Differential Activities of Antioxidant Enzymes, Superoxide Dismutase, Peroxidase, and Catalase vis-à-vis Phosphine Resistance in Field Populations of Lesser Grain Borer (Rhyzopertha dominica) from India
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Insect
2.2. Bioassay
3. Phosphine Treatment and Determination of Resistance Ratio
4. Enzyme Assays
5. Catalase Assay
6. Peroxidase Assay
7. Superoxide Dismutase Assay
8. Protein Estimation
9. Statistical Analysis
10. Results
10.1. Susceptibility of Field-Collected Populations to Phosphine
10.2. The Specific Activity of Antioxidant Enzymes in R. dominica
Peroxidase Activity
10.3. Superoxide Dismutase Activity
10.4. Catalase Activity
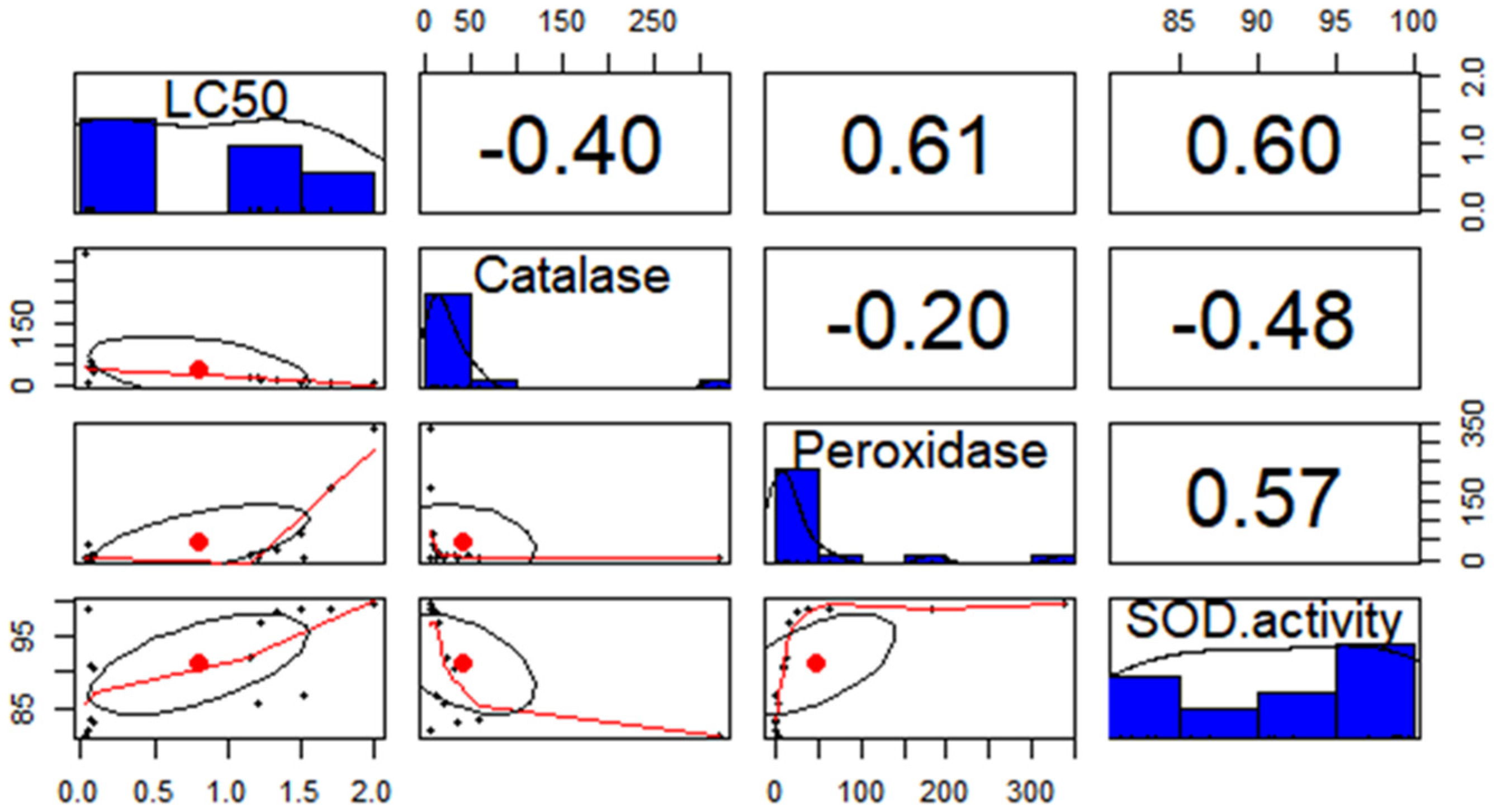
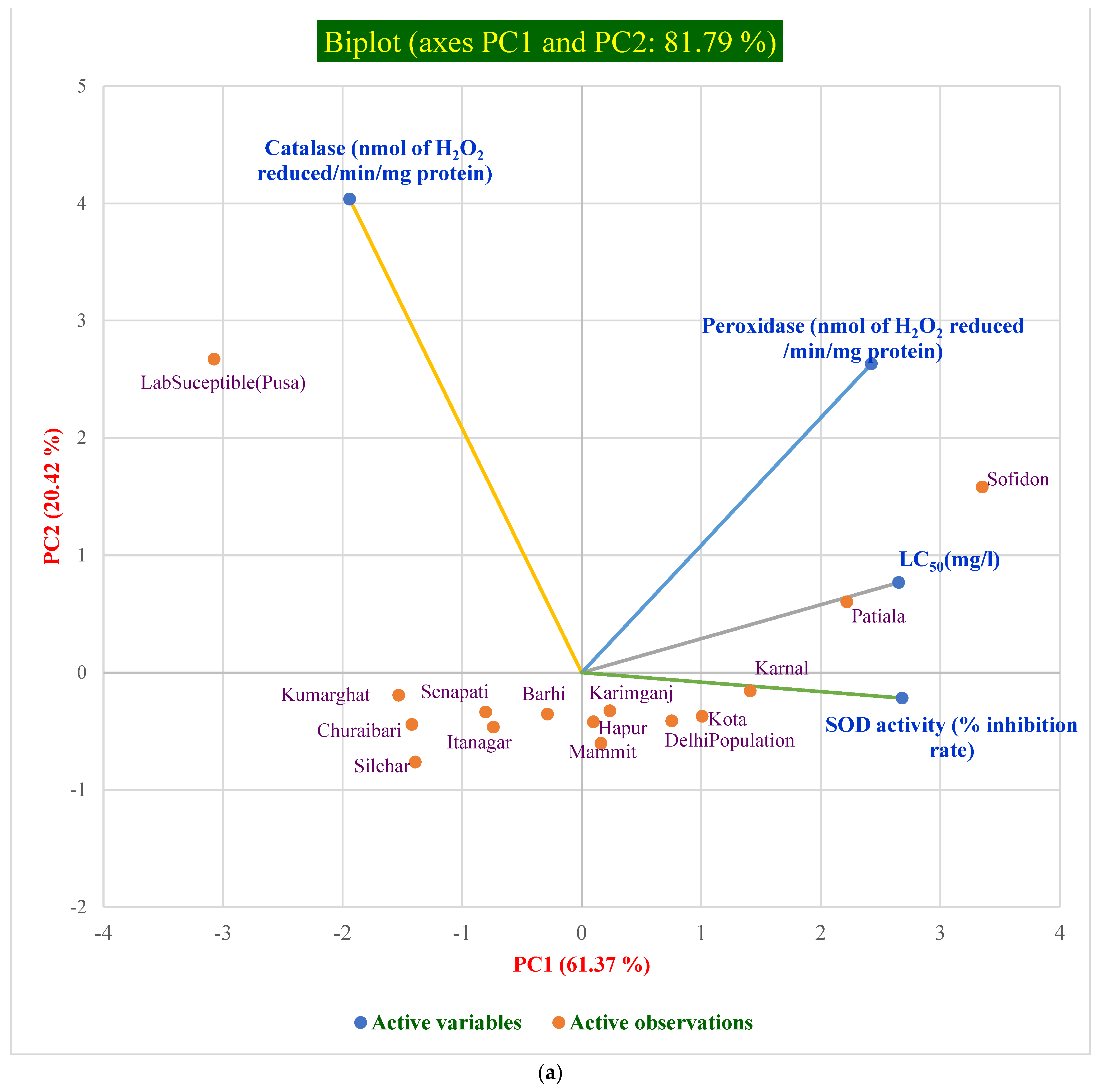
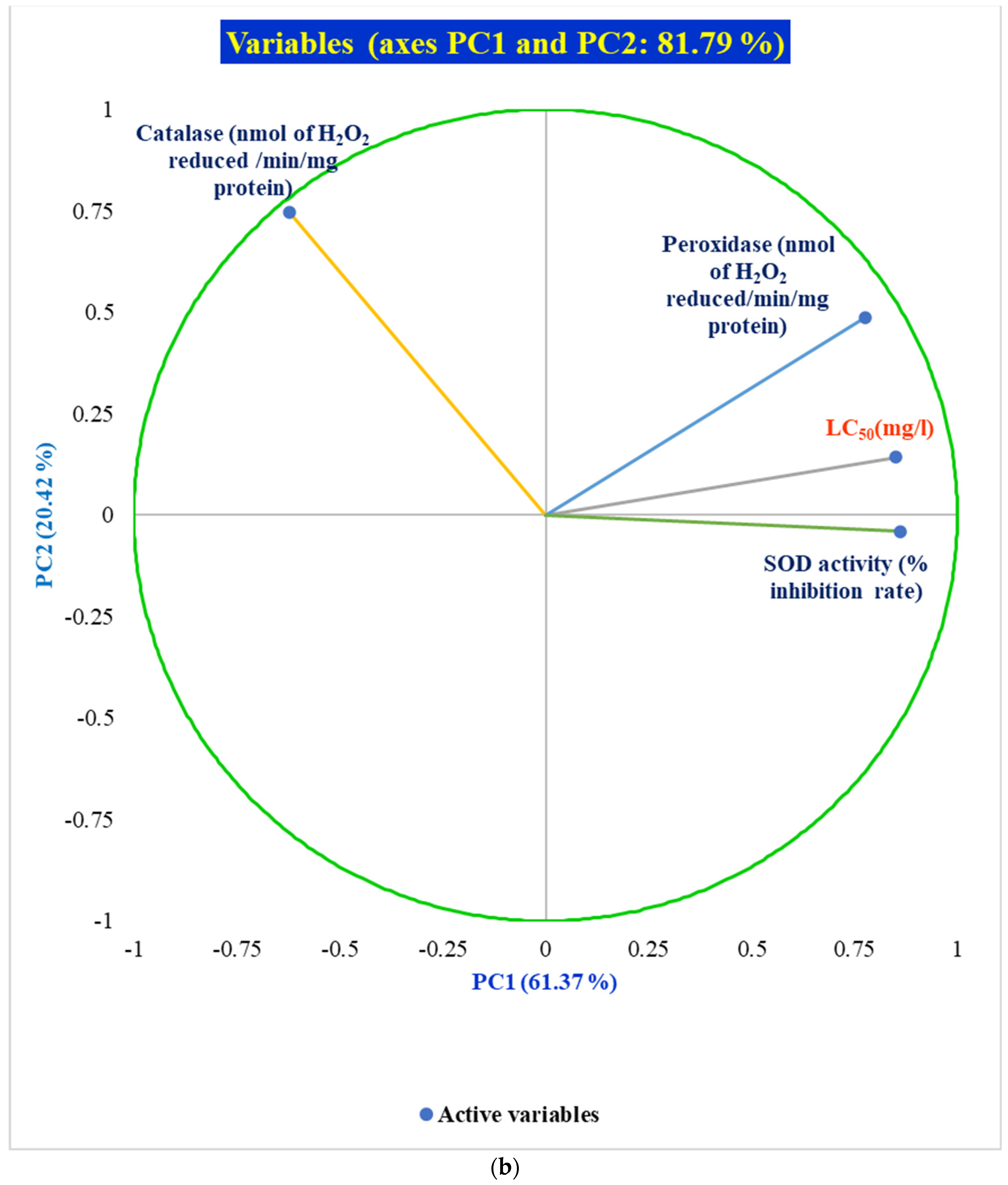
10.5. Pearson Correlation and PCA of Antioxidant Enzyme Activities
11. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chittenden, F.H. The lesser grain borer and the larger grain borer. Bull. U. S. Bur. Entomol. 1911, 96, 29–47. [Google Scholar]
- Potter, C. The biology and distribution of Rhyzopertha dominica (FAB.). Trans. R. Entomol. Soc. Lond. 1935, 83, 449–482. [Google Scholar] [CrossRef]
- Fields, P.G.; Phillips, T.W. The Distribution and PCR-Based Fingerprints of Rhyzopertha dominica (F.) in Canada. In Proceedings of the 6th International Working Conference on Stored-Product Protection, Canberra, Australia, 17–23 April 1994; pp. 517–522. [Google Scholar]
- Campbell, A.; Sinha, R.N. Damage of wheat by feeding of some stored product beetles. J. Econ. Entomol. 1976, 69, 11–13. [Google Scholar] [CrossRef]
- Park, S.H.; Arthur, F.H.; Bean, S.R.; Schober, T.J. Impact of differing population levels of Rhyzopertha dominica (F.) on milling and physicochemical properties of sorghum kernel and flour. J. Stored Prod. Res. 2008, 44, 322–327. [Google Scholar] [CrossRef]
- Rao, H.R.G.; Wilbur, D.A. Loss of wheat weight from the feeding of lesser grain borer. J. Kans. Entomol. Soc. 1972, 45, 238–241. [Google Scholar]
