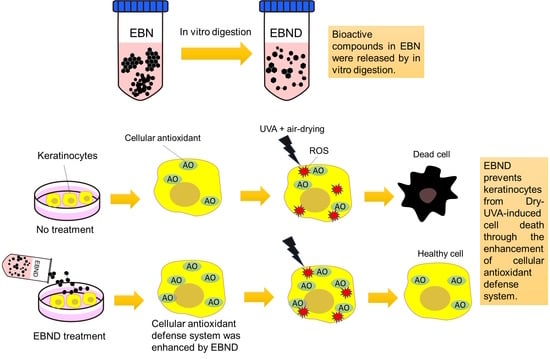
Enzyme-Digested Edible Bird’s Nest (EBND) Prevents UV and Arid Environment-Induced Cellular Oxidative Stress, Cell Death and DNA Damage in Human Skin Keratinocytes and Three-Dimensional Epithelium Equivalents
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Edible Bird’s Nest and SA
2.2. SDS-PAGE Analysis
2.3. Scanning Electron Microscope (SEM) Analysis
2.4. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.5. Cell Culture
2.6. 3D-Cultured Epithelium Equivalents
2.7. Air-Drying and UV Irradiation
2.8. Cell Viability Assay
2.9. Apoptosis Detection Methods
2.9.1. Annexin V/PI Staining
2.9.2. Terminal Deoxynucleotidyl Transferase Deoxyuridine Triphosphate Nick End Labeling (TUNEL) Assay
2.10. Enzyme-Linked Immuno-Sorbent Assay (ELISA)
2.11. Cellular ROS Detection
2.12. Determination of γ-H2A.X Foci Formation
2.13. Statistical Analysis
3. Results
3.1. Features of EBN Material and Solutions of EBN and EBND
3.2. ORAC Capacity of EBN, EBND and SA under Cell-Free Conditions
3.3. Effects of EBND and SA on Cell Viability and Cellular ORAC Capacity in HaCaT Cells
3.4. EBND Protects Air-Drying- and UV- Induced Membrane Damage and Cell Death in HaCaT Cells
3.5. EBND Diminishes Dryness and UVA-Induced Intracellular ROS and DNA Double-Strand Break in HaCaT Cells
3.6. EBND Diminishes Dryness and UVA-Induced Apoptosis and ROS Production in 3D Epithelium Equivalents
3.7. EBND Suppresses UVB-Induced Inflammatory Cytokines Secretion in HaCaT Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tobi, S.E.; Gilbert, M.; Paul, N.; McMillan, T.J. The green tea polyphenol, epigallocatechin-3-gallate, protects against the oxidative cellular and genotoxic damage of UVA radiation. Int. J. Cancer 2002, 102, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Valencia, A.; Kochevar, I.E. Nox1-based NADPH oxidase is the major source of UVA-induced reactive oxygen species in human keratinocytes. J. Investig. Dermatol. 2008, 128, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Karran, P.; Brem, R. Protein oxidation, UVA and human DNA repair. DNA Repair (Amst.) 2016, 44, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Greinert, R.; Volkmer, B.; Henning, S.; Breitbart, E.W.; Greulich, K.O.; Cardoso, M.C.; Rapp, A. UVA-induced DNA double-strand breaks result from the repair of clustered oxidative DNA damages. Nucleic Acids Res. 2012, 40, 10263–10273. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, C. Cellular aspects of photocarcinogenesis. Photochem. Photobiol. Sci. 2006, 5, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K. UV-induced immune suppression and photocarcinogenesis: Chemoprevention by dietary botanical agents. Cancer Lett. 2007, 255, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Yang, A.Y.; Huang, M.T.; Liu, Y.; Lee, J.H.; Khor, T.O.; Su, Z.Y.; Shu, L.; Lu, Y.; Conney, A.H.; et al. Nrf2 null enhances UVB-induced skin inflammation and extracellular matrix damages. Cell Biosci. 2014, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Miwa, N. Hydrogen-Rich Water Prevents Dehydration-Induced Cellular Oxidative Stress and Cell Death in Human Skin Keratinocytes. Hydrogen 2022, 3, 62–71. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Hou, Z.; Abdullah, M.A.; Ideris, A.; Ismail, N. Edible Bird’s Nest attenuates high fat diet-induced oxidative stress and inflammation via regulation of hepatic antioxidant and inflammatory genes. BMC Complement. Altern. Med. 2015, 15, 310. [Google Scholar] [CrossRef]
- Hu, Q.; Li, G.; Yao, H.; He, S.; Li, H.; Liu, S.; Wu, Y.; Lai, X. Edible bird’s nest enhances antioxidant capacity and increases lifespan in Drosophila Melanogaster. Cell Mol. Biol. (Noisy-le-Grand) 2016, 62, 116–122. [Google Scholar] [PubMed]
- Vimala, B.; Hussain, H.; Wan Nazaimoon, W.M. Effects of edible bird’s nest on tumour necrosis factor-α secretion, nitric oxide production and cell viability of lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Agric. Immunol. 2012, 23, 303–314. [Google Scholar] [CrossRef]
