COVID-19 Causes Ferroptosis and Oxidative Stress in Human Endothelial Cells
Abstract
1. Introduction
2. Results
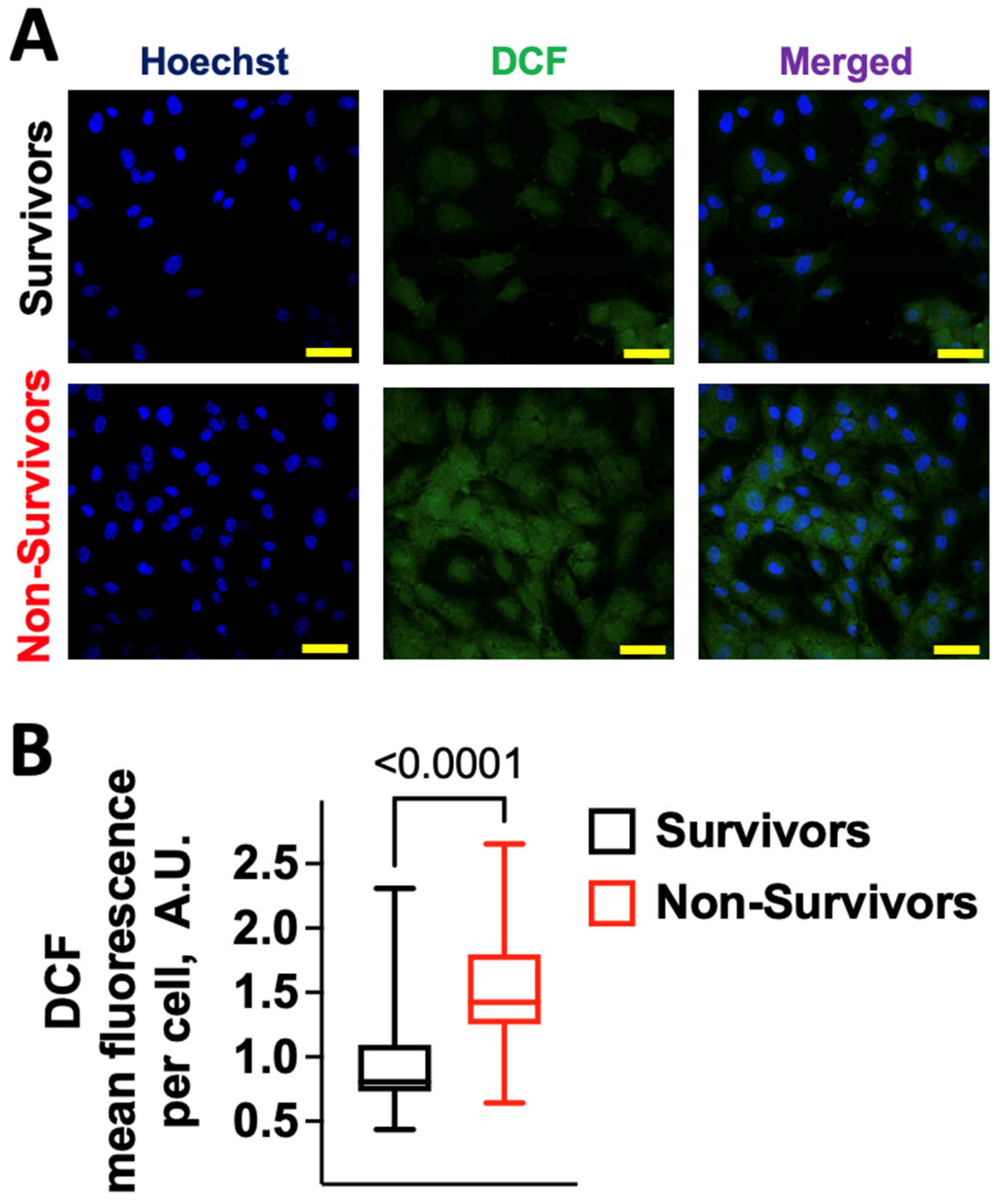
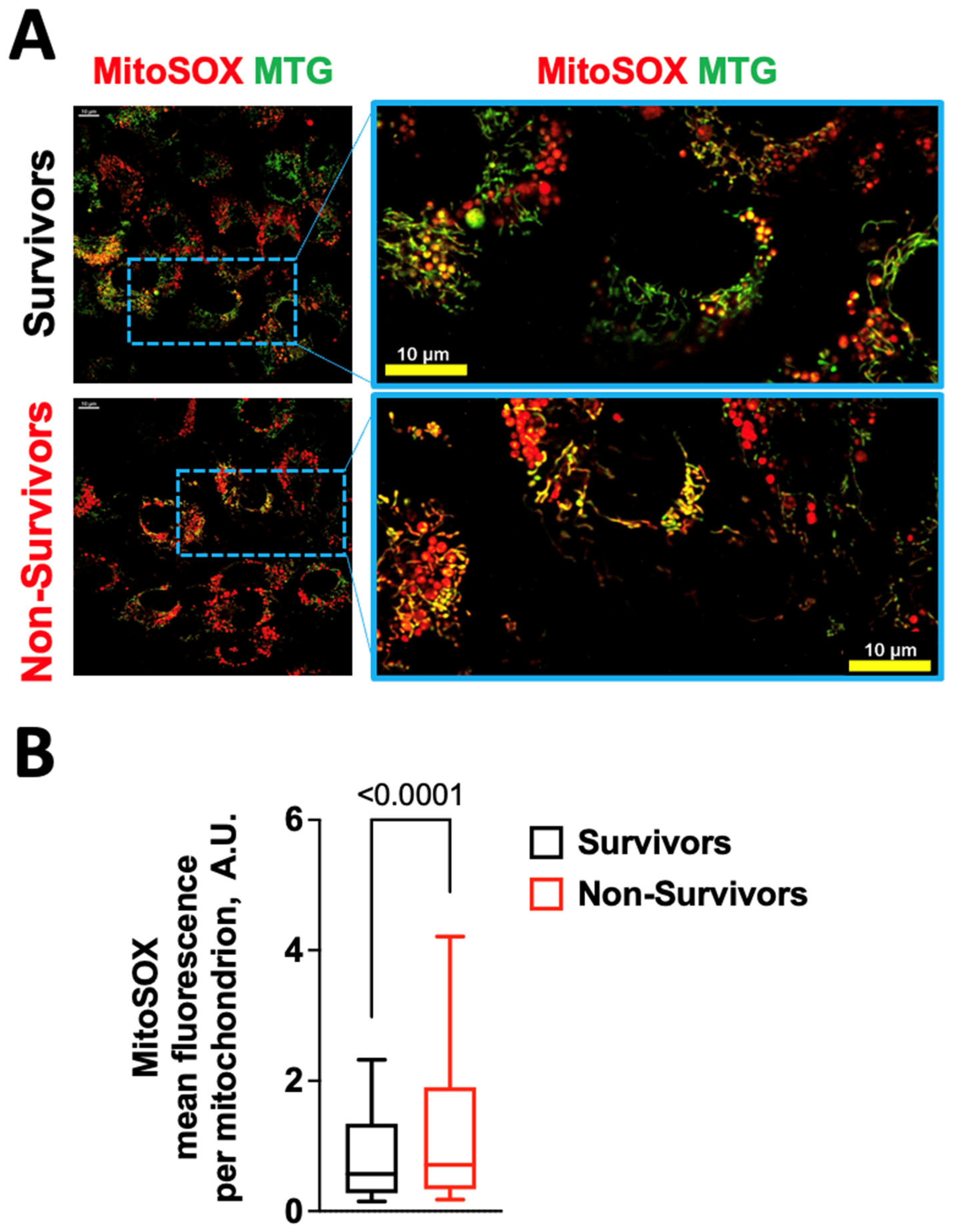
2.1. COVID-19 Induces Oxidative Stress in Human Endothelial Cells
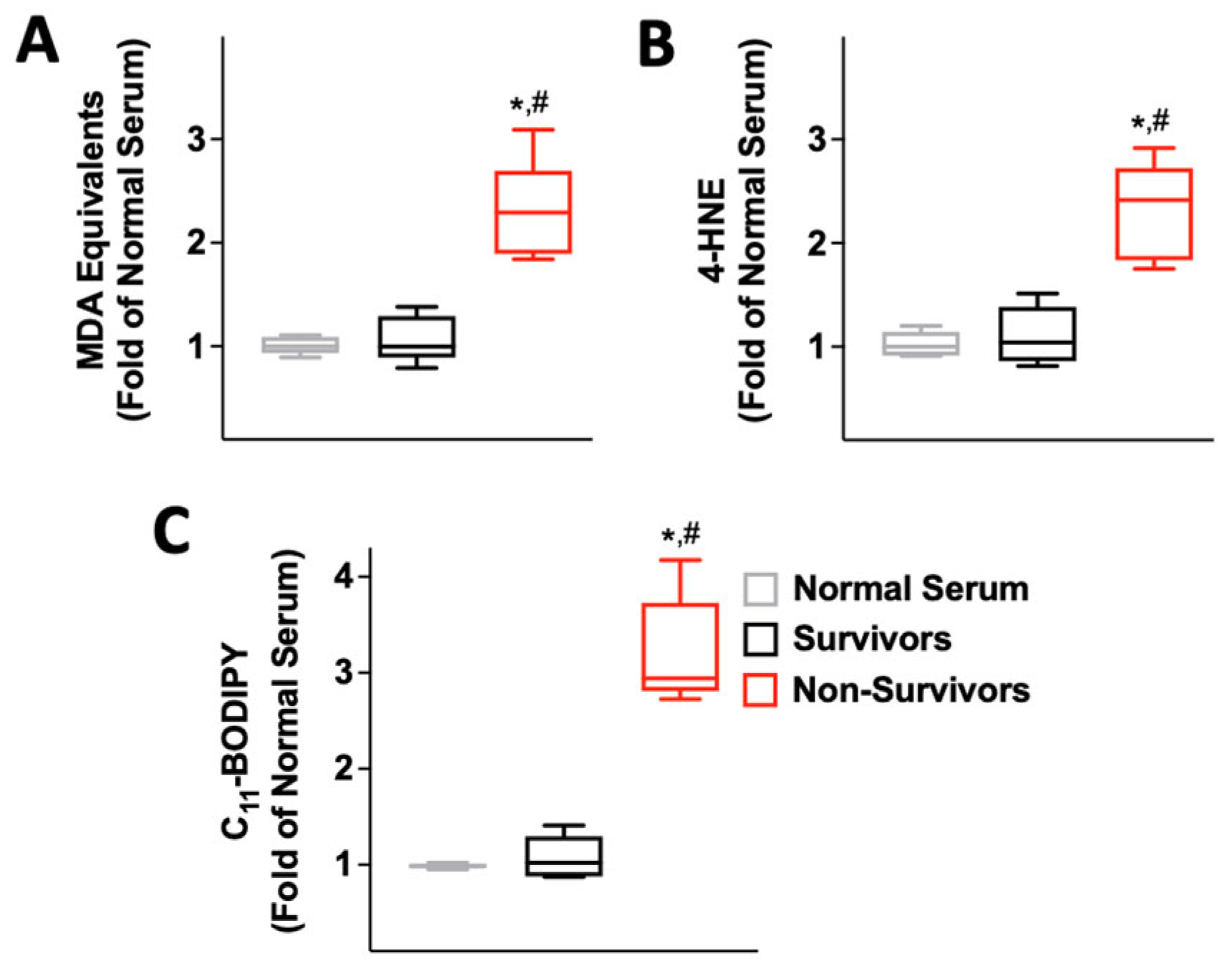
2.2. Serum from COVID-19 Non-Survivors Causes Lipid Peroxidation
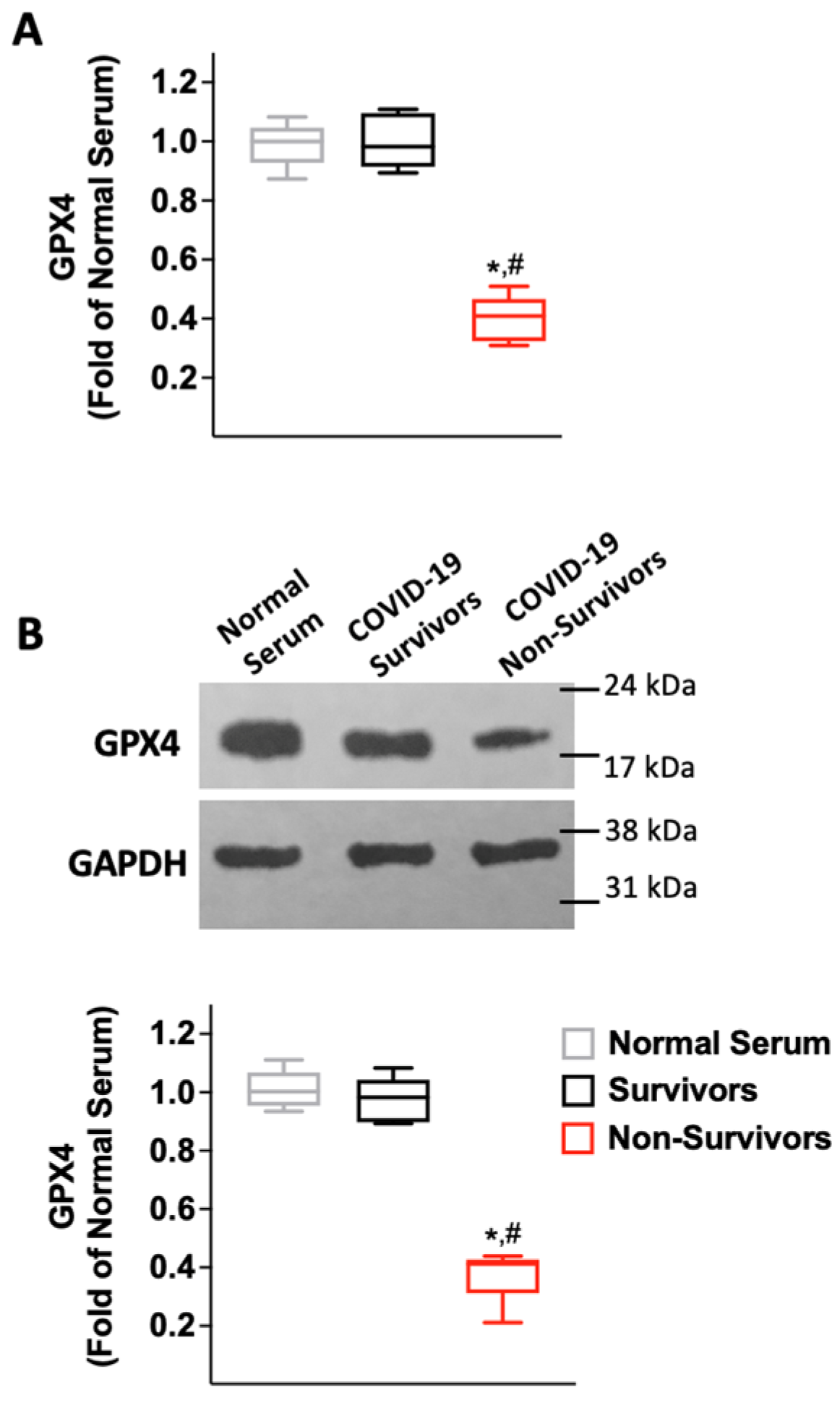
2.3. Serum from COVID-19 Non-Survivors Reduces the Expression of the Antioxidant Enzyme Glutathione Peroxidase 4 (GPX4) in HUVECs
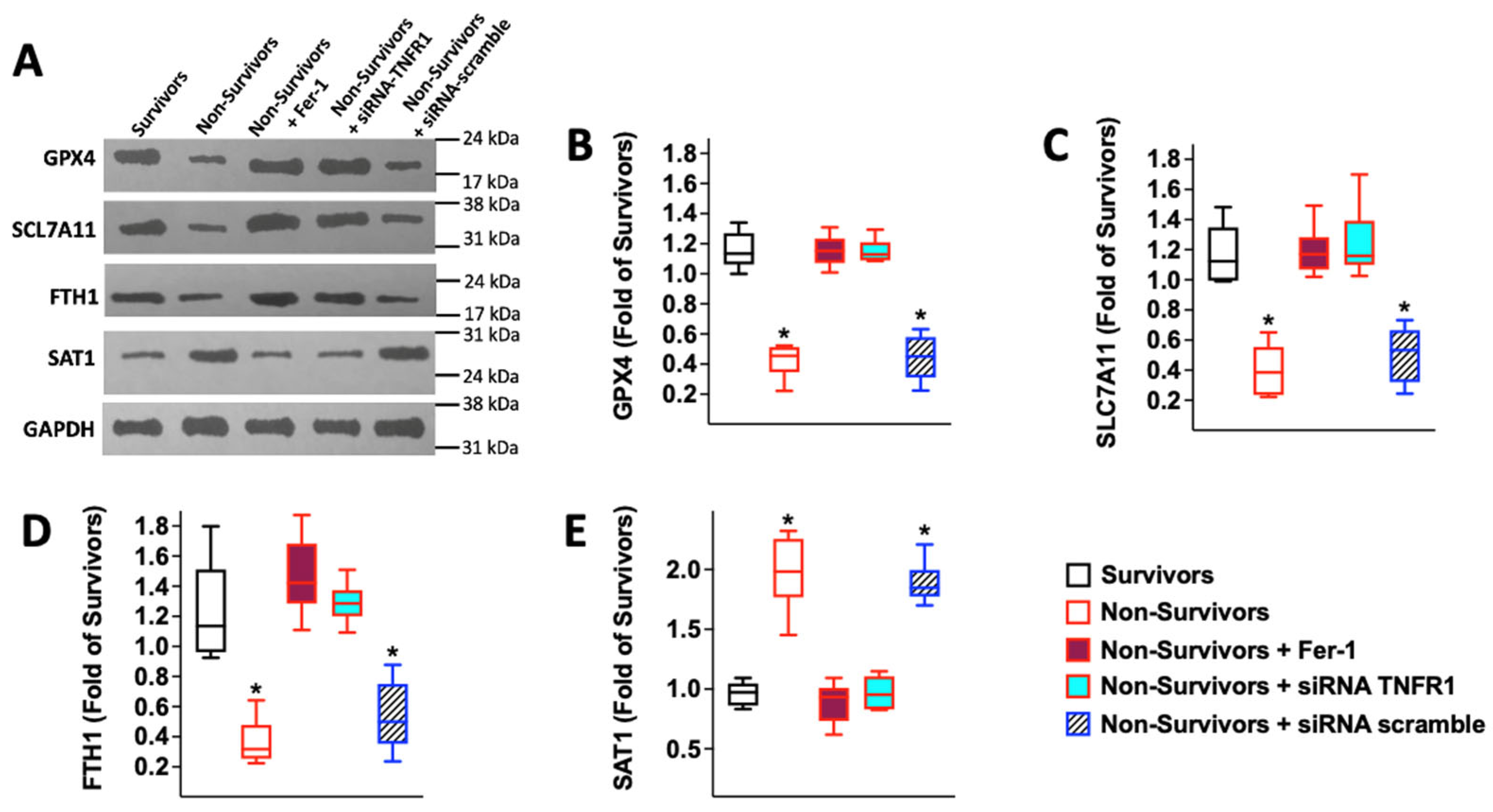
2.4. Serum from COVID-19 Non-Survivors Triggers Ferroptosis in Human Endothelial Cells
2.5. COVID-19-Induced Ferroptosis in HUVECs Is Mediated by TNFα
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Assays Measuring Reactive Oxygen Species (ROS) and Lipid Oxidation
4.3. Immunoblotting and RT-qPCR
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sardu, C.; Gambardella, J.; Morelli, M.B.; Wang, X.; Marfella, R.; Santulli, G. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence. J. Clin. Med. 2020, 9, 1417. [Google Scholar] [CrossRef] [PubMed]
- Matarese, A.; Gambardella, J.; Sardu, C.; Santulli, G. miR-98 Regulates TMPRSS2 Expression in Human Endothelial Cells: Key Implications for COVID-19. Biomedicines 2020, 8, 462. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, H.K.; Libby, P.; Ridker, P.M. COVID-19—A vascular disease. Trends Cardiovasc. Med. 2020, 31, 1–5. [Google Scholar] [CrossRef]
- Perico, L.; Benigni, A.; Casiraghi, F.; Ng, L.F.P.; Renia, L.; Remuzzi, G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2020, 17, 46–64. [Google Scholar] [CrossRef]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and endotheliopathy: Crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2020, 18, 194–209. [Google Scholar] [CrossRef]
- Ionescu, M.; Stoian, A.P.; Rizzo, M.; Serban, D.; Nuzzo, D.; Mazilu, L.; Suceveanu, A.I.; Dascalu, A.M.; Parepa, I.R. The Role of Endothelium in COVID-19. Int. J. Mol. Sci. 2021, 22, 11920. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Enjyoji, K.; Schmaier, A.A. Vasculopathy in COVID-19. Blood 2022, 140, 222–235. [Google Scholar] [CrossRef]
- Faria, D.; Moll-Bernardes, R.J.; Testa, L.; Moniz, C.M.; Rodrigues, E.C.; Rodrigues, A.G.; Araujo, A.; Alves, M.J.; Ono, B.E.; Izaias, J.E.; et al. Sympathetic Neural Overdrive, Aortic Stiffening, Endothelial Dysfunction, and Impaired Exercise Capacity in Severe COVID-19 Survivors: A Mid-Term Study of Cardiovascular Sequelae. Hypertension 2023, 80, 470–481. [Google Scholar] [CrossRef]
- Montiel, V.; Lobysheva, I.; Gérard, L.; Vermeersch, M.; Perez-Morga, D.; Castelein, T.; Mesland, J.-B.; Hantson, P.; Collienne, C.; Gruson, D.; et al. Oxidative stress-induced endothelial dysfunction and decreased vascular nitric oxide in COVID-19 patients. Ebiomedicine 2022, 77, 103893. [Google Scholar] [CrossRef]
- Trimarco, V.; Izzo, R.; Zanforlin, A.; Tursi, F.; Scarpelli, F.; Santus, P.; Pennisi, A.; Pelaia, G.; Mussi, C.; Mininni, S.; et al. Endothelial dysfunction in long-COVID: New insights from the nationwide multicenter LINCOLN Study. Pharmacol. Res. 2022, 185, 106486. [Google Scholar] [CrossRef] [PubMed]
- Kansakar, U.; Gambardella, J.; Varzideh, F.; Avvisato, R.; Jankauskas, S.S.; Mone, P.; Matarese, A.; Santulli, G. miR-142 Targets TIM-1 in Human Endothelial Cells: Potential Implications for Stroke, COVID-19, Zika, Ebola, Dengue, and Other Viral Infections. Int. J. Mol. Sci. 2022, 23, 10242. [Google Scholar] [CrossRef] [PubMed]
- Mone, P.; Gambardella, J.; Wang, X.; Jankauskas, S.S.; Matarese, A.; Santulli, G. miR-24 Targets the Transmembrane Glycoprotein Neuropilin-1 in Human Brain Microvascular Endothelial Cells. Non-Coding RNA 2021, 7, 9. [Google Scholar] [CrossRef]
- Gambardella, J.; Santulli, G. What is linking COVID-19 and endothelial dysfunction? Updates on nanomedicine and bioengineering from the 2020 AHA Scientific Sessions. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, e2–e3. [Google Scholar] [CrossRef]
