Scanning Electron Microscopy and Triple TOF-LC-MS-MS Analysis of Polyphenols from PEF-Treated Edible Mushrooms (L. edodes, A. brunnescens, and P. ostreatus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Sample Preparation
2.3. Pulsed Electric Field-Assisted Extraction
2.4. Chemical Analysis
2.4.1. Total Antioxidant Capacity
2.4.2. Polyphenol Analyses
2.5. Scanning Electron Microscopy
2.6. Statistical Analysis
3. Results and Discussion
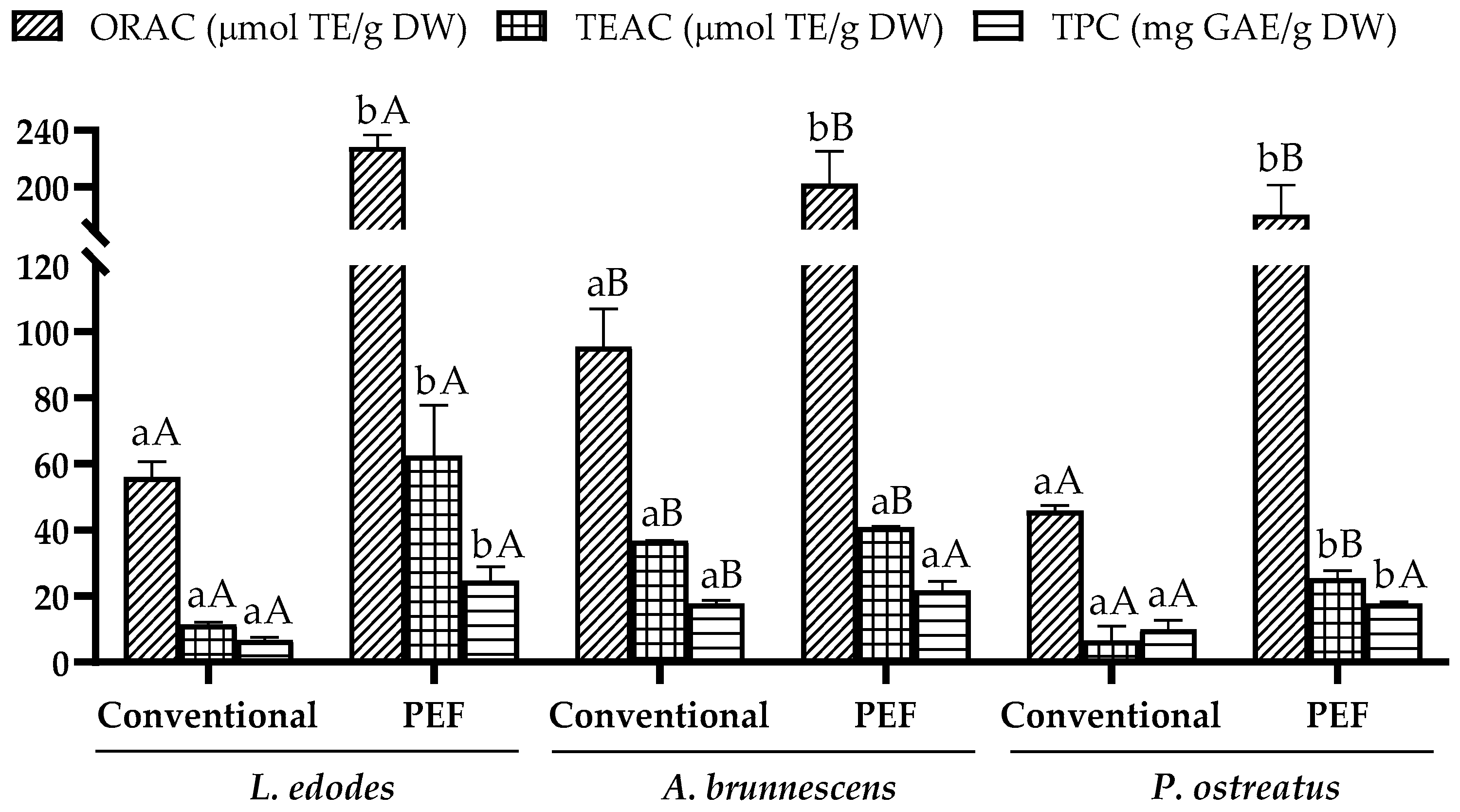
3.1. Extraction of Total Antioxidant Compounds under PEF Optimal Conditions
| Extraction | Mushroom | Treatment | ORAC | TEAC | TPC | Ref. |
|---|---|---|---|---|---|---|
| Conv. (Water) | Lentinula edodes Pleurotus ostreatus Agaricus brunnescens | 200 mL water, 25 °C, 6 h stirring | 56.1 µmol TE/g DW 45.99 µmol TE/g DW 95.44 µmol TE/g DW | 11.5 µmol TE/g DW 6.67 µmol TE/g DW 36.71 µmol TE/g DW | 6.71 mg GAE/g DW 9.99 mg GAE/g DW 17.86 mg GAE/g DW | Present study |
