Antiperiodontitis Effects of Siegesbeckia glabrescens In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of S. glabrescens Extraction
2.2. HPLC Analysis of SGE
2.3. Disc Diffusion Assay
2.4. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration Assay
2.5. Scanning Electron Microscope Analysis
2.6. Inhibition of Glucosyltransferase Activity
2.7. Inhibition of Biofilm Formation
2.8. Inhibition of Acid Production
2.9. Total Phenolic and Flavonoid Contents
2.10. 1,1-Diphenyl-2-Picrylhydrazyl and 2,2′-Azino-Bis-(3-Ethylbenzothiazoline)-6-Sulfonic Acid Radical Scavenging Assay
2.11. Superoxide Dismutase-Like Assay
2.12. Cytotoxicity
2.13. NO Assay
2.14. Enzyme-Linked Immunosorbent Assay
2.15. Statistical Analysis
3. Results
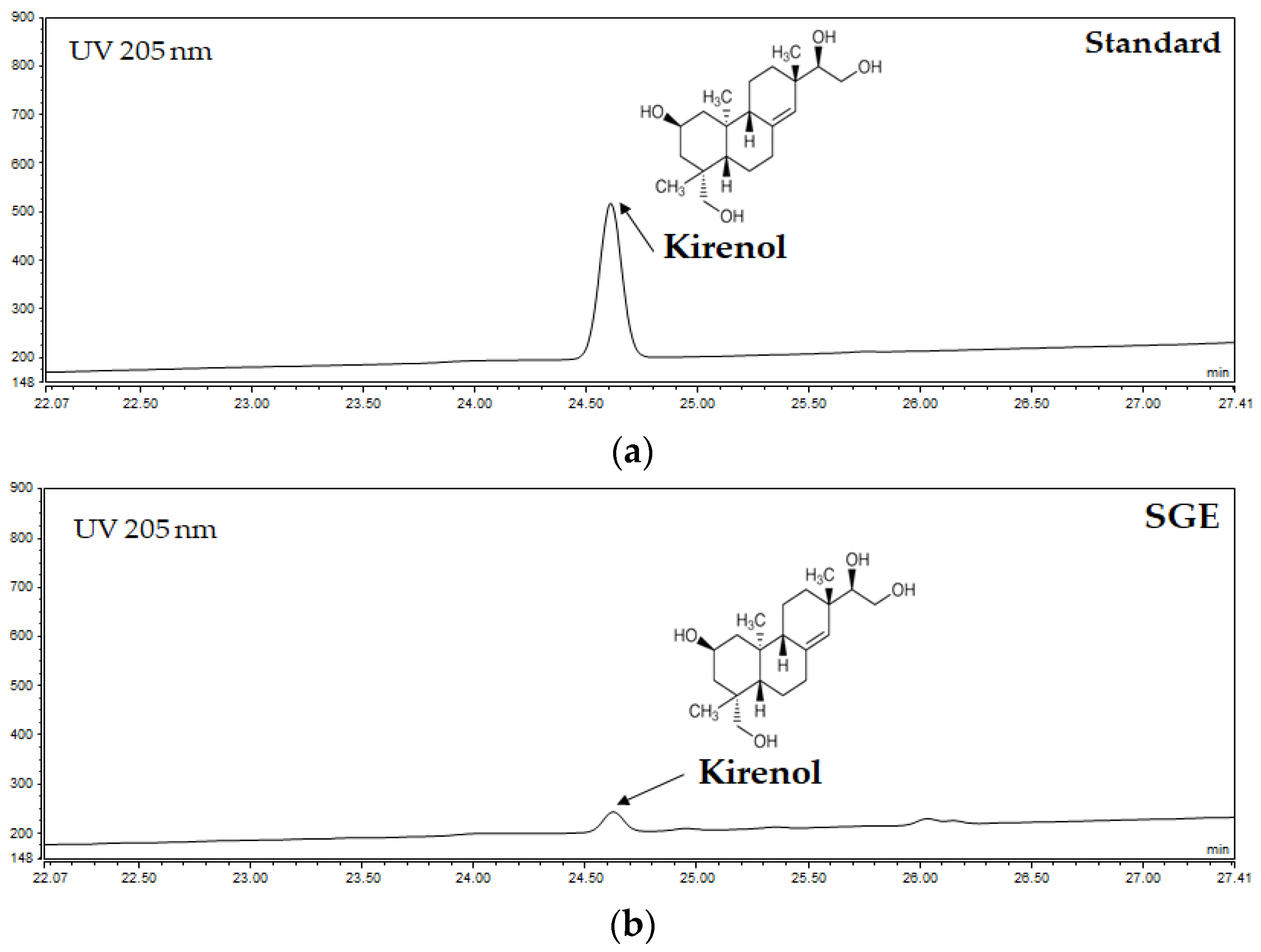
3.1. Quantitative Analysis of Kirenol from SGE
3.2. Antimicrobial Activity
3.3. MIC and MBC Assays
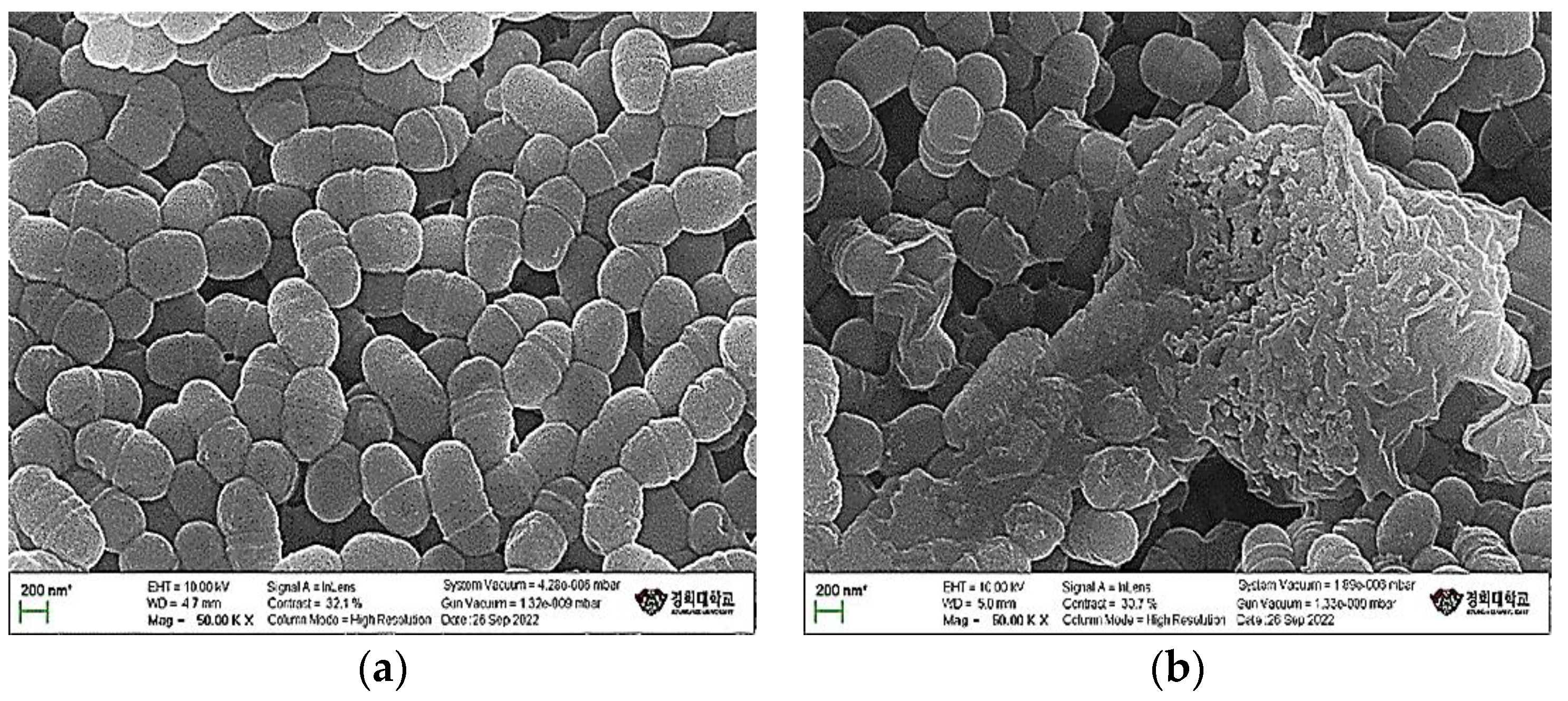
3.4. Morphological Changes in Streptococcus Mutans
3.5. Inhibition of GTFs’ Activity
3.6. Inhibition of Biofilm Formation
3.7. Inhibition of Acid Production
3.8. Total Phenolic and Flavonoid Contents
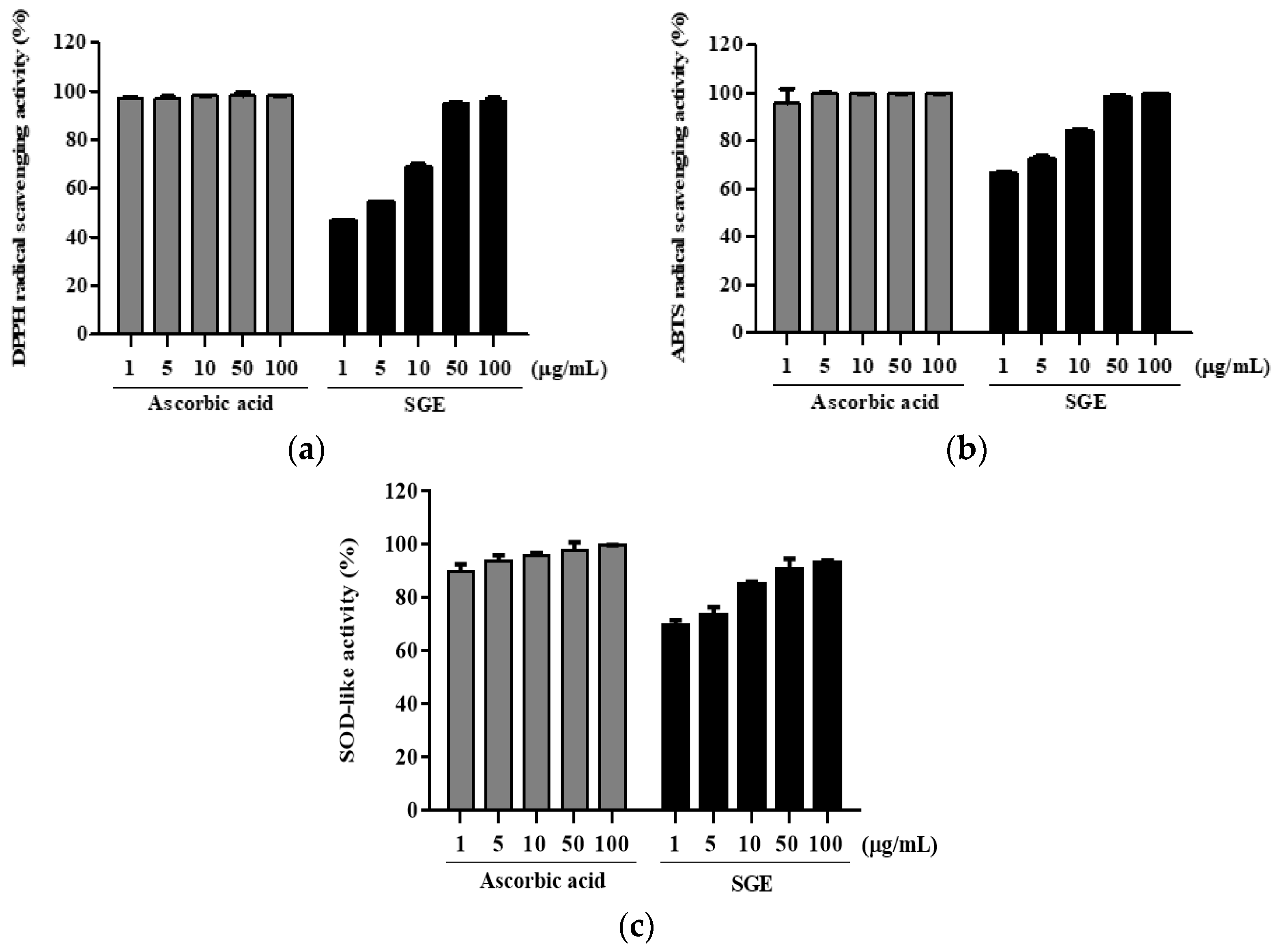
3.9. Antioxidant Activity
3.10. Cytotoxicity and Regulation of NO Production of SGE
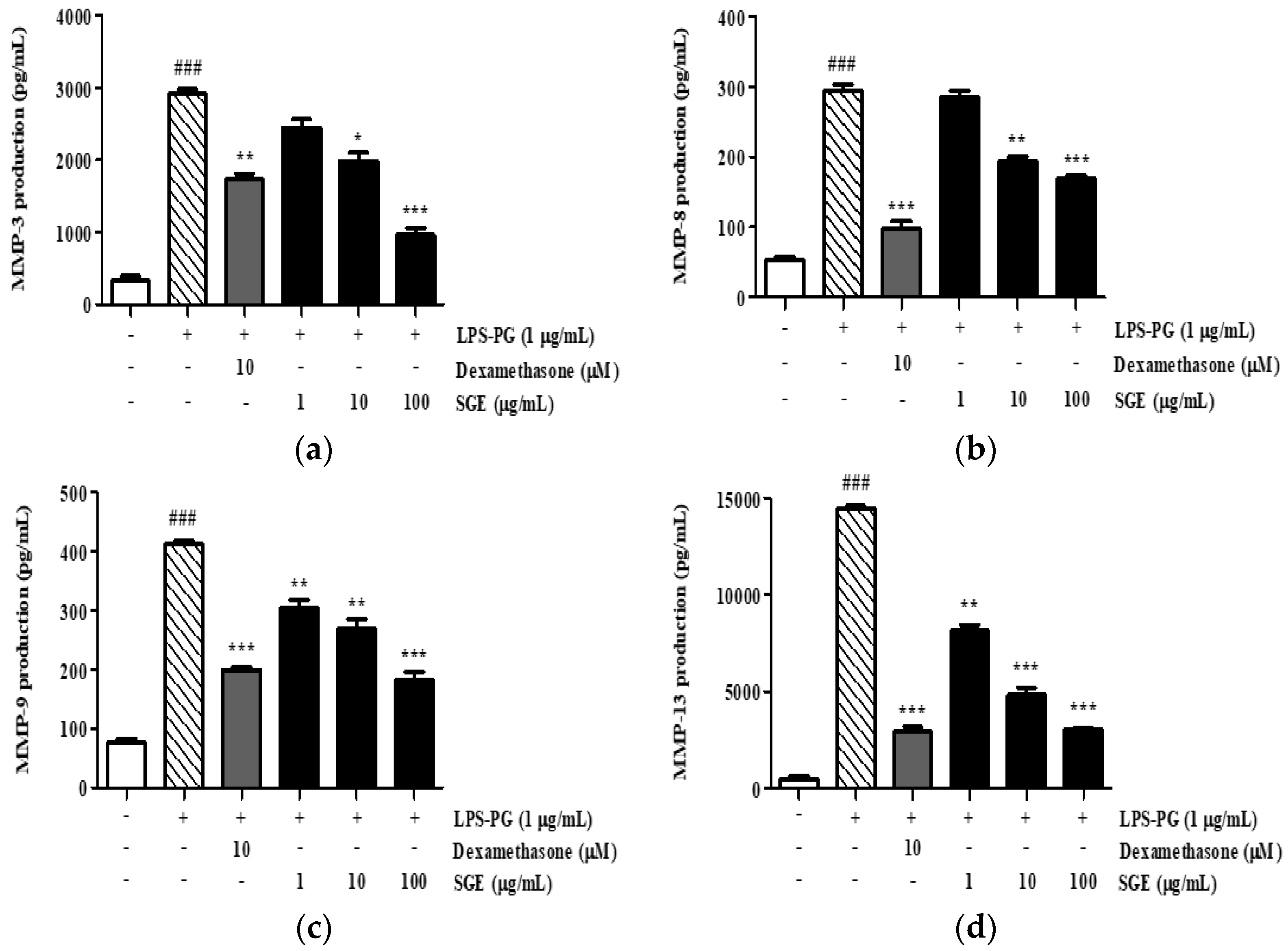
3.11. Effect of SGE on the Secretion of MMP-3, MMP-8, MMP-9, and MMP-13