- Gambardella, J.; Kansakar, U.; Sardu, C.; Messina, V.; Jankauskas, S.S.; Marfella, R.; Maggi, P.; Wang, X.; Mone, P.; Paolisso, G.; et al. Exosomal miR-145 and miR-885 Regulate Thrombosis in COVID-19. J. Pharmacol. Exp. Ther. 2023, 384, 109–115. [Google Scholar] [CrossRef]
- Jiang, Z.; Wu, L.; van der Leeden, B.; van Rossum, A.C.; Niessen, H.W.; Krijnen, P.A. NOX2 and NOX5 are increased in cardiac microvascular endothelium of deceased COVID-19 patients. Int. J. Cardiol. 2022, 370, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Plazak, W.; Drabik, L. SARS-CoV-2 infection and SLE: Endothelial dysfunction, atherosclerosis, and thrombosis. Clin. Rheumatol. 2023, 1–12. [Google Scholar] [CrossRef]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020, 9, 624. [Google Scholar] [CrossRef]
- Vardakas, P.; Skaperda, Z.; Tekos, F.; Kouretas, D. ROS and COVID. Antioxidants 2022, 11, 339. [Google Scholar] [CrossRef]
- Chernyak, B.V.; Popova, E.N.; Prikhodko, A.S.; Grebenchikov, O.A.; Zinovkina, L.A.; Zinovkin, R.A. COVID-19 and Oxidative Stress. Biochem. (Moscow) 2020, 85, 1543–1553. [Google Scholar] [CrossRef]
- Carnevale, R.; Cammisotto, V.; Bartimoccia, S.; Nocella, C.; Castellani, V.; Bufano, M.; Loffredo, L.; Sciarretta, S.; Frati, G.; Coluccia, A.; et al. Toll-Like Receptor 4-Dependent Platelet-Related Thrombosis in SARS-CoV-2 Infection. Circ. Res. 2023, in press. [Google Scholar] [CrossRef]
- Jana, S.; Heaven, M.R.; Stauft, C.B.; Wang, T.T.; Williams, M.C.; D’Agnillo, F.; Alayash, A.I. HIF-1α-Dependent Metabolic Reprogramming, Oxidative Stress, and Bioenergetic Dysfunction in SARS-CoV-2-Infected Hamsters. Int. J. Mol. Sci. 2022, 24, 558. [Google Scholar] [CrossRef] [PubMed]
- Munguía, L.; Nájera, N.; Martínez, F.D.J.; Díaz-Chiguer, D.; Jiménez-Ponce, F.; Ortiz-Flores, M.; Villarreal, F.; Ceballos, G. Correlation of Biomarkers of Endothelial Injury and Inflammation to Outcome in Hospitalized COVID-19 Patients. J. Clin. Med. 2022, 11, 7436. [Google Scholar] [CrossRef] [PubMed]
- Aparisi, Á.; Martín-Fernández, M.; Ybarra-Falcón, C.; Gil, J.F.; Carrasco-Moraleja, M.; Martínez-Paz, P.; Cusácovich, I.; Gonzalo-Benito, H.; Fuertes, R.; Marcos-Mangas, M.; et al. Dyslipidemia and Inflammation as Hallmarks of Oxidative Stress in COVID-19: A Follow-Up Study. Int. J. Mol. Sci. 2022, 23, 15350. [Google Scholar] [CrossRef] [PubMed]
- Tavassolifar, M.J.; Aghdaei, H.A.; Sadatpour, O.; Maleknia, S.; Fayazzadeh, S.; Mohebbi, S.R.; Montazer, F.; Rabbani, A.; Zali, M.R.; Izad, M.; et al. New insights into extracellular and intracellular redox status in COVID-19 patients. Redox Biol. 2023, 59, 102563. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Goff, D.C., Jr.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 51. [Google Scholar] [CrossRef]
- Sardu, C.; Santulli, G.; Savarese, G.; Trotta, M.C.; Sacra, C.; Santamaria, M.; Volpicelli, M.; Ruocco, A.; Mauro, C.; Signoriello, G.; et al. Endothelial Dysfunction Drives CRTd Outcome at 1-Year Follow-Up: A Novel Role as Biomarker for miR-130a-5p. Int. J. Mol. Sci. 2023, 24, 1510. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Guzmán, R.R.; Centofanti, F.; Doldo, E.; Miranda, E.M.C.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Santulli, G.; Campanile, A.; Galasso, G.; Cervèro, P.; Altobelli, G.G.; Cimini, V.; Pastore, L.; Piscione, F.; Trimarco, B.; et al. Endothelial α1 -adrenoceptors regulate neo-angiogenesis. Br. J. Pharmacol. 2008, 153, 936–946. [Google Scholar] [CrossRef]
- Avvisato, R.; Jankauskas, S.S.; Varzideh, F.; Kansakar, U.; Mone, P.; Santulli, G. Sortilin and Hypertension. Curr. Opin. Nephrol. Hypertens. 2023, 32, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Gui, F.; You, Z.; Fu, S.; Wu, H.; Zhang, Y. Endothelial Dysfunction in Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Varzideh, F.; Jankauskas, S.S.; Kansakar, U.; Mone, P.; Gambardella, J.; Santulli, G. Sortilin drives hypertension by modulating sphingolipid/ceramide homeostasis and by triggering oxidative stress. J. Clin. Investig. 2022, 132, e156624. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Li, S.; Lv, J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 851941. [Google Scholar] [CrossRef]
