Enzymatic and Non-Enzymatic Antioxidant Responses of Young Tomato Plants (cv. Micro-Tom) to Single and Combined Mild Nitrogen and Water Deficit: Not the Sum of the Parts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Biometric Traits
2.3. Analysis of Total Antioxidant Capacity and Nonenzymatic Component
2.3.1. Preparation of the Extracts
2.3.2. Total Antioxidant Capacity: ABTS Assay
2.3.3. Non-Enzymatic Component: Total Phenolics, Flavonoids, Carotenoids, Anthocyanins, and Lignin
Determination of Total Phenolic Content (TPC)
Determination of Total Flavonoids
Determination of Total Monomeric Anthocyanins
Determination of Lignin
2.4. Oxidative Stress Biomarkers
2.4.1. Lipid Peroxidation
2.4.2. Hydrogen Peroxide (H2O2) Quantification
2.5. Enzymatic Component
2.5.1. Enzyme Extraction and Quantification: SOD (EC.1.15.1.1), CAT (EC.1.11.1.6) and APX (EC.1.11.1.11)
2.5.2. SOD, APX, and CAT Expression Profile
2.6. Statistical Analysis
3. The Results
3.1. Plant Growth
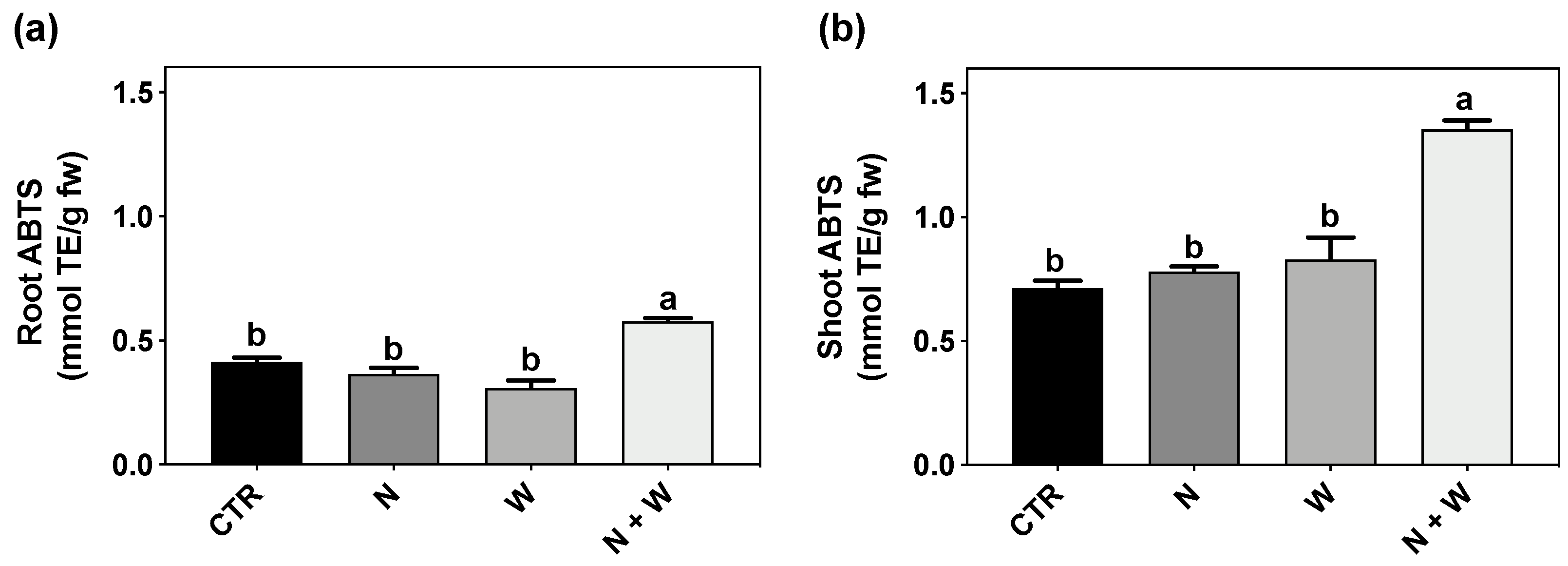
3.2. AOX Activity
3.3. Non-Enzymatic Component: Total Phenolics, Flavonoids, Carotenoids, Anthocyanins, and Lignin
3.4. Oxidative Stress Biomarkers—MDA and H2O2 Levels
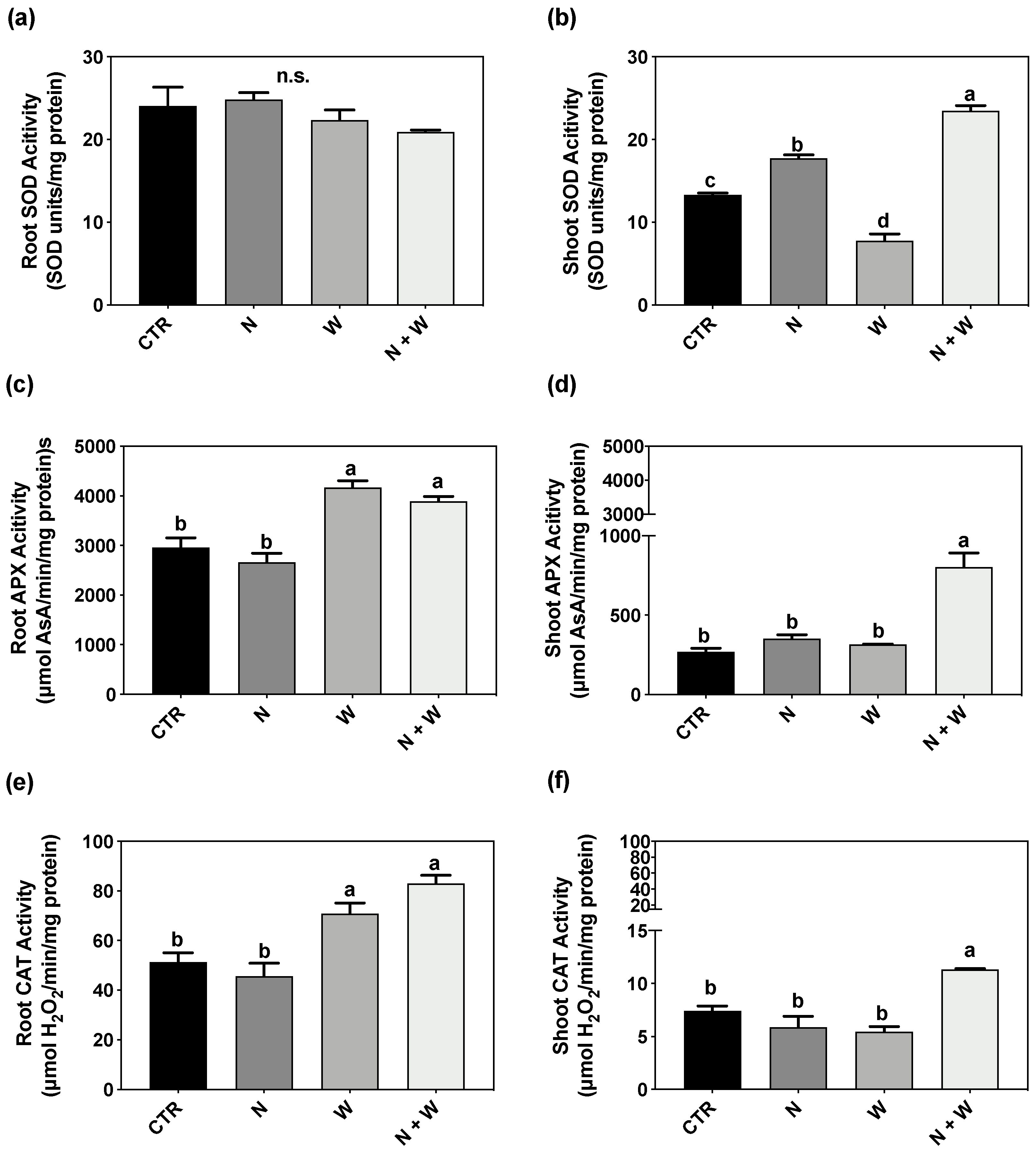
3.5. Enzymatic Component: SOD, CAT, APX
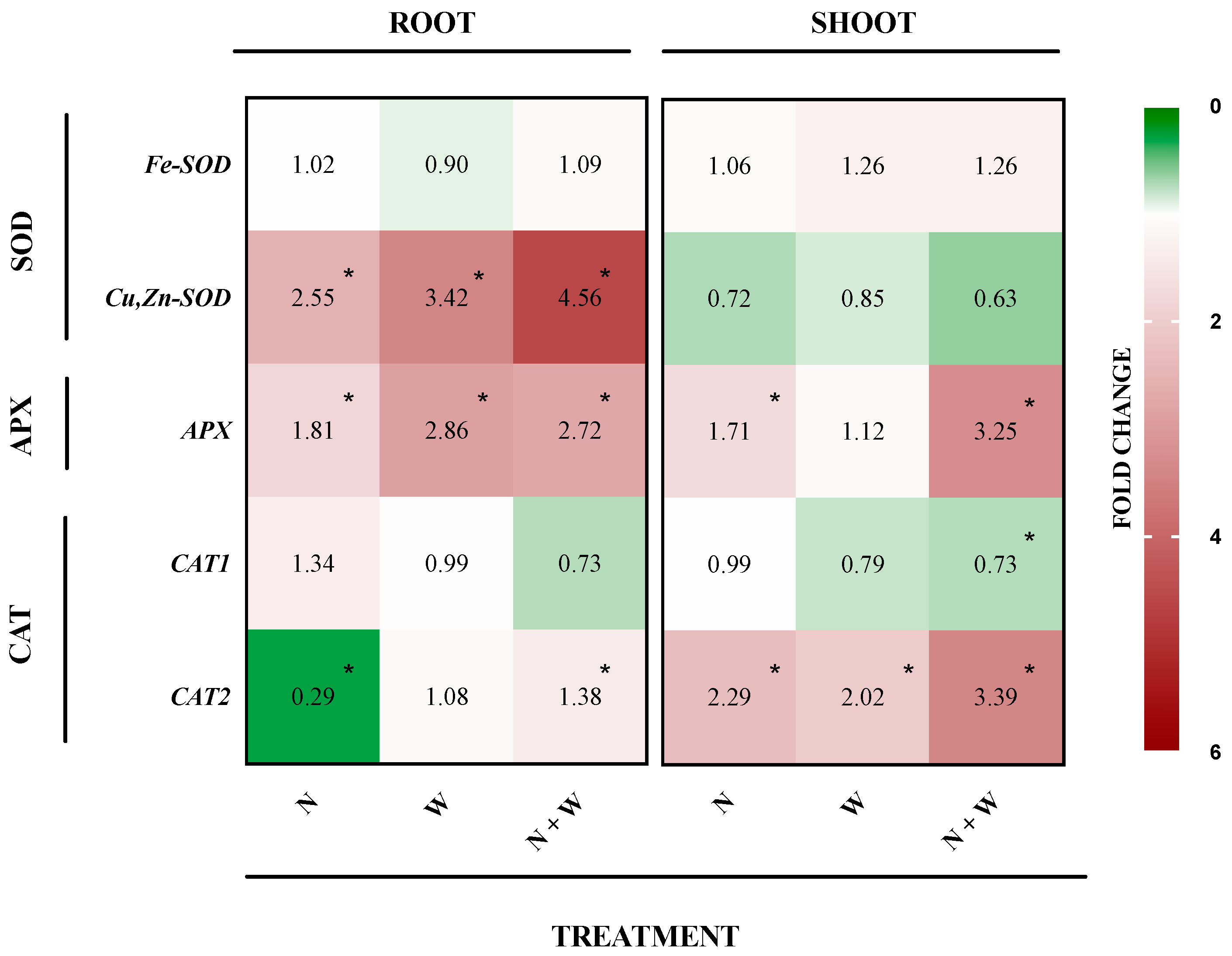
3.6. SOD, APX, and CAT Expression Profile
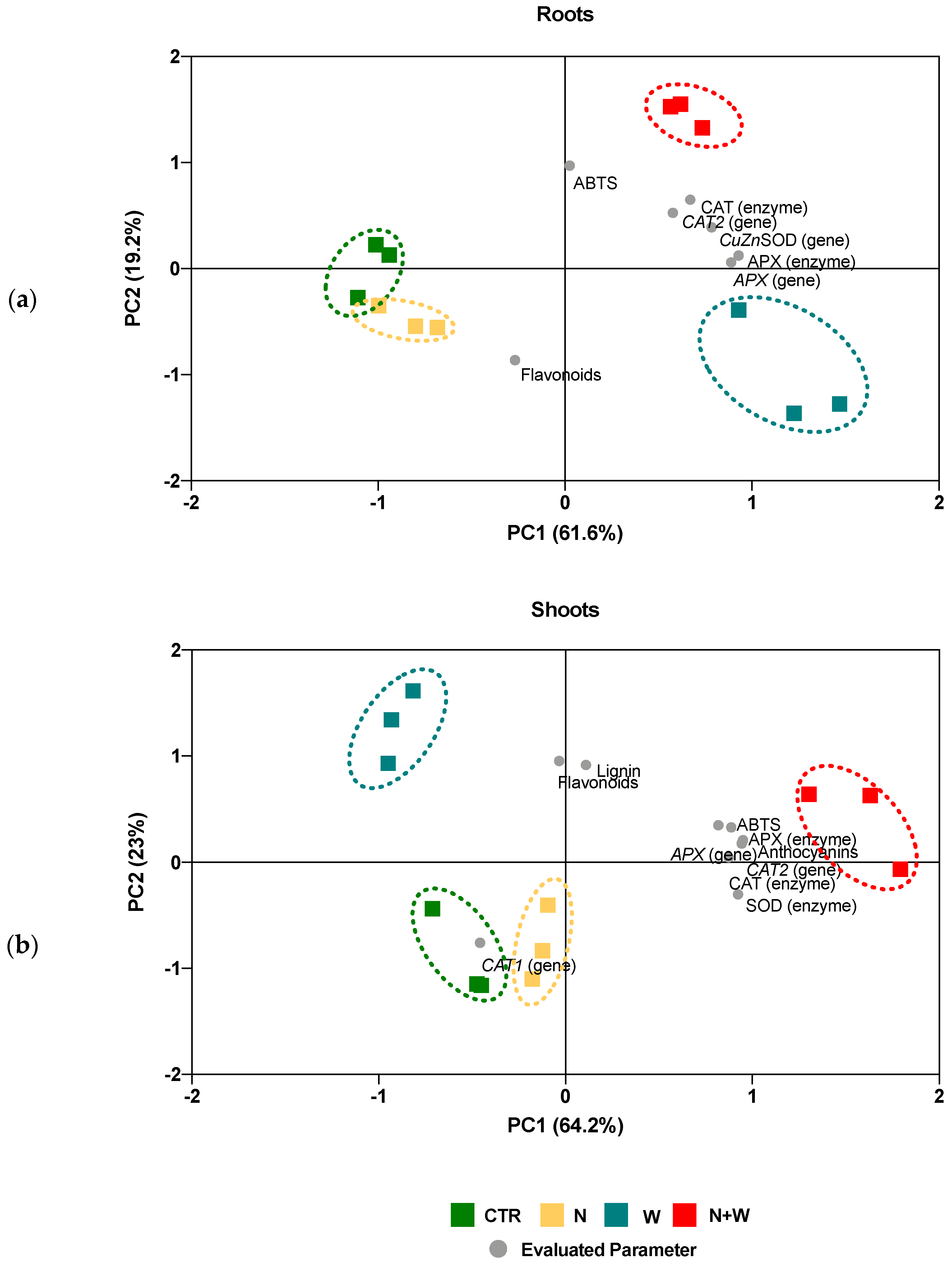
3.7. Principal Component Analysis (PCA)
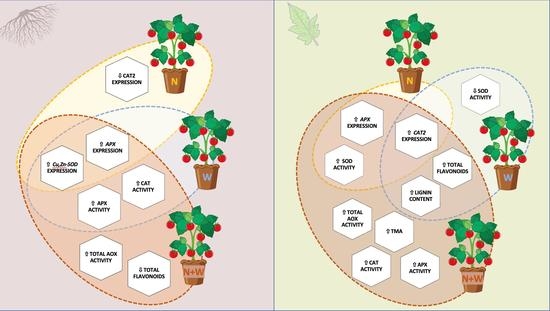
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elbehri, A. Climate Change and Food Systems: Global Assessments and Implications for Food Security and Trade; Food and Agriculture Organization of the United Nations (FAO): Roma, Italy, 2015. [Google Scholar]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Ridoutt, B.G.; Lal, R.; Wang, D.; Wu, W.; Peng, P.; Hang, S.; Wang, L.; Zhao, G. Nitrogen footprint and nitrogen use efficiency of greenhouse tomato production in North China. J. Clean. Prod. 2019, 208, 285–296. [Google Scholar] [CrossRef]
