Protective Effects of Early Caffeine Administration in Hyperoxia-Induced Neurotoxicity in the Juvenile Rat
Abstract
1. Introduction

2. Materials and Methods
2.1. Animal Welfare
2.2. Oxygen Exposure and Drug Administration
2.3. Tissue Preparation
2.4. RNA Extraction and Quantitative Real-Time PCR
2.5. Immunohistochemistry
2.6. Statistical Analyses
3. Results
3.1. Caffeine Determines the Proliferative Capacity of Near-Birth Hyperoxia-Impaired Intermediate Neurons
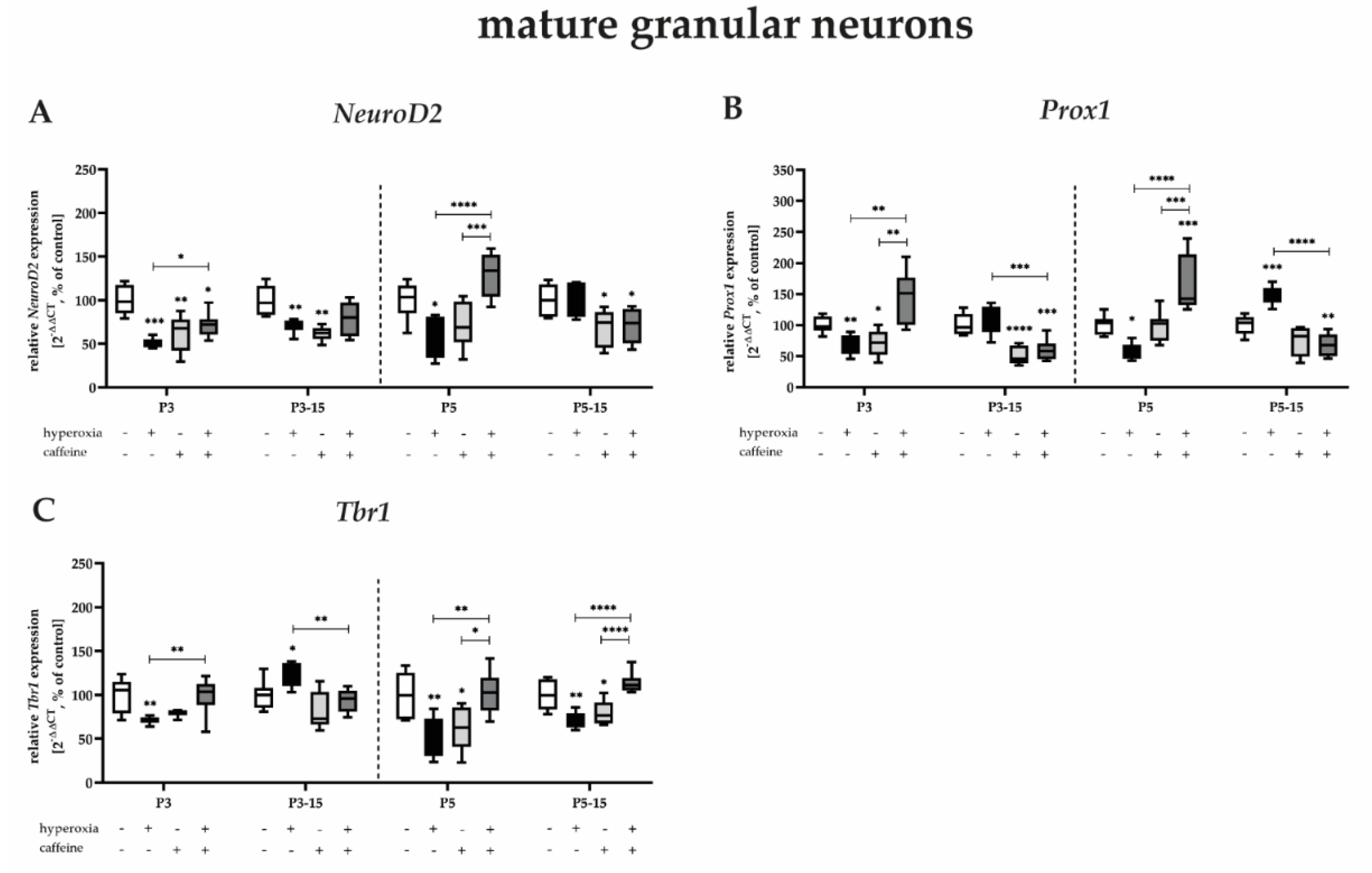
3.2. Caffeine Rescues NeuN-Positive Mature Granule Neurons Damaged by Near-Birth Short-Term Hyperoxia
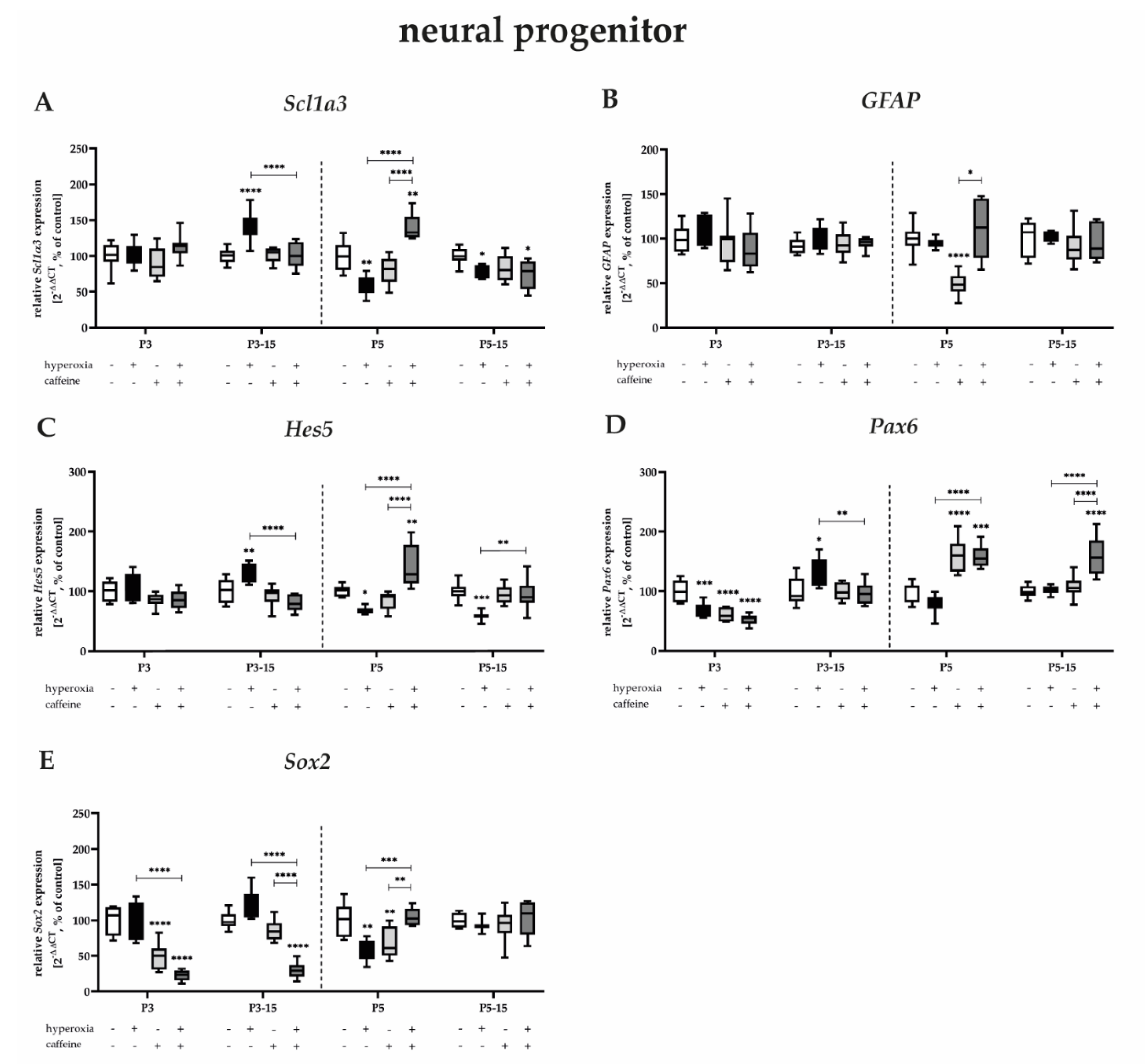
3.3. Caffeine Differentially Modulates Neuronal Transcription Factors of Neurogenic Developmental Stages under Toxic and Non-Toxic Oxygen Concentrations
3.3.1. Neuronal Progenitors Benefit from Prolonged Caffeine Administration after Hyperoxic Injury
3.3.2. Caffeine Protects Proliferation Capacity and Tends to Protect More the Late Stages of Intermediate Progenitor Cells after Hyperoxic Injury
3.3.3. Highest Protection for Mature Granular Neurons by Caffeine after Near-Birth Hyperoxic Injury
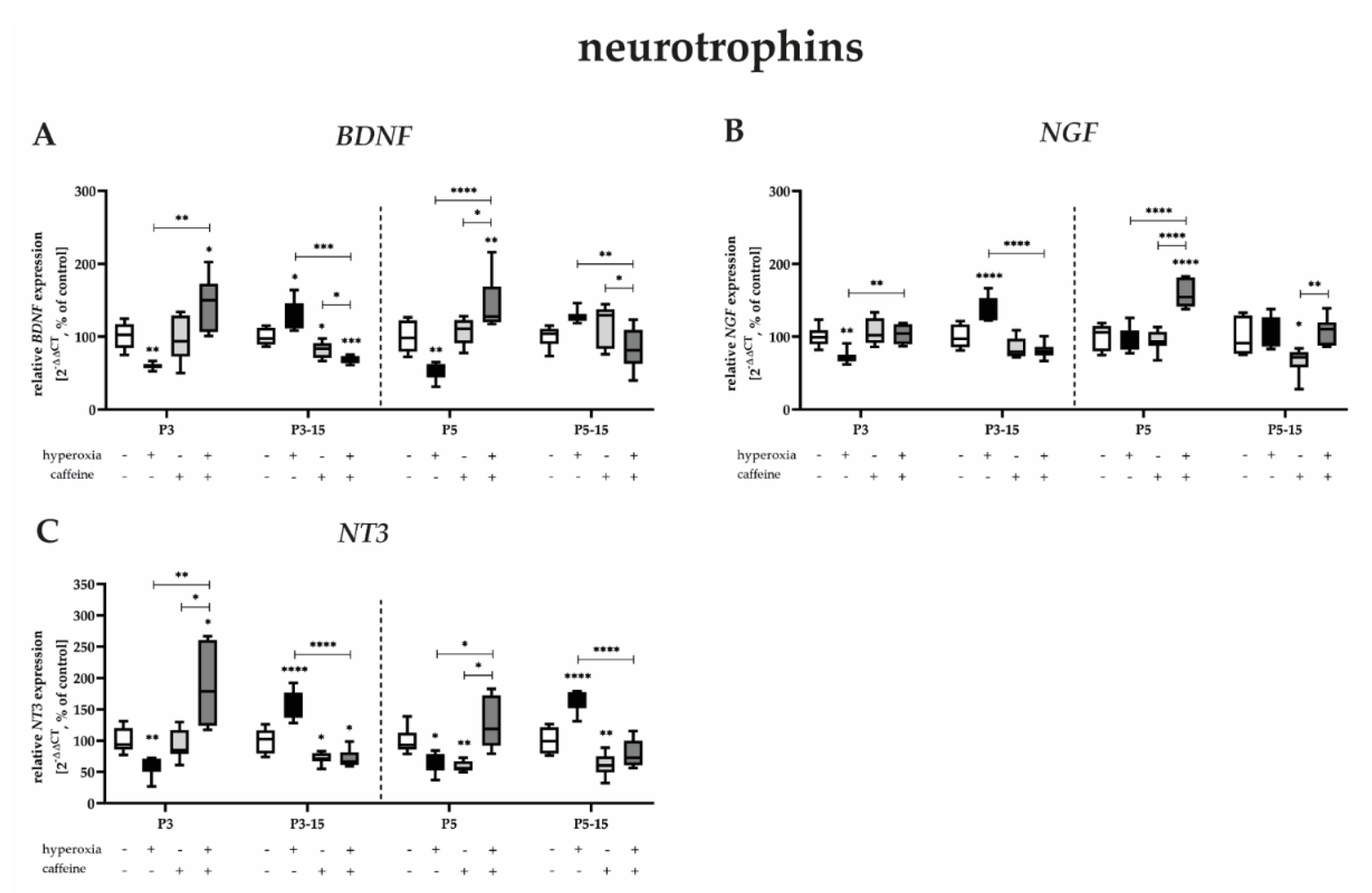
3.3.4. Caffeine Is Protective against Acute-Hyperoxia-Induced Downregulation of Neurotrophins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, D.K.; Kelly, C.E.; Chen, J.; Beare, R.; Alexander, B.; Seal, M.L.; Lee, K.; Matthews, L.G.; Anderson, P.J.; Doyle, L.W.; et al. Early life predictors of brain development at term-equivalent age in infants born across the gestational age spectrum. NeuroImage 2019, 185, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Morsing, E.; Lundgren, P.; Hård, A.L.; Rakow, A.; Hellström-Westas, L.; Jacobson, L.; Johnson, M.; Nilsson, S.; Smith, L.E.H.; Sävman, K.; et al. Neurodevelopmental disorders and somatic diagnoses in a national cohort of children born before 24 weeks of gestation. Acta Paediatr. 2022, 111, 1167–1175. [Google Scholar] [CrossRef]
- Pascal, A.; Govaert, P.; Oostra, A.; Naulaers, G.; Ortibus, E.; Van den Broeck, C. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: A meta-analytic review. Dev. Med. Child Neurol. 2018, 60, 342–355. [Google Scholar] [CrossRef]
- Doyle, L.W.; Spittle, A.; Anderson, P.J.; Cheong, J.L.Y. School-aged neurodevelopmental outcomes for children born extremely preterm. Arch. Dis. Child. 2021, 106, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.L.Y.; Olsen, J.E.; Lee, K.J.; Spittle, A.J.; Opie, G.F.; Clark, M.; Boland, R.A.; Roberts, G.; Josev, E.K.; Davis, N.; et al. Temporal Trends in Neurodevelopmental Outcomes to 2 Years After Extremely Preterm Birth. JAMA Pediatr. 2021, 175, 1035–1042. [Google Scholar] [CrossRef]
- Kanel, D.; Vanes, L.D.; Pecheva, D.; Hadaya, L.; Falconer, S.; Counsell, S.J.; Edwards, D.A.; Nosarti, C. Neonatal White Matter Microstructure and Emotional Development during the Preschool Years in Children Who Were Born Very Preterm. eNeuro 2021, 8. [Google Scholar] [CrossRef]
- Laverty, C.; Surtees, A.; O’Sullivan, R.; Sutherland, D.; Jones, C.; Richards, C. The prevalence and profile of autism in individuals born preterm: A systematic review and meta-analysis. J. Neurodev. Disord. 2021, 13, 41. [Google Scholar] [CrossRef]
- Götz, M.; Nakafuku, M.; Petrik, D. Neurogenesis in the Developing and Adult Brain-Similarities and Key Differences. Cold Spring Harb. Perspect. Biol. 2016, 8, a018853. [Google Scholar] [CrossRef] [PubMed]
- Roybon, L.; Hjalt, T.; Stott, S.; Guillemot, F.; Li, J.Y.; Brundin, P. Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS ONE 2009, 4, e4779. [Google Scholar] [CrossRef]
- Kumar, A.; Pareek, V.; Faiq, M.A.; Ghosh, S.K.; Kumari, C. Adult neurogenesis in humans: A Review of Basic Concepts, History, Current Research, and Clinical Implications. Innov. Clin. Neurosci. 2019, 16, 30–37. [Google Scholar]
- Catavero, C.; Bao, H.; Song, J. Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis. Cell Tissue Res. 2018, 371, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Endesfelder, S.; Weichelt, U.; Strauss, E.; Schlor, A.; Sifringer, M.; Scheuer, T.; Buhrer, C.; Schmitz, T. Neuroprotection by Caffeine in Hyperoxia-Induced Neonatal Brain Injury. Int. J. Mol. Sci. 2017, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Abbah, J.; Vacher, C.-M.; Goldstein, E.Z.; Li, Z.; Kundu, S.; Talbot, B.; Bhattacharya, S.; Hashimoto-Torii, K.; Wang, L.; Banerjee, P.; et al. Oxidative Stress-Induced Damage to the Developing Hippocampus Is Mediated by GSK3β. J. Neurosci. 2022, 42, 4812–4827. [Google Scholar] [CrossRef] [PubMed]
- Reich, B.; Hoeber, D.; Bendix, I.; Felderhoff-Mueser, U. Hyperoxia and the Immature Brain. Dev. Neurosci. 2016, 38, 311–330. [Google Scholar] [CrossRef]