- Santulli, G. Endothelial cells: The heart attack of the clones. Sci. Transl. Med. 2018, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Saenz-Medina, J.; Muñoz, M.; Rodriguez, C.; Contreras, C.; Sánchez, A.; Coronado, M.J.; Ramil, E.; Santos, M.; Carballido, J.; Prieto, D. Hyperoxaluria Induces Endothelial Dysfunction in Preglomerular Arteries: Involvement of Oxidative Stress. Cells 2022, 11, 2306. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Mone, P.; Kansakar, U.; Jankauskas, S.S.; Donkor, K.; Adebayo, A.; Varzideh, F.; Eacobacci, M.; Gambardella, J.; Lombardi, A.; et al. Diabetes and restenosis. Cardiovasc. Diabetol. 2022, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, K.D.S.; Stefanon, I.; Junior, R.F.R. Tributyltin and Vascular Dysfunction: The Role of Oxidative Stress. Front. Endocrinol. 2018, 9, 354. [Google Scholar] [CrossRef]
- Iaccarino, G.; Ciccarelli, M.; Sorriento, D.; Cipolletta, E.; Cerullo, V.; Iovino, G.L.; Paudice, A.; Elia, A.; Santulli, G.; Campanile, A.; et al. AKT Participates in Endothelial Dysfunction in Hypertension. Circulation 2004, 109, 2587–2593. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Li, M.; Zhou, H.; Zhang, H.; Zhang, Y.; Xu, J.; Xu, Y.; Zhang, J.; Xia, B.; et al. Endothelial Shp2 deficiency controls alternative activation of macrophage preventing radiation-induced lung injury through notch signaling. Iscience 2022, 25, 103867. [Google Scholar] [CrossRef]
- Gambardella, J.; Sorriento, D.; Bova, M.; Rusciano, M.; Loffredo, S.; Wang, X.; Petraroli, A.; Carucci, L.; Mormile, I.; Oliveti, M.; et al. Role of Endothelial G Protein-Coupled Receptor Kinase 2 in Angioedema. Hypertension 2020, 76, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- McElwain, C.; Tuboly, E.; McCarthy, F.P.; McCarthy, C.M. Mechanisms of Endothelial Dysfunction in Pre-eclampsia and Gestational Diabetes Mellitus: Windows into Future Cardiometabolic Health? Front. Endocrinol. 2020, 11, 655. [Google Scholar] [CrossRef] [PubMed]
- Mone, P.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; Lombardi, A.; Frullone, S.; Santulli, G. SGLT2 Inhibition via Empagliflozin Improves Endothelial Function and Reduces Mitochondrial Oxidative Stress: Insights from Frail Hypertensive and Diabetic Patients. Hypertension 2022, 79, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Colombo, B.M.; Cagnati, P.; Gulli, R.; Spanò, F.; Puppo, F. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis 2012, 224, 309–317. [Google Scholar] [CrossRef]
- Mone, P.; Lombardi, A.; Kansakar, U.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; Marzocco, S.; De Gennaro, S.; Famiglietti, M.; Macina, G.; et al. Empagliflozin Improves the MicroRNA Signature of Endothelial Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Diabetes. J. Pharmacol. Exp. Ther. 2023, 384, 116–122. [Google Scholar] [CrossRef]
- Duică, F.; Dănilă, C.A.; Boboc, A.E.; Antoniadis, P.; Condrat, C.E.; Onciul, S.; Suciu, N.; Creţoiu, S.M.; Varlas, V.N.; Creţoiu, D. Impact of Increased Oxidative Stress on Cardiovascular Diseases in Women with Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 614679. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Cipolletta, E.; Santulli, G.; Campanile, A.; Pumiglia, K.; Cervero, P.; Pastore, L.; Astone, D.; Trimarco, B.; Iaccarino, G. Endothelial β2 adrenergic signaling to AKT: Role of Gi and SRC. Cell. Signal. 2007, 19, 1949–1955. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Mone, P.; Santulli, G. From resveratrol to ISIDE11: How to activate SIRT1 and improve endothelial function? New therapeutic insights for methylenetetrahydrofolate reductase deficiency. Cell. Mol. Life Sci. 2022, 79, 451. [Google Scholar] [CrossRef]
- Yuan, W.; Xia, H.; Xu, Y.; Xu, C.; Chen, N.; Shao, C.; Dai, Z.; Chen, R.; Tao, A. The role of ferroptosis in endothelial cell dysfunction. Cell Cycle 2022, 21, 1897–1914. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, S.; Sun, M.; Hua, M.; Liu, Z.; Mu, G.; Wang, Z.; Xiang, Q.; Cui, Y. Ferroptosis of Endothelial Cells in Vascular Diseases. Nutrients 2022, 14, 4506. [Google Scholar] [CrossRef]
