Abstract
Stroke represents one of the main causes of death and disability in the world; despite this, pharmacological therapies against stroke remain insufficient. Ischemic stroke is the leading etiology of stroke. Different molecular mechanisms, such as excitotoxicity, oxidative stress, and inflammation, participate in cell death and tissue damage. At a preclinical level, different garlic compounds have been evaluated against these mechanisms. Additionally, there is evidence supporting the participation of garlic compounds in other mechanisms that contribute to brain tissue recovery, such as neuroplasticity. After ischemia, neuroplasticity is activated to recover cognitive and motor function. Some garlic-derived compounds and preparations have shown the ability to promote neuroplasticity under physiological conditions and, more importantly, in cerebral damage models. This work describes damage/repair mechanisms and the importance of garlic as a source of antioxidant and anti-inflammatory agents against damage. Moreover, we examine the less-explored neurotrophic properties of garlic, culminating in proposals and observations based on our review of the available information. The aim of the present study is to propose that garlic compounds and preparations could contribute to the treatment of ischemic stroke through their neurotrophic effects.
1. Introduction
Stroke significantly impacts a large segment of the population and stands as one of the leading causes of death and disability. Currently, fibrinolytics and endovascular therapies that induce reperfusion are the only treatments available, yet they are often insufficient and can even result in further brain damage. Consequently, research is focused on identifying new therapeutic targets and protective molecules. Key mechanisms implicated in ischemic stroke-related injury include excitotoxicity, oxidative stress, and inflammation. Numerous molecules demonstrate potent antioxidant and anti-inflammatory properties; however, they frequently fail in clinical trials as effective stroke treatments. On the other hand, there are repair mechanisms such as neuroplasticity that are potential targets for ischemic stroke treatment. Neuroplasticity is a repair mechanism that comprises changes that generate new cells and synaptic connections. Thus, the discovery of novel mechanisms related to recovery in therapeutic stroke research is essential. Garlic and its preparations are a source of antioxidant, anti-inflammatory, and neurotrophic molecules. Hence, the aim of this review is to analyze the neurotrophic properties of garlic compounds and preparations as a possible management method for ischemic stroke.
2. Stroke
2.1. Stroke Epidemiology and Risk Factors
Amongst neurological diseases, stroke represents one of the leading causes of death and disability worldwide [1]. Furthermore, people affected by stroke require temporary or lifelong assistance, resulting in a huge burden at the human and economic cost levels [2,3].
Stroke is classified into ischemic and hemorrhagic, with a higher prevalence of the ischemic condition. Ischemic stroke occurs when the blood supply decreases under the tissue demand requirements for normal function, resulting in deficiencies in oxygen, glucose, and other molecules required for brain metabolism [4].
Despite the heterogeneity of this disease, some non-modifiable risk factors such as age and gender contribute importantly to the incidence of ischemic stroke. Aging is the strongest non-modifiable risk factor; three quarters of all strokes occur in persons aged >65 years, and the risk doubles every 10 years after the age of 55 [5,6,7]. Moreover, aged patients with stroke have higher mortality and morbidity rates and present poorer functional recovery than their young counterparts [5,6,7]. It is estimated that the increase in the size of the aged population represents an important factor that will contribute to the increase in ischemic stroke cases in the future [8]. Additionally, gender also is an important factor contributing to the incidence, mortality, and after-effects associated with stroke [9]. After the age of 65, the risk of suffering a stroke is increased in women compared with men of the same age [9,10,11]. Other clinical studies observed that older women experience more severe strokes, longer periods of hospitalization, more severe sequelae, and lower quality of life relative to men of similar ages [10,11].
2.2. Damage Mechanisms in Ischemic Stroke
Ischemic tissue damage is caused by a disruption in blood supply (ischemia) to the brain, whereas the restoration of blood flow (reperfusion) sometimes leads to an additional form of damage called reperfusion injury [12,13,14]. These two phases trigger a rapid loss of brain function and the development of an infarct region, caused by excitotoxicity, oxidative stress, inflammation, synaptic deficits, the disintegration of neural networks, cell death, and ultimately, failure of neurological functions [12,13,14].
Excitotoxicity is one of the first mechanisms activated after blood vessel occlusion [15,16,17]. This process is mediated by the excitatory neurotransmitter glutamate [15,16,17]. During ischemia, the decrease in ATP levels promotes neuron depolarization, causing a rapid and massive release of glutamate to the synaptic cleft. Additionally, its clearance, mediated by astrocytes, is compromised due to a decrease in cell energy. Glutamate accumulation induces the overactivation of the N-methyl-D-aspartate receptor (NMDAR), resulting in an increase in neuron cytoplasmic calcium levels. These (1) promote the activation of different enzymes such as endonucleases, lipases, and proteases; (2) increase reactive oxygen species (ROS) production; and (3) induce cell damage and death [15,16,17].
Oxidative stress is an imbalance between prooxidants and antioxidants due to an increase in oxidant agents, a decrease in antioxidant systems, or a combination of both conditions [18]. During ischemia, ROS production increases due to the activation of calcium-dependent enzymes such as xanthine oxidase. Additionally, during reperfusion, the increases in tissue oxygen level promote a second and major burst of ROS, generated mainly by mitochondria and NADPH oxidase [19,20,21]. The increase in ROS levels promotes their interaction with biomolecules, leading to the aberrant regulation, altering, or destroying of the functions of cellular lipids, proteins, and nucleic acids, and inducing inflammation, cell death, and senescence [18,20,21].
Finally, inflammation occurs at the ischemic core, due to dying cells, which release damage-associated molecular patterns (DAMPs), like purines, lipids, and other proteins, that activate the immune system. It starts immediately after stroke; leukocytes are activated and release proinflammatory molecules from the endothelium and parenchyma, contributing to cell death and injury [22].
2.3. Neuroprotective Mechanisms in Ischemic Stroke: Neuroplasticity
Several weeks or months after suffering an ischemic stroke, some patients show improvement in their neurological sequelae. This could be associated with the natural recovery of the brain after injury as result of neuroplasticity, which is defined as the ability to make adaptive changes related to the structure and function of the nervous system, such as synaptogenesis and neurogenesis [23], together with neurobiochemical transformations that include changes in the release of chemical mediators and changes in the receptor sensitivity and activation of postsynaptic mechanisms [24,25].
2.3.1. Synaptogenesis
Synaptic plasticity appears during animal development and continues throughout life, but is decreased in aging [26]. Even during ischemic stroke, the formation of new synapses occurs in the damaged tissue (Figure 1) [27,28]. Two mechanisms have been described. First, dendritic spines undergo remodeling in the peri-infarct zone [27,28]. Within the first two weeks, there is an increase in the number and the turnover of dendritic spines [27]. Then, in the peri-infarct area, neurons develop branches and establish new connections [29]. The new synapses can be developed at the local level or reach longer distances, forming new circuits [29,30]. Synaptogenesis and axonal sprouting occur simultaneously [29,30].
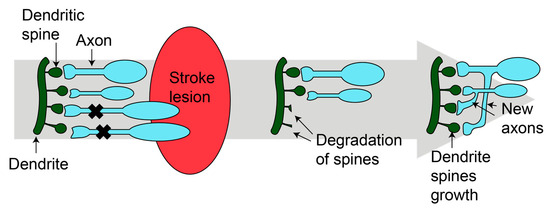
Figure 1.
Synaptogenesis after stroke. After injury, new dendritic spines grow and new axons are formed, resulting in new mature synapses. Figure was made in Illustrator 2022.
The phosphoinositide 3-kinase/serine/threonine protein kinase/glycogen synthase kinase-3 beta (PI3K/AKT/GSK3β) pathway controls axonal growth and dendritic changes [31]. Moreover, Leucine-rich repeat and IgG domain-containing protein 1 (LINGO1) and Nogo receptor (NogoR) determine the direction and location of fibers [31]. Additionally, netrin is a highly conserved laminin-associated secreted protein that attracts or repels axons. If it binds to the deleted in colorectal cancer (DCC) or neogenin receptors, it attracts the axon, whereas if it binds to the DCC/uncoordinated A–D receptor complex, the result is the repulsion of axons [32].
On the other hand, synapse formation occurs via the following steps [30]: (1) the damaged tissue is removed by glia; (2) an increase in neurotrophic factor (neurotrophins, NT) levels occurs in the damaged area, which are secreted by neurons and glia; (3) the extracellular matrix is modified an increase in cell adhesion molecules (e.g., laminin, fibronectin), produced by the surrounding neurons and glia; and finally (4) the neurotransmitter delivery system and postsynaptic receptors accomplish the maturation of the synapses.
2.3.2. Neurogenesis
The other mechanism of repair is neurogenesis, leading to the generation of new functional neurons from the neural stem and precursor cells (NS/PS). Like synaptogenesis, neurogenesis occurs in mammals throughout life in restricted brain regions. It is activated after stroke and starts at the neurogenic niches where the NS/PC are located (Figure 2) [33].

Figure 2.
Neurogenesis after stroke. Neural stem and precursor cells (NS/PC) reside in two neurogenic regions in the adult mammalian brain: the subventricular zone (SVZ) and subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus. After stroke, neurogenesis could be activated, generating new mature neurons that migrate to CA3 or the stroke lesion. Arrows indicated the direction cell migration. Figure was made in Illustrator 2022.
In the adult brain, there are two principal neurogenic regions, which reside in the subventricular zone (SVZ) and subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus [34,35]. The NS/PC in these areas have two characteristics: (1) the capacity to produce a new copy of themselves and (2) the ability to generate neurons, astrocytes, or oligodendrocytes [34]. Ischemic stroke is a strong stimulant of neurogenesis toward the damaged area [35].
