Oxidative Stress and Cardiovascular Risk Factors: The Coronary Artery Risk Development in Young Adults (CARDIA) Study
Abstract
:1. Introduction
2. Materials and Methods
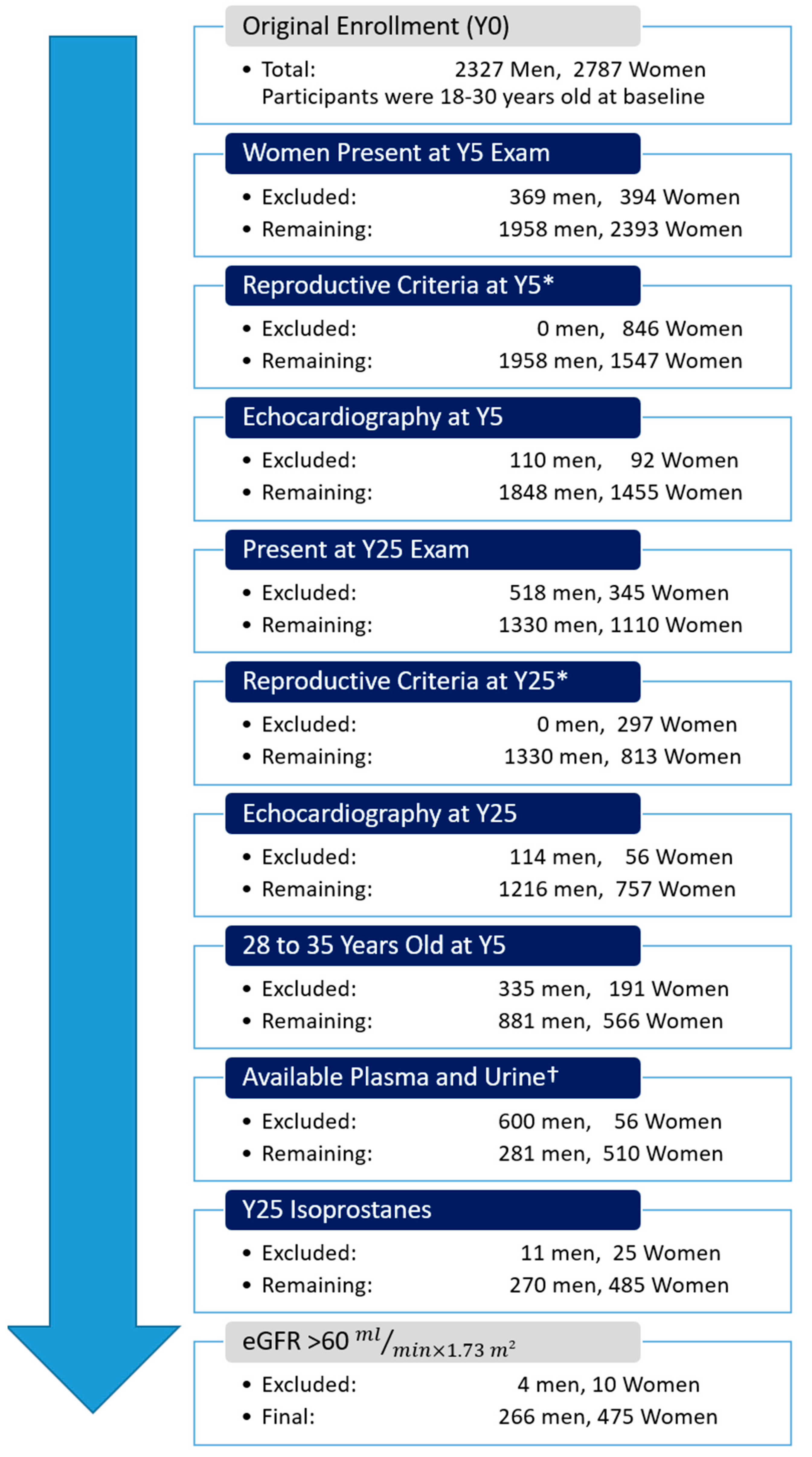
2.1. Study Population
2.2. Measurement of Exposure Variables
2.3. Isoprostane Measurements
2.4. Statistical Analysis
2.5. Sensitivity Analysis
3. Results
3.1. Population Characteristics and Urinary Assays
3.2. Urinary IsoP vs. Cardiovascular Risk Factors
3.3. Urinary IsoP m vs. Cardiovascular Risk Factors
3.4. Plasma Isoprostanes vs. Cardiovascular Risk Factors
3.5. Secondary and Sensitivity Analyses
4. Discussion
4.1. Female Sex
4.2. Smoking and Physical Activity
4.3. BMI
4.4. Diabetes
4.5. Creatinine and Kidney Function
4.6. Hypertension
4.7. Plasma Lipids and Cholesterol Medications
4.8. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.; Haskard, D.; Khamis, R. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis—Novel insights and future directions in diagnosis and therapy. Trends Cardiovasc. Med. 2019, 29, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Vaart, H.; Postma, D.S.; Timens, W.; ten Hacken, N.H. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax 2004, 59, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, Y.; Noma, K.; Yoshizumi, M.; Kihara, Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ. J. 2009, 73, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Sartori-Valinotti, J.C.; Iliescu, R.; Fortepiani, L.A.; Yanes, L.L.; Reckelhoff, J.F. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2007, 34, 938–945. [Google Scholar] [CrossRef]
- White, R.E.; Gerrity, R.; Barman, S.A.; Han, G. Estrogen and oxidative stress: A novel mechanism that may increase the risk for cardiovascular disease in women. Steroids 2010, 75, 788–793. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, E.; Domanico, F.; La Russa, D.; Pellegrino, D. Sex differences in oxidative stress biomarkers. Curr. Drug. Targets 2014, 15, 811–815. [Google Scholar] [CrossRef]
- Ide, T.; Tsutsui, H.; Ohashi, N.; Hayashidani, S.; Suematsu, N.; Tsuchihashi, M.; Tamai, H.; Takeshita, A. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 438–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.W.; Bruno, R.S.; Traber, M.G. Women and smokers have elevated urinary F(2)-isoprostane metabolites: A novel extraction and LC-MS methodology. Lipids 2008, 43, 925–936. [Google Scholar] [CrossRef] [Green Version]
- Milne, G.L.; Dai, Q.; Roberts, L.J., 2nd. The isoprostanes—25 years later. Biochim. Biophys. Acta 2015, 1851, 433–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadiiska, M.B.; Gladen, B.C.; Baird, D.D.; Germolec, D.; Graham, L.B.; Parker, C.E.; Nyska, A.; Wachsman, J.T.; Ames, B.N.; Basu, S.; et al. Biomarkers of oxidative stress study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005, 38, 698–710. [Google Scholar] [CrossRef]
