Ethyl Gallate Isolated from Castanopsis cuspidata var. sieboldii Branches Inhibits Melanogenesis and Promotes Autophagy in B16F10 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of CCS Extracts and Solvent Fractions
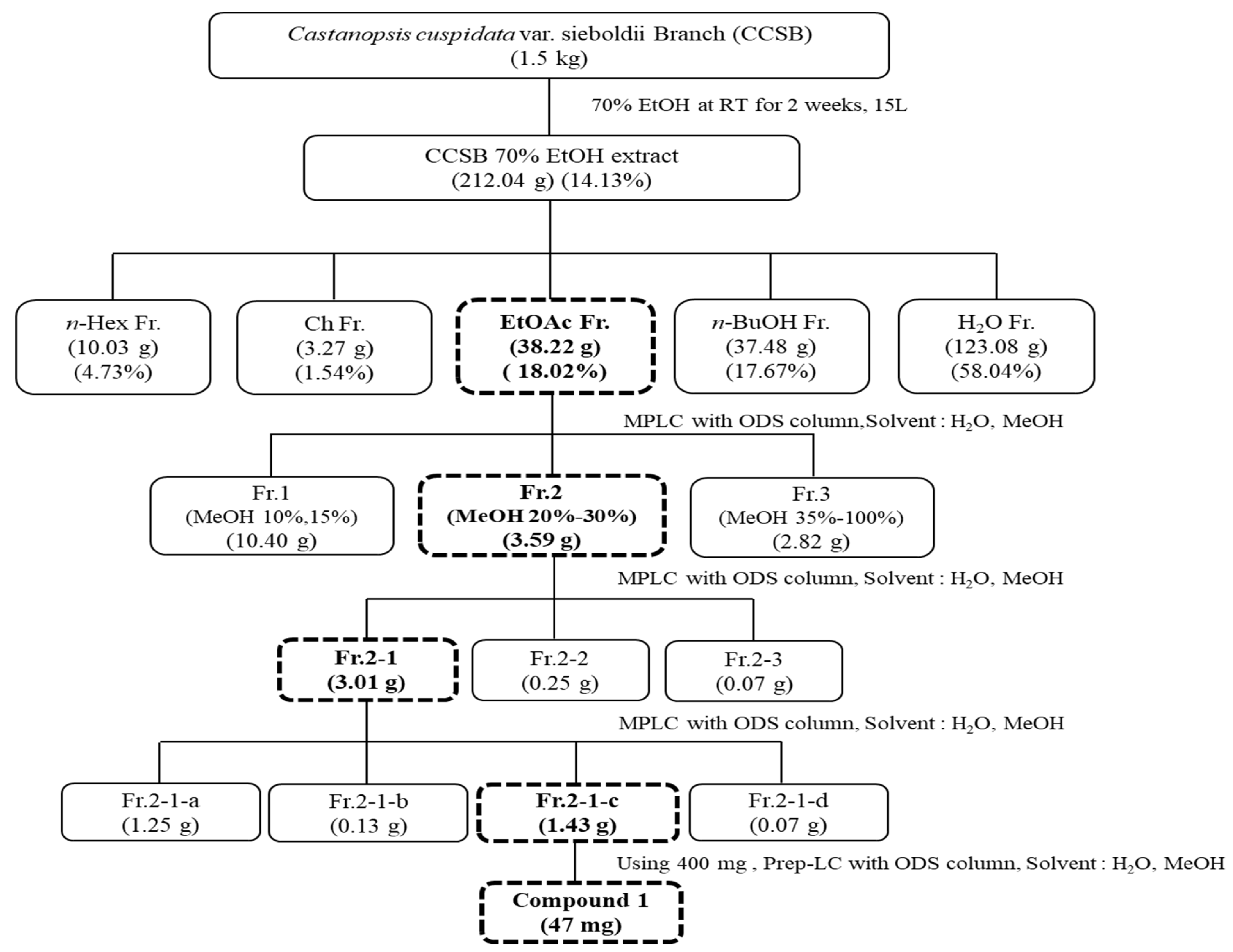
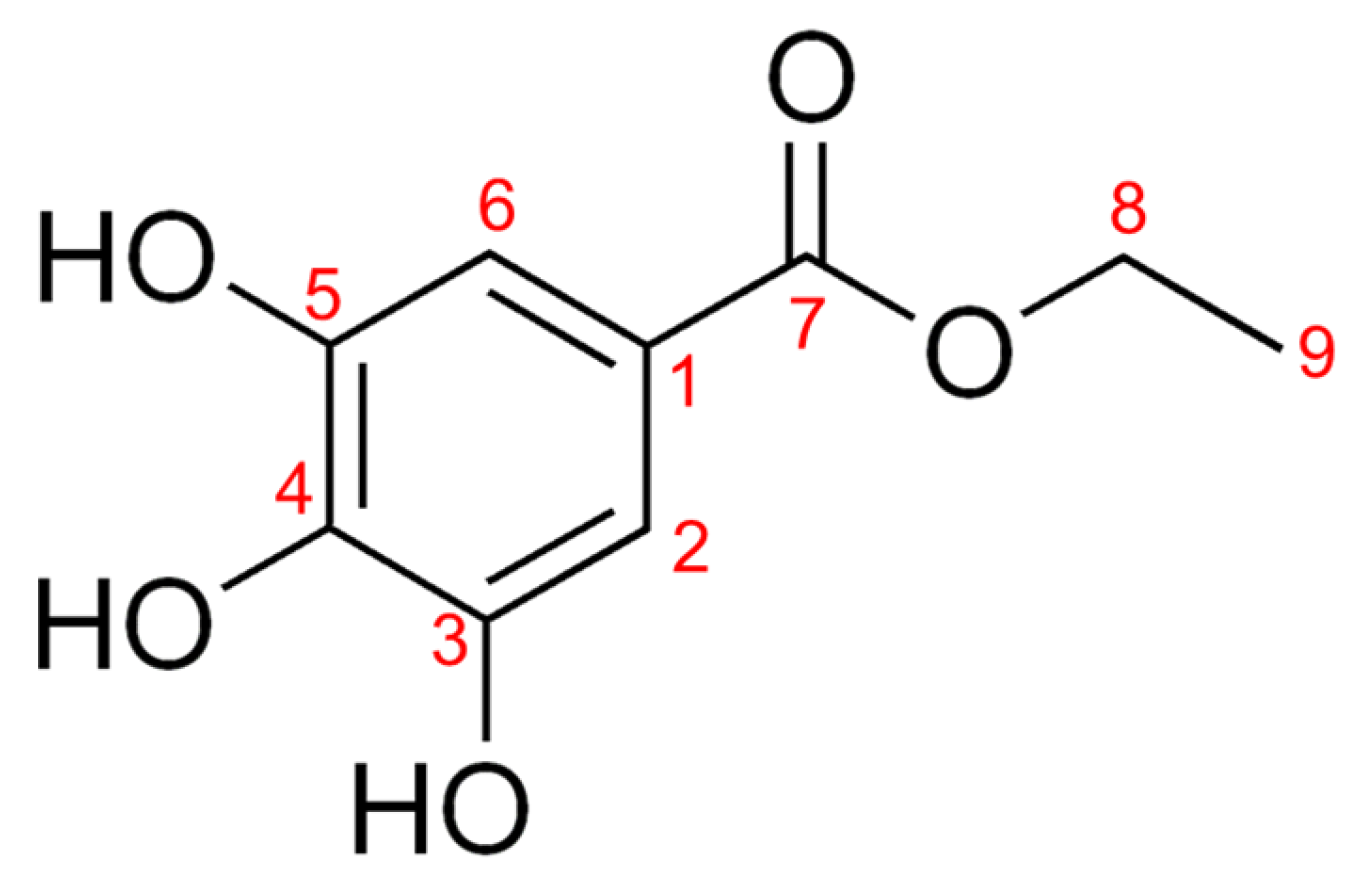
2.3. Separation of Active Components from EtOAc Fractions of CCS Branches
2.4. Antioxidant Activity Assay
2.4.1. Measurement of DPPH Free Radical Scavenging Activity
2.4.2. Measurement of ABTS Cation Radical Scavenging Activity
2.5. Total Polyphenol Content Analysis
2.6. Analysis of Total Flavonoid Content
2.7. Quantitative Analysis of Polyphenols Using HPLC MS/MS
2.8. Antimelanogenic Activity Assay
2.8.1. Tyrosinase Inhibition Assay
2.8.2. Enzyme Kinetics Assay
2.9. Molecular Docking Procedure
2.10. Cell Culture
2.11. MTT Cell Viability Assay
2.12. Measurement of Melanin Content
2.13. Measurement of ROS Production
2.14. Immunoblot Analysis
2.15. RNA Isolation and RT-PCR
2.16. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Yield Results of CCSB
3.2. Antioxidant Activity Results of CCSB and Other Fractions
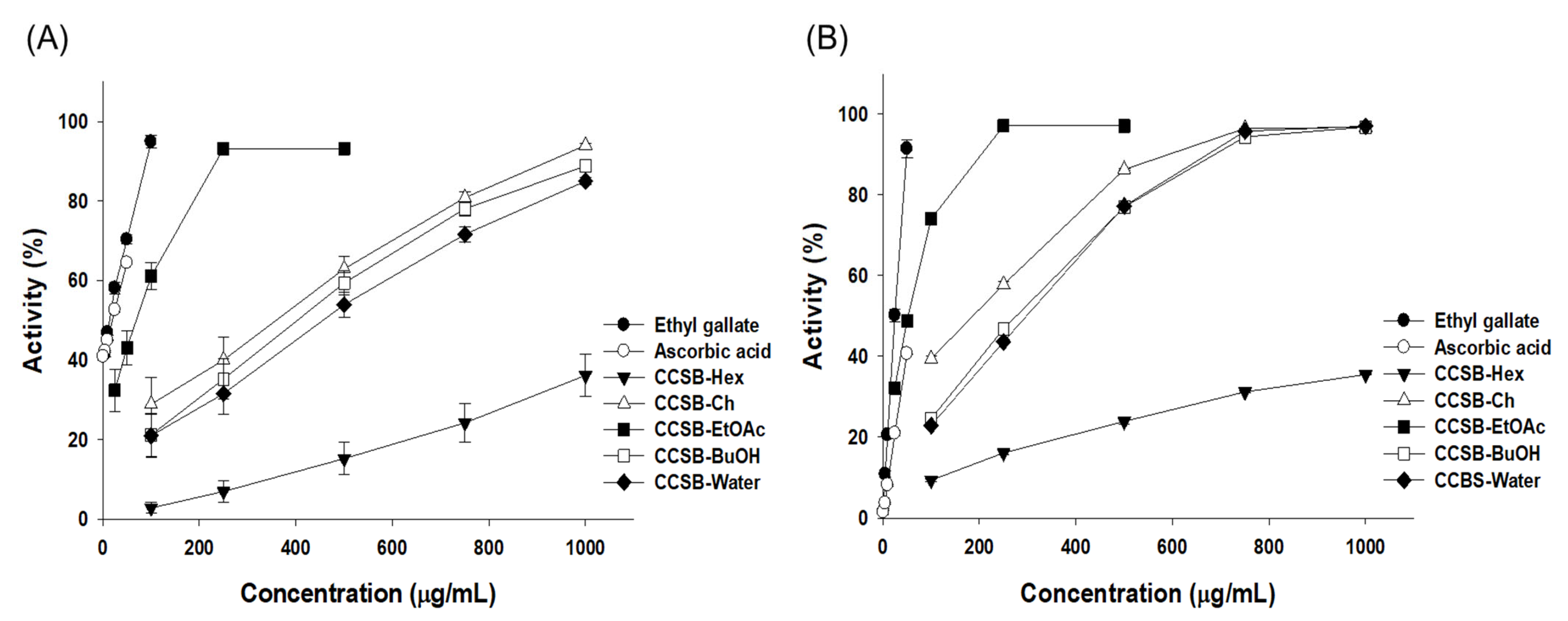
3.2.1. DPPH Radical Scavenging Activity
3.2.2. ABTS Radical Scavenging Activity
3.3. Total Polyphenol and Total Flavonoid Contents
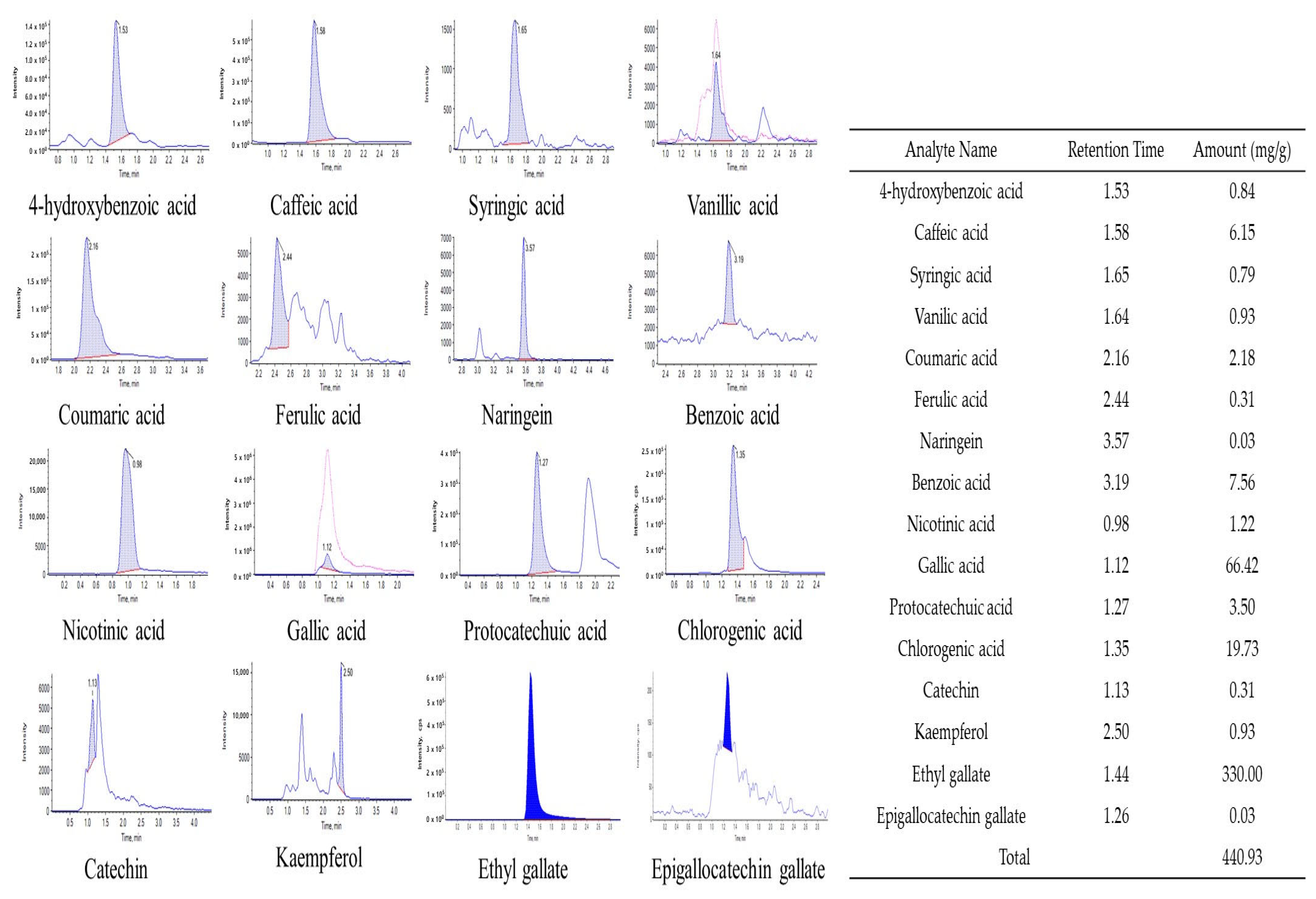
3.4. Polyphenol Content Analysis Using HPLC MS/MS
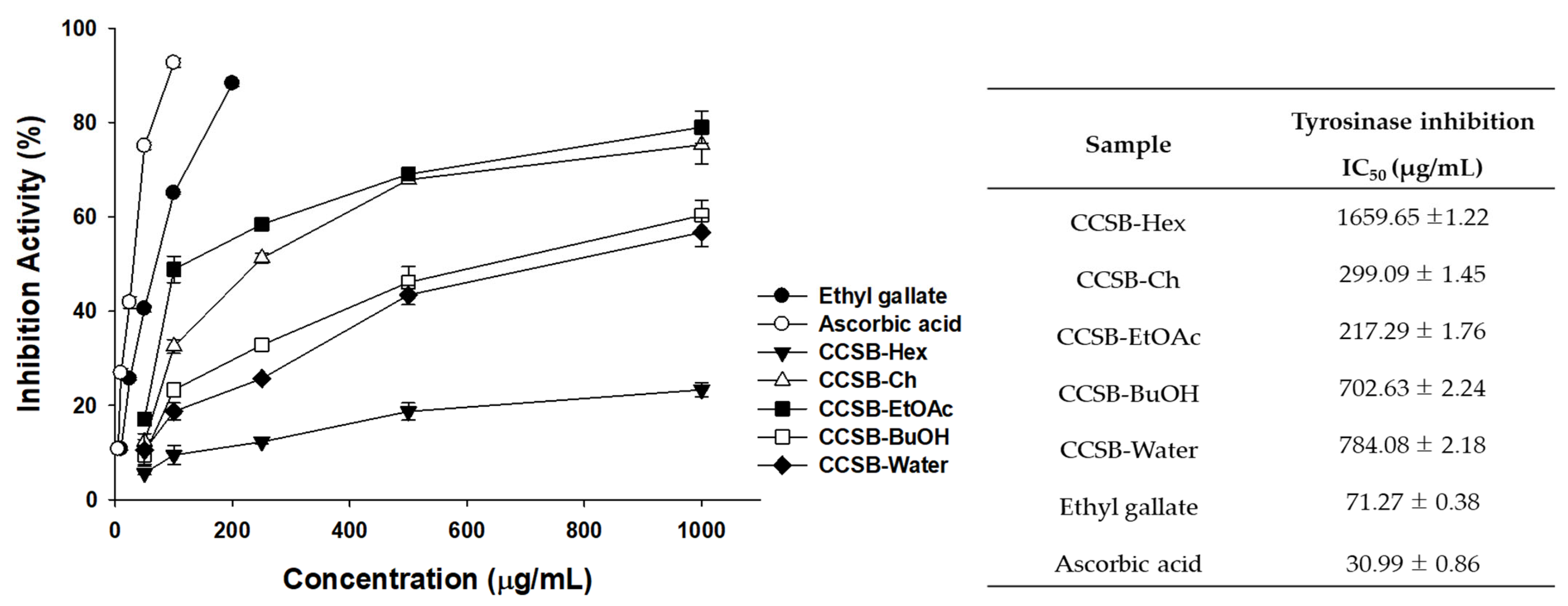
3.5. Results of EG Tyrosinase Inhibitory Activity Using a Cell-Free System
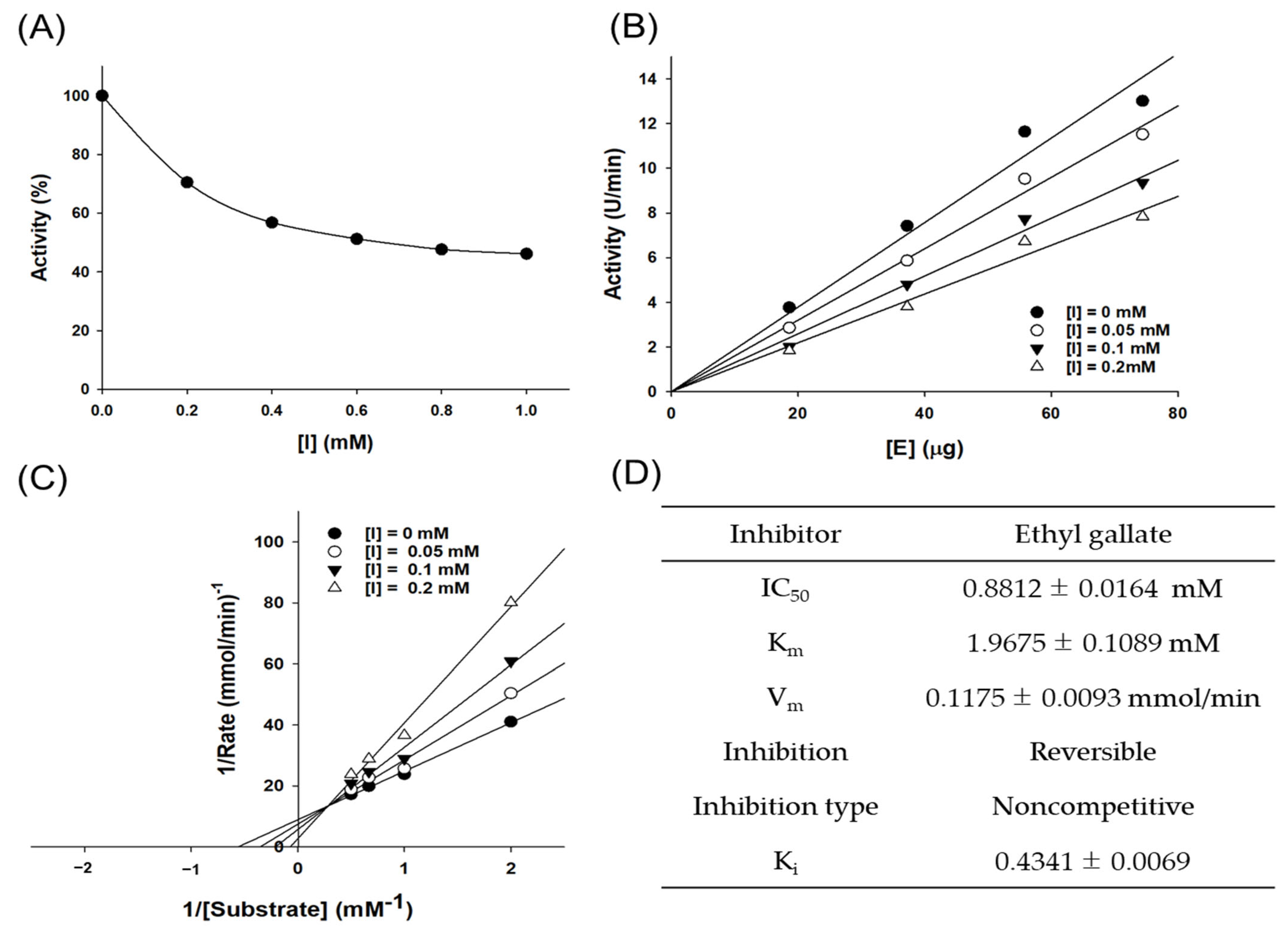
3.6. Enzyme Kinetics Analysis of Tyrosinase
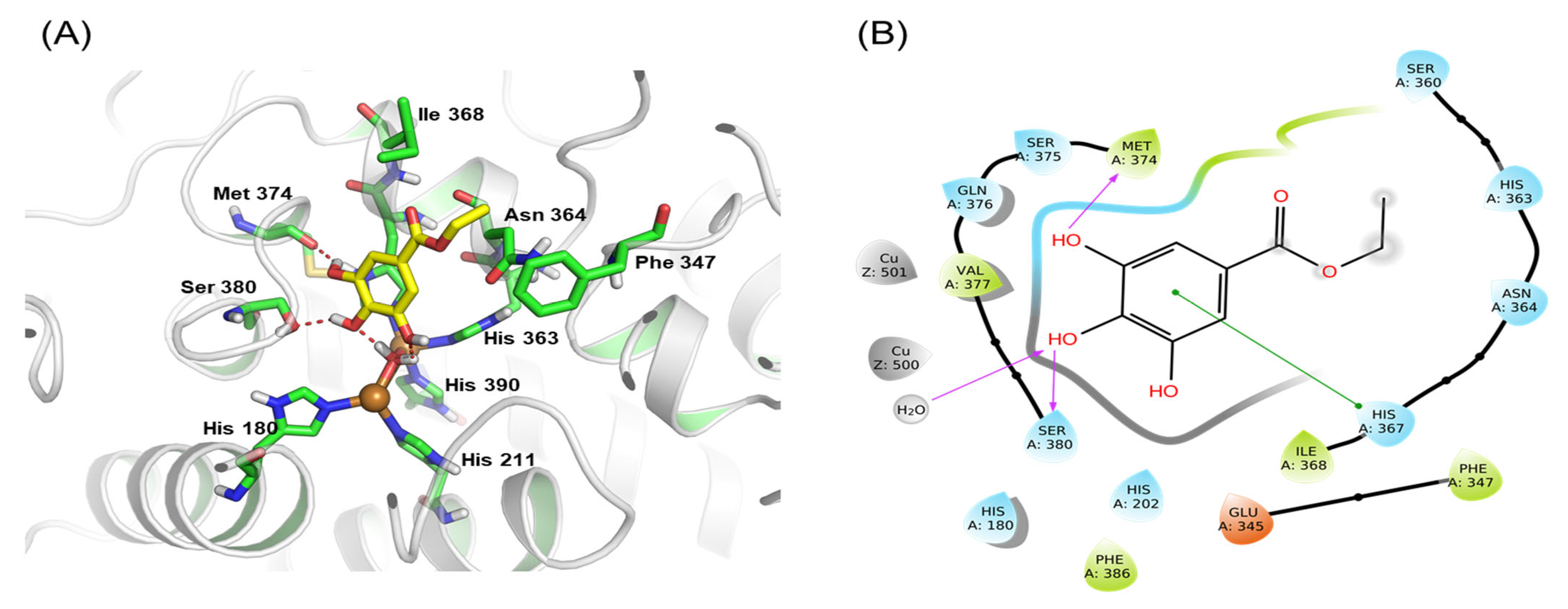
3.7. Molecular Docking Study
3.8. Cytotoxicity Evaluation and Quantitative Analysis of Intracellular ROS
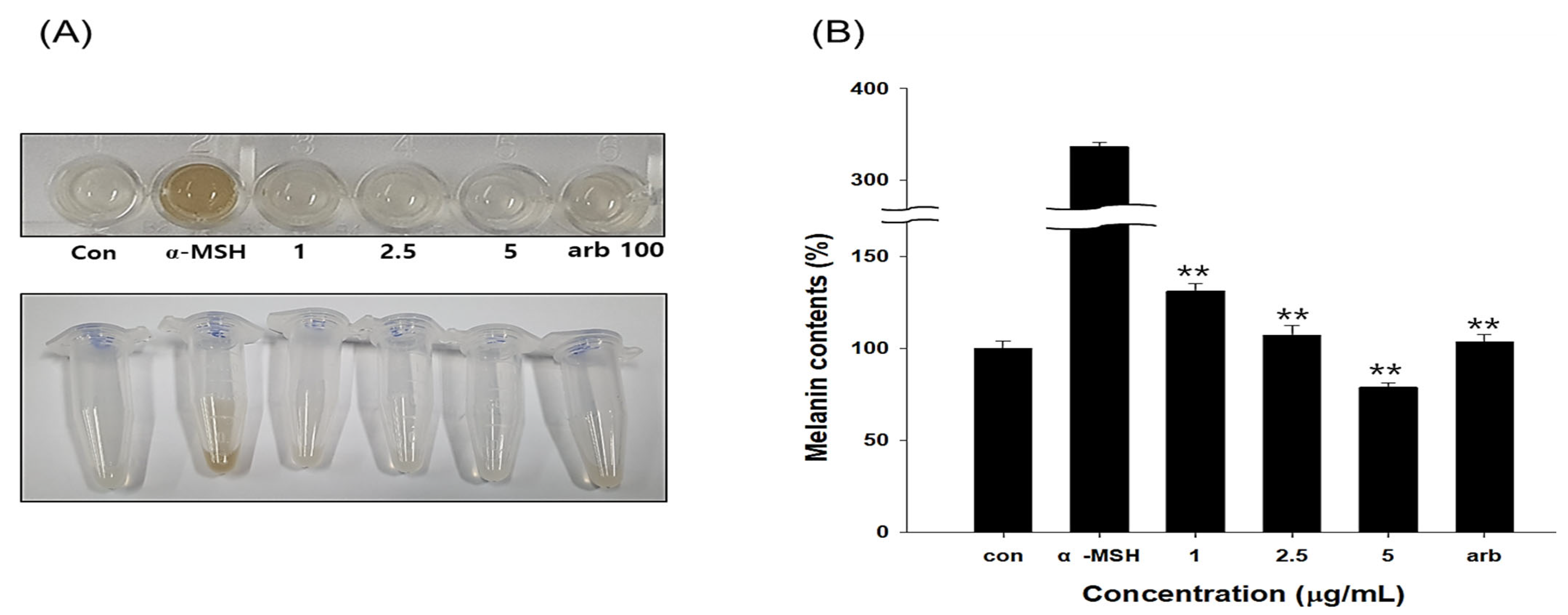
3.9. Results of Melanin Content by EG in B16F10 Cells