At a molecular level, neurogenesis is induced by intrinsic (neurotrophic factors, transcriptional programs, inflammatory cytokines, neurotransmitters, and hormones) and extrinsic (physical activity, dietary intake, stem cell transplantation, and the intake of some compounds) factors [36].
The neurogenic niche represents a specialized microenvironment that functionally contributes to maintaining and regulating NS/PC proliferation, producing several intrinsic factors as trophic factors [33]. This neurogenic niche in adults is composed mainly of endothelial cells, astrocytes, ependymal cells, microglia, mature neurons, and the progeny of adult neural precursors [33]. After stroke, glia and the vasculature resident in the niche have considerable importance [36]. They release complex arrays of signals that stimulate proliferation and guide new cells to the damaged area [37]. Brain capillary cells are capable of sprouting, and neural precursor cells proliferate and migrate along cerebral micro-vessels to the ischemic lesion [37]. Glia cells promote the restoration of functional micro-vessels while controlling the buildup of the extracellular matrix, creating a favorable environment for neuronal plasticity [37].
2.3.3. Neurotrophic Factors
Neurotrophic factors are a group of soluble polypeptides delivered by cells, with a wide range of functions in the nervous system, including neuronal survival and repair, synaptic plasticity, and the formation of long-lasting memories [38]. They are divided into different families according to their structure and function:
- (1)
- NTs promote neuronal survival, neuronal differentiation, axonal and dendritic growth, synaptic plasticity, and synaptogenesis [39]. Some examples are nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3).
- (2)
- Members of the transforming growth factor family (TGF) stimulate astrocyte proliferation, migration, and transformation to the axon growth-supportive phenotype [40]. They stimulate neural cell proliferation and differentiation and the synthesis of NGF in astrocytes [40]. After stroke, they promote neurogenesis, angiogenesis, and provide oligodendrocyte protection [41], e.g., glia-derived neurotrophic factor (GDNF).
- (3)
- Neurokines, such as interleukin 6 (IL6), play critical roles in immunity, brain-regulating neurodevelopment, food intake, body temperature, learning, and memory [42].
- (4)
- Non-neuronal factor families have neurotrophic and angiogenic activity [43]. They act as neuroprotective signals against acute ischemic brain injury [43], e.g., insulin growth factor (IGF).
Other proteins called angioneurins act as neurotrophic factors and regulate angiogenesis. They act on neurons and vascular cells directly (promoting their proliferation and migration and altering the composition of the extracellular matrix to facilitate angiogenesis) or indirectly (recruiting pro-angiogenic cells like mesenchymal stem cells and promoting the release of angiogenic factors by neurons and astrocytes) [44]. Moreover, they protect the blood–brain barrier’s integrity, promote vascular perfusion, and induce neuroprotection, neuroregeneration, and synaptic plasticity. Some examples of angioneurins are brain-derived neurotrophic factor (BDNF), neurotrophin-4 (NT4), and nerve growth factor (NGF).
Neurotrophic factors exert their biological activities through tyrosine kinase activity receptors. The tropomyosin-related kinase (Trk) receptor family is the main target of NTs (each NT has a preference for a specific Trk receptor). The binding NT/Trk receptor activates different pathways, including (A) mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK/ERK), (B) phospholipase C gamma (PLCγ), and (C) PI3K/AKT. This activation induces transcription factors such as CREB that increase the expression of proteins involved in promoting neuronal survival, differentiation, cytoskeletal rearrangement, synapse formation, and synaptic plasticity (Figure 3) [45].

Figure 3.
Cellular pathways activated by the union of neurotrophin to the tropomyosin-related kinase (Trk) receptor that induces neuroplasticity. (A) MEK/ERK: The adapter protein GRB2 binds to the phosphorylated Trk receptor. GRB2 is associated with the SOS protein, which promotes the activation of RAS, which initiates the kinase cascade that includes RAF, MEK, and ERK. ERK can get into the nucleus to activate transcription factors (CREB), promoting neuronal differentiation and synapse maturation. (B) PLCγ: The phosphorylated Trk receptor activates PLCγ, which hydrolyzes PIP2 into two secondary messengers: IP3 and DAG. The first binds to its receptor on the endoplasmic reticulum, causing the release of calcium into the cytoplasm, whereas DAG activates PKC. Calcium and PKC modulate ion channels, affecting membrane potential and excitability and modulating synaptic plasticity. (C) PI3K/AKT: The phosphorylated Trk receptor recruits PI3K, which, in turn, phosphorylates PIP2 to generate PIP3. PIP3 serves as a secondary messenger that recruits AKT. AKT activation leads to the activation of the mTOR pathway, which plays a role in protein synthesis and impacts axonal growth. AKT can phosphorylate GSK3β, inhibiting its kinase activity; this could favor the survival and differentiation of oligodendrocytes, which are critical for myelination, or affect neuronal structure, stimulating the axonal growth cone. (D) Other transcription factors that regulate neuroplasticity after stroke are Nrf2 and HIF-2. Both transcription factors are stabilized and translocated into the nucleus, where they induce the transcription of genes involved in proliferation (Nrf2) and differentiation (HIF-2). AKT: serine/threonine protein kinase; CREB: cyclic AMP response-element-binding protein; DAG: diacylglycerol; 4E-BP1: eukaryotic translation initiation factor 4E-binding protein 1; ERK1/2: extracellular signal-regulated kinase; FRS2: factor receptor substrate 2; GRB2: growth factor receptor-bound protein-2; GSK3 β: glycogen synthase kinase-3β; HIF-2: hypoxia-inducible factor 2; IP3: inositol 1,4,5-trisphosphate; MEK: mitogen-activated protein kinase kinase; mTOR: mechanistic target of rapamycin; Nrf2: nuclear factor erythroid 2-related factor 2; PI3K: phosphoinositide 3-kinase; PIP2: phosphatidylinositol 4,5-bisphosphate; PIP3: phosphatidylinositol 3,4,5-trisphosphate; PKC: protein kinase C; PLCγ: phospholipase C gamma; RAF: rapidly accelerated fibrosarcoma kinases; RAS: rat sarcoma virus proteins; Shc: Src homology and collagen; SOS: RAS activator son of sevenless; Trk: tropomyosin-related kinase. Figure was made in Illustrator 2022.
MEK/ERK pathway activation occurs after ligand–receptor dimerization, leading to the phosphorylation of tyrosine residues of the carboxyl terminal of the receptor, which acts as docking site for Shc (Src homology and collagen) and fibroblast growth factor receptor substrate 2 (FRS2) and forms a complex with growth factor receptor binding protein 2 (GRB2). This complex is constitutively associated with rat sarcoma virus proteins (RAS) and the activator son of sevenless (SOS), forming the GRB2/SOS complex [46]. The recruitment of this complex activates RAS and rapidly accelerates the fibrosarcoma kinase (RAF)/MEK/ERK cascade [47]. Finally, the ERK pathway induces local axonal growth and increases CREB-mediated transcriptional events such as cell proliferation, neural differentiation, synapse formation, and new circuit formation (Figure 3A) [47].
Alternatively, the interaction of NT/Trk receptors activates PLC-γ, which regulates synaptic plasticity through the activation of protein kinase C (PKC) or through the generation of inositol triphosphate (IP3), which releases calcium from internal stores [48]. Calcium and PKC have effects on synaptic plasticity and memory storage, as calcium influx is crucial for the generation of action potentials, neurotransmitter release, and gene expression. These effects are related to changes in long-term potentiation and long-term depression, changing the activity and strength of neural circuits (Figure 3B) [48].
Moreover, the dimerization and autophosphorylation of Trk receptors lead to the activation of the PI3K/AKT pathway. AKT activation increases protein translation via the mammalian target of rapamycin (mTOR)-p70S6 kinase and eukaryotic translation factor 4E-binding protein 1 (4E-BP1), resulting in axonal growth. Furthermore, the phosphorylation and inactivation of GSK3β by AKT regulates cellular morphogenesis, depending on the phosphorylation site. Phosphorylation at Ser21/9 induces the accumulation of beta-catenin (β-cat)/N-cadherin, which guide the microtubule for cellular interaction or initiate myelination [49]. However, if GSK3β is phosphorylated at Tyr279/216, microtubule assembly is initiated at the axonal growth cone (Figure 3C) [49].
Additionally, other transcription factors are activated and participate in the regulation of neuroplasticity after ischemia, such as hypoxia-inducible factor 2 (HIF-2) and nuclear factor erythroid 2-related factor 2 (Nrf2) (Figure 3D). In vitro, cell cultures of neurospheres subjected to oxygen and glucose deprivation (OGD) show that HIF-2 induce the expression of NTs (VEGF and NGF) and others transcription factors, such as differentiation factor 1 (NeuroD1), that promote the differentiation of NS/PC towards neurons. The loss of HIF-2 diminishes the number of differentiated neurons and cellular migration [50], playing a vital role in the maintenance of the proliferation, differentiation, and regeneration of NS/PC [51,52,53]. In Nrf2 knock-out, cell proliferation and endogenous neurogenesis are decreased in the hippocampus [54]. Also, in Nrf2 knock-out, NS/PC [54] and oligodendrocyte precursor cell differentiation [55] are diminished.
2.4. Treatments for Ischemic Stroke
The main objective of ischemic stroke treatment is to provide safe revascularization and, therefore, limit the neuronal damage. Additionally, the proper management of patients is mandatory and includes early hemodynamic stabilization and monitoring of possible complications. Revascularization of the affected brain area could be carried out by intravenous drug thrombolysis and endovascular thrombectomy under imaging guidance [56].
