Sustainable Green Extraction of Carotenoid Pigments: Innovative Technologies and Bio-Based Solvents
Abstract
:1. Introduction
2. Methodology
3. Green Chemistry and Green Extractions: The Concepts
4. Technologies Amenable to Green Extractions
4.1. Ultrasounds
4.2. Microwaves
4.3. Pulsed Electric Fields
4.4. Pressurized Liquid Extraction
4.5. Supercritical Fluid Extraction
4.6. Subcritical Fluid Extraction
4.7. Enzyme-Assisted Extraction
5. Extraction of Carotenoids with Bio-Based Solvents
5.1. Ethyl Lactate
5.2. 2-Methyltetahydrofuran
5.3. Natural Deep Eutectic Solvents and Deep Eutectic Solvents
5.4. Ionic Liquids
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meléndez-Martínez, A.J.; Böhm, V.; Borge, G.I.; Cano, M.P.; Fikselová, M.; Gruskiene, R.; Lavelli, V.; Loizzo, M.R.; Mandić, A.; Mapelli-Brahm, P.; et al. Carotenoids: Considerations for their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals and Novel Foods in the Context of Sustainability, Circular Economy and Climate Change. Annu. Rev. Food Sci. Technol. 2021, 12, 433–460. [Google Scholar] [CrossRef] [PubMed]
- Kolašinac, S.M.; Stevanović, Z.P.D.; Kilibarda, S.N.; Kostić, A. Carotenoids: New applications of “old” pigments. Phyton 2021, 90, 1041–1062. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef] [PubMed]
- Mapelli-Brahm, P.; Barba, F.J.; Remize, F.; Garcia, C.; Fessard, A.; Mousavi Khaneghah, A.; Sant’Ana, A.S.; Lorenzo, J.M.; Montesano, D.; Meléndez-Martínez, A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Technol. 2020, 99, 389–401. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.S.F. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC—Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Nanda, B.; Sailaja, M.; Mohapatra, P.; Pradhan, R.K.; Nanda, B.B. Green solvents: A suitable alternative for sustainable chemistry. Mater. Today Proc. 2021, 47, 1234–1240. [Google Scholar] [CrossRef]
- Soro, A.B.; Garcia-Vaquero, M.; Tiwari, B.K. Equipment and recent advances in ultrasound technology. In Innovative and Emerging Technologies in the Bio-Marine Food Sector: Applications, Regulations, and Prospects; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A critical review on pulsed electric field: A novel technology for the extraction of phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized liquid extraction. In Liquid-Phase Extraction; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Ahmad, T.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gull, A. Supercritical Fluid Extraction: A Review. J. Biol. Chem. Chronicles 2019, 5, 114–122. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical water extraction of natural products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed]
- Majik, M.S.; Gawas, U.B. Recent Advances in Extraction of Natural Compounds. In New Horizons in Natural Compound Research; Elsevier Inc.: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Coelho, T.L.S.; Silva, D.S.N.; dos Santos, J.M., Jr.; Dantas, C.; Nogueira, A.R.d.A.; Lopes Júnior, C.A.; Vieira, E.C. Multivariate optimization and comparison between conventional extraction (CE) and ultrasonic-assisted extraction (UAE) of carotenoid extraction from cashew apple. Ultrason. Sonochem. 2022, 84, 105980. [Google Scholar] [CrossRef] [PubMed]
- Pani, M.; Radoj, I. Using novel hydrophobic deep eutectic solvents to improve a sustainable carotenoid extraction from orange peels. Food Biosci. 2023, 53, 102570. [Google Scholar] [CrossRef]
- Nie, J.; Chen, D.; Ye, J.; Lu, Y.; Dai, Z. Optimization and kinetic modeling of ultrasonic-assisted extraction of fucoxanthin from edible brown algae Sargassum fusiforme using green solvents. Ultrason. Sonochem. 2021, 77, 105671. [Google Scholar] [CrossRef]
- Song, J.; Yang, Q.; Huang, W.; Xiao, Y.; Li, D.; Liu, C. Optimization of trans lutein from pumpkin (Cucurbita moschata) peel by ultrasound-assisted extraction. Food Bioprod. Process. 2018, 107, 104–112. [Google Scholar] [CrossRef]
- Benmeziane, A.; Boulekbache-Makhlouf, L.; Mapelli-Brahm, P.; Khaled Khodja, N.; Remini, H.; Madani, K.; Meléndez-Martínez, A.J. Extraction of carotenoids from cantaloupe waste and determination of its mineral composition. Food Res. Int. 2018, 111, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.S.N.; Silva, M.d.S.; Coelho, T.L.S.; Dantas, C.; Lopes Júnior, C.A.; Caldas, N.M.; Vieira, E.C. Combining high intensity ultrasound and experimental design to improve carotenoid extraction efficiency from Buriti (Mauritia flexuosa). Ultrason. Sonochem. 2022, 88, 106076. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Jabbar, S.; Nasiru, M.M.; Lu, Z.; Zhang, J.; Abid, M.; Murtaza, M.A.; Kieliszek, M.; Zhao, L. Ultrasound-assisted extraction of carotenoids from carrot pomace and their optimization through response surface methodology. Molecules 2021, 26, 6763. [Google Scholar] [CrossRef] [PubMed]
- Morón-Ortiz, A.; Mapelli-brahm, P.; León-Vaz, A.; Benítez-González, A.M.; Meléndez-Martínez, A.J.; León, R. Ultrasound-assisted extraction of carotenoids from phytoene-accumulating Chlorella sorokiniana microalgae: Effect of milling and performance of the green biosolvents 2-methyltetrahydrofuran and ethyl lactate. Food Chem. 2024, 434, 137437. [Google Scholar] [CrossRef]
- Mary Leema, J.T.; Persia Jothy, T.; Dharani, G. Rapid green microwave assisted extraction of lutein from Chlorella sorokiniana (NIOT-2)—Process optimization. Food Chem. 2022, 372, 131151. [Google Scholar] [CrossRef] [PubMed]
- Elik, A.; Yanık, D.K.; Göğüş, F. Microwave-assisted extraction of carotenoids from carrot juice processing waste using flaxseed oil as a solvent. Lwt 2020, 123, 109100. [Google Scholar] [CrossRef]
- Tsiaka, T.; Zoumpoulakis, P.; Sinanoglou, V.J.; Makris, C.; Heropoulos, G.A.; Calokerinos, A.C. Response surface methodology toward the optimization of high-energy carotenoid extraction from Aristeus antennatus shrimp. Anal. Chim. Acta 2015, 877, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Delso, C.; Angulo, J.; Álvarez, I.; Raso, J. Pulsed electric field-assisted extraction of carotenoids from fresh biomass of Rhodotorula glutinis. Innov. Food Sci. Emerg. Technol. 2018, 47, 421–427. [Google Scholar] [CrossRef]
