Interaction between Dietary Fibre and Bioactive Compounds in Plant By-Products: Impact on Bioaccessibility and Bioavailability
Abstract
1. Introduction
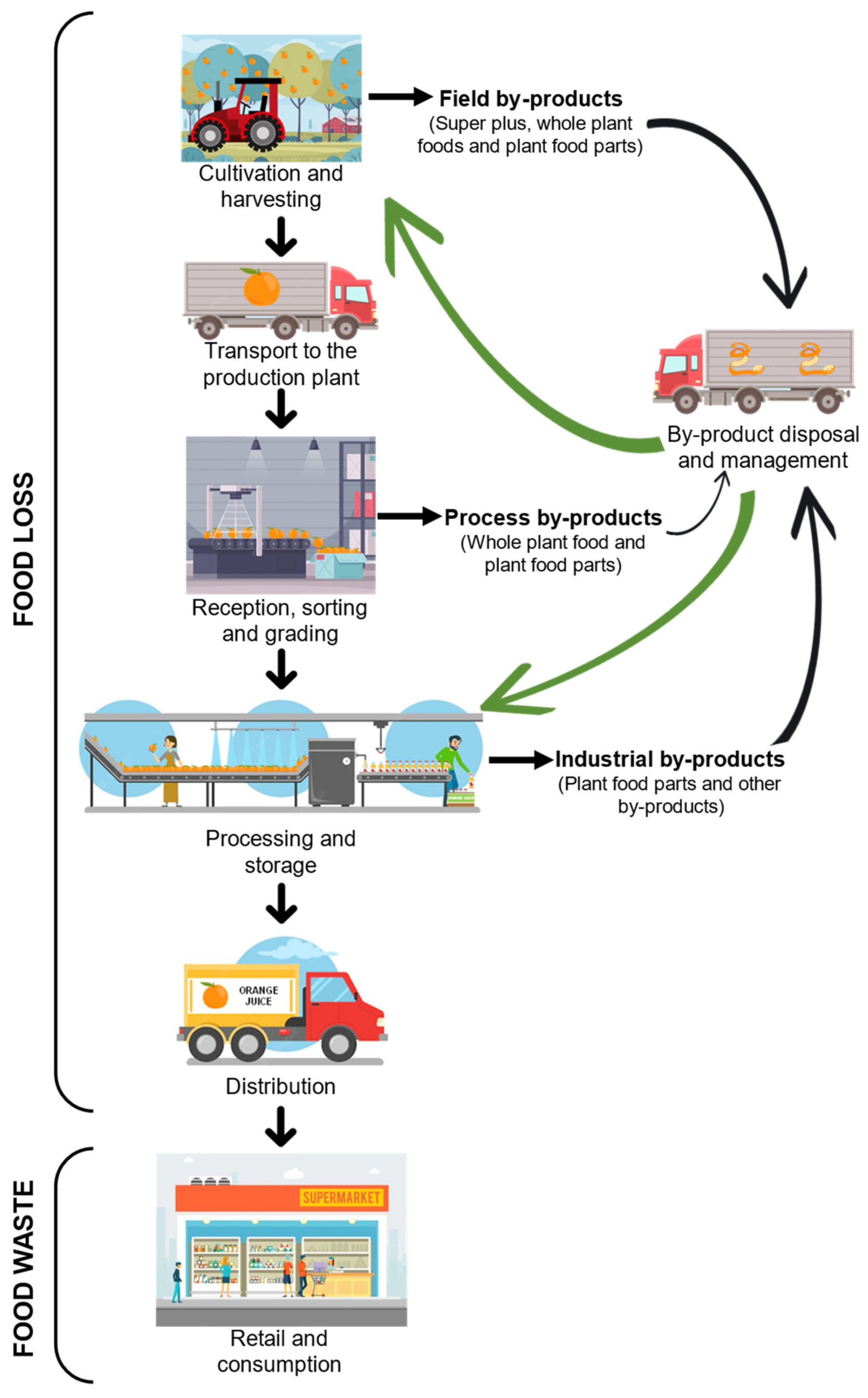
2. Importance of By-Products for the Agri-Food Industry
2.1. Generation of By-Products and Their Implications
2.2. Composition of Agro-Industrial By-Products
2.3. Industrial Applications
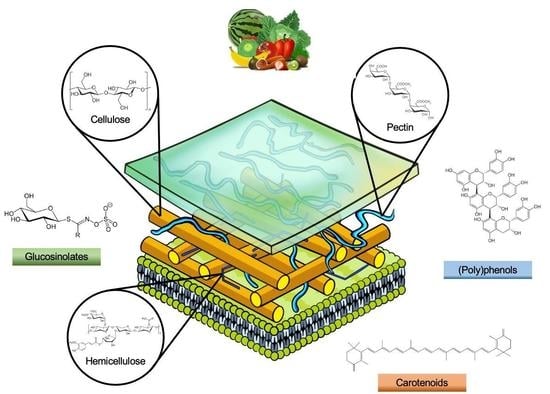
3. Main By-Product Components
3.1. Dietary Fibre
3.2. (Poly)phenols
3.3. Carotenoids
3.4. Glucosinolates
3.5. Antioxidant Dietary Fibre
| Fruit/Vegetable | FRAP | DPPH | ABTS | ORAC | Reference |
|---|---|---|---|---|---|
| Avocado peel | - | 52–190 | - | 58–631 | [115] |
| Avocado seed | - | 128–240 | - | 229–464 | [115] |
| Papaya peel fibre | 25 | 0.6 | - | - | [116] |
| Orange peel | 75–155 | 134–269 | 489–810 | - | [40] |
| Broccoli stalk fibres | 1–2 | - | - | 17–19 | [30] |
| Tomato peel fibre | - | - | 3.9 | - | [25] |
| Raspberry fibres | 2–38 | - | - | 31–81 | [117] |
| Chokeberry pomace | - | 3 | 8 | - | [118] |
| Persimmon flour | 22 | 13 | 10 | - | [119] |
4. Bioaccessibility and Bioavailability of Bioactive Compounds
4.1. (Poly)phenol Bioavailability
4.2. Bioaccessibility and Bioavailability of Other Bioactive Compounds
5. Dietary Fibre-Rich Functional Foods and Claims
6. Conclusions
Funding
Conflicts of Interest
References
- Harris, J.; van Zonneveld, M.; Achigan-Dako, E.G.; Bajwa, B.; Brouwer, I.D.; Choudhury, D.; de Jager, I.; de Steenhuijsen Piters, B.; Ehsan Dulloo, M.; Guarino, L.; et al. Fruit and vegetable biodiversity for nutritionally diverse diets: Challenges, opportunities, and knowledge gaps. Glob. Food Sec. 2022, 33, 100618. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A.; Rome, F. Cutting Food Waste to Feed the World; FAO-News: Rome, Italy, 2011; pp. 1–37. [Google Scholar]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef]
- Schulz, R.; Slavin, J. Fiber intake and resulting health benefits. Ref. Modul. Food Sci. 2022, 239–246. [Google Scholar] [CrossRef]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary Fiber from Underutilized Plant Resources—A Positive Approach for Valorization of Fruit and Vegetable Wastes. Sustainablity 2020, 12, 5401. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef][Green Version]
- Pathania, S.; Kaur, N. Utilization of fruits and vegetable by-products for isolation of dietary fibres and its potential application as functional ingredients. Bioact. Carbohydr. Diet. Fibre. 2022, 27, 100295. [Google Scholar] [CrossRef]
- EUR-Lex. Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2008/98/EC on Waste (Text with EEA relevance). Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/?uri=CELEX%3A32018L0851 (accessed on 9 June 2021).
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1–15. [Google Scholar] [CrossRef][Green Version]
- Murray, A.; Skene, K.; Haynes, K. The Circular Economy: An Interdisciplinary Exploration of the Concept and Application in a Global Context. J. Bus. Ethics. 2017, 140, 369–380. [Google Scholar] [CrossRef][Green Version]
- Sheridan, K. Making the Bioeconomy Circular: The Biobased Industries’ Next Goal? Ind. Biotechnol. 2016, 12, 339–340. [Google Scholar] [CrossRef]
- Morales-Moreno, A.B. Estudio De Los Residuos y Subproductos Agroindustriales De La Región De Murcia: Opciones De Valorización Mediante Compostaje y Biometanización. Ph.D. Thesis, Universidad Politécnica de Cartagena, Cartagena, Spain, 2015. [Google Scholar]
- F.A.O. of the U.N. Food Loss and Waste Database | FAO | Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/platform-food-loss-waste/flw-data/en (accessed on 18 March 2022).
- European Comission Food Waste. Available online: https://ec.europa.eu/food/food/food-waste_en (accessed on 8 June 2021).
- Del Rio Osorio, L.L.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food Wastage Footprint & Climate Change; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Otles, S.; Despoudi, S.; Bucatariu, C.; Kartal, C. Food Waste Management, Valorization, and Sustainability in the Food Industry; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128004197. [Google Scholar]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Mallek-Ayadi, S.; Bahloul, N.; Kechaou, N. Chemical composition and bioactive compounds of Cucumis melo L. seeds: Potential source for new trends of plant oils. Process Saf. Environ. Prot. 2018, 113, 68–77. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, K.; Tian, M. Chemical composition and nutritive value of hot pepper seed (Capsicum annuum) grown in Northeast Region of China. Food Sci. Technol. 2015, 35, 659–663. [Google Scholar] [CrossRef][Green Version]