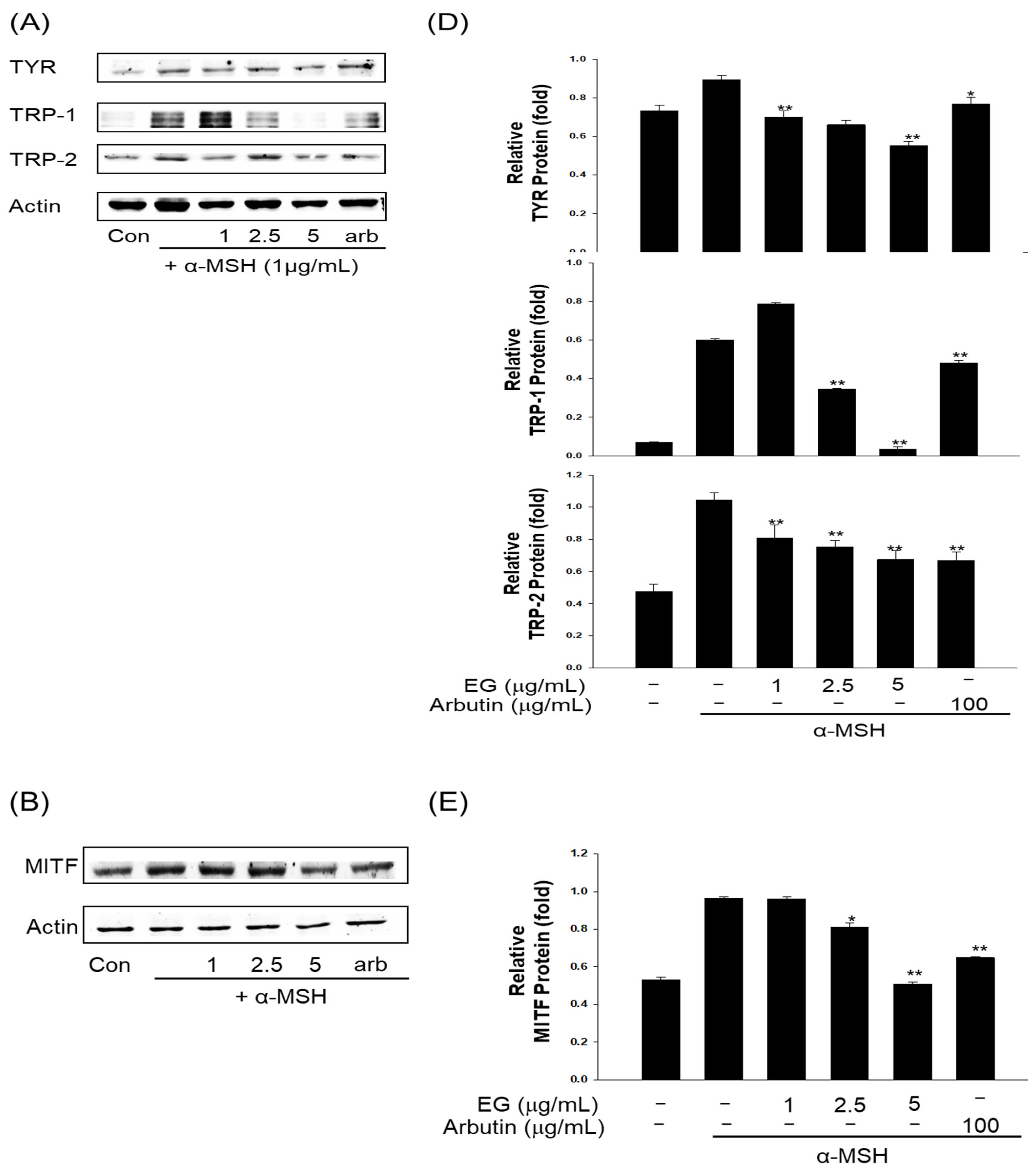
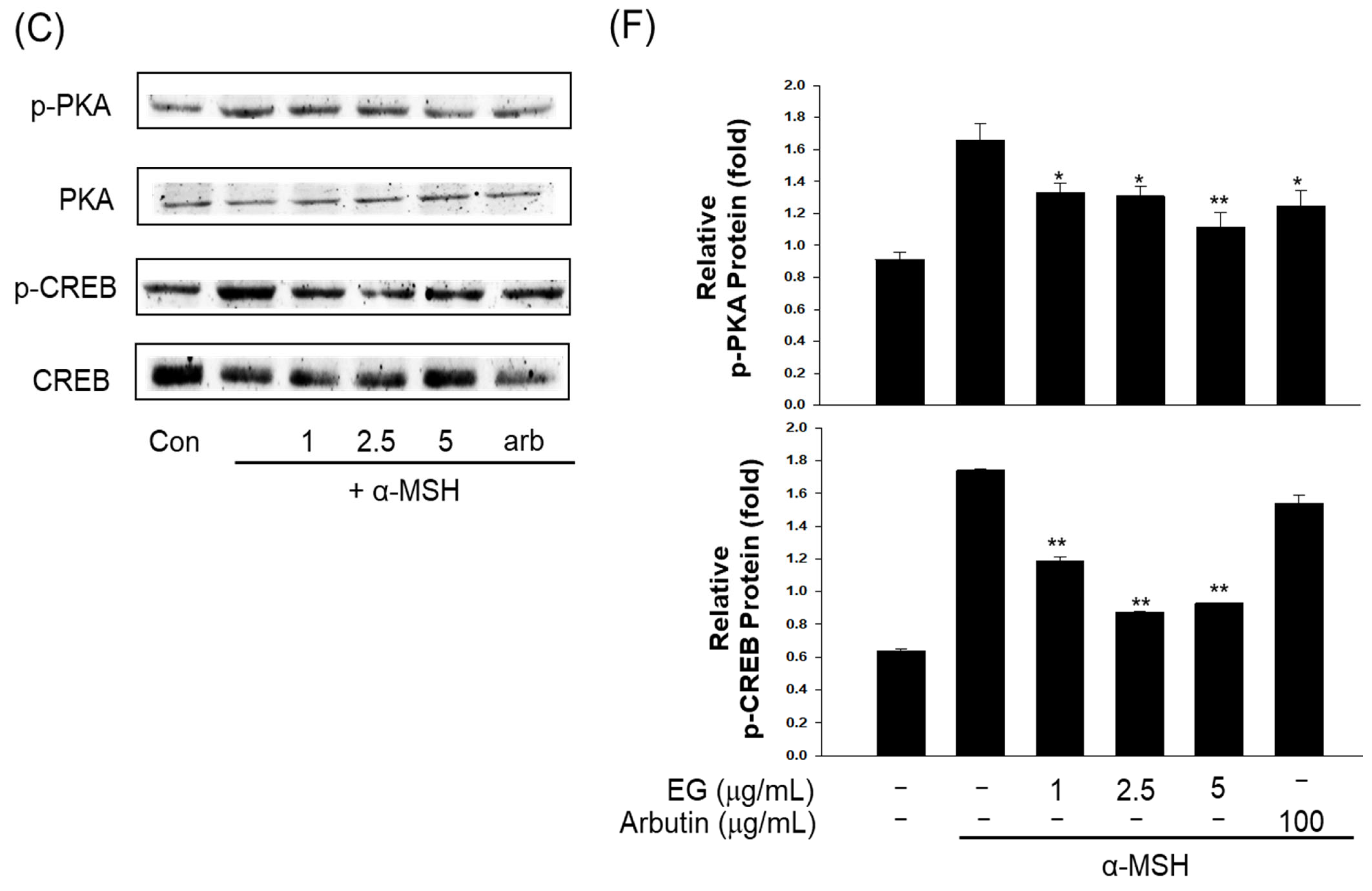
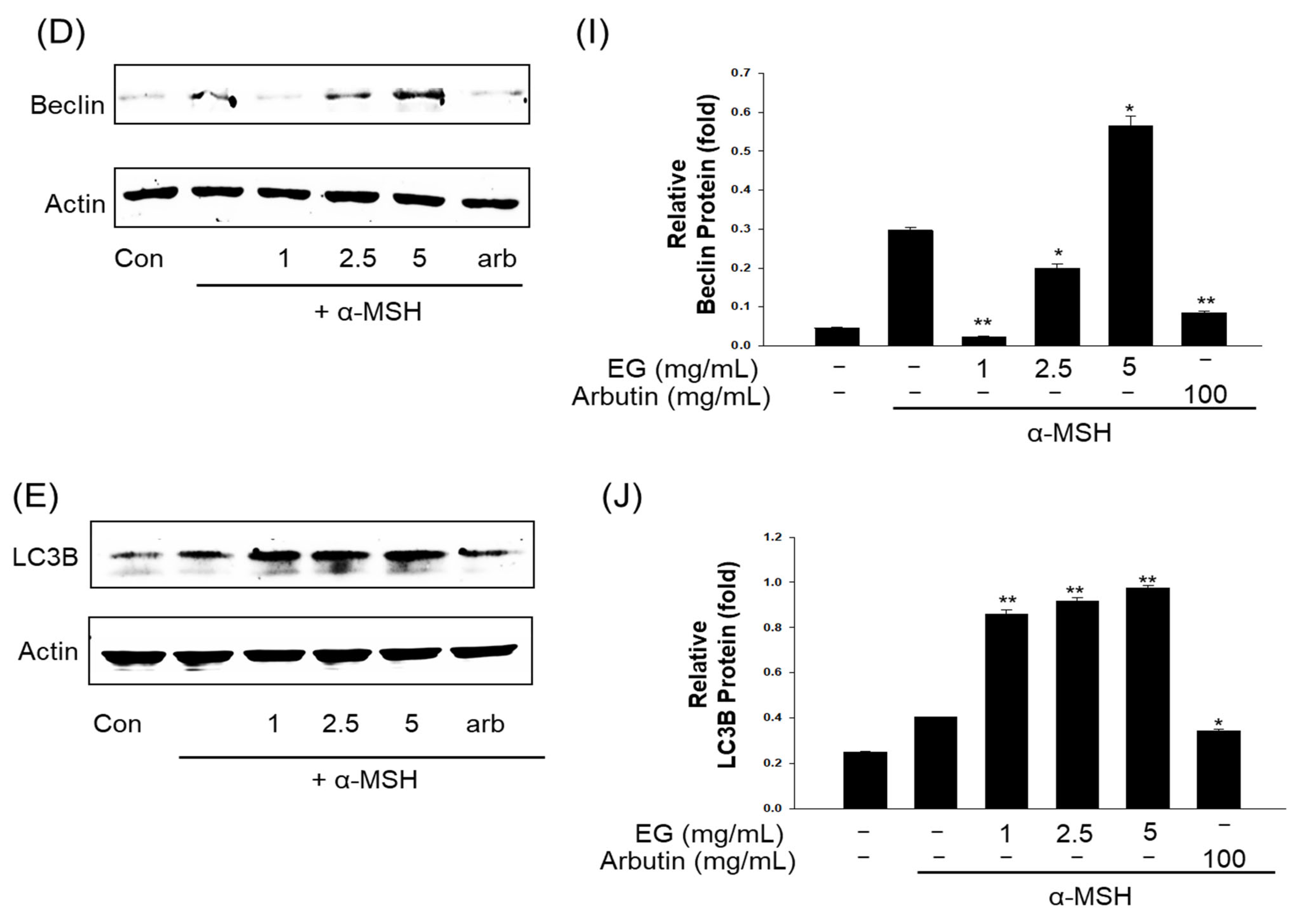
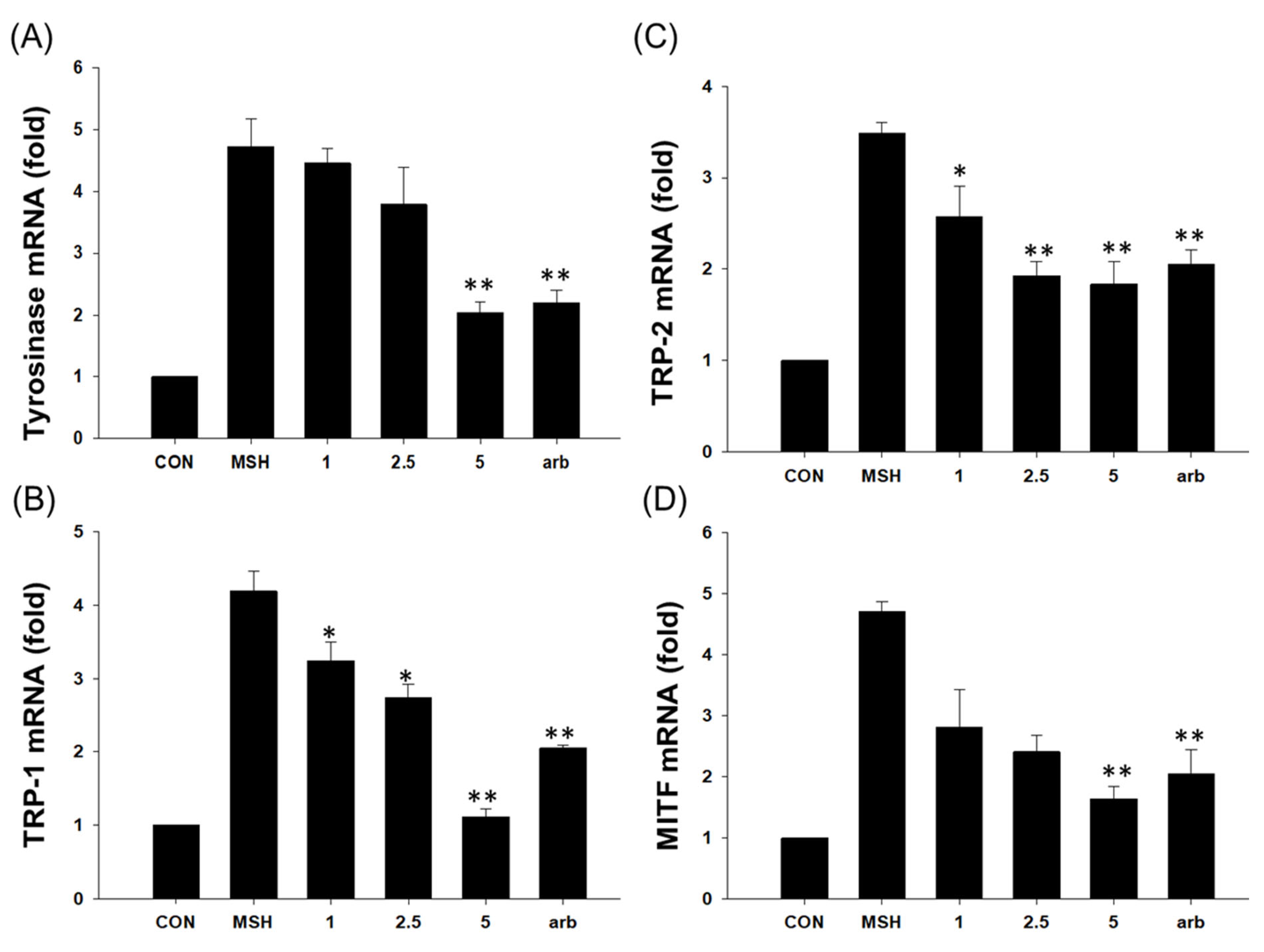
3.10. Effect of EG on Antimelanogenesis-Related Proteins/Genes and Autophagy-Related Proteins in the B16F10 Cell Line
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yun, M.E.; Lee, Y.S.; Lee, Y.J.; Park, Y.M.; Park, S.N. Antimicrobial, antioxidant and cellular protective effects of Houttuynia cordata extract and fraction. Appl. Chem. Eng. 2018, 29, 452–460. [Google Scholar]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Afonso, V.; Champy, R.; Mitrovic, D.; Collin, P.; Lomri, A. Reactive oxygen species and superoxide dismutases: Role in joint diseases. Jt. Bone Spine 2007, 74, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N. Protective effect of isoflavone, genistein from soybean on singlet oxygen induced photohemolysis of human erythrocytes. Korean J. Food Sci. Technol. 2003, 35, 510–518. [Google Scholar]
- Slater, T.F. Free radical mechanisms in tissue injury. Biochem. J. 1988, 222, 209–218. [Google Scholar]
- Oh, S.I. Effect of melatonin on rat skeletal muscles of oophorectomized rat: Oxidative stress and anti of oophorectomized rat: Oxidative stress and antioxidative enzyme activities. Korean J. Gerontol. 2002, 12, 1–10. [Google Scholar]
- Kohen, R. Skin antioxidants: Their role in aging and in oxidative stress—New approaches for their evaluation. Biomed. Pharmacother. 1999, 53, 181–192. [Google Scholar] [CrossRef]
- Schatrffetter-Kochanek, K. Photoaging of the connective tissue of skin: Its prevention and therapy. Adv. Pharmacol. 1996, 38, 639–655. [Google Scholar]