- Crombie, A.C. On intraspecific and interspecific competition in larvae of graminivorous insects. J. Exp. Biol. 1944, 20, 135–151. [Google Scholar] [CrossRef]
- Edde, P.A.; Phillips, T.W. Longevity and pheromone output in stored-product Bostrichidae. Bull. Entomol. Res. 2006, 96, 547–554. [Google Scholar] [CrossRef]
- Singh, P.K. A decentralized and holistic approach for grain management in India. Curr. Sci. 2010, 99, 1179–1180. [Google Scholar]
- Taylor, R. The Use of Phosphine as a MB Alternative in Post-Harvest Treatments. In Proceedings of the UNEP-Regional Workshop on Methyl Bromide Alternatives for Post-Harvest Treatments in Eastern and Central Europe, Sofia, Bulgaria, 28–30 May 2002; pp. 28–30. [Google Scholar]
- Chaudhry, M.Q. A review of the mechanisms involved in the action of phosphine as an insecticide and phosphine resistance in stored-product insects. Pestic. Sci. 1997, 49, 213–228. [Google Scholar] [CrossRef]
- Lorini, I.; Collins, P.J.; Daglish, G.J.; Nayak, M.K.; Pavic, H. Detection and characterization of strong resistance to phosphine in Brazilian Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae). Pest. Manag. Sci. 2007, 63, 358–364. [Google Scholar] [CrossRef]
- Champ, B.R.; Dyte, C.E. FAO global survey of pesticide susceptibility of stored grain pests. FAO Plant Prot. Bull. 1976, 25, 49–67. [Google Scholar]
- Zettler, L.J.; Cuperus, G.W. Pesticide resistance in Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) in wheat. J. Econ. Entomol. 1990, 83, 1677–1681. [Google Scholar] [CrossRef]
- Collins, P.J. Resistance to Grain Protectants and Fumigation in Insect Pests of Stored Products in Australia. In Stored Grain in Australia, Proceedings of the Australian Postharvest Technical Conference, Canberra, Australia, 26–29 May 1998; Banks, H.J., Wright, E.J., Damcevski, K.A., Eds.; CSIRO: Campbell, Australia, 1998; pp. 55–57. [Google Scholar]
- Bora, G.; Chahal, B.S. Development of resistance in Trogoderma granarium Everts to phosphine in Punjab. FAO Plant Prot. Bull. 1979, 27, 77–80. [Google Scholar]