- Reißner, A.M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and physicochemical properties of dried berry pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef]
- Azabou, S.; Louati, I.; Ben Taheur, F.; Nasri, M.; Mechichi, T. Towards sustainable management of tomato pomace through the recovery of valuable compounds and sequential production of low-cost biosorbent. Environ. Sci. Pollut. Res. Int. 2020, 27, 39402–39412. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Jayalaxmi, B.; Vijayalakshmi, D.; Kapale, M. Extraction of Total Polyphenols and Dietary Fiber from Mango Peel-As Potential Sources of Natural Phytonutrients. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1196–1205. [Google Scholar] [CrossRef]
- Pacheco, M.T.; Moreno, F.J.; Villamiel, M. Chemical and physicochemical characterization of orange by-products derived from industry. J. Sci. Food Agric. 2019, 99, 868–876. [Google Scholar] [CrossRef]
- Cassani, L.; Gomez-Zavaglia, A. Sustainable Food Systems in Fruits and Vegetables Food Supply Chains. Front. Nutr. 2022, 9, 829061. [Google Scholar] [CrossRef]
- Blancas-Benitez, F.J.; Mercado-Mercado, G.; Quirós-Sauceda, A.E.; Montalvo-González, E.; González-Aguilar, G.A.; Sáyago-Ayerdi, S.G. Bioaccessibility of polyphenols associated with dietary fiber and in vitro kinetics release of polyphenols in Mexican ‘Ataulfo’ mango (Mangifera indica L.) by-products. Food Funct. 2015, 6, 859–868. [Google Scholar] [CrossRef]
- Núñez-Gómez, V.; González-Barrio, R.; Baenas, N.; Moreno, D.A.; Periago, M.J. Dietary-Fibre-Rich Fractions Isolated from Broccoli Stalks as a Potential Functional Ingredient with Phenolic Compounds and Glucosinolates. Int. J. Mol. Sci. 2022, 23, 13309. [Google Scholar] [CrossRef] [PubMed]
- Casarotti, S.N.; Borgonovi, T.F.; Batista, C.L.F.M.; Penna, A.L.B. Guava, orange and passion fruit by-products: Characterization and its impacts on kinetics of acidification and properties of probiotic fermented products. LWT 2018, 98, 69–76. [Google Scholar] [CrossRef][Green Version]
- Batista, K.S.; Alves, A.F.; Lima, M.D.S.; Da Silva, L.A.; Lins, P.P.; De Sousa Gomes, J.A.; Silva, A.S.; Toscano, L.T.; De Albuquerque Meireles, B.R.L.; De Magalhães Cordeiro, A.M.T.; et al. Beneficial effects of consumption of acerola, cashew or guava processing by-products on intestinal health and lipid metabolism in dyslipidaemic female Wistar rats. Br. J. Nutr. 2018, 119, 30–41. [Google Scholar] [CrossRef][Green Version]
- Abbasi-Parizad, P.; De Nisi, P.; Scaglia, B.; Scarafoni, A.; Pilu, S.; Adani, F. Recovery of phenolic compounds from agro-industrial by-products: Evaluating antiradical activities and immunomodulatory properties. Food Bioprod. Process. 2021, 127, 338–348. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M.W.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- San Martin, D.; Ramos, S.; Zufía, J. Valorisation of food waste to produce new raw materials for animal feed. Food Chem. 2016, 198, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Jõgi, K.; Bhat, R. Valorization of food processing wastes and by-products for bioplastic production. Sustain. Chem. Pharm. 2020, 18, 100326. [Google Scholar] [CrossRef]
- Kumar, K. Nutraceutical potential and utilization aspects of food industry by-products and wastes. In Food Industry Wastes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–111. [Google Scholar]
- Dienaitė, L.; Baranauskienė, R.; Rimantas Venskutonis, P. Lipophilic extracts isolated from European cranberry bush (Viburnum opulus) and sea buckthorn (Hippophae rhamnoides) berry pomace by supercritical CO2-Promising bioactive ingredients for foods and nutraceuticals. Food Chem. 2021, 348, 129047. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vázquez, L.; Lozano, M.V.; Rodríguez-Robledo, V.; González-Fuentes, J.; Marcos, P.; Villaseca, N.; Arroyo-Jiménez, M.M.; Santander-Ortega, M.J. Pressurized Extraction as an Opportunity to Recover Antioxidants from Orange Peels: Heat treatment and Nanoemulsion Design for Modulating Oxidative Stress. Molecules 2021, 26, 5928. [Google Scholar] [CrossRef] [PubMed]