- Masaki, H.; Sskaki, S.; Atsumi, T.; Sakurai, H. Active-oxygen scavenging activity of plant extracts. Biol. Pharm. Bull. 1995, 18, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.; Park, S.H.; Kim, M.H.; Lee, S.; Cho, Y.K.; Kim, Y.A.; Park, B.J.; Kang, H.; Cho, J.Y. Anti-melanogenic effects of ethanol extracts of the leaves and roots of Patrinia villosa (thunb.) Juss through their inhibition of CREB and induction of ERK and autophagy. Molecules 2020, 25, 5375. [Google Scholar] [CrossRef]
- Nonaka, G.; Ageta, M.; Nishioka, I. Tannins and related compounds XXV. A new class of gallotannins possessing a (-)-shikimic acid core from Castanopsis cuspidata var. sieboldii NAKAI (1). Chem. Pharm. Bull. 1985, 33, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.F.; He, R.J.; Li, D.P.; Huang, Y.L. Phytochemical and chemotaxonomic study on Castanopsis fargesii Franch. Biochem. Syst. Ecol. 2018, 78, 113–115. [Google Scholar] [CrossRef]
- Ageta, M.; Nonaka, G.; Nishioka, I. Tannins and related compounds. LXVII: Isolation and characterization of castanopsinins A–H, novel ellagitannins containing a triterpenoid glycoside core, from Castanopsis cuspidata var. sieboldii NAKAI (3). Chem. Pharm. Bull. 1988, 36, 1646–1663. [Google Scholar] [CrossRef] [Green Version]
- Ageta, M.; Ishimaru, K.; Nonaka, G.I.; Nishioka, I. Tannins and related compounds. LXIV: Six new phenol glucoside gallates from Castanopsis cuspidata var. sieboldii NAKAI (2). Chem. Pharm. Bull. 1987, 36, 870–876. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.W.; Park, E.J.; Cho, H.M.; An, J.P.; Chin, Y.W.; Kim, J.; Sung, S.H.; Oh, W.K. Glucose uptake-stimulating galloyl ester triterpenoids from Castanopsis sieboldii. J. Nat. Prod. 2020, 83, 3093–3101. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Liang, J.H.; Lin, H.R.; Yang, C.S.; Liaw, C.C.; Wang, I.C.; Chen, J.J. Bioactive components from Ampelopsis japonica with antioxidant, anti-α-glucosidase, and antiacetylcholinesterase activities. Antioxidants 2022, 11, 1228. [Google Scholar] [CrossRef]