- Endesfelder, S.; Weichelt, U.; Schiller, C.; Winter, K.; von Haefen, C.; Buhrer, C. Caffeine Protects Against Anticonvulsant-Induced Impaired Neurogenesis in the Developing Rat Brain. Neurotox. Res. 2018, 34, 173–187. [Google Scholar] [CrossRef]
- Ehlting, A.; Zweyer, M.; Maes, E.; Schleehuber, Y.; Doshi, H.; Sabir, H.; Bernis, M.E. Impact of Hypoxia-Ischemia on Neurogenesis and Structural and Functional Outcomes in a Mild-Moderate Neonatal Hypoxia-Ischemia Brain Injury Model. Life 2022, 12, 1164. [Google Scholar] [CrossRef]
- Van Steenwinckel, J.; Schang, A.L.; Sigaut, S.; Chhor, V.; Degos, V.; Hagberg, H.; Baud, O.; Fleiss, B.; Gressens, P. Brain damage of the preterm infant: New insights into the role of inflammation. Biochem. Soc. Trans. 2014, 42, 557–563. [Google Scholar] [CrossRef]
- Vicidomini, C.; Guo, N.; Sahay, A. Communication, Cross Talk, and Signal Integration in the Adult Hippocampal Neurogenic Niche. Neuron 2020, 105, 220–235. [Google Scholar] [CrossRef]
- Novak, C.M.; Ozen, M.; Burd, I. Perinatal Brain Injury: Mechanisms, Prevention, and Outcomes. Clin. Perinatol. 2018, 45, 357–375. [Google Scholar] [CrossRef]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef]
- Bührer, C.; Heller, G.; Thome, U.H. Population-Based Outcome Data of Extremely Preterm Infants in Germany during 2010–2017. Neonatology 2022, 119, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Thome, U.H.; Dreyhaupt, J.; Genzel-Boroviczeny, O.; Bohnhorst, B.; Schmid, M.; Fuchs, H.; Rohde, O.; Avenarius, S.; Topf, H.G.; Zimmermann, A.; et al. Influence of PCO2 Control on Clinical and Neurodevelopmental Outcomes of Extremely Low Birth Weight Infants. Neonatology 2018, 113, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Panfoli, I.; Candiano, G.; Malova, M.; De Angelis, L.; Cardiello, V.; Buonocore, G.; Ramenghi, L.A. Oxidative Stress as a Primary Risk Factor for Brain Damage in Preterm Newborns. Front. Pediatr. 2018, 6, 369. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Pellegrino, S.; D’Arrigo, S.; Barberi, I.; Reiter, R.J. Oxidative stress in resuscitation and in ventilation of newborns. Eur. Respir. J. 2009, 34, 1461–1469. [Google Scholar] [CrossRef]
- Lee, Y.S.; Chou, Y.H. Antioxidant profiles in full term and preterm neonates. Chang Gung Med. J. 2005, 28, 846–851. [Google Scholar]
- Hoffman, S.B.; Cheng, Y.J.; Magder, L.S.; Shet, N.; Viscardi, R.M. Cerebral autoregulation in premature infants during the first 96 h of life and relationship to adverse outcomes. Arch. Dis. Childhood. Fetal Neonatal Ed. 2019, 104, F473–F479. [Google Scholar] [CrossRef]
- Torres-Cuevas, I.; Parra-Llorca, A.; Sanchez-Illana, A.; Nunez-Ramiro, A.; Kuligowski, J.; Chafer-Pericas, C.; Cernada, M.; Escobar, J.; Vento, M. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017, 12, 674–681. [Google Scholar] [CrossRef]
- Perez, M.; Robbins, M.E.; Revhaug, C.; Saugstad, O.D. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free Radic. Biol. Med. 2019, 142, 61–72. [Google Scholar] [CrossRef]
- Juul, S.E.; Comstock, B.A.; Wadhawan, R.; Mayock, D.E.; Courtney, S.E.; Robinson, T.; Ahmad, K.A.; Bendel-Stenzel, E.; Baserga, M.; LaGamma, E.F.; et al. A Randomized Trial of Erythropoietin for Neuroprotection in Preterm Infants. N. Engl. J. Med. 2020, 382, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Falsaperla, R.; Lombardo, F.; Filosco, F.; Romano, C.; Saporito, M.A.N.; Puglisi, F.; Piro, E.; Ruggieri, M.; Pavone, P. Oxidative Stress in Preterm Infants: Overview of Current Evidence and Future Prospects. Pharmaceuticals 2020, 13, 145. [Google Scholar] [CrossRef]
- Tataranno, M.L.; Perrone, S.; Longini, M.; Buonocore, G. New antioxidant drugs for neonatal brain injury. Oxidative Med. Cell. Longev. 2015, 2015, 108251. [Google Scholar] [CrossRef]
- Gustorff, C.; Scheuer, T.; Schmitz, T.; Bührer, C.; Endesfelder, S. GABAB Receptor-Mediated Impairment of Intermediate Progenitor Maturation During Postnatal Hippocampal Neurogenesis of Newborn Rats. Front. Cell. Neurosci. 2021, 15, 295. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2007, 357, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Roberts, R.S.; Anderson, P.J.; Asztalos, E.V.; Costantini, L.; Davis, P.G.; Dewey, D.; D’Ilario, J.; Doyle, L.W.; Grunau, R.E.; et al. Academic Performance, Motor Function, and Behavior 11 Years After Neonatal Caffeine Citrate Therapy for Apnea of Prematurity: An 11-Year Follow-up of the CAP Randomized Clinical Trial. JAMA Pediatr. 2017, 171, 564–572. [Google Scholar] [CrossRef]
- DeMauro, S.B. Neurodevelopmental outcomes of infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 2021, 56, 3509–3517. [Google Scholar] [CrossRef]