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wolf, T.G.; Cagetti, M.G.; Fisher, J.-M.; Seeberger, G.K.; Campus, G. Non-Communicable Diseases and Oral Health: An Overview. Front. Oral. Health. 2021, 2, 725460. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A. Prevalence of Periodontal Disease, Its Association with Systemic Diseases and Prevention. Int. J. Health Sci. (Qassim) 2017, 11, 72–80. [Google Scholar] [PubMed]
- Sondorová, M.; Kučera, J.; Kačírová, J.; Krchová Nagyová, Z.; Šurín Hudáková, N.; Lipták, T.; Maďar, M. Prevalence of Periodontal Pathogens in Slovak Patients with Periodontitis and Their Possible Aspect of Transmission from Companion Animals to Humans. Biology 2022, 11, 1529. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. The Bacterial Etiology of Destructive Periodontal Disease: Current Concepts. J. Periodontol. 1992, 63, 322–331. [Google Scholar] [CrossRef]
- Loomer, P.M. Microbiological Diagnostic Testing in the Treatment of Periodontal Diseases. Periodontology 2004, 34, 49–56. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef]
- Jeon, C.-M.; Shin, I.-S.; Shin, N.-R.; Hong, J.-M.; Kwon, O.-K.; Kim, H.-S.; Oh, S.-R.; Myung, P.-K.; Ahn, K.-S. Siegesbeckia Glabrescens Attenuates Allergic Airway Inflammation in LPS-Stimulated RAW 264.7 Cells and OVA Induced Asthma Murine Model. Int. Immunopharmacol. 2014, 22, 414–419. [Google Scholar] [CrossRef]
- Lee, D.-S.; Lee, M.; Sung, S.H.; Jeong, G.S. Involvement of Heme Oxygenase-1 Induction in the Cytoprotective and Neuroinflammatory Activities of Siegesbeckia Pubescens Isolated from 5,3′-Dihydroxy-3,7,4′-Trimethoxyflavone in HT22 Cells and BV2 Cells. Int. Immunopharmacol. 