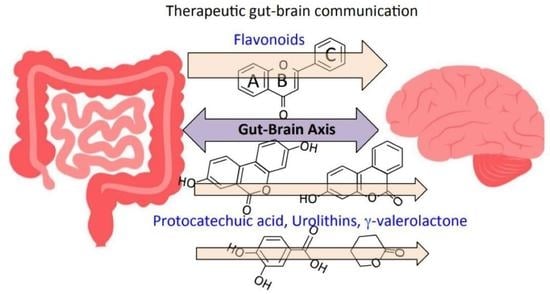
The Potential of Flavonoids and Flavonoid Metabolites in the Treatment of Neurodegenerative Pathology in Disorders of Cognitive Decline
Abstract
1. Introduction
2. Therapeutic Vagus Nerve Stimulation
3. Transporter Proteins in the Afferent Fibres of the Vagus Nerve
4. Neuroregulatory Properties of Flavonoids
5. Natural Flavonoids Used in the Treatment of Neurodegenerative Conditions
6. Traditional Chinese Medicinal Formulations Used to Treat Alzheimer’s Disease
6.1. LeZhe
6.2. Shuang-Huang-Lian Herbal Preparations
6.3. Chaihu-Shugan-San
6.4. Qingfei Paidu and Ma Xing Shi Gan
7. Complex Heterocyclic Polyphenolic Precursor Compounds That Are Processed by the Gut Microbiome Releasing Bioactive Metabolites
7.1. Eligatannins
7.2. The Urolithins
7.3. Hydroxybenzoic Acids
8. Vasodilatory Flavonoids
The Anthocyanidins
9. Natural Flavonoids and Multifunctional Analog Derivatives Used in Western Medicine to Treat Neurodegenerative Conditions
9.1. Hesperidin/Hesperitin
9.2. Kaempferol and Luteolin
10. The Potential of Flavonoids in Tissue Repair Processes in a Biodiverse Range of Diseases in Linked Organ Systems
11. Flavonoid Clinical Trials
11.1. Hesperidin/Hesperitin Trials
11.2. Epigallocatechin Gallate Clinical Trials
11.3. Anthocyanin Clinical Trials
11.4. Quercetin Clinical Trials
12. Bioactive Quercetin Metabolites
13. Catechin Metabolites
14. Bioactive Flavonoid Metabolites and Regulation of Microbiome Bacterial Populations
15. Metabolites Generated from Ellagic Acid with Bioactive Properties
16. Bioactive Quercetin Metabolites
17. Flavonoids can Induce Neuroinflammation
18. Cellular Transport of Flavonoids by ATP-Binding Cassette (ABC)
Transporter Proteins and their Potential Roles in the Modulation of Cellular Influx/Efflux in Disease Processes
19. Bioavailability of Flavonoids
20. Conversion of Quercetin to Bioactive Metabolites by the Gut Microbiome
21. Processing of Epigallocatechin Gallate by the Gut Microbiome
22. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, H.; Silberstein, S.D. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part III. Headache 2016, 56, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Silberstein, S.D. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I. Headache 2016, 56, 71–78. [Google Scholar] [CrossRef]
- Yuan, H.; Silberstein, S.D. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part II. Headache 2016, 56, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- George, M.; Sackeim, H.A.; Rush, A.J.; Marangell, L.B.; Nahas, Z.; Husain, M.M.; Lisanby, S.; Burt, T.; Goldman, J.; Ballenger, J.C. Vagus nerve stimulation: A new tool for brain research and therapy. Biol. Psychiatry 2000, 47, 287–295. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Nahas, Z.; Borckardt, J.J.; Anderson, B.; Burns, C.; Kose, S.; Short, E.B. Vagus nerve stimulation for the treatment of depression and other neuropsychiatric disorders. Expert Rev. Neurother. 2007, 7, 63–74. [Google Scholar]
- Vargas, M.; Meyer, R.; Avanes, A.A.; Rus, M.; Olson, D.E. Psychedelics and Other Psychoplastogens for Treating Mental Illness. Front. Psychiatry 2021, 12, 727117. [Google Scholar] [CrossRef]
- Calder, A.; Hasler, G. Towards an understanding of psychedelic-induced neuroplasticity. Neuropsychopharmacology 2023, 48, 104–112. [Google Scholar] [CrossRef]
- Kwan, A.; Olson, D.E.; Preller, K.H.; Roth, B.L. The neural basis of psychedelic action. Nat. Neurosci. 2022, 25, 1407–1419. [Google Scholar] [CrossRef]
- Olson, D. Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. Exp. Neurosci. 2018, 12, 1179069518800508. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh, E.; Esmaeili, A.; Rahgozar, S.; Nourbakhshnia, M. Application of quercetin in neurological disorders: From nutrition to nanomedicine. Rev. Neurosci. 2019, 30, 555–572. [Google Scholar] [CrossRef]
- Antoniolli, G.; Almeida, W.P.; Frias, C.C.; de Oliveira, T.B. Chalcones Acting as Inhibitors of Cholinesterases, β-Secretase and β- Amyloid Aggregation and other Targets for Alzheimer’s Disease: A Critical Review. Curr. Med. Chem. 2021, 28, 4259–4282. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. The Impact of Ellagitannins and Their Metabolites through Gut Microbiome on the Gut Health and Brain Wellness within the Gut-Brain Axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Patanè, G.T.; Tellone, E.; Barreca, D.; Ficarra, S.; Misiti, F.; Laganà, G. The Neuroprotective Potentiality of Flavonoids on Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 14835. [Google Scholar] [CrossRef] [PubMed]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The Roles of Flavonols/Flavonoids in Neurodegeneration and Neuroinflammation. Mini. Rev. Med. Chem. 2020, 20, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Carlessi, A.; Borba, L.A.; Zugno, A.I.; Quevedo, J.; Réus, G.Z. Gut microbiota-brain axis in depression: The role of neuroinflammation. Eur. J. Neurosci. 2021, 53, 222–235. [Google Scholar] [CrossRef]
- Evans, J.; Mendonca, P.; Soliman, K.F.A. Neuroprotective Effects and Therapeutic Potential of the Citrus Flavonoid Hesperetin in Neurodegenerative Diseases. Nutrients 2022, 14, 2228. [Google Scholar] [CrossRef]
- Flanagan, E.; Müller, M.; Hornberger, M.; Vauzour, D. Impact of Flavonoids on Cellular and Molecular Mechanisms Underlying Age-Related Cognitive Decline and Neurodegeneration. Curr. Nutr. Rep. 2018, 7, 49–57. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, e2101019. [Google Scholar] [CrossRef]