- Schebesta, H.; Candel, J.J.L. Game-changing potential of the EU’s Farm to Fork Strategy. Nat. Food 2020, 1, 586–588. [Google Scholar] [CrossRef]
- Machado, J.; Fernandes, A.; Fernandes, T.; Heuvelink, E.; Vasconcelos, M.; Carvalho, S.M.P. Drought and nitrogen stress effects and tolerance mechanisms in tomato: A review. In Plant Nutrition and Food Security in the Era of Climate Change; Academic Press Elsevier: Amsterdam, The Netherlands, 2022; pp. 315–359. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Kumar, S.; Sachdeva, S.; Bhat, K.; Vats, S. Plant responses to drought stress: Physiological, biochemical and molecular basis. In Biotic and Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–25. [Google Scholar]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta 2020, 251, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Cervilla, L.M.; Blasco, B.; Rios, J.J.; Rosales, M.A.; Romero, L.; Ruiz, J.M. Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci. 2010, 178, 30–40. [Google Scholar] [CrossRef]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.-O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 1–11. [Google Scholar] [CrossRef]
- Dong, Y.; Zhai, J.; Yan, J.; Li, K.; Xu, H. Physiological and transcriptomic responses of antioxidant system and nitrogen metabolism in tomato seedlings treated with nitrogen starvation and re-supply. J. Hortic. Sci. Biotechnol. 2022, 98, 57–71. [Google Scholar] [CrossRef]