- Rajendran, S. Fumigation resistance—Problems and its implications in the control of stored product insects in India. Pestic. Res. J. 1989, 1, 111–115. [Google Scholar]
- Ramya, R.S.; Srivastava, C.; Subramanian, S. Monitoring of phosphine resistance in Indian populations of Tribolium castaneum (Herbst) from stored wheat. Indian J. Entomol. 2018, 80, 19–23. [Google Scholar] [CrossRef]
- Yadav, S.K.; Srivastava, C.; Sabtharishi, S. Phosphine resistance and antioxidant enzyme activity in Trogoderma granarium Everts. J. Stored Prod. Res. 2020, 87, 1016–1036. [Google Scholar] [CrossRef]
- Schlipalius, D.I.; Cheng, Q.; Reilly, P.E.B.; Collins, P.J.; Ebert, P.R. Genetic linkage analysis of the lesser grain borer Rhyzopertha dominica identifies two loci that confer high-level resistance to the fumigant phosphine. Genetics 2002, 161, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Mau, Y.S.; Collins, P.J.; Daglish, G.J.; Nayak, M.K.; Ebert, P.R. The rph2 gene is responsible for high-level resistance to phosphine in independent field strains of Rhyzopertha dominica. PLoS ONE 2012, 7, e34027. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Schlipalius, D.; Opit, G.; Subramanyam, B.; Phillips, T.W. Diagnostic molecular markers for phosphine resistance in U.S. populations of Tribolium castaneum and Rhyzopertha dominica. PLoS ONE 2015, 10, e0121343. [Google Scholar] [CrossRef]
- Kaur, R.; Emily, V.; Daniels Manoj, K.N.; Ebert, P.R.; Schlipalius, D.I. Determining changes in the distribution and abundance of a Rhyzopertha dominica phosphine resistance allele in farm grain storages using a DNA marker. Pest. Manag. Sci. 2013, 69, 685–688. [Google Scholar] [CrossRef]
- Kocak, E.; Schlipalius, D.; Kaur, R.; Tuck, A.; Ebert, P.R.; Collins, P.; Yilmaz, A. Determining phosphine resistance in rust flour beetle, Tribolium castaneum (Herbst.) (Coleoptera: Tenebrionidae) populations from Turkey. Turk. Entomol. Derg. 2015, 39, 129–136. [Google Scholar] [CrossRef]
