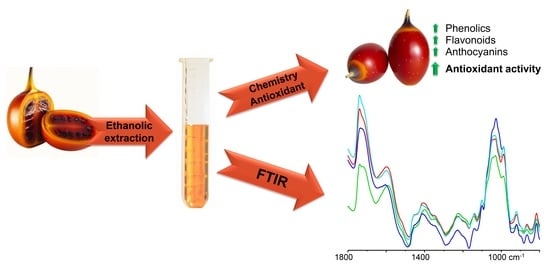
Antioxidant Potential of Tamarillo Fruits—Chemical and Infrared Spectroscopy Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biological Material
2.3. Physical Properties
2.4. Aqueous Ethanolic Extraction
2.5. Antioxidant Properties
2.5.1. Total Phenolic Compounds (TPC), Total Flavonoids Content (TFC), and Total Monomeric Anthocyanin Content (TMAC)
2.5.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS)
2.5.3. β-Carotene–Linoleic Acid Bleaching Method and Inhibition of Lipid Peroxidation in Buffered Egg Yolk
2.5.4. Metal Chelating Ability, Ferric Reducing Antioxidant Power Assay (FRAP), and Cupric Ion Reducing Antioxidant Capacity Assay (CUPRAC)
2.5.5. Enzymatic Activity (Cholinesterase Inhibition)
2.6. Fourier-Transform Infrared Spectroscopy
2.7. Statistical Analysis
3. Results
3.1. Physical Properties
3.2. Total Phenolic, Flavonoid, and Monomeric Anthocyanin Content
3.3. DPPH and ABTS
3.4. β-Carotene Oxidation Inhibition and Lipid Peroxidation Inhibition in Buffered Egg Yolk
3.5. Metal Chelating Ability, FRAP, and CUPRAC
3.6. Enzymatic Activity (AChE Inhibition)
3.7. Spectroscopic Analysis
4. Discussion
4.1. Physical Properties
4.2. Total Phenolic Compounds (TPC), Flavonoids (TFC), and Anthocyanins (TMAC)
4.3. Antioxidant and Enzymatic Activities
4.4. Spectroscopic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mueller, L.A.; Solow, T.H.; Taylor, N.; Skwarecki, B.; Buels, R.; Binns, J.; Lin, C.; Wright, M.; Ahrens, R.; Wang, Y.; et al. The SOL Genomics Network. A Comparative Resource for Solanaceae Biology and Beyond. Plant Physiol. 2005, 138, 1310–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canhoto, J.M.; Lopes, M.; Cruz, G. Protocol of Somatic Embryogenesis: Tamarillo (Cyphomandra betacea (Cav.) Sendtn.). In Protocol for Somatic Embryogenesis in Woody Plants, 1st ed.; Jain, S.M., Gupta, P.K., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 77, pp. 379–389. [Google Scholar] [CrossRef]
- Ramírez, F.; Kallarackal, J. Tree tomato (Solanum betaceum Cav.) reproductive physiology: A review. Sci. Hortic. 2019, 248, 206–215. [Google Scholar] [CrossRef]
- Prohens, J.; Nuez, F. The Tamarillo (Cyphomandra betacea). Small Fruits Rev. 2001, 1, 43–68. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Tamarillo (Solanum betaceum): Chemical composition, biological properties, and product innovation. Trends Food Sci. Technol. 2020, 95, 45–58. [Google Scholar] [CrossRef]
- Kou, M.-C.; Yen, J.-H.; Hong, J.-T.; Wang, C.-L.; Lin, C.-W.; Wu, M.-J. Cyphomandra betacea Sendt. phenolics protect LDL from oxidation and PC12 cells from oxidative stress. LWT—Food Sci. Tech. 2008, 42, 458–463. [Google Scholar] [CrossRef]
- Hurtado, N.H.; Morales, A.L.; González-Miret, M.L.; Escudero-Gilete, M.L.; Heredia, F.J. Colour, pH stability and antioxidant activity of anthocyanin rutinosides isolated from tamarillo fruit (Solanum betaceum Cav.). Food Chem. 2009, 117, 88–93. [Google Scholar] [CrossRef]
- Mertz, C.; Gancel, A.-L.; Gunata, Z.; Alter, P.; Dhuique-Mayer, C.; Vaillant, F.; Perez, A.M.; Ruales, J.; Brat, P. Phenolic compounds, carotenoids and antioxidant activity of three tropical fruits. J. Food Compos. Anal. 2009, 22, 381–387. [Google Scholar] [CrossRef]
- Diep, T.; Pook, C.; Yoo, M. Phenolic and Anthocyanin Compounds and Antioxidant Activity of Tamarillo (Solanum betaceum Cav.). Antioxidants 2020, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.; Lopes, T.; Correia, S.; Canhoto, J.; Marques, M.P.M.; de Carvalho, L.A.B. Nutraceutical properties of tamarillo fruits: A vibrational study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119501. [Google Scholar] [CrossRef]