- Dunford, N.T.; Gumus, Z.P.; Gur, C.S. Chemical composition and antioxidant properties of pecan shell water extracts. Antioxidants 2022, 11, 1127. [Google Scholar] [CrossRef]
- Park, Y.K.; Koo, M.H.; Ikegaki, M.; Contado, J.L. Comparison of the flavonoid aglycone contents of Apis mellifera propolis from various regions of Brazil. Arq. Biol. Tecnol. 1997, 40, 97–106. [Google Scholar]
- Choi, M.H.; Jo, H.G.; Yang, J.H.; Ki, S.H.; Shin, H.J. Antioxidative and anti-melanogenic activities of bamboo stems (Phyllostachys nigra variety henosis) via PKA/CREB-mediated MITF downregulation in B16F10 melanoma cells. Int. J. Mol. Sci. 2018, 19, 409. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.H.; Yang, S.H.; Kim, D.S.; Kim, N.D.; Shin, H.J.; Liu, K. Novel quercetin derivative of 3,7-dioleylquercetin shows less toxicity and highly potent tyrosinase inhibition activity. Int. J. Mol. Sci. 2021, 22, 4264. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Matthew, P.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins 2004, 55, 351–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, K.; Wu, C.; Damm, W.; Reboul, M.; Stevenson, J.M.; Lu, C.; Dahlgren, M.K.; Mondal, S.; Chen, W.; Wang, L.; et al. OPLS3e: Extending force field coverage for drug-like small molecules. J. Chem. Theory Comput. 2019, 15, 1863–1874. [Google Scholar] [CrossRef]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure of human tyrosinase related protein 1 reveals a binuclear zinc active site important for melanogenesis. Angew. Chem. Int. Ed. 2017, 56, 9812–9815. [Google Scholar] [CrossRef] [Green Version]
- Dou, J.; Pan, M.; Wen, P.; Li, Y.; Tang, Q.; Chu, L.; Zhao, F.; Jiang, C.; Hu, W.; Hu, K.; et al. Isolation and identification of cancer stem-like cells from murine melanoma cell lines. Cell Mol. Immunol. 2007, 4, 467–472. [Google Scholar]