- Kaur, R.; Subbarayalu, M.; Jagadeesan, R.; Daglish, G.J.; Nayak, M.K.; Naik, H.R.; Ramasamy, S.; Subramanian, C.; Ebert, P.R.; Schlipalius, D.I. Phosphine resistance in India is characterized by a dihydrolipoamide dehydrogenase variant that is otherwise unobserved in eukaryotes. J. Hered. 2015, 115, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Price, N.R.; Dance, S.J. Some biochemical aspects of phosphine action and resistance in three species of storage product beetles. Comp. Biochem. Physiol. 1983, 16, 277–281. [Google Scholar]
- Akhagari, M.; Abdollahi, M.; Kebryaeezadeh, A.; Hosseini, R.; Sabzevari, O. Biochemical evidence for free radical-induced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum. Exp. Toxicol. 2003, 22, 205–211. [Google Scholar] [CrossRef]
- Kolawole, A.O.; Olajuyigbe, F.M.; Ajele, J.O.; Adedire, C.O. Activity of the antioxidant defense system in a typical bioinsecticide- and synthetic insecticide-treated cowpea storage beetle Callosobrochus maculatus F. (Coleoptera: Chrysomelidae). Int. J. Insect Sci. 2014, 6, 99–108. [Google Scholar] [CrossRef]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef]
- Price, N.R.; Mills, K.A.; Humphries, L.A. Phosphine toxicity and catalase activity in susceptible and resistant strains of the lesser grain borer (Rhyzopertha dominica). Comp. Biochem. Physiol. 1982, 73C, 411–413. [Google Scholar] [CrossRef]
- Bolter, C.J.; Chefurka, W. Extramitochondrial release of hydrogen peroxide from insect and mouse liver mitochondria using the respiratory inhibition phosphine, myxothiazol, and antimycin and spectral analysis of inhibited cytochromes. Arch. Biochem. Biophys. 1990, 278, 65–72. [Google Scholar] [CrossRef]
- Chaudhry, M.Q.; Price, N.R. Comparison of the oxidant damage induced by phosphine and the uptake and tracheal exchange of 32P-radiolabelled phosphine in the susceptible and resistant strains of Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae). Pestic. Biochem. Physiol. 1992, 42, 167–179. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Zhang, F.; Wang, Y. Transcriptional inhibition of the catalase gene in phosphine-induced oxidative stress in Drosophila melanogaster. Pestic. Biochem. Physiol. 2015, 124, 1–7. [Google Scholar] [CrossRef]
