Chemical Characterization of Different Extracts of Justicia secunda Vahl and Determination of Their Anti-Oxidant, Anti-Enzymatic, Anti-Viral, and Cytotoxic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Extraction
2.2. Determination of Total Phenolic, Flavonoid and Antioxidant and Enzyme Inhibitory Effects
2.3. LC-MS Analysis
2.4. Cytotoxicity Testing and Anticancer Selectivity
2.5. Evaluation of the Anti-Herpesvirus Effect
2.6. Data Analysis
3. Results and Discussion
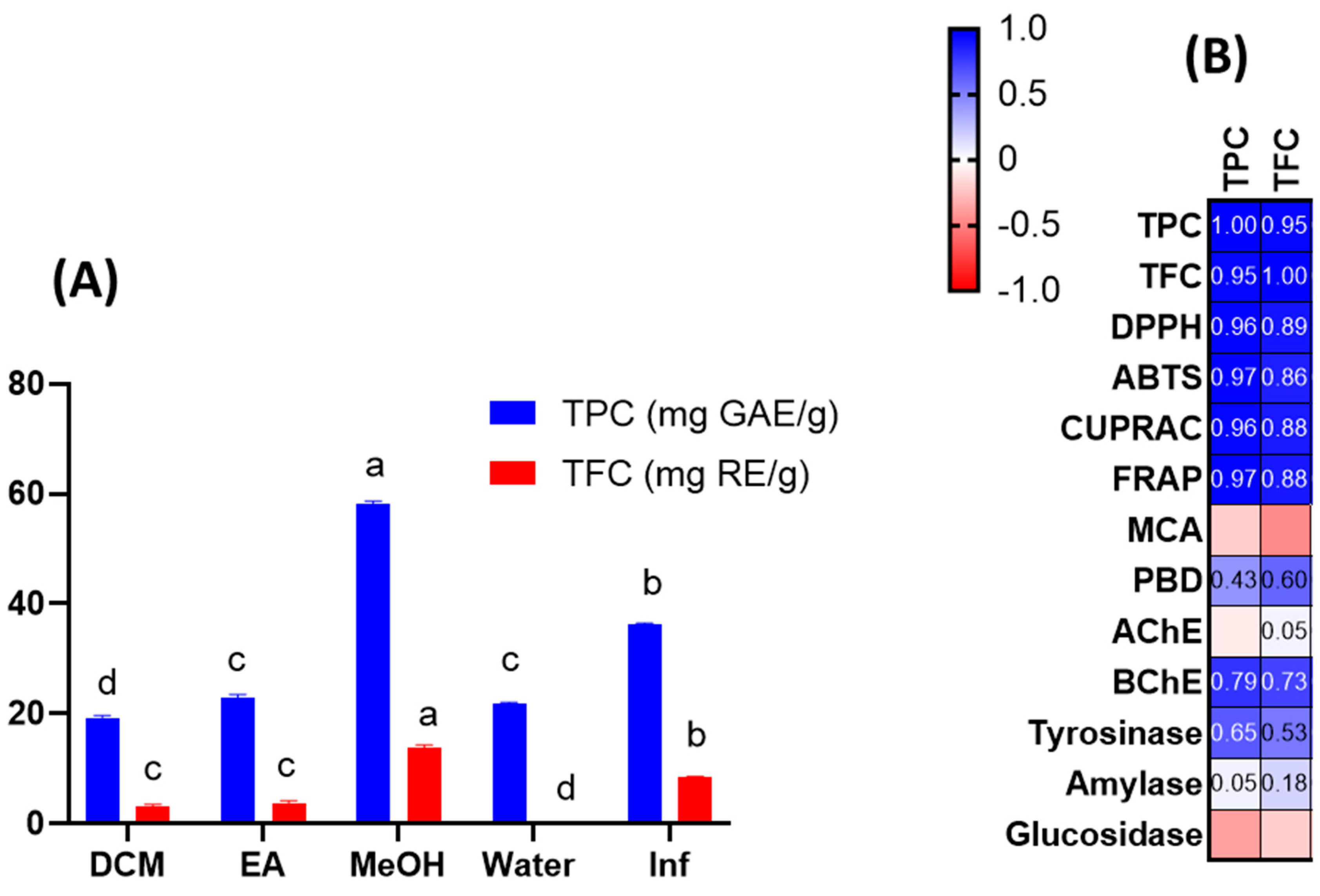
3.1. Total Phenolic and Flavonoid Contents
3.2. Chemical Characterization
3.3. Antioxidant Capacity
3.4. Enzyme Inhibitory Properties
3.5. Cytotoxicity Evaluation and Anticancer Selectivity
3.6. Antiviral Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.-F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health; American College of Cardiology Foundation: Washington, DC, USA, 2022; Volume 80, pp. 2361–2371. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Carrington, S.; Cohall, D.; Gossell-Williams, M.; Lindo, J. The antimicrobial screening of a Barbadian medicinal plant with indications for use in the treatment of diabetic wound infections. West Indian Med. J. 2012, 61, 861. [Google Scholar] [CrossRef] [PubMed]
- Lans, C.; Harper, T.; Georges, K.; Bridgewater, E. Medicinal and ethnoveterinary remedies of hunters in Trinidad. BMC Complement. Altern. Med. 2001, 1, 10. [Google Scholar] [CrossRef]
- Kitadi, J.M.; Mazasa, P.P.; Sha-Tshibey Tshibangu, D.; Kasali, F.M.; Tshilanda, D.D.; Ngbolua, K.-T.-N.; Mpiana, P.T. Ethnopharmacological survey and antisickling activity of plants used in the management of sickle cell disease in Kikwit city, DR Congo. Evid.-Based Complement. Altern. Med. 2020, 2020, 1346493. [Google Scholar] [CrossRef]
- Mpiana, P.T.; Ngbolua, K.N.N.; Bokota, M.T.; Kasonga, T.K.; Atibu, E.K.; Tshibangu, D.S.; Mudogo, V. In vitro effects of anthocyanin extracts from Justicia secunda Vahl on the solubility of haemoglobin S and membrane stability of sickle erythrocytes. Blood Transfus. 2010, 8, 248. [Google Scholar] [PubMed]
- Theiler, B.A.; Istvanits, S.; Zehl, M.; Marcourt, L.; Urban, E.; Caisa, L.O.E.; Glasl, S. HPTLC bioautography guided isolation of α-glucosidase inhibiting compounds from Justicia secunda Vahl (Acanthaceae). Phytochem. Anal. 2017, 28, 87–92. [Google Scholar] [CrossRef]
- Rojas, J.J.; Ochoa, V.J.; Ocampo, S.A.; Muñoz, J.F. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complement. Altern. Med. 2006, 6, 1–6. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Maciejewska-Turska, M.; Boguszewska, A.; Polz-Dacewicz, M.; Senkardes, I.; Guler, G.O.; Bibi Sadeer, N.; Mahomoodally, M.F. LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure. Int. J. Mol. Sci. 2021, 22, 7621. [Google Scholar] [CrossRef] [PubMed]