- Vasco, C.; Avila, J.; Ruales, J.; Svanberg, U.; Kamal-Eldin, A. Physical and chemical characteristics of golden-yellow and purple-red varieties of tamarillo fruit (Solanum betaceum Cav.). Int. J. Food Sci. Nutr. 2009, 60, 278–288. [Google Scholar] [CrossRef]
- Noor Atiqah, A.A.K.; Maisarah, A.M.; Asmah, R. Comparison of antioxidant properties of tamarillo (Cyphomandra betacea), cherry tomato (Solanum lycopersicum var. cerasiform) and tomato (Lycopersicon esculentum). Int. Food Res. J. 2014, 21, 2355–2362. [Google Scholar]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-Based Antioxidants and the In Vitro Methods Used for Their Assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Tavares-Da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Blomhoff, R. Dietary antioxidants and cardiovascular disease. Curr. Opin. Infect. Dis. 2015, 16, 47–54. [Google Scholar] [CrossRef]
- Marques, J.; Martin, D.; Amado, A.M.; Lysenko, V.; Osório, N.; de Carvalho, L.A.E.B.; Marques, M.P.M.; Barroca, M.J.; da Silva, A.M. Novel Insights into Corema album Berries: Vibrational Profile and Biological Activity. Plants 2021, 10, 1761. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2‘-Diphenyl-1-picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. Influence of some experimental variables and matrix components in the determination of antioxidant properties by β-carotene bleaching assay: Experiments with BHT used as standard antioxidant. Eur. Food Res. Technol. 2010, 231, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Sakanaka, S.; Tachibana, Y. Active oxygen scavenging activity of egg-yolk protein hydrolysates and their effects on lipid oxidation in beef and tuna homogenates. Food Chem. 2006, 95, 243–249. [Google Scholar] [CrossRef]
- Wong, F.C.; Yong, A.L.; Ting, E.P.S.; Khoo, S.C.; Ong, H.C.; Chai, T.T. Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iran. J. Pharm. Res. 2014, 13, 1409. [Google Scholar] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Talić, S.; Dragičević, I.; Ćorajević, L.; Bevanda, A.M. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of extracts from medicinal plants. Glas. Hem. Tehnol. Bosne Herceg. 2014, 43, 11–14. [Google Scholar]
- Bekiaris, G.; Lindedam, J.; Peltre, C.; Decker, S.R.; Turner, G.B.; Magid, J.; Bruun, S. Rapid estimation of sugar release from winter wheat straw during bioethanol production using FTIR-photoacoustic spectroscopy. Biotechnol. Biofuels 2015, 8, 85. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Ferrari, C.; Angiuli, M.; Yao, J.; Raspi, C.; Bramanti, E. Qualitative and quantitative analysis of wood samples by Fourier transform infrared spectroscopy and multivariate analysis. Carbohydr. Polym. 2010, 82, 772–778. [Google Scholar] [CrossRef]
- Deng, Z.; Xia, A.; Liao, Q.; Zhu, X.; Huang, Y.; Fu, Q. Laccase pretreatment of wheat straw: Effects of the physicochemical characteristics and the kinetics of enzymatic hydrolysis. Biotechnol. Biofuels 2019, 12, 159. [Google Scholar] [CrossRef] [Green Version]