- Yang, J.; Park, J.S.; Lee, H.; Kwon, M.; Kim, G.H.; Kim, J. Identification of a phosphine resistance mechanism in Rhyzopertha dominica based on transcriptome analysis. J. Asia Pac. Entomol. 2018, 21, 1450–1456. [Google Scholar] [CrossRef]
- FAO. Recommended methods for the detection and measurement of resistance of agricultural pests to pesticides. Tentative method for adults of some major pest species of stored cereals with methyl bromide and phosphine. FAO Plant Prot. Bull. 1975, 23, 12–25. [Google Scholar]
- Beer, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Armstrong, D.; Rinehart, R.; Dixon, L.; Reigh, D. Changes of peroxidase with age in Drosophila. J. Age 1978, 1, 8–12. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971; p. 333. [Google Scholar]
- Rajendran, S. Insecticide and fumigation resistance of stored product insects in India. Bull. Grain Technol. 1992, 30, 164–169. [Google Scholar]
- Schlipalius, D.I.; Valmas, N.; Tuck, A.G.; Jagadeesan, R.; Ma, L.; Kaur, R.; Goldinger, A.; Anderson, C.; Kuang, J.; Zuryn, S.; et al. A Core metabolic enzyme mediates resistance to phosphine gas. J. Sci. 2012, 338, 807–810. [Google Scholar] [CrossRef]
- Nakakita, H.; Saito, T.; Iyatomi, K. Effect of phosphine on the respiration of adult Sitophilus zeamais Motsch. (Coleoptera, Curculionidae). J. Stored Prod. Res. 1974, 10, 87–92. [Google Scholar] [CrossRef]
- Pimentel, M.A.G.; Faroni, L.R.D.A.; Totola, M.R.; Guedes, R.N.C. Phosphine resistance, respiration rate and fitness consequences in stored-product insects. Pest. Manag. Sci. 2007, 63, 876–881. [Google Scholar] [CrossRef]
- Pratt, S.J. A new measure of uptake: Desorption of unreacted phosphine from susceptible and resistant strains of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2003, 39, 507–520. [Google Scholar] [CrossRef]
- Quistad, G.B.; Sparks, S.E.; Casida, J.E. Chemical model for phosphine-induced lipid peroxidation. Pest. Manag. Sci. 2000, 56, 779–783. [Google Scholar] [CrossRef]
