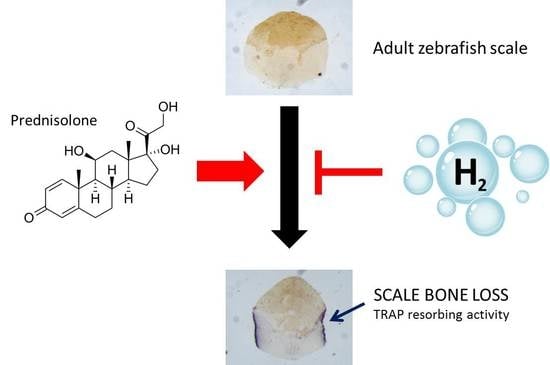
Molecular Hydrogen Prevents Osteoclast Activation in a Glucocorticoid-Induced Osteoporosis Zebrafish Scale Model
Abstract
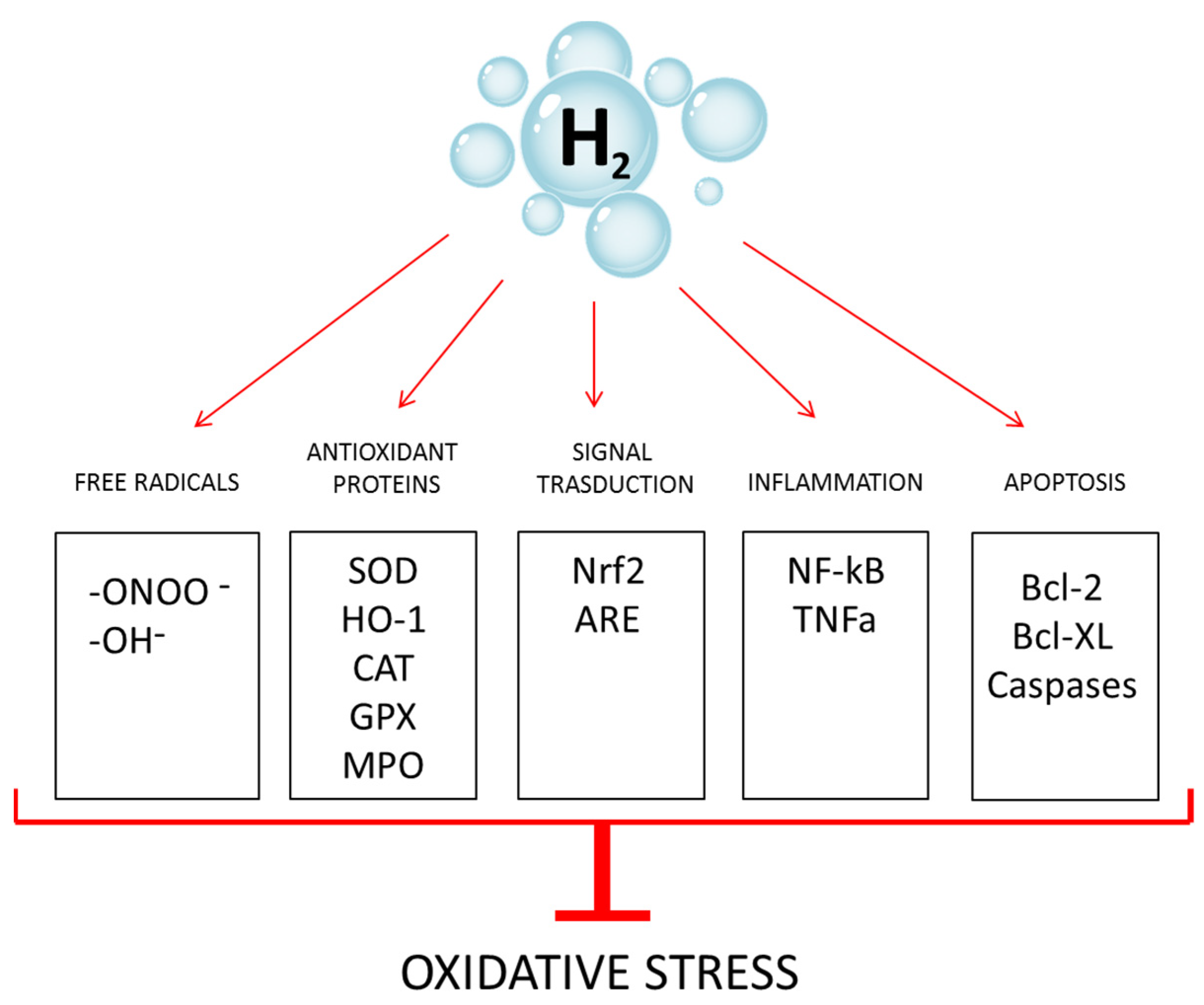
1. Introduction
2. Materials and Methods
2.1. Ethic Statement
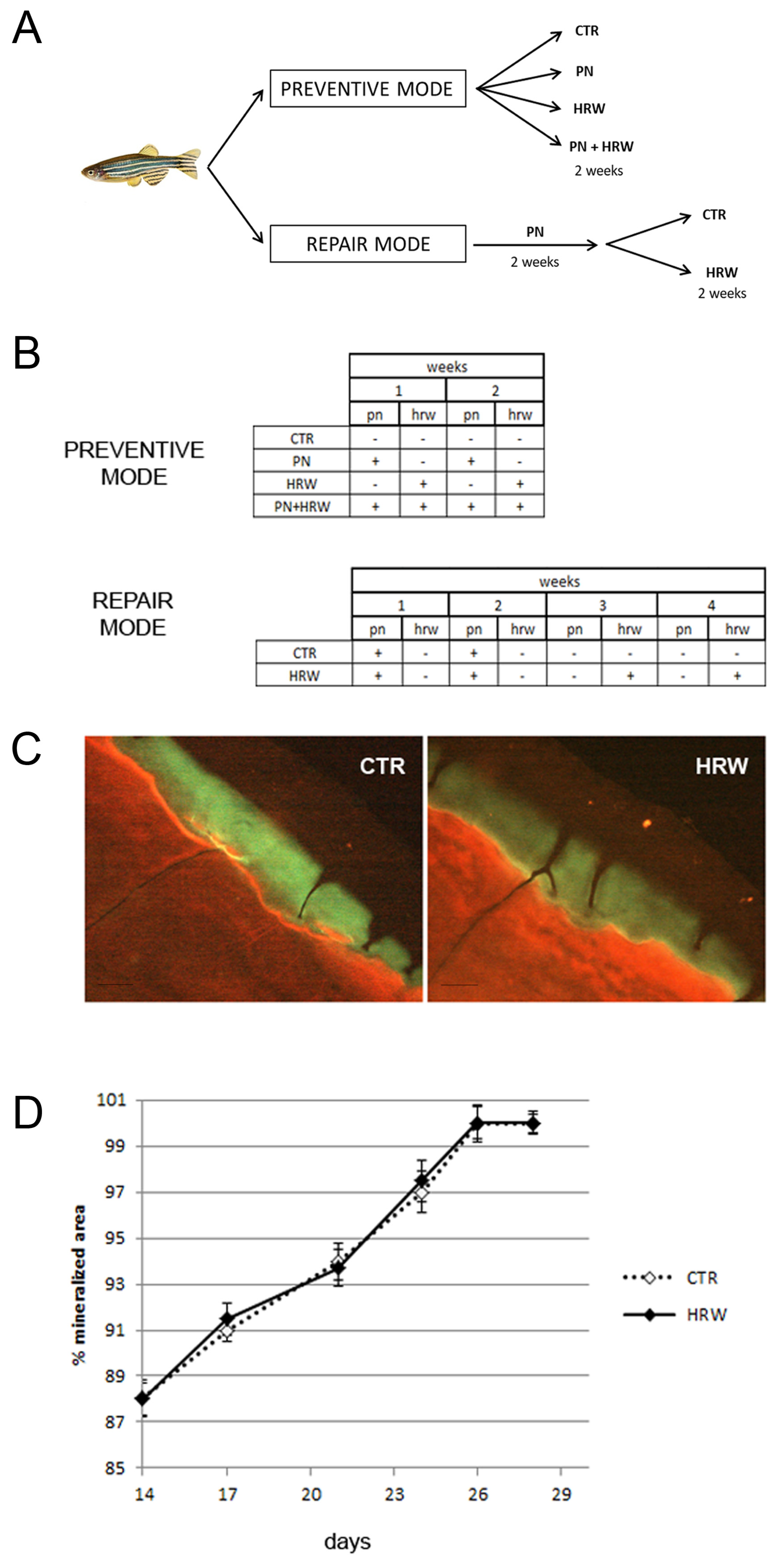
2.2. Animals and Treatments
2.3. Oxidation–Reduction Potential Measurements
2.4. Scale Collection
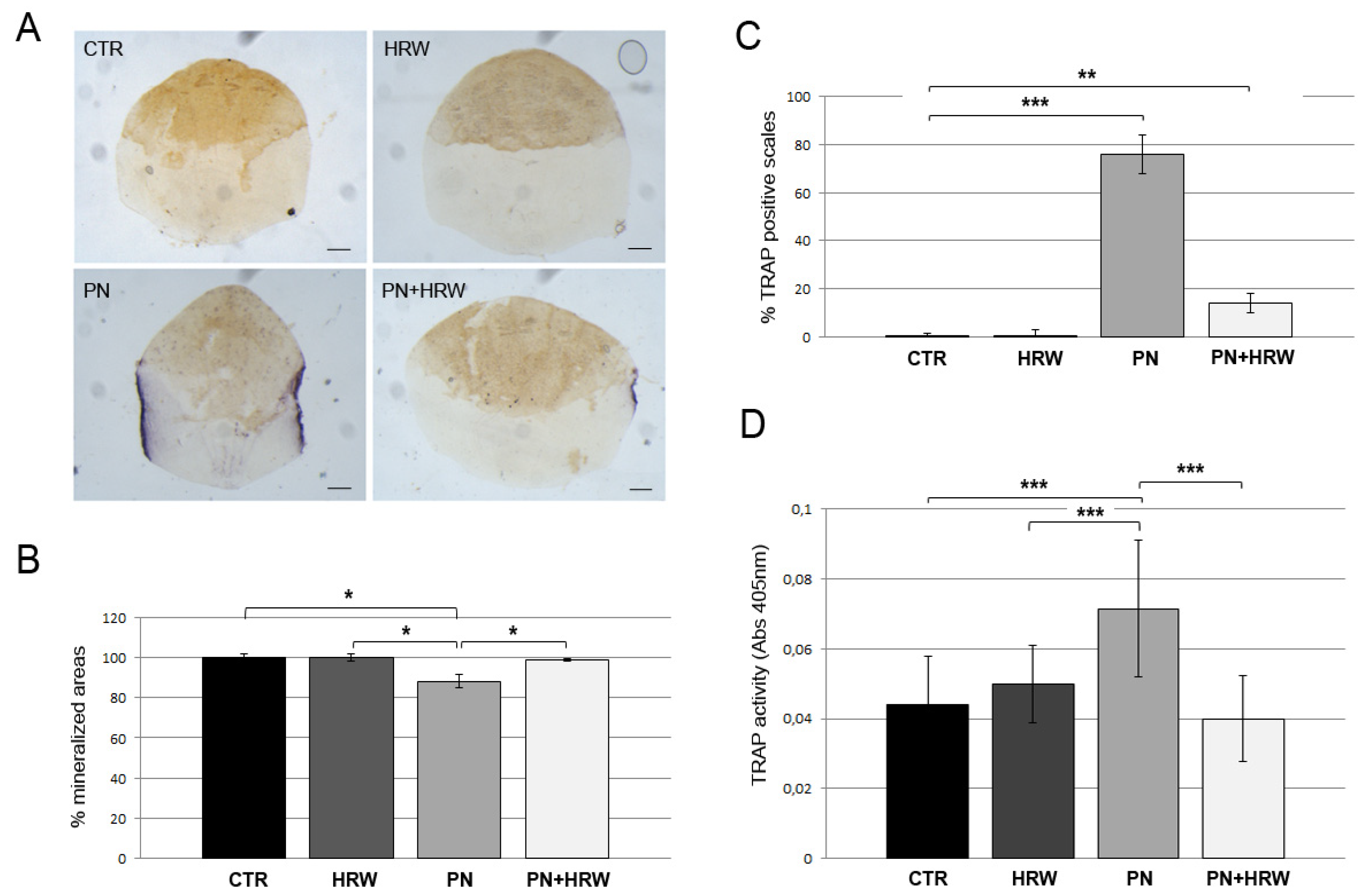
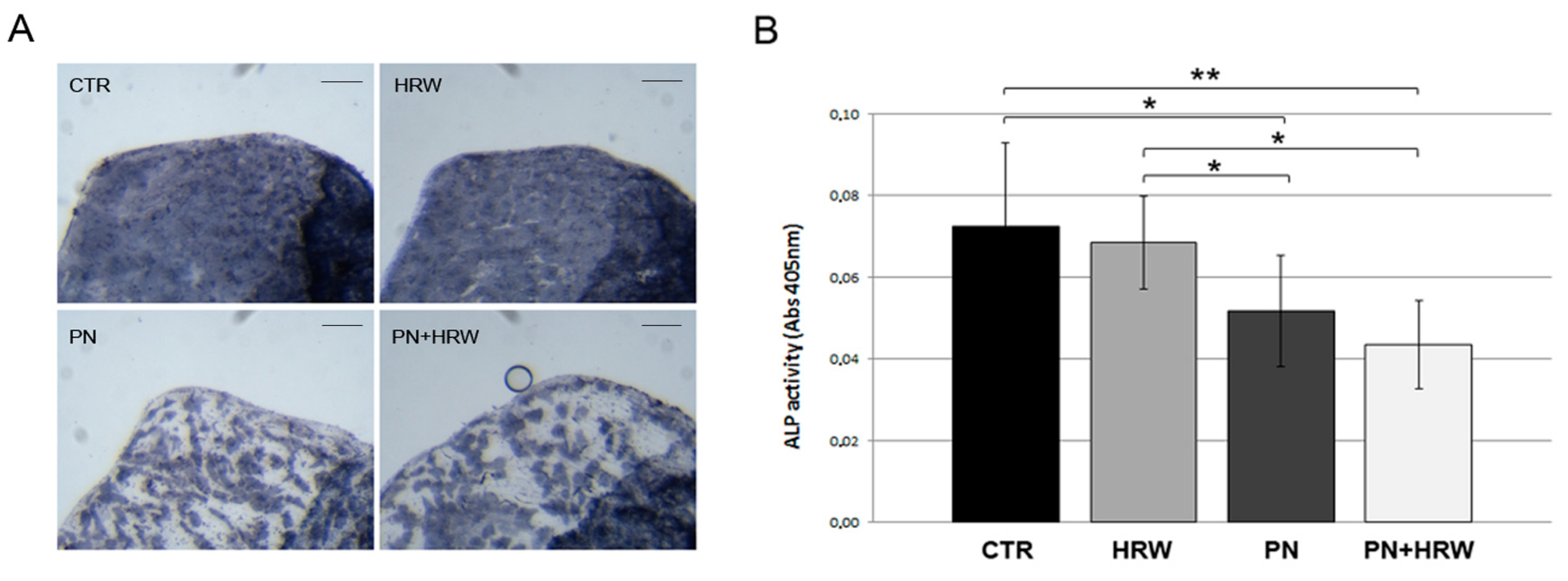
2.5. Histological TRAP and ALP Assays in Scales
2.6. Biochemical TRAP and ALP Assays in Scales
2.7. Double Bone Matrix Vital Staining
2.8. Statistics
3. Results
3.1. ORP Measurements
3.2. HRW Treatment Prevents Osteoclast Activation in PN-Treated Fish Scales
3.3. HRW Treatment Does Not Prevent PN-Dependent Downregulation of ALP Activity in Scale Osteoblasts
3.4. HRW Does Not Facilitate Bone Tissue Repair in Scales after PN Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdollahi, M.; Larjani, B.; Rahimi, R.; Salari, P. Role of oxidative stress in osteoporosis. Therapy 2005, 2, 787–796. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive oxygen species in osteoclast differentiation and possible osteoclast diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef] [PubMed]