- Agmon, E.; Solon, J.; Bassereau, P.; Stockwell, B.R. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci. Rep. 2018, 8, 5155. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Kapanova, G.; Kalmakhanov, S.; Kussainov, A.Z.; Datkhayeva, Z. Regulation of Ferroptosis by Non-Coding RNAs: Mechanistic Insights. J. Pharmacol. Exp. Ther. 2023, 384, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-C.; Chen, C.-M. Hyperoxia Induces Ferroptosis and Impairs Lung Development in Neonatal Mice. Antioxidants 2022, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Musheshe, N.; Oun, A.; Sabogal-Guáqueta, A.M.; Trombetta-Lima, M.; Mitchel, S.C.; Adzemovic, A.; Speek, O.; Morra, F.; van der Veen, C.H.J.T.; Lezoualc’H, F.; et al. Pharmacological Inhibition of Epac1 Averts Ferroptosis Cell Death by Preserving Mitochondrial Integrity. Antioxidants 2022, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.; Zhang, C.; Meng, Z.; Lv, X.; Li, Z.; Wei, J.; Wu, H. Integrative analysis links ferroptosis to necrotizing enterocolitis and reveals the role of ACSL4 in immune disorders. iScience 2022, 25, 105406. [Google Scholar] [CrossRef]
- Martín-Fernández, M.; Aller, R.; Heredia-Rodríguez, M.; Gómez-Sánchez, E.; Martínez-Paz, P.; Gonzalo-Benito, H.; Prada, L.S.-D.; Gorgojo, Ó.; Carnicero-Frutos, I.; Tamayo, E.; et al. Lipid peroxidation as a hallmark of severity in COVID-19 patients. Redox Biol. 2021, 48, 102181. [Google Scholar] [CrossRef]
- Tang, X.-H.; Gambardella, J.; Jankauskas, S.S.; Wang, X.; Santulli, G.; Gudas, L.J.; Levi, R. A Retinoic Acid Receptor β2 Agonist Improves Cardiac Function in a Heart Failure Model. J. Pharmacol. Exp. Ther. 2021, 379, 182–190. [Google Scholar] [CrossRef]
- Mihalas, B.P.; De Iuliis, G.N.; Redgrove, K.A.; McLaughlin, E.A.; Nixon, B. The lipid peroxidation product 4-hydroxynonenal contributes to oxidative stress-mediated deterioration of the ageing oocyte. Sci. Rep. 2017, 7, 6247. [Google Scholar] [CrossRef]
- Wiernicki, B.; Dubois, H.; Tyurina, Y.Y.; Hassannia, B.; Bayir, H.; Kagan, V.E.; Vandenabeele, P.; Wullaert, A.; Berghe, T.V. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis. 2020, 11, 922. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Grocin, A.G.; da Silva, T.N.X.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Ufer, C.; Wang, C.C.; Fähling, M.; Schiebel, H.; Thiele, B.J.; Billett, E.E.; Kuhn, H.; Borchert, A. Translational regulation of glutathione peroxidase 4 expression through guanine-rich sequence-binding factor 1 is essential for embryonic brain development. Genes Dev. 2008, 22, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Pabisz, P.; Bazak, J.; Girotti, A.W.; Korytowski, W. Anti-steroidogenic effects of cholesterol hydroperoxide trafficking in MA-10 Leydig cells: Role of mitochondrial lipid peroxidation and inhibition thereof by selenoperoxidase GPx4. Biochem. Biophys. Res. Commun. 2022, 591, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Comish, P.B.; Tang, D.; Kang, R. Characteristics and Biomarkers of Ferroptosis. Front. Cell Dev. Biol. 2021, 9, 637162. [Google Scholar] [CrossRef] [PubMed]
- Turchi, R.; Faraonio, R.; Lettieri-Barbato, D.; Aquilano, K. An Overview of the Ferroptosis Hallmarks in Friedreich’s Ataxia. Biomolecules 2020, 10, 1489. [Google Scholar] [CrossRef] [PubMed]
- Miotto, G.; Rossetto, M.; Di Paolo, M.L.; Orian, L.; Venerando, R.; Roveri, A.; Vučković, A.-M.; Travain, V.B.; Zaccarin, M.; Zennaro, L.; et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 2019, 28, 101328. [Google Scholar] [CrossRef] [PubMed]
- Zilka, O.; Shah, R.; Li, B.; Angeli, J.P.F.; Griesser, M.; Conrad, M.; Pratt, D.A. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Central Sci. 2017, 3, 232–243. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Y.; Bai, L.; Zhi, L.; Yang, Y.; Zhao, Q.; Chen, C.; Qi, Y.; Gao, W.; He, W.; et al. RBMS1 regulates lung cancer ferroptosis through translational control of SLC7A11. J. Clin. Investig. 2021, 131, e152067. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Ye, T.; Yang, L.; Shen, Y.; Li, H. Ferroptosis Signaling and Regulators in Atherosclerosis. Front. Cell Dev. Biol. 2021, 9, 809457. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef]