- Hole, K.; Williams, R.J. Flavonoids as an Intervention for Alzheimer’s Disease: Progress and Hurdles Towards Defining a Mechanism of Action. Brain Plast. 2021, 6, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Islam, M.M.; Khan Meem, A.F.; Nafady, M.H.; Islam, M.R.; Akter, A.; Mitra, S.; Alhumaydhi, F.A.; Emran, T.B.; Khusro, A.; et al. Multifaceted role of polyphenols in the treatment and management of neurodegenerative diseases. Chemosphere 2022, 307 Pt 3, 136020. [Google Scholar] [CrossRef]
- Islam, M.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.; Kim, S.R. Beneficial Effects of Flavonoids Against Parkinson’s Disease. J. Med. Food 2018, 21, 421–432. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N.; et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. Biofactors 2021, 47, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Tundis, R.; Ullah, H.; Aschner, M.; Belwal, T.; Mirzaei, H.; Akkol, E.K. Flavonoids targeting NRF2 in neurodegenerative disorders. Food Chem. Toxicol. 2020, 146, 111817. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Zhai, K.; Al-Ishaq, R.K.; Bugos, O.; Šudomová, M.; Biringer, K.; Pec, M.; Adamkov, M.; et al. Protective Effects of Flavonoids Against Mitochondriopathies and Associated Pathologies: Focus on the Predictive Approach and Personalized Prevention. Int. J. Mol. Sci. 2021, 22, 8649. [Google Scholar] [CrossRef]
- Magni, G.; Riboldi, B.; Petroni, K.; Ceruti, S. Flavonoids bridging the gut and the brain: Intestinal metabolic fate, and direct or indirect effects of natural supporters against neuroinflammation and neurodegeneration. Biochem. Pharmacol. 2022, 205, 115257. [Google Scholar] [CrossRef]
- Meng-Zhen, S.; Ju, L.; Lan-Chun, Z.; Cai-Feng, D.; Shu-da, Y.; Hao-Fei, Y.; Wei-Yan, H. Potential therapeutic use of plant flavonoids in AD and PD. Heliyon 2022, 8, e11440. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; El-Senduny, F.F.; Sanadgol, N.; Abd-ElGhani, G.E.; Farzaei, M.H.; Chen, J.T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules 2019, 9, 690. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Cho, K.S.; Yi, I.; To, C.H.; Chen, D.F.; Do, C.W. Baicalein, Baicalin, and Wogonin: Protective Effects against Ischemia-Induced Neurodegeneration in the Brain and Retina. Oxid. Med. Cell. Longev. 2021, 2021, 8377362. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.; Beltrán, D.; Luna, M.C.; Romo-Vaquero, M.; García-Villalba, R.; Mira, A.; Espín, J.C.; Tomás-Barberán, F.A. Isolation of Human Intestinal Bacteria Capable of Producing the Bioactive Metabolite Isourolithin A from Ellagic Acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C. Epigallocatechin gallate for Parkinson’s disease. Clin. Exp. Pharmacol. Physiol. 2022, 49, 1029–1041. [Google Scholar] [CrossRef]
- Winter, A.; Bickford, P.C. Anthocyanins and Their Metabolites as Therapeutic Agents for Neurodegenerative Disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef]
- D’Amico, D.; Olmer, M.; Fouassier, A.M.; Valdés, P.; Andreux, P.A.; Rinsch, C.; Lotz, M. Urolithin A improves mitochondrial health, reduces cartilage degeneration, and alleviates pain in osteoarthritis. Aging Cell. 2022, 21, e13662. [Google Scholar] [CrossRef]
- D’Amico, D.; Andreux, P.A.; Valdes, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef]
- Garcia-VIllalba, R.; Tomas-Berberan, F.A.; Iglesias-Aguire, C.E.; Gimenez-Bastida, J.A.; Gonzales-Sarrias, A.; Selma, M.V.; Espin, J.C. Ellagitannins, urolithins, and neuroprotection: Human evidence and the possible link to the gut microbiota. Mol. Asp. Med. 2023, 89, 101109. [Google Scholar] [CrossRef]
- Millstine, D.; Chen, C.Y.; Bauer, B. Complementary and integrative medicine in the management of headache. BMJ 2017, 357, j1805. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.; Xia, S.; Chen, X.; Sun, H.; Xu, Z. Ameliorative Effect of Parishin C Against Cerebral Ischemia-Induced Brain Tissue Injury by Reducing Oxidative Stress and Inflammatory Responses in Rat Model. Neuropsychiatr. Dis. Treat. 2021, 17, 1811–1823. [Google Scholar] [CrossRef]
- Ma, L.; Liu, H.M.; Luo, C.H.; He, Y.N.; Wang, F.; Huang, H.Z.; Han, L.; Yang, M.; Xu, R.C.; Zhang, D.K. Fever and Antipyretic Supported by Traditional Chinese Medicine: A Multi-Pathway Regulation. Front. Pharmacol. 2021, 12, 583279. [Google Scholar] [CrossRef]
- Friedemann, T.; Schumacher, U.; Tao, Y.; Leung, A.K.; Schröder, S. Neuroprotective Activity of Coptisine from Coptis chinensis (Franch). Evid. Based Complement. Altern. Med. 2015, 2015, 827308. [Google Scholar] [CrossRef]
- Srivastava, V.; Mathur, D.; Rout, S.; Mishra, B.K.; Pannu, V.; Rao, R.; Anand, A. Ayurvedic Herbal Therapies: A Review of Treatment and Management of Dementia. Curr. Alzheimer Res. 2022, 19, 568–584. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.S.; Chen, C.; Tang, X.Y.; Cheng, G.Q.; Li, X. Protective effect of Shouwu Yizhi decoction against vascular dementia by promoting angiogenesis. Chin. J. Nat. Med. 2017, 15, 740–750. [Google Scholar] [CrossRef]
- Chang, S. The meridian system and mechanism of acupuncture: A comparative review. Part 3: Mechanisms of acupuncture therapies. Taiwan. J. Obstet. Gynecol. 2013, 52, 171–184. [Google Scholar] [CrossRef]