- Koffi, E.N.; Le Guernevé, C.; Lozano, P.R.; Meudec, E.; Adjé, F.A.; Bekro, Y.-A.; Lozano, Y.F. Polyphenol extraction and characterization of Justicia secunda Vahl leaves for traditional medicinal uses. Ind. Crops Prod. 2013, 49, 682–689. [Google Scholar] [CrossRef]
- Koffi, E.; Kassi, A.B.B.; Adje, F.A.; Lozano, Y.F.; Bekro, Y.-A. Effect of freeze-drying and spray-drying on total phenolics content and antioxidant activity from aqueous extract of Justicia secunda leaves. Trends Phytochem. Res. 2020, 4, 69–76. [Google Scholar]
- John, B.; Reddy, V.; Sulaiman, C. Total phenolics and flavonoids in selected Justicia species. J. Pharmacogn. Phytochem. 2013, 2, 72–73. [Google Scholar]
- Sepúlveda-Jiménez, G.; Reyna-Aquino, C.; Chaires-Martínez, L.; Bermúdez-Torres, K.; Rodríguez-Monroy, M. Antioxidant activity and content of phenolic compounds and flavonoids from Justicia spicigera. J. Biol. Sci. 2009, 9, 629–632. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Waseem, R.; Mahmood, N.; Hussain, Z.; Khan, Z.A.; Shahzad, S.A.; Yar, M.; Amin, R.; Bukhari, S.A.; Zahoor, A.F. Phenolic acid content, antioxidant properties, and antibacterial potential of flowers and fruits from selected Pakistani indigenous medicinal plants. Sci. Asia 2013, 39, 340–345. [Google Scholar] [CrossRef]
- Corrêa, G.M.; Alcântara, A.F.d.C. Chemical constituents and biological activities of species of Justicia: A review. Rev. Bras. Farmacogn. 2012, 22, 220–238. [Google Scholar] [CrossRef]
- e Silva, J.P.; Pereira, L.C.; Abreu, L.S.; Lins, F.S.; de Souza, T.A.; do Espírito-Santo, R.F.; Barros, R.P.; Villarreal, C.F.; de Melo, J.I.; Scotti, M.T. Targeted Isolation of Anti-inflammatory Lignans from Justicia aequilabris by Molecular Networking Approach. J. Nat. Prod. 2022, 85, 2184–2191. [Google Scholar] [CrossRef]
- Calderón, A.I.; Hodel, A.; Wolfender, J.-L.; Gupta, M.P.; Correa, M.; Hostettmann, K. LC–DAD–MS-based metabolite profiling of three species of Justicia (Acanthaceae). Nat. Prod. Res. 2013, 27, 1335–1342. [Google Scholar] [CrossRef]
- Theiler, B.A.; Revoltella, S.; Zehl, M.; Dangl, C.; Caisa, L.O.E.; König, J.; Winkler, J.; Urban, E.; Glasl, S. Secundarellone A, B, and C from the leaves of Justicia secunda Vahl. Phytochem. Lett. 2014, 10, cxxix–cxxxii. [Google Scholar] [CrossRef]
- Gonçalves-Filho, D.; De Souza, D. Detection of Synthetic Antioxidants: What Factors Affect the Efficiency in the Chromatographic Analysis and in the Electrochemical Analysis? Molecules 2022, 27, 7137. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Mazhar, M.S.; Quddus, S.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS profiling of phenolic compounds in Australian native plums and their potential antioxidant activities. Food Biosci. 