| Agaricus bisporus | 200 mL, 9% (w/w), 95 °C, 1 h | - | - | 1.3 mg GAE/g mushroom | [26] | |
| Lentinula edodes | 1 L, 80% (v/v), 80°C, 30 min | - | 290 µmol/g DW | - | [33] | |
| Conv. (Methanol) | Morchella elata | 300 mL (1:3 w/v) 3500 r/min, 30 min + 24 h | - | 240 µmol TE/g extract | 0.46 mg GAE/g DW | [34] |
| Suillus luteus | - | 154 µmol TE/g extract | 0.67 mg GAE/g DW | |||
| Pleurotus eryngii | - | 67.0 µmol TE/g extract | 0.180 mg GAE/g DW | |||
| Cyttaria gunnii | - | 77.0 µmol TE/g extract | 0.200 mg GAE/g DW | |||
| Flammulina velutipes | - | 221 µmol TE/g extract | 0.283 mg GAE/g DW | |||
| Lentinula edodes | 100 mL (1:20 w/v), 25 °C, 6 h | - | - | 5.06 mg GAE/g DE | [35] | |
| Lentinula edodes | 1 mL (80% v/v), 80 °C, 30 min | - | 0.28 µmol/g DW | - | [33] | |
| Pleurotusostreatus | 100 mL (80% v/v), 8 h | - | - | 9.64 mg/g extract | [36] | |
