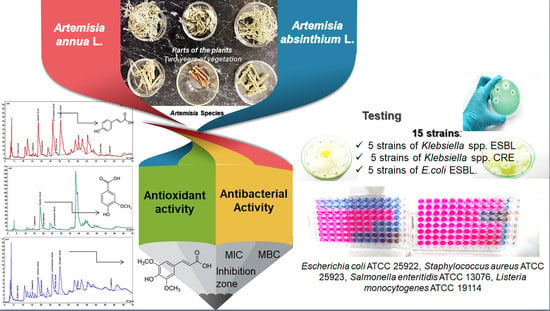
Antibacterial and Phytochemical Screening of Artemisia Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Herb Samples and Ethanolic Extraction
2.2. Reagents
2.3. Total Phenolic Content (TPC)
2.4. Assessment of the Antioxidant Activity
2.5. Quantitative Determination of Phenolic Compounds
2.6. Antibacterial Activity
2.6.1. Determination of the Minimum Inhibitory Concentration (MIC)
2.6.2. Assessment of the Minimum Bactericidal Concentration (MBC)
2.7. Bacterial Samples
2.8. Statistical Analysis
3. Results
3.1. Total Phenolic Content of Different Tissues of Artemisia Extracts
3.2. Antioxidant Activity
3.3. Phenolic Compound Profile by HPLC
3.4. Antibacterial Activity of Different Tissues of Artemisia
4. Discussion
4.1. Phytochemical Characteristics of Extracts
4.2. Antibacterial Effects
4.3. Limits of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z. Artemisinin anti-malarial drugs in China. Acta Pharm. Sin. B 2016, 6, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Babacana, Ü.; Fatih, M.; Mariem, C.; Timur, B.; Songül, T.; Mutluc, S.; Gülmez, E. Determination, solvent extraction, and purification of artemisinin from Artemisia annua L. J. Appl. Res. Med. Aromat. Plants 2022, 28, 100363. [Google Scholar] [CrossRef]
- Klayman, D.L. Artemisia annua. In Human Medicinal Agents from Plants; ACS Symposium Series; ACS Publications: Washington, DC, USA, 1993; Volume 534, pp. 242–255. [Google Scholar] [CrossRef]
- Liu, C.-X. Discovery and Development of Artemisinin and Related Compounds. Chin. Herb. Med. 2017, 9, 101–114. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.J.; Chia, W.N.; Loh, C.C.; Li, Z.; Lee, Y.M.; He, Y.; Yuan, L.X.; Lim, T.K.; Liu, M.; et al. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat. Commun. 2015, 6, 10111. [Google Scholar] [CrossRef]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef]
- Alesaeidi, S.; Miraj, S. A Systematic Review of Anti-malarial Properties, Immunosuppressive Properties, Anti-inflammatory Properties, and Anti-cancer Properties of Artemisia Annua. Electron. Physician 2016, 8, 3150–3155. [Google Scholar] [CrossRef]
- Moacă, E.A.; Pavel, I.Z.; Danciu, C.; Crainiceanu, Z.; Minda, D.; Ardelean, F.; Antal, D.S.; Ghiulai, R.; Cioca, A.; Derban, M.; et al. Romanian Wormwood (Artemisia absinthium L.): Physicochemical and Nutraceutical Screening. Molecules 2019, 24, 3087. [Google Scholar] [CrossRef]
- Ivanov, M.; Gasic, U.; Stojkovic, D.; Kostic, M.; Misic, D.; Sokovic, M. New Evidence for Artemisia absinthium L. Application in Gastrointestinal Ailments: Ethnopharmacology, Antimicrobial Capacity, Cytotoxicity, and Phenolic Profile. Evid. Based Complement. Med. 2021, 2021, 9961089. [Google Scholar] [CrossRef]
- Ahamad, J.; Mir, S.R.; Amin, S. A Pharmacognostic Review on Artemisia Absinthium. Int. Res. J. Pharm. 2019, 10, 25–31. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Ebrahimzadeh, M.A.; Ansaroudi, F.; Nabavi, S.F.; Nabavi, S.M. Antidepressant and antioxidant activities of Artemisia absinthium L. at flowering stage. Afr. J. Biotechnol. 2009, 8, 7170–7175. [Google Scholar]
- Batiha, G.E.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Zahoor, M.; Magdy Beshbishy, A.; Murata, T.; Zaragoza-Bastida, A.; et al. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef]