Intravenous Thrombolysis
The only drug approved by the United States Food and Drug Administration (FDA) for the treatment of acute ischemic stroke is alteplase, a recombinant tissue plasminogen activator (rtPA). rtPA is an enzyme that converts plasminogen to plasmin, dissolving the blood clot responsible for blood flow obstruction. However, its use is limited due to the exclusion criteria defined by each country [57,58]. Due to the differences in criteria and other cultural and economic factors, there are variations between countries and the percentage of patients who receive thrombolytic therapy. Nevertheless, various studies and meta-analyses have shown a clinical benefit when alteplase administration occurs within the first 4.5 h. Patients who cannot receive rtPA treatment have the option to undergo endovascular thrombectomy [59].
Primary prevention includes strategies to prevent a first stroke or transient ischemic attack (TIA) in patients. There are modifiable risk factors and non-modifiable risk factors. Nevertheless, 90% of risk for stroke worldwide is attributable to modifiable risk factors. Hence, the management of these risk factors is the best strategy for preventing first-ever stroke [60].
Secondary prevention involves therapeutics to prevent stroke in patients who previously suffered a stroke or TIA. According to the etiology, doctors will apply one of the strategies summarized in Table 1 [61].

Table 1.
Secondary prevention of stroke according to its etiology or risk factors [60,61].
As mentioned above, there are many people affected by ischemic stroke, and there are few therapeutic options. Hence, it is of utmost importance to seek therapeutic options that help reduce damage or that stimulate the prompt and efficient recovery of patients. In this context, preparations and compounds derived from garlic have shown beneficial effects against ischemic stroke injury; furthermore, they have effects related to regeneration and neuroplasticity. Therefore, its therapeutic potential could be broader. The findings are described below.
3. Garlic
Garlic (Allium sativum L.) is a vegetable that has been used worldwide since ancient times in folk medicine and gastronomy in many cultures [62,63]. Garlic cloves are commonly used for the treatment of fungal and bacterial infectious diseases, and as a cardiovascular protective measure for the prevention of stroke. Garlic extracts have been used for blood sugar maintenance, to reduce serum cholesterol levels, and for the treatment of rheumatism, toothache, and earache [64].
Garlic cloves contain (1) 62–68% water; (2) 26–30% carbohydrates (it has a high content of fructans, such as fructose polymers); (3) 1.5–2.1% proteins; (4) 1–1.5% free amino acids (which is like its protein content); (5) 1.5% fibers; and (6) 1.1–3.5% organosulfur compounds (OSCs) [65,66].
The medicinal properties of garlic are mainly associated with its OSC, like allicin, diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), and S-allylcysteine (SAC). The effects of these compounds have been evaluated in several preclinical models and some clinical trials in the treatment of different diseases [67,68].
In fresh garlic, the principal OSCs are S-allylcysteine sulfoxide (alliin, 6–14 mg/g fresh weight), γ-glutamyl-S-trans-1-propenylcysteine (3–9 mg/g fresh weight), γ-glutamyl-S-allylcysteine (2–6 mg/g fresh weight), methylcysteine sulfoxide (methiin, 0.5–2 mg/g fresh weight), cycloalliin (0.5–1.5 mg/g fresh weight), and trans-1-propenylcysteine sulfoxide (isoalliin, 0.1–1.2 mg/g fresh weight) (Figure 4A) [69,70,71,72]. These cysteine sulfoxides are odorless compounds; however, when garlic cloves are cut, crushed, or chewed, they are transformed to thiosulfinates [72,73]. The formation of these compounds occurs when cysteine sulfoxides, located in clove mesophyll storage cells, are metabolized by allinase or alliin lyase (10 mg/g fresh), an enzyme localized in the vacuoles of vascular bundle sheath cells. Due to the abundance of alliin in cloves, the main thiosulfinate formed is allicin (Figure 4B). Thiosulfinates are reactive and unstable compounds, and when they are processed in oils or by aging (commercial garlic products), other OSCs are produced [67,70].

Figure 4.
Main organosulfur compounds (OSCs) in garlic cloves and garlic products. (A) In garlic cloves, the main OSCs are γ-glutamyl-S-cysteines (γ-glutamyl-S-allylcysteine and γ-glutamyl-S-t-1-propenylcysteine) and cysteine sulfoxides (alliin, methiin, and cycloallin). (B) When garlic cloves are cut, cooked, or crushed, new compounds are formed such as the thiosulfinates (allicin and allylmethanethiosulfinate) by the interaction between alliin and alliinase. (C) In commercial garlic products, the transformation of OSCs (γ-glutamyl-S-cysteines and thiosulfinates) depends on the enzymatic reactions and extraction conditions. Adapted from [71]. The image was made in Inkscape.
3.1. Garlic Preparations
In addition to garlic cloves, several commercial garlic products are consumed: (1) garlic powder (dried garlic), (2) aged garlic extract (AGE), (3) steam-distilled garlic oils, and (4) garlic oil macerate. The OSC content in each product is different, and its transformation depends on the enzymatic reactions and extraction conditions (Figure 4C) [71].
Garlic powder is the most identical product to garlic cloves since it dehydrated at low oven temperatures (50–60 °C) and pulverized. The amount of alliin will depend on the care used in slicing and handling the cloves [71].
AGEs are obtained from the prolonged (aging) extraction (18–24 months) of chopped garlic in 20% ethanol (12 mL/g) in a closed stainless-steel container at room temperature [71,74]. Under these conditions, the main changes are: (1) the complete loss of thiosulfinates after 3 months, converted to volatile allyl sulfides, and (2) the complete hydrolysis of γ-glutamyl-S-alkylcysteines to form SAC (7.2 mg/g dry extract) and S-1-propenylcysteine (4.4 mg/g dry extract), the main OSCs in AGE. SAC content remains constant after 3 months, but S-1-propenylcysteine decreases from 12 months. Additionally, the cysteine (1.2 mg/g dry extract) and S-allylmercaptocysteine (1.9 mg/g dry extract) content increases at 24 months [71]. In fresh garlic, the γ-glutamyl-S-allylcysteine (localized in vacuoles) is metabolized by γ-glutamyltranspeptidase (bound to cell membranes) to form SAC [67,75]. In fresh garlic cloves, the SAC levels are low (0.27–0.68 mg/g of dry weight) [72]; however, it is the main OSC in AGE [67].
Steam-distilled garlic oils and garlic oil macerate are the result of converting the thiosulfinates (water-soluble compounds) in sulfides (oil-soluble compounds) using steam-distilled oil or by incubation in a common plant oil (oil macerate) [71]. In steam-distilled garlic oils, the transformation of thiosulfinates depends on temperature and occurs when garlic is homogenized in water or alcohol [73]. In these conditions, DADS (1 mg/g product), DATS (0.7 mg/g product), and allyl methyl trisulfide (0.6 mg/g product) are the principal OSCs [66,72]. In oil-macerated products, the incubation of garlic cloves in organic solvents (hexane, ether) or oils (soybean oil) at room temperature generates two additional compounds: (1) vinyldithiins (2-vinyl-4H-1,3-dithiin and 3-vinyl-4H-1,2-dithiin), which are the main compounds formed (70–80%, 1.1 mg/g product), and (2) ajoene (E,Z-4,5,9,-trithiadodeca-1,6,11-triene-9-oxide) in lower amounts (12–16%, 0.2 mg/g product) [71].
3.2. Garlic Compounds as Treatment for Ischemic Stroke
Compounds derived from garlic are known to have antioxidant and anti-inflammatory properties. They can scavenge different ROS [76,77], and some (SAC, DATS, and DADS) show the ability to promote the activation of Nrf2 transcription factor, increasing endogenous antioxidant defense [51,78,79,80]. Also, SAC, DATS, and DAS inhibit the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcription factor, decreasing the expression of different proinflammatory cytokines, such as tumor necrosis factor alfa (TNFα), interleukin (IL)1β, IL6, monocyte chemoattractant protein-1 (MCP-1), and IL-12 [81,82]. Due to these properties, garlic-derived compounds have been evaluated in different ischemic stroke models, showing a neuroprotective effect against the damage induced by brain ischemia [76,82].
In vitro models, SAC shows protection against OGD/reoxygenation insult, increasing viability [83] and decreasing apoptosis [80] through the inhibition of the ERK [76], c-Jun N-terminal kinase (JNK), and 38-kDa mitogen-activated protein kinase (p38) pathways and the activation of the Nrf2 pathway [80]. Allicin and alliin prevent the decline of cellular viability induced by OGD/reoxygenation [83,84]. Additionally, allicin decreases apoptosis, and the protective mechanism involved has bene associated with the increase in the expression of sphingosine kinase 2 (Sphk2) [84]. Sphk2 induces protection through neuronal and microvascular mechanisms [84]. DATS prevents the decrease in cellular viability, apoptosis, and lipoperoxidation, possibly through the activation of the PI3K/Nrf2/heme oxygenase 1 (HO-1) pathway in the same model [85] (Table 2).

Table 2.
Protective effect of garlic compounds in oxygen and glucose deprivation (OGD) model associated with its antioxidant and anti-inflammatory properties.
In models of global ischemia, pretreatment with SAC decreases cellular loss in the hippocampal CA1 region [76,83], edema, infarct volume, and ROS levels [86]. Moreover, E- and Z-ajoene decrease cell death in the hippocampal CA1 region, and reactivate astrogliosis and microgliosis, through a decrease in lipoperoxidation [87]. Also, the therapeutic administration of DATS decreases brain inflammation and malondialdehyde levels and preserves the activity of the antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT) in cardiac arrest models (Table 3) [88].