- Fernandes, A.; Machado, J.; Fernandes, T.; Vasconcelos, M.; Carvalho, S.M.P. Water and nitrogen fertilization management in light of climate change: Impacts on food security and product quality. In Plant Nutrition and Food Security in the Era of Climate Change; Academic Press Elsevier: Amsterdam, The Netherlands, 2022; pp. 147–178. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef]
- Sousa, B.; Rodrigues, F.; Soares, C.; Martins, M.; Azenha, M.; Lino-Neto, T.; Santos, C.; Cunha, A.; Fidalgo, F. Impact of Combined Heat and Salt Stresses on Tomato Plants—Insights into Nutrient Uptake and Redox Homeostasis. Antioxidants 2022, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef]
- García-Martí, M.; Piñero, M.C.; García-Sanchez, F.; Mestre, T.C.; López-Delacalle, M.; Martínez, V.; Rivero, R.M. Amelioration of the oxidative stress generated by simple or combined abiotic stress through the K+ and Ca2+ supplementation in tomato plants. Antioxidants 2019, 8, 81. [Google Scholar] [CrossRef]
- Spormann, S.; Soares, C.; Martins, V.; Azenha, M.; Gerós, H.; Fidalgo, F. Early Activation of Antioxidant Responses in Ni-Stressed Tomato Cultivars Determines Their Resilience Under Co-exposure to Drought. J. Plant Growth Regul. 2022, 1–15. [Google Scholar] [CrossRef]
- Joshi, S.; Thoday-Kennedy, E.; Daetwyler, H.D.; Hayden, M.; Spangenberg, G.; Kant, S. High-throughput phenotyping to dissect genotypic differences in safflower for drought tolerance. PLoS ONE 2021, 16, e0254908. [Google Scholar] [CrossRef]
- Gonçalves, B.; Falco, V.; Moutinho-Pereira, J.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of elevated CO2 on grapevine (Vitis vinifera L.): Volatile composition, phenolic content, and in vitro antioxidant activity of red wine. J. Agric. Food Chem. 2009, 57, 265–273. [Google Scholar] [CrossRef]