2023, 52, 102331. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, D.; Liu, X.; Cheng, J.; Wang, X.; Guo, D.; Zou, J.; Yang, H. Phenolic profile and antioxidant activity of longan pulp of different cultivars from South China. LWT 2022, 165, 113698. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Y.; Li, C.; Yu, Q.; Xie, J.; Dong, R.; Xie, Y.; Li, B.; Tian, J.; Chen, Y. Natural variation on free, esterified, glycosylated and insoluble-bound phenolics of Rubus chingii Hu: Correlation between phenolic constituents and antioxidant activities. Food Res. Int. 2022, 162, 112043. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and in vitro and in vivo studies: A review. J. Food Biochem. 2022, 46, e13950. [Google Scholar] [CrossRef]
- Abramovič, H. Antioxidant properties of hydroxycinnamic acid derivatives: A focus on biochemistry, physicochemical parameters, reactive species, and biomolecular interactions. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 843–852. [Google Scholar]
- Anyasor, G.N.; Moses, N.; Kale, O. Hepatoprotective and hematological effects of Justicia secunda Vahl leaves on carbon tetrachloride induced toxicity in rats. Biotech. Histochem. 2020, 95, 349–359. [Google Scholar] [CrossRef]
- Onoja, S.O.; Ezeja, M.I.; Omeh, Y.N.; Onwukwe, B.C. Antioxidant, anti-inflammatory and antinociceptive activities of methanolic extract of Justicia secunda Vahl leaf. Alex. J. Med. 2017, 53, 207–213. [Google Scholar] [CrossRef]
- Locatelli, M.; Yerlikaya, S.; Baloglu, M.C.; Zengin, G.; Altunoglu, Y.C.; Cacciagrano, F.; Campestre, C.; Mahomoodally, M.F.; Mollica, A. Investigations into the therapeutic potential of Asphodeline liburnica roots: In vitro and in silico biochemical and toxicological perspectives. Food Chem. Toxicol. 2018, 120, 172–182. [Google Scholar] [CrossRef] [PubMed]
- YALÇIN, H.; KAVUNCUOGLU, H.; CAPAR, T.D. Total Phenolic Contents, Antioxidant Activities and Antioxidant Capacities of Some Selected Pepper (Capsicum annuum L.) Pulps and Seeds. Avrupa Bilim Teknol. Derg. 2021, 21, 581–586. [Google Scholar]
- Holdgate, G.A.; Meek, T.D.; Grimley, R.L. Mechanistic enzymology in drug discovery: A fresh perspective. Nat. Rev. Drug Discov. 2018, 17, 115–132. [Google Scholar] [CrossRef]
- Seetaloo, A.; Aumeeruddy, M.; Kannan, R.R.; Mahomoodally, M. Potential of traditionally consumed medicinal herbs, spices, and food plants to inhibit key digestive enzymes geared towards diabetes mellitus management—A systematic review. S. Afr. J. Bot. 2019, 120, 3–24. [Google Scholar] [CrossRef]
- Mishra, P.; Kumar, A.; Panda, G. Anti-cholinesterase hybrids as multi-target-directed ligands against Alzheimer’s disease (1998–2018). Bioorganic Med. Chem. 2019, 27, 895–930. [Google Scholar]