- Kimball, J.S.; Jhonson, J.P.; Carlson, D.A. Oxidative stress and osteoporosis. J. Bone Joint Surg. Am. 2021, 103, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Ge, G.; Liang, X.; Zhang, W.; Sun, H.; Li, M.; Geng, D. ROS signaling cascades: Dual regulations for osteoclast and osteoblast. Acta Biochim. Biophys. Sin. 2020, 52, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Tarantino, U.; Iolascon, G.; Cianferotti, L.; Masi, L.; Marcucci, G.; Giusti, F.; Marini, F.; Parri, S.; Feola, M.; Rao, C. Clinical guidelines for the prevention and treatment of osteoporosis: Summary statements and recommendation from the Italian Society for Orthopaedics and Traumatology. J. Orthop. Traumatol. 2017, 18 (Suppl. 19), 3–36. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, T.W.; Sharpe, R.; Ohno, K. Electrolyzed-Reduced Water: Review II: Safety Concerns and Effectiveness as a Source of Hydrogen Water. Int. J. Mol. Sci. 2022, 23, 14508. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Sharpe, R.; Ohno, K. Electrolyzed-Reduced Water: Review I. Molecular Hydrogen Is the Exclusive Agent Responsible for the Therapeutic Effects. Int. J. Mol. Sci. 2022, 23, 14750. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Ohta, S. Recent progress toward hydrogen medicine: Potential of molecular hydrogen for preventive and therapeutic applications. Curr. Pharm. Des. 2011, 17, 2241–2252. [Google Scholar] [CrossRef]