- Silvan, J.M.; Martinez-Rodriguez, A.J. Food by-products as natural source of bioactive compounds against campylobacter. Encycl. Food Secur. Sustain. 2018, 1, 336–350. [Google Scholar] [CrossRef]
- Sanchez-Martinez, J.D.; Bueno, M.; Alvarez-Rivera, G.; Tudela, J.; Ibanez, E.; Cifuentes, A. In vitro neuroprotective potential of terpenes from industrial orange juice by-products. Food Funct. 2021, 12, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef][Green Version]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R.N.A. Potential application of grape (Vitis vinifera L.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Oreopoulou, V.; Tzia, C. Utilization of plant by-products for the recovery of proteins, dietary fibers, antioxidants, and colorants. In Utilization of By-Products and Treatment of Waste in the Food Industry; Springer: New York, NY, USA, 2007; pp. 209–232. ISBN 0387335110. [Google Scholar]
- Amaya-Cruz, D.M.; Pérez-Ramírez, I.F.; Delgado-García, J.; Mondragón-Jacobo, C.; Dector-Espinoza, A.; Reynoso-Camacho, R. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities. Food Chem. 2019, 278, 568–578. [Google Scholar] [CrossRef]
- Majerska, J.; Michalska, A.; Figiel, A. A review of new directions in managing fruit and vegetable processing by-products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- Ma, S.; Han, W. Application in Bakery Products; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128164952. [Google Scholar]
- Zielinski, G.; Rozema, B. Review of fiber methods and applicability to fortified foods and supplements: Choosing the correct method and interpreting results. Anal. Bioanal. Chem. 2013, 405, 4359–4372. [Google Scholar] [CrossRef]
- Eskicioglu, V.; Kamiloglu, S.; Nilufer-Erdil, D. Antioxidant dietary fibres: Potential functional food ingredients from plant processing by-products. Czech J. Food Sci. 2015, 33, 487–499. [Google Scholar] [CrossRef][Green Version]
- Williams, P.G. The Benefits of Breakfast Cereal Consumption: A Systematic Review of the Evidence Base. Adv. Nutr. 2014, 5, 636S–673S. [Google Scholar] [CrossRef][Green Version]
- Soukoulis, C.; Lebesi, D.; Tzia, C. Enrichment of ice cream with dietary fibre: Effects on rheological properties, ice crystallisation and glass transition phenomena. Food Chem. 2009, 115, 665–671. [Google Scholar] [CrossRef]
- Brennan, C.; Mustafa, R.; Boukid, F.; Gagaoua, M. Vegan Egg: A Future-Proof Food Ingredient? Foods 2022, 11, 161. [Google Scholar] [CrossRef]
- Iriondo-Dehond, M.; Miguel, E.; Del Castillo, M.D. Food Byproducts as Sustainable Ingredients for Innovative and Healthy Dairy Foods. Nutrients 2018, 10, 1358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- EFSA (European Food Safety Authority). Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2016, 8, 1462. [Google Scholar] [CrossRef][Green Version]
- FDA. The Declaration of Certain Isolated or Synthetic Non-Digestible Carbohydrates as Dietary Fiber on Nutrition and Supplement Facts Labels: Guidance for Industry; Food and Drug Administration: Montgomery, MD, USA, 2018. [Google Scholar]
- Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary fibers as beneficial microbiota modulators: A proposed classification by prebiotic categories. Nutrition 2021, 89, 111217. [Google Scholar] [CrossRef]
- Ahmad Khorairi, A.N.S.; Sofian-Seng, N.S.; Othaman, R.; Abdul Rahman, H.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. A Review on Agro-industrial Waste as Cellulose and Nanocellulose Source and Their Potentials in Food Applications. Food Rev. Int. 2023, 2, 663–688. [Google Scholar] [CrossRef]
- Rivas-Cantu, R.C.; Jones, K.D.; Mills, P.L. A citrus waste-based biorefinery as a source of renewable energy: Technical advances and analysis of engineering challenges. Waste Manag. Res. 2013, 31, 413–420. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Quick, A.; Taha, M.; Mignard, N.; Becquart, F.; Ayoub, A. Hemicellulose extraction and characterization for applications in paper coatings and adhesives. Ind. Crops Prod. 2017, 107, 370–377. [Google Scholar] [CrossRef]