- Ramos, P.A.; Moreirinha, C.; Silva, S.; Costa, E.M.; Veiga, M.; Coscueta, E.; Santos, S.A.; Almeida, A.; Pintado, M.M.; Freire, C.S. The health-promoting potential of Salix spp. bark polar extracts: Key insights on phenolic composition and in vitro bioactivity and biocompatibility. Antioxidants 2019, 8, 609. [Google Scholar] [CrossRef]
- Zafar, H.; Ali, A.; Ali, J.S.; Haq, I.U.; Zia, M. Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: Growth dynamics and antioxidative response. Front. Plant Sci. 2016, 7, 535. [Google Scholar] [CrossRef] [PubMed]
- Nicoue, E.E.; Savard, S.; Belkacemi, K. Anthocyanins in wild blueberries of Quebec: Extraction and identification. J. Agric. Food Chem. 2007, 55, 5626–5635. [Google Scholar] [CrossRef]
- Fukushima, R.S.; Hatfield, R.D. Extraction and isolation of lignin for utilization as a standard to determine lignin concentration using the acetyl bromide spectrophotometric method. J. Agric. Food Chem. 2001, 49, 3133–3139. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Fidalgo, F.; Freitas, R.; Ferreira, R.; Pessoa, A.M.; Teixeira, J. Solanum nigrum L. antioxidant defence system isozymes are regulated transcriptionally and posttranslationally in Cd-induced stress. Environ. Exp. Bot. 2011, 72, 312–319. [Google Scholar] [CrossRef]
- Soares, C.; Pereira, R.; Spormann, S.; Fidalgo, F. Is soil contamination by a glyphosate commercial formulation truly harmless to non-target plants?–Evaluation of oxidative damage and antioxidant responses in tomato. Environ. Pollut. 2019, 247, 256–265. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Donahue, J.L.; Okpodu, C.M.; Cramer, C.L.; Grabau, E.A.; Alscher, R.G. Responses of antioxidants to paraquat in pea leaves (relationships to resistance). Plant Physiol. 1997, 113, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vaerman, J.; Saussoy, P.; Ingargiola, I. Evaluation of real-time PCR data. J. Biol. Regul. Homeost. Agents 2004, 18, 212–214. [Google Scholar]