- Mamatova, A.S.; Korona-Glowniak, I.; Skalicka-Wozniak, K.; Jozefczyk, A.; Wojtanowski, K.K.; Baj, T.; Sakipova, Z.B.; Malm, A. Phytochemical composition of wormwood (Artemisia gmelinii) extracts in respect of their antimicrobial activity. BMC Complement. Altern. Med. 2019, 19, 288. [Google Scholar] [CrossRef] [PubMed]
- Minda, D.; Ghiulai, R.; Banciu, C.D.; Pavel, I.Z.; Danciu, C.; Racoviceanu, R.; Soica, C.; Budu, O.D.; Muntean, D.; Diaconeasa, Z.; et al. Phytochemical Profile, Antioxidant and Wound Healing Potential of Three Artemisia Species: In Vitro and In Ovo Evaluation. Appl. Sci. 2022, 12, 1359. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Ungur, R.A.; Borda, I.M.; Codea, R.A.; Ciortea, V.M.; Nasui, B.A.; Muste, S.; Sarpataky, O.; Filip, M.; Irsay, L.; Craciun, E.C.; et al. A Flavonoid-Rich Extract of Sambucus nigra L. Reduced Lipid Peroxidation in a Rat Experimental Model of Gentamicin Nephrotoxicity. Materials 2022, 15, 772. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Mavi, H.; Kilic, H.; Yildirm, A. Screening of Chemical Composition and Antifungal and Antioxidant Activities of the Essential Oils from Three Turkish Artemisia Species. J. Agric. Food Chem. 2005, 53, 1408–1416. [Google Scholar] [CrossRef]
- Stanojevic, L.; Stankovic, M.; Nikolic, V.; Nikolic, L.; Ristic, D.; Canadanovic-Brunet, J.; Tumbas, V. Antioxidant Activity and Total Phenolic and Flavonoid Contents of Hieracium pilosella L. Extracts. Sensors 2009, 9, 5702–5714. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. Evaluation of antioxidant and free-radical scavenging potential of Artemisia absinthium. Pharm. Biol. 2011, 49, 1216–1223. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H. Light-induced fluctuations in biomass accumulation, secondary metabolites production and antioxidant activity in cell suspension cultures of Artemisia absinthium L. J. Photochem. Photobiol. B 2014, 140, 223–227. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H.; Ihsan ul, H. Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Ind. Crops Prod. 2013, 49, 400–406. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Wormwood (Artemisia absinthium L.)—A curious plant with both neurotoxic and neuroprotective properties? J. Ethnopharmacol. 2010, 131, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.J.; Anand, U.; Altemimi, A.B.; Tripathi, V.; Guo, Y.; Pratap-Singh, A. Phenolic Composition, Antioxidant Capacity and Antibacterial Activity of White Wormwood (Artemisia herba-alba). Plants 2021, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Hoseinian, A.; Moslemi, H.R.; Sedaghat, R. Antioxidant properties of Artemisia absinthium accelerate healing of experimental Achilles tendon injury in rabbits. Herba Pol. 2018, 64, 36–43. [Google Scholar] [CrossRef]
- Amat, N.; Upur, H.; Blazekovic, B. In vivo hepatoprotective activity of the aqueous extract of Artemisia absinthium L. against chemically and immunologically induced liver injuries in mice. J. Ethnopharmacol. 2010, 131, 478–484. [Google Scholar] [CrossRef]
- Hatziieremia, S.; Gray, A.I.; Ferro, V.A.; Paul, A.; Plevin, R. The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFkappaB signalling pathways in monocytes/macrophages. Br. J. Pharmacol. 2006, 149, 188–198. [Google Scholar] [CrossRef]