- van’t Erve, T.J.; Kadiiska, M.B.; London, S.J.; Mason, R.P. Classifying oxidative stress by F2-isoprostane levels across human diseases: A meta-analysis. Redox Biol. 2017, 12, 582–599. [Google Scholar] [CrossRef]
- Halliwell, B.; Lee, C.Y. Using isoprostanes as biomarkers of oxidative stress: Some rarely considered issues. Antioxid. Redox Signal 2010, 13, 145–156. [Google Scholar] [CrossRef]
- Friedman, G.D.; Cutter, G.R.; Donahue, R.P.; Hughes, G.H.; Hulley, S.B.; Jacobs, D.R., Jr.; Liu, K.; Savage, P.J. CARDIA: Study design, recruitment, and some characteristics of the examined subjects. J. Clin. Epidemiol. 1988, 41, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Gross, M.; Steffes, M.; Jacobs, D.R., Jr.; Yu, X.; Lewis, L.; Lewis, C.E.; Loria, C.M. Plasma F2-isoprostanes and coronary artery calcification: The CARDIA Study. Clin. Chem. 2005, 51, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, D.R., Jr.; Hahn, L.P.; Haskell, W.L.; Pirie, P.; Sidney, S. Validity and Reliability of Short Physical Activity History: Cardia and the Minnesota Heart Health Program. J. Cardiopulm. Rehabil. 1989, 9, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Jenner, A.M.; Halliwell, B. Rapid preparation of human urine and plasma samples for analysis of F2-isoprostanes by gas chromatography-mass spectrometry. Biochem. Biophys. Res. Commun. 2004, 320, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R., Jr.; Murtaugh, M.A.; Steffes, M.; Yu, X.; Roseman, J.; Goetz, F.C. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: The Coronary Artery Risk Development in Young Adults Study. Am. J. Epidemiol. 2002, 155, 1114–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Keaney, J.F., Jr.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J.; et al. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Il’yasova, D.; Wang, F.; Spasojevic, I.; Base, K.; D’Agostino, R.B., Jr.; Wagenknecht, L.E. Racial differences in urinary F2-isoprostane levels and the cross-sectional association with BMI. Obesity 2012, 20, 2147–2150. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Niemann, B.; Rohrbach, S.; Miller, M.R.; Newby, D.E.; Fuster, V.; Kovacic, J.C. Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution: Part 3 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 230–251. [Google Scholar] [CrossRef]
- Sack, M.N.; Fyhrquist, F.Y.; Saijonmaa, O.J.; Fuster, V.; Kovacic, J.C. Basic Biology of Oxidative Stress and the Cardiovascular System: Part 1 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 196–211. [Google Scholar] [CrossRef]
- Munzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef]