2016, 40, 65–72. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, Q.; Tao, H.; Sang, W.; Cui, L.; Qiang, W.; Cheang, W.S.; Hu, Y.; Yu, H.; Wang, Y. Anti-Inflammatory Activities of Sigesbeckia Glabrescens Makino: Combined in Vitro and in Silico Investigations. Chin. Med. 2019, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.-B.; Yun, J.G.; Hwang, J.K. Protective Effects of Standardized Siegesbeckia Glabrescens Extract and Its Active Compound Kirenol against UVB-Induced Photoaging through Inhibition of MAPK/NF-κB Pathways. J. Microbiol. Biotechnol. 2017, 27, 242–250. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, H.; Jung, E.; Kim, J.-H.; Hwang, W.; Kang, E.-J.; Lee, S.; Ha, B.-J.; Lee, J.; Park, D. A Novel Antibacterial Compound from Siegesbeckia Glabrescens. Molecules 2012, 17, 12469–12477. [Google Scholar] [CrossRef]
- Shim, S.-Y.; Lee, Y.E.; Lee, M. Antioxidant Compounds, Kirenol and Methyl Ent-16α, 17-Dihydroxy-Kauran-19-Oate Bioactivity-Guided Isolated from Siegesbeckia Glabrescens Attenuates MITF-Mediated Melanogenesis via Inhibition of Intracellular ROS Production. Molecules 2021, 26, 1940. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- M, M. Antieurodontic Effect of Various Fractions Extracted from the Leaves of Gymnema Sylvestre. J. Yonago Med. Assoc. 1987, 38, 127–137. [Google Scholar]