- Ong, Y.T.; Andrade, J.; Armbruster, M.; Shi, C.; Castro, M.; Costa, A.S.H.; Sugino, T.; Eelen, G.; Zimmermann, B.; Wilhelm, K.; et al. A YAP/TAZ-TEAD signalling module links endothelial nutrient acquisition to angiogenic growth. Nat. Metab. 2022, 4, 672–682. [Google Scholar] [CrossRef]
- Xie, S.-S.; Deng, Y.; Guo, S.-L.; Li, J.-Q.; Zhou, Y.-C.; Liao, J.; Wu, D.-D.; Lan, W.-F. Endothelial cell ferroptosis mediates monocrotaline-induced pulmonary hypertension in rats by modulating NLRP3 inflammasome activation. Sci. Rep. 2022, 12, 3056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bai, X.; Lin, T.; Wang, X.; Zhang, B.; Dai, L.; Shi, J.; Zhang, Y.; Zhao, X. HMOX1 Promotes Ferroptosis in Mammary Epithelial Cells via FTH1 and Is Involved in the Development of Clinical Mastitis in Dairy Cows. Antioxidants 2022, 11, 2221. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Cai, Z.; Wang, H.; Han, D.; Cheng, Q.; Zhang, P.; Gao, F.; Yu, Y.; Song, Z.; Wu, Q.; et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 2020, 127, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; He, Q.; Yang, S.; Sun, H.; Liu, Y.; Li, W.; Tang, Y.; Zheng, Y.; Wu, T. FTH1- and SAT1-Induced Astrocytic Ferroptosis Is Involved in Alzheimer’s Disease: Evidence from Single-Cell Transcriptomic Analysis. Pharmaceuticals 2022, 15, 1177. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Wang, S.-J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef]
- Mou, Y.; Zhang, L.; Liu, Z.; Song, X. Abundant expression of ferroptosis-related SAT1 is related to unfavorable outcome and immune cell infiltration in low-grade glioma. BMC Cancer 2022, 22, 215. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Tabarsi, P.; Jamaati, H.; Roofchayee, N.D.; Dezfuli, N.K.; Hashemian, S.M.; Moniri, A.; Marjani, M.; Malekmohammad, M.; Mansouri, D.; et al. Increased Serum Levels of Soluble TNF-α Receptor Is Associated With ICU Mortality in COVID-19 Patients. Front. Immunol. 2021, 12, 592727. [Google Scholar] [CrossRef]
- Halim, C.; Mirza, A.F.; Sari, M.I. The Association between TNF-α, IL-6, and Vitamin D Levels and COVID-19 Severity and Mortality: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 195. [Google Scholar] [CrossRef]
- Kaur, S.; Tripathi, D.M.; Yadav, A. The Enigma of Endothelium in COVID-19. Front. Physiol. 2020, 11, 989. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Delorey, T.M.; Ziegler, C.G.K.; Heimberg, G.; Normand, R.; Yang, Y.; Segerstolpe, Å.; Abbondanza, D.; Fleming, S.J.; Subramanian, A.; Montoro, D.T.; et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 2021, 595, 107–113. [Google Scholar] [CrossRef]
- Tan, S.; Schubert, D.; Maher, P. Oxytosis: A Novel Form of Programmed Cell Death. Curr. Top. Med. Chem. 2001, 1, 497–506. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Kan, X.; Yin, Y.; Song, C.; Tan, L.; Qiu, X.; Liao, Y.; Liu, W.; Meng, S.; Sun, Y.; Ding, C. Newcastle-disease-virus-induced ferroptosis through nutrient deprivation and ferritinophagy in tumor cells. Iscience 2021, 24, 102837. [Google Scholar] [CrossRef]
- Maschalidi, S.; Mehrotra, P.; Keçeli, B.N.; De Cleene, H.K.L.; Lecomte, K.; Van der Cruyssen, R.; Janssen, P.; Pinney, J.; van Loo, G.; Elewaut, D.; et al. Targeting SLC7A11 improves efferocytosis by dendritic cells and wound healing in diabetes. Nature 2022, 606, 776–784. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Giraudi, P.J.; Bellarosa, C.; Coda-Zabetta, C.D.; Peruzzo, P.; Tiribelli, C. Functional Induction of the Cystine-Glutamate Exchanger System Xc− Activity in SH-SY5Y Cells by Unconjugated Bilirubin. PLoS ONE 2011, 6, e29078. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Roh, J.-L. SLC7A11 as a Gateway of Metabolic Perturbation and Ferroptosis Vulnerability in Cancer. Antioxidants 2022, 11, 2444. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Chen, L.-J.; Chen, G.; Chao, T.-I.; Wang, C.-Y. SHP-1/STAT3-Signaling-Axis-Regulated Coupling between BECN1 and SLC7A11 Contributes to Sorafenib-Induced Ferroptosis in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 11092. [Google Scholar] [CrossRef] [PubMed]