- Martínez, J.M.; Gojkovic, Z.; Ferro, L.; Maza, M.; Álvarez, I.; Raso, J.; Funk, C. Use of pulsed electric field permeabilization to extract astaxanthin from the Nordic microalga Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121694. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving Carotenoid Extraction from Tomato Waste by Pulsed Electric Fields. Front. Nutr. 2014, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Luengo, E.; Condón-Abanto, S.; Álvarez, I.; Raso, J. Effect of Pulsed Electric Field Treatments on Permeabilization and Extraction of Pigments from Chlorella vulgaris. J. Membr. Biol. 2014, 247, 1269–1277. [Google Scholar] [CrossRef]
- De Aguiar Saldanha Pinheiro, A.C.; Martí-Quijal, F.J.; Barba, F.J.; Benítez-González, A.M.; Meléndez-Martínez, A.J.; Castagnini, J.M.; Tappi, S.; Rocculi, P. Pulsed Electric Fields (PEF) and Accelerated Solvent Extraction (ASE) for Valorization of Red (Aristeus antennatus) and Camarote (Melicertus kerathurus) Shrimp Side Streams: Antioxidant and HPLC Evaluation of the Carotenoid Astaxanthin Recovery. Antioxidants 2023, 12, 406. [Google Scholar] [CrossRef]
- Wang, M.; Morón-ortiz, Á.; Zhou, J.; Benítez-gonzález, A.; Mapelli-brahm, P.; Meléndez-martínez, A.J.; Barba, F.J. Effects of Pressurized Liquid Extraction with dimethyl sulfoxide on the recovery of carotenoids and other dietary valuable compounds from the microalgae Spirulina, Chlorella and Phaeodactylum tricornutum. Food Chem. 2022, 405, 134885. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, M.; Berrada, H.; Zhu, Z.; Grimi, N.; Barba, F.J. Pulsed electric fields (PEF), pressurized liquid extraction (PLE) and combined PEF + PLE process evaluation: Effects on Spirulina microstructure, biomolecules recovery and Triple TOF-LC-MS-MS polyphenol composition. Innov. Food Sci. Emerg. Technol. 2022, 77, 102989. [Google Scholar] [CrossRef]
- Derwenskus, F.; Metz, F.; Gille, A.; Schmid-Staiger, U.; Briviba, K.; Schließmann, U.; Hirth, T. Pressurized extraction of unsaturated fatty acids and carotenoids from wet Chlorella vulgaris and Phaeodactylum tricornutum biomass using subcritical liquids. GCB Bioenergy 2019, 11, 335–344. [Google Scholar] [CrossRef]
- Nunes, A.N.; Roda, A.; Gouveia, L.F.; Fernández, N.; Bronze, M.R.; Matias, A.A. Astaxanthin Extraction from Marine Crustacean Waste Streams: An Integrate Approach between Microwaves and Supercritical Fluids. ACS Sustain. Chem. Eng. 2021, 9, 3050–3059. [Google Scholar] [CrossRef]
- Georgiopoulou, I.; Tzima, S. applied sciences Process Optimization of Microwave-Assisted Extraction of Chlorophyll, Carotenoid and Phenolic Compounds from Chlorella vulgaris and Comparison with Conventional and Supercritical Fluid Extraction. Appl. Sci. 2023, 13, 2740. [Google Scholar] [CrossRef]
- Lad, J.D.; Kar, A. Supercritical CO2 extraction of lycopene from pink grapefruit (Citrus paradise Macfad) and its degradation studies during storage. Food Chem. 2021, 361, 130113. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Lu, J.; Feng, X.; Han, Y.; Xue, C. Optimization of subcritical fluid extraction of carotenoids and chlorophyll a from Laminaria japonica Aresch by response surface methodology. J. Sci. Food Agric. 2014, 94, 139–145. [Google Scholar] [CrossRef]
- Aslanbay Guler, B.; Deniz, I.; Demirel, Z.; Yesil-Celiktas, O.; Imamoglu, E. A novel subcritical fucoxanthin extraction with a biorefinery approach. Biochem. Eng. J. 2020, 153, 107403. [Google Scholar] [CrossRef]
- Turola Barbi, R.C.; de Souza, A.R.C.; Hamerski, F.; Lopes Teixeira, G.; Corazza, M.L.; Hoffmann Ribani, R. Subcritical propane extraction of high-quality inajá (Maximiliana maripa) pulp oil. J. Supercrit. Fluids 2019, 153, 104576. [Google Scholar] [CrossRef]
- Ghafoor, K.; Sarker, M.Z.I.; Al-Juhaimi, F.Y.; Babiker, E.E.; Alkaltham, M.S.; Almubarak, A.K.; Ahmed, I.A.M. Innovative and Green Extraction Techniques for the Optimal Recovery of Phytochemicals from Saudi Date Fruit Flesh. Processes 2022, 10, 2224. [Google Scholar] [CrossRef]
- Zuorro, A.; Fidaleo, M.; Lavecchia, R. Enzyme-assisted extraction of lycopene from tomato processing waste. Enzyme Microb. Technol. 2011, 49, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Adadi, P.; Barakova, N.V.; Adadi, P. Application of surface response methodology for an enzyme-assisted extraction of carotenoids. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 195. [Google Scholar] [CrossRef]
- Catalkaya, G.; Kahveci, D. Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep. Purif. Technol. 2019, 219, 55–63. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Banakar, V.V.; Sabnis, S.S.; Gogate, P.R.; Raha, A.; Saurabh. Ultrasound assisted continuous processing in microreactors with focus on crystallization and chemical synthesis: A critical review. Chem. Eng. Res. Des. 2022, 182, 273–289. [Google Scholar] [CrossRef]
- Geow, C.H.; Tan, M.C.; Yeap, S.P.; Chin, N.L. A Review on Extraction Techniques and Its Future Applications in Industry. Eur. J. Lipid Sci. Technol. 2021, 123, 2000302. [Google Scholar] [CrossRef]
- Mandal, V.; Hemalatha, S. Microwave Assisted Extraction-An Innovative and Promising Extraction Tool for Medicinal Plant Research Natural product Extraction View project Polyaromatic hydrocarbon Extraction View project. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Puértolas, E.; Luengo, E.; Álvarez, I.; Raso, J. Improving mass transfer to soften tissues by pulsed electric fields: Fundamentals and applications. Annu. Rev. Food Sci. Technol. 2012, 3, 263–282. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.d.P.; Parada-Alonso, F.; Ibáñez, E.; Cifuentes, A. Recent applications of on-line supercritical fluid extraction coupled to advanced analytical techniques for compounds extraction and identification. J. Sep. Sci. 2019, 42, 243–257. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Xu, Y.; Hu, M.; Hu, Z.; Wang, J.; Pan, Z. Reactor for biomass conversion and waste treatment in supercritical water: A review. Renew. Sustain. Energy Rev. 2023, 171, 113031. [Google Scholar] [CrossRef]