- Zainal Abidin, F.; Hui, C.K.; Luan, N.S.; Mohd Ramli, E.S.; Hun, L.T.; Abd Ghafar, N. Effects of edible bird’s nest (EBN) on cultured rabbit corneal keratocytes. BMC Complement. Altern. Med. 2011, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Guo, M.; Wu, K.Q.; Liao, Z.; Guan, D.; Dong, T.T.; Tong, P.; Tsim, K. Edible Bird’s Nest, an Asian Health Food Supplement, Possesses Moisturizing Effect by Regulating Expression of Filaggrin in Skin Keratinocyte. Front. Pharmacol. 2021, 12, 685982. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liao, F.; Ide, R.; Horie, T.; Fan, Y.; Saiki, C.; Miwa, N. Enzyme-Digested Colla Corii Asini (E’jiao) prevents hydrogen peroxide-induced cell death and accelerates amyloid beta clearance in neuronal-like PC12 cells. Neural Regen. Res. 2020, 15, 2270–2272. [Google Scholar] [CrossRef]
- Xiao, L.; Liao, F.; Fan, Y.; Miwa, N. Enzyme-Digested Colla Corii Asini (E’jiao) accelerates wound healing and prevents ultraviolet A-induced collagen synthesis decline and wrinkle formation in three-dimensional skin equivalents. Hum. Cell 2020, 33, 1056–1067. [Google Scholar] [CrossRef]
- Xiao, L.; Mochizuki, M.; Nakahara, T.; Miwa, N. Hydrogen-Generating Silica Material Prevents UVA-Ray-Induced Cellular Oxidative Stress, Cell Death, Collagen Loss and Melanogenesis in Human Cells and 3D Skin Equivalents. Antioxidants 2021, 10, 76. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Xiao, L.; Saiki, C.; Okamura, H. Oxidative Stress-Tolerant Stem Cells from Human Exfoliated Deciduous Teeth Decrease Hydrogen Peroxide-Induced Damage in Organotypic Brain Slice Cultures from Adult Mice. Int. J. Mol. Sci. 2019, 20, 1858. [Google Scholar] [CrossRef]
- Burgess, A.; Vigneron, S.; Brioudes, E.; Labbé, J.C.; Lorca, T.; Castro, A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl. Acad. Sci. USA 2010, 107, 12564–12569. [Google Scholar] [CrossRef]
- Xiao, L.; Miwa, N. Hydrogen-rich water achieves cytoprotection from oxidative stress injury in human gingival fibroblasts in culture or 3D-tissue equivalents, and wound-healing promotion, together with ROS-scavenging and relief from glutathione diminishment. Hum. Cell 2017, 30, 72–87. [Google Scholar] [CrossRef]
- Xiao, L.; Sakagami, H.; Miwa, N. A New Method for Testing Filtration Efficiency of Mask Materials Under Sneeze-Like Pressure. In Vivo 2020, 34, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wang, D.L. Nutrient composition and active components of edible bird nest. New Food Indust. 2022, 64, 579–582. [Google Scholar]
- Kong, H.K.; Chan, Z.; Yan, S.W.; Lo, P.Y.; Wong, W.T.; Wong, K.H.; Lo, C.L. Revealing the species-specific genotype of the edible bird’s nest-producing swiftlet, Aerodramus fuciphagus and the proteome of edible bird’s nest. Food Res. Int. 2022, 160, 111670. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cao, J.; Wang, Y.; Chen, Y.; Jiang, L. A comprehensive review of edible bird’s nest. Food Res. Int. 2021, 140, 109875. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Liu, D. Sketch of the edible bird’s nest and its important bioactivities. Food Res. Int. 2012, 48, 559–567. [Google Scholar] [CrossRef]
- Halimi, N.M.; Kasim, Z.M.; Babji, A.S. Nutritional Composition and Solubility of Edible Bird Nest (Aerodramus fuchiphagus). AIP Conf. Proc. 2014, 1614, 476. [Google Scholar] [CrossRef]
- Hun Lee, T.; Hau Lee, C.; Alia Azmi, N.; Kavita, S.; Wong, S.; Znati, M.; Ben Jannet, H. Characterization of Polar and Non-Polar Compounds of House Edible Bird’s Nest (EBN) from Johor, Malaysia. Chem. Biodivers. 2020, 17, e1900419. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M. In vitro bioaccessibility and antioxidant properties of edible bird’s nest following simulated human gastro-intestinal digestion. BMC Complement. Altern. Med. 2014, 14, 468. [Google Scholar] [CrossRef]