- Kubo, S.; Kadla, J.F. Hydrogen Bonding in Lignin: A Fourier Transform Infrared Model Compound Study. Biomacromolecules 2005, 6, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- España, L.; Heredia-Guerrero, J.A.; Segado, P.; Benítez, J.J.; Heredia, A.; Domínguez, E. Biomechanical properties of the tomato (Solanum lycopersicum) fruit cuticle during development are modulated by changes in the relative amounts of its components. New Phytol. 2014, 202, 790–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef] [Green Version]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Application of FTIR spectroscopy to the characterization of archeological wood. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 63–70. [Google Scholar] [CrossRef]
- McLean, J.P.; Jin, G.; Brennan, M.; Nieuwoudt, M.K.; Harris, P.J. Using NIR and ATR-FTIR spectroscopy to rapidly detect compression wood in Pinus radiata. Can. J. For. Res. 2014, 44, 820–830. [Google Scholar] [CrossRef]
- Ramirez, F.J.; Luque, P.; Heredia, A.; Bukovac, M.J. Fourier transform IR study of enzymatically isolated tomato fruit cuticular membrane. Biopolymers 1992, 32, 1425–1429. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Singh, S.; Parthasarathi, R.; Simmons, B.A.; Henry, R.J. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sustain. Energy Rev. 2015, 49, 871–906. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Zhang, M.; Lapierre, C.; Nouxman, N.L.; Nieuwoudt, M.K.; Smith, B.G.; Chavan, R.R.; McArdle, B.H.; Harris, P.J. Location and characterization of lignin in tracheid cell walls of radiata pine (Pinus radiata D. Don) compression woods. Plant Physiol. Biochem. 2017, 118, 187–198. [Google Scholar] [CrossRef]
- Martin, D.; Marques, J.; Amado, A.; Barroca, M.; da Silva, A.M.; de Carvalho, L.B.; Marques, M. Shedding light into the health-beneficial properties of Corema album—A vibrational spectroscopy study. J. Raman Spectrosc. 2020, 51, 313–322. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables Along a Fraction Process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Evans, P. Differentiating “hard” from “soft” woods using Fourier transform infrared and Fourier transform spectroscopy. Spectrochim. Acta Part A Mol. Spectrosc. 1991, 47, 1441–1447. [Google Scholar] [CrossRef]
- Mutalib, M.A.; Rahmat, A.; Ali, F.; Othman, F.; Ramasamy, R. Nutritional Compositions and Antiproliferative Activities of Different Solvent Fractions from Ethanol Extract of Cyphomandra betacea (Tamarillo) Fruit. Malays. J. Med Sci. 2017, 24, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Akanitapichat, P.; Phraibung, K.; Nuchklang, K.; Prompitakkul, S. Antioxidant and hepatoprotective activities of five eggplant varieties. Food Chem. Toxicol. 2010, 48, 3017–3021. [Google Scholar] [CrossRef] [PubMed]
- Balík, J.; Kyseláková, M.; Vrchotová, N.; Tříska, J.; Kumšta, M.; Veverka, J.; Híc, P.; Totušek, J.; Lefnerová, D. Relations between polyphenols content and antioxidant activity in vine grapes and leaves. Czech J. Food Sci. 2009, 26, S25–S32. [Google Scholar] [CrossRef] [Green Version]
- Fidrianny, I.; Natalia, S.; Insanu, M. Antioxidant capacities of various fruit extracts from three varieties of tomato and correlation with total phenolic, flavonoid, carotenoid content. Int. J. Pharm. Clin. Res. 2015, 7, 283–289. [Google Scholar]