Furthermore, garlic OSCs also promote brain protection in focal brain ischemia models. SAC administered before ischemia decreases neurological deficit and infarct volume, preventing the activation of the ERK1/2 [76], JNK, and p38 pathways [80]. Additionally, it reduces oxidative stress, and increases glutathione (GSH) [89] and antioxidant defense levels (HO-1, glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase regulatory subunit (GCLM)) through the Nrf2 pathway [80], as well as the activity of the antioxidant enzymes glutathione reductase (GR), glutathione peroxidase (GPx), SOD, and CAT [90]. Also, SAC reduces the increase in glial fribillary acidic protein (GFAP) and inducible nitric oxide synthase (iNOS) levels [90], resulting in the improvement of neurological deficits [80,89] and a reduction in infarct volume and brain edema [80,86,89,90]. Moreover, SAC can regulate the energy content of the cell after ischemia, enhancing glucose transport by glucose transporter 3 (GLUT3) [91]. Allicin preserves neurons and diminishes neurological impairment, brain edema, infarct volume, and apoptosis by increasing antioxidant defense (glutathione S-transferase (GST), GPx, SOD, and CAT activities), reducing inflammation (TNFα levels and myeloperoxidase (MPO) activity) [92,93], and increasing Sphk2 levels [84]. Pretreatment with DAS reduces neurological deficit, infarct volume, and apoptosis in the brain [94]. DATS protects brain tissue when it is administered at the onset of reperfusion or at a later time, augmenting antioxidant defense (CAT and GPx activities and SOD and GST levels) through the Nrf2 pathway, and reducing oxidative stress and cerebral inflammation (decreasing metalloproteinase 9 levels) [95] (Table 3).

Table 3.
Protective effect of garlic compounds in ischemia and reperfusion injury models associated with its antioxidant and anti-inflammatory properties.
Table 3.
Protective effect of garlic compounds in ischemia and reperfusion injury models associated with its antioxidant and anti-inflammatory properties.
| Garlic Compound | Animal | Ischemia Model | Doses | Effect |
|---|---|---|---|---|
| SAC |   SD SD250–300 g | Global brain ischemia I: 20 min R: 5, 10, and 20 min | 300 mg/Kg i.p. 1 dose. 30 min before I | ↓ Edema and infarct volume ↓ ROS levels [86] |
  Mongolian 60–80 g | Global brain ischemia I: 5 min R: 7 days | 300 mg/Kg i.p. 3 doses. 30 min before I, and at the onset and 2 h after R | ↑ Survival of neurons in hippocampal CA1 region [76] | |
  Mongolian 60–80 g | Global brain ischemia I: 5 min R: 7 days | 300 mg/Kg i.p. 2 doses. 30 min before I and 2 h after R | ↑ Survival of neurons in hippocampal CA1 region [83] | |
  SD SD270–290 g | Focal brain ischemia I: 2 h R: 3 and 24 h | 300 mg/Kg i.p. 1 dose. Onset I 300 mg/Kg i.p. 2 doses. 30 min before I and at onset of R | ↓ Neurological deficit ↓ Infarct volume ↓ ERK1/2 levels [76] | |
  Wistar WistarUnspecified weight | Focal brain ischemia I: 2 h R: 22 h | 300 mg/Kg i.p. 2 doses. 15 min before I and 2 h after I onset | ↓ Edema and infarct area ↓ Neurological deficits ↑ GSH level and G6PDH activity ↓ Mitochondrial dysfunction (complex I-IV, ATP levels, and cytochrome c release) [89] | |
  Wistar Wistar250–300 g | Focal brain ischemia I: 2 h R: 22 h | 100 mg/Kg i.p. 4 doses. 30 min before I onset and 0, 6, and 12 h after R | ↓ Infarct volume and histological abnormalities in neurons ↓ Neurological deficits ↓ TBARS levels ↑ GSH levels and GR, GPx, SOD, and CAT activities ↓ GFAP and iNOS levels [90] | |
  Wistar Wistar280–320 g | Focal brain ischemia I: 2 h R: 0, 1, 2, 3, 4, 6, 10, 24, and 48 h | 300 mg/Kg i.p. 1 dose. At onset of R | ↑ GLUT3 and GCLC mRNA levels [91] | |
  Nrf2−/− and Nrf2+/+ Unspecified weight | Focal brain ischemia I: 2 h R: 24 h | 300 mg/Kg i.p. 1 dose. 30 min before I | ↓ Neurological deficit, infarct volume, histological damage, and apoptosis ↑ p-JNK and p-p38 levels ↑ Nrf2 levels and activation, and HO-1, GCLC and GCLM levels [80] | |
| Allicin |   SD SD250–300 g | Focal brain ischemia I: 1.5 h R: 24 h | 50 mg/Kg i.p. 1 dose. 3 h after R | ↓ Neurological impairment, edema, infarct volume, and caspase-3 levels Preserved neurons ↓ Inflammation (TNFα levels and MPO activity) [93] |
  SD SD280–300 g | Focal brain ischemia I: 1 h R: 24 h | 50 mg/Kg i.p. 1 dose. 3, 6, or 9 h after R | ↓ Neurological deficit, edema, infarct volume, and apoptosis. ↑ Sphk2 levels [84] | |
  C57 C5713–15 weeks old | Focal brain ischemia I: 2 h R: 0, 1, 2, 3, 4, 6, 10, 24, and 48 h | 50 mg/Kg i.p. 1 dose. 3 h after R | ↓ Cell apoptosis ↑ GST, GPx, SOD, and CAT activities [92] | |
| DAS |   SD SD250–300 g | Focal brain ischemia I: 2 h R: 24 h | 200 mg/Kg i.p. 7 doses. 24 h before | ↓ Neurological deficit and infarct volume. ↓ Apoptosis (DNA fragmentation levels and caspase-3 levels) ↑ Antiapoptotic markers (Bcl-2 levels) [94] |
| DATS |   Wistar Wistar280–320 g | Focal brain ischemia I: 1 h R: 7 days | 15 mg/Kg i.p. 4 doses. Before and 24, 48, and 72 h after R onset | ↓ Infarct area, and MDA and metalloproteinase 9 levels ↑ Nrf2 activation, CAT, and GPx activities, and SOD and GST levels [95] |
  SD SD250–280 g | Cardiac arrest cardiopulmonary resuscitation I: 5 min R: 24 h | 10 mg/Kg in tail vein. 1 dose. After successful resuscitation | ↓ Cerebral inflammation and MDA levels Preserve: SOD and CAT activity [88] | |
| E-ajoene and Z-ajone |   Unspecified weight | Global brain ischemia I: 5 min R: 3, 12, and 24 h and 5 days | 25 mg/kg p.o. 1 dose. 30 min before I | ↓ Cell damage in hippocampus ↓ Reactive astrogliosis and microgliosis ↓ LPO levels [87] |
ATP: adenosine triphosphate; Bcl-2: B cell lymphoma 2; CAT: catalase; DAS: diallyl sulfide; DATS: diallyl trisulfide; ERK1/2: extracellular signal-regulated kinase; GCLC: glutamate-cysteine ligase catalytic subunit; GCLM: glutamate-cysteine ligase regulatory subunit; GFAP: glial fibrillary acidic protein; GLUT3: glucose transporter 3; G6PDH: glucose 6-phosphate dehydrogenase; GPx: glutathione peroxidase; GR: glutathione reductase; GSH: reduced glutathione; GST: glutathione S-transferase; HO-1: heme oxygenase 1; I: ischemia; iNOS: inducible nitric oxide synthase; LPO: lipoperoxidation; MDA: malondialdehyde; MPO: myeloperoxidase; Nrf2: nuclear factor erythroid 2-related factor 2; p38: 38-kDa mitogen-activated protein kinase; p-JNK: phosphorylated c-Jun N-terminal kinase; R: reperfusion; ROS: reactive oxygen species; SAC: S-allylcysteine; SD: Sprague Dawley; SOD: superoxide dismutase; Sphk2: sphingosine kinase 2; TBARS: thiobarbituric acid-reactive substances; TNFα: tumor necrosis factor alpha.  : rat;
: rat;  : mouse;
: mouse;  : gerbil;
: gerbil;  : male. The antioxidant and anti-inflammatory effects and cognitive deficit are highlighted in orange, blue, and green, respectively.
: male. The antioxidant and anti-inflammatory effects and cognitive deficit are highlighted in orange, blue, and green, respectively.
 : rat;
: rat;  : mouse;
: mouse;  : gerbil;
: gerbil;  : male. The antioxidant and anti-inflammatory effects and cognitive deficit are highlighted in orange, blue, and green, respectively.
: male. The antioxidant and anti-inflammatory effects and cognitive deficit are highlighted in orange, blue, and green, respectively.3.3. Garlic Preparations as Treatment for Ischemic Stroke
Commercial garlic products, which contain a mixture of different OSCs, also show protection against global brain ischemia. Pretreatment with aqueous garlic extract reduces inflammation [96], whereas garlic oil decreases infarct volume and lipoperoxidation, and improves short-term memory and motor coordination [97]. In focal brain ischemia, AGE, aqueous garlic extract, and garlic clove and skin extracts (GCE and GSE) show brain tissue protection. AGE decreases neurological impairment, infarct area, and brain edema by reducing oxidative stress and inflammation [86,98,99] and increasing GLUT3 transporter [91]. Aqueous garlic extract improves neurobehavioral problems, diminishes cell death, and enhances antioxidant defense [100]. GCE and GSE reduce cell damage and increase mitochondrial membrane potential and ATP levels, which could be associated with its scavenging activity against superoxide anions, peroxynitrite, hydroxyl radicals, and peroxyl radicals (Table 4) [101].

Table 4.
Protective effect of garlic preparation in ischemia and reperfusion injury models associated with its antioxidant and anti-inflammatory properties.
3.4. Garlic Compounds and Neuroplasticity
At the time of writing this review, the neurotrophic effect of garlic and its compounds has only been assessed in models of neurological damage and aging, but not in stroke.