- Ananth, S.; Miyauchi, S.; Thangaraju, M.; Jadeja, R.N.; Bartoli, M.; Ganapathy, V.; Martin, P.M. Selenomethionine (Se-Met) Induces the Cystine/Glutamate Exchanger SLC7A11 in Cultured Human Retinal Pigment Epithelial (RPE) Cells: Implications for Antioxidant Therapy in Aging Retina. Antioxidants 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Pasini, A.M.F.; Stranieri, C.; Girelli, D.; Busti, F.; Cominacini, L. Is Ferroptosis a Key Component of the Process Leading to Multiorgan Damage in COVID-19? Antioxidants 2021, 10, 1677. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Eagle, H. Nutrition Needs of Mammalian Cells in Tissue Culture. Science 1955, 122, 501–504. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, K.; Li, Y.; Lu, C.; Ling, K.; Cai, C.; Wang, W.; Ye, D. Targeting TNF-α for COVID-19: Recent Advanced and Controversies. Front. Public Health 2022, 10, 833967. [Google Scholar] [CrossRef]
- Rarani, F.Z.; Rashidi, B.; Abadi, M.H.J.N.; Hamblin, M.R.; Hashemian, S.M.R.; Mirzaei, H. Cytokines and microRNAs in SARS-CoV-2: What do we know? Mol. Ther.—Nucleic Acids 2022, 29, 219–242. [Google Scholar] [CrossRef]
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00299-20. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, L.; Pink, I.; Kühne, J.F.; Beushausen, K.; Keil, J.; Christoph, S.; Sauer, A.; Boblitz, L.; Schmidt, J.; David, S.; et al. Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduct. Target. Ther. 2021, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fara, A.; Mitrev, Z.; Rosalia, R.A.; Assas, B.M. Cytokine storm and COVID-19: A chronicle of pro-inflammatory cytokines. Open Biol. 2020, 10, 200160. [Google Scholar] [CrossRef] [PubMed]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Attiq, A.; Yao, L.J.; Afzal, S.; Khan, M.A. The triumvirate of NF-κB, inflammation and cytokine storm in COVID-19. Int. Immunopharmacol. 2021, 101, 108255. [Google Scholar] [CrossRef]
- Morris, G.; Bortolasci, C.C.; Puri, B.K.; Marx, W.; O’Neil, A.; Athan, E.; Walder, K.; Berk, M.; Olive, L.; Carvalho, A.F.; et al. The cytokine storms of COVID-19, H1N1 influenza, CRS and MAS compared. Can one sized treatment fit all? Cytokine 2021, 144, 155593. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Muhammad, J.; Khan, A.; Sule, A.A.; Tirupathi, R.; Mutair, A.A.; Alhumaid, S.; Al-Omari, A.; Dhawan, M.; et al. Role of Inflammatory Cytokines in COVID-19 Patients: A Review on Molecular Mechanisms, Immune Functions, Immunopathology and Immunomodulatory Drugs to Counter Cytokine Storm. Vaccines 2021, 9, 436. [Google Scholar] [CrossRef]
- Cure, E.; Cure, M.C. Prolonged NHE Activation may be Both Cause and Outcome of Cytokine Release Syndrome in COVID-19. Curr. Pharm. Des. 2022, 28, 1815–1822. [Google Scholar] [CrossRef]
- Peter, A.E.; Sandeep, B.V.; Rao, B.G.; Kalpana, V.L. Calming the Storm: Natural Immunosuppressants as Adjuvants to Target the Cytokine Storm in COVID-19. Front. Pharmacol. 2021, 11, 583777. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Sagara, Y.; Liu, Y.; Maher, P.; Schubert, D. The Regulation of Reactive Oxygen Species Production during Programmed Cell Death. J. Cell Biol. 1998, 141, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2018, 73, 354–363.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Zhao, Y.; Gao, G. Mitochondria regulation in ferroptosis. Eur. J. Cell Biol. 2019, 99, 151058. [Google Scholar] [CrossRef]
- Battaglia, A.M.; Chirillo, R.; Aversa, I.; Sacco, A.; Costanzo, F.; Biamonte, F. Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells 2020, 9, 1505. [Google Scholar] [CrossRef]
- Gan, B. Mitochondrial regulation of ferroptosis. J. Cell Biol. 2021, 220, e202105043. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Fang, K.; Du, S.; Shen, D.; Xiong, Z.; Jiang, K.; Liang, D.; Wang, J.; Xu, H.; Hu, L.; Zhai, X.; et al. SUFU suppresses ferroptosis sensitivity in breast cancer cells via Hippo/YAP pathway. Iscience 2022, 25, 104618. [Google Scholar] [CrossRef]