| Pleurotus eryngii | - | - | 7.91 mg/g extract | |||
| Conv. (Ethanol) | Agaricus brasiliensis Agaricus campestris Agaricus silvaticus Agaricus bisporus | Ethanol 70%, 3 h shaking, room temperature | - | - | 1154.7 mg GAE/100 g DW 767.3 mg GAE/100 g DW 638.3 mg GAE/100 g DW 132.7 mg GAE/100 g DW | [27] |
| PEF | Lentinula edodes Pleurotus ostreatus Agaricus brunnescens | 200 mL water, specific energy 50 kJ/kg, 2.5 kV/cm + 6 h stirring | 228.07 µmol TE/g DW 180.41 µmol TE/g DW 202.18 µmol TE/g DW | 62.50 µmol TE/g DW 25.33 µmol TE/g DW 41.01 µmol TE/g DW | 24.68 mg GAE/g DW 17.69 µmol TE/g DW 21.68 µmol TE/g DW | Present study |
| Agaricus bisporus | Specific energy 50 kJ/kg 2.5 and 3 kV/cm (TPC) + 5.6 h in water | 161.41 µmol TE/g DW | 67.94 µmol TE/g DW | 22.16 mg GAE/g DW | [15] | |
| 38.4 kV/cm; 85 °C | - | - | 1.6 mg GAE/g mushroom | [26] | ||