- Kura, B.; Szantova, M.; LeBaron, T.W.; Mojto, V.; Barancik, M.; Szeiffova Bacova, B.; Kalocayova, B.; Sykora, M.; Okruhlicova, L.; Tribulova, N.; et al. Biological Effects of Hydrogen Water on Subjects with NAFLD: A Randomized, Placebo-Controlled Trial. Antioxidants 2022, 11, 1935. [Google Scholar] [CrossRef] [PubMed]
- Asgharzadeh, F.; Tarnava, A.; Mostafapour, A.; Khazaei, M.; LeBaron, T.W. Hydrogen-rich water exerts anti-tumor effects comparable to 5-fluorouracil in a colorectal cancer xenograft model. World J. Gastrointest. Oncol. 2022, 14, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Alwazeer, D.; Liu, F.F.; Wu, X.Y.; LeBaron, T.W. Combating Oxidative Stress and Inflammation in COVID-19 by Molecular Hydrogen Therapy: Mechanisms and Perspectives. Oxid. Med. Cell. Longev. 2021, 2021, 5513868. [Google Scholar] [CrossRef]
- Barancik, M.; Kura, B.; LeBaron, T.W.; Bolli, R.; Buday, J.; Slezak, J. Molecular and Cellular Mechanisms Associated with Effects of Molecular Hydrogen in Cardiovascular and Central Nervous Systems. Antioxidants 2020, 9, 1281. [Google Scholar] [CrossRef]
- Huang, L. Molecular hydrogen: A therapeutic antioxidant and beyond. Med. Gas Res. 2016, 6, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Z.; Zhang, Q.X.; Dong, X.X.; Li, H.D.; Ma, X. Treatment with hydrogen molecules prevents RANKL-induced osteoclast differentiation associated with inhibition of ROS formation and inactivation of MAPK, AKT and NF-Kappa B pathways in murine RAW264.7 cells. J. Bone Min. Metab. 2014, 32, 494–504. [Google Scholar] [CrossRef]