The neurotrophic effects of SAC include an increase in axonal branching, neurite length, and the number of neurites in hippocampal neuron cultures. The changes in the morphology of neurons are related to better efficiency of the transmission and information processing ability of the neural network [102,103]. Also, after cell damage triggered by excitotoxic insult with quinolinic acid, SAC treatment increases the levels of the neurotrophin BDNF, antioxidant defenses (HO-1) through the Nrf2 pathway, and ERK1/2 phosphorylation levels [104]. Furthermore, in vitro, SAC induces the neovasculogenesis of endothelial precursor cells, and this effect is comparable to that produced by the stem cell factor. SAC increases the positive cell number of the hematopoietic stem cell KIT proto-oncogene, receptor tyrosine kinase (c-kit), which is important for blood vessel formation, and activates the PI3K/AKT/endothelial nitric oxide synthase (eNOS) pathway. Moreover, the treatment of endothelial precursor cells with SAC induces the phosphorylation of GSK-3β, leading to a reduction in β-cat phosphorylation. β-cat translocates to the nucleus and enhances the expression of cyclin D1 and the proliferation of endothelial precursor cells [105].
Alliin has neurotrophic effects in hippocampal neurons, since it increases the number of branching points per axon and survival [102]. In contrast, DADS diminishes the proliferation of neuronal precursor cells [106] (Table 5).

Table 5.
Trophic effect of garlic compounds in vitro.
SAC is the OSC that is most studied in vivo, and its trophic effects have been proven in different models. Treatment administered for 21 days to young healthy animals increases the number of positive cells to marker of proliferation Ki67 (Ki67) and the marker of neuroblast differentiation (doublecortin) in the SGZ of the dentate gyrus in the hippocampus. Furthermore, SAC increases serotonin 1 A receptor levels, and the activation of these receptors increases neurogenesis in the dentate gyrus [107]. Also, it improves memory in senescence-accelerated animals, or damage due to streptozotocin or lipopolysaccharide [103,108,109]. Senescence-accelerated mouse prone is a model for aging and age-related disorders that has a short lifespan and age-dependent pathologies like impairment in learning and memories. The improvement in memory in senescence-accelerated mouse prone treated with SAC was accompanied by the preservation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), NMDAR, and phosphorylated α-calcium/calmodulin-dependent protein kinase II (CaMKII) in the hippocampus; these proteins are related to the maintenance of learning and memory functions [103].
The intraventricular streptozotocin administration model produces cognitive deficits and oxidative damage in the hippocampus. SAC prevents cognitive and neurobehavioral impairments, increases the antioxidant state (GSH, GPx and GR), and diminishes thiobarbituric acid-reactive substances (TBARS) and apoptotic parameters (DNA fragmentation, the expression of B cell lymphoma 2 (Bcl-2) and tumor protein p53 (p53)) [108]. Similarly, lipopolysaccharide administration induces learning and memory impairment and neuroinflammation. SAC improves memory, mitigates lipid peroxidation (malondyaldehyde) and augments SOD, GSH, and acetylcholinesterase activity. Furthermore, it downregulated hippocampal NF-κB, Toll like receptor 4 (TLR4), GFAP, IL-1β, and ionized calcium-binding adaptor molecule (Iba1) and upregulated Nrf2 [109]. Additionally, there are two studies that show the trophic SAC effect. The first one is a model of hind-limb ischemia, performed through the removal of the femoral artery. After surgery, SAC improves blood flow recovery in ischemic tissue through neovasculogenesis mediated by the increase in endothelial precursor cells (c-Kit-positive cells levels) [105]. The neovasculogenic effect of SAC has not been studied in the brain yet, but improvement in blood flow after ischemic stroke is relevant for maintaining collateral flow supply and facilitating the migration of new cells and NTs. In research conducted by Kurihara and collaborators [110], SAC shows hepatocyte proliferation through the increase in IGF-1 and its receptor after partial hepatectomy. IGF-1 is an NTs that promotes neuroplasticity; hence, this effect should be assessed in the brain (Table 6).

Table 6.
Neurotrophic effects of garlic compounds in vivo.
The other OSCs that have shown an increase in memory performance after injury are the allicin and Z-ajone [111,112]. The effects of allicin have been mainly related to morphological modifications, increasing the density of the dendritic spine, and synaptophysin and glutamate receptor-1 levels, indicating the formation of new synapses [111]. As mentioned before, the formation of new synapses after stroke has been linked to functional and cognitive recovery. Z-ajone has inhibitory effects against memory impairment induced by scopolamine [112] (Table 6).
Finally, DADS (10 or 20 mg/kg) administered for 28 or 35 days diminishes depressive behavior by increasing serotonin and dopamine levels through the activation of the BDNF/AKT/CREB pathway in rats with mild stress-induced depression [113]. However, in mice with lower doses (1 or 10 mg/kg for 14 days), DADS causes memory defects and diminishes cell proliferation, BDNF levels, and the phosphorylation of CREB and ERK, and these effects were also observed in vitro [106] (Table 6).
3.5. Garlic Preparations and Neuroplasticity
Garlic preparations have shown effects on neuroplasticity in vivo, improving the memory [114,115,116,117,118,119,120] of healthy young mice [114] and animals with cognitive deficits induced by lead [115], diabetes [116], monosodium glutamate [117,118], amyloid-β [119], and senescence acceleration [120] (Table 7).

Table 7.
Neurotrophic effects of garlic preparations in vivo.
Essential oils from two Allium species administered for 21 days to healthy animals increase memory, cell proliferation, and neuroblast differentiation in the dentate gyrus by increasing BDNF and acetylcholinesterase levels [114]. Also, after chronic mild stress, treatment with garlic oil diminishes depressive-like behavior, increasing serotonin and dopamine levels through the activate BDNF/AKT/CREB pathway in the hippocampus [113]. Aqueous garlic extract decreased blood lead levels and increased the neuroblast number (doublecortin-positive cells) in the dentate gyrus of 21-day-old offspring rats [115]. In the case of memory deficits caused by diabetes, cognitive impairment was related to the alteration of the fluidity of the membranes, inhibiting Na+/K+ ATPase and Ca2+ATPase. In that work, ethanolic garlic extract augmented the activity of both ATPases and glutamine synthetase in animals with diabetes [116]. Glutamine synthetase is an enzyme that is important in controlling the intracellular concentration of glutamate. The accumulation of glutamate in the extracellular fluid decreases the levels of glutamine synthetase, which may lead to seizures [116]. In addition, black garlic ethanol extract induces neurogenesis after injury caused by monosodium glutamate in the hippocampus, but not in the prefrontal cortex [117,118]. Finally, AGE diminishes the cognitive dysfunction caused by amyloid-β, ameliorating the loss of cholinergic neurons and increasing vesicular glutamate transporters and glutamate decarboxylase levels in the hippocampus [119]. Also, in senescence-accelerated mice, AGE increases lifespan and improves memory [120].
4. Final Remarks
This review focuses mainly on garlic, which was chosen for its low toxicity, ease of acquisition, and high bioavailability. Different garlic OSCs and preparations have been extensively utilized in preclinical studies for treating stroke. Their protective properties are principally attributed to their antioxidant and anti-inflammatory capacities assessed during short periods of ischemia and/or reperfusion. However, the mechanisms activated over longer periods, such as neuroplasticity, that are essential for effective patient recovery have not been studied. Despite this, both garlic compounds and preparations can stimulate neuroplasticity in healthy animals and models of neurological damage, suggesting that garlic compounds and preparations might stimulate neuroplasticity in ischemic stroke. Although this is a process that occurs after ischemic stroke, it requires an antioxidant and anti-inflammatory environment to ensure the survival of the new neurons and the proper functioning of connections between pre-existing neurons. Therefore, we propose studying the relationships among antioxidant, anti-inflammatory, and neuroplasticity mechanisms, since these mechanisms are activated in ischemic stroke and could offer a broader therapeutic window for intervention.
Finally, these investigations were conducted using young male animals, whereas ischemic stroke predominantly affects the older population and has a higher prevalence in women. Therefore, it is important that future research on the use of OSCs and garlic preparations as treatment for ischemic stroke include models with the characteristics of the affected population.
5. Conclusions
The research on the treatments for stroke using preparations and compounds derived from garlic are focused only on reducing damage through their antioxidant and anti-inflammatory properties, mainly in short times. However, garlic-derived preparations and compounds induce NT production, neovasculogenesis, and neuroplasticity in healthy animals and pathological models, suggesting that they could improve cognitive and motor function after stroke. For this reason, we propose that the induction of neuroplasticity using garlic compounds and preparations could represent an important therapeutic target. Hence, we assert that clinical research with garlic derivates must be carried out.
6. Future Directions
- Studies should be designed that focus on understanding the mechanisms through which garlic compounds and preparations can activate neuroplasticity processes and how this could produce an impact on the recovery of post-stroke patients.
- It is imperative that future works using garlic as a treatment for ischemic stroke include aged animals, both sexes, and animals with comorbidities.
- Preclinical findings associated with neuroplasticity though garlic derivates should be evaluated at a clinical level in future research.