- Gupta, A.K.; Seth, K.; Maheshwari, K.; Baroliya, P.K.; Meena, M.; Kumar, A.; Vinayak, V. Harish Biosynthesis and extraction of high-value carotenoid from algae. Front. Biosci.-Landmark 2021, 26, 171–190. [Google Scholar] [CrossRef]
- Essien, S.O.; Young, B.; Baroutian, S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.R.; Tonon, R.V.; Cabral, L.; Gottschalk, L.; Pastrana, L.; Pintado, M.E. Valorization of Agricultural Lignocellulosic Plant Byproducts through Enzymatic and Enzyme-Assisted Extraction of High-Value-Added Compounds: A Review. ACS Sustain. Chem. Eng. 2020, 8, 13112–13125. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Monteagudo-Arrebola, M.J.; Dobado, J.A.; Isac-García, J. Green and Bio-Based Solvents. Top. Curr. Chem. 2018, 376, 18. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wu, J.; Wang, H.; Feng, J.; Zheng, X.; Wang, X.; Wang, S. Bio-derived solvent-based dispersive liquid-liquid microextraction followed by smartphone digital image colorimetry for the detection of carbofuran in cereals. J. Food Compos. Anal. 2022, 114, 104782. [Google Scholar] [CrossRef]
- De Jong, E.; Higson, A.; Walsh, P.; Wellisch, M. Product developments in the bio-based chemicals arena. Biofuels Bioprod. Biorefining 2012, 6, 606–624. [Google Scholar] [CrossRef]
- Perrone, S.; Messa, F.; Salomone, A. Towards Green Reductions in Bio-Derived Solvents. Eur. J. Org. Chem. 2023, 26, e202201494. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Effect of extraction parameters on the carotenoid recovery from tomato waste. Int. J. Food Sci. Technol. 2011, 46, 23–29. [Google Scholar] [CrossRef]
- Kua, Y.L.; Gan, S.; Morris, A.; Ng, H.K. Simultaneous Recovery of Carotenes and Tocols from Crude Palm Olein Using Ethyl Lactate and Ethanol. J. Phys. Conf. Ser. 2018, 989, 012005. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.E.; Ranga, F.; Roman, I.; Khaoula, H.; Boudaya, E.; Ltaief, A.B.; Aouani, W.; Thiamrat, M.; Vodnar, D.C. Carotenoid Recovery from Tomato Processing By-Products through Green Chemistry. Molecules 2022, 27, 3771. [Google Scholar] [CrossRef]
- Ishida, B.K.; Chapman, M.H. Carotenoid extraction from plants using a novel, environmentally friendly solvent. J. Agric. Food Chem. 2009, 57, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, K.; Simińska, D.; Kunicka-Styczyńska, A. Bioactivities and Microbial Quality of Lycium Fruits (Goji) Extracts Derived by Various Solvents and Green Extraction Methods. Molecules 2022, 27, 7856. [Google Scholar] [CrossRef] [PubMed]
- Yara-Varón, E.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Chemat, F. Is it possible to substitute hexane with green solvents for extraction of carotenoids? A theoretical versus experimental solubility study. RSC Adv. 2016, 6, 27750–27759. [Google Scholar] [CrossRef]
- Kumar Kashyap, P.; Singh, S.; Kumar Singh, M.; Gupta, A.; Tandon, S.; Shanker, K.; Kumar Verma, R.; Swaroop Verma, R. An efficient process for the extraction of lutein and chemical characterization of other organic volatiles from marigold (Tagetes erecta L.) flower. Food Chem. 2022, 396, 133647. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, C.; Fabiano-Tixier, A.S.; Claux, O.; Rapinel, V.; Tomao, V.; Stathopoulos, P.; Skaltsounis, A.L.; Tabasso, S.; Jacques, L.; Chemat, F. Higher Yield and Polyphenol Content in Olive Pomace Extracts Using 2-Methyloxolane as Bio-Based Solvent. Foods 2022, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, W.; Liu, L.; Wen, W.; Jing, X.; Wang, X. Switchable deep eutectic solvents for sustainable extraction of β-carotene from millet. Microchem. J. 2023, 187, 108369. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsiouras, A.; Mourtzinos, I. Extraction of Lycopene from Tomato Using Hydrophobic Natural Deep Eutectic Solvents Based on Terpenes and Fatty Acids. Foods 2022, 11, 2645. [Google Scholar] [CrossRef] [PubMed]
- Mussagy, C.U.; Farias, F.O.; Bila, N.M.; Giannini, M.J.S.M.; Pereira, J.F.B.; Santos-Ebinuma, V.C.; Pessoa, A. Recovery of β-carotene and astaxanthin from Phaffia rhodozyma biomass using aqueous solutions of cholinium-based ionic liquids. Sep. Purif. Technol. 2022, 290, 120852. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chong, Y.M.; Chang, W.S.; Yap, J.M.; Foo, S.C.; Khoiroh, I.; Lau, P.L.; Chew, K.W.; Ooi, C.W.; Show, P.L. Permeabilization of Chlorella sorokiniana and extraction of lutein by distillable CO2-based alkyl carbamate ionic liquids. Sep. Purif. Technol. 2021, 256, 117471. [Google Scholar] [CrossRef]
- Paliwal, C.; Rehmanji, M.; Shaikh, K.M.; Zafar, S.U.; Jutur, P.P. Green extraction processing of lutein from Chlorella saccharophila in water-based ionic liquids as a sustainable innovation in algal biorefineries. Algal Res. 2022, 66, 102809. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Román-Ramírez, L.A.; Powders, M.; McKeown, P.; Jones, M.D.; Wood, J. Ethyl Lactate Production from the Catalytic Depolymerisation of Post-consumer Poly(lactic acid). J. Polym. Environ. 2020, 28, 2956–2964. [Google Scholar] [CrossRef]
- Milaniak, N.; Laroche, G.; Massines, F. Fourier-transform infrared spectroscopy of ethyl lactate decomposition and thin-film coating in a filamentary and a glow dielectric barrier discharge. Plasma Process. Polym. 2021, 18, 2000248. [Google Scholar] [CrossRef]
- Stenutz, n.d. Available online: https://www.stenutz.eu/ (accessed on 20 December 2023).
- ChemicalBook, n.d. Available online: https://www.chemicalbook.com/ (accessed on 20 December 2023).
- ChemSpider, n.d. Available online: https://www.chemspider.com/ (accessed on 20 December 2023).