- Jakaria, M.; Belaidi, A.A.; Bush, A.I.; Ayton, S. Ferroptosis as a mechanism of neurodegeneration in Alzheimer’s disease. J. Neurochem. 2021, 159, 804–825. [Google Scholar] [CrossRef]
- Mahoney-Sánchez, L.; Bouchaoui, H.; Ayton, S.; Devos, D.; Duce, J.A.; Devedjian, J.-C. Ferroptosis and its potential role in the physiopathology of Parkinson’s Disease. Prog. Neurobiol. 2020, 196, 101890. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.K.; Zelic, M.; Han, Y.; Teeple, E.; Chen, L.; Sadeghi, M.; Shankara, S.; Guo, L.; Li, C.; Pontarelli, F.; et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 2022, 26, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.; Wu, C.-H.; Wu, C.-Y.; Luo, S.-F.; Lai, J.-H. Ferroptosis and Autoimmune Diseases. Front. Immunol. 2022, 13, 916664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Y.; Du, C.; Sang, L.; Liu, L.; Li, Y.; Wang, F.; Fan, W.; Tang, P.; Zhang, S.; et al. Evidence of pyroptosis and ferroptosis extensively involved in autoimmune diseases at the single-cell transcriptome level. J. Transl. Med. 2022, 20, 363. [Google Scholar] [CrossRef]
- Hao, L.; Mi, J.; Song, L.; Guo, Y.; Li, Y.; Yin, Y.; Zhang, C. SLC40A1 Mediates Ferroptosis and Cognitive Dysfunction in Type 1 Diabetes. Neuroscience 2021, 463, 216–226. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Xiang, M.; Wang, Y.; Zhang, Z.; Liang, J.; Xu, J. The Potential Role of Ferroptosis in Systemic Lupus Erythematosus. Front. Immunol. 2022, 13, 855622. [Google Scholar] [CrossRef]
- Yang, X.-D.; Yang, Y.-Y. Ferroptosis as a Novel Therapeutic Target for Diabetes and Its Complications. Front. Endocrinol. 2022, 13, 853822. [Google Scholar] [CrossRef]
- Ye, H.; Wang, R.; Wei, J.; Wang, Y.; Zhang, X.; Wang, L. Bioinformatics Analysis Identifies Potential Ferroptosis Key Gene in Type 2 Diabetic Islet Dysfunction. Front. Endocrinol. 2022, 13, 904312. [Google Scholar] [CrossRef]
- Yin, M.; Zhou, L.; Ji, Y.; Lu, R.; Ji, W.; Jiang, G.; Ma, J.; Song, X. In silico identification and verification of ferroptosis-related genes in type 2 diabetic islets. Front. Endocrinol. 2022, 13, 946492. [Google Scholar] [CrossRef]
- Du, S.; Shi, H.; Xiong, L.; Wang, P.; Shi, Y. Canagliflozin mitigates ferroptosis and improves myocardial oxidative stress in mice with diabetic cardiomyopathy. Front. Endocrinol. 2022, 13, 1011669. [Google Scholar] [CrossRef]
- Zhao, W.-K.; Zhou, Y.; Xu, T.-T.; Wu, Q. Ferroptosis: Opportunities and Challenges in Myocardial Ischemia-Reperfusion Injury. Oxidative Med. Cell. Longev. 2021, 2021, 9929687. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nature 2020, 22, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, X.; Liu, X.; Shen, L.; Chen, Q.; Shu, Q. Targeting Ferroptosis as a Promising Therapeutic Strategy for Ischemia-Reperfusion Injury. Antioxidants 2022, 11, 2196. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Jia, J.; Zheng, J.; Zhou, Y.; Jia, D.; Wang, J. Recent Progress of Ferroptosis in Lung Diseases. Front. Cell Dev. Biol. 2021, 9, 789517. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Yu, M. Bioinformatics Analysis Identifies Potential Ferroptosis Key Genes in the Pathogenesis of Pulmonary Fibrosis. Front. Genet. 2022, 12, 919187. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Yang, Y. Multifaceted Roles of Ferroptosis in Lung Diseases. Front. Mol. Biosci. 2022, 9, 919187. [Google Scholar] [CrossRef]
- Jacobs, W.; Lammens, M.; Kerckhofs, A.; Voets, E.; Van San, E.; Van Coillie, S.; Peleman, C.; Mergeay, M.; Sirimsi, S.; Matheeussen, V.; et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): Autopsy reveals a ferroptosis signature. ESC Hear. Fail. 2020, 7, 3772–3781. [Google Scholar] [CrossRef]
- Kwon, M.-Y.; Park, E.; Lee, S.-J.; Chung, S.W. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget 2015, 6, 24393–24403. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2015, 63, 173–184. [Google Scholar] [CrossRef]