- Zhou, R.; Kong, L.; Wu, Z.; Rosenqvist, E.; Wang, Y.; Zhao, L.; Zhao, T.; Ottosen, C.O. Physiological response of tomatoes at drought, heat and their combination followed by recovery. Physiol. Plant. 2019, 165, 144–154. [Google Scholar] [CrossRef]
- Liang, G.; Liu, J.; Zhang, J.; Guo, J. Effects of drought stress on photosynthetic and physiological parameters of tomato. J. Am. Soc. Hortic. Sci. 2020, 145, 12–17. [Google Scholar] [CrossRef]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Rubio-Wilhelmi, M.; Sanchez-Rodriguez, E.; Rosales, M.; Begona, B.; Rios, J.; Romero, L.; Blumwald, E.; Ruiz, J. Effect of cytokinins on oxidative stress in tobacco plants under nitrogen deficiency. Environ. Exp. Bot. 2011, 72, 167–173. [Google Scholar] [CrossRef]
- Kubalt, K. The Role of Phenolic Compounds in Plant Resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; del Mar Rubio-Wilhelmi, M.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: Changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Klokić, I.; Parađiković, N.; Kukavica, B. Biostimulant prevents yield loss and reduces oxidative damage in tomato plants grown on reduced NPK nutrition. J. Plant Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Otegui, M.S.; Grotewold, E. Abiotic stresses induce different localizations of anthocyanins in Arabidopsis. Plant Signal Behav. 2015, 10, e1027850. [Google Scholar] [CrossRef]
- Sanchez-Casas, P.; Klessig, D.F. A salicylic acid-binding activity and a salicylic acid-inhibitable catalase activity are present in a variety of plant species. Plant Physiol. 1994, 106, 1675–1679. [Google Scholar] [CrossRef]