- Cai, W.W.; Zhang, M.H.; Yu, Y.S.; Cai, J.H. Treatment with hydrogen molecule alleviates TNFα-induced cell injury in osteoblast. Mol. Cell. Biochem. 2013, 373, 1–9. [Google Scholar] [CrossRef]
- Sun, Y.; Shuang, F.; Chen, D.M.; Zhou, R.B. Treatment of hydrogen molecule abates oxidative stress and alleviates bone loss induced by modeled microgravity in rats. Osteoporos. Int. 2013, 24, 969–978. [Google Scholar] [CrossRef]
- Guo, J.D.; Li, L.; Shi, Y.M.; Wang, H.D.; Hou, S.X. Hydrogen water consumption prevents osteopenia in ovariectomized rats. Br. J. Pharmacol. 2013, 168, 1412–1420. [Google Scholar] [CrossRef]
- Ishibashi, T.; Sato, B.; Rikitake, M.; Seo, T.; Kurokawa, R.; Hara, Y.; Naritomi, Y.; Hara, H.; Nagao, T. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: An open-label pilot study. Med. Gas Res. 2012, 2, 27. [Google Scholar] [CrossRef]
- Ishibashi, T.; Sato, B.; Shibata, S.; Sakai, T.; Hara, Y.; Naritomi, Y.; Koyanagi, S.; Hara, H.; Nagao, T. Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: A randomized, double-blind, placebo-controlled pilot study. Int. Immunopharmacol. 2014, 21, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kurokawa, R.; Hirano, S.I.; Imai, J. Hydrogen Indirectly Suppresses Increases in Hydrogen Peroxide in Cytoplasmic Hydroxyl Radical-Induced Cells and Suppresses Cellular Senescence. Int. J. Mol. Sci. 2019, 20, 456. [Google Scholar] [CrossRef] [PubMed]