- Inanç, B. A New Theory on the Evaluation of Traditional Chinese Acupuncture Mechanisms from the Latest Medical Scientific Point of View. Acupunct. Electrother. Res. 2015, 40, 189–204. [Google Scholar] [CrossRef]
- Guo, Z. Chinese Confucian culture and the medical ethical tradition. J. Med. Ethics 1995, 21, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Chinese values, health and nursing. J. Adv. Nurs. 2001, 36, 270–273. [Google Scholar] [CrossRef]
- Wang, Q. Individualized medicine, health medicine, and constitutional theory in Chinese medicine. Front. Med. 2012, 6, 1–7. [Google Scholar] [CrossRef]
- Ye, X.; Dong, M.H. A review on different English versions of an ancient classic of Chinese medicine: Huang Di Nei Jing. J. Integr. Med. 2017, 15, 11–18. [Google Scholar] [CrossRef]
- Fairbrass, K.; Lovatt, J.; Barberio, B.; Yuan, Y.; Gracie, D.J.; Ford, A.C. Bidirectional brain-gut axis effects influence mood and prognosis in IBD: A systematic review and meta-analysis. Gut 2021, 71, 325985. [Google Scholar] [CrossRef] [PubMed]
- Mogilevski, T. The bi-directional role of the gut-brain axis in inflammatory and other gastrointestinal diseases. Curr. Opin. Gastroenterol. 2021, 37, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Frausto, D.; Forsyth, C.B.; Keshavarzian, A.; Voigt, R.M. Dietary Regulation of Gut-Brain Axis in Alzheimer’s Disease: Importance of Microbiota Metabolites. Front. Neurosci. 2021, 15, 736814. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, E.; Macnaughtan, J.; Schapira, A.H.V. The gut-brain axis and Parkinson disease: Clinical and pathogenetic relevance. Ann. Med. 2021, 53, 611–625. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Q.; Liu, J. Microbiota-gut-brain axis: A novel potential target of ketogenic diet for epilepsy. Curr. Opin. Pharmacol. 2021, 61, 36–41. [Google Scholar] [CrossRef]
- Matsubara, Y.; Kiyohara, H.; Teratani, T.; Mikami, Y.; Kanai, T. Organ and brain crosstalk: The liver-brain axis in gastrointestinal, liver, and pancreatic diseases. Neuropharmacology 2021, 205, 108915. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, B. The Gut-Liver Axis in Health and Disease: The Role of Gut Microbiota-Derived Signals in Liver Injury and Regeneration. Front. Immunol. 2021, 12, 775526. [Google Scholar] [CrossRef]
- Gokulan, K.; Joshi, M.; Khare, S.; Bartter, T. Lung microbiome, gut-lung axis and chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2021, 28, 134–138. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, L.; Chang, L.; Pu, Y.; Qu, Y.; Hashimoto, K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl. Psychiatry 2020, 10, 186. [Google Scholar] [CrossRef]
- Kaelberer, M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef]
- Ko, D. Transcutaneous vagus nerve stimulation (tVNS) as a potential therapeutic application for neurodegenerative disorders—A focus on dysautonomia in Parkinson’s disease. Auton. Neurosci. 2021, 235, 102858. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, H.; Raw, R.; Hunter, H.; Baker, M.R.; Taylor, J.P.; Rochester, L.; Yarnall, A.J. Noninvasive vagus nerve stimulation in Parkinson’s disease: Current status and future prospects. Expert Rev. Med. Devices 2021, 18, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhan, G.; Cai, Z.; Jiao, B.; Zhao, Y.; Li, S.; Luo, A. Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci. Biobehav. Rev. 2021, 127, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Abiega-Franyutti, P.; Freyre-Fonseca, V. Chronic consumption of food-additives lead to changes via microbiota gut-brain axis. Toxicology 2021, 464, 153001. [Google Scholar] [CrossRef]
- Srihagulang, C.; Vongsfak, J.; Vaniyapong, T.; Chattipakorn, N.; Chattipakorn, S.C. Potential roles of vagus nerve stimulation on traumatic brain injury: Evidence from in vivo and clinical studies. Exp. Neurol. 2022, 247, 113887. [Google Scholar] [CrossRef]
- Tang, Y.; Dong, X.; Chen, G.; Ye, W.; Kang, J.; Tang, Y.; Feng, Z. Vagus Nerve Stimulation Attenuates Early Traumatic Brain Injury by Regulating the NF-κB/NLRP3 Signaling Pathway. Neurorehabil Neural Repair 2020, 34, 831–843. [Google Scholar] [CrossRef]
- Neren, D.; Johnson, M.D.; Legon, W.; Bachour, S.P.; Ling, G.; Divani, A.A. Vagus Nerve Stimulation and Other Neuromodulation Methods for Treatment of Traumatic Brain Injury. Neurocrit Care 2016, 24, 308–319. [Google Scholar] [CrossRef]
- Lopez, N.; Krzyzaniak, M.J.; Costantini, T.W.; Putnam, J.; Hageny, A.M.; Eliceiri, B.; Coimbra, R.; Bansal, V. Vagal nerve stimulation decreases blood-brain barrier disruption after traumatic brain injury. J. Trauma Acute Care Surg. 2012, 72, 1562–1566. [Google Scholar] [CrossRef]
- Tang, H.; Li, J.; Zhou, Q.; Li, S.; Xie, C.; Niu, L.; Ma, J.; Li, C. Vagus nerve stimulation alleviated cerebral ischemia and reperfusion injury in rats by inhibiting pyroptosis via α7 nicotinic acetylcholine receptor. Cell. Death Discov. 2022, 8, 54. [Google Scholar] [CrossRef]
- Pitra, S.; Smith, B.N. Musings on the wanderer: What’s new in our understanding of vago-vagal reflexes? VI. Central vagal circuits that control glucose metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G175–G182. [Google Scholar] [CrossRef]
- Travagli, R.; Hermann, G.E.; Browning, K.N.; Rogers, R.C. Musings on the wanderer: What’s new in our understanding of vago-vagal reflexes? III. Activity-dependent plasticity in vago-vagal reflexes controlling the stomach. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G180. [Google Scholar] [CrossRef]