- Aghaie, P.; Tafreshi, S.A.H.; Ebrahimi, M.A.; Haerinasab, M. Tolerance evaluation and clustering of fourteen tomato cultivars grown under mild and severe drought conditions. Sci. Hortic. 2018, 232, 1–12. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, Z.; Li, Y.; Liu, Q.; Han, W. Effects of different levels of water stress on leaf photosynthetic characteristics and antioxidant enzyme activities of greenhouse tomato. Photosynthetica 2016, 54, 28–39. [Google Scholar] [CrossRef]
- Rai, G.K.; Parveen, A.; Jamwal, G.; Basu, U.; Kumar, R.R.; Rai, P.K.; Sharma, J.P.; Alalawy, A.I.; Al-Duais, M.A.; Hossain, M.A. Leaf Proteome Response to Drought Stress and Antioxidant Potential in Tomato (Solanum lycopersicum L.). Atmosphere 2021, 12, 1021. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Polesskaya, O.; Kashirina, E.; Alekhina, N. Changes in the activity of antioxidant enzymes in wheat leaves and roots as a function of nitrogen source and supply. Russ. J. Plant Physiol. 2004, 51, 615–620. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Modulation of Antioxidant Defense System Is Associated with Combined Drought and Heat Stress Tolerance in Citrus. Front. Plant Sci 2017, 8, 953. [Google Scholar] [CrossRef]
- Pandey, P.; Ramegowda, V.; Senthil-Kumar, M. Shared and unique responses of plants to multiple individual stresses and stress combinations: Physiological and molecular mechanisms. Front. Plant Sci. 2015, 6, 723. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Song, T. Heat and drought priming induce tolerance to subsequent heat and drought stress by regulating leaf photosynthesis, root morphology, and antioxidant defense in maize seedlings. Environ. Exp. Bot. 2022, 202, 105010. [Google Scholar] [CrossRef]
- Khapte, P.; Kumar, P.; Burman, U.; Kumar, P. Deficit irrigation in tomato: Agronomical and physio-biochemical implications. Sci. Hortic. 2019, 248, 256–264. [Google Scholar] [CrossRef]



| Gene (Accession Number) | Primer Sequence (5′-3′) | Tann. | |
|---|---|---|---|
