after Fermentation (µg·g−1) | Seed after Both Fermentation and Distillation (µg·g−1) |
|---|---|---|---|
| 2,3-Dihydroxybenzoic acid A | 38 ± 3 a | 64 ± 8 b | 84 ± 9 b |
| Neochlorogenic acid B | 36 ± 1 a | 12 ± 4 b | 3.9 ± 0.4 c |
| Chlorogenic acid B | 2.2 ± 0.4 a | 7.7 ± 0.5 b | 1.7 ± 0.8 a |
| Vanillic acid A | n.q. | 1.2 ± 0.3 a | 1.7 ± 0.2 a |
| Caffeic acid B | 25.4 ± 0.4 a | 13.5 ± 0.5 b | 3 ± 1 c |
| Syringic acid A | n.d. | n.d. | n.q. |
| p-Coumaric acid C | n.d. | 2.7 ± 0.4 a | 1.8 ± 0.1 b |
| trans-Ferulic acid D | n.q. | n.d. | n.d. |
| Kaempferol 3-rutinoside E | 0.27 ± 0.03 a | n.d. | n.d. |
| Isorhamnetin 3-rutinoside F | 0.48 ± 0.03 a | 0.015 ± 0.001 b | 0.035 ± 0.005 b |
| Quercetin F | 16 ± 2 a | 8 ± 2 b | 5.8 ± 0.1 b |
| Kaempferol E | 2.1 ± 0.1 a | 0.95 ± 0.02 b | 2.2 ± 0.1 a |
| Total phenolic acids | 78 ± 4 a | 92 ± 3 a | 96 ± 9 a |
| Total flavonoids | 16 ± 2 a | 8 ± 2 b | 5.8 ± 0.1 b |
| Total phenolics | 96 ± 7 a | 100 ± 4 a | 104 ± 9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Blázquez, S.; Fernández-Ávila, L.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Miranda, R. Valorization of Defatted Cherry Seed Residues from Liquor Processing by Matrix Solid-Phase Dispersion Extraction: A Sustainable Strategy for Production of Phenolic-Rich Extracts with Antioxidant Potential. Antioxidants 2023, 12, 2041. https://doi.org/10.3390/antiox12122041
Rodríguez-Blázquez S, Fernández-Ávila L, Gómez-Mejía E, Rosales-Conrado N, León-González ME, Miranda R. Valorization of Defatted Cherry Seed Residues from Liquor Processing by Matrix Solid-Phase Dispersion Extraction: A Sustainable Strategy for Production of Phenolic-Rich Extracts with Antioxidant Potential. Antioxidants. 2023; 12(12):2041. https://doi.org/10.3390/antiox12122041
Chicago/Turabian StyleRodríguez-Blázquez, Sandra, Lorena Fernández-Ávila, Esther Gómez-Mejía, Noelia Rosales-Conrado, María Eugenia León-González, and Rubén Miranda. 2023. "Valorization of Defatted Cherry Seed Residues from Liquor Processing by Matrix Solid-Phase Dispersion Extraction: A Sustainable Strategy for Production of Phenolic-Rich Extracts with Antioxidant Potential" Antioxidants 12, no. 12: 2041. https://doi.org/10.3390/antiox12122041