- Binnie, C. Vagus nerve stimulation for epilepsy: A review. Seizure 2000, 9, 161–169. [Google Scholar] [CrossRef]
- Van Laere, K.; Vonck, K.; Boon, P.; Brans, B.; Vandekerckhove, T.; Dierckx, R. Vagus nerve stimulation in refractory epilepsy: SPECT activation study. J. Nucl. Med. 2000, 41, 1145–1154. [Google Scholar]
- Aaronson, S.; Sears, P.; Ruvuna, F.; Bunker, M.; Conway, C.R.; Dougherty, D.D.; Reimherr, F.W.; Schwartz, T.L.; Zajecka, J.M. A 5-Year Observational Study of Patients with Treatment-Resistant Depression Treated With Vagus Nerve Stimulation or Treatment as Usual: Comparison of Response, Remission, and Suicidality. Am. J. Psychiatry 2017, 174, 640–648. [Google Scholar] [CrossRef]
- Rush, A.; George, M.S.; Sackeim, H.A.; Marangell, L.B.; Husain, M.M.; Giller, C.; Nahas, Z.; Haines, S.; Simpson, R.K., Jr.; Goodman, R. Vagus nerve stimulation (VNS) for treatment-resistant depressions: A multicenter study. Biol. Psychiatry 2000, 47, 276–286. [Google Scholar] [CrossRef]
- Clark, K.; Naritoku, D.K.; Smith, D.C.; Browning, R.A.; Jensen, R.A. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat. Neurosci. 1999, 2, 94–98. [Google Scholar] [CrossRef]
- Gershon, M.; Ratcliffe, E.M. Developmental biology of the enteric nervous system: Pathogenesis of Hirschsprung’s disease and other congenital dysmotilities. Semin. Pediatr. Surg. 2004, 13, 224–235. [Google Scholar] [CrossRef]
- Mayer, E.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Lynch, S.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Cerovic, M.; Forloni, G.; Balducci, C. Neuroinflammation and the Gut Microbiota: Possible Alternative Therapeutic Targets to Counteract Alzheimer’s Disease? Front. Aging Neurosci. 2019, 11, 284. [Google Scholar] [CrossRef]
- Cenit, M.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.-Z. Therapeutic potential of bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef]
- Quigley, E. Microbiota-brain-gut axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Sublette, M.; Cheung, S.; Lieberman, E.; Hu, S.; Mann, J.J.; Uhlemann, A.C.; Miller, J.M. Bipolar disorder and the gut microbiome: A systematic review. Bipolar Disord. 2021, 23, 544–564. [Google Scholar] [CrossRef]
- Beopoulos, A.; Gea, M.; Fasano, A.; Iris, F. Autonomic Nervous System Neuroanatomical Alterations Could Provoke and Maintain Gastrointestinal Dysbiosis in Autism Spectrum Disorder (ASD): A Novel Microbiome-Host Interaction Mechanistic Hypothesis. Nutrients 2021, 14, 65. [Google Scholar] [CrossRef]
- Generoso, J.; Giridharan, V.V.; Lee, J.; Macedo, D.; Barichello, T. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz. J. Psychiatry 2021, 43, 293–305. [Google Scholar] [CrossRef]
- Cryan, J.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Iannone, L.F.; Preda, A.; Blottière, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C.; et al. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev. Neurother. 2019, 19, 1037–1050. [Google Scholar] [CrossRef]
- Schächtle, M.; Rosshart, S.P. The Microbiota-Gut-Brain Axis in Health and Disease and Its Implications for Translational Research. Front. Cell. Neurosci. 2021, 15, 698172. [Google Scholar] [CrossRef]
- Larroya, A.; Pantoja, J.; Codoñer-Franch, P.; Cenit, M.C. Towards Tailored Gut Microbiome-Based and Dietary Interventions for Promoting the Development and Maintenance of a Healthy Brain. Front. Pediatr. 2021, 9, 705859. [Google Scholar] [CrossRef]
- Dicks, L.; Hurn, D.; Hermanus, D. Gut Bacteria and Neuropsychiatric Disorders. Microorganisms 2021, 9, 2583. [Google Scholar] [CrossRef] [PubMed]
- Barrio, C.; Arias-Sánchez, S.; Martín-Monzón, I. The gut microbiota-brain axis, psychobiotics and its influence on brain and behaviour: A systematic review. Psychoneuroendocrinology 2021, 137, 105640. [Google Scholar] [CrossRef]
- Wang, Y.; de Lartigue, G.; Page, A.J. Dissecting the Role of Subtypes of Gastrointestinal Vagal Afferents. Front. Physiol. 2020, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Caravaca, A.; Gallina, A.L.; Tarnawski, L.; Tracey, K.J.; Pavlov, V.A.; Levine, Y.A.; Olofsson, P.S. An Effective Method for Acute Vagus Nerve Stimulation in Experimental Inflammation. Front. Neurosci. 2019, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Mastitskaya, S.; Thompson, N.; Holder, D. Selective Vagus Nerve Stimulation as a Therapeutic Approach for the Treatment of ARDS: A Rationale for Neuro-Immunomodulation in COVID-19 Disease. Front. Neurosci. 2021, 15, 667036. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Edwards, R.H. The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annu. Rev. Neurosci. 1997, 20, 125–156. [Google Scholar] [CrossRef]
- Varoqui, H.; Erickson, J.D. Vesicular neurotransmitter transporters. Potential sites for the regulation of synaptic function. Mol. Neurobiol. 1997, 15, 165–191. [Google Scholar] [CrossRef]
- Jordi, J.; Herzog, B.; Camargo, S.M.; Boyle, C.N.; Lutz, T.A.; Verrey, F. Specific amino acids inhibit food intake via the area postrema or vagal afferents. J. Physiol. 2013, 59, 5611–5621. [Google Scholar] [CrossRef]
- Tomé, D.; Schwarz, J.; Darcel, N.; Fromentin, G. Protein, amino acids, vagus nerve signaling, and the brain. Am. J. Clin. Nutr. 2009, 90, 838S–843S. [Google Scholar] [CrossRef]
- Yuan, T.; Ma, H.; Liu, W.; Niesen, D.B.; Shah, N.; Crews, R.; Rose, K.N.; Vattem, D.A.; Seeram, N.P. Pomegranate’s Neuroprotective Effects against Alzheimer’s Disease Are Mediated by Urolithins, Its Ellagitannin-Gut Microbial Derived Metabolites. ACS Chem. Neurosci. 2016, 7, 26–33. [Google Scholar] [CrossRef]