- Bashary, R.; Vyas, M.; Nayak, S.K.; Suttee, A.; Verma, S.; Narang, R.; Khatik, G.L. An insight of alpha-amylase inhibitors as a valuable tool in the management of type 2 diabetes mellitus. Curr. Diabetes Rev. 2020, 16, 117–136. [Google Scholar]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative Properties and Effect of Quercetin and Its Glycosylated Form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid.-Based Complement. Altern. Med. 2016, 21, Np11–Np17. [Google Scholar] [CrossRef]
- Li, N.; Yang, J.; Wang, C.; Wu, L.; Liu, Y. Screening bifunctional flavonoids of anti-cholinesterase and anti-glucosidase by in vitro and in silico studies: Quercetin, kaempferol and myricetin. Food Biosci. 2023, 51, 102312. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D. Anticholinesterase activity of selected phenolic acids and flavonoids-interaction testing in model solutions. Ann. Agric. Environ. Med. 2015, 22, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Rawa, M.S.A.; Hassan, Z.; Murugaiyah, V.; Nogawa, T.; Wahab, H.A. Anti-cholinesterase potential of diverse botanical families from Malaysia: Evaluation of crude extracts and fractions from liquid-liquid extraction and acid-base fractionation. J. Ethnopharmacol. 2019, 245, 112160. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, R. A survey of plants for presence of cholinesterase activity. Phytochemistry 1997, 46, 827–831. [Google Scholar] [CrossRef]
- Yuan, E.; Liu, B.; Wei, Q.; Yang, J.; Chen, L.; Li, Q. Structure activity relationships of flavonoids as potent α-amylase inhibitors. Nat. Prod. Commun. 2014, 9, 1934578X1400900829. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Ayeni, P.O.; Omojokun, O.S.; Bello, F. Comparative effect of quercetin and rutin on α-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidation in rat pancreas. Comp. Clin. Pathol. 2015, 24, 1103–1110. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Anigboro, A.A.; Avwioroko, O.J.; Ohwokevwo, O.A.; Pessu, B.; Tonukari, N.J. Phytochemical profile, antioxidant, α-amylase inhibition, binding interaction and docking studies of Justicia carnea bioactive compounds with α-amylase. Biophys. Chem. 2021, 269, 106529. [Google Scholar] [CrossRef]
- Ani, O.N.; Udedi, S.C.; Asogwa, K.K.; Enemali, M.O.; Onwelumadu, C.M.; Ikedife, K.S. Inhibitory Potential and Antidiabetic Activity of Leaf Extracts of Justicia carnea. Int. J. Biochem. Res. Rev. 2020, 29, 34–45. [Google Scholar] [CrossRef]