- Chuai, Y.; Gao, F.; Li, B.; Zhao, L.; Qian, L.; Cao, F.; Wang, L.; Sun, X.; Cui, J.; Cai, J. Hydrogen-rich saline attenuates radiation-induced male germ cell loss in mice through reducing hydroxyl radicals. Biochem. J. 2012, 442, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Liu, Y.; Zhang, J. Recent Advances in Studies of Molecular Hydrogen against Sepsis. Int. J. Biol. Sci. 2019, 15, 1261–1275. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.S.; Masutani, K.; Tanaka, Y.; Noda, K.; Peng, X.; Takahashi, T.; Billiar, T.R.; et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L646–L656. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T. Therapeutic Efficacy of Molecular Hydrogen: A New Mechanistic Insight. Curr. Pharm. Des. 2019, 25, 946–955. [Google Scholar] [CrossRef]
- Yoritaka, A.; Kobayashi, Y.; Hayashi, T.; Saiki, S.; Hattori, N. Randomized double-blind placebo-controlled trial of hydrogen inhalation for Parkinson’s disease: A pilot study. Neurol. Sci. 2021, 42, 4767–4770. [Google Scholar] [CrossRef]
- Carnovali, M.; Banfi, G.; Mariotti, M. Zebrafish models of human skeletal disorders: Embryo and adult swimming together. Biomed Res. Int. 2019, 2019, 1253710. [Google Scholar] [CrossRef]