- Farzaneh, F.; Ebrahim, H.S.; Akbar, V. Investigating on Effect of Wormwood Extract on Reduction of Renal Toxicity in Treated Rats by Azathioprine. Biomed. Pharmacol. J. 2015, 8, 291–299. [Google Scholar] [CrossRef]
- Kim, M.H.; Seo, J.Y.; Liu, K.H.; Kim, J.S. Protective effect of Artemisia annua L. extract against galactose-induced oxidative stress in mice. PLoS ONE 2014, 9, e101486. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef]
- Moslemi, H.R.; Hoseinzadeh, H.; Badouei, M.A.; Kafshdouzan, K.; Fard, R.M. Antimicrobial Activity of Artemisia absinthium Against Surgical Wounds Infected by Staphylococcus aureus in a Rat Model. Indian J. Microbiol. 2012, 52, 601–604. [Google Scholar] [CrossRef]
- Khan, M.F.; Tang, H.; Lyles, J.T.; Pineau, R.; Mashwani, Z.U.; Quave, C.L. Antibacterial Properties of Medicinal Plants From Pakistan Against Multidrug-Resistant ESKAPE Pathogens. Front. Pharm. 2018, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Marțiș, G.S.; Muresan, V.; Marc Vlaic, R.M.; Muresan, C.C.; Pop, C.R.; Buzgau, G.; Muresan, A.E.; Ungur, R.A.; Muste, S. The Physicochemical and Antioxidant Properties of Sambucus nigra L. and Sambucus nigra Haschberg during Growth Phases: From Buds to Ripening. Antioxidants 2021, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Hatamia, T.; Emamic, S.A.; Miraghaeea, S.S.; Mojarraba, M. Total Phenolic Contents and Antioxidant Activities of Different Extracts and Fractions from the Aerial Parts of Artemisia biennis Willd. Iran. J. Pharm. Res. 2014, 12, 551–558. [Google Scholar]
- Szydlowska-Czerniak, A.; Trokowski, K.; Karlovits, G.; Szlyk, E. Determination of antioxidant capacity, phenolic acids, and fatty acid composition of rapeseed varieties. J. Agric. Food Chem. 2010, 58, 7502–7509. [Google Scholar] [CrossRef] [PubMed]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O.; Mawson, A.J.; He, R.; Girgih, A.; Aluko, R.E. Antioxidant properties of Australian canola meal protein hydrolysates. Food Chem. 2014, 146, 500–506. [Google Scholar] [CrossRef]
- Filip, M.; Silaghi-Dumitrescu, L.; Prodan, D.; Sarosi, C.; Moldovan, M.; Cojocaru, I. Analytical Approaches for Characterization of Teeth Whitening Gels Based on Natural Extracts. Key Eng. Mater. 2017, 752, 24–28. [Google Scholar] [CrossRef]
- Camorlinga-Ponce, M.; Gomez-Delgado, A.; Aguilar-Zamora, E.; Torres, R.C.; Giono-Cerezo, S.; Escobar-Ogaz, A.; Torres, J. Phenotypic and Genotypic Antibiotic Resistance Patterns in Helicobacter pylori Strains From Ethnically Diverse Population in Mexico. Front. Cell Infect. Microbiol. 2020, 10, 539115. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef]
- Cazella, L.N.; Glamoclija, J.; Sokovic, M.; Goncalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial Activity of Essential Oil of Baccharis dracunculifolia DC (Asteraceae) Aerial Parts at Flowering Period. Front. Plant Sci. 2019, 10, 27. [Google Scholar] [CrossRef]
- Lee, Y.J.; Thiruvengadam, M.; Chung, I.M.; Praveen, N. Polyphenol composition and antioxidant activity from the vegetable plant Artemisia absinthium L. Aust. J. Crop Sci. 2013, 7, 1921–1926. [Google Scholar]
- Sembiring, B.; Gusmaini; Nurhayati, H.; Kurniasari, I. Antioxidant activity of Artemisia (Artemisia annua) extract on several concentrations and solvents. IOP Conf. Ser. Earth Environ. Sci. 2022, 974, 012119. [Google Scholar] [CrossRef]