| MAE | Terfezia boudieri | Methanol 80%, 80 °C, 5 min | - | - | 182.2 µmol TE/g | [37] |
| Boletus edulis | - | - | 357.7 µmol TE/g | |||
| Lactarius volemu | - | - | 230.2 µmol TE/g | |||
| USN | Boletus edulis | 50% ethanol 45 °C, 40 min | - | - | 41.82 mg GAE/g | [28] |
| Boletus auranticus | - | - | 36.43 mg GAE/g |
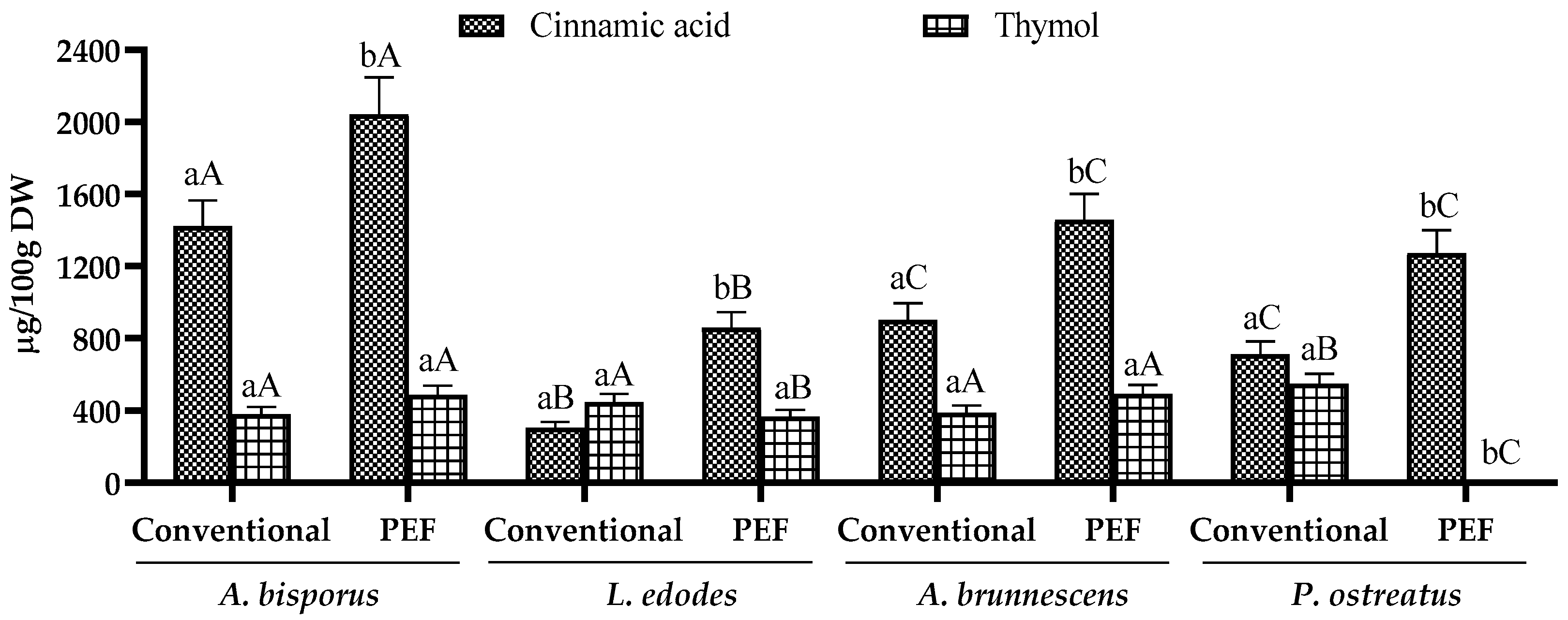
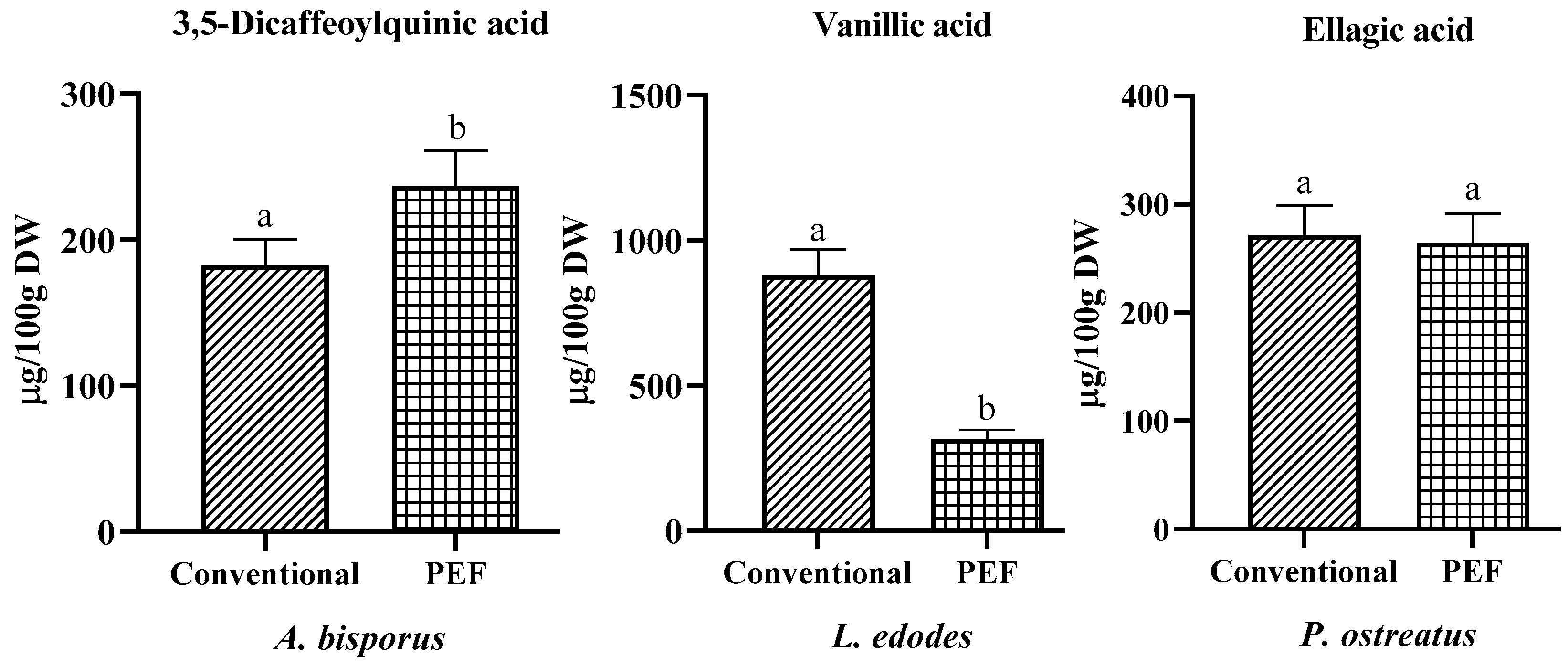
3.2. Phenolic Profile
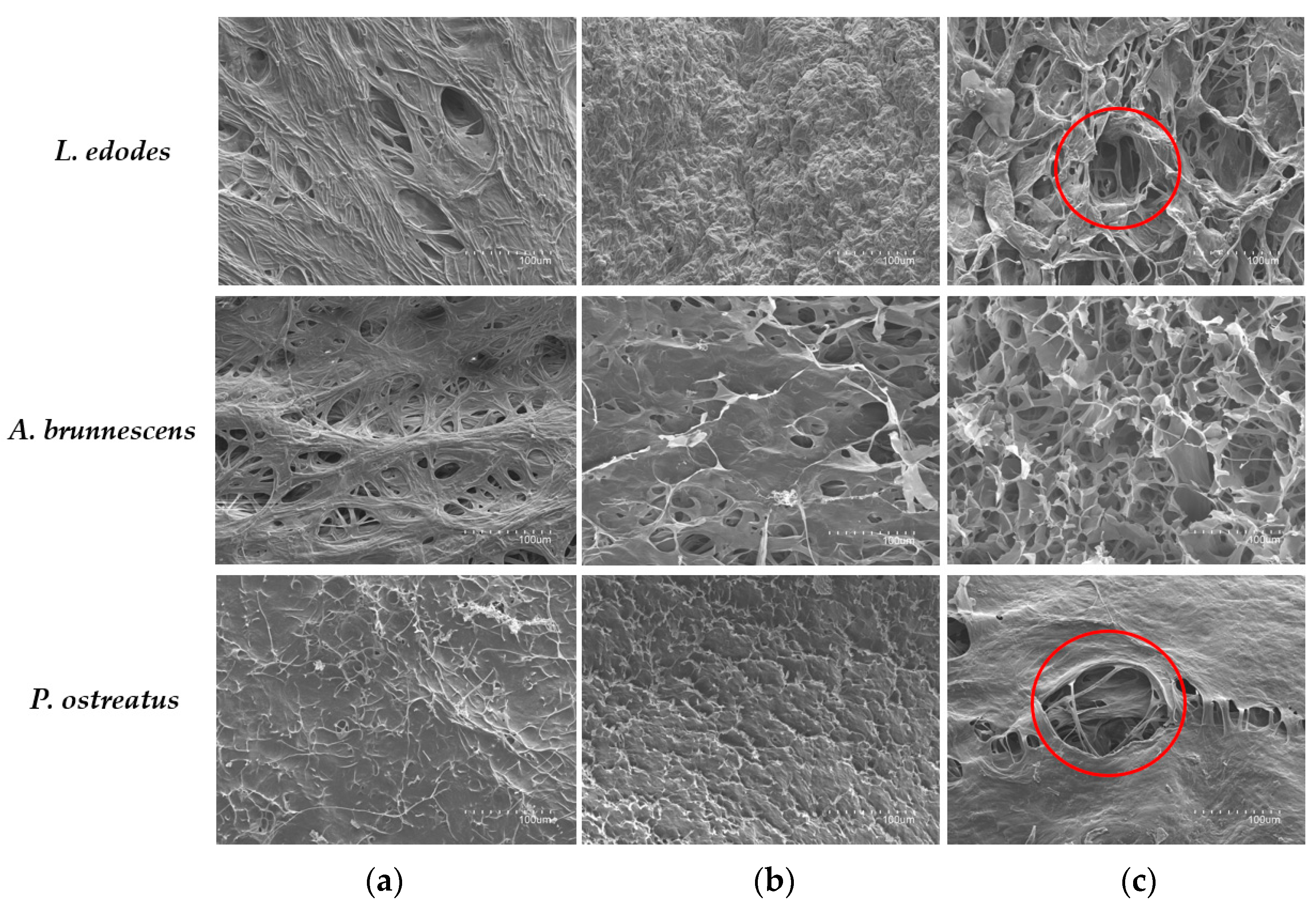
3.3. Evaluation of Mushroom Surface after PEF Pre-Treatment by Scanning Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front. Immunol. 2014, 5, 116722. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; De Cosmi, V.; Risé, P.; Milani, G.P.; Turolo, S.; Syrén, M.L.; Sala, A.; Agostoni, C. bioactive compounds in edible oils and their role in oxidative stress and inflammation. Front. Physiol. 2021, 12, 659551. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhu, Y.C.; Lu, J.; Gu, N. Effects of traditional chinese medication-based bioactive compounds on cellular and molecular mechanisms of oxidative stress. Oxid. Med. Cell. Longev. 2021, 2021, 3617498. [Google Scholar] [CrossRef] [PubMed]
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Singh, M.; Kamal, S.; Sharma, V.P. Status and trends in world mushroom production-III -World production of different mushroom species in 21st century. Mushroom Res. 2021, 26, 1–20. [Google Scholar] [CrossRef]
- De Cianni, R.; Pippinato, L.; Mancuso, T. A Systematic review on drivers influencing consumption of edible mushrooms and innovative mushroom containing products. Appetite 2023, 182, 106454. [Google Scholar] [CrossRef] [PubMed]
- Interreg Europe. Mushroom Sector as Part of Circular Economy. Available online: https://www.interregeurope.eu/good-practices/mushroom-sector-as-part-of-circular-economy (accessed on 17 May 2023).