- Henderson-Smart, D.J.; Steer, P.A. Caffeine versus theophylline for apnea in preterm infants. Cochrane Database Syst. Rev. 2010, 1, CD000273. [Google Scholar] [CrossRef]
- Kua, K.P.; Lee, S.W. Systematic review and meta-analysis of clinical outcomes of early caffeine therapy in preterm neonates. Br. J. Clin. Pharmacol. 2017, 83, 180–191. [Google Scholar] [CrossRef]
- Lodha, A.; Entz, R.; Synnes, A.; Creighton, D.; Yusuf, K.; Lapointe, A.; Yang, J.; Shah, P.S. Early Caffeine Administration and Neurodevelopmental Outcomes in Preterm Infants. Pediatrics 2019, 143, e20181348. [Google Scholar] [CrossRef]
- Endesfelder, S.; Zaak, I.; Weichelt, U.; Bührer, C.; Schmitz, T. Caffeine protects neuronal cells against injury caused by hyperoxia in the immature brain. Free Radic. Biol. Med. 2014, 67, 221–234. [Google Scholar] [CrossRef]
- Giszas, V.; Strauß, E.; Bührer, C.; Endesfelder, S. The Conflicting Role of Caffeine Supplementation on Hyperoxia-Induced Injury on the Cerebellar Granular Cell Neurogenesis of Newborn Rats. Oxidative Med. Cell. Longev. 2022, 2022, 5769784. [Google Scholar] [CrossRef] [PubMed]
- Endesfelder, S.; Strauß, E.; Scheuer, T.; Schmitz, T.; Bührer, C. Antioxidative effects of caffeine in a hyperoxia-based rat model of bronchopulmonary dysplasia. Respir. Res. 2019, 20, 88. [Google Scholar] [CrossRef]
- Endesfelder, S.; Strauß, E.; Bendix, I.; Schmitz, T.; Bührer, C. Prevention of Oxygen-Induced Inflammatory Lung Injury by Caffeine in Neonatal Rats. Oxidative Med. Cell. Longev. 2020, 2020, 3840124. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pérez-Domínguez, M.; Tovar, Y.R.L.B.; Zepeda, A. Neuroinflammation and physical exercise as modulators of adult hippocampal neural precursor cell behavior. Rev. Neurosci. 2018, 29, 1–20. [Google Scholar] [CrossRef]
- Gao, Z.; Ure, K.; Ables, J.L.; Lagace, D.C.; Nave, K.-A.; Goebbels, S.; Eisch, A.J.; Hsieh, J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat. Neurosci. 2009, 12, 1090–1092. [Google Scholar] [CrossRef]
- Duan, W.; Zhang, Y.P.; Hou, Z.; Huang, C.; Zhu, H.; Zhang, C.Q.; Yin, Q. Novel Insights into NeuN: From Neuronal Marker to Splicing Regulator. Mol. Neurobiol. 2016, 53, 1637–1647. [Google Scholar] [CrossRef]
- Götz, M.; Sirko, S.; Beckers, J.; Irmler, M. Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and Genome-wide expression analysis. Glia 2015, 63, 1452–1468. [Google Scholar] [CrossRef]
- Englund, C.; Fink, A.; Lau, C.; Pham, D.; Daza, R.A.; Bulfone, A.; Kowalczyk, T.; Hevner, R.F. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 247–251. [Google Scholar] [CrossRef]
- Hevner, R.F.; Hodge, R.D.; Daza, R.A.; Englund, C. Transcription factors in glutamatergic neurogenesis: Conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci. Res. 2006, 55, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Takashima, K.; Nakajima, K.; Shimizu, S.; Ojiro, R.; Tang, Q.; Okano, H.; Takahashi, Y.; Ozawa, S.; Jin, M.; Yoshinari, T.; et al. Disruption of postnatal neurogenesis and adult-stage suppression of synaptic plasticity in the hippocampal dentate gyrus after developmental exposure to sterigmatocystin in rats. Toxicol. Lett. 2021, 349, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Endesfelder, S.; Makki, H.; von Haefen, C.; Spies, C.D.; Buhrer, C.; Sifringer, M. Neuroprotective effects of dexmedetomidine against hyperoxia-induced injury in the developing rat brain. PLoS ONE 2017, 12, e0171498. [Google Scholar] [CrossRef] [PubMed]
- Pilz, G.A.; Bottes, S.; Betizeau, M.; Jörg, D.J.; Carta, S.; Simons, B.D.; Helmchen, F.; Jessberger, S. Live imaging of neurogenesis in the adult mouse hippocampus. Science 2018, 359, 658–662. [Google Scholar] [CrossRef]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef]
- Gage, F.H. Adult neurogenesis in mammals. Science 2019, 364, 827–828. [Google Scholar] [CrossRef]
- Song, J.; Zhong, C.; Bonaguidi, M.A.; Sun, G.J.; Hsu, D.; Gu, Y.; Meletis, K.; Huang, Z.J.; Ge, S.; Enikolopov, G.; et al. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 2012, 489, 150–154. [Google Scholar] [CrossRef]
- Velloso, F.J.; Shankar, S.; Parpura, V.; Rakic, P.; Levison, S.W. Neural Stem Cells in Adult Mammals are not Astrocytes. ASN Neuro 2022, 14, 17590914221134739. [Google Scholar] [CrossRef]
- Miranda-Negrón, Y.; García-Arrarás, J.E. Radial glia and radial glia-like cells: Their role in neurogenesis and regeneration. Front. Neurosci. 2022, 16, 1006037. [Google Scholar] [CrossRef]