- Bijoy, R.; Agarwala, P.; Roy, L.; Thorat, B.N. Unconventional Ethereal Solvents in Organic Chemistry: A Perspective on Applications of 2-Methyltetrahydrofuran, Cyclopentyl Methyl Ether, and 4-Methyltetrahydropyran. Org. Process Res. Dev. 2022, 26, 480–492. [Google Scholar] [CrossRef]
- Sajid, M.; Farooq, U.; Bary, G.; Azim, M.M.; Zhao, X. Sustainable production of levulinic acid and its derivatives for fuel additives and chemicals: Progress, challenges, and prospects. Green Chem. 2021, 23, 9198–9238. [Google Scholar] [CrossRef]
- Monticelli, S.; Castoldi, L.; Murgia, I.; Senatore, R.; Mazzeo, E.; Wackerlig, J.; Urban, E.; Langer, T.; Pace, V. Recent advancements on the use of 2-methyltetrahydrofuran in organometallic chemistry. Monatshefte Chem. 2017, 148, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; et al. Safety assessment of 2-methyloxolane as a food extraction solvent. EFSA J. 2022, 20, e07138. [Google Scholar] [CrossRef]
- Kovács, A.; Neyts, E.C.; Cornet, I.; Wijnants, M.; Billen, P. Modeling the Physicochemical Properties of Natural Deep Eutectic Solvents. ChemSusChem 2020, 13, 3789–3804. [Google Scholar] [CrossRef] [PubMed]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.L.; Chen, C.W.; Sun, P.P.; Patel, A.K.; Singhania, R.R.; Nargotra, P.; Dong, C. Di Deep eutectic solvents as promising pretreatment agents for sustainable lignocellulosic biorefineries: A review. Bioresour. Technol. 2022, 360, 127631. [Google Scholar] [CrossRef] [PubMed]
- Mehariya, S.; Fratini, F.; Lavecchia, R.; Zuorro, A. Green extraction of value-added compounds form microalgae: A short review on natural deep eutectic solvents (NaDES) and related pre-treatments. J. Environ. Chem. Eng. 2021, 9, 105989. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep eutectic solvents for the extraction of bioactive compounds from natural sources and agricultural by-products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- de Jesus, S.S.; Maciel Filho, R. Are ionic liquids eco-friendly? Renew. Sustain. Energy Rev. 2022, 157, 112039. [Google Scholar] [CrossRef]
- Quintana, A.A.; Sztapka, A.M.; Santos Ebinuma, V.d.C.; Agatemor, C. Enabling Sustainable Chemistry with Ionic Liquids and Deep Eutectic Solvents: A Fad or the Future? Angew. Chem. 2022, 134, e202205609. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; López-Malo, D.; Esteve, M.J.; Blesa, J.; Frígola, A. Green Solvents: Emerging Alternatives for Carotenoid Extraction from Fruit and Vegetable By-products. Foods 2023, 12, 863. [Google Scholar] [CrossRef]
- Murador, D.C.; Braga, A.R.C.; Martins, P.L.G.; Mercadante, A.Z.; de Rosso, V.V. Ionic liquid associated with ultrasonic-assisted extraction: A new approach to obtain carotenoids from orange peel. Food Res. Int. 2019, 126, 108653. [Google Scholar] [CrossRef] [PubMed]
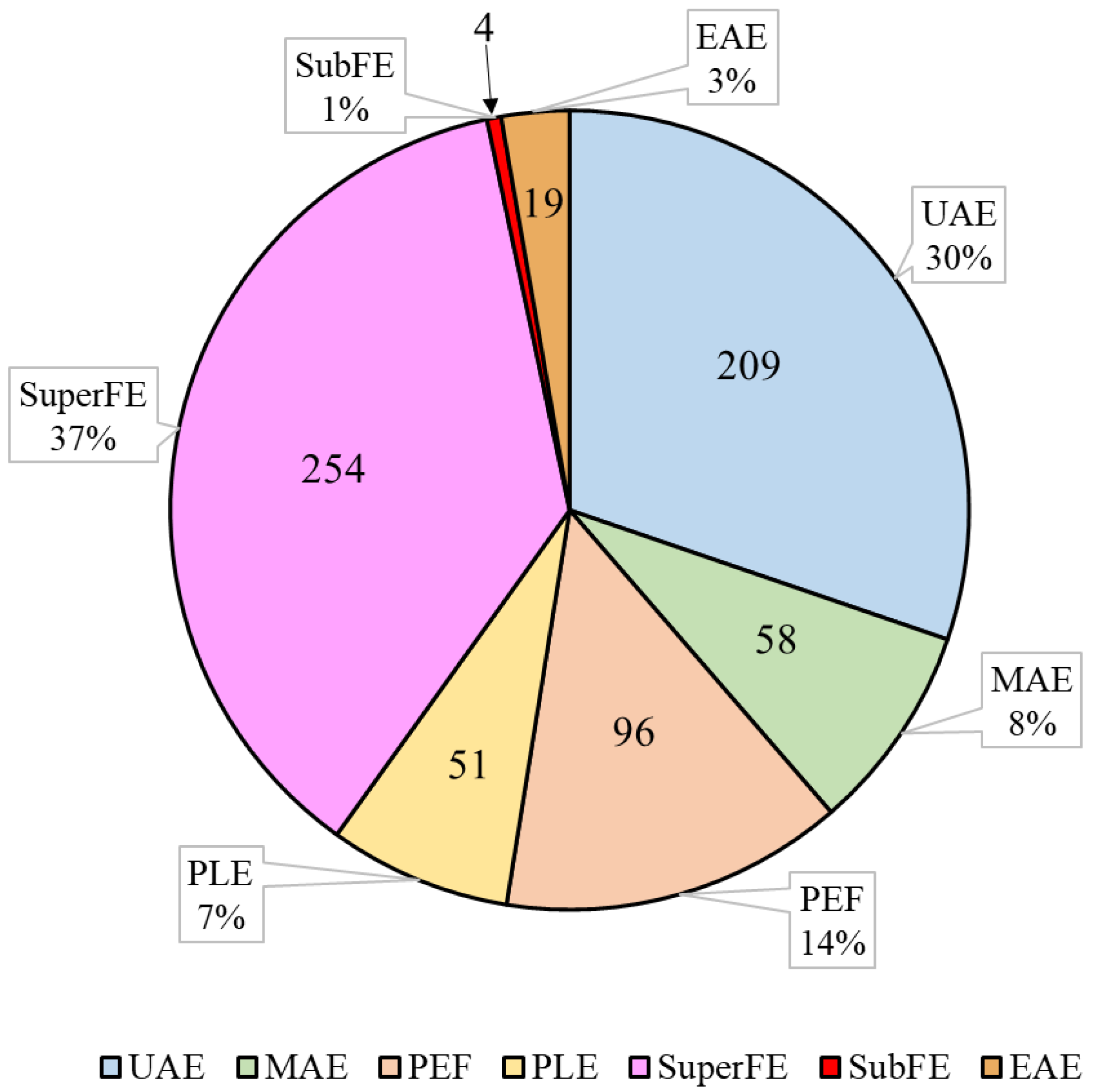

| Extraction Method | Mechanism | Ref |
|---|---|---|
| UAE | Utilizes sound waves for inducing cavitation in the solution, thereby facilitating the extraction process. | [11] |
| MAE | Generates heat within the solvent by employing ionic conduction of the dipole rotation and dissolved ions in the polar solvent. | [12] |
| PEF | Applies high-voltage microsecond pulses to induce pores in cell membranes, resulting in the disruption of barrier function and leakage of intracellular content. | [13] |
| PLE | Utilizes organic solvents under high pressures and temperatures, exceeding the boiling point, amplifying the solubility of analytes, and reducing solvent viscosity. This approach minimizes the required time and solvent volume. | [14] |
| SuperFE | Utilizes CO2 in supercritical state as extraction solvent, segregating the analytes according to their relative solubility. Supercritical CO2 has a high density and solvent power, similar to that of a liquid. | [15] |
| SubFE | Extracts less-polar compounds using water or other fluids in subcritical conditions under high pressures and high temperatures, sustaining subcritical fluids in a liquid state for a brief extraction period. | [16] |
| EAE | Encompasses the binding of cells to the active site of an enzyme, which induces a transformation of the enzyme form to adapt to the substrate. Consequently, the active components are released from the cells into the extraction medium. | [17] |
| Extraction Method | Conditions | Solvent | Matrix | Results | Ref |
|---|---|---|---|---|---|
| CSE and UAE (bath) | CSE (290 rpm) and UAE (40 kHz, 80 W): time (6, 10, 20, 30, 34 min), SSR (6 mL/59, 80, 130, 180, 201 mg). | Acetone, ethanol, petroleum ether, and methanol | Cashew apple | Optimal conditions: CSE: 38% acetone, 30% ethanol, and 32% petroleum ether, 23 min, and 136 mg; UAE: 44% acetone and 56% methanol, 19 min, and 153 mg. UAE achieved a ~21% faster and higher carotenoid yield in all samples compared to CSE. | [18] |