Author Contributions
Conceptualization, S.M.B.-P. and P.D.M.; investigation, S.M.B.-P., C.A.S.-I., O.U.S.-M., J.T.-T., J.M.B.-M., D.B.-O. and P.D.M.; writing original draft preparation, S.M.B.-P., C.A.S.-I., O.U.S.-M., J.T.-T., J.M.B.-M., D.B.-O. and P.D.M.; writing-review and editing, S.M.B.-P., C.A.S.-I., O.U.S.-M., J.T.-T., J.M.B.-M., D.B.-O. and P.D.M.; funding acquisition, P.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by a CONAHCYT grant (number A1-S-21433) awarded to PDM. Sandra Monserrat Bautista-Perez received a scholarship from CONAHCYT (number 778387).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Sandra Monserrat Bautista-Perez is a PhD student from the Programa de Maestría y Doctorado en Ciencias Bioquímicas, Universidad Nacional Autónoma de México (UNAM), and this publication is derived from their doctoral thesis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, X.; Wang, Y.; Ding, Z.J.; Yue, B.; Zhang, P.Z.; Chen, X.D.; Chen, X.; Chen, J.; Chen, F.Q.; Chen, Y.; et al. The role of RIP3 mediated necroptosis in ouabain-induced spiral ganglion neurons injuries. Neurosci. Lett. 2014, 2014, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Digaleh, H.; di Napoli, M.; Stranges, S.; Behrouz, R.; Shojaeianbabaei, G.; Amiri, A.; Tabrizi, R.; Mokhber, N.; Spence, J.D.; et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Godwin, K.M.; Wasserman, J.; Ostwald, S.K. Cost associated with stroke: Outpatient rehabilitative services and medication. Top. Stroke Rehabil. 2011, 18, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Cowled, P.; Fitridge, R. Pathophysiology of reperfusion injury. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists, 1st ed.; Fitridge, R., Matthew, T., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; pp. 331–350. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Roy-O’Reilly, M.A.; Ahnstedt, H.; Spychala, M.S.; Munshi, Y.; Aronowski, J.; Sansing, L.H.; McCullough, L.D. Aging exacerbates neutrophil pathogenicity in ischemic stroke. Aging 2020, 12, 436–461. [Google Scholar] [CrossRef] [PubMed]
- Yousufuddin, M.; Young, N. Aging and ischemic stroke. Aging 2019, 11, 2542–2544. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.M.E.E.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Dotson, A.L.; Offner, H. Sex differences in the immune response to experimental stroke: Implications for translational research. J. Neurosci. Res. 2017, 95, 437–446. [Google Scholar] [CrossRef]
- Kaidonis, G.; Rao, A.N.; Ouyang, Y.B.; Stary, C.M. Elucidating sex differences in response to cerebral ischemia: Immunoregulatory mechanisms and the role of microRNAs. Prog. Neurobiol. 2019, 176, 73–85. [Google Scholar] [CrossRef]
- Manwani, B.; Liu, F.; Scranton, V.; Hammond, M.D.; Sansing, L.H.; McCullough, L.D. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp. Neurol. 2013, 249, 120–131. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion-from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Chin, J.; Mucke, L. A network dysfunction perspective on neurodegenerative diseases. Nature 2006, 443, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jaffer, T.; Eguchi, S.; Wang, Z.; Linkermann, A.; Ma, D. Role of necroptosis in the pathogenesis of solid organ injury. Cell Death Dis. 2015, 6, e1975. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Domercq, M.; Matute, C. Excitotoxicity therapy for stroke patients still alive. EBioMedicine 2019, 39, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef]
- Crack, P.J.; Taylor, J.M. Reactive oxygen species and the modulation of stroke. Free Radic. Biol. Med. 2005, 38, 1433–1444. [Google Scholar] [CrossRef]
- Rodrigo, R.; Fernandez-Gajardo, R.; Gutierrez, R.; Matamala, J.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative Stress and Pathophysiology of Ischemic Stroke: Novel Therapeutic Opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef]
- Yang, J. The role of reactive oxygen species in angiogenesis and preventing tissue injury after brain ischemia. Microvasc. Res. 2019, 123, 62–67. [Google Scholar] [CrossRef]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Flügge, G. Adult neuroplasticity: More than 40 years of research. Neural Plast. 2014, 2014, 541870. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E.; Antila, H. Neuronal plasticity and neurotrophic factors in drug responses. Mol. Psychiatry 2017, 22, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Toricelli, M.; Pereira, A.; Souza Abrao, G.; Malerba, H.; Maia, J.; Buck, H.; Viel, T. Mechanisms of neuroplasticity and brain degeneration: Strategies for protection during the aging process. Neural Regen. Res. 2021, 16, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.; Barnes, C. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Li, P.; Boyd, J.D.; Delaney, K.R.; Murphy, T.H. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J. Neurosci. 2007, 27, 4101–4109. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Wong, C.; Murphy, T.H. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke 2008, 39, 1286–1291. [Google Scholar] [CrossRef]
- Cotman, C.W. Axon Sprouting and Reactive Synaptogenesis. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects; Siegel, G.J., Agranoff, B.W., Albers, R.W., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999. Available online: https://www.ncbi.nlm.nih.gov/books/NBK28183/ (accessed on 17 July 2023).
- Koh, S.H.; Park, H.H. Neurogenesis in Stroke Recovery. Transl. Stroke Res. 2017, 8, 3–13. [Google Scholar] [CrossRef]
- Zhang, J.; Chopp, M. Cell-based therapy for ischemic stroke. Expert Opin. Biol. Ther. 2013, 13, 1229–1240. [Google Scholar] [CrossRef]
- Dun, X.P.; Parkinson, D.B. Role of Netrin-1 Signaling in Nerve Regeneration. Int. J. Mol. Med. Sci. 2017, 18, 491. [Google Scholar] [CrossRef]
- Ming, G.-L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2012, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, R.M.; Morshead, C.M. Home sweet home: The neural stem cell niche throughout development and after injury. Cell Tissue Res. 2018, 371, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Kokaia, Z. Neurogenesis following stroke affecting the adult brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a019034. [Google Scholar] [CrossRef] [PubMed]
- Shohayeb, B.; Diab, M.; Ahmed, M.; Ng, D.C.H. Factors that influence adult neurogenesis as potential therapy. Transl. Neurodegener. 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.M.; Buga, A.M.; Popa-Wagner, A. Neurovascular remodeling in the aged ischemic brain. J. Neural Transm. 2015, 122, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.P.; Teixeira, A.L. Neurotrophic Factors in Aging. In Encyclopedia of Geropsychology; Pachana, N.A., Ed.; Springer: Singapore, 2017; pp. 1628–1638. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Al-Dwairi, A. Mechanisms and regulation of neurotrophin synthesis and secretion. Neurosciences 2016, 21, 306–313. [Google Scholar] [CrossRef] [PubMed]
- White, R.E.; Rao, M.; Gensel, J.C.; Mctigue, D.M.; Kaspar, B.K.; Jakeman, L.B. Transforming Growth Factor α Transforms Astrocytes to a Growth-Supportive Phenotype after Spinal Cord Injury. J. Neurosci. 2011, 31, 15173–15187. [Google Scholar] [CrossRef]
- Dai, X.; Chen, J.; Xu, F.; Zhao, J.; Cai, W.; Sun, Z.; Hitchens, T.K.; Foley, L.M.; Leak, R.K.; Chen, J.; et al. TGFα preserves oligodendrocyte lineage cells and improves white matter integrity after cerebral ischemia. J. Cereb. Blood Flow Metab. 2020, 40, 639–655. [Google Scholar] [CrossRef]
- Sarver, D.C.; Lei, X.; Wong, G.W. FAM19A (TAFA): An Emerging Family of Neurokines with Diverse Functions in the Central and Peripheral Nervous System. ACS Chem. Neurosci. 2021, 12, 945–958. [Google Scholar] [CrossRef]
- Lanfranconi, S.; Locatelli, F.; Corti, S.; Candelise, L.; Comi, G.P.; Baron, P.L.; Strazzer, S.; Bresolin, N.; Bersano, A. Growth factors in ischemic stroke. J. Cell. Mol. Med. 2011, 15, 1645–1687. [Google Scholar] [CrossRef]
- Zacchigna, S.; Lambrechts, D.; Carmeliet, P. Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Yanuar, I.; Sulistio, A.; Heese, K. Neurotrophin Signaling and Stem Cells-Implications for Neurodegenerative Diseases and Stem Cell Therapy. Mol. Neurobiol. 2017, 54, 7401–7459. [Google Scholar] [CrossRef] [PubMed]
- Minichiello, L. Long-term potentiation Synaptic plasticity TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009, 10, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, D.E.; Josselyn, S.A. Plasticity-related genes in brain development and amygdala-dependent learning. Genes Brain Behav. 2016, 15, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.J.; Sun, M.K.; Hongpaisan, J.; Alkon, D.L. Insulin, PKC signaling pathways and synaptic remodeling during memory storage and neuronal repair. Eur. J. Pharmacol. 2008, 585, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.M.; Wang, Y.C.; Lee TT, Y.; Tsai, C.F.; Chen, H.S.; Lai, F.J.; Yokoyama, K.K.; Hsieh, T.H.; Wu, R.M.; Lee, J.N. Dynamic Trk and G Protein Signalings Regulate Dopaminergic Neurodifferentiation in Human Trophoblast Stem Cells. PLoS ONE 2015, 10, e0143852. [Google Scholar] [CrossRef] [PubMed]