- Cruz-Requena, M.; Escobedo-García, S.; Salas-Tovar, J.A.; Mora-Cura, Y.; Chávez-González, M.L.; Castillo-Reyes, F.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Definitions and regulatory perspectives of dietary fibers. In Dietary Fiber: Properties, Recovery, and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 1–25. ISBN 9780128164952. [Google Scholar]
- Nawirska, A.; Kwaśniewska, M. Dietary fibre fractions from fruit and vegetable processing waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Venkatanagaraju, E.; Bharathi, N.; Sindhuja, R.H.; Chowdhury, R.R.; Sreelekha, Y. Extraction and Purification of Pectin from Agro-Industrial Wastes. Pectins-Extr. Purif. Charact. Appl. 2020. [Google Scholar] [CrossRef][Green Version]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Waterman, P.G. Roles for secondary metabolites in plants. Ciba Found. Symp. 1992, 171, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef][Green Version]
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef][Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef][Green Version]
- Carboni Martins, C.; Rodrigues, R.C.; Domeneghini Mercali, G.; Rodrigues, E. New insights into non-extractable phenolic compounds analysis. Food Res. Int. 2022, 157, 111487. [Google Scholar] [CrossRef] [PubMed]
- Hümmer, W.; Schreier, P. Analysis of proanthocyanidins. Mol. Nutr. Food Res. 2008, 52, 1381–1398. [Google Scholar] [CrossRef]
- Durazzo, A. Extractable and Non-extractable Polyphenols: An Overview. In Non-Extractable Polyphenols and Carotenoids; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 37–45. [Google Scholar]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ludwig, I.A.; Rubió, L.; Mosele, J.I.; Motilva, M.J. Metabolic Fate of Extractable and Non-extractable Polyphenols. In Non-Extractable Polyphenols and Carotenoids; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 220–240. [Google Scholar]
- Pérez-Jiménez, J.; Saura-Calixto, F. Fruit peels as sources of non-extractable polyphenols or macromolecular antioxidants: Analysis and nutritional implications. Food Res. Int. 2018, 111, 148–152. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Torres, J.L. Analysis of nonextractable phenolic compounds in foods: The current state of the art. J. Agric. Food Chem. 2011, 59, 12713–12724. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Meza, Y.; Reynoso-Camacho, R.; Pérez-Jiménez, J. Nonextractable Polyphenols: A Relevant Group with Health Effects; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 31–83. [Google Scholar]
- Mourtzinos, I.; Goula, A. Polyphenols in Agricultural Byproducts and Food Waste; Academic Press: Cambridge, MA, USA, 2019; pp. 23–44. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O.; Francis, F.J. Natural pigments: Carotenoids, anthocyanins, and betalains-Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Rivera-Madrid, R.; Carballo-Uicab, V.M.; Cárdenas-Conejo, Y.; Aguilar-Espinosa, M.; Siva, R. Overview of carotenoids and beneficial effects on human health. In Carotenoids: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–40. [Google Scholar]
- Lindbergh, C.A.; Renzi-Hammond, L.M.; Hammond, B.R.; Terry, D.P.; Mewborn, C.M.; Puente, A.N.; Miller, L.S. Lutein and Zeaxanthin Influence Brain Function in Older Adults: A Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2018, 24, 77–90. [Google Scholar] [CrossRef][Green Version]
- Navarro-González, I.; Pérez-Sánchez, H.; Martín-Pozuelo, G.; García-Alonso, J.; Periago, M.J. The Inhibitory Effects of Bioactive Compounds of Tomato Juice Binding to Hepatic HMGCR: In Vivo Study and Molecular Modelling. PLoS ONE 2014, 9, 83968. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional importance of carotenoids and their effect on liver health: A review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tiwari, S.; Upadhyay, N.; Singh, A.K.; Meena, G.S.; Arora, S. Organic solvent-free extraction of carotenoids from carrot bio-waste and its physico-chemical properties. J. Food Sci. Technol. 2019, 56, 4678–4687. [Google Scholar] [CrossRef]