- Mone, P.; Gambardella, J.; Pansini, A.; de Donato, A.; Martinelli, G.; Boccalone, E.; Matarese, A.; Frullone, S.; Santulli, G. Cognitive Impairment in Frail Hypertensive Elderly Patients: Role of Hyperglycemia. Cells 2021, 10, 2115. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Chupyrkina, A.A.; Jankauskas, S.S.; Pevzner, I.B.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim. et Biophys. Acta (BBA)—Mol. Basis Dis. 2011, 1812, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Santulli, G.; Reiken, S.R.; Yuan, Q.; Osborne, B.W.; Chen, B.-X.; Marks, A.R. Mitochondrial oxidative stress promotes atrial fibrillation. Sci. Rep. 2015, 5, 11427. [Google Scholar] [CrossRef] [PubMed]
- Mone, P.; Pansini, A.; Jankauskas, S.S.; Varzideh, F.; Kansakar, U.; Lombardi, A.; Trimarco, V.; Frullone, S.; Santulli, G. L-Arginine Improves Cognitive Impairment in Hypertensive Frail Older Adults. Front. Cardiovasc. Med. 2022, 9, 868521. [Google Scholar] [CrossRef]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef]
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.-I.; et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. J. Clin. Investig. 2020, 5, e132747. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, A.; Santulli, G.; Reiken, S.R.; Coromilas, E.; Godfrey, S.J.; Brunjes, D.L.; Colombo, P.C.; Yuzefpolskaya, M.; Sokol, S.I.; Kitsis, R.N.; et al. Ryanodine Receptor Calcium Leak in Circulating B-Lymphocytes as a Biomarker in Heart Failure. Circulation 2018, 138, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Amgalan, D.; Garner, T.P.; Pekson, R.; Jia, X.F.; Yanamandala, M.; Paulino, V.; Liang, F.G.; Corbalan, J.J.; Lee, J.; Chen, Y.; et al. A small-molecule allosteric inhibitor of BAX protects against doxorubicin-induced cardiomyopathy. Nat. Cancer 2020, 1, 315–328. [Google Scholar] [CrossRef]
- Santulli, G.; Wronska, A.; Uryu, K.; Diacovo, T.G.; Gao, M.; Marx, S.O.; Kitajewski, J.; Chilton, J.M.; Akat, K.M.; Tuschl, T.; et al. A selective microRNA-based strategy inhibits restenosis while preserving endothelial function. J. Clin. Investig. 2014, 124, 4102–4114. [Google Scholar] [CrossRef]
- Cipolletta, E.; Rusciano, M.R.; Maione, A.S.; Santulli, G.; Sorriento, D.; Del Giudice, C.; Ciccarelli, M.; Franco, A.; Crola, C.; Campiglia, P.; et al. Targeting the CaMKII/ERK Interaction in the Heart Prevents Cardiac Hypertrophy. PLoS ONE 2015, 10, e0130477. [Google Scholar] [CrossRef]
- Gambardella, J.; Coppola, A.; Izzo, R.; Fiorentino, G.; Trimarco, B.; Santulli, G. Role of endothelial miR-24 in COVID-19 cerebrovascular events. Crit. Care 2021, 25, 306. [Google Scholar] [CrossRef]
| Primer | Sequence (5′-3′) | Amplicon (bp) | |
|---|---|---|---|
| GPX4 | Forward | GAG ATC AAA GAG TTC GCC GC | 102 |
| Reverse | CTT CAT CCA CTT CCA CAG CG | ||
| TNFR1 | Forward | TTG TAT GGC CCC AAC TGT CT | 99 |
| Reverse | CTG GCT CAA GTC CTT CCT CA | ||
| GAPDH | Forward | GGC TCC CTT GGG TAT ATG GT | 94 |
| Reverse | TTG ATT TTG GAG GGA TCT CG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankauskas, S.S.; Kansakar, U.; Sardu, C.; Varzideh, F.; Avvisato, R.; Wang, X.; Matarese, A.; Marfella, R.; Ziosi, M.; Gambardella, J.; et al. COVID-19 Causes Ferroptosis and Oxidative Stress in Human Endothelial Cells. Antioxidants 2023, 12, 326. https://doi.org/10.3390/antiox12020326
Jankauskas SS, Kansakar U, Sardu C, Varzideh F, Avvisato R, Wang X, Matarese A, Marfella R, Ziosi M, Gambardella J, et al. COVID-19 Causes Ferroptosis and Oxidative Stress in Human Endothelial Cells. Antioxidants. 2023; 12(2):326. https://doi.org/10.3390/antiox12020326
Chicago/Turabian StyleJankauskas, Stanislovas S., Urna Kansakar, Celestino Sardu, Fahimeh Varzideh, Roberta Avvisato, Xujun Wang, Alessandro Matarese, Raffaele Marfella, Marcello Ziosi, Jessica Gambardella, and et al. 2023. "COVID-19 Causes Ferroptosis and Oxidative Stress in Human Endothelial Cells" Antioxidants 12, no. 2: 326. https://doi.org/10.3390/antiox12020326
APA StyleJankauskas, S. S., Kansakar, U., Sardu, C., Varzideh, F., Avvisato, R., Wang, X., Matarese, A., Marfella, R., Ziosi, M., Gambardella, J., & Santulli, G. (2023). COVID-19 Causes Ferroptosis and Oxidative Stress in Human Endothelial Cells. Antioxidants, 12(2), 326. https://doi.org/10.3390/antiox12020326