- Leu, T.; Fandrey, J.; Schreiber, T. (H)IF applicable: Promotion of neurogenesis by induced HIF-2 signalling after ischaemia. Pflug. Arch. 2021, 473, 1287–1299. [Google Scholar] [CrossRef]
- Kärkkäinen, V.; Pomeshchik, Y.; Savchenko, E.; Dhungana, H.; Kurronen, A.; Lehtonen, S.; Naumenko, N.; Tavi, P.; Levonen, A.L.; Yamamoto, M.; et al. Nrf2 Regulates Neurogenesis and Protects Neural Progenitor Cells Against Aβ Toxicity. Stem Cells 2014, 32, 1904–1916. [Google Scholar] [CrossRef]
- Ray, S.; Corenblum, M.J.; Anandhan, A.; Reed, A.; Ortiz, F.O.; Zhang, D.D.; Barnes, C.A.; Madhavan, L. A Role for Nrf2 Expression in Defining the Aging of Hippocampal Neural Stem Cells. Cell Transplant. 2018, 27, 589–606. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Rojo, A.I.; Ferreiro, E.; Núñez, Á.; Krause, K.H.; Jaquet, V.; Cuadrado, A. Transcription factor NRF2 controls the fate of neural stem cells in the subgranular zone of the hippocampus. Redox Biol. 2017, 13, 393–401. [Google Scholar] [CrossRef]
- Corenblum, M.J.; Ray, S.; Remley, Q.W.; Long, M.; Harder, B.; Zhang, D.D.; Barnes, C.A.; Madhavan, L. Reduced Nrf2 expression mediates the decline in neural stem cell function during a critical middle-age period. Aging Cell 2016, 15, 725–736. [Google Scholar] [CrossRef]
- Li, Q.; Lou, J.; Yang, T.; Wei, Z.; Li, S.; Zhang, F. Ischemic Preconditioning Induces Oligodendrogenesis in Mouse Brain: Effects of Nrf2 Deficiency. Cell. Mol. Neurobiol. 2022, 42, 1859–1873. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, S.; Ruff, I.; Bernstein, R.S. Acute Stroke Intervention: A Systematic Review. JAMA 2015, 313, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.E.; Middleton, S.; Hamilton, H.; Cudlip, F.; Swatzell, V.; Alexandrov, A.V.; Lightbody, E.; Watkins, D.C.; Philip, S.; Cadilhac, D.A.; et al. Does the Addition of Non-Approved Inclusion and Exclusion Criteria for rtPA Impact Treatment Rates? Findings in Australia, the UK, and the USA. Interv. Neurol. 2020, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Demaerschalk, B.M.; Kleindorfer, D.O.; Adeoye, O.M.; Demchuk, A.M.; Fugate, J.E.; Grotta, J.C.; Khalessi, A.A.; Levy, E.I.; Palesch, Y.Y.; Prabhakaran, S.; et al. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, 581–641. [Google Scholar] [CrossRef] [PubMed]
- Campbell BC, V.; de Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Caprio, F.Z.; Sorond, F.A. Cerebrovascular Disease Primary and Secondary Stroke Prevention. Med. Clin. North Am. 2019, 103, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; Thomalla, G. Causes and secondary prevention of acute ischemic stroke in adults. Hamostaseologie 2020, 40, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B.; Cekovska, S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010, 4, 106–110. [Google Scholar] [CrossRef]
- Rivlin, R.S. Historical perspective on the use of garlic. J Nutr. 2001, 131, 951–954. [Google Scholar] [CrossRef]
- Ekşi, G.; Mine, A.; Özkan, G.; Koyuncu, M. Garlic and onions: An eastern tale. J. Ethnopharmacol. 2020, 253, 112675. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H.; Al-Wabel, N.A. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm. J. 2010, 18, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H. Clarifying the Real Bioactive Constituents of Garlic. J. Nutr. 2006, 136, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Alvarenga, L.; Cardozo, L.F.M.F.; Chermut, T.R.; Sequeira, J.; de Souza Gouveia Moreira, L.; Teixeira KT, R.; Shiels, P.G.; Stenvinkel, P.; Mafra, D. From the distinctive smell to therapeutic effects: Garlic for cardiovascular, hepatic, gut, diabetes and chronic kidney disease. Clin. Nutr. 2021, 40, 4807–4819. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Hori, Y.; Myoda, Y. Volatile compounds of fresh and processed garlic. Exp. Ther. Med. 2020, 19, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Abenavoli, L.; Borrelli, F.; Capasso, R.; Izzo, A.A.; Lembo, F.; Romano, B.; Capasso, F. Garlic: Empiricism or science? Nat. Prod. Commun. 2009, 4, 1785–1796. [Google Scholar] [CrossRef]
- Lawson, L.D. Garlic: A Review of Its Medicinal Effects and Indicated Active Compounds. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [CrossRef]
- Yoo, M.; Lee, S.; Kim, S.; Hwang, J.B.; Choe, J.; Shin, D. Composition of organosulfur compounds from cool- and warm-type garlic (Allium sativum L.) in Korea. Food Sci. Biotechnol. 2014, 23, 337–344. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kumagai, H. Characteristics, biosynthesis, decomposition, metabolism and functions of the garlic odour precursor, S-allyl-L-cysteine sulfoxide. Exp. Ther. Med. 2020, 19, 1528–1535. [Google Scholar] [CrossRef]
- Borek, C. Antioxidant Health Effects of Aged Garlic Extract. J. Nutr. 2001, 131, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Miao, Y.; Chen, J.Y.; Zhang, Q.; Wang, J. Effective production of S-allyl-L-cysteine through a homogeneous reaction with activated endogenous γ-glutamyltranspeptidase in garlic (Allium Sativum). J. Food Sci. Technol. 2015, 52, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, J.C.; Chang, N.; Chun, H.S.; Kim, W.K. S-Allyl-l-cysteine attenuates cerebral ischemic injury by scavenging peroxynitrite and inhibiting the activity of extracellular signal-regulated kinase. Free Radic. Res. 2006, 40, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, P.D.; Alvarez-Idaboy, J.R.; Aguilar-González, A.; Lira-Rocha, A.; Jung-Cook, H.; Medina-Campos, O.N.; Pedraza-Chaverrí, J.; Galano, A. Role of allyl group in the hydroxyl and peroxyl radical scavenging activity of S-allylcysteine. J. Phys. Chem. B 2011, 115, 13408–13417. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Pung, D.; Leong, V.; Hebbar, V.; Shen, G.; Nair, S.; Li, W.; Tony Kong, A.N. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: Effect of chemical structure and stress signals. Free Radic. Biol. Med. 2004, 37, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.D.; Augustine, L.M.; Maher, J.M.; Nelson, D.M.; Slitt, A.L.; Klaassen, C.D.; Lehman-McKeeman, L.D.; Cherrington, N.J. Induction of Drug-Metabolizing Enzymes by Garlic and Allyl Sulfide Compounds via Activation of Constitutive Androstane Receptor and Nuclear Factor E2-Related Factor 2. Drug Metab. Dispos. 2007, 35, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Jing, X.; Wei, X.; Perez, R.G.; Ren, M.; Zhang, X.; Lou, H. S-allyl cysteine activates the Nrf2-dependent antioxidant response and protects neurons against ischemic injury in vitro and in vivo. J. Neurochem. 2015, 133, 298–308. [Google Scholar] [CrossRef]
- Arreola, R.; Quintero-Fabián, S.; Lopez-Roa, R.L.; Flores-Gutierrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuno-Sahagun, D. Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef]
- Moutia, M.; Habti, N.; Badou, A. In Vitro and In Vivo Immunomodulator Activities of Allium sativum L. Evid. Based Complement. Alternat. Med. 2018, 2018, 4984659. [Google Scholar] [CrossRef]
- Kim, J.M.; Hyun, J.C.; Kim, W.K.; Chang, N.; Hyang, S.C. Structure−Activity Relationship of Neuroprotective and Reactive Oxygen Species Scavenging Activities for Allium Organosulfur Compounds. J. Agric. Food Chem. 2006, 54, 6547–6553. [Google Scholar] [CrossRef]
- Lin, J.J.; Chang, T.; Cai, W.K.; Zhang, Z.; Yang, Y.X.; Sun, C.; Li, X.Y.; Li, W.X. Post-injury administration of allicin attenuates ischemic brain injury through sphingosine kinase 2: In vivo and in vitro studies. Neurochem. Int. 2015, 89, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Li, G.L.; Wang, B.A.; Qin, Y.; Bai, S.R.; Rong, J.; Deng, T.; Li, Q. Diallyl trisufide protects against oxygen glucose deprivation -induced apoptosis by scavenging free radicals via the PI3K/Akt -mediated Nrf2/HO-1 signaling pathway in B35 neural cells. Brain Res. 2015, 1614, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Numagami, Y.; Sato, S.; Ohnishi, S.T. Attenuation of rat ischemic brain damage by aged garlic extracts: A possible protecting mechanism as antioxidants. Neurochem. Int. 1996, 29, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.Y.; Kim, W.; Nam, S.M.; Yoo, M.; Lee, S.; Yoon, Y.S.; Won, M.H.; Hwang, I.K.; Choi, J.H. Neuroprotective effects of Z-ajoene, an organosulfur compound derived from oil-macerated garlic, in the gerbil hippocampal CA1 region after transient forebrain ischemia. Food Chem. Toxicol. 2014, 72, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Wen, S.; Huang, K.; Huang, J.; Chu, X.; Wang, F.; Pang, L. Novel controlled and targeted releasing hydrogen sulfide system exerts combinational cerebral and myocardial protection after cardiac arrest. J. Nanobiotechnology 2021, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Atif, F.; Yousuf, S.; Agrawal, S.K. S-Allyl L-cysteine diminishes cerebral ischemia-induced mitochondrial dysfunctions in hippocampus. Brain Res. 2009, 1265, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Ashafaq, M.; Khan, M.M.; Shadab Raza, S.; Ahmad, A.; Khuwaja, G.; Javed, H.; Khan, A.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. S-allyl cysteine mitigates oxidative damage and improves neurologic deficit in a rat model of focal cerebral ischemia. Nutr. Res. 2012, 32, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.D.; Aguilera, P.; Ortiz-Plata, A.; López, F.N.; Chánez-Cárdenas, M.E.; Flores-Alfaro, E.; Ruiz-Tachiquín, M.E.; Espinoza-Rojo, M. Aged garlic extract and S-allylcysteine increase the GLUT3 and GCLC expression levels in cerebral ischemia. Adv. Clin. Exp. Med. 2019, 28, 1609–1614. [Google Scholar] [CrossRef]