- Esposto, B.S.; Pinho, S.G.B.; Thomazini, M.; Ramos, A.P.; Tapia-Blácido, D.R.; Martelli-Tosi, M. TPP-chitosomes as potential encapsulation system to protect carotenoid-rich extract obtained from carrot by-product: A comparison with liposomes and chitosomes. Food Chem. 2022, 397, 133857. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-Camacho, R.; Rodríguez-Villanueva, L.D.; Sotelo-González, A.M.; Ramos-Gómez, M.; Pérez-Ramírez, I.F. Citrus decoction by-product represents a rich source of carotenoid, phytosterol, extractable and non-extractable polyphenols. Food Chem. 2021, 350, 129239. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Castro-Torres, I.G.; Castro-Torres, V.A.; Hernández-Lozano, M.; Naranjo-Rodríguez, E.B.; Domínguez-Ortiz, M.Á. Glucosinolates and metabolism. In Glucosinolates: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 107–141. ISBN 9780128164938. [Google Scholar]
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre- and Post-harvest Factors Affecting Glucosinolate Content in Broccoli. Front. Nutr. 2020, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, F.; Pinela, J.; Bailón, A.D.H.; Fereira, I.C.F.R.; Petropoulos, S.A. The dilemma of “good” and “bad” glucosinolates and the potential to regulate their content. In Glucosinolates: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–45. ISBN 9780128164938. [Google Scholar]
- Possenti, M.; Baima, S.; Raffo, A.; Durazzo, A.; Giusti, A.M.; Natella, F. Glucosinolates in Food. In Glucosinolates; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–46. [Google Scholar]
- Huang, H.; Wang, J.; Mao, S.; Wu, Q.; Tian, Y.; Wang, F.; Wang, P.; Huang, K.; Wu, Q. Variation Characteristics of Glucosinolate Contents in Leaf Mustard (Brassica juncea). Agronomy 2022, 12, 2287. [Google Scholar] [CrossRef]
- Cleemput, S.; Becker, H.C. Genetic variation in leaf and stem glucosinolates in resynthesized lines of winter rapeseed (Brassica napus L.). Genet. Resour. Crop. Evol. 2012, 59, 539–546. [Google Scholar] [CrossRef][Green Version]
- Andréasson, E.; Jørgensen, L.B. Chapter four Localization of plant myrosinases and glucosinolates. Recent Adv. Phytochem. 2003, 37, 79–99. [Google Scholar] [CrossRef]
- Del Martínez-Ballesta, M.C.; Moreno, D.A.; Carvajal, M. The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef][Green Version]
- Akram, M.; Jabeen, F.; Riaz, M.; Khan, F.S.; Okushanova, E.; Imran, M.; Shariati, M.A.; Riaz, T.; Egbuna, C.; Ezeofor, N.J. Health benefits of glucosinolate isolated from cruciferous and other vegetables. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, The Netherlands, 2021; pp. 361–371. [Google Scholar]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates-A review. LWT-Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health-Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Hedayati, M.; Hosseinpour-Niazi, S.; Azizi, F. Broccoli sprouts reduce oxidative stress in type 2 diabetes: A randomized double-blind clinical trial. Eur. J. Clin. Nutr. 2011, 65, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.B.; Raes, K.; Vanhoutte, H.; Coelus, S.; Smagghe, G.; Van Camp, J. Liquid chromatography-mass spectrometry coupled with multivariate analysis for the characterization and discrimination of extractable and nonextractable polyphenols and glucosinolates from red cabbage and Brussels sprout waste streams. J. Chromatogr. A 2015, 1402, 60–70. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.M. Comparative phytonutrient analysis of broccoli by-products: The potentials for broccoli by-product utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef][Green Version]
- Welti-Chanes, J.; Serna-Saldívar, S.O.; Campanella, O.; Tejada-Ortigoza, V. Science and Technology of Fibers in Food Systems; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Antioxidant Dietary Fiber Product: A New Concept and a Potential Food Ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar] [CrossRef][Green Version]
- Shahidi, F.; Chandrasekara, A. Interaction of Phenolics and their Association with Dietary Fiber. In Dietary Fiber Functionality in Food and Nutraceuticals: From Plant to Gut; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2016; pp. 21–44. [Google Scholar]
- Renard, C.M.G.C.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Fărcaș, A.; Drețcanu, G.; Pop, T.D.; Enaru, B.; Socaci, S.; Diaconeasa, Z. Cereal processing by-products as rich sources of phenolic compounds and their potential bioactivities. Nutrients 2021, 13, 3934. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polyphenols and antioxidant activity of citrus fiber/blackberry juice complexes. Molecules 2021, 26, 4400. [Google Scholar] [CrossRef] [PubMed]