- Bhat, K.M.; Gul, M.Z.; Lohamror, L.R.; Qureshi, I.A.; Ghazi, I.A. An in vitro Study of the Antioxidant and Antiproliferative Properties of Artemisia absinthium—A Potent Medicinal Plant. Free. Radic. Antioxid. 2018, 8, 18–25. [Google Scholar] [CrossRef]
- Sengul, M.; Ercisli, S.; Erzurum, T.; Yildizb, H.; Gungorc, N.; Kavaza, A.; Çetina, B. Antioxidant, Antimicrobial Activity and Total Phenolic Content within the Aerial Parts of Artemisia absinthum, Artemisia santonicum and Saponaria officinalis. Iran. J. Pharm. Res. 2011, 10, 49–56. [Google Scholar] [PubMed]
- Briars, R.; Paniwnyk, L. Effect of ultrasound on the extraction of artemisinin from Artemisia annua. Ind. Crops Prod. 2013, 42, 595–600. [Google Scholar] [CrossRef]
- Craciunescu, O.; Constantin, D.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chem. Cent. J. 2012, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Baiceanu, E.; Vlase, L.; Baiceanu, A.; Nanes, M.; Rusu, D.; Crisan, G. New Polyphenols Identified in Artemisiae abrotani herba Extract. Molecules 2015, 20, 11063–11075. [Google Scholar] [CrossRef] [PubMed]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant Properties of Ferulic Acid and Its Related Compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Thiyam, U.; Stöckmann, H.; Zum Felde, T.; Schwarz, K. Antioxidative effect of the main sinapic acid derivatives from rapeseed and mustard oil by-products. Eur. J. Lipid Sci. Technol. 2006, 108, 239–248. [Google Scholar] [CrossRef]
- Zou, Y.; Him, A.R.; Kim, J.E.; Choi, J.S.; Chung, H.Y. Peroxynitrite Scavenging Activity of Sinapic Acid (3,5-Dimethoxy-4-hydroxycinnamic Acid) Isolated from Brassica juncea. J. Agric. Food Chem. 2002, 50, 5884–5890. [Google Scholar] [CrossRef]
- Yun, K.J.; Koh, D.J.; Kim, S.H.; Park, S.J.; Ryu, J.H.; Kim, D.G.; Lee, J.Y.; Lee, K.T. Anti-Inflammatory Effects of Sinapic Acid through the Suppression of Inducible Nitric Oxide Synthase, Cyclooxygase-2, and Proinflammatory Cytokines Expressions via Nuclear Factor-KB Inactivation. J. Agric. Food Chem. 2008, 56, 10265–10272. [Google Scholar] [CrossRef]
- Silambarasan, T.; Manivannan, J.; Krishna Priya, M.; Suganya, N.; Chatterjee, S.; Raja, B. Sinapic acid prevents hypertension and cardiovascular remodeling in pharmacological model of nitric oxide inhibited rats. PLoS ONE 2014, 9, e115682. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Jung, J.W.; Lee, J.J.; Cho, Y.W.; Jang, C.G.; Jin, C.; Oh, T.H.; Ryu, J.H. Anxiolytic-like effects of sinapic acid in mice. Life Sci. 2007, 81, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxid. Med. Cell Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Huttemann, M. Molecular Mechanisms and Therapeutic Effects of (-)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Escandón, R.A.; del Campo, M.; López-Solis, R.; Obreque-Slier, E.; Toledo, H. Antibacterial effect of kaempferol and (−)-epicatechin on Helicobacter pylori. Eur. Food Res. Technol. 2016, 242, 1495–1502. [Google Scholar] [CrossRef]