- Ishii, Y.; Muta, O.; Teshima, T.; Hirasima, N.; Odaka, M.; Fushimi, T.; Fujii, Y.; Osakabe, N. Repeated Oral Administration of Flavan-3-ols Induces Browning in Mice Adipose Tissues through Sympathetic Nerve Activation. Nutrients 2021, 13, 4214. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Lu, C.W.; Hsieh, P.W.; Chiu, K.M.; Lee, M.Y.; Wang, S.J. Natural Product Isoliquiritigenin Activates GABAB Receptors to Decrease Voltage-Gate Ca2+ Channels and Glutamate Release in Rat Cerebrocortical Nerve Terminals. Biomolecules 2021, 11, 1537. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Galván, M.; Rangel, A.; Romero-Méndez, C.; Dávila, E.M.; Castro, M.E.; Caballero, N.A.; Meléndez Bustamante, F.J.; Sanchez-Gaytan, B.L.; Meza, U.; Perez-Aguilar, J.M. Inhibitory Mechanism of the Isoflavone Derivative Genistein in the Human CaV3.3 Channel. ACS Chem. Neurosci. 2021, 12, 651–659. [Google Scholar] [CrossRef]
- Wang, Z.; Ling, D.; Wu, C.; Han, J.; Zhao, Y. Baicalin prevents the up-regulation of TRPV1 in dorsal root ganglion and attenuates chronic neuropathic pain. Vet. Med. Sci. 2020, 6, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wang, J.; Han, X.; Ma, B.; Yuan, H.; Song, Y. Baicalin regulates the dopamine system to control the core symptoms of ADHD. Mol. Brain 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cai, S.; Moutal, A.; Yu, J.; Gómez, K.; Madura, C.L.; Shan, Z.; Pham, N.Y.N.; Serafini, M.J.; Dorame, A.; et al. The Natural Flavonoid Naringenin Elicits Analgesia through Inhibition of NaV1.8 Voltage-Gated Sodium Channels. ACS Chem. Neurosci. 2019, 10, 4834–4846. [Google Scholar] [CrossRef]
- Peixoto-Neves, D.; Soni, H.; Adebiyi, A. CGRPergic Nerve TRPA1 Channels Contribute to Epigallocatechin Gallate-Induced Neurogenic Vasodilation. ACS Chem. Neurosci. 2019, 10, 216–220. [Google Scholar] [CrossRef]
- Li, P.; Xiong, D.L.; Sun, W.P.; Xu, S.Y. Effects of baicalin on diabetic neuropathic pain involving transient receptor potential vanilloid 1 in the dorsal root ganglia of rats. Neuroreport 2018, 29, 1492–1498. [Google Scholar] [CrossRef]
- Chang, C.; Lin, T.Y.; Lu, C.W.; Wang, C.C.; Wang, Y.C.; Chou, S.S.; Wang, S.J. Apigenin, a natural flavonoid, inhibits glutamate release in the rat hippocampus. Eur. J. Pharmacol. 2015, 762, 72–81. [Google Scholar] [CrossRef]
- Chang, Y.; Chang, C.Y.; Wang, S.J.; Huang, S.K. Myricetin inhibits the release of glutamate in rat cerebrocortical nerve terminals. J. Med. Food 2015, 18, 516–523. [Google Scholar] [CrossRef]
- Lin, T.; Hung, C.F.; Weng, J.R.; Hsieh, T.Y.; Wang, S.J. Kaempferol 3-Rhamnoside on Glutamate Release from Rat Cerebrocortical Nerve Terminals Involves P/Q-Type Ca2+ Channel and Ca2+/Calmodulin-Dependent Protein Kinase II-Dependent Pathway Suppression. Molecules 2022, 27, 1342. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hsieh, P.W.; Kuo, J.R.; Wang, S.J. Rosmarinic Acid, a Bioactive Phenolic Compound, Inhibits Glutamate Release from Rat Cerebrocortical Synaptosomes through GABAA Receptor Activation. Biomolecules 2021, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, T.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Hiebert, P. The Nrf2 transcription factor: A multifaceted regulator of the extracellular matrix. Matrix Biol Plus 2021, 10, 100057. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Heurtaux, T.; Bouvier, D.S.; Benani, A.; Helgueta Romero, S.; Frauenknecht, K.B.M.; Mittelbronn, M.; Sinkkonen, L. Normal and Pathological NRF2 Signalling in the Central Nervous System. Antioxidants 2022, 11, 1426. [Google Scholar] [CrossRef]
- Cores, Á.; Piquero, M.; Villacampa, M.; León, R.; Menéndez, J.C. NRF2 Regulation Processes as a Source of Potential Drug Targets against Neurodegenerative Diseases. Biomolecules 2020, 10, 904. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, J.; Wu, X.; Song, L.; Wang, Y.; Gong, M.; Li, B. Quercetin reverses chronic unpredictable mild stress-induced depression-like behavior in vivo by involving nuclear factor-E2-related factor 2. Brain Res. 2021, 1772, 147661. [Google Scholar] [CrossRef]
- Lei, L.; Chai, Y.; Lin, H.; Chen, C.; Zhao, M.; Xiong, W.; Zhuang, J.; Fan, X. Dihydroquercetin Activates AMPK/Nrf2/HO-1 Signaling in Macrophages and Attenuates Inflammation in LPS-Induced Endotoxemic Mice. Front. Pharmacol. 2020, 11, 662. [Google Scholar] [CrossRef]
- Sun, L.; Xu, G.; Dong, Y.; Li, M.; Yang, L.; Lu, W. Quercetin Protects Against Lipopolysaccharide-Induced Intestinal Oxidative Stress in Broiler Chickens through Activation of Nrf2 Pathway. Molecules 2020, 25, 1053. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Alshehri, A.; El-kott, A.F.; El-Gerbed, M.S.A.; El-Kenawy, A.E.; Albadrani, G.M.; Khalifa, H.S. Kaempferol prevents cadmium chloride-induced liver damage by upregulating Nrf2 and suppressing NF-κB and keap1. Environ. Sci. Pollut. Res. 2022, 29, 13917–13929. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, C.; Yue Jin Meng, Q.; Wu, J.; Sun, H. Kaempferol induced GPER upregulation attenuates atherosclerosis via the PI3K/AKT/Nrf2 pathway. Pharm. Biol. 2021, 59, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Mishra, G.; İlgün, S.; Samarghandian, S. New insights into the role of the Nrf2 signaling pathway in green tea catechin applications. Phytother. Res. 2021, 35, 3078–3112. [Google Scholar] [CrossRef]