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Mujić, I.; Zeković, Z.; Lepojević, Ž.; Vidović, S.; Živković, J. Antioxidant properties of selected edible mushroom species. JCEA 2010, 11, 387–392. [Google Scholar]
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R.M.V. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef]
- Jayakumar, T.; Thomas, P.A.; Sheu, J.R.; Geraldine, P. In-vitro and in-vivo antioxidant effects of the oyster mushroom Pleurotus ostreatus. Food Res. Int. 2011, 44, 851–861. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Pavo-Caballero, C.; Baeza, E.; Baenas, N.; Garcia-Viguera, C.; Marín, F.R.; Soler-Rivas, C. Mushrooms do not contain flavonoids. J. Funct. Foods 2016, 25, 1–13. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N.; et al. Application of non-conventional extraction methods: Toward a sustainable and green production of valuable compounds from mushrooms. Food Eng. Rev. 2016, 8, 214–234. [Google Scholar] [CrossRef]
- Calleja-Gómez, M.; Castagnini, J.M.; Carbó, E.; Ferrer, E.; Berrada, H.; Barba, F.J. Evaluation of pulsed electric field-assisted extraction on the microstructure and recovery of nutrients and bioactive compounds from mushroom (Agaricus bisporus). Separations 2022, 9, 302. [Google Scholar] [CrossRef]
- Parniakov, O.; Lebovka, N.I.; Van Hecke, E.; Vorobiev, E. Pulsed electric field assisted pressure extraction and solvent extraction from mushroom (Agaricus bisporus). Food Bioproc. Tech. 2014, 7, 174–183. [Google Scholar] [CrossRef]
- Nowacka, M.; Tappi, S.; Wiktor, A.; Rybak, K.; Miszczykowska, A.; Czyzewski, J.; Drozdzal, K.; Witrowa-Rajchert, D.; Tylewicz, U. The impact of pulsed electric field on the extraction of bioactive compounds from beetroot. Foods 2019, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Al Jbawi, E.A.; Saewan, S. Traditional and innovative approaches for the extraction of bioactive compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Collado, M.C.; Barba, F.J. Accelerated solvent extraction and pulsed electric fields for valorization of rainbow trout (Oncorhynchus mykiss) and sole (Dover sole) by-products: Protein content, molecular weight distribution and antioxidant potential of the extracts. Mar. Drugs 2021, 19, 207. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Martí-Quijal, F.J.; Cilla, A.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F.; Barba, F.J. Influence of temperature, solvent and pH on the selective extraction of phenolic compounds from tiger nuts by-products: Triple-TOF-LC-MS-MS characterization. Molecules 2019, 24, 797. [Google Scholar] [CrossRef] [PubMed]
- Redondo, D.; Venturini, M.E.; Luengo, E.; Raso, J.; Arias, E. Pulsed electric fields as a green technology for the extraction of bioactive compounds from thinned peach by-products. Innov. Food Sci. Emerg. Technol. 2018, 45, 335–343. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Castagnini, J.M.; Ruiz, M.J.; Barba, F.J. Sea Bass Side Streams Extracts Obtained by Pulsed Electric Fields: Nutritional Characterization and Effect on SH-SY5Y Cells. Foods 2023, 12, 2717. [Google Scholar] [CrossRef]
- Xue, D.; Farid, M.M. Pulsed electric field extraction of valuable compounds from white button mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2015, 29, 178–186. [Google Scholar] [CrossRef]
- Gąsecka, M.; Magdziak, Z.; Siwulski, M.; Mleczek, M. Profile of phenolic and organic acids, antioxidant properties and ergosterol content in cultivated and wild growing species of Agaricus. Eur. Food Res. Technol. 2018, 244, 259–268. [Google Scholar] [CrossRef]
- Vidović, S.S.; Mujić, I.O.; Zeković, Z.P.; Lepojević, Ž.D.; Tumbas, V.T.; Mujić, A.I. Antioxidant properties of selected Boletus mushrooms. Food Biophys. 2010, 5, 49–58. [Google Scholar] [CrossRef]
- Barba, F.J.; Galanakis, C.M.; Esteve, M.J.; Frigola, A.; Vorobiev, E. Potential use of pulsed electric technologies and ultrasounds to improve the recovery of highadded value compounds from blackberries. J. Food Eng. 2015, 167, 38–44. [Google Scholar] [CrossRef]
- Anticona, M.; Blesa, J.; Frigola, A.; Esteve, M.J. High biological value compounds extraction from citrus waste with non-conventional methods. Foods 2020, 9, 811. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Wu, X.J.; Hansen, C. Antioxidant capacity, phenolic content, and polysaccharide content of Lentinus edodes grown in whey permeate-based submerged culture. J. Food Sci. 2008, 73, M1–M8. [Google Scholar] [CrossRef]