- Bajpai, B.; Patil, S. A new approach to microbial production of gallic acid. Braz. J. Microbiol. 2008, 39, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Mourtzinos, I.; Konteles, S.; Kalogeropoulos, N.; Karathanos, V.T. Thermal oxidation of vanillin affects its antioxidant and antimicrobial properties. Food Chem. 2009, 114, 791–797. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Marimuthu, S.; Adluri, R.S.; Venugopal, P.M. Ferulic Acid: Therapeutic Potential Through Its Antioxidant Property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Zdunska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharm. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Su, K.Y.; Yu, C.Y.; Chen, Y.W.; Huang, Y.T.; Chen, C.T.; Wu, H.F.; Chen, Y.L. Rutin, a flavonoid and principal component of saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. Int. J. Med. Sci. 2014, 11, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Patel, D.K. The Beneficial Role of Rutin, A Naturally Occurring Flavonoid in Health Promotion and Disease Prevention: A Systematic Review and Update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Academic Press: Cambridge, MA, USA, 2019; pp. 457–479. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Cruz-Zuniga, J.M.; Soto-Valdez, H.; Peralta, E.; Mendoza-Wilson, A.M.; Robles-Burgueno, M.R.; Auras, R.; Gamez-Meza, N. Development of an antioxidant biomaterial by promoting the deglycosylation of rutin to isoquercetin and quercetin. Food Chem. 2016, 204, 420–426. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Keman, D.; Soyer, F. Antibiotic-Resistant Staphylococcus aureus Does Not Develop Resistance to Vanillic Acid and 2-Hydroxycinnamic Acid after Continuous Exposure in Vitro. ACS Omega 2019, 4, 15393–15400. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Al Sane, K.; Ben Ammar, W.; Azeiz, S.; Haj Brahim, A.; et al. Chemical Composition and Antioxidant and Antimicrobial Activities of Wormwood (Artemisia absinthium L.) Essential Oils and Phenolics. J. Chem. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Riahi, L.; Chograni, H.; Elferchichi, M.; Zaouali, Y.; Zoghlami, N.; Mliki, A. Variations in Tunisian wormwood essential oil profiles and phenolic contents between leaves and flowers and their effects on antioxidant activities. Ind. Crops Prod. 2013, 46, 290–296. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Radácsi, P.; Gosztola, B.; Németh, É.Z. Effects of temperature and light intensity on morphological and phytochemical characters and antioxidant potential of wormwood (Artemisia absinthium L.). Biochem. Syst. Ecol. 2018, 79, 1–7. [Google Scholar] [CrossRef]
- Lommen, W.J.; Schenk, E.; Bouwmeester, H.J.; Verstappen, F.W. Trichome dynamics and artemisinin accumulation during development and senescence of Artemisia annua leaves. Planta Med. 2006, 72, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.F.; Martinez, J.M.; Lizama, R.S.; Vermeersch, M.; Cos, P.; Maes, L. In vitro anti-microbial activity of the Cuban medicinal plants Simarouba glauca DC, Melaleuca leucadendron L and Artemisia absinthium L. Mem. Inst. Oswaldo. Cruz. 2008, 103, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Erel, Ş.B.; Reznicek, G.; Șenol, S.G.; Yavașogulu, N.Ü.K.; Konyalioglu, S.; Zeybek, A.U. Antimicrobial and antioxidant properties of Artemisia L. species from western Anatolia. Turk. J. Biol. 2012, 36, 75–84. [Google Scholar] [CrossRef]
- Mihajilov-Krstev, T.; Jovanovic, B.; Jovic, J.; Ilic, B.; Miladinovic, D.; Matejic, J.; Rajkovic, J.; Dordevic, L.; Cvetkovic, V.; Zlatkovic, B. Antimicrobial, antioxidative, and insect repellent effects of Artemisia absinthium essential oil. Planta Med. 2014, 80, 1698–1705. [Google Scholar] [CrossRef]