- Bolter, C.J.; Chefurka, W. The effect of phosphine treatment on superoxide dismutase, catalase, and peroxidase in the granary weevil, Sitophilus granarius. Pestic. Biochem. Physiol. 1990, 36, 52–60. [Google Scholar] [CrossRef]
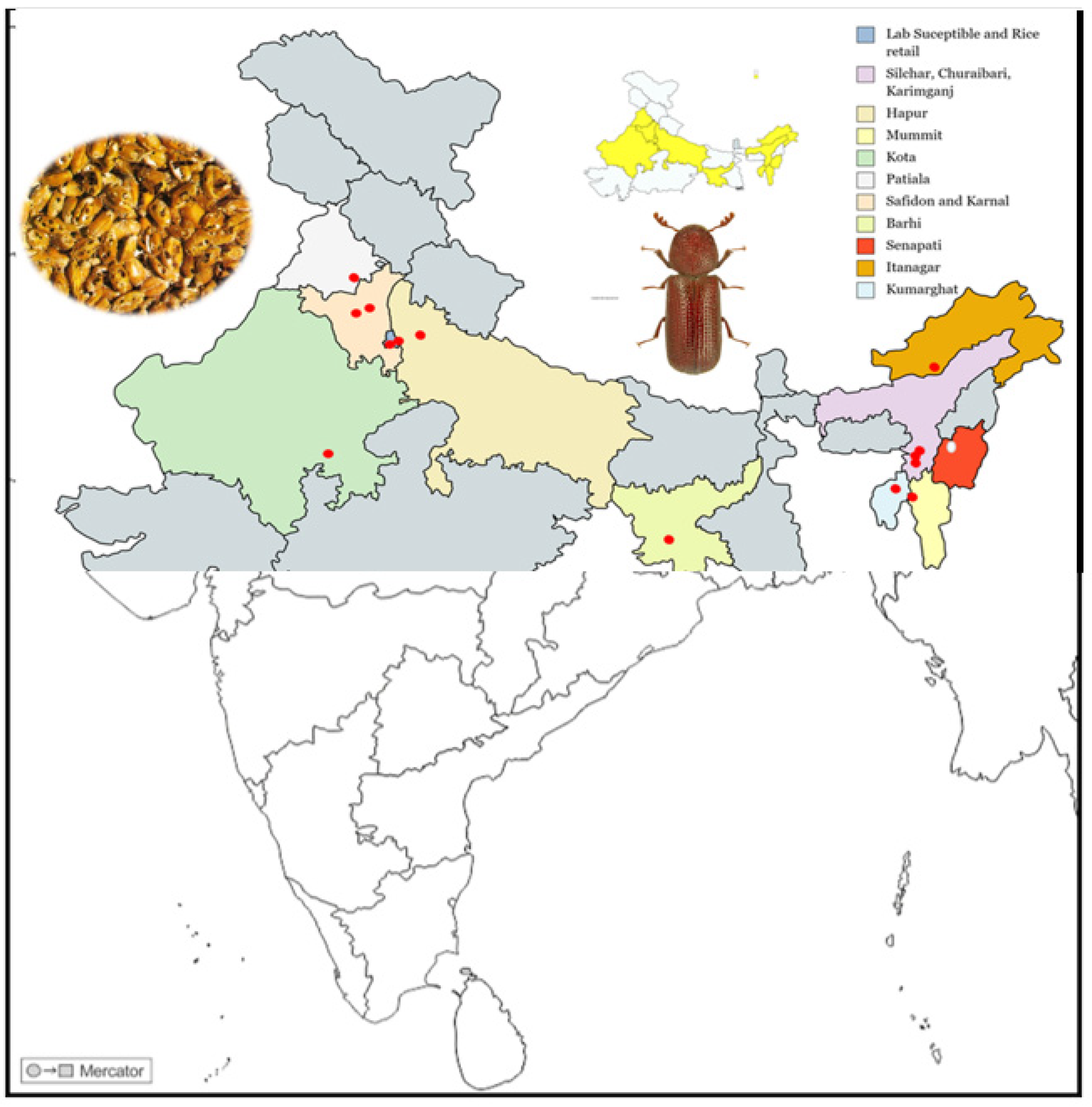
| Sl. No | Region | Location | Storage Details | Food Grain | GPS Coordinates |
|---|---|---|---|---|---|
| 1 | Haryana | Safidon | Bulk grain storage | Wheat | 29°23′48″ N 76°39′13″ E |
| 2 | Karnal | Bulk grain storage | Wheat | 29°40′47″ N 76°57′13″ E | |
| 3 | Punjab | Patiala | Bulk grain storage | Wheat | 30°21′25″ N 76°24′59″ E |
| 4 | Rajasthan | Kota | Farm storage | Wheat | 25°07′48″ N 75°48′34″ E |
| 5 | Uttar Pradesh | Hapur | Bulk grain storage | Wheat | 28°44′25″ N 77°46′09″ E |
| 6 | Delhi | Pusa | Laboratory population | Wheat | 28°36′ N 77°13′ E |
| 7 | Delhi | Farm storage | Rice | 28°37′55″ N 77°08′42″ E | |
| 8 | Jharkhand | Barhi | Farm storage | Wheat | 24°18′11″ N 85°24′48″ E |
| 9 | Assam | Karimganj | Farm storage | Wheat | 24°52′18″ N 92°22′18″ E |
| 10 | Silchar | Farm storage | Rice | 24°49′ N 92°48′ E | |
| 11 | Churaibari | Farm storage | Wheat | 24°26′07″ N 92°14′28″ E | |
| 12 | Manipur | Senapati | Bulk grain storage | Rice | 25°11′53″ N 93°59′23″ E |
| 13 | Tripura | Kumarghat | Bulk grain storage | Wheat | 24°09′49″ N 92°02′18″ E |
| 14 | Arunachal Pradesh | Itanagar | Farm storage | Wheat | 27°05′ N 93°37′ E |
| 15 | Mizoram | Mammit | Bulk grain storage | Rice | 23°54′40″ N 92°29′23″ E |
| Sl. No | State | Population | LC50 (mg/L) | Fiducial Limit (95%) | Slope ± SE | χ2 | H | d.f | RR (%) | ‘p’ Value |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Haryana | Safidon | 1.991 | 1.555–2.546 | 4.00 ± 1.28 | 0.008 | 0.003 | 3 | 82.96 | 0.999 |
| 2 | Punjab | Patiala | 1.708 | 1.129–2.124 | 3.44 ± 1.15 | 0.311 | 0.104 | 3 | 71.17 | 0.957 |
| 3 | Uttar Pradesh | Hapur | 1.514 | 1.029–1.789 | 4.86 ± 1.43 | 0.537 | 0.179 | 3 | 63.08 | 0.910 |