- Carmichael, J.; Mitchell, J.B.; DeGraff, W.G.; Gamson, J.; Gazdar, A.F.; Johnson, B.E.; Glatstein, E.; Minna, J.D. Chemosensitivity testing of human lung cancer cell lines using the MTT assay. Br. J. Cancer 1988, 57, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1α, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985, 45, 1474–1478. [Google Scholar]
- Yang, J.H.; Choi, M.H.; Yang, S.H.; Cho, S.S.; Park, S.J.; Shin, H.J.; Ki, S.H. Potent anti-inflammatory and antiadipogenic properties of bamboo (Sasa coreana Nakai) leaves extract and its major constituent flavonoids. J. Agric. Food Chem. 2017, 65, 6665–6673. [Google Scholar] [CrossRef]
- Lee, Y.; Im, E. Regulation of miRNAs by natural antioxidants in cardiovascular diseases: Focus on SIRT1 and eNOS. Antioxidants 2021, 10, 377. [Google Scholar] [CrossRef]
- Zhanga, Z.; Liaoc, L.; Moored, J.; Wua, T.; Wanga, Z. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.). Food Chem. 2009, 113, 160–165. [Google Scholar] [CrossRef]
- Marzouk, M.S.; El-Toumy, S.A.; Moharram, F.A.; Nagwa, M.S.; Amany, A.A. Pharmacologically active ellagitannins from terminalia myriocarpa. Planta Med. 2002, 68, 523–527. [Google Scholar] [CrossRef]
- Moreno, M.I.N.; Isla, M.I.; Sampietro, A.R.; Vattuone, M.A. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J. Ethnopharmacol. 2000, 71, 109–114. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yoon, W.J.; Yim, E.Y.; Park, S.Y.; Kim, Y.J.; Song, G.P. Antioxidative and antimicrobial activities of Castanopsis cuspidata var. sieboldii extracts. Korean J. Plant Res. 2011, 24, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Kalaivani, T.; Rajasekaran, C.; Mathew, L. Free radical scavenging, cytotoxic, and hemolytic activities of an active antioxidant compound ethyl gallate from leaves of Acacia Nilotica (L.) Wild. Ex. Delile Subsp. Indica Benth. Brenan. J. Food Sci. 2011, 76, 144–149. [Google Scholar] [CrossRef]