- Zeng, X.; Suwandi, J.; Fuller, J.; Doronila, A.; Ng, K. Antioxidant capacity and mineral contents of edible wild Australian mushrooms. Food Sci. Technol. Int. 2012, 18, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.; Choi, J.Y.; Kim, M.S. Metabolic profiles, bioactive compounds, and antioxidant capacity in Lentinula edodes cultivated on log versus sawdust substrates. Biomolecules 2021, 11, 1654. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur. Food Res. Technol. 2016, 242, 723–732. [Google Scholar] [CrossRef]
- Özyürek, M.; Bener, M.; Güçlü, K.; Apak, R. Antioxidant/antiradical properties of microwave-assisted extracts of three wild edible mushrooms. Food Chem. 2014, 157, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.; Zielinski, A.A.F.; Helm, C.V.; Maciel, G.M.; Pedro, A.C.; Stafussa, A.P.; Ávila, S.; Haminiuk, C.W.I. Bio compounds of edible mushrooms: In vitro antioxidant and antimicrobial activities. LWT 2019, 107, 214–220. [Google Scholar] [CrossRef]
- Sabino Ferrari, A.B.; Azevedo de Oliveira, G.; Mannochio Russo, H.; de Carvalho Bertozo, L.; da Silva Bolzani, V.; Cunha Zied, D.; Farias Ximenes, V.; Zeraik, M.L. Pleurotus ostreatus and Agaricus subrufescens: Investigation of chemical composition and antioxidant properties of these mushrooms cultivated with different handmade and commercial supplements. Int. J. Food Sci. Technol. 2021, 56, 452–460. [Google Scholar] [CrossRef]
- Gogoi, P.; Chutia, P.; Singh, P.; Mahanta, C.L. Effect of optimized ultrasoundassisted aqueous and ethanolic extraction of Pleurotus citrinopileatus mushroom on total phenol, flavonoids and antioxidant properties. J. Food Process Eng. 2019, 42, e13172. [Google Scholar] [CrossRef]
- de Souza Campos Junior, F.A.; Petrarca, M.H.; Meinhart, A.D.; de Jesus Filho, M.; Godoy, H.T. Multivariate optimization of extraction and validation of phenolic acids in edible mushrooms by capillary electrophoresis. Food Res. Int. 2019, 126, 108685. [Google Scholar] [CrossRef]
- Contato, A.G.; Inácio, F.D.; de Araújo, C.A.V.; Brugnari, T.; Maciel, G.M.; Haminiuk, C.W.I.; Bracht, A.; Peralta, R.M.; de Souza, C.G.M. Comparison between the aqueous extracts of mycelium and basidioma of the edible mushroom Pleurotus pulmonarius: Chemical composition and antioxidant analysis. J. Food Meas. Charact. 2020, 14, 830–837. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A comprehensive review on phenolic compounds from edible mushrooms: Occurrence, biological activity, application and future prospective. Crit. Rev. Food Sci. Nutr. 2021, 62, 6204–6224. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, S.; Dalar, A.; Uzun, Y. Determination of antioxidant activities and chemical composition of sequential fractions of five edible mushrooms from Turkey. J. Food Sci. Technol. 2020, 57, 1866–1876. [Google Scholar] [CrossRef] [PubMed]
- Çayan, F.; Deveci, E.; Tel-Çayan, G.; Duru, M.E. Identification and quantification of phenolic acid compounds of twenty-six mushrooms by HPLC–DAD. J. Food Meas. Charact. 2020, 14, 1690–1698. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wang, R.; Rahaman, A.; Zeng, X.A.; Brennan, C.S. Combined effects of pulsed electric field and ultrasound pretreatments on mass transfer and quality of mushrooms. LWT 2021, 150, 112008. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calleja-Gómez, M.; Roig, P.; Rimac Brnčić, S.; Barba, F.J.; Castagnini, J.M. Scanning Electron Microscopy and Triple TOF-LC-MS-MS Analysis of Polyphenols from PEF-Treated Edible Mushrooms (L. edodes, A. brunnescens, and P. ostreatus). Antioxidants 2023, 12, 2080. https://doi.org/10.3390/antiox12122080
Calleja-Gómez M, Roig P, Rimac Brnčić S, Barba FJ, Castagnini JM. Scanning Electron Microscopy and Triple TOF-LC-MS-MS Analysis of Polyphenols from PEF-Treated Edible Mushrooms (L. edodes, A. brunnescens, and P. ostreatus). Antioxidants. 2023; 12(12):2080. https://doi.org/10.3390/antiox12122080
Chicago/Turabian StyleCalleja-Gómez, Mara, Patricia Roig, Suzana Rimac Brnčić, Francisco J. Barba, and Juan Manuel Castagnini. 2023. "Scanning Electron Microscopy and Triple TOF-LC-MS-MS Analysis of Polyphenols from PEF-Treated Edible Mushrooms (L. edodes, A. brunnescens, and P. ostreatus)" Antioxidants 12, no. 12: 2080. https://doi.org/10.3390/antiox12122080