| 4 | Haryana | Karnal | 1.498 | 0.801–1.871 | 3.39 ± 1.16 | 0.543 | 0.181 | 3 | 62.42 | 0.909 |
| 5 | Rajasthan | Kota | 1.33 | 0.809–1.783 | 2.31 ± 0.65 | 1.029 | 0.343 | 3 | 55.42 | 0.794 |
| 6 | Delhi | Delhi | 1.221 | 0.825–1.557 | 2.59 ± 0.62 | 2.176 | 0.725 | 3 | 50.88 | 0.536 |
| 7 | Jharkhand | Barhi | 1.207 | 0.569–1.484 | 4.36 ± 1.43 | 2.016 | 0.672 | 3 | 50.29 | 0.569 |
| 8 | Assam | Karimganj | 1.146 | 0.783–1.439 | 2.85 ± 0.64 | 1.844 | 0.615 | 3 | 47.75 | 0.605 |
| 9 | Arunachal Pradesh | Itanagar | 0.092 | 0.061–0.133 | 1.37 ± 0.27 | 0.292 | 0.100 | 3 | 3.83 | 0.961 |
| 10 | Assam | Chauribari | 0.085 | 0.054–0.15 | 1.10 ± 0.21 | 0.346 | 0.120 | 3 | 3.54 | 0.951 |
| 11 | Manipur | Senapati | 0.065 | 0.036–0.106 | 1.45 ± 0.29 | 0.129 | 0.040 | 3 | 2.71 | 0.988 |
| 12 | Tripura | Kumarghat | 0.059 | 0.032–0.104 | 1.05 ± 0.22 | 0.135 | 0.050 | 3 | 2.46 | 0.987 |
| 13 | Assam | Silchar | 0.044 | 0.024–0.070 | 1.34 ± 0.27 | 0.115 | 0.040 | 3 | 1.83 | 0.990 |
| 14 | Mizoram | Mammit | 0.039 | 0.023–0.059 | 1.32 ± 0.24 | 1.654 | 0.550 | 3 | 1.63 | 0.647 |
| 15 | Delhi | Lab susceptible (Pusa) | 0.024 | 0.016–0.032 | 2.30 ± 0.41 | 2.113 | 0.700 | 3 | 1 | 0.547 |
| Sl. No | Location | Catalase (nmol/min/mg Protein) | Peroxidase (nmol/min/mg Protein) | SOD Activity (Inhibition Rate%) |
|---|---|---|---|---|
| 1 | Barhi | 22.221 ± 0.208 bc | 2.583 ± 0.678 a | 85.759 ± 0.919 d |
| 2 | Silchar | 6.287 ± 0.113 a | 1.281 ± 0.320 a | 81.930 ± 0.403 ab |
| 3 | Karimganj | 23.833 ± 0.394 c | 13.487 ± 0.885 bc | 92.174 ± 0.382 g |
| 4 | Hapur | 11.104 ± 0.285 a | 1.999 ± 0.219 a | 86.975 ± 0.180 e |
| 5 | Churaibari | 35.223 ± 0.922 d | 2.104 ± 0.389 a | 83.004 ± 0.090 bc |
| 6 | Kumarghat | 59.061 ± 0.777 f | 1.805 ± 0.00 a | 83.456 ± 0.013 c |
| 7 | Itanagar | 32.991 ± 0.642 d | 10.430 ± 1.323 abc | 90.713 ± 0.093 f |
| 8 | Kota | 13.114 ± 0.212 ab | 25.278 ± 0.623 d | 98.531 ± 0.510 i |
| 9 | Mummit | 10.501 ± 0.108 a | 38.833 ± 0.987 e | 98.963 ± 0.299 i |
| 10 | Patiala | 7.701 ± 0.202 a | 183.720 ± 1.482 g | 98.778 ± 0.815 i |