- Sultan, M.H.; Zuwaiel, A.A.; Moni, S.S.; Alshahrani, S.; Alqahtani, S.S.; Madkhali, O.; Elmobark, M.E. Bioactive Principles and Potentiality of Hot Methanolic Extract of the Leaves from Artemisia absinthium L “in vitro Cytotoxicity Against Human MCF-7 Breast Cancer Cells, Antibacterial Study and Wound Healing Activity”. Curr. Pharm. Biotechnol. 2020, 21, 1711–1721. [Google Scholar] [CrossRef]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef]
- Joshi, R.K. Volatile composition and antimicrobial activity of the essential oil of Artemisia absinthium growing in Western Ghats region of North West Karnataka, India. Pharm. Biol. 2013, 51, 888–892. [Google Scholar] [CrossRef]
- Jouteau, S.; Cornuéjols, A.; Sebag, M.; Tarroux, P.; Liénard, J.S. Nouveaux résultats en classification à l’aide d’un codage par motifs fréquents. Rev. D’intell. Artif. Lavoisier 2003, 17, 521–532. [Google Scholar]
- Ryu, J.-H.; Kim, R.-J.; Lee, S.-J.; Kim, I.-S.; Lee, H.-J.; Sung, N.-J. Nutritional Properties and Biological Activities of Artemisia annua L. J. Korean Soc. Food Sci. Nutr. 2011, 40, 163–170. [Google Scholar] [CrossRef][Green Version]
- Qian, W.; Yang, M.; Wang, T.; Sun, Z.; Liu, M.; Zhang, J.; Zeng, Q.; Cai, C.; Li, Y. Antibacterial Mechanism of Vanillic Acid on Physiological, Morphological, and Biofilm Properties of Carbapenem-Resistant Enterobacter hormaechei. J. Food Prot. 2020, 83, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Sethupathy, S.; Ananthi, S.; Selvaraj, A.; Shanmuganathan, B.; Vigneshwari, L.; Balamurugan, K.; Mahalingam, S.; Pandian, S.K. Vanillic acid from Actinidia deliciosa impedes virulence in Serratia marcescens by affecting S-layer, flagellin and fatty acid biosynthesis proteins. Sci. Rep. 2017, 7, 16328. [Google Scholar] [CrossRef] [PubMed]
- Yemis, G.P.; Pagotto, F.; Bach, S.; Delaquis, P. Effect of vanillin, ethyl vanillin, and vanillic acid on the growth and heat resistance of Cronobacter species. J. Food Prot. 2011, 74, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Huang, C.Y. Inhibition of Klebsiella pneumoniae DnaB helicase by the flavonol galangin. Protein J. 2011, 30, 59–65. [Google Scholar] [CrossRef]
- Niciforovic, N.; Abramovic, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Barber, M.S.; McConnell, V.S.; DeCaux, B.S. Antimicrobial intermediates of the general phenylpropanoid and lignin specifc pathways. Phytochemistry 2000, 54, 53–56. [Google Scholar] [CrossRef]
- Singh, K.; Coopoosamy, R.M.; Gumede, N.J.; Sabiu, S. Computational Insights and In Vitro Validation of Antibacterial Potential of Shikimate Pathway-Derived Phenolic Acids as NorA Efflux Pump Inhibitors. Molecules 2022, 27, 2601. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol 2020, 94, 651–715. [Google Scholar] [CrossRef]
| Botanical Family | Herb | Year of Vegetation | Sample | TPC [mg GAE/mL Extract] | TPC [mg GAE/100 g DW] | DPPH [µM Trolox/g DW] | ABTS [µM Trolox/g DW] |
|---|---|---|---|---|---|---|---|
| Asteraceae | Artemisia annua L. leaf | I | AnL | 518.09 ± 0.01 a | 2089.07 ± 0.03 b | 250.51 ± 0.01 b | 816.55 ± 0.05 d |
| Artemisia annua L. stem | I | AnS | 135.34 ± 0.08 d | 390.77 ± 0.03 c | 60.87 ± 0.02 e | 659.57 ± 0.02 e | |
| Artemisia absinthium L. leaf | II | AbL2 | 487.36 ± 0.08 b | 3778.512 ± 0.02 a | 735.77 ± 0.02 a | 1360.51 ± 0.04 a | |