- Kong, X.; Gong, S.; Su, L.; Li, C.; Kong, Y. Neuroprotective effects of allicin on ischemia-reperfusion brain injury. Oncotarget 2017, 8, 104492–104507. [Google Scholar] [CrossRef]
- Zhang, B.; Li, F.; Zhao, W.; Li, J.; Li, Q.; Wang, W. Protective effects of allicin against ischemic stroke in a rat model of middle cerebral artery occlusion. Mol. Med. Rep. 2015, 12, 3734–3738. [Google Scholar] [CrossRef]
- Lin, X.; Yu, S.; Chen, Y.; Wu, J.; Zhao, J.; Zhao, Y. Neuroprotective effects of diallyl sulfide against transient focal cerebral ischemia via anti-apoptosis in rats. Neurol. Res. 2013, 34, 32–37. [Google Scholar] [CrossRef]
- Silva-Islas, C.A.; Chánez-Cárdenas, M.E.; Barrera-Oviedo, D.; Ortiz-Plata, A.; Pedraza-Chaverri, J.; Maldonado, P.D. Diallyl Trisulfide Protects Rat Brain Tissue against the Damage Induced by Ischemia-Reperfusion through the Nrf2 Pathway. Antioxidants 2019, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Batirel, H.F.; Aktan, S.; Aykut, C.; Yeǧen, B.C.; Coşkun, T. The effect of aqueous garlic extract on the levels of arachidonic acid metabolites (leukotriene C4 and prostaglandin E2) in rat forebrain after ischemia-reperfusion injury. Prostaglandins Leukot. Essent. Fat. Acids 1996, 54, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Singh, M.; Sharma, A. Neuroprotective effect of antioxidants on ischaemia and reperfusion-induced cerebral injury. Pharmacol. Res. 2003, 48, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, P.; Chánez-Cárdenas, M.E.; Ortiz-Plata, A.; León-Aparicio, D.; Barrera, D.; Espinoza-Rojo, M.; Villeda-Hernández, J.; Sánchez-García, A.; Maldonado, P.D. Aged garlic extract delays the appearance of infarct area in a cerebral ischemia model, an effect likely conditioned by the cellular antioxidant systems. Phytomedicine 2010, 17, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Colín-González, A.L.; Ortiz-Plata, A.; Villeda-Hernández, J.; Barrera, D.; Molina-Jijón, E.; Pedraza-Chaverrí, J.; Maldonado, P.D. Aged Garlic Extract Attenuates Cerebral Damage and Cyclooxygenase-2 Induction after Ischemia and Reperfusion in Rats. Plant Foods Hum. Nut. 2011, 66, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Ahmad, M.; Ahmad, A.S.; Yousuf, S.; Ansari, M.A.; Khan, M.B.; Ishrat, T.; Islam, F. Behavioral and Histologic Neuroprotection of Aqueous Garlic Extract After Reversible Focal Cerebral Ischemia. J. Med. Food 2007, 9, 537–544. [Google Scholar] [CrossRef]
- Cervantes, M.I.; De Oca Balderas, P.M.; de Jesús Gutiérrez-Baños, J.; Orozco-Ibarra, M.; Fernández-Rojas, B.; Medina-Campos, O.M.; Espinoza-Rojo, M.; Ruiz-Tachiquín, M.; Ortiz-Plata, A.; Salazar, M.I.; et al. Comparison of antioxidant activity of hydroethanolic fresh and aged garlic extracts and their effects on cerebral ischemia. Food Chem. 2013, 140, 343–352. [Google Scholar] [CrossRef]
- Moriguchi, T.; Matsuura, H.; Kodera, Y.; Itakura, Y.; Katsuki, H.; Saito, H.; Nishiyama, N. Neurotrophic activity of organosulfur compounds having a thioallyl group on cultured rat hippocampal neurons. Neurochem. Res. 1997, 22, 1449–1452. [Google Scholar] [CrossRef]
- Hashimoto, M.; Nakai, T.; Masutani, T.; Unno, K.; Akao, Y. Improvement of Learning and Memory in Senescence-Accelerated Mice by S-Allylcysteine in Mature Garlic Extract. Nutrients 2020, 12, 1834. [Google Scholar] [CrossRef]
- Reyes-Soto, C.Y.; Rangel-López, E.; Galván-Arzate, S.; Colín-González, A.L.; Silva-Palacios, A.; Zazueta, C.; Pedraza-Chaverri, J.; Ramírez, J.; Chavarria, A.; Túnez, I.; et al. S-Allylcysteine Protects Against Excitotoxic Damage in Rat Cortical Slices Via Reduction of Oxidative Damage, Activation of Nrf2/ARE Binding, and BDNF Preservation. Neurotox. Res. 2020, 38, 929–940. [Google Scholar] [CrossRef]
- Syu, J.N.; Yang, M.D.; Tsai, S.Y.; Chiang, E.P.I.; Chiu, S.C.; Chao, C.Y.; Rodriguez, R.L.; Tang, F.Y. S-allylcysteine Improves Blood Flow Recovery and Prevents Ischemic Injury by Augmenting Neovasculogenesis. Cell Transplant. 2017, 26, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Taek Ji, S.; Kim, M.S.; Ra Park, H.; Lee Lee, E.Y.; Jung Jang, Y.; Sik Kim, H.; Lee, J. Diallyl disulfide impairs hippocampal neurogenesis in the young adult brain. Toxicol. Lett. 2013, 221, 31–38. [Google Scholar] [CrossRef]
- Nam, S.M.; Yoo, Y.; Kim, W.; Yoo, M.; Kim, D.W.; Won, M.H.; Hwang, I.K.; Yoon, Y.S. Effects of S-Allyl-L-Cysteine on Cell Proliferation and Neuroblast Differentiation in the Mouse Dentate Gyrus. J. Vet. Med. Sci. 2011, 73, 1071–1075. [Google Scholar] [CrossRef][Green Version]
- Javed, H.; Khan, M.M.; Khan, A.; Vaibhav, K.; Ahmad, A.; Khuwaja, G.; Ahmed, M.E.; Raza, S.S.; Ashafaq, M.; Tabassum, R.; et al. S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2011, 1389, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, M.; Baluchnejadmojarad, T.; Kiasalari, Z.; Afshin-Majd, S.; Roghani, M. Garlic active constituent s-allyl cysteine protects against lipopolysaccharide-induced cognitive deficits in the rat: Possible involved mechanisms. Eur. J. Pharmacol. 2017, 795, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K.; Moteki, H.; Natsume, H.; Ogihara, M.; Kimura, M. The Enhancing Effects of S-Allylcysteine on Liver Regeneration Are Associated with Increased Expression of mRNAs Encoding IGF-1 and Its Receptor in Two-Thirds Partially Hepatectomized Rats. Biol. Pharm. Bull. 2020, 43, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Li, X.; Yang, B.; Fang, X.; Jia, J.; Ren, J.; Dong, Y.; Ou-Yang, C.; Wang, G. Allicin attenuates tunicamycin-induced cognitive deficits in rats via its synaptic plasticity regulatory activity. Iran. J. Basic Med. Sci. 2017, 20, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Hattori, A.; Hayashi, T.; Nishikawa, T.; Fukuda, H.; Fujino, T. Improvement of scopolamine-induced memory impairment by Z-ajoene in the water maze in mice. Pharmacol. Biochem. Behav. 2004, 78, 787–791. [Google Scholar] [CrossRef]
- Huang, Y.J.; Lu, K.H.; Lin, Y.E.; Panyod, S.; Wu, H.Y.; Chang, W.T.; Sheen, Y. Garlic essential oil mediates acute and chronic mild stress-induced depression in rats via modulation of monoaminergic neurotransmission and brain-derived neurotrophic factor levels. Food Funct. 2019, 10, 8094–8105. [Google Scholar] [CrossRef]
- Jung, H.Y.; Lee, K.Y.; Yoo, D.Y.; Kim, J.W.; Yoo, M.; Lee, S.; Yoo, K.Y.; Yoon, Y.S.; Hoon Choi, J.; Hwang, I.K. Essential oils from two Allium species exert effects on cell proliferation and neuroblast differentiation in the mouse dentate gyrus by modulating brain-derived neurotrophic factor and acetylcholinesterase. BMC Complement. Med. Ther. 2016, 16, 431. [Google Scholar] [CrossRef] [PubMed]
- Alipour, F.; Bideskan, A.E.; Fazel, A.; Sadeghi, A.; Hami, J.; Kheradmand, H.; Haghir, H. Protective effects of ascorbic acid and garlic extract against neurogenesis inhibition caused by developmental lead exposure in the dentate gyrus of rat. Comp. Clin. Pathol. 2014, 23, 1681–1687. [Google Scholar] [CrossRef]
- Semuyaba, I.; Alao Safiriyu, A.; Ayikobua Tiyo, E.; Figueredo Niurka, R. Memory Improvement Effect of Ethanol Garlic (A. sativum) Extract in Streptozotocin-Nicotinamide Induced Diabetic Wistar Rats Is Mediated through Increasing of Hippocampal Sodium-Potassium ATPase, Glutamine Synthetase, and Calcium ATPase Activities. Evid. Based Complement. Alternat. Med. 2017, 2017, 3720380. [Google Scholar] [CrossRef] [PubMed]
- Nurmasitoh, T.; Sari, D.C.R.; Partadiredja, G. The effects of black garlic on the working memory and pyramidal cell number of medial prefrontal cortex of rats exposed to monosodium glutamate. Drug Chem. Toxicol. 2017, 41, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Hermawati, E.; Dwi, C.; Ratna, C.; Ginus Partadiredja, S. The effects of black garlic ethanol extract on the spatial memory and estimated total number of pyramidal cells of the hippocampus of monosodium glutamate-exposed adolescent male Wistar rats. Anat. Sci. Int. 2015, 90, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Thorajak, P.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Effects of Aged Garlic Extract on Cholinergic, Glutamatergic and GABAergic Systems with Regard to Cognitive Impairment in Aβ-Induced Rats. Nutrients 2017, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Takashina, K.; Chu, P.J.; Saito, H.; Nishiyama, N. Prolongation of Life Span and Improved Learning in the Senescence Accelerated Mouse Produced by Aged Garlic Extract. Biol. Pharm. Bull. 1994, 17, 1589–1594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