- Vendidandala, N.; Yin, T.P.; Nelli, G.; Pasupuleti, V.R.; Nyamathulla, S.; Mokhtar, S.I. Gallocatechin-silver nanoparticle impregnated cotton gauze patches enhance wound healing in diabetic rats by suppressing oxidative stress and inflammation via modulating the Nrf2/HO-1 and TLR4/NF-κB pathways. Life Sci. 2021, 286, 120019. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Yu, H.; Li, M.; Hang, L.; Xu, X. Apigenin Protects Mouse Retina against Oxidative Damage by Regulating the Nrf2 Pathway and Autophagy. Oxidative Med. Cell. Longev. 2020, 2020, 9420704. [Google Scholar] [CrossRef]
- Albarakati, A.; Baty, R.S.; Aljoudi, A.M.; Habotta, O.A.; Elmahallawy, E.K.; Kassab, R.B.; Moneim, A.E.A. Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, antiinflammatory, anti-apoptotic, and Nrf2/HO-1 signaling pathways. Mol. Biol. Rep. 2020, 47, 2591–2603. [Google Scholar] [CrossRef]
- Alekhya Sita, G.; Gowthami, M.; Srikanth, G.; Krishna, M.M.; Rama Sireesha, K.; Sajjarao, M.; Nagarjuna, K.; Nagarjuna, M.; Chinnaboina, G.K.; Mishra, A. Protective role of luteolin against bisphenol A-induced renal toxicity through suppressing oxidative stress, inflammation, and upregulating Nrf2/ARE/ HO-1 pathway. IUBMB Life 2019, 71, 1041–1047. [Google Scholar] [CrossRef]
- Kang, K.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Dai, J.; Guo, W.; Tan, Y.; Niu, K.; Zhang, J.; Liu, C.; Yang, X.-M.; Tao, K.-S.; Chen, Z.-N.; Dai, J.-Y. Wogonin alleviates liver injury in sepsis through Nrf2-mediated NF-κB signalling suppression. J. Cell. Mol. Med. 2021, 25, 5782–5798. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xu, Z.; Gao, Q.; Xu, Y.; Wang, B.; Dai, Y. Protective role of wogonin against cadmium induced testicular toxicity: Involvement of antioxidant, anti-inflammatory and anti-apoptotic pathways. Life Sci. 2020, 258, 118192. [Google Scholar] [CrossRef] [PubMed]
- Xingyue, L.; Shuang, L.; Qiang, W.; Jinjuan, F.; Yongjian, Y. Chrysin Ameliorates Sepsis-Induced Cardiac Dysfunction through Upregulating Nfr2/Heme Oxygenase 1 Pathway. J. Cardiovasc. Pharmacol. 2021, 77, 491–500. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Ramprasath, T.; Saravanan, B.; Vasudevan, V.; Sasikumar, S.; Selvam, G.S. Chrysin attenuates high-fat-diet-induced myocardial oxidative stress via upregulating eNOS and Nrf2 target genes in rats. Mol. Cell. Biochem. 2021, 476, 2719–2727. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, L.; Deng, S.; Liang, M. Daidzein ameliorates LPSinduced hepatocyte injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2020, 885, 173399. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, G.; He, Y.; Xu, H.; Fan, H.; An, J.; Zhang, L.; Zhang, R.; Cao, G.; Hao, D.; et al. Involvement of α7nAChR in the Protective Effects of Genistein against β-Amyloid-Induced Oxidative Stress in Neurons via a PI3K/Akt/Nrf2 Pathway-Related Mechanism. Cell. Mol. Neurobiol. 2021, 41, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, A.; Liu, Y.; Zhan, S.; Zhong, L.; Du, Y.; Xu, D.; Wang, W.; Huang, W. Genistein protects against acetaminophen-induced liver toxicity through augmentation of SIRT1 with induction of Nrf2 signalling. Biochem. Biophys. Res. Commun. 2020, 527, 90–97. [Google Scholar] [CrossRef]
- Yi, S.; Chen, S.; Xiang, J.; Tan, J.; Huang, K.; Zhang, H.; Wang, Y.; Wu, H. Genistein exerts a cell-protective effect via Nrf2/HO-1/ /PI3K signaling in Ab25-35-induced Alzheimer’s disease models in vitro. Folia Histochem. Cytobiol. 2021, 59, 49–56. [Google Scholar] [CrossRef]
- Guo, K.; Ren, J.; Gu, G.; Wang, G.; Gong, W.; Wu, X.; Ren, H.; Hong, Z.; Li, J. Hesperidin Protects against Intestinal Inflammation by Restoring Intestinal Barrier Function and up-Regulating Treg Cells. Mol. Nutr. Food Res. 2019, 63, 1800975. [Google Scholar] [CrossRef]
- Xin, X.; Li, Y.; Liu, H. Hesperidin ameliorates hypobaric hypoxiainduced retinal impairment through activation of Nrf2/HO-1 pathway and inhibition of apoptosis. Sci. Rep. 2020, 10, 19426. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, L.; Tang, Z.; Zhang, Y.; Liu, Y.; Wang, T.; Liu, Y. Hesperetin ameliorates diabetic nephropathy in rats by activating Nrf2/are/glyoxalase 1 pathway. Biomed. Pharmacother. 2019, 111, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef]
- Lin, Z.; Fu, C.; Yan, Z.; Wu, Y.; Zhan, J.; Lou, Z.; Liao, X.; Pan, J. The protective effect of hesperetin in osteoarthritis: An in vitro and in vivo study. Food Funct. 2020, 11, 2654–2666. [Google Scholar] [CrossRef]
- Chen, W.; Ye, Y.; Wu, Z.; Lin, J.; Wang, Y.; Ding, Q.; Yang, X.; Yang, W.; Lin, B.; Lin, B. Temporary Upregulation of Nrf2 by Naringenin Alleviates Oxidative Damage in the Retina and ARPE-19 Cells. Oxidative Med. Cell. Longev. 2021, 2021, 4053276. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.; Hsu, H.; Lee, T.; Chang, W.; Lo, Y. Naringenin, a dietary flavanone, enhances insulin-like growth factor 1 receptormediated antioxidant defense and attenuates methylglyoxalinduced neurite damage and apoptotic death. Nutr. Neurosci. 2021, 24, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Sharath Babu, G.; Anand, T.; Ilaiyaraja, N.; Khanum, F.; Gopalan, N. Pelargonidin Modulates Keap1/Nrf2 Pathway Gene Expression and Ameliorates Citrinin-Induced Oxidative Stress in HepG2 Cells. Front. Pharmacol. 2017, 8, 868. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Mathew, S.; Nair, P.; Ramadan, W.S.; Vazhappilly, C.G. Health benefits of cyanidin-3-glucoside as a potent modulator of Nrf2-mediated oxidative stress. Inflammopharmacology 2021, 29, 907–923. [Google Scholar] [CrossRef]