- Myung, S.K.; Ju, W.; Cho, B.; Oh, S.W.; Park, S.M.; Koo, B.K.; Park, B.J.; Korean Meta-Analysis Study Group. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 346, f10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.; Milne, G.L.; Sandler, D.P.; Nichols, H.B. Oxidative stress in relation to diet and physical activity among premenopausal women. Br. J. Nutr. 2016, 116, 1416–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savini, I.; Catani, M.V.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-associated oxidative stress: Strategies finalized to improve redox state. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Il’yasova, D.; Wang, F.; Spasojevic, I.; Base, K.; D’Agostino, R.B., Jr.; Wagenknecht, L.E. Urinary F2-isoprostanes, obesity, and weight gain in the IRAS cohort. Obesity 2012, 20, 1915–1921. [Google Scholar] [CrossRef] [Green Version]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal 2017, 26, 501–518. [Google Scholar] [CrossRef] [Green Version]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; de Bittencourt, P.I., Jr.; Newsholme, P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and beta-Cell Dysfunction. Oxid. Med. Cell Longev. 2015, 2015, 181643. [Google Scholar] [CrossRef] [Green Version]
- Feillet-Coudray, C.; Chone, F.; Michel, F.; Rock, E.; Thieblot, P.; Rayssiguier, Y.; Tauveron, I.; Mazur, A. Divergence in plasmatic and urinary isoprostane levels in type 2 diabetes. Clin. Chim. Acta 2002, 324, 25–30. [Google Scholar] [CrossRef]
- Ma, E.; Ingram, K.H.; Milne, G.L.; Garvey, W.T. F2-Isoprostanes Reflect Oxidative Stress Correlated With Lean Mass and Bone Density but Not Insulin Resistance. J. Endocr. Soc. 2017, 1, 436–448. [Google Scholar] [CrossRef] [Green Version]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Goff, D.C., Jr.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 51. [Google Scholar] [CrossRef] [Green Version]
- Il’yasova, D.; Morrow, J.D.; Wagenknecht, L.E. Urinary F2-isoprostanes are not associated with increased risk of type 2 diabetes. Obes. Res. 2005, 13, 1638–1644. [Google Scholar] [CrossRef]
- Nerpin, E.; Helmersson-Karlqvist, J.; Riserus, U.; Sundstrom, J.; Larsson, A.; Jobs, E.; Basu, S.; Ingelsson, E.; Arnlov, J. Inflammation, oxidative stress, glomerular filtration rate, and albuminuria in elderly men: A cross-sectional study. BMC Res. Notes 2012, 5, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehouse, W.; Quimby, J.; Wan, S.; Monaghan, K.; Robbins, R.; Trepanier, L.A. Urinary F2-Isoprostanes in Cats with International Renal Interest Society Stage 1-4 Chronic Kidney Disease. J. Vet. Intern. Med. 2017, 31, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Roberts, L.J., 2nd. The isoprostanes. Current knowledge and directions for future research. Biochem. Pharmacol. 1996, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Loperena, R.; Harrison, D.G. Oxidative Stress and Hypertensive Diseases. Med. Clin. N. Am. 2017, 101, 169–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munzel, T.; Keaney, J.F., Jr. Are ACE inhibitors a “magic bullet” against oxidative stress? Circulation 2001, 104, 1571–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pignatelli, P.; Carnevale, R.; Pastori, D.; Cangemi, R.; Napoleone, L.; Bartimoccia, S.; Nocella, C.; Basili, S.; Violi, F. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation 2012, 126, 92–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cangemi, R.; Loffredo, L.; Carnevale, R.; Perri, L.; Patrizi, M.P.; Sanguigni, V.; Pignatelli, P.; Violi, F. Early decrease of oxidative stress by atorvastatin in hypercholesterolaemic patients: Effect on circulating vitamin E. Eur. Heart J. 2008, 29, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Sinzinger, H.; Oguogho, A. Variable influence of statins on isoprostanes in hyperlipidemia. Adv. Exp. Med. Biol. 2003, 525, 209–212. [Google Scholar] [CrossRef]
- Negi, S.; Shukrullah, I.; Veledar, E.; Bloom, H.L.; Jones, D.P.; Dudley, S.C. Statin therapy for the prevention of atrial fibrillation trial (SToP AF trial). J. Cardiovasc. Electrophysiol. 2011, 22, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, S.T.; Andersen, J.T.; Nielsen, T.K.; Cejvanovic, V.; Petersen, K.M.; Henriksen, T.; Weimann, A.; Lykkesfeldt, J.; Poulsen, H.E. Simvastatin and oxidative stress in humans: A randomized, double-blinded, placebo-controlled clinical trial. Redox Biol. 2016, 9, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Scheffer, P.G.; Schindhelm, R.K.; van Verschuer, V.M.; Groenemeijer, M.; Simsek, S.; Smulders, Y.M.; Nanayakkara, P.W. No effect of atorvastatin and simvastatin on oxidative stress in patients at high risk for cardiovascular disease. Neth J. Med. 2013, 71, 359–365. [Google Scholar] [PubMed]
- Sathyapalan, T.; Shepherd, J.; Atkin, S.L.; Kilpatrick, E.S. The effect of atorvastatin and simvastatin on vitamin D, oxidative stress and inflammatory marker concentrations in patients with type 2 diabetes: A crossover study. Diabetes Obes. Metab. 2013, 15, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Kei, A.; Tellis, C.; Liberopoulos, E.; Tselepis, A.; Elisaf, M. Effect of switch to the highest dose of rosuvastatin versus add-on-statin fenofibrate versus add-on-statin nicotinic acid/laropiprant on oxidative stress markers in patients with mixed dyslipidemia. Cardiovasc. Ther. 2014, 32, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voutilainen, S.; Morrow, J.D.; Roberts, L.J., 2nd; Alfthan, G.; Alho, H.; Nyyssonen, K.; Salonen, J.T. Enhanced in vivo lipid peroxidation at elevated plasma total homocysteine levels. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1263–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, J.M.; Barden, A.E.; Loke, W.M.; Croft, K.D.; Puddey, I.B.; Mori, T.A. HDL is the major lipoprotein carrier of plasma F2-isoprostanes. J. Lipid Res. 2009, 50, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, O.S.; Galano, J.M.; Pavlickova, T.; Revol-Cavalier, J.; Vigor, C.; Lee, J.C.; Oger, C.; Durand, T. Moving forward with isoprostanes, neuroprostanes and phytoprostanes: Where are we now? Essays Biochem. 2020, 64, 463–484. [Google Scholar] [CrossRef]