- Khadayat, K.; Marasini, B.P.; Gautam, H.; Ghaju, S.; Parajuli, N. Evaluation of the alpha-amylase inhibitory activity of Nepalese medicinal plants used in the treatment of diabetes mellitus. Clin. Phytosci. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Sari, S.; Milewski, R.; Supuran, C.T.; Şöhretoğlu, D.; Tomczyk, M. Flavonoids as tyrosinase inhibitors in in silico and in vitro models: Basic framework of SAR using a statistical modelling approach. J. Enzym. Inhib. Med. Chem. 2022, 37, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Angelis, A.; Antosiewicz, B.; Sakipova, Z.; Kozhanova, K.; Głowniak, K.; Kukula-Koch, W. Identification of mushroom and murine tyrosinase inhibitors from Achillea biebersteinii Afan. extract. Molecules 2021, 26, 964. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Ahmad, S.; Sherif, A.E.; Aati, H.Y.; Ovatlarnporn, C.; Khan, M.A.; Rao, H.; Ahmad, I.; Shahzad, M.N.; Ghalloo, B.A. New mechanistic insights on Justicia vahlii Roth: UPLC-Q-TOF-MS and GC–MS based metabolomics, in-vivo, in-silico toxicological, antioxidant based anti-inflammatory and enzyme inhibition evaluation. Arab. J. Chem. 2022, 15, 104135. [Google Scholar] [CrossRef]
- Ito, J.; Hara, K.; Someya, T.; Myoda, T.; Sagane, Y.; Watanabe, T.; Wijesekara, R.; Toeda, K.; Nojima, S. Data on the inhibitory effect of traditional plants from Sri Lanka against tyrosinase and collagenase. Data Brief 2018, 20, 573–576. [Google Scholar] [CrossRef]
- Łaska, G.; Sieniawska, E.; Świątek, Ł.; Zjawiony, J.; Khan, S.; Boguszewska, A.; Stocki, M.; Angielczyk, M.; Polz-Dacewicz, M. Phytochemistry and biological activities of Polemonium caeruleum L. Phytochem. Lett. 2019, 30, 314–323. [Google Scholar] [CrossRef]
- Onochie, A.U.; Oli, A.H.; Oli, A.N.; Ezeigwe, O.C.; Nwaka, A.C.; Okani, C.O.; Okam, P.C.; Ihekwereme, C.P.; Okoyeh, J.N. The pharmacobiochemical effects of ethanol extract of Justicia secunda vahl leaves in rattus norvegicus. J. Exp. Pharmacol. 2020, 12, 423. [Google Scholar] [CrossRef]
- Asano, J.; Chiba, K.; Tada, M.; Yoshii, T. Antiviral activity of lignans and their glycosides from Justicia procumbens. Phytochemistry 1996, 42, 713–717. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Wang, D.-Y.; Li, Y.-P.; Deyrup, S.T.; Zhang, H.-J. Plant-derived lignans as potential antiviral agents: A systematic review. Phytochem. Rev. 2022, 21, 239–289. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Rumschlag-Booms, E.; Guan, Y.-F.; Liu, K.-L.; Wang, D.-Y.; Li, W.-F.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H.; Rong, L. Anti-HIV diphyllin glycosides from Justicia gendarussa. Phytochemistry 2017, 136, 94–100. [Google Scholar] [CrossRef]