| 11 | Safidon | 7.764 ± 0.094 a | 336.8 ± 10.024 h | 99.661 ± 0.068 i |
| 12 | Karnal | 8.771 ± 0.195 a | 63.520 ± 1.464 f | 99.050 ± 0.180 i |
| 13 | Delhi | 14.821 ± 0.444 abc | 18.015 ± 2.756 cd | 97.013 ± 0.339 h |
| 14 | Senapati | 46.337 ± 1.354 e | 9.014 ± 2.410 ab | 91.107 ± 0.068 fg |
| 15 | Lab susceptible (Pusa) | 320.13 ± 11.555 g | 5.350 ± 0.261 ab | 81.296 ± 0.358 a |
| Correlation with a Resistance Ratio | Coefficient (b) | |
|---|---|---|
| Catalase | −0.707 ** | −1.34 ** |
| Peroxidase | 0.632 ** | 0.205 ** |
| Superoxide Dismutase | 0.689 ** | 3.151 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranjith, H.V.; Sagar, D.; Kalia, V.K.; Dahuja, A.; Subramanian, S. Differential Activities of Antioxidant Enzymes, Superoxide Dismutase, Peroxidase, and Catalase vis-à-vis Phosphine Resistance in Field Populations of Lesser Grain Borer (Rhyzopertha dominica) from India. Antioxidants 2023, 12, 270. https://doi.org/10.3390/antiox12020270
Ranjith HV, Sagar D, Kalia VK, Dahuja A, Subramanian S. Differential Activities of Antioxidant Enzymes, Superoxide Dismutase, Peroxidase, and Catalase vis-à-vis Phosphine Resistance in Field Populations of Lesser Grain Borer (Rhyzopertha dominica) from India. Antioxidants. 2023; 12(2):270. https://doi.org/10.3390/antiox12020270
Chicago/Turabian StyleRanjith, Hagalavadi Vijaykumar, Doddachowdappa Sagar, Vinay Kumari Kalia, Anil Dahuja, and Sabtharishi Subramanian. 2023. "Differential Activities of Antioxidant Enzymes, Superoxide Dismutase, Peroxidase, and Catalase vis-à-vis Phosphine Resistance in Field Populations of Lesser Grain Borer (Rhyzopertha dominica) from India" Antioxidants 12, no. 2: 270. https://doi.org/10.3390/antiox12020270
APA StyleRanjith, H. V., Sagar, D., Kalia, V. K., Dahuja, A., & Subramanian, S. (2023). Differential Activities of Antioxidant Enzymes, Superoxide Dismutase, Peroxidase, and Catalase vis-à-vis Phosphine Resistance in Field Populations of Lesser Grain Borer (Rhyzopertha dominica) from India. Antioxidants, 12(2), 270. https://doi.org/10.3390/antiox12020270