| Women (n = 475) | Men (n = 266) | p | |
|---|---|---|---|
| Age, years | 52.2 ± 2.2 | 52.1 ± 2.3 | 0.46 |
| Postmenopausal | 279 (58.7%) | - | - |
| Black Race | 183 (38.5%) | 106 (39.8%) | 0.72 |
| Body Mass Index (kg/m2) | 30.0 ± 7.8 | 29.0 ± 5.8 | 0.06 |
| College Attainment | 280 (59.1%) | 139 (52.3%) | 0.07 |
| Smoking | 0.68 | ||
| Never | 288 (61.0%) | 169 (63.8%) | |
| Former | 118 (25.3%) | 59 (22.3%) | |
| Current | 63 (13.6%) | 37 (13.8%) | |
| Cumulative Pack years | 1.0 ± 3.4 | 1.6 ± 5.0 | 0.06 |
| Physical Activity, EU * | 304 ± 230 | 404 ± 286 | <0.01 |
| Systolic Blood Pressure, mmHg | 116 ± 16 | 121 ± 15 | <0.01 |
| Total Cholesterol, mg/dL | 198 ± 36 | 189 ± 35 | <0.01 |
| HDL Cholesterol, mg/dL | 65 ± 17 | 51 ± 16 | <0.01 |
| Triglycerides, mg/dL | 98 ± 59 | 136 ± 138 | <0.01 |
| Serum Creatinine, mg/dL | 0.75 ± 0.12 | 0.97 ± 0.16 | <0.01 |
| Diabetes | 54 (11.4%) | 47 (17.7%) | 0.02 |
| Fasting Glucose, mg/dL | 95 ± 21 | 105 ± 31 | <0.01 |
| Antihypertensive Medication Use | 101 (21.2%) | 76 (28.6%) | 0.03 |
| Cholesterol Lowering Medication Use | 62 (13.2%) | 49 (18.6%) | 0.05 |
| Urinary Creatinine, mg/dL | 105 ± 72 | 150 ± 86 | <0.01 |
| Urinary IsoP, ng/g Creatinine † | 880 [75, 1379] | 704 [464, 1019] | <0.01 |
| Urinary IsoP-M, ng/g Creatinine ‡ | 1675 [1118, 2726] | 1284 [788, 2037] | <0.01 |
| Model 1 (n = 738) | Model 2 (n = 738) | Model 3 (n = 731) | Model 4 (n = 723) | |
|---|---|---|---|---|
| Age (per SD) | 1.04 (0.98 to 1.10) | 1.05 (0.99 to 1.11) | 1.05 (0.99 to 1.12) | 1.05 (0.99 to 1.11) |
| Female Sex (vs. Male) | 1.28 (1.14 to 1.45) | 1.04 (0.89 to 1.22) | 1.07 (0.91 to 1.25) | 1.00 (0.84 to 1.19) |
| Black Race (vs. White) | 0.83 (0.72 to 0.94) | 0.88 (0.77 to 1.01) | 0.87 (0.76 to 1.00) | 0.84 (0.72 to 0.97) |
| Body Mass Index (per SD) | 1.01 (0.95 to 1.07) | 1.00 (0.95 to 1.07) | 1.00 (0.94 to 1.07) | 1.02 (0.95 to 1.09) |
| Current Smoking (vs. Never) * | 1.23 (1.03 to 1.47) | 1.20 (1.01 to 1.43) | - | 1.21 (1.01 to 1.44) |
| Former Smoking (vs. Never) * | 0.93 (0.81 to 1.08) | 0.94 (0.82 to 1.09) | - | 0.95 (0.82 to 1.10) |
| Cumulative Pack years (per SD) * | 1.10 (1.04 to 1.17) | 1.09 (1.03 to 1.15) | - | 1.09 (1.02 to 1.15) |
| Diabetes | 0.94 (0.79 to 1.12) | 0.91 (0.77 to 1.09) | 0.90 (0.75 to 1.07) | 0.95 (0.79 to 1.15) |
| Blood Pressure Medication | 0.92 (0.80 to 1.07) | 0.92 (0.80 to 1.07) | 0.91 (0.79 to 1.05) | 0.92 (0.79 to 1.08) |
| Systolic BP (per SD) | 1.06 (1.00 to 1.13) | 1.05 (0.98 to 1.12) | 1.05 (0.98 to 1.12) | 1.05 (0.99 to 1.12) |
| Cholesterol-lowering Medication | 0.84 (0.71 to 0.99) | 0.84 (0.71 to 0.99) | 0.84 (0.72 to 1.00) | 0.86 (0.72 to 1.03) |
| Total Cholesterol (per SD) | 1.01 (0.95 to 1.07) | 1.02 (0.96 to 1.08) | 1.02 (0.96 to 1.08) | 0.97 (0.91 to 1.05) |