- Kuo, H.; Wu, R.; Li, S.; Yang, A.Y.; Kong, A.N. Anthocyanin Delphinidin Prevents Neoplastic Transformation of Mouse Skin JB6 P+ Cells: Epigenetic Re-activation of Nrf2-ARE Pathway. AAPS J. 2019, 21, 83. [Google Scholar] [CrossRef]
- Zheng, S.; Deng, Z.; Chen, F.; Zheng, L.; Pan, Y.; Xing, Q.; Tsao, R.; Li, H. Synergistic antioxidant effects of petunidin and lycopene in H9c2 cells submitted to hydrogen peroxide: Role of Akt/Nrf2 pathway. J. Food Sci. 2020, 85, 1752–1763. [Google Scholar] [CrossRef]
- Qi, W.; Boliang, W.; Xiaoxi, T.; Guoqiang, F.; Jianbo, X.; Gang, W. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed. Pharmacother. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Xie, C.; Ma, H.; Shi, Y.; Li, J.; Wu, H.; Wang, B.; Shao, Z.; Huang, C.; Chen, J.; Sun, L.; et al. Cardamonin protects nucleus pulposus cells against IL-1β-induced inflammation and catabolism via Nrf2/NF-κB axis. Food Funct. J. 2021, 12, 2703–2714. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Z.; Hong, H.; Wang, N.; Chen, J.; Lu, S.; Zhang, H.; Zhang, X.; Bei, C. Xanthohumol suppresses inflammation in chondrocytes and ameliorates osteoarthritis in mice. Biomed. Pharmacother. 2021, 137, 111238. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, Z.; Zheng, T.; Zhang, M. Xanthohumol alleviates T2DM-induced liver steatosis and fibrosis by mediating the NRF2/RAGE/NF-κB signaling pathway. Future Med. Chem. 2021, 13, 2069–2081. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Q.; Zhang, M.; Yin, T.; Xu, R.; Xiao, W.; Wu, J.; Deng, B.; Gao, X.; Gong, W.; et al. Isoliquiritigenin Ameliorates Acute Pancreatitis in Mice via Inhibition of Oxidative Stress and Modulation of the Nrf2/HO-1 Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 7161592. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Jia, H.W.; Xiao, C.; Lu, Q.P. Theory of traditional Chinese medicine and therapeutic method of diseases. World J. Gastroenterol. 2004, 10, 1854–1856. [Google Scholar] [CrossRef]
- Jiang, W. Therapeutic wisdom in traditional Chinese medicine: A perspective from modern science. Trends Pharmacol. Sci. 2005, 26, 558–563. [Google Scholar] [CrossRef]
- Choudhry, N.; Zhao, X.; Xu, D.; Zanin, M.; Chen, W.; Yang, Z.; Chen, J. Chinese Therapeutic Strategy for Fighting COVID-19 and Potential Small-Molecule Inhibitors against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). J. Med. Chem. 2020, 63, 13205–13227. [Google Scholar] [CrossRef]
- Li, Y.; Chu, F.; Li, P.; Johnson, N.; Li, T.; Wang, Y.; An, R.; Wu, D.; Chen, J.; Su, Z.; et al. Potential effect of Maxing Shigan decoction against coronavirus disease 2019 (COVID-19) revealed by network pharmacology and experimental verification. J. Ethnopharmacol. 2021, 271, 113854. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, L.; Siu, W.; Jin, Y.; Cao, M.; Li, W.; Chen, J.; Cong, W.; Ma, M.; Chen, K.; et al. A combined molecular biology and network pharmacology approach to investigate the multi-target mechanisms of Chaihu Shugan San on Alzheimer’s disease. Biomed. Pharmacother. 2019, 120, 109370. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; Chen, W.; Shi, W.; Zhao, Q.; Zhao, J.; Li, L. Network Pharmacology and Experimental Evidence: PI3K/AKT Signaling Pathway is Involved in the Antidepressive Roles of Chaihu Shugan San. Drug. Des. Devel. Ther. 2021, 15, 3425–34441. [Google Scholar] [CrossRef]
- Zhou, F.; He, K.; Guan, Y.; Yang, X.; Chen, Y.; Sun, M.; Qiu, X.; Yan, F.; Huang, H.; Yao, L.; et al. Network pharmacology-based strategy to investigate pharmacological mechanisms of Tinospora sinensis for treatment of Alzheimer’s disease. J. Ethnopharmacol. 2020, 259, 112940. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Saadeldeen, F.S.A.; Xu, L.; Zhao, Y.; Wei, J.; Wang, H.D.; Liu, Z.; Kang, W. The Mechanism of Phillyrin from the Leaves of Forsythia suspensa for Improving Insulin Resistance. Biomed. Res. Int. 2019, 2019, 3176483. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pathak, N.; Fatima, E.; Negi, A.S. Plant isoquinoline alkaloids: Advances in the chemistry and biology of berberine. Eur. J. Med. Chem. 2021, 226, 113839. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Chen, Y. Synergetic protective effect of berberine and ginsenoside Rb1 against tumor necrosis factor alpha-induced inflammation in adipocytes. Bioengineered 2021, 12, 11784–11796. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Lu, E.; Sheng, Q.; Zhao, Y. Berberine exerts neuroprotective activities against cerebral ischemia/reperfusion injury through up-regulating PPAR-γ to suppress NF-κB-mediated pyroptosis. Brain Res. Bull. 2021, 177, 22–30. [Google Scholar] [CrossRef]
- Xin, Z.; Fang, Y.; Du, L.; Zhu, T.; Duan, L.; Chen, J.; Gu, Q.Q.; Zhu, W.M. Aurantiomides A-C, quinazoline alkaloids from the sponge-derived fungus Penicillium aurantiogriseum SP0-19. J. Nat. Prod. 2007, 70, 853–855. [Google Scholar] [CrossRef]
- Dong, H.; Wu, M.; Wang, Y.; Du, W.; He, Y.; Shi, Z. Total Syntheses and Anti-inflammatory Activities of Syringin and Its Natural Analogues. J. Nat. Prod. 2021, 84, 2866–2874. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Zhang, L.; Guo, Y.; Lu, Y.; Lin, D. Phillyrin, a natural lignan, attenuates tumor necrosis factor α-mediated insulin resistance and lipolytic acceleration in 3T3-L1 adipocytes. Planta Med. 2014, 80, 880–886. [Google Scholar] [CrossRef]