| Artemisia absinthium L. stem | II | AbS2 | 60.59 ± 0.20 e | 299.21 ± 0.02 e | 129.49 ± 0.01 c | 1118.12 ± 0.02 c | |
| Artemisia absinthium L. leaf | I | AbL1 | 229.68 ± 0.16 c | 323.66 ± 0.02 d | 57.09 ± 0.01 e | 253.39 ± 0.01 f | |
| Artemisia absinthium L. stem | I | AbS1 | 51.73 ± 0.11 f | 293.51 ± 0.01 e | 110.77 ± 0.03 d | 1314.38 ± 0.01 b | |
| Sig. | *** | *** | *** | *** |
| Correlation between | R | p | N | |
|---|---|---|---|---|
| TPC total samples | DPPH total samples | 0.959 | 0.0001 *** | 18 |
| TPC total samples | ABTS total samples | 0.421 | 0.082 ns | 18 |
| TPC leaf samples | DPPH leaf samples | 0.967 | 0.0001 *** | 9 |
| TPC stem samples | DPPH stem samples | −0.949 | 0.0001 *** | 9 |
| Sample | Gallic Acid | Catechin | Vanillic Acid | Caffeic Acid | Epicatechin | p-Coumaric Acid | Ferulic Acid | Sinapic Acid | Rutin | Quercetin | Luteolin | Sig. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AnL | 1.132 ± 0.001 a I | 16.603 ± 0.001 a E | 46.863 ± 0.002 b C | nd | 4.957 ± 0.002 d F | 51.267 ± 0.002 a B | 1.575 ± 0.002 a G | 285.694 ± 0.002 a A | 17.320 ± 0.000 a D | 1.653 ± 0.003 b G | 1.218 ± 0.002 a H | *** |
| AnS | 0.086 ± 0.004 b H | 8.003 ± 0.003 b B | 6.502 ± 0.003 e C | nd | 1.914 ± 0.001 e D | 6.549 ± 0.001 b C | 0.669 ± 0.001 b F | 44.155 ± 0.001 b A | 0.519 ± 0.001 b G | nd | 0.703 ±0.001 c E | *** |
| AbL2 | nd | 1.275 ± 0.001 d C | 66.777 ± 0.002 a A | nd | 10.136 ± 0.002 c B | 1.375 ± 0.002 d C | nd | nd | nd | nd | nd | *** |
| AbS2 | nd | nd | 11.913 ± 0.002 d B | nd | 21.123 ± 0.001 a A | 0.248 ± 0.001 e D | 0.145 ± 0.002 e E | 0.267 ± 0.002 c D | nd | nd | 1.035 ± 0.002 b C | *** |
| AbL1 | nd | 1.262 ± 0.002 d D | 42.241 ± 0.001 c A | nd | 13.488 ± 0.001 b B | 2.565 ± 0.002 c C | 0.227 ± 0.002 d E | nd | nd | nd | nd | *** |
| AbS1 | nd | 2.438 ± 0.002 c B | 2.420 ± 0.002 f B | nd | 1.600 ± 0.002 e C | 0.359 ± 0.002 e E | 0.121 ± 0.002 e F | nd | nd | 8.492 ± 0.002 a A | 1.046 ± 0.002 b D | *** |
| Sig. | *** | ** | *** | *** | *** | ** | *** | *** | *** | ** |
| Sample | Staphylococcus aureus ATCC 25923 | Escherichia coli ATCC 25922 | Listeria monocytogenes ATCC 19114 | Salmonella enteritidis ATCC 13076 | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| AnL | 2.00 ± 0.014 f | 5.00 ± 0.014 e | 5.00 ± 0.014 e | 12.00 ± 0.014 f | 5.00 ± 0.014 e | 5.00 ± 0.014 f | 5.00 ± 0.014 e | 12.00 ± 0.014 c |
| AnS | 54.00 ± 0.002 d | 114.00 ± 0.014 c | 54.00 ± 0.014 d | 114.00 ± 0.014 d | 114.00 ± 0.014 c | 114.00 ± 0.014 d | 54.00 ± 0.002 d | 241.00 ± 0.014 a |
| AbL2 | 25.00 ± 0.002 e | 54.00 ± 0.014 d | 54.00 ± 0.014 d | 54.00 ± 0.002 e | 54.00 ± 0.002 d | 54.00 ± 0.002 e | 54.00 ± 0.002 d | 54.00 ± 0.002 b |
| AbS2 | 114.00 ± 0.014 a | 114.00 ± 0.014 c | 240.00 ± 0.014 c | 240.00 ± 0.014 c | 114.00 ± 0.014 c | 240.00 ± 0.014 b | 240.00 ± 0.014 c | - |
| AbL1 | 89.50 ± 0.028 b | 255.00 ± 0.014 b | 255.00 ± 0.014 b | 255.00 ± 0.014 b | 121.00 ± 0.014 b | 121.00 ± 0.014 c | 255.00 ± 0.014 b | - |
| AbS1 | 85.00 ± 0.002 c | 375.00 ± 0.014 a | 375.00 ± 0.014 a | 375.00 ± 0.014 a | 178.00 ± 0.014 a | 375.00 ± 0.014 a | 375.00 ± 0.014 a | - |
| Gentamicin | 0.0005 | 0.00024 | 0.00152 | 0.00024 | ||||
| Sign. | *** | *** | *** | *** | *** | *** | *** | ** |