- Gomes, S.M.; Ghica, M.E.; Rodrigues, I.A.; Gil, E.S.; Oliveira-Brett, A.M. Flavonoids electrochemical detection in fruit extracts and total antioxidant capacity evaluation. Talanta 2016, 154, 284–291. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Muy-Rangel, D.; Gardea-Béjar, A.; González-Aguilar, G.; Heredia, B.; Báez-Sañudo, M.; Siller-Cepeda, J.; DE LA Rocha1, R.V. Nutritional and Nutraceutical Components of Commercial Eggplant Types Grown in Sinaloa, Mexico. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Espin, S.; Gonzalez-Manzano, S.; Taco, V.; Poveda, C.; Ayuda-Durán, B.; Gonzalez-Paramas, A.M.; Santos-Buelga, C. Phenolic composition and antioxidant capacity of yellow and purple-red Ecuadorian cultivars of tree tomato (Solanum betaceum Cav.). Food Chem. 2016, 194, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vargas, H.I.; Benelli, P.; Ferreira, S.R.; Parada-Alfonso, F. Supercritical fluid extracts from tamarillo (Solanum betaceum Sendtn) epicarp and its application as protectors against lipid oxidation of cooked beef meat. J. Supercrit. Fluids 2013, 76, 17–23. [Google Scholar] [CrossRef]
- Braidy, N.; Poljak, A.; Jayasena, T.; Sachdev, P. Natural Plant-Derived Acetylcholinesterase Inhibitors: Relevance for Alzheimer’s Disease. In Natural Products Targeting Clinically Relevant Enzymes, 1st ed.; Andrade, P.B., Valentão, P., Pereira, D.M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 297–318. [Google Scholar] [CrossRef]
- Suárez-Montenegro, Z.J.; Ballesteros-Vivas, D.; Gallego, R.; Valdés, A.; Sánchez-Martínez, J.D.; Parada-Alfonso, F.; Ibáñez, E.; Cifuentes, A. Neuroprotective Potential of Tamarillo (Cyphomandra betacea) Epicarp Extracts Obtained by Sustainable Extraction Process. Front. Nutr. 2021, 8, 769617. [Google Scholar] [CrossRef] [PubMed]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Lam, H.S.; Proctor, A.; Howard, L.; Cho, M.J. Rapid Fruit Extracts Antioxidant Capacity Determination by Fourier Transform Infrared Spectroscopy. J. Food Sci. 2005, 70, C545–C549. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crop. Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- A Villa-Rodriguez, J.; Palafox-Carlos, H.; Yahia, E.M.; Zavala, J.F.A.; Gonzalez-Aguilar, G.A. Maintaining Antioxidant Potential of Fresh Fruits and Vegetables After Harvest. Crit. Rev. Food Sci. Nutr. 2015, 55, 806–822. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Ullevig, S.; Short, J.; Wang, L.; Ahn, Y.; Asmis, R. Ursolic Acid and Related Analogues: Triterpenoids with Broad Health Benefits. Antioxidants 2021, 10, 1161. [Google Scholar] [CrossRef] [PubMed]


| Assay/Extract | TMI | TVM | CR | CO |
|---|---|---|---|---|
| Weight (g) | 94.41 ± 11.29 a | 57.16 ± 5.84 b | 47.36 ± 4.01 b | 57.71 ± 7.34 b |
| Fruit diameter (cm) | 5.17 ± 0.26 a | 4.24 ± 0.19 b | 3.86 ± 0.10 b | 4.29 ± 0.21 c |
| Fruit length (cm) | 6.46 ± 0.45 a,b | 6.79 ± 0.15 b | 6.65 ± 0.34 a,b | 6.25 ± 0.28 a |
| Fruit + peduncle length (cm) | 10.99 ± 0.69 a,b | 11.49 ± 0.30 b | 11.01 ± 0.46 a,b | 10.51 ± 0.62 a |
| Peduncle diameter (cm) | 0.74 ± 0.14 a | 0.70 ± 0.04 a | 0.68 ± 0.05 a | 0.63 ± 0.06 a |
| Hardness (N/cm2) | 17.46 ± 1.18 a | 18.14 ± 1.17 a | 15.30 ± 2.35 a | 17.55 ± 1.86 a |
| Soluble solid content (°Brix) | 8.17 ± 0.92 c | 10.35 ± 0.24 a,b | 11.28 ± 0.46 a | 9.77 ± 0.12 b |
| Acidity (% of total acidity to citric acid conversion) | 1.83 ± 0.13 c | 1.86 ± 0.05 b,c | 2.32 ± 0.15 a | 2.04 ± 0.08 b |
| Assay/Extract | TMI | TVM | CR | CO |
|---|---|---|---|---|
| TPC (GAE mg g−1 DW) | 1.82 ± 0.05 c | 3.47 ± 0.18 a | 3.17 ± 0.11 a | 2.52 ± 0.06 b |
| TFC (QCTE mg g−1 DW) | 3.39 ± 0.11 b | 4.20 ± 0.37 b | 5.55 ± 0.43 a | 5.33 ± 0.33 a |
| TMAC (C3GE mg g−1 DW) | 0.18 ± 0.05 b,c | 0.62 ± 0.05 a | 0.33 ± 0.05 b | 0.11 ± 0.04 d |
| Assay/Extract | TMI | TVM | CR | CO |
|---|---|---|---|---|
| DPPH (IC50 mg mL−1) | 1.79 ± 0.08 c | 1.33 ± 0.04 d | 2.03 ± 0.08 b | 3.34 ± 0.08 a |