- Zhao, Y.; Yi Tsang, N.; Xu, X.Y.; Zhao, C.L.; Ku, C.F.; Li, W.F.; Zhu, Y.; Liu, K.L.; Rong, L.; Zhang, H.J. Axial Chirality and Antiviral Activity Evaluation of Arylnaphthalene Lignan Glycosides from Justicia procumbens. Asian J. Org. Chem. 2022, 11, e202200267. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Widiyanti, P.; Prajogo EW, B. In vitro anti-HIV activity of ethanol extract from gandarusa (Justicia gendarussa Burm. f) leaves. Infect. Dis. Rep. 2020, 12, 8730. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Chakraborty, A.; Biswas, A.; Chowdhuri, S. Identification of alkaloids from Justicia adhatoda as potent SARS-CoV-2 main protease inhibitors: An in silico perspective. J. Mol. Struct. 2021, 1229, 129489. [Google Scholar] [CrossRef] [PubMed]





| No. | Molecular Formula | Identification | Extracts |
|---|---|---|---|
| 1. | C5H10O6 | Carboxylic acid derivative | MeOH, H2O, INF |
| 2. | C5H8O6 | Carboxylic acid derivative | H2O, INF |
| 3. | C6H10O8 | Unknown | MeOH, INF |
| 4. | C12H15NO6 | Secundarellone B/C (racemate) | DCM |
| 5. | C6H8O7 | Citric acid | MeOH, INF |
| 6. | C12H15NO5 | Secundarellone A isomer 1 | DCM |
| 7. | C12H15NO5 | Secundarellone A isomer 2 | DCM, EA, MeOH, H2O, INF |
| 8. | C10H11NO6 | Unknown | INF |
| 9. | C22H12O5 | Lignan derivative | INF |
| 10. | C15H20O10 | Syringic acid glucoside | H2O, INF |
| 11. | C7H12O5 | Carboxylic acid derivative | H2O, INF |
| 12. | C13H16O8 | Salicylic acid glucoside | MeOH, INF |
| 13. | C13H16O9 | Dihydroxybenzoic acid O-glucoside (2,4-dihydroxybenzoic acid O-glucoside) | INF |
| 14. | C12H14O8 | Dihydroxybenzoic acid O-pentoside | MeOH, H2O, INF |
| 15. | C22H12O5 | Lignan derivative (10-(1,3-benzodioxol-5-yl)-5H-benzo[c]furo [3,2-g]chromen-5-one isomer) | INF |
| 16. | C13H16O9 | Unknown | MeOH, H2O, INF |
| 17. | C22H12O5 | Lignan derivative (10-(1,3-benzodioxol-5-yl)-5H-benzo[c]furo [3,2-g]chromen-5-one isomer) | INF |
| 18. | C15H18O9 | Caffeoyl glucoside | MeOH, INF |
| 19. | C18H28O9 | Hydroxyjasmonic acid glucoside | MeOH, INF |
| 20. | C19H30O8 | Roseoside | DCM, EA, MeOH, INF |
| 21. | C20H26O12 | Hydroxycinnamic acid O-pentoside-glucoside | MeOH, INF |
| 22. | C22H12O5 | Lignan derivative (10-(1,3-benzodioxol-5-yl)-5H-benzo[c]furo [3,2-g]chromen-5-one isomer) | INF |
| 23. | C17H22O13 | Trihydroxybenzoic acid O-dipentoside (Gallic acid O-dipentoside) | INF |
| 24. | C12H14O9 | Trihydroxybenzoic acid O-pentoside (Gallic acid O-pentoside) | MeOH, INF |
| 25. | C19H32O8 | Cyclohexanone derivative glucoside (Dihydroroseoside) | DCM, EA, MeOH, INF |
| 26. | C14H14O8 | Caffeic acid derivative | MeOH |
| 27. | C9H8O3 | Hydroxycinnamic acid | INF |
| 28. | C11H16O3 | Unknown | DCM, EA, MeOH, INF |
| 29. | C33H40O20 | Luteolin 7-O-[β-glucopyranosyl-(1→2)-β-rhamnosyl-(1→6)] β-glucopyranoside | EA, MeOH, INF |
| 30. | C33H40O19 | Trihydroxyflavone di-O-hexoside-O-rhamnoside (apigenin 7-O-glucoside-glucoside-rhamnoside) | MeOH, INF |
| 31. | C21H36O8 | Decalin derivative | DCM, EA, MeOH, INF |
| 32. | C34H42O20 | Trihydroxymethoxyflavone di-O-hexoside-O-rhamnoside (diosmetin 7-O-glucoside-rhamnoside-glucoside) | MeOH, INF |