| CSE, MAE, and UAE (probe) | CSE: 30 min, RT, SSR: 10 mL/g, 150 rpm; MAE: 100 W, 30 min, 60 °C, SSR: 1 g/10 mL. UAE: 40 kHz, 50 W, 60%, 30 min, SSR: 10 mL/g; UAE + MAE (same conditions). | Menthol/camphor (1:1, n:n) | Orange peel | Significantly higher extraction with UAE (~2-fold) compared to CE, no statistical differences between CE and MAE or between UAE and UAE+MAE. | [19] |
| UAE (probe) | 20 kHz, 500 W, amplitude (20, 30, 40, 50, 60, 70%), time (10, 15, 20, 25, 30, 35 min), temperature (55, 60, 65, 70, 75, 80 ℃), SSR (10, 20, 30, 40, 50, 60 mL/g). | Ethyl lactate, limonene, soybean oil, and sunflower oil | Sargassum fusiforme | Optimal conditions: ethyl lactate, 20 kHz, 500 W, 53%, 27 min, 75 ℃, and SSR: 40 mL/g. | [20] |
| CSE and UAE (bath) | CSE: 90 min, 40 °C, SSR: 40 mL/g; UAE: 45 kHz, ultrasonic power (150, 180, 210 W), time (30, 40, 50 min), and SSR (30, 35, 40 mL/g). | Ethanol–petroleum ether mixture (2:1, v/v) | Cucurbita moschata | Optimal UAE conditions: 45 kHz, 203 W, 30 min, and 31 mL/g. Compared to the CSE, UAE avoided degradation and isomerization, resulting in a higher yield. | [21] |
| UAE (probe) | 20 kHz, amplitude (20, 40, 60, 80, 100%), time (10, 20, 30, 40 min), SSR (30, 40, 50, 60 mL/g), and hexane/acetone ratio (50/50, 70/30, 90/10). | Hexane/acetone mixture | Cantaloupe waste | Optimal UAE conditions: 20 kHz, 100%, 10 min, 55 mL/g, and hexane/acetone ratio of 80/20. | [22] |
| UAE (bath) | 40 kHz, 80 W, time (14, 20, 30, 40, 44 min), and SSR (10 mL/10, 30, 80, 130, 150 mg). | Acetone/ethanol (3:1) | Buriti (Mauritia flexuosa) | Optimal UAE conditions: 40 kHz, 80 W, 30 min, 80 mg/10 mL, acetone/ethanol (75:25). | [23] |
| UAE (probe) | 20 kHz, 70% amplitude, time (3, 10, 20, 30, 37 min), temperature (10, 20, 35, 50, 60 °C), SSR (30, 50, 70 mL/g), solvent percentage (13, 30, 55, 80, 97%). | Ethanol, methanol, acetone, acetonitirile, and n-hexane | Carrot pomace | Optimal UAE conditions for total carotenoid content: 17 min, 32 °C, 50 mL/g, and 51% ethanol. | [24] |
| UAE (probe) | UAE: 20 kHz, 30%, 2 min, SSR: 2 mL/0.1 g. | Ethanol, methanol, ethyl lactate, MeTHF, and DMSO | Chlorella sorokiniana | The best solvent depended on the matrix (fresh: ethanol; freeze-dried: methanol; encapsulated: MeTHF and ethyl lactate). | [25] |
| CSE and MAE | CSE: 30 min, 60 °C, 100 mg/5 mL (10 M aqueous KOH with 2.5% ascorbic acid); MAE: time (0, 15, 30, 45, 60, 180, 300, 600 s), 60 °C, SSR (1 mL/10, 20, 30, 40, 50, 60 mg), KOH concentration (0, 2, 4, 6, 8, 10, 12 M). | Ethanol | Chlorella sorokiniana | Optimal alkali-assisted MAE pretreatment conditions for lutein: 850 W, 1.47 min, 8.16 M KOH, and SSR of 36.8:1 mg/mL. Lutein yield obtained via MAE was 3.26 folds higher than that obtained via CSE. | [26] |
| MAE | Power (50, 80, 125, 170, 200 W), time (1, 3.14, 6.3, 9.46, 12 min), and oil-to-waste ratio (5:1, 8:1, 12.5:1, 17:1, 20:1 g/g). | Flaxseed oil | Carrot juice | Optimal conditions: 165 W, 9.39 min, and SSR 8.06:1 g/g. | [27] |
| MAE and UAE (probe) | MAE: power (30, 40, 50 W), time (5, 7, and 9 min), and SSR (10, 20, 30 mL/g); UAE: 20 kHz, power (375, 562.5, 750 W), time (2, 9, 16 min), and SSR (10, 30, 50 mL/g). | MAE: acetone, ethanol, petroleum, ether/acetone/ethanol mixture (2:1:1), and n-hexane/acetone/ethanol mixture (2:1:1); UAE: acetone, N,N-dimethylformamide, isopropanol/n-hexane mixture (1:1), petroleum ether/acetone mixture (1:1), and petroleum ether/acetone/ethanol mixture (2:1:1) | Aristeus antennatus shrimp | Optimal MAE conditions: 30 W, 7 min, 20:1 mL/g, and n-hexane/acetone/ethanol 2:1:1 (v/v/v); Optimal UAE conditions: 20 kHz, 600 W, 5 min, 10:1 mL/g, and acetone. No differences between UAE and MAE in carotenoid extraction from the head of the shrimp. UAE resulted in a ~2 times higher total carotenoid extraction compared to that of MAE in the body of the shrimp. | [28] |
| PEF | 3.5 kW of power (15 kV/cm, 150 μs). | Ethanol | Rhodotorula glutinis | PEF treatment without incubation did not recover carotenoids; however, PEF with 1 h incubation (20 °C) permits the extraction of carotenoids from fresh biomass. | [29] |
| Bead beating, freeze-thawing, thermal treatment, PEF, and UAE (probe) | PEF: 1 Hz, 1 kV/cm, 10 pulses, 50 kJ/Kg; Bead beating: 4800 rpm (5–10 cycles of 60 s), 6 h incubation time; UAE: 450 W, 80%, 10 times for 10 s; Thermal treatment: 1 h, 70 °C; Freeze-thawing: in liquid nitrogen and left to melt on ice (repeated 5 times). | Ethanol, acetone, and methanol | Haematococcus pluvialis and Chlorella vulgaris | The best extraction yields were achieved after PEF pretreatment with 6 h incubation and ethanol. Statistically significant differences in the extracted carotenoid yields after PEF pretreatment compared to other treatments in H. pluvialis cells grown in the control BBM medium or in N-free BBM medium supplemented with 6 g/L glucose. | [30] |