| ABTS (IC50 mg mL−1) | 5.89 ± 0.02 a,b | 3.70 ± 0.04 c | 5.62 ± 0.09 b | 10.17 ± 0.17 d |
| β-carotene/linoleic acid (IC50 mg mL−1) | 3.69 ± 0.37 b,c | 5.20 ± 0.33 a | 4.62 ± 0.33 a,c | 3.79 ± 0.24 c |
| Lipid peroxidation (IC50 mg mL−1) | 3.38 ± 0.51 a | 3.22 ± 0.57 a | 3.06 ± 0.63 a | 3.23 ± 0.27 a |
| Metal chelating ability (EDTA mg g−1 DW) | 1.22 ± 0.04 c | 2.17 ± 0.11 a | 1.71 ± 0.02 b | 1.98 ± 0.04 a |
| FRAP (TE mg g−1 DW) | 2.56 ± 0.06 d | 5.67 ± 0.17 a | 4.74 ± 0.07 b | 2.96 ± 0.06 c |
| CUPRAC (TE mg g−1 DW) | 5.26 ± 0.07 c | 11.11 ± 0.35 a | 10.54 ± 0.19 a | 7.53 ± 0.04 b |
| AChE (GALAE mg g−1 DW) | 0.10 ± 0.02 c | 0.95 ± 0.10 a | 0.44 ± 0.05 b | 0.49 ± 0.12 b |
| Assay/Reference Antioxidant | Trolox | BHT |
|---|---|---|
| DPPH (IC50 mg mL−1) | 0.065 ± 0.003 | – |
| ABTS (IC50 mg mL−1) | 0.084 ± 0.001 | – |
| β-carotene/linoleic acid (IC50 mg mL−1) | – | 0.125 ± 0.015 |
| Lipid peroxidation (IC50 mg mL−1) | – | 0.009 ± 0.005 [17] |
| Wavenumber (cm−1) | Reference | Assignment a | Compositional Feature |
|---|---|---|---|
| 1739/1720 | 1735 [29] 1739 [30] 1734 [31] 1735 [10] | ν(C=O) | Polysaccharides |
| 1592 | 1595 [30] 1594 [32] 1595 [33] | Aromatic ring vibration and ν(C=O) | Phenolics |
| 1454 | 1455 [10] 1456 [34] 1457 [35] | δ(CH2) scissoring | Polysaccharides |
| 1414 | 1419 [36] 1419 [37] | Aromatic skeletal vibration combined with CH in plane deformation | Phenolics |
| 1346 | 1344 [35] 1344 [38] | δ(CH2) wagging and twisting | Cutin, waxes |
| 1222 | 1220 [29] 1226 [39] | CC, CO, C=O stretches | Polysaccharides and phenolics |
| 1144 | 1142 [39] 1140 [40] 1136 [41] | aromatic CH in-plane deformation | Phenolics |
| 1100 | 1098, 1105 [10] 1090 [42] 1103 [38] | ν(C-O-C)ester | Polysaccharides (esters) |
| 1045 | 1047 [42] 1054 [35] 1052 [10] | ν(C-O-C)glycosidic | Polysaccharides |
| 1032 | 1032 [42] 1034 [10] 1035 [43] 1030 [44] | ν(C-O) and ν(C-C) | Polysaccharides (pectins) |
| 988 | 993 [45] 995 [10] 985 [36] | ν(CO) and ν(CC) | Polysaccharides |
| 922 | 918 [42] | ρ(CH3) | Triterpenoids |
| 865 | 866 [40] 869 [41] | C-H out-of-plane | - |
| 816 | 816 [35] 810 [46] | Aromatic C-H out-of-plane deformations | Phenolics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rito, M.; Marques, J.; da Costa, R.M.F.; Correia, S.; Lopes, T.; Martin, D.; Canhoto, J.M.P.L.; Batista de Carvalho, L.A.E.; Marques, M.P.M. Antioxidant Potential of Tamarillo Fruits—Chemical and Infrared Spectroscopy Analysis. Antioxidants 2023, 12, 536. https://doi.org/10.3390/antiox12020536
Rito M, Marques J, da Costa RMF, Correia S, Lopes T, Martin D, Canhoto JMPL, Batista de Carvalho LAE, Marques MPM. Antioxidant Potential of Tamarillo Fruits—Chemical and Infrared Spectroscopy Analysis. Antioxidants. 2023; 12(2):536. https://doi.org/10.3390/antiox12020536
Chicago/Turabian StyleRito, Miguel, Joana Marques, Ricardo M. F. da Costa, Sandra Correia, Tércia Lopes, Daniel Martin, Jorge M. P. L. Canhoto, Luís A. E. Batista de Carvalho, and Maria Paula M. Marques. 2023. "Antioxidant Potential of Tamarillo Fruits—Chemical and Infrared Spectroscopy Analysis" Antioxidants 12, no. 2: 536. https://doi.org/10.3390/antiox12020536
APA StyleRito, M., Marques, J., da Costa, R. M. F., Correia, S., Lopes, T., Martin, D., Canhoto, J. M. P. L., Batista de Carvalho, L. A. E., & Marques, M. P. M. (2023). Antioxidant Potential of Tamarillo Fruits—Chemical and Infrared Spectroscopy Analysis. Antioxidants, 12(2), 536. https://doi.org/10.3390/antiox12020536