| 33. | C27H30O15 | Tetrahydroxyflavone O-hexoside-O-rhamnoside (luteolin-7-O-rutinoside) | EA, MeOH, INF |
| 34. | C27H30O16 | Rutin | MeOH |
| 35. | C19H32O7 | Cyclohexanone derivative glucoside (9-Hydroxy-7-megastigmen-3-one glucoside) | DCM, EA, MeOH, INF |
| 36. | C26H34O10 | Lignan derivative | MeOH |
| 37. | C19H28O7 | Cyclohexanone derivative | DCM, EA, MeOH |
| 38. | C37H38O19 | Tetrahydroxyflavone O-hexoside-O-rhamnoside derivative (luteolin O-hydroxyferuloyl -O-rutinoside) | INF |
| 39. | C21H38O8 | Decalin glucoside derivative (Ophiopogonoside A isomer) | EA, MeOH, INF |
| 40. | C26H28O15 | Tetrahydroxyflavone O-pentoside-hexoside; (graveobioside A = luteolin 7-O-(2-apiosyl)glucoside) | EA, MeOH, INF |
| 41. | C13H15NO3 | Unknown | DCM |
| 42. | C25H26O14 | Luteolin 7-O-[β-apiofuranosyl-(1→2)]-β-xylopyranoside | EA, MeOH, INF |
| 43. | C19H34O10 | Cyclohexanone derivative glucoside | DCM, EA, MeOH, INF |
| 44. | C26H28O14 | Tetrahydroxyflavone O-pentoside-O-rhamnoside (luteolin O-pentoside-O-rhamnoside) | EA, MeOH, INF |
| 45. | C17H30O7 | Unknown | DCM, EA, MeOH, INF |
| 46. | C21H38O8 | Decalin glucoside derivative (Ophiopogonoside A isomer) | DCM, EA, MeOH, INF |
| 47. | C19H36O10 | Rhodiooctanoside | DCM, EA, MeOH, |
| 48. | C27H28O15 | Tetrahydroxyflavone O-pentoside-O-acetylpentoside (luteolin O- apiofuranosyl -O-acetylapiofuranosyl) | MeOH |
| 49. | C25H26O13 | Trihydroxyflavone di-O-pentoside (apigenin di-O-pentoside) | MeOH, INF |
| 50. | C26H28O14 | Trihydroxymethoxyflavone di-O-pentoside (diosmetin 7-O-apiofuranosyl-xylopyranoside) | EA, MeOH, INF |
| 51. | C27H30O14 | Trihydroxymethoxyflavone O-pentoside O-rhamnoside (diosmetin O-apiofuranosyl- O-rhamnoside) | EA, MeOH, H2O, INF |
| 52. | C28H30O15 | Trihydroxymethoxyflavone O-pentoside-O-acetylpentoside (diosmetin O- apiofuranosyl -O-acetylapiofuranosyl) | EA, MeOH |
| 53. | C15H10O6 | Luteolin | MeOH, INF |
| 54. | C31H28O14 | Trihydroxymethoxyflavone O-pentoside O-hydroxyferuloyl (diosmetin O-apiofuranosyl O-hydroxyferuloyl) | INF |
| 55. | C18H32O5 | Fatty acid | DCM, EA, MeOH, H2O, INF |
| 56. | C11H16O2 | Unknown | DCM, EA, MeOH |
| 57. | C18H34O5 | Fatty acid | DCM, EA, MeOH, H2O, INF |
| 58. | C16H12O6 | Trihydroxymethoxyflavone (diosmetin) | EA |
| Extracts | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | Phosphomolybdenum Assay (mmol TE/g) | MCA (mg EDTAE/g) |
|---|---|---|---|---|---|---|
| Dichloromethane | 5.20 ± 0.33 d | 29.53 ± 0.32 d | 65.07 ± 1.53 c | 17.68 ± 0.54 d | 1.23 ± 0.08 b | 10.20 ± 0.96 c |
| Ethyl acetate | 10.47 ± 0.91 c | 32.65 ± 0.25 d | 70.71 ± 0.77 c | 19.12 ± 0.15 d | 1.66 ± 0.04 a | 17.52 ± 0.38 b |
| Methanol | 92.32 ± 0.80 a | 227.90 ± 3.36 a | 375.36 ± 8.66 a | 173.92 ± 3.04 a | 1.63 ± 0.15 a | 17.38 ± 1.66 b |