- Vikas, D.; Shriya, B.; Kanika Sonkhla, R.J.; Mahesh, G. Quantification of Free and Bound Phenolics in Bio-Waste Pomegranate Peel and Formulation of Punicalagin Rich Rice Extruded Snacks | Semantic Scholar. Int. J. Food Nutr. Sci. 2017, 4, 98–104. [Google Scholar]
- Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of agri-food waste from pistachio hard shells: Extraction of polyphenols as natural antioxidants. Resources 2021, 10, 45. [Google Scholar] [CrossRef]
- Wang, W.; Bostic, T.R.; Gu, L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010, 122, 1193–1198. [Google Scholar] [CrossRef]
- Nieto Calvache, J.; Cueto, M.; Farroni, A.; de Escalada Pla, M.; Gerschenson, L.N. Antioxidant characterization of new dietary fiber concentrates from papaya pulp and peel (Carica papaya L.). J. Funct. Foods 2016, 27, 319–328. [Google Scholar] [CrossRef]
- Baenas, N.; Nuñez-Gómez, V.; Navarro-González, I.; Sánchez-Martínez, L.; García-Alonso, J.; Periago, M.J.; González-Barrio, R. Raspberry dietary fibre: Chemical properties, functional evaluation and prebiotic in vitro effect. LWT 2020, 134, 110140. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A.; Navarro-Cruz, A.R.; Corona-Jiménez, E.; Ochoa-Velasco, C.E. Optimization of Antioxidant Compounds Extraction from Fruit By-Products: Apple Pomace, Orange and Banana Peel. J. Food Process. Preserv. 2016, 40, 103–115. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez Álvarez, J.A.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef]
- McRorie, J.W.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blaut, M. Composition and function of the gut microbiome. In The Gut Microbiome in Health and Disease; Springer International Publishing: Cham, Switzerland, 2018; pp. 5–30. ISBN 9783319905457. [Google Scholar]
- Yegin, S.; Kopec, A.; Kitts, D.D.; Zawistowski, J. Dietary Fiber: A Functional Food Ingredient with Physiological Benefits; Academic Press: Cambridge, MA, USA, 2020; pp. 531–555. [Google Scholar]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6. [Google Scholar] [CrossRef][Green Version]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- D’archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Neilson, A.P.; Goodrich, K.M.; Ferruzzi, M.G. Bioavailability and Metabolism of Bioactive Compounds from Foods, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128029282. [Google Scholar]
- Gioxari, A.; Kogiannou, D.A.A.; Kalogeropoulos, N.; Kaliora, A.C. Phenolic Compounds: Bioavailability and Health Effects, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9780123849533. [Google Scholar]
- Shubina, V.S.; Kozina, V.I.; Shatalin, Y.V. Comparison of antioxidant properties of a conjugate of taxifolin with glyoxylic acid and selected flavonoids. Antioxidants 2021, 10, 1262. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ito, T.; Yano, H.; Kita, E.; Mikasa, K.; Okada, M.; Furutani, A.; Murono, Y.; Shibata, M.; Nishii, Y.; et al. Antioxidant potential in non-extractable fractions of dried persimmon (Diospyros kaki Thunb.). Food Chem. 2016, 202, 99–103. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Cao, J.; Ma, L. Evaluation of antioxidant properties of extractable and nonextractable polyphenols in peel and flesh tissue of different peach varieties. J. Food Process. Preserv. 2018, 42, e13624. [Google Scholar] [CrossRef]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef]
- Dueñas, M.; Muñoz-González, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef][Green Version]
- González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A. Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends Food Sci. Technol. 2017, 69, 281–288. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Havlik, J.; Edwards, C.A. Non-extractable Polyphenols and the Gut Microbiome. Food Chem. Funct. Anal. 2018, 69, 241–262. [Google Scholar] [CrossRef]
- González-Barrio, R.; Truchado, P.; García-Villalba, R.; Hervás, G.; Frutos, P.; Espín, J.C.; Tomás-Barberán, F.A. Metabolism of oak leaf ellagitannins and urolithin production in beef cattle. J. Agric. Food Chem. 2012, 60, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Borges, G.; Crozier, A. Berry flavonoids and phenolics: Bioavailability and evidence of protective effects. Br. J. Nutr. 2010, 104, 67–90. [Google Scholar] [CrossRef][Green Version]