| Forward | Reverse | ||
| Fe-SOD | TAA ATA GAG ACT TTG GTT CC | TAT ATT TGC CTC TTA ACC CT | 45.6 |
| Cu,Zn-SOD | GGC CAA TCT TTG ACC CTT TA | AGT CCA GGA GCA AGT CCA GT | 54.7 |
| APX | TCT GAA TTG GGA TTT GCT GA | CGT CTA ACG TAG CTG CCA AA | 55.4 |
| CAT 1 | CAA ACA ATG GAC CCC GAG GA | ACT GGG ATC AAC GGC AAG AG | 60.3 |
| CAT 2 | GGG TCT GGT GTC CAC ACA TT | GCA TGG CTG TGA TTT GCT CC | 59.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, J.; Vasconcelos, M.W.; Soares, C.; Fidalgo, F.; Heuvelink, E.; Carvalho, S.M.P. Enzymatic and Non-Enzymatic Antioxidant Responses of Young Tomato Plants (cv. Micro-Tom) to Single and Combined Mild Nitrogen and Water Deficit: Not the Sum of the Parts. Antioxidants 2023, 12, 375. https://doi.org/10.3390/antiox12020375
Machado J, Vasconcelos MW, Soares C, Fidalgo F, Heuvelink E, Carvalho SMP. Enzymatic and Non-Enzymatic Antioxidant Responses of Young Tomato Plants (cv. Micro-Tom) to Single and Combined Mild Nitrogen and Water Deficit: Not the Sum of the Parts. Antioxidants. 2023; 12(2):375. https://doi.org/10.3390/antiox12020375
Chicago/Turabian StyleMachado, Joana, Marta W. Vasconcelos, Cristiano Soares, Fernanda Fidalgo, Ep Heuvelink, and Susana M. P. Carvalho. 2023. "Enzymatic and Non-Enzymatic Antioxidant Responses of Young Tomato Plants (cv. Micro-Tom) to Single and Combined Mild Nitrogen and Water Deficit: Not the Sum of the Parts" Antioxidants 12, no. 2: 375. https://doi.org/10.3390/antiox12020375
APA StyleMachado, J., Vasconcelos, M. W., Soares, C., Fidalgo, F., Heuvelink, E., & Carvalho, S. M. P. (2023). Enzymatic and Non-Enzymatic Antioxidant Responses of Young Tomato Plants (cv. Micro-Tom) to Single and Combined Mild Nitrogen and Water Deficit: Not the Sum of the Parts. Antioxidants, 12(2), 375. https://doi.org/10.3390/antiox12020375