| Water | 8.65 ± 0.90 c | 65.75 ± 0.89 c | 72.70 ± 0.23 c | 36.61 ± 1.04 c | 0.25 ± 0.01 c | 35.91 ± 0.11 a |
| Infusion | 20.75 ± 1.91 b | 83.37 ± 1.49 b | 108.52 ± 1.74 b | 57.24 ± 2.27 b | 1.13 ± 0.02 b | na |
| Extracts | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|
| Dichloromethane | 1.51 ± 0.03 a | na | 39.24 ± 1.66 b | 0.53 ± 0.01 b | 2.02 ± 0.10 a |
| Ethyl acetate | 1.63 ± 0.22 a | 0.45 ± 0.01 b | 24.31 ± 1.60 c | 0.58 ± 0.03 a | 2.12 ± 0.01 a |
| Methanol | 1.09 ± 0.08 b | 1.02 ± 0.09 a | 49.83 ± 2.04 a | 0.48 ± 0.03 b | 0.67 ± 0.02 b |
| Water | na | na | 40.92 ± 2.02 b | 0.06 ± 0.01 c | na |
| Infusion | na | na | 38.57 ± 0.99 b | 0.09 ± 0.01 c | 0.19 ± 0.02 c |
| Tested Extracts | VERO | FaDu | Detroit 562 | ||
|---|---|---|---|---|---|
| CC50 * | CC50 | SI ** | CC50 | SI | |
| Dichloromethane | 198.33 ± 11.42 | 70.18 ± 5.89 | 2.83 | 113.53 ± 7.77 | 1.75 |
| Ethyl acetate | 68.00 ± 4.80 | 61.18 ± 7.83 | 1.11 | 79.57 ± 7.34 | 0.85 |
| Methanol | 947.93 ± 40.10 | 556.90 ± 38.82 | 1.70 | 408.33 ± 26.65 | 2.32 |
| Water | >1000 | 81.69 ± 5.88 | >12.24 | 127.98 ± 13.75 | >7.81 |
| Infusion | >1000 | 70.67 ± 7.95 | >14.15 | 96.81 ± 13.06 | >10.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Zengin, G.; Boguszewska, A.; Hryć, B.; Bene, K.; Polz-Dacewicz, M.; Dall’Acqua, S. Chemical Characterization of Different Extracts of Justicia secunda Vahl and Determination of Their Anti-Oxidant, Anti-Enzymatic, Anti-Viral, and Cytotoxic Properties. Antioxidants 2023, 12, 509. https://doi.org/10.3390/antiox12020509
Świątek Ł, Sieniawska E, Sinan KI, Zengin G, Boguszewska A, Hryć B, Bene K, Polz-Dacewicz M, Dall’Acqua S. Chemical Characterization of Different Extracts of Justicia secunda Vahl and Determination of Their Anti-Oxidant, Anti-Enzymatic, Anti-Viral, and Cytotoxic Properties. Antioxidants. 2023; 12(2):509. https://doi.org/10.3390/antiox12020509
Chicago/Turabian StyleŚwiątek, Łukasz, Elwira Sieniawska, Kouadio Ibrahime Sinan, Gokhan Zengin, Anastazja Boguszewska, Benita Hryć, Kouadio Bene, Małgorzata Polz-Dacewicz, and Stefano Dall’Acqua. 2023. "Chemical Characterization of Different Extracts of Justicia secunda Vahl and Determination of Their Anti-Oxidant, Anti-Enzymatic, Anti-Viral, and Cytotoxic Properties" Antioxidants 12, no. 2: 509. https://doi.org/10.3390/antiox12020509
APA StyleŚwiątek, Ł., Sieniawska, E., Sinan, K. I., Zengin, G., Boguszewska, A., Hryć, B., Bene, K., Polz-Dacewicz, M., & Dall’Acqua, S. (2023). Chemical Characterization of Different Extracts of Justicia secunda Vahl and Determination of Their Anti-Oxidant, Anti-Enzymatic, Anti-Viral, and Cytotoxic Properties. Antioxidants, 12(2), 509. https://doi.org/10.3390/antiox12020509