- D’Amico, R.; Gugliandolo, E.; Cordaro, M.; Fusco, R.; Genovese, T.; Peritore, A.F.; Crupi, R.; Interdonato, L.; Di Paola, D.; Cuzzocrea, S.; et al. Toxic Effects of Endocrine Disruptor Exposure on Collagen-Induced Arthritis. Biomolecules 2022, 12, 564. [Google Scholar] [CrossRef]
- Pasqualetti, S.; Congiu, T.; Banfi, G.; Mariotti, M. Alendronate rescued osteoporotic phenotype in a model of glucocorticoid-induced osteoporosis in adult zebrafish scale. Int. J. Exp. Path. 2015, 96, 11–20. [Google Scholar] [CrossRef]
- Li, C.; Cao, Y.; Kohei, F.; Hao, H.; Peng, G.; Cheng, C.; Ye, J. Nano-bubble hydrogen water: An effective therapeutic agent against inflammation related disease caused by viral infection in zebrafish model. Virol. Sin. 2022, 37, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wu, B.; Meng, F.; Zhou, Z.; Lu, H.; Zhao, H. Impact of molecular hydrogen treatments on the innate immune activity and survival of zebrafish (Danio rerio) challenged with Aeromonas hydrophila. Fish Sellfish Immunol. 2017, 67, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Carnovali, M.; Mariotti, M.; Banfi, G. Molecular hydrogen enhances osteogenesis in Danio rerio embryos. J. Fish Biol. 2021, 98, 1471–1474. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th ed.; University of Oregon Press: Eugene, OR, USA, 2007; Available online: https://zfin.org/zf_info/zfbook/zfbk.html (accessed on 15 December 2022).