- Frödin, T.; Molin, L.; Skogh, M. Effects of single doses of UVA, UVB, and UVC on skin blood flow, water content, and barrier function measured by laser-Doppler flowmetry, optothermal infrared spectrometry, and evaporimetry. Photodermatology 1988, 5, 187–195. [Google Scholar]
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral Intake of Collagen Peptide Attenuates Ultraviolet B Irradiation-Induced Skin Dehydration In Vivo by Regulating Hyaluronic Acid Synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009, 19, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef]
- Bennett, E.S. Isoform-specific effects of sialic acid on voltage-dependent Na+ channel gating: Functional sialic acids are localized to the S5-S6 loop of domain I. J. Physiol. 2002, 538, 675–690. [Google Scholar] [CrossRef]
- Schwetz, T.A.; Norring, S.A.; Ednie, A.R.; Bennett, E.S. Sialic acids attached to O-glycans modulate voltage-gated potassium channel gating. J. Biol. Chem. 2011, 286, 4123–4132. [Google Scholar] [CrossRef]
- Böhm, S.; Schwab, I.; Lux, A.; Nimmerjahn, F. The role of sialic acid as a modulator of the anti-inflammatory activity of IgG. Semin. Immunopathol. 2012, 34, 443–453. [Google Scholar] [CrossRef]
- Payazdan, M.; Khatami, S.; Galehdari, H.; Delfan, N.; Shafiei, M.; Heydaran, S. The anti-inflammatory effects of sialic acid on the human glia cells by the upregulation of IL-4 and IL-10 genes’ expressions. Gene Rep. 2021, 24, 101218. [Google Scholar] [CrossRef]
- Babji, A.S.; Etty Syarmila, I.K.; D’Aliah, N.; Nurul Nadia, M.; Hadi Akbar, D.; Norrakiah, A.S.; Ghassem, M.; Najafian, L.; Salma, M.Y. Assessment on bioactive components of hydrolysed edible bird nest. Int. Food Res. J. 2018, 25, 1936–1941. [Google Scholar]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Biochem. Soc. Trans. 2005, 33, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Hu, C.-A.A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef]








| Experimental Group (n = 8) | ORAC Value [Trolox Equivalents (µM) (Mean ± SD)] | p Value (vs. NC) |
|---|---|---|
| NC | 166.7 ± 3.51 | |
| SA 250 µg/mL | 169.9 ± 3.03 | 1.000 |
| SA 500 µg/mL | 172.4 ± 2.65 | 0.055 |
| SA 1250 µg/mL | 176.1 ± 2.87 | 0.001 ** |
| EBND 50 µg/mL | 177.5 ± 1.69 | 0.000 *** |
| EBND 125 µg/mL | 176.7 ± 2.22 | 0.000 *** |
| EBND 250 µg/mL | 172.8 ± 3.56 | 0.088 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Shimamura, N.; Mochizuki, M.; Nakahara, T.; Sunada, K.; Xiao, L. Enzyme-Digested Edible Bird’s Nest (EBND) Prevents UV and Arid Environment-Induced Cellular Oxidative Stress, Cell Death and DNA Damage in Human Skin Keratinocytes and Three-Dimensional Epithelium Equivalents. Antioxidants 2023, 12, 609. https://doi.org/10.3390/antiox12030609
Wang D, Shimamura N, Mochizuki M, Nakahara T, Sunada K, Xiao L. Enzyme-Digested Edible Bird’s Nest (EBND) Prevents UV and Arid Environment-Induced Cellular Oxidative Stress, Cell Death and DNA Damage in Human Skin Keratinocytes and Three-Dimensional Epithelium Equivalents. Antioxidants. 2023; 12(3):609. https://doi.org/10.3390/antiox12030609
Chicago/Turabian StyleWang, Dongliang, Naohiro Shimamura, Mai Mochizuki, Taka Nakahara, Katsuhisa Sunada, and Li Xiao. 2023. "Enzyme-Digested Edible Bird’s Nest (EBND) Prevents UV and Arid Environment-Induced Cellular Oxidative Stress, Cell Death and DNA Damage in Human Skin Keratinocytes and Three-Dimensional Epithelium Equivalents" Antioxidants 12, no. 3: 609. https://doi.org/10.3390/antiox12030609
APA StyleWang, D., Shimamura, N., Mochizuki, M., Nakahara, T., Sunada, K., & Xiao, L. (2023). Enzyme-Digested Edible Bird’s Nest (EBND) Prevents UV and Arid Environment-Induced Cellular Oxidative Stress, Cell Death and DNA Damage in Human Skin Keratinocytes and Three-Dimensional Epithelium Equivalents. Antioxidants, 12(3), 609. https://doi.org/10.3390/antiox12030609