| Sample | Klebsiella spp. ESBL1 | Klebsiella spp. ESBL 2 | Klebsiella spp. ESBL 3 | Klebsiella spp. ESBL 4 | Klebsiella spp. ESBL 5 | Klebsiella spp. CRE 1 | Klebsiella spp. CRE 2 | Klebsiella spp. CRE 3 | Klebsiella spp. CRE 4 | Klebsiella spp. CRE 5 | E. coli spp. ESBL 1 | E. coli spp. ESBL 2 | E. coli spp. ESBL 3 | E. coli spp. ESBL 4 | E. coli spp. ESBL 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AnL | 10.863 ± 0.308 a | 5.473 ± 0.756 a | 8.866 ± 2.0304 ab | 6.846 ± 0.170 b | 5.840 ± 0.215 bc | 12.756 ± 0.993 a | 11.120 ± 3.187 a | 7.210 ± 0.441 a | 7.210 ± 0.891 a | 6.876 ± 0.732 a | 5.117 ± 1.366 a | 8.610 ± 1.861 a | 4.753 ± 1.467 a | 7.946 ± 1.788 a | 8.130 ± 1.731 a |
| AnS | 2.523 ± 0.606 c | 1.920 ± 0.13 ef | 3.593 ± 0.472 cd | 5.106 ± 0.783 bc | 4.993 ± 0.248 c | 9.843 ± 0.945 b | 7.180 ± 0.669 b | 1.796 ± 0.248 ab | 3.286 ± 0.177 b | 5.020 ± 0.157 b | 4.473 ± 0.315 a | 4.100 ± 0.845 b | 5.67± 0.682 a | 3.656 ± 0.708 b | 4.583 ± 0.724 ab |
| AbL2 | 1.083 ± 0.145 d | 3.026 ± 0.248 de | 2.360 ± 0.964 d | 2.330 ± 1.598 d | 1.926 ± 0.516 d | 1.756 ± 0.273 c | 0.593 ± 0.639 c | 5.340 ± 4.624 ab | 1.146 ± 1.986 b | 0.423 ± 0.733 c | 3.486 ± 3.080 a | 0.423 ± 0.733 c | 0.403 ± 0.698 b | 0.310 ± 0.536 c | 0.056 ± 0.098 b |
| AbS2 | 4.493 ± 0.609 b | 1.410 ± 0.75 f | 5.610 ± 0.745 bcd | 3.950 ± 1.565 cd | 3.353 ± 0.606 cd | 0.313 ± 0.542 c | 0.206 ± 0.357 c | 2.016 ± 0.919 ab | 0.400 ± 0.560 b | 0.123 ± 0.213 c | 3.136 ± 2.738 a | 0.426 ± 0.739 c | 0.170 ± 0.294 b | 0.243 ± 0.421 c | 0.466 ± 0.808 b |
| AbL1 | 1.583 ± 0.282 cd | 5.183 ± 0.620 ab | 6.793 ± 1.116 bc | 3.803 ± 0.329 cd | 10.110 ± 1.68 a | 0.313 ± 0.542 c | 0.136 ± 0.236 c | 1.206 ± 0.780 b | 1.003 ± 0.976 b | 0.053 ± 0.092 c | 2.626 ± 2.277 a | 0.336 ± 0.583 c | 0.430 ± 0.744 b | 0.683 ± 1.183 c | 3.430 ± 5.94 ab |
| AbS1 | 2.180 ± 0.448 cd | 3.833 ± 0.225 bc | 11.246 ± 1.71 a | 11.000 ± 0.245 a | 8.436 ± 1.699 ab | 0.210 ± 0.363 c | 0.126 ± 0.219 c | 0.390 ± 0.675 b | 1.476 ± 0.958 b | 0.096 ± 0.167 c | 4.723 ± 0.895 a | 1.270 ± 0.208 c | 0.053 ± 0.092 b | 0.466 ± 0.808 c | 0.170 ± 0.294 b |
| Sig. | ns | ns | ns | ** | ns | ns | *** | *** | * | ** | ns | * | * | ns | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordean, M.-E.; Ungur, R.A.; Toc, D.A.; Borda, I.M.; Marțiș, G.S.; Pop, C.R.; Filip, M.; Vlassa, M.; Nasui, B.A.; Pop, A.; et al. Antibacterial and Phytochemical Screening of Artemisia Species. Antioxidants 2023, 12, 596. https://doi.org/10.3390/antiox12030596
Bordean M-E, Ungur RA, Toc DA, Borda IM, Marțiș GS, Pop CR, Filip M, Vlassa M, Nasui BA, Pop A, et al. Antibacterial and Phytochemical Screening of Artemisia Species. Antioxidants. 2023; 12(3):596. https://doi.org/10.3390/antiox12030596
Chicago/Turabian StyleBordean, Maria-Evelina, Rodica Ana Ungur, Dan Alexandru Toc, Ileana Monica Borda, Georgiana Smaranda Marțiș, Carmen Rodica Pop, Miuța Filip, Mihaela Vlassa, Bogdana Adriana Nasui, Anamaria Pop, and et al. 2023. "Antibacterial and Phytochemical Screening of Artemisia Species" Antioxidants 12, no. 3: 596. https://doi.org/10.3390/antiox12030596
APA StyleBordean, M.-E., Ungur, R. A., Toc, D. A., Borda, I. M., Marțiș, G. S., Pop, C. R., Filip, M., Vlassa, M., Nasui, B. A., Pop, A., Cinteză, D., Popa, F. L., Marian, S., Szanto, L. G., & Muste, S. (2023). Antibacterial and Phytochemical Screening of Artemisia Species. Antioxidants, 12(3), 596. https://doi.org/10.3390/antiox12030596