- Hodge, R.D.; Nelson, B.R.; Kahoud, R.J.; Yang, R.; Mussar, K.E.; Reiner, S.L.; Hevner, R.F. Tbr2 is essential for hippocampal lineage progression from neural stem cells to intermediate progenitors and neurons. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 6275–6287. [Google Scholar] [CrossRef]
- Mihalas, A.B.; Hevner, R.F. Control of Neuronal Development by T-Box Genes in the Brain. Curr. Top. Dev. Biol. 2017, 122, 279–312. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, F.F.; Castro, D.S. Transcriptional control of vertebrate neurogenesis by the proneural factor Ascl1. Front. Cell. Neurosci. 2014, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Nicola, Z.; Fabel, K.; Kempermann, G. Development of the adult neurogenic niche in the hippocampus of mice. Front. Neuroanat. 2015, 9, 53. [Google Scholar] [CrossRef]
- Kuwabara, T.; Hsieh, J.; Muotri, A.; Yeo, G.; Warashina, M.; Lie, D.C.; Moore, L.; Nakashima, K.; Asashima, M.; Gage, F.H. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 2009, 12, 1097–1105. [Google Scholar] [CrossRef]
- Hodge, R.D.; Hevner, R.F. Expression and actions of transcription factors in adult hippocampal neurogenesis. Dev. Neurobiol. 2011, 71, 680–689. [Google Scholar] [CrossRef]
- Faigle, R.; Song, H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Et Biophys. Acta 2013, 1830, 2435–2448. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Bramham, C.R.; Duarte, C.B. BDNF and Hippocampal Synaptic Plasticity. Vitam. Horm. 2017, 104, 153–195. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Chauhan, L.; Bhardwaj, A.; Sharma, A.; Fayaz, F.; Kumar, B.; Alhashmi, M.; AlHajri, N.; Alam, M.S.; Pottoo, F.H. Brain-Derived Neurotropic Factor in Neurodegenerative Disorders. Biomedicines 2022, 10, 1143. [Google Scholar] [CrossRef]
- Gibon, J.; Barker, P.A. Neurotrophins and Proneurotrophins: Focus on Synaptic Activity and Plasticity in the Brain. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2017, 23, 587–604. [Google Scholar] [CrossRef]
- Pergolizzi, J.V., Jr.; Fort, P.; Miller, T.L.; LeQuang, J.A.; Raffa, R.B. The epidemiology of apnoea of prematurity. J. Clin. Pharm. Ther. 2022, 47, 685–693. [Google Scholar] [CrossRef]
- Abdel-Hady, H.; Nasef, N.; Shabaan, A.E.; Nour, I. Caffeine therapy in preterm infants. World J. Clin. Pediatr. 2015, 4, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Pournami, F.; Prabhakar, J.; Nandakumar, A.; Nair, P.M.C.; Jain, N. Duration of Caffeine for Apnea of Prematurity-A Randomized Controlled Trial. Indian J. Pediatr. 2021, 88, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Henderson-Smart, D.J.; De Paoli, A.G. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst. Rev. 2010, 12, CD000140. [Google Scholar] [CrossRef] [PubMed]
- Seppä-Moilanen, M.; Andersson, S.; Rantakari, K.; Mikkola, K.; Kirjavainen, T. Caffeine and supplemental oxygen effectively suppress periodic breathing with only minor effects during long episodes of apnoea in preterm infants. Acta Paediatr. 2019, 108, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.; Kraus, A.; Magnusson, P.; Breve, F.; Mitchell, K.; Raffa, R.; LeQuang, J.A.K.; Varrassi, G. Treating Apnea of Prematurity. Cureus 2022, 14, e21783. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.H.S.; Lipshultz, S.E. Caffeine and Clinical Outcomes in Premature Neonates. Children 2019, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.; Poorun, R.; Hartley, C. Apnoea of Prematurity and Neurodevelopmental Outcomes: Current Understanding and Future Prospects for Research. Front. Pediatr. 2021, 9, 755677. [Google Scholar] [CrossRef]
- Faudone, G.; Arifi, S.; Merk, D. The Medicinal Chemistry of Caffeine. J. Med. Chem. 2021, 64, 7156–7178. [Google Scholar] [CrossRef]
- Teng, R.J.; Jing, X.; Michalkiewicz, T.; Afolayan, A.J.; Wu, T.J.; Konduri, G.G. Attenuation of endoplasmic reticulum stress by caffeine ameliorates hyperoxia-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L586–L598. [Google Scholar] [CrossRef]
- Poets, C.F.; Roberts, R.S.; Schmidt, B.; Whyte, R.K.; Asztalos, E.V.; Bader, D.; Bairam, A.; Moddemann, D.; Peliowski, A.; Rabi, Y.; et al. Association Between Intermittent Hypoxemia or Bradycardia and Late Death or Disability in Extremely Preterm Infants. JAMA 2015, 314, 595–603. [Google Scholar] [CrossRef]
- Shi, X.; Dalal, N.S.; Jain, A.C. Antioxidant behaviour of caffeine: Efficient scavenging of hydroxyl radicals. Food Chem Toxicol 1991, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Park, T.J.; Ali, T.; Kim, M.O. Antioxidant and Neuroprotective Effects of Caffeine against Alzheimer’s and Parkinson’s Disease: Insight into the Role of Nrf-2 and A2AR Signaling. Antioxidants 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Moschino, L.; Zivanovic, S.; Hartley, C.; Trevisanuto, D.; Baraldi, E.; Roehr, C.C. Caffeine in preterm infants: Where are we in 2020? ERJ Open Res. 2020, 6, 00330–02019. [Google Scholar] [CrossRef] [PubMed]