- Zayed, S.M.; Aboulwafa, M.M.; Hashem, A.M.; Saleh, S.E. Biofilm Formation by Streptococcus Mutans and Its Inhibition by Green Tea Extracts. AMB Express 2021, 11, 73. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.; de Mello, J. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-Throughput Micro Plate Assays for Screening Flavonoid Content and DPPH-Scavenging Activity in Sorghum Bran and Flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From Microbial Immune Subversion to Systemic Inflammation. Nat. Rev. Immunol. 2014, 15, 30–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatipoğlu, M.; Barutcigil, Ç.; Harorlı, O.T.; Ulug, B. Effect of the Lasers Used in Periodontal Therapy on the Surfaces of Restorative Materials. Scanning 2015, 38, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas Gingivalis: An Invasive and Evasive Opportunistic Oral Pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef]
- Frédéric, L.; Michel, B.; Selena, T. Oral Microbes, Biofilms and Their Role in Periodontal and Peri-Implant Diseases. Materials 2018, 11, 1802. [Google Scholar] [CrossRef]
- Dahiya, P.; Bhardwaj, R.; Chaudhary, K.; Kamal, R.; Gupta, R.; Kaur, S. Reactive Oxygen Species in Periodontitis. J. Indian. Soc. Periodontol. 2013, 17, 411. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Jacoby, B.H.; Davis, W.L. The Electron Microscopic Immunolocalization of a Copper-Zinc Superoxide Dismutase in Association with Collagen Fibers of Periodontal Soft Tissues. Periodontology 1991, 62, 413–420. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, T.; Ghosh, P.; Bandyopadhyay, P.; Swarnakar, S.; Sarkar, P.; Varatharajan, A. Expression of Matrix Metalloproteinase-9 in Gingival Tissue Biopsy in Patients with Slowly/Moderately and Rapidly Progressing Periodontitis: An Observational Study. J. Indian Soc. Periodontol. 2021, 25, 386–392. [Google Scholar] [CrossRef]
- Hernandez, M.; Valenzuela, M.A.; Lopez-Otin, C.; Alvarez, J.; Lopez, J.M.; Vernal, R.; Gamonal, J. Matrix Metalloproteinase-13 Is Highly Expressed in Destructive Periodontal Disease Activity. J. Periodontol. 2006, 77, 1863–1870. [Google Scholar] [CrossRef] [PubMed]




| Indicators | Inhibition Zone | |
|---|---|---|
| Amoxicillin | SGE | |
| Streptococcus mutans KACC 16833 | ++ ** | ++ |
| Streptococcus sanguinis KACC 11301 | ++ | ++ |
| Streptococcus downei KACC 13827 | ++ | ++ |
| Streptococcus gordonii KACC 13829 | ++ | ++ |
| Streptococcus ferus KACC 13881 | ++ | ++ |
| Streptococcus mitis KACC 16832 | ++ | ++ |
| Indicators | Inhibition Zone | |
|---|---|---|
| Amoxicillin | SGE | |
| Porphyromonas gingivalis KCTC 5352 | ++ ** | ++ |
| Treponema denticola KCTC 15104 | ++ | ++ |
| Campylobacter gracilis KCTC 15224 | ++ | ++ |
| Campylobacter rectus KCTC 5636 | ++ | ++ |
| Fusobacterium nucleatum KCTC 2640 | ++ | ++ |
| Parvimonas micra KCTC 15021 | ++ | ++ |
| Prevotella intermedia KCTC 15693 | ++ | ++ |
| Prevotella nigrescens KCTC 15081 | ++ | ++ |
| Aggregatibacter actinomycetemcomitans KCTC 2581 | ++ | ++ |
| Eikenella corrodens KCTC 15198 | ++ | ++ |
| Indicators | SGE (mg/mL) | |