- Di Pede, G.; Bresciani, L.; Brighenti, F.; Clifford, M.N.; Crozier, A.; Del Rio, D.; Mena, P.; Di Pede, G.; Bresciani, L.; Brighenti, F.; et al. In Vitro Faecal Fermentation of Monomeric and Oligomeric Flavan-3-ols: Catabolic Pathways and Stoichiometry. Mol. Nutr. Food Res. 2022, 66, 2101090. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Jalil, A.M.M.; Combet, E.; Edwards, C.A.; Garcia, A.L. Effect of β-Glucan and Black Tea in a Functional Bread on Short Chain Fatty Acid Production by the Gut Microbiota in a Gut Digestion/Fermentation Model. Int. J. Environ. Res. Public Health 2019, 16, 227. [Google Scholar] [CrossRef][Green Version]
- Bazzocco, S.; Mattila, I.; Guyot, S.; Renard, C.M.G.C.; Aura, A.M. Factors affecting the conversion of apple polyphenols to phenolic acids and fruit matrix to short-chain fatty acids by human faecal microbiota in vitro. Eur. J. Nutr. 2008, 47, 442–452. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends Food Sci. Technol. 2017, 69, 270–280. [Google Scholar] [CrossRef][Green Version]
- Bohn, T. Metabolic Fate of Bioaccessible and Non-bioaccessible Carotenoids. In Non-Extractable Polyphenols and Carotenoids; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 165–200. [Google Scholar] [CrossRef]
- Walayat, N. A review: Carotenoid types, sources and bioavailability in fruits and vegetables. J. Innov. Bioresearch 2017, 1, 79–85. [Google Scholar]
- Periago, M.J.; Martín-Pozuelo, G.; González-Barrio, R.; Santaella, M.; Gómez, V.; Vázquez, N.; Navarro-González, I.; García-Alonso, J. Effect of tomato juice consumption on the plasmatic lipid profile, hepatic HMGCR activity, and fecal short chain fatty acid content of rats. Food Funct. 2016, 7, 4460–4467. [Google Scholar] [CrossRef]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; De Lera, A.R.; Desmarchelier, C.; et al. From carotenoid intake to carotenoid blood and tissue concentrations-implications for dietary intake recommendations. Nutr. Rev. 2021, 79, 544–573. [Google Scholar] [CrossRef]
- Steiger, S.; Perez-Fons, L.; Fraser, P.D.; Sandmann, G. Biosynthesis of a novel C30 carotenoid in Bacillus firmus isolates. J. Appl. Microbiol. 2012, 113, 888–895. [Google Scholar] [CrossRef]
- Perez-Fons, L.; Steiger, S.; Khaneja, R.; Bramley, P.M.; Cutting, S.M.; Sandmann, G.; Fraser, P.D. Identification and the developmental formation of carotenoid pigments in the yellow/orange Bacillus spore-formers. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2011, 1811, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, R.; Mazzoli, A.; Cancelliere, R.; Bucci, A.; Naclerio, G.; Baccigalupi, L.; Cutting, S.M.; Ricca, E.; Iossa, S. Beneficial effects of carotenoid-producing cells of Bacillus indicus HU16 in a rat model of diet-induced metabolic syndrome. Benef. Microbes 2017, 8, 823–831. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Sun, X.; Sun, X.; Wang, S.; Xu, Y. Fucoxanthin Isolated from Undaria pinnatifida Can Interact with Escherichia coli and lactobacilli in the Intestine and Inhibit the Growth of Pathogenic Bacteria. JOUC 2019, 18, 926–932. [Google Scholar] [CrossRef]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Suárez-Martínez, C.; García-Viguera, C.; Moreno, D.A. Bioavailability and new biomarkers of cruciferous sprouts consumption. Food Res. Int. 2017, 100, 497–503. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef][Green Version]
- Luang-In, V.; Narbad, A.; Nueno-Palop, C.; Mithen, R.; Bennett, M.; Rossiter, J.T. The metabolism of methylsulfinylalkyl- and methylthioalkyl-glucosinolates by a selection of human gut bacteria. Mol. Nutr. Food Res. 2014, 58, 875–883. [Google Scholar] [CrossRef]
- Sikorska-Zimny, K.; Beneduce, L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients 2021, 13, 2750. [Google Scholar] [CrossRef]
- Martirosyan, D.; Lampert, T.; Ekblad, M. Classification and regulation of functional food proposed by the Functional Food Center. Funct. Food Sci. 2022, 2, 25–46. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Santos, A.P.; de Almeida de Melo, E.; Campos, A.; Lins, L.; Boyano-Orozco, L.C. Bioactive Compounds and Their Potential Use as Ingredients for Food and Its Application in Food Packaging. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 143–156. ISBN 9780128147757. [Google Scholar]
- Mohanty, S.; Singhal, K. Functional foods as personalised nutrition: Definitions and genomic insights. In Functional Food and Human Health; Springer: Singapore, 2018; pp. 513–535. ISBN 9789811311239. [Google Scholar]