| CSE and PEF | CSE: 25 °C, 120 rpm, SSR: 100 mL/5 g, hexane/ethanol/acetone (50:25:25); PEF: 1 Hz, 5 kV/cm, 90 µs, time (20, 160, 300 min), SSR: 5 g/100 mL, hexane/ethanol (25:75, 50:50, 75:25). | Acetone, hexane, and ethanol mixtures | Tomato waste | Optimal PEF conditions: 1 Hz, 5 kV/cm, 90 µs, 150 min, and 30% hexane. PEF treatment improved the carotenoid extraction by 39% as compared with CSE in a mixture of hexane/ethanol/acetone (50:25:25). | [31] |
| CSE and PEF | CSE: 100 µL sample/1 mL solvent, vortexed; PEF: 0.5 Hz, 20 kV/cm, 75 µs, with or without 1 h incubation (20 °C). | Ethanol (96%) | Chlorella vulgaris | Extraction yield with PEF was significantly higher than that with CSE. Pre-incubation for 1 h after PEF treatment improved extraction yields. | [32] |
| ASE, CSE, PEF, and PEF+ASE | CSE: 400 rpm, 30 min, RT, SSR (1:10, w/v); PEF: 100 kJ/kg, 74 pulses, 300 mL of tap water/30 g; PEF+ASE: 1 min of preheating period, 5 min of heating period, 60% of flush volume, 10 MPa of extraction pressure (for 15 min) and 60 s of nitrogen purge, 50 °C. | DMSO and ethanol | Shrimp by-products | PEF+ASE with DMSO resulted in the highest carotenoid recovery from Aristeus antennatus. The antioxidant capacity varied depending on solvent. | [33] |
| CSE and PLE | CSE: 15 min, 40 °C, SSR (20 mL/0.5 g); PLE: preheating time: 1 min, heating time: 5 min, 40 °C, SSR (0.5 g/20 mL). | DMSO (0, 30, 50, and 100%) | Spirulina, Chlorella and Phaeodactylum tricornutum powder | Carotenoid recovery with PLE was significantly higher than that with CSE; 100% DMSO enhanced the extraction yields of carotenoids significantly. | [34] |
| CSE, PEF, PLE, and PEF+PLE | CSE: 6 h, 40 °C, SSR: 20 mL/g (chloroform/methanol (5:2, v/v)); PEF: 3 kV/cm, 44 pulses, 99 kJ/kg energy input, SSR: 200 mL H2O/2 g; PLE: 15 min, 40 °C, 1 min preheating period, 5 min heating period, flush volume of 60%, nitrogen purge of 60 s, 103.4 bars,1000–2000 μS/cm, SSR: 20 mL/0.5 g (H2O, 50% DMSO, 100% DMSO). | H2O, 50% DMSO, 100% DMSO | Spirulina biomass | The extraction yield with PEF + PLE (50% DMSO) was significantly higher than those with PEF, PLE, or CSE. PEF + PLE increased efficiency and reduced the extraction time. | [35] |
| PLE | 20 min, temperature (50, 100, 150 °C), 103.4 bar, nitrogen purge 300 s. | Ethanol, ethyl acetate, and n-hexane | Chlorella vulgaris and Phaeodactylum tricornutum (wet and dry biomasses) | Best conditions for total lipid yield: ethyl acetate and n-hexane (temperature did not affect) for dry C. vulagris; ethyl lactate at 150 °C for wet C. vulgaris; ethanol at 100 or 150 °C for dry P. tricornutum; and ethanol at 150 °C for wet P. tricornutum. | [36] |
| CSE and SuperFE | CSE: 30 min, 40 °C, 1 mL/g; SuperFE: 40−60 °C, CO2 flow rate 16.5 g/min, equilibration time (0−30 min), extraction pressure (200−500 bar), and ethanol content in supercritical fluid mixture (8−13 wt %), with or without MW pretreatment (time (30–90 s), temperature (41–140 °C), up to 300 W). | Acetone | Brown crab (Cancer pagurus) processing waste | Optimal SuperFE conditions: 500 bar, 40 °C, and 13 wt % ethanol content after an optimized MW pretreatment (140 °C, 90 s, 300 W). | [37] |
| CSE, MAE, and SuperFE | CSE: 24 h, 30 °C, 500 rpm, SSR: 37 mL/g; MAE: time (5–25 min), temperature (40–60 °C), power (300–800 W), SSR (20–90 mL/g); SuperFE: 60 °C, 250 bar, flow rate 40 g/min, total solvent consumption 100 kg CO2/kg biomass. | Ethanol 90% (v/v) | Chlorella vulgaris | Optimal MAE conditions: 14 min, 60 °C, 300 W, and 22 mL/g. CSE presented the highest yield. MAE and SFE led to an antioxidant capacity similar to or better than CSE in a significantly shorter extraction time. | [38] |
| SuperFE | Extraction time (60, 90, 135, 180, 210 min), temperature (55, 60, 70, 80, 85 °C), pressure (250, 300, 375, 450, 500 bar), SSR 1 mL/0.1 g, flow rate CO2 (35 g/min), and rice bran oil (3%, w/w). | CO2 with rice bran oil as co-solvent | Citrus paradise Macfad | Optimal conditions for lycopene extraction: 143 min, 64 °C, and 325 bar. Extraction temperatures higher than 80 °C and time lower than 180 min led to lycopene isomerization. | [39] |
| SuperFE | 80 min, temperature (50, 60, 70 °C), pressure (150, 250, 350 bar), CO2 flow rate 15 g/min, and co-solvent concentration (5, 10, 15%, v/v). | CO2 with ethanol as co-solvent | Carrot peels | Optimal conditions: 59 °C, 349 bar, and 15.5% ethanol. | [40] |
| SubFE | 15 min, temperature (303, 318, 333 K), pressure (5, 11, 17 MPa), co-solvent percentage (2, 4, 6%), 50 min with a constant flow rate of 10 g/min. | 1,1,1,2-Tetrafluoroethane with ethanol as co-solvent | Laminaria japonica | Optimal conditions: 324.13 K, 17 Mpa, and a co-solvent amount of 4.73%. | [41] |