- Murner-Lavanchy, I.M.; Doyle, L.W.; Schmidt, B.; Roberts, R.S.; Asztalos, E.V.; Costantini, L.; Davis, P.G.; Dewey, D.; D’Ilario, J.; Grunau, R.E.; et al. Neurobehavioral Outcomes 11 Years After Neonatal Caffeine Therapy for Apnea of Prematurity. Pediatrics 2018, 141, e20174047. [Google Scholar] [CrossRef]
- Doyle, L.W.; Schmidt, B.; Anderson, P.J.; Davis, P.G.; Moddemann, D.; Grunau, R.E.; O’Brien, K.; Sankaran, K.; Herlenius, E.; Roberts, R. Reduction in developmental coordination disorder with neonatal caffeine therapy. J. Pediatr. 2014, 165, 356–359.e352. [Google Scholar] [CrossRef]
- Bruschettini, M.; Moreira, A.; Pizarro, A.B.; Mustafa, S.; Romantsik, O. The effects of caffeine following hypoxic-ischemic encephalopathy: A systematic review of animal studies. Brain Res. 2022, 1790, 147990. [Google Scholar] [CrossRef]
- Back, S.A.; Craig, A.; Luo, N.L.; Ren, J.; Akundi, R.S.; Ribeiro, I.; Rivkees, S.A. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann. Neurol. 2006, 60, 696–705. [Google Scholar] [CrossRef]
- Silva, C.G.; Metin, C.; Fazeli, W.; Machado, N.J.; Darmopil, S.; Launay, P.S.; Ghestem, A.; Nesa, M.P.; Bassot, E.; Szabo, E.; et al. Adenosine receptor antagonists including caffeine alter fetal brain development in mice. Sci. Transl. Med. 2013, 5, 197ra104. [Google Scholar] [CrossRef]
- Soontarapornchai, K.; Cai, C.L.; Ahmad, T.; Aranda, J.V.; Hand, I.; Beharry, K.D. Pharmacodynamic Effects of Standard versus High Caffeine Doses in the Developing Brain of Neonatal Rats Exposed to Intermittent Hypoxia. Int. J. Mol. Sci. 2021, 22, 3473. [Google Scholar] [CrossRef]
- Tchekalarova, J.D.; Kubová, H.; Mareš, P. Early caffeine exposure: Transient and long-term consequences on brain excitability. Brain Res. Bull. 2014, 104, 27–35. [Google Scholar] [CrossRef]
- Atik, A.; Harding, R.; De Matteo, R.; Kondos-Devcic, D.; Cheong, J.; Doyle, L.W.; Tolcos, M. Caffeine for apnea of prematurity: Effects on the developing brain. Neurotoxicology 2017, 58, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Eichenwald, E.C. National and international guidelines for neonatal caffeine use: Are they evidenced-based? Semin. Fetal Neonatal Med. 2020, 25, 101177. [Google Scholar] [CrossRef]
- Chavez, L.; Bancalari, E. Caffeine: Some of the Evidence behind Its Use and Abuse in the Preterm Infant. Neonatology 2022, 119, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Borszewska-Kornacka, M.K.; Hozejowski, R.; Rutkowska, M.; Lauterbach, R. Shifting the boundaries for early caffeine initiation in neonatal practice: Results of a prospective, multicenter study on very preterm infants with respiratory distress syndrome. PLoS ONE 2017, 12, e0189152. [Google Scholar] [CrossRef]
- Park, H.W.; Lim, G.; Chung, S.H.; Chung, S.; Kim, K.S.; Kim, S.N. Early Caffeine Use in Very Low Birth Weight Infants and Neonatal Outcomes: A Systematic Review and Meta-Analysis. J. Korean Med. Sci. 2015, 30, 1828–1835. [Google Scholar] [CrossRef]
- Patel, R.M.; Zimmerman, K.; Carlton, D.P.; Clark, R.; Benjamin, D.K.; Smith, P.B. Early Caffeine Prophylaxis and Risk of Failure of Initial Continuous Positive Airway Pressure in Very Low Birth Weight Infants. J. Pediatr. 2017, 190, 108–111.e101. [Google Scholar] [CrossRef]
- Henderson-Smart, D.J.; De Paoli, A.G. Prophylactic methylxanthine for prevention of apnoea in preterm infants. Cochrane Database Syst. Rev. 2010, 12, CD000432. [Google Scholar] [CrossRef]
- Ikonomidou, C.; Turski, L. Antiepileptic drugs and brain development. Epilepsy Res. 2010, 88, 11–22. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Env. Health Perspect 2000, 108 (Suppl. S3), 511–533. [Google Scholar]
- Altman, J.; Bayer, S.A. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J. Comp. Neurol. 1990, 301, 365–381. [Google Scholar] [CrossRef]
- Ikonomidou, C.; Kaindl, A.M. Neuronal death and oxidative stress in the developing brain. Antioxid. Redox Signal. 2011, 14, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.F.; Ouyang, S.H.; Zhang, Q.Y.; Wu, Y.P.; Wang, G.E.; Tu, L.F.; Luo, Z.; Li, W.X.; Kurihara, H.; Li, Y.F.; et al. New insights into the effects of caffeine on adult hippocampal neurogenesis in stressed mice: Inhibition of CORT-induced microglia activation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 10998–11014. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Ozsurekci, Y.; Aykac, K. Oxidative Stress Related Diseases in Newborns. Oxidative Med. Cell. Longev. 2016, 2016, 2768365. [Google Scholar] [CrossRef]