|---|---|---|
| MIC | MBC | |
| S. mutans KACC 16833 | ≥0.63 | 1.30 |
| S. sanguinis KACC 11301 | ≥0.40 | 0.80 |
| S. downei KACC 13827 | ≥0.20 | 0.40 |
| S. gordonii KACC 13829 | ≥0.20 | 0.31 |
| S. ferus KACC 13881 | ≥1.30 | 3.00 |
| S. mitis KACC 16832 | ≥0.40 | 0.80 |
| Indicators | SGE (mg/mL) | |
|---|---|---|
| MIC | MBC | |
| P. gingivalis KCTC 5352 | ≥0.20 | 0.40 |
| T. denticola KCTC 15104 | ≥0.20 | 0.40 |
| C. gracilis KCTC 15224 | ≥0.31 | 0.63 |
| C. rectus KCTC 5636 | ≥0.31 | 0.63 |
| F. nucleatum KCTC 2640 | ≥0.20 | 0.40 |
| P. micra KCTC 15021 | ≥0.31 | 0.63 |
| P. intermedia KCTC 15693 | ≥0.31 | 0.63 |
| P. nigrescens KCTC 15081 | ≥0.31 | 0.63 |
| A. actinomycetemcomitans KCTC 2581 | ≥0.20 | 0.40 |
| E. corrodens KCTC 15198 | ≥0.20 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellere, A.D.; Yu, D.; Oh, S.; Kim, M.; Jung, J.; Fang, M.; Zheng, S.; Yi, T.-H. Antiperiodontitis Effects of Siegesbeckia glabrescens In Vitro. Antioxidants 2023, 12, 471. https://doi.org/10.3390/antiox12020471
Bellere AD, Yu D, Oh S, Kim M, Jung J, Fang M, Zheng S, Yi T-H. Antiperiodontitis Effects of Siegesbeckia glabrescens In Vitro. Antioxidants. 2023; 12(2):471. https://doi.org/10.3390/antiox12020471
Chicago/Turabian StyleBellere, Arce Defeo, Duna Yu, Sarang Oh, Myeongju Kim, Jeyong Jung, Minzhe Fang, Shengdao Zheng, and Tae-Hoo Yi. 2023. "Antiperiodontitis Effects of Siegesbeckia glabrescens In Vitro" Antioxidants 12, no. 2: 471. https://doi.org/10.3390/antiox12020471