| HDL Cholesterol (per SD) | 1.05 (0.98 to 1.12) | 1.05 (0.98 to 1.12) | 1.06 (0.99 to 1.13) | 1.07 (0.99 to 1.16) |
| Log Triglycerides (per SD) | 1.00 (0.94 to 1.06) | 1.00 (0.94 to 1.07) | 0.99 (0.93 to 1.06) | 1.02 (0.94 to 1.10) |
| Serum Creatinine (per SD) | 0.85 (0.79 to 0.92) | - | 0.86 (0.80 to 0.93) | 0.87 (0.80 to 0.94) |
| Physical Activity (per SD) | 0.92 (0.87 to 0.98) | 0.92 (0.87 to 0.98) | 0.93 (0.87 to 0.98) | 0.92 (0.87 to 0.98) |
| Model 1 (n = 738) | Model 2 (n = 738) | Model 3 (n = 731) | Model 4 (n = 723) | |
|---|---|---|---|---|
| Age (per SD) | 1.04 (0.98 to 1.10) | 1.05 (0.99 to 1.11) | 1.05 (0.99 to 1.11) | 1.04 (0.98 to 1.10) |
| Female Sex (vs. Male Sex) | 1.36 (1.20 to 1.54) | 1.18 (1.01 to 1.38) | 1.20 (1.02 to 1.40) | 1.16 (0.97 to 1.39) |
| BlackRace (vs. White) | 0.92 (0.80 to 1.05) | 0.96 (0.83 to 1.10) | 0.94 (0.82 to 1.08) | 0.94 (0.81 to 1.09) |
| BMI (per SD) | 1.13 (1.06 to 1.20) | 1.13 (1.06 to 1.20) | 1.13 (1.06 to 1.20) | 1.14 (1.06 to 1.22) |
| Current Smoking (vs. Never)* | 1.61 (1.35 to 1.93) | 1.59 (1.33 to 1.90) | - | 1.55 (1.22 to 1.87) |
| Former Smoking (vs. Never)* | 1.14 (0.99 to 1.31) | 1.15 (0.99 to 1.32) | - | 1.13 (0.98 to 1.31) |
| Cumulative Pack years (per SD)* | 1.13 (1.07 to 1.20) | 1.13 (1.06 to 1.20) | - | 1.12 (1.06 to 1.19) |
| Diabetes | 0.88 (0.72 to 1.08) | 0.86 (0.70 to 1.06) | 0.86 (0.70 to 1.06) | 0.84 (0.70 to 1.02) |
| Blood Pressure Medication | 1.10 (0.95 to 1.28) | 1.10 (0.96 to 1.28) | 1.09 (0.95 to 1.26) | 1.08 (0.93 to 1.26) |
| Systolic BP (per SD) | 1.04 (0.97 to 1.10) | 1.03 (0.96 to 1.09) | 1.02 (0.96 to 1.09) | 1.01 (0.95 to 1.08) |
| Cholesterol-lowering Meds | 1.06 (0.89 to 1.25) | 1.06 (0.90 to 1.25) | 1.05 (0.89 to 1.24) | 1.03 (0.87 to 1.24) |
| Total Cholesterol (per SD) | 1.00 (0.94 to 1.06) | 1.01 (0.95 to 1.07) | 1.00 (0.94 to 1.06) | 0.94 (0.87 to 1.01) |
| HDL Cholesterol (per SD) | 1.06 (0.99 to 1.14) | 1.06 (0.99 to 1.13) | 1.06 (0.99 to 1.13) | 1.12 (1.04 to 1.22) |
| Log Triglycerides (per SD) | 1.07 (1.00 to 1.14) | 1.07 (1.01 to 1.14) | 1.05 (0.99 to 1.12) | 1.12 (1.03 to 1.21) |
| Serum Creatinine (per SD) | 0.90 (0.83 to 0.97) | 0.90 (0.83 to 0.97) | 0.91 (0.84 to 0.98) | 0.91 (0.84 to 0.98) |
| Physical Activity (per SD) | 0.98 (0.92 to 1.04) | 0.98 (0.92 to 1.04) | 0.98 (0.93 to 1.05) | 0.99 (0.93 to 1.05) |
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Age (per SD) | 0.98 (0.97 to 1.00) | 0.98 (0.97 to 1.00) | 0.98 (0.97 to 1.00) | 0.97 (0.96 to 0.99) |
| Female Sex (vs. Male Sex) | 1.26 (1.23 to 1.30) | 1.27 (1.23 to 1.31) | 1.28 (1.24 to 1.32) | 1.21 (1.17 to 1.26) |
| BlackRace (vs. White) | 0.90 (0.87 to 0.93) | 0.89 (0.86 to 0.92) | 0.89 (0.86 to 0.92) | 0.89 (0.86 to 0.92) |
| BMI (per SD) | 1.16 (1.14 to 1.18) | 1.16 (1.14 to 1.18) | 1.17 (1.15 to 1.18) | 1.18 (1.16 to 1.20) |