- Venkatesh, K.K.; Meeker, J.D.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Association of antenatal depression with oxidative stress and impact on spontaneous preterm birth. J. Perinatol. 2019, 39, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, L.; Meyer, A.K.; Wagenführ, L.; Storch, A. Oxygen regulates proliferation of neural stem cells through Wnt/β-catenin signalling. Mol. Cell. Neurosci. 2015, 67, 84–92. [Google Scholar] [CrossRef]
- Mennen, R.H.; de Leeuw, V.C.; Piersma, A.H. Oxygen tension influences embryonic stem cell maintenance and has lineage specific effects on neural and cardiac differentiation. Differentiation 2020, 115, 1–10. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hsia, C.-W.; Ho, C.-W.; Liang, C.-M.; Chen, C.-M.; Huang, K.-L.; Kang, B.-H.; Chen, Y.-H. Hypoxia and hyperoxia differentially control proliferation of rat neural crest stem cells via distinct regulatory pathways of the HIF1α–CXCR4 and TP53–TPM1 proteins. Dev. Dyn. 2017, 246, 162–185. [Google Scholar] [CrossRef] [PubMed]
- Kaindl, A.M.; Sifringer, M.; Zabel, C.; Nebrich, G.; Wacker, M.A.; Felderhoff-Mueser, U.; Endesfelder, S.; von der Hagen, M.; Stefovska, V.; Klose, J.; et al. Acute and long-term proteome changes induced by oxidative stress in the developing brain. Cell Death Differ. 2006, 13, 1097–1109. [Google Scholar] [CrossRef]
- Wentz, C.T.; Magavi, S.S. Caffeine alters proliferation of neuronal precursors in the adult hippocampus. Neuropharmacology 2009, 56, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Houghton, V.; Du Preez, A.; Lefèvre-Arbogast, S.; de Lucia, C.; Low, D.Y.; Urpi-Sarda, M.; Ruigrok, S.R.; Altendorfer, B.; González-Domínguez, R.; Andres-Lacueva, C.; et al. Caffeine Compromises Proliferation of Human Hippocampal Progenitor Cells. Front. Cell Dev. Biol. 2020; 8, 806. [Google Scholar] [CrossRef]
- Ősz, B.E.; Jîtcă, G.; Ștefănescu, R.E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C.E. Caffeine and Its Antioxidant Properties-It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef] [PubMed]
- Mateus, J.M.; Ribeiro, F.F.; Alonso-Gomes, M.; Rodrigues, R.S.; Marques, J.M.; Sebastião, A.M.; Rodrigues, R.J.; Xapelli, S. Neurogenesis and Gliogenesis: Relevance of Adenosine for Neuroregeneration in Brain Disorders. J. Caffeine Adenosine Res. 2019, 9, 129–144. [Google Scholar] [CrossRef]
- Gaytan, S.P.; Pasaro, R. Neonatal caffeine treatment up-regulates adenosine receptors in brainstem and hypothalamic cardio-respiratory related nuclei of rat pups. Exp. Neurol. 2012, 237, 247–259. [Google Scholar] [CrossRef]
- Miyagi, S.; Masui, S.; Niwa, H.; Saito, T.; Shimazaki, T.; Okano, H.; Nishimoto, M.; Muramatsu, M.; Iwama, A.; Okuda, A. Consequence of the loss of Sox2 in the developing brain of the mouse. FEBS Lett. 2008, 582, 2811–2815. [Google Scholar] [CrossRef]
- Peltier, J.; Conway, A.; Keung, A.J.; Schaffer, D.V. Akt increases sox2 expression in adult hippocampal neural progenitor cells, but increased sox2 does not promote proliferation. Stem Cells Dev. 2011, 20, 1153–1161. [Google Scholar] [CrossRef]
- Bath, K.G.; Akins, M.R.; Lee, F.S. BDNF control of adult SVZ neurogenesis. Dev. Psychobiol. 2012, 54, 578–589. [Google Scholar] [CrossRef]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef]
- Jeon, S.J.; Rhee, S.Y.; Ryu, J.H.; Cheong, J.H.; Kwon, K.; Yang, S.-I.L.; Park, S.H.; Lee, J.; Kim, H.Y.; Han, S.-H.; et al. Activation of Adenosine A2A Receptor Up-Regulates BDNF Expression in Rat Primary Cortical Neurons. Neurochem. Res. 2011, 36, 2259. [Google Scholar] [CrossRef]
- Quesseveur, G.; David, D.J.; Gaillard, M.C.; Pla, P.; Wu, M.V.; Nguyen, H.T.; Nicolas, V.; Auregan, G.; David, I.; Dranovsky, A.; et al. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl. Psychiatry 2013, 3, e253. [Google Scholar] [CrossRef]
- DeCarolis, N.A.; Mechanic, M.; Petrik, D.; Carlton, A.; Ables, J.L.; Malhotra, S.; Bachoo, R.; Götz, M.; Lagace, D.C.; Eisch, A.J. In vivo contribution of nestin- and GLAST-lineage cells to adult hippocampal neurogenesis. Hippocampus 2013, 23, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.A.; Belnoue, L.; Song, H.; Simon, A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 2013, 140, 2548–2561. [Google Scholar] [CrossRef] [PubMed]
- Bansod, S.; Kageyama, R.; Ohtsuka, T. Hes5 regulates the transition timing of neurogenesis and gliogenesis in mammalian neocortical development. Development 2017, 144, 3156–3167. [Google Scholar] [CrossRef] [PubMed]
- Favaro, R.; Valotta, M.; Ferri, A.L.; Latorre, E.; Mariani, J.; Giachino, C.; Lancini, C.; Tosetti, V.; Ottolenghi, S.; Taylor, V.; et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat. Neurosci. 2009, 12, 1248–1256. [Google Scholar] [CrossRef]