| SubFE and UAE (bath) | UAE (to select best solvent): 15 min, 35 °C, SSR: 10 mL/0.5 g. SubFE: extraction time (30, 60, 90 min), extraction temperature (35, 50, 75 °C), pressure of 20 MPa, SSR (200:1, 100:1, 20:1 (v/wet weight). | Hexane, DCM, ethanol, methanol, THF, ultrapure water, and THF:DCM (1:1). | Phaeodactylum tricornutum | Optimal conditions: methanol, 60 min, 35 °C, 20 MPa, 120 rpm, and SSR 200:1. | [42] |
| SOX and SubFE | SOX: 360 min, temperature (341.15 K for n-hexane, and 333.15 K for petroleum ether), SSR: 200 mL/ 5 g, solvent removed at 316.15 K; SubFE: 60 min, temperature (293.15, 313.15, 333.15 K), pressure (2, 6, 10 MPa), propane flow rate of ~2 cm3/min. | SOX: n-hexane or petroleum ether; SubFE: propane | (Maximiliana maripa) pulp oil | Optimal SubFE conditions: 293.15 K and 2 MPa. Extraction yield with SOX (n-hexane) was significantly higher than that with SubFE. | [43] |
| SOX, SubFE, and SuperFE | SuperFE: 52.5 °C, 27.50 MPa, 5 mL CO2/min flow rate; SubFE: 12 h, 29 °C, 6.8 MPa, 250 extraction cycles; SOX: 12 h, 70 °C. | SuperFE: CO2; SubFE: ethanol; SOX: n-hexane | Saudi date fruit flesh (Sukari, Ambara, Majdool, and Sagai date fruit) | The highest carotenoid extraction was found as follows: Sukari (via SubFE) and Ambara, Majdool, and Sagai date fruit (via SuperFE). | [44] |
| EAE | Pretreatment time (0.5, 2, 3.5, 5, 6.5 h), extraction time (0.5, 1.5, 2.5, 3.5, 4.5 h), extraction temperature (10, 20, 30, 40, 50 °C), enzyme solution-to-solid ratio (10, 20, 30, 40, 50 dm3/kg), and enzyme load (0, 0.05, 0.1, 0.15, 0.2 kg/kg); SSR: 30 mL/0.5 g. | Hexane, enzyme/Peclyve PR and Cellulyve 50LC (50:50) | Tomato waste | Optimal conditions: 3.18 h, 30 °C, and 0.16 kg/kg enzyme load. | [45] |
| EAE | Time (12, 18, 24 h), temperature (30, 33.5, 37 °C), enzyme dose (0.15, 0.3, 0.45, 0.6, 0.75 mL), pH (4.6, 6, 7.4). | 95% Ethanol, enzyme/fructozym® MA | Carrot | Optimal conditions: 24 h, 37 °C, pH 7.4, and 0.3 mL enzyme dose. | [46] |
| CSE and EAE | CSE (to select the best solvent): 30 min, RT, SSR: 20 mL/4 g; EAE: time (1, 2.5, 4 min), temperature (40, 50, 60 °C), SSR (1 mL/5, 17.5, 30 g), enzymatic reaction time (1, 3, 5 h), enzyme/substrate ratio (0.2, 1.1, 2 mL/g), enzyme/enzyme ratio (1, 2, 3). | CSE: acetone, ethyl acetate, ethanol, and 1:1 combinations, and ethanol/water mixture (1:1); EAE: ethyl acetate, enzymes: pectinolytic enzyme (P), cellulolytic enzyme (C), and a combination of carbohydrases, including arabanase, cellulase, β-glucanase, hemicellulase, and xylanase (V) | Tomato waste | Optimal EAE conditions: C-P combination for enzymatic pretreatment, ethyl acetate as solvent, 1 h extraction time, 40 °C, 5 h enzymatic reaction time, 0.2 mL/g enzyme/substrate ratio, 5 mL/g solvent/substrate ratio, and 1 enzyme/enzyme ratio. | [47] |
| Solvent | Extraction Method | Conditions | Matrix | Results | Ref |
|---|---|---|---|---|---|
| Ethyl lactate, acetone, ethyl acetate, hexane, and ethanol | CSE | SSR: 10 mL/g, extract removed at different time intervals (5–40 min), and temperatures (25, 50, 70 °C). | Tomato waste | Optimal conditions: ethyl lactate, 30 min, and 70 °C. | [65] |
| Ethyl lactate/ethanol (3:2) | CSE | Time (10–40 min), temperature (10–30 °C), 360 rpm, and crude palm oil proportion (20–60%). | Palm olein | Optimal conditions: 10 min, 20 °C, and 50% of crude palm oil. | [66] |
| Hexane, ethyl acetate, ethyl lactate, and ethyl acetate/ethyl lactate (1:3, v/v). | UAE (bath) | 10 min, 35 °C, and 1 g/20 mL. | Tomato by-products | The highest lycopene extraction was obtained with ethyl lactate in the wet sample. | [67] |
| Ethyl lactate and ethyl lactate/ethanol mixtures (0–100%) | CSE | Time (0–350 min), temperature (30, 45, and 60 °C), and SSR (0.25–1.0 g/10 mL). | Tangerine and red tomato, corn, and carrot | Optimal conditions: lutein (2 h, 30 °C), β-carotene (0.5 h, 30 °C), and lycopene (1 h, 45 °C). The addition of R-tocopherol or R-lipoic acid improves the extraction efficiency. Ethyl lactate is an excellent solvent for extracting trans- and cis-lycopene isomers from dried tomato powder. | [68] |
| Ethanol, acetone, ethyl lactate, sunflower oil, and water | UAE (bath) and heating | UAE: 35 kHz, 30 min, and 40 °C; Heating: 30 and 60 min and 45 and 100 °C. | Lycium fruits (Goji, naturally dried and freeze-dried) | The highest carotenoid levels were obtained via UAE (with water and ethyl lactate) and heating (with ethyl lactate). The lowest content was obtained with sunflower oil, ethanol, and acetone. Water was a better solvent for naturally dried berries and ethyl lactate and sunflower oil for the freeze-dried sample. | [69] |
| MeTHF, dimethyl carbonate, cyclopentyl methyl ether, isopropyl alcohol, ethyl acetate, and n-hexane | CSE | 1 h maceration, 65 °C, and 30 g/125 mL. | Daucus carota | The best green solvents were as follows in descending order: cyclopentylmethyl ether, MeTHF, and ethyl acetate. | [70] |