- Ahn, G.Y.; Bae, S.H. Strategies for the safe use of non-steroidal anti-inflammatory drugs. J. Korean Med. Assoc. 2018, 61, 367–375. [Google Scholar] [CrossRef]
- Cano, A.; Acosta, M.; Arnao, M.B. A method to measure antioxidant activity in organic media: Application to lipophilic vitamins. Redox Rep. 2000, 5, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Tatullo, M.; Simone, G.M.; Tarullo, F.; Irlandese, G.; Vito, D.D.; Marrelli, M.; Luigi, S.; Tiziana, C.; Andrea, B.; Scacco, S. Antioxidant and antitumor activity of a bioactive polyphenolic fraction isolated from the brewing process. Sci. Rep. 2016, 6, 36042. [Google Scholar] [CrossRef] [Green Version]
- Dimitrios, B. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512. [Google Scholar] [CrossRef]
- Lee, S.J.; An, K.W.; Choi, T.S.; Jung, H.S.; Moon, J.H.; Park, K.H. Component analysis and antioxidative activity of Castanopsis cuspidata var. sieboldii nut. Korean J. Food Preserv. 2010, 17, 139–144. [Google Scholar]
- Oh, J.M.; Jang, H.J.; Kang, M.G.; Song, S.; Kim, D.Y.; Kim, J.H.; Noh, J.I.; Park, J.E.; Park, D.; Yee, S.T.; et al. Acetylcholinesterase and monoamine oxidase-B inhibitory activities by ellagic acid derivatives isolated from Castanopsis cuspidata var. sieboldii. Sci. Rep. 2021, 11, 13953. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, H.; Tanaka, S.; Matsuo, Y.; Saito, Y.; Nishida, K.; Tanaka, T. Reductive metabolism of ellagitannins in the young leaves of Castanopsis sieboldii. Molecules 2019, 24, 4279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent advances in the production and applications of ellagic acid and its derivatives. A review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Choi, M.H.; Yang, S.H.; Oh, D.S.; Shin, H.J. Evaluation of bioactivity of Castanopsis cuspidata var. sieboldii leaves extract and isolation of polyphenolic compounds. Korean Soc. Biotechnol. Bioeng. J. 2022, 37, 64–70. [Google Scholar] [CrossRef]