- Hulme, A.J.; Maksour, S.; St-Clair Glover, M.; Miellet, S.; Dottori, M. Making neurons, made easy: The use of Neurogenin-2 in neuronal differentiation. Stem Cell Rep. 2022, 17, 14–34. [Google Scholar] [CrossRef]
- Pataskar, A.; Jung, J.; Smialowski, P.; Noack, F.; Calegari, F.; Straub, T.; Tiwari, V.K. NeuroD1 reprograms chromatin and transcription factor landscapes to induce the neuronal program. EMBO J. 2016, 35, 24–45. [Google Scholar] [CrossRef]
- Tutukova, S.; Tarabykin, V.; Hernandez-Miranda, L.R. The Role of Neurod Genes in Brain Development, Function, and Disease. Front. Mol. Neurosci. 2021, 14, 662774. [Google Scholar] [CrossRef]
- Uda, M.; Ishido, M.; Kami, K. Features and a possible role of Mash1-immunoreactive cells in the dentate gyrus of the hippocampus in the adult rat. Brain Res. 2007, 1171, 9–17. [Google Scholar] [CrossRef]
- Castro, D.S.; Skowronska-Krawczyk, D.; Armant, O.; Donaldson, I.J.; Parras, C.; Hunt, C.; Critchley, J.A.; Nguyen, L.; Gossler, A.; Göttgens, B.; et al. Proneural bHLH and Brn proteins coregulate a neurogenic program through cooperative binding to a conserved DNA motif. Dev. Cell 2006, 11, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.; Urbán, N.; Achimastou, A.; Ito, A.; Simic, M.; Ullom, K.; Martynoga, B.; Lebel, M.; Göritz, C.; Frisén, J.; et al. A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron 2014, 83, 1085–1097. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hsieh, P.F.; Huang, D.F.; Chin, P.S.; Chou, C.H.; Tung, C.C.; Chen, S.Y.; Lee, L.J.; Gau, S.S.; Huang, H.S. RBFOX3/NeuN is Required for Hippocampal Circuit Balance and Function. Sci. Rep. 2015, 5, 17383. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Wang, H.Y.; Huang, D.F.; Hsieh, P.F.; Lin, M.Y.; Chou, C.H.; Wu, I.J.; Huang, G.J.; Gau, S.S.; Huang, H.S. Neuronal Splicing Regulator RBFOX3 (NeuN) Regulates Adult Hippocampal Neurogenesis and Synaptogenesis. PLoS ONE 2016, 11, e0164164. [Google Scholar] [CrossRef] [PubMed]
- Lavado, A.; Lagutin, O.V.; Chow, L.M.; Baker, S.J.; Oliver, G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 2010, 8, e1000460. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.; Sebastiao, A.M. Caffeine and adenosine. J. Alzheimer’s Dis. JAD 2010, 20 (Suppl. 1), S3–S15. [Google Scholar] [CrossRef]
- Cao, T.; Ma, T.; Xu, Y.; Tian, Y.; Cai, Q.; Li, B.; Li, H. Caffeine Treatment Promotes Differentiation and Maturation of Hypoxic Oligodendrocytes via Counterbalancing Adenosine 1 Adenosine Receptor-Induced Calcium Overload. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 1729–1739. [Google Scholar] [CrossRef]
- Maraula, G.; Traini, C.; Mello, T.; Coppi, E.; Galli, A.; Pedata, F.; Pugliese, A.M. Effects of oxygen and glucose deprivation on synaptic transmission in rat dentate gyrus: Role of A2A adenosine receptors. Neuropharmacology 2013, 67, 511–520. [Google Scholar] [CrossRef]
- Nakaso, K.; Ito, S.; Nakashima, K. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells. Neurosci. Lett. 2008, 432, 146–150. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Chen, J.F.; Cunha, R.A.; Svenningsson, P.; Vaugeois, J.M. Adenosine and brain function. Int. Rev. Neurobiol. 2005, 63, 191–270. [Google Scholar] [CrossRef]
- Migita, H.; Kominami, K.; Higashida, M.; Maruyama, R.; Tuchida, N.; McDonald, F.; Shimada, F.; Sakurada, K. Activation of adenosine A1 receptor-induced neural stem cell proliferation via MEK/ERK and Akt signaling pathways. J. Neurosci. Res. 2008, 86, 2820–2828. [Google Scholar] [CrossRef] [PubMed]
- Benito-Muñoz, M.; Matute, C.; Cavaliere, F. Adenosine A1 receptor inhibits postnatal neurogenesis and sustains astrogliogenesis from the subventricular zone. Glia 2016, 64, 1465–1478. [Google Scholar] [CrossRef]
- Stafford, M.R.; Bartlett, P.F.; Adams, D.J. Purinergic receptor activation inhibits mitogen-stimulated proliferation in primary neurospheres from the adult mouse subventricular zone. Mol. Cell. Neurosci. 2007, 35, 535–548. [Google Scholar] [CrossRef]
- Moscoso-Castro, M.; López-Cano, M.; Gracia-Rubio, I.; Ciruela, F.; Valverde, O. Cognitive impairments associated with alterations in synaptic proteins induced by the genetic loss of adenosine A(2A) receptors in mice. Neuropharmacology 2017, 126, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, J.; Jakova, E.; Cayabyab, F.S. Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration. Molecules 2017, 22, 676. [Google Scholar] [CrossRef]
- Bruzzese, L.; Rostain, J.-C.; Née, L.; Condo, J.; Mottola, G.; Adjriou, N.; Mercier, L.; Berge-Lefranc, J.-L.; Fromonot, J.; Kipson, N.; et al. Effect of hyperoxic and hyperbaric conditions on the adenosinergic pathway and CD26 expression in rat. J. Appl. Physiol. 2015, 119, 140–147. [Google Scholar] [CrossRef]