- Viscione, L. Fibre-enriched beverages. In Fibre-Rich and Wholegrain Foods: Improving Quality; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 369–388. ISBN 9780857090386. [Google Scholar]
- Belović, M.; Torbica, A.; Pajić-Lijaković, I.; Mastilović, J. Development of low calorie jams with increased content of natural dietary fibre made from tomato pomace. Food Chem. 2017, 237, 1226–1233. [Google Scholar] [CrossRef]

| Concept | Definition | Reference |
|---|---|---|
| By-product | A substance or object resulting from a production process whose primary purpose is not the production of that substance or object, and which is not considered as waste, for which different conditions must be fulfilled | [9] |
| Field by-products | Plant materials that remain after harvesting | [10] |
| Process by-products | Plant materials discarded during processing because quality standards are not met | [10] |
| Industrial by-products | By-products generated from different industrial transformation processes of different physical and chemical materials | [10] |
| Food losses | Losses that take place at production, postharvest, and processing stages in the food supply chain | [2] |
| Food waste | Losses that take place at the retail and consumption stages at the end of the food supply chain | [2] |
| Circular economy | An economic model wherein planning, resourcing, procurement, production, and reprocessing are designed and managed, as both process and output, to maximise ecosystem functioning and human well-being | [11] |
| Bioeconomy | Encompasses the sustainable production of renewable resources from land, fisheries, and aquaculture environments and their conversion into food, feed, fibre, ingredients, bio-based products, and bioenergy, as well as related public goods | [12] |
| Fruit/Vegetable | TDF * | IDF | SDF | (Poly)phenols | Carotenoids | Glucosinolates | Reference |
|---|---|---|---|---|---|---|---|
| Pomace | |||||||
| Apple | 51.1 | 36.5 | 14.6 | 10.2 mg GAE/g | - | - | [28] |
| Redcurrant | 58.1 | 51.1 | 7.1 | 20.0 mAU min/g | - | - | [28] |
| Rowanberry | 67.2 | 59.5 | 7.7 | 37.0 mAU min/g | - | - | [28] |
| Tomato | 64.1 | 58.5 | 5.6 | 55.1 mg GAE/g | - | - | [28] |
| Mango | 15 | 7 | 8 | 130 mg GAE/g | - | - | [29] |
| Peel | |||||||
| Citrus | 67.4 | 62.5 | 4.9 | 1.0 mg GAE/g | - | - | [28] |
| Mango | 69.9 | 44.2 | 24.6 | 0.1 mg GAE/g | 5.6 µg β-carotene/g | - | [28] |
| Plantain | 64.3 | 56.9 | 7.5 | 15.2 mg QE/g | - | - | [28] |
| Tomato | 86.2 | 71.8 | 14.3 | 1.6 mg GAE/g | 30–40 µg lycopene/g | - | [25] |
| Seed | |||||||
| Avocado | 3–26 | - | - | 3–5 mg GAE/g | - | - | [28] |
| Grapes | 8.2 | - | - | 5 mg/g | - | - | [28] |
| Papaya | 8–9 | 2.5–3.4 | 5.2–5.4 | 34–92 mg GAE/g | - | - | [28] |
| Stalk | |||||||
| Broccoli | 38.2 | 35 | 3.2 | 1.6 mg GAE/g | - | 0.7 mg/g | [30] |
| Others | |||||||
| Orange | 58.2 | 46.9 | 11.3 | 4.2 mg GAE/g | 57.7 µg carotenoids/g | - | [31] |
| Guava | 89.8 | 86.1 | 3.7 | 2.5 mg GAE/g | 12.7 µg carotenoids/g | - | [31] |
| Passion fruit | 64.2 | 44.8 | 19.4 | 3.8 mg GAE/g | 84.6 µg carotenoids/g | - | [31] |
| Acerola | 48.6 | 34.4 | 14.2 | 19 mg/g | - | - | [32] |
| Cashew | 35 | 27 | 8 | 8 mg/g | - | - | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Gómez, V.; González-Barrio, R.; Periago, M.J. Interaction between Dietary Fibre and Bioactive Compounds in Plant By-Products: Impact on Bioaccessibility and Bioavailability. Antioxidants 2023, 12, 976. https://doi.org/10.3390/antiox12040976
Núñez-Gómez V, González-Barrio R, Periago MJ. Interaction between Dietary Fibre and Bioactive Compounds in Plant By-Products: Impact on Bioaccessibility and Bioavailability. Antioxidants. 2023; 12(4):976. https://doi.org/10.3390/antiox12040976
Chicago/Turabian StyleNúñez-Gómez, Vanesa, Rocío González-Barrio, and María Jesús Periago. 2023. "Interaction between Dietary Fibre and Bioactive Compounds in Plant By-Products: Impact on Bioaccessibility and Bioavailability" Antioxidants 12, no. 4: 976. https://doi.org/10.3390/antiox12040976
APA StyleNúñez-Gómez, V., González-Barrio, R., & Periago, M. J. (2023). Interaction between Dietary Fibre and Bioactive Compounds in Plant By-Products: Impact on Bioaccessibility and Bioavailability. Antioxidants, 12(4), 976. https://doi.org/10.3390/antiox12040976