- Lin, Y.F.; Hu, Y.H.; Lin, H.T.; Liu, X.; Chen, Y.H.; Zhang, S.; Chen, Q.X. Inhibitory effects of propyl gallate on tyrosinase and its application in controlling pericarp browning of harvested longan fruits. J. Agric. Food Chem. 2013, 61, 2889–2895. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Raphael, T.J.; Kuttan, G. Nomilin inhibits metastasis via induction of apoptosis and regulates the activation of transcription factors and the cytokine profile in B16F-10 cells. Integr. Cancer Ther. 2012, 11, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.Y.; Min, B.S.; Youn, U.J.; Hung, T.M.; Song, K.S.; Seong, Y.H.; Bae, K.H. Chemical constituents from the leaf and twig of Acer okamotoanum Nakai and their cytotoxicity. Korean J. Med. Crop Sci. 2006, 14, 77–81. [Google Scholar]
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K.; Kuriki, T. Syntheses of arbutin-α-glycosides and a comparison of their inhibitory effects with those of α-arbutin and arbutin on human tyrosinase. Chem. Pharm. Bull. 2003, 51, 798–801. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Jung, T.K.; Kim, M.H.; Yoon, K.S. In vitro screening of Jeju island plants for cosmetic ingredients. Korean Soc. Biotechnol. Bioeng. J. 2018, 33, 76–82. [Google Scholar]
- Li, H.X.; Park, J.U.; Su, X.D.; Kim, K.T.; Kang, J.S.; Kim, Y.R.; Kim, Y.H.; Yang, S.Y. Identification of anti-melanogenesis constituents from Morus alba L. leaves. Molecules 2018, 23, 2559. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, T.; Barros, A.O.; Nogueira, J.R.; Fruet, A.C.; Rodrigues, I.C.; Calcagno, D.Q.; Smith, M.A.C.; Souza, T.P.; Barros, S.B.M.; Vasconcellos, M.C.; et al. Anti-wrinkle and anti-whitening effects of jucá (Libidibia ferrea Mart.) extracts. Arch. Dermatol. Res. 2016, 308, 643–654. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.W.; Kim, M.; Lee, S.H.; Kim, K.D. The function of autophagy as a regulator of melanin homeostasis. Cells 2022, 11, 2085. [Google Scholar] [CrossRef]



| Sample | DPPH IC50 (μg/mL) | ABTS IC50 (μg/mL) | TPC (GAE mg/g) | TFC (QUE mg/g) |
|---|---|---|---|---|
| CCSB-Hex | 2038.59 ± 43.88 | 1443.51 ± 12.74 | 189.45 ± 0.14 | 21.78 ± 2.55 |
| CCSB-Ch | 366.90 ± 4.28 | 176.35 ± 5.09 | 181.66 ± 6.51 | 33.59 ± 1.67 |
| CCSB-EtOAc | 77.77 ± 1.79 | 57.43 ± 0.40 | 368.87 ± 3.22 | 45.14 ± 0.73 |
| CCSB-BuOH | 434.72 ± 1.24 | 300.63 ± 1.36 | 123.22 ± 0.72 | 13.00 ± 0.36 |
| CCSB-Water | 483.58 ± 2.17 | 313.20 ± 1.44 | 102.92 ± 0.14 | 13.63 ± 0.36 |
| Ethyl gallate | 15.45 ± 0.26 | 25.55 ± 0.78 | - | - |
| Ascorbic acid | 20.26 ± 0.33 | 59.63 ± 0.19 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.-H.; Yang, S.-H.; Kim, D.-S.; Kim, N.-D.; Shin, H.-J. Ethyl Gallate Isolated from Castanopsis cuspidata var. sieboldii Branches Inhibits Melanogenesis and Promotes Autophagy in B16F10 Cells. Antioxidants 2023, 12, 269. https://doi.org/10.3390/antiox12020269
Choi M-H, Yang S-H, Kim D-S, Kim N-D, Shin H-J. Ethyl Gallate Isolated from Castanopsis cuspidata var. sieboldii Branches Inhibits Melanogenesis and Promotes Autophagy in B16F10 Cells. Antioxidants. 2023; 12(2):269. https://doi.org/10.3390/antiox12020269
Chicago/Turabian StyleChoi, Moon-Hee, Seung-Hwa Yang, Da-Song Kim, Nam-Doo Kim, and Hyun-Jae Shin. 2023. "Ethyl Gallate Isolated from Castanopsis cuspidata var. sieboldii Branches Inhibits Melanogenesis and Promotes Autophagy in B16F10 Cells" Antioxidants 12, no. 2: 269. https://doi.org/10.3390/antiox12020269