- Stefovska, V.G.; Uckermann, O.; Czuczwar, M.; Smitka, M.; Czuczwar, P.; Kis, J.; Kaindl, A.M.; Turski, L.; Turski, W.A.; Ikonomidou, C. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann. Neurol. 2008, 64, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Zeiss, C.J. Comparative Milestones in Rodent and Human Postnatal Central Nervous System Development. Toxicol. Pathol. 2021, 49, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef]
- Abu-Sa’da, O.S.; Armstrong, E.A.; Scott, O.; Shaw, O.; Nguyen, A.I.; Shen, K.; Cheung, P.; Baker, G.; Yager, J.Y. The Effect of Caffeine on the Neuropathological and Neurobehavioral Outcome in the Newborn Rat. J. Caffeine Adenosine Res. 2018, 8, 143–152. [Google Scholar] [CrossRef]



| Oligonucleotide Sequence 5′-3′ | Accession No. | |
|---|---|---|
| Ascl1 (Mash1) | ||
| Forward | AACTTCAGTGGCTTCGGCTA | NM_022384.1 |
| Reverse | GCCCAGGTTAACCAACTTGA | |
| Probe | AGCCTTCCACAGCAGCAG | |
| BDNF | ||
| Forward | TCAGCAGTCAAGTGCCTTTGG | NM_012513.4 |
| Reverse | CGCCGAACCCTCATAGACATG | |
| Probe | CCTCCTCTGCTCTTTCTGCTGGAGGAATACAA | |
| CycD2 | ||
| Forward | CGTACATGCGCAGGATGGT | NM_199501.1 |
| Reverse | AATTCATGGCCAGAGGAAAGAC | |
| Probe | TGGATGCTAGAGGTCTGTGA | |
| GFAP | ||
| Forward | TCTGGACCAGCTTACTACCAACAG | NM_017009.2 |
| Reverse | TGGTTTCATCTTGGAGCTTCTG | |
| Probe | AGAGGGACAATCTCACACAG | |
| Hes5 | ||
| forward | ATGCTCAGTCCCAAGGAGAA | NM_024383.1 |
| reverse | TAGTCCTGGTGCAGGCTCTT | |
| probe | CCCAACTCCAAACTGGAGAA | |
| HPRT | ||
| forward | GGAAAGAACGTCTTGATTGTTGAA | NM_012583.2 |
| reverse | CCAACACTTCGAGAGGTCCTTTT | |
| probe | CTTTCCTTGGTCAAGCAGTACAGCCCC | |
| NeuroD1 | ||
| forward | TCAGCATCAATGGCAACTTC | NM_019218.2 |
| reverse | AAGATTGATCCGTGGCTTTG | |
| probe | TTACCATGCACTACCCTGCA | |
| NeuroD2 | ||
| forward | TCTGGTGTCCTACGTGCAGA | NM_019326.1 |
| reverse | CCTGCTCCGTGAGGAAGTTA | |
| probe | TGCCTGCAGCTGAACTCTC | |
| NGF | ||
| forward | ACCCAAGCTCACCTCAGTGTCT | NM_001277055.1 |
| reverse | GACATTACGCTATGCACCTCAGAGT | |
| probe | CAATAAAGGCTTTGCCAAGG | |
| Ngn2 | ||
| forward | AGGCTCAAAGCCAACAACC | XM_008775262.2 |
| reverse | GATGTAATTGTGGGCGAAGC | |
| probe | CTCACGAAGATCGAGACGCT | |
| NT3 | ||
| forward | AGAACATCACCACGGAGGAAA | NM_031073.3 |
| reverse | GGTCACCCACAGGCTCTCA | |
| probe | AGAGCATAAGAGTCACCGAG | |
| Pax6 | ||
| forward | TCCCTATCAGCAGCAGTTTCAGT | NM_013001.2 |
| reverse | GTCTGTGCGGCCCAACAT | |
| probe | CTCCTCCTTTACATCGGGTT | |
| Prox1 | ||
| forward | TGCCTTTTCCAGGAGCAACTAT | NM_001107201.1 |
| reverse | CCGCTGGCTTGGAAACTG | |
| probe | ACATGAACAAAAACGGTGGC | |
| Scl1a3 (GLAST) | ||
| forward | CCCTGCCCATCACTTTCAAG | NM_001289942.1 |
| reverse | GCGGTCCCATCCATGTTAA | |
| probe | CTGGAAGAAAACAATGGTGTGG | |
| Sox2 | ||
| forward | ACAGATGCAGCCGATGCA | NM_001109181.1 |
| reverse | GGTGCCCTGCTGCGAGTA | |
| probe | CAGTACAACTCCATGACCAG | |
| Tbr1 | ||
| forward | TCCCAATCACTGGAGGTTTCA | NM_001191070.1 |
| reverse | GGATGCATATAGACCCGGTTTC | |
| probe | AAATGGGTTCCTTGTGGCAA | |
| Tbr2 | ||
| forward | ACGCAGATGATAGTGTTGCAGTCT | XM_006226608.2 |
| reverse | ATTCAAGTCCTCCACACCATCCT | |
| probe | CACAAATACCAACCTCGACT | |
| Effects on Neuronal Cells in the Developing DG | |
|---|---|
| Hyperoxia |
|
| Hyperoxia and Caffeine |
|
| Caffeine |
|
| Effects on transcription factors in the developing brain | |
| Hyperoxia |
|
| Hyperoxia and Caffeine |
|
| Caffeine |
|
| Effects on neurotrophic factors in the developing brain | |
| Hyperoxia |
|
| Hyperoxia and Caffeine |
|
| Caffeine |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heise, J.; Schmitz, T.; Bührer, C.; Endesfelder, S. Protective Effects of Early Caffeine Administration in Hyperoxia-Induced Neurotoxicity in the Juvenile Rat. Antioxidants 2023, 12, 295. https://doi.org/10.3390/antiox12020295
Heise J, Schmitz T, Bührer C, Endesfelder S. Protective Effects of Early Caffeine Administration in Hyperoxia-Induced Neurotoxicity in the Juvenile Rat. Antioxidants. 2023; 12(2):295. https://doi.org/10.3390/antiox12020295
Chicago/Turabian StyleHeise, Julia, Thomas Schmitz, Christoph Bührer, and Stefanie Endesfelder. 2023. "Protective Effects of Early Caffeine Administration in Hyperoxia-Induced Neurotoxicity in the Juvenile Rat" Antioxidants 12, no. 2: 295. https://doi.org/10.3390/antiox12020295
APA StyleHeise, J., Schmitz, T., Bührer, C., & Endesfelder, S. (2023). Protective Effects of Early Caffeine Administration in Hyperoxia-Induced Neurotoxicity in the Juvenile Rat. Antioxidants, 12(2), 295. https://doi.org/10.3390/antiox12020295