| Current Smoking (vs. Never) | 1.12 (1.08 to 1.16) | 1.12 (1.08 to 1.16) | 1.12 (1.08 to 1.16) | 1.11 (1.07 to 1.15) |
| Former Smoking (vs. Never) | 1.00 (0.96 to 1.04) | 1.00 (0.96 to 1.04) | 1.00 (0.96 to 1.04) | 0.99 (0.95 to 1.03) |
| Cumulative Pack years* | Not Available | |||
| Diabetes | 0.90 (0.84 to 0.93) | 0.90 (0.83 to 0.97) | 0.89 (0.83 to 0.96) | 0.89 (0.83 to 0.96) |
| Blood Pressure Medication | 0.95 (0.90 to 1.00) | 0.94 (0.89 to 1.00) | 0.95 (0.89 to 1.00) | 0.92 (0.87 to 0.98) |
| Systolic BP (per SD) | 1.02 (1.00 to 1.04) | 1.02 (1.00 to 1.03) | 1.02 (1.00 to 1.03) | 1.01 (1.00 to 1.03) |
| Cholesterol-lowering Meds | 1.07 (0.97 to 1.18) | 1.07 (0.97 to 1.18) | 1.07 (0.97 to 1.19) | 1.11 (1.01 to 1.23) |
| Total Cholesterol (per SD) | 1.02 (1.00 to 1.03) | 1.02 (1.00 to 1.03) | 1.02 (1.00 to 1.03) | 0.98 (0.96 to 1.00) |
| HDL Cholesterol (per SD) | 1.07 (1.05 to 1.09) | 1.07 (1.05 to 1.09) | 1.07 (1.06 to 1.09) | 1.11 (1.09 to 1.13) |
| Log Triglycerides (per SD) | 1.03 (1.01 to 1.04) | 1.03 (1.01 to 1.05) | 1.02 (1.01 to 1.04) | 1.07 (1.05 to 1.10) |
| Serum Creatinine (per SD) | 1.01 (1.00 to 1.03) | 1.01 (1.00 to 1.03) | 1.01 (1.00 to 1.03) | 1.02 (1.00 to 1.03) |
| Physical Activity (per SD) | 0.97 (0.96 to 0.99) | 0.97 (0.96 to 0.99) | 0.98 (0.96 to 0.99) | 0.97 (0.95 to 0.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heravi, A.S.; Zhao, D.; Michos, E.D.; Doria De Vasconcellos, H.; Ambale-Venkatesh, B.; Lloyd-Jones, D.; Schreiner, P.J.; Reis, J.P.; Shikany, J.M.; Lewis, C.E.; et al. Oxidative Stress and Cardiovascular Risk Factors: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Antioxidants 2023, 12, 555. https://doi.org/10.3390/antiox12030555
Heravi AS, Zhao D, Michos ED, Doria De Vasconcellos H, Ambale-Venkatesh B, Lloyd-Jones D, Schreiner PJ, Reis JP, Shikany JM, Lewis CE, et al. Oxidative Stress and Cardiovascular Risk Factors: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Antioxidants. 2023; 12(3):555. https://doi.org/10.3390/antiox12030555
Chicago/Turabian StyleHeravi, Amir S., Di Zhao, Erin D. Michos, Henrique Doria De Vasconcellos, Bharath Ambale-Venkatesh, Donald Lloyd-Jones, Pamela J. Schreiner, Jared P. Reis, James M. Shikany, Cora E. Lewis, and et al. 2023. "Oxidative Stress and Cardiovascular Risk Factors: The Coronary Artery Risk Development in Young Adults (CARDIA) Study" Antioxidants 12, no. 3: 555. https://doi.org/10.3390/antiox12030555
APA StyleHeravi, A. S., Zhao, D., Michos, E. D., Doria De Vasconcellos, H., Ambale-Venkatesh, B., Lloyd-Jones, D., Schreiner, P. J., Reis, J. P., Shikany, J. M., Lewis, C. E., Ndumele, C. E., Guallar, E., Ouyang, P., Hoogeveen, R. C., Lima, J. A. C., Post, W. S., & Vaidya, D. (2023). Oxidative Stress and Cardiovascular Risk Factors: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Antioxidants, 12(3), 555. https://doi.org/10.3390/antiox12030555