- Pasqualetti, S.; Banfi, G.; Mariotti, M. Osteoblast and osteoclast behavior in zebrafish cultured scales. Cell Tissue Res. 2012, 350, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Perrson, P.; Takagi, Y.; Björnsson, B.T. Tartrate resistant acid phosphatases as a marker for scale resorption in rainbow trout, Oncorhynchus mykiss: Effects of estradiol-17 treatment and refeeding. Fish Physiol. Biochem. 1995, 14, 329–339. [Google Scholar] [CrossRef]
- Jin, L.Y.; Huo, S.C.; Guo, C.; Liu, H.Y.; Xu, S.; Li, X.F. GSK 650394 Inhibits Osteoclasts Differentiation and Prevents Bone Loss via Promoting the Activities of Antioxidant Enzymes In Vitro and In Vivo. Oxid. Med. Cell. Longev. 2022, 2022, 3458560. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Ke, K.; Safder, M.A.; Sul, O.J.; Kim, W.K.; Suh, J.H.; Joe, Y.; Chung, H.T.; Choi, H.S. Hemeoxygenase-1 maintains bone mass via attenuating a redox imbalance in osteoclast. Mol. Cell. Endocrinol. 2015, 409, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, L.; Zhang, H.; Jie, Q.; Li, X.; Shi, Q.; Huang, Q.; Gao, B.; Han, Y.; Guo, K.; et al. Glucocorticoids: Dose-related effects on osteoclast formation and function via reactive oxygen species and autophagy. Bone 2015, 79, 222–232. [Google Scholar] [CrossRef]
- Kratschmar, D.V.; Calabrese, D.; Walsh, J.; Lister, A.; Birk, J.; Appenzeller-Herzog, C.; Moulin, P.; Goldring, C.E.; Odermatt, A. Suppression of the Nrf2-dependent antioxidant response by glucocorticoids and 11β-HSD1-mediated glucocorticoid activation in hepatic cells. PLoS ONE 2012, 7, e36774. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Sun, H.; Xue, Y.; Zhang, W.; Wang, H.; Tao, H.; Liang, X.; Li, M.; Xu, Y.; Chen, L.; et al. Inhibition of MAGL activates the Keap1/Nrf2 pathway to attenuate glucocorticoid-induced osteonecrosis of the femoral head. Clin. Transl. Med. 2021, 11, e447. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Song, D.; Su, Y.; Chen, J.; Wu, L.; Lian, H.; Hai, N.; Li, J.; Jiang, J.; Zhao, J.; et al. Pharmacology-based molecular docking of 4-methylcatechol and its role in RANKL-mediated ROS/Keap1/Nrf2 signalling axis and osteoclastogenesis. Biomed Pharm. 2023, 159, 114101. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J. Reprod. Dev. 2012, 58, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gado, M.; Baschant, U.; Hofbauer, L.C.; Henneicke, H. Bad to the Bone: The Effects of Therapeutic Glucocorticoids on Osteoblasts and Osteocytes. Front. Endocrinol. 2022, 13, 835720. [Google Scholar] [CrossRef]
- Hildebrandt, S.; Baschant, U.; Thiele, S.; Tuckermann, J.; Hofbauer, L.C.; Rauner, M. Glucocorticoids suppress Wnt16 expression in osteoblasts in vitro and in vivo. Sci. Rep. 2018, 8, 8711. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnovali, M.; Banfi, G.; Mariotti, M. Molecular Hydrogen Prevents Osteoclast Activation in a Glucocorticoid-Induced Osteoporosis Zebrafish Scale Model. Antioxidants 2023, 12, 345. https://doi.org/10.3390/antiox12020345
Carnovali M, Banfi G, Mariotti M. Molecular Hydrogen Prevents Osteoclast Activation in a Glucocorticoid-Induced Osteoporosis Zebrafish Scale Model. Antioxidants. 2023; 12(2):345. https://doi.org/10.3390/antiox12020345
Chicago/Turabian StyleCarnovali, Marta, Giuseppe Banfi, and Massimo Mariotti. 2023. "Molecular Hydrogen Prevents Osteoclast Activation in a Glucocorticoid-Induced Osteoporosis Zebrafish Scale Model" Antioxidants 12, no. 2: 345. https://doi.org/10.3390/antiox12020345
APA StyleCarnovali, M., Banfi, G., & Mariotti, M. (2023). Molecular Hydrogen Prevents Osteoclast Activation in a Glucocorticoid-Induced Osteoporosis Zebrafish Scale Model. Antioxidants, 12(2), 345. https://doi.org/10.3390/antiox12020345