| MeTHF, hexane, tetrahydrofuran, acetone, and 1,4- dioxane | CSE | First maceration overnight with SSR 1:10 (w/v) and second maceration with SSR 1:2 (w/v). | Tagetes erecta L. | MeTHF is a potential green alternative solvent to hexane and tetrahydrofuran for lutein extraction. | [71] |
| Hexane, dry MeTHF, and MeTHF 95.5% | SOX | 4.5 h. | Olive pomace extracts | Dry MeTHF and MeTHF 95.5% recovered carotenoid yields ~11.3 and 12.4 folds higher, respectively, than that of hexane. | [72] |
| HBA:HBD (3:1), being HBA ethanol, N,N-dimethylcyclohexylamine, N,N-dimethyloctylamine, and N,N-dimethylbenzylamine and HBD n-butanol | GAE, WBE, and UAE | GAE: 30 Hz, 40 s, and SSR 1:4; WBE: 120 min, 50 °C, and SSR 1:4; UAE: 40 kHz, 15 min, 50 °C, and SSR 4 mL/g. | Millet | DESs extracted a significantly higher carotenoid yield compared to ethanol. GAE resulted in the best extraction method. | [73] |
| Acetone, DL-menthol or thymol, and capric acid as HBAs; capric acid and lauric acid as HBDs | CSE | 60 min, RT, 750 rpm, and SSR 40 mL/g. | Tomato | The combination of capric acid and lauric acid exhibited extraction capacities comparable to that of acetone. | [74] |
| Acetone; cholinium-based ILs: choline bicarbonate (Ch), octanoic acid (Oct), hexanoic acid (Hex); butyric acid (But), and lactic acid (Lac) | CSE | Time (0, 20, 60, 120, and 144 min), temperature (11.4, 25, 45, 65, and 78.4 °C), enzyme concentration (0, 20, 50, 80, and 100 wt %), and SSR: 0.2 g/1 mL. | Phaffia rhodozyma | Optimal conditions: [Ch][Oct], 60 min, 45 °C, and 50 wt % in water. | [75] |
| Acetone, methanol, dipropylammonium dipropylcarbamate (DPCARB), diallylammonium diallylcarbamate (DACARB), and dibutylammonium dibutylcarbamate (DBCARB) | CSE | Time (30, 45, 60, 75, and 90 min), temperature (25, 35, 45, and 55 °C), and DPCARB/methanol ratio (9:1, 8:2, 7:3, 6:4). | Chlorella sorokiniana | Optimal conditions: DPCARB/methanol (9:1), 45 min, and 25 °C. | [76] |
| Methanol, IL1 [1-decyl 3 methyl imidazolium chloride], IL2 [tetrabutyl phosphonium hydroxide], IL3 [tetrabutyl hexadecyl phosphonium bromide], and IL4 [tetrabutyl ammonium hydroxide] | CSE | Time (5, 10, and 15 min), temperature (25, 40, and 55 °C), IL concentration (5, 22.5, and 40%), SSR (0.1, 0.3, and 0.5 mL/mg). | Chlorella saccharophila | Optimal conditions: IL2, 5 min, 25 °C, 40% IL concentration, and 0.5 mL/mg SSR. | [77] |
| Solvent | 2-Methyltetrahydrofuran | Ethyl Lactate |
|---|---|---|
| Molecular formula | C5H10O | C5H10O3 |
| Molecular weight (g/mol) | 86.132 | 118.131 |
| Appearance | Colourless | Colourless |
| Stability | Stable, but highly flammable. Not suitable for use with oxidizing agents, potent acids, or strong bases. There is a risk of developing explosive peroxides during storage, which is why it is frequently supplied with an inhibitor as a precaution. | Stable, flammable, and not compatible with powerful oxidizing agents. |
| Toxicity | LD50 orally in Rabbit: >300–2000 mg/kg. LD50 dermal Rat > 2000 mg/kg | LD50 orally in Rat (female): >2.000 mg/kg. LD50 dermal Rabbit: >5.000 mg/kg |
| Safety | Flammable, corrosive, and irritant. Acute toxicity. It requires safety glasses, good ventilation, a test for the presence of peroxides before use, and the removal of ignition sources from the working area. | Flammable, corrosive, and irritant for eyes and skin. |
| Melting point (°C) | −136 | −26 |
| Boiling point (°C) | 82 | 154 |
| Organic solvent solubility | Miscible | Miscible |
| Water solubility | Immiscible | Miscible (with partial decomposition) |
| Density (g/mL) | 0.855 | 1.03 |
| Refraction index | 1.407 | 1.415 |
| Dielectric constant | 6.97 | 13.1 |
| Molar refraction (mL/mol) | 24.8 | 28.72 |
| Dipole moment (D) | 1.38 | 2.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morón-Ortiz, Á.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J. Sustainable Green Extraction of Carotenoid Pigments: Innovative Technologies and Bio-Based Solvents. Antioxidants 2024, 13, 239. https://doi.org/10.3390/antiox13020239
Morón-Ortiz Á, Mapelli-Brahm P, Meléndez-Martínez AJ. Sustainable Green Extraction of Carotenoid Pigments: Innovative Technologies and Bio-Based Solvents. Antioxidants. 2024; 13(2):239. https://doi.org/10.3390/antiox13020239
Chicago/Turabian StyleMorón-Ortiz, Ángeles, Paula Mapelli-Brahm, and Antonio J. Meléndez-Martínez. 2024. "Sustainable Green Extraction of Carotenoid Pigments: Innovative Technologies and Bio-Based Solvents" Antioxidants 13, no. 2: 239. https://doi.org/10.3390/antiox13020239
APA StyleMorón-Ortiz, Á., Mapelli-Brahm, P., & Meléndez-Martínez, A. J. (2024). Sustainable Green Extraction of Carotenoid Pigments: Innovative Technologies and Bio-Based Solvents. Antioxidants, 13(2), 239. https://doi.org/10.3390/antiox13020239