- Fang, Z.; Wei, L.; Lv, Y.; Wang, T.; Hamezah, H.S.; Han, R.; Tong, X. Phillyrin restores metabolic disorders in mice fed with high-fat diet through inhibition of interleukin-6-mediated basal lipolysis. Front Nutr. 2022, 9, 956218. [Google Scholar] [CrossRef]
- Su, H.-x.; Yao, S.; Zhao, W.-f.; Li, M.-j.; Liu, J.; Shang, W.-j.; Xie, H.; Ke, C.-q.; Hu, H.-c.; Gao, M.-n.; et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol. Sin. 2020, 41, 1167–1177. [Google Scholar] [CrossRef]
- Wang, H.; Hui, K.M.; Xu, S.; Chen, Y.; Wong, J.T.; Xue, H. Two flavones from Scutellaria baicalensis Georgi and their binding affinities to the benzodiazepine site of the GABAA receptor complex. Pharmazie 2002, 57, 857–858. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.; Wang, X.H.; Xue, H. Interaction of flavones from the roots of Scutellaria baicalensis with the benzodiazepine site. Planta Med. 2000, 66, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, F.; Tsang, S.Y.; Ho, K.H.; Zheng, H.; Yuen, C.T.; Chow, C.Y.; Xue, H. Anxiolytic-Like Effect of baicalin and its additivity with other anxiolytics. Planta Med. 2006, 72, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Hung, W.Y.; Chen, C.F. Anxiolytic-like effects of baicalein and baicalin in the Vogel conflict test in mice. Eur. J. Pharmacol. 2003, 464, 141–146. [Google Scholar] [CrossRef]
- Awad, R.; Arnason, J.T.; Trudeau, V.; Bergeron, C.; Budzinski, J.W.; Foster, B.C.; Merali, Z. Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): A medicinal plant with anxiolytic properties. Phytomedicine 2003, 10, 640–649. [Google Scholar] [CrossRef]
- de Carvalho, R.; Duarte, F.S.; de Lima, T.C. Involvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in mice. Behav. Brain Res. 2011, 221, 75–82. [Google Scholar] [CrossRef]
- Park, H.; Yoon, S.Y.; Choi, J.Y.; Lee, G.S.; Choi, J.H.; Shin, C.Y.; Son, K.H.; Lee, Y.S.; Kim, W.K.; Ryu, J.H.; et al. Anticonvulsant effect of wogonin isolated from Scutellaria baicalensis. Eur. J. Pharmacol. 2007, 574, 112–119. [Google Scholar] [CrossRef]
- Hu, Z.; Guan, Y.; Hu, W.; Xu, Z.; Ishfaq, M. An overview of pharmacological activities of baicalin and its aglycone baicalein: New insights into molecular mechanisms and signaling pathways. Iran. J. Basic Med. Sci. 2022, 25, 14–26. [Google Scholar]
- Tarragó, T.; Kichik, N.; Claasen, B.; Prades, R.; Teixidó, M.; Giralt, E. Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor. Bioorganic Med. Chem. 2008, 16, 7516–7524. [Google Scholar] [CrossRef]
- Svarcbahs, R.; Julku, U.; Kilpeläinen, T.; Kyyrö, M.; Jäntti, M.; Myöhänen, T.T. New tricks of prolyl oligopeptidase inhibitors—A common drug therapy for several neurodegenerative diseases. Biochem. Pharmacol. 2019, 161, 113–120. [Google Scholar] [CrossRef]
- Szeltner, Z.; Polgár, L. Structure, function and biological relevance of prolyl oligopeptidase. Curr. Protein. Pept. Sci. 2008, 9, 96–107. [Google Scholar] [PubMed]
- Dethe, S.; Deepak, M.; Agarwal, A. Elucidation of Molecular Mechanism(s) of Cognition Enhancing Activity of Bacomind®: A Standardized Extract of Bacopa Monnieri. Pharmacogn. Mag. 2016, 12 (Suppl. S4), S482–S487. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Kilpeläinen, T.; Zouzoula, L.; Auno, S.; Trontti, K.; Kurvonen, S.; Norrbacka, S.; Hovatta, I.; Jensen, P.H.; Myöhänen, T.T. Prolyl oligopeptidase inhibition reduces alpha-synuclein aggregation in a cellular model of multiple system atrophy. J. Cell. Mol. Med. 2021, 25, 9634–9646. [Google Scholar] [CrossRef] [PubMed]
- Rostami, J.; Jäntti, M.; Cui, H.; Rinne, M.K.; Kukkonen, J.P.; Falk, A.; Erlandsson, A.; Myöhänen, T. Prolyl oligopeptidase inhibition by KYP-2407 increases alpha-synuclein fibril degradation in neuron-like cells. Biomed. Pharmacother. 2020, 131, 110788. [Google Scholar] [CrossRef]
- Kumar, R.; Bavi, R.; Jo, M.G.; Arulalapperumal, V.; Baek, A.; Rampogu, S.; Kim, M.O.; Lee, K.W. New compounds identified through in silico approaches reduce the α-synuclein expression by inhibiting prolyl oligopeptidase in vitro. Sci. Rep. 2017, 7, 10827. [Google Scholar] [CrossRef]
- Höfling, C.; Kulesskaya, N.; Jaako, K.; Peltonen, I.; Männistö, P.T.; Nurmi, A.; Vartiainen, N.; Morawski, M.; Zharkovsky, A.; Võikar, V.; et al. Deficiency of prolyl oligopeptidase in mice disturbs synaptic plasticity and reduces anxiety-like behaviour, body weight, and brain volume. Eur. Neuropsychopharmacol. 2016, 26, 1048–1061. [Google Scholar] [CrossRef]
- Yang, R.; Liu, H.; Bai, C.; Wang, Y.; Zhang, X.; Guo, R.; Wu, S.; Wang, J.; Leung, E.; Chang, H.; et al. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In silico and experimental study. Pharmacol. Res. 2020, 157, 104820. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell. Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef]
- Yamada, H.; Wakamori SHirokane, T.; Ikeuchi, K.; Matsumoto, S. Structural revisions in natural ellagitannins. Molecules 2018, 23, 1901. [Google Scholar] [CrossRef]
- Gong, Q.; Cai, L.; Jing, Y.; Wang, W.; Yang, D.X.; Chen, S.W.; Tian, H.L. Urolithin A alleviates blood-brain barrier disruption and attenuates neuronal apoptosis following traumatic brain injury in mice. Neural Regen. Res. 2022, 17, 2007–2013. [Google Scholar]
- Larrosa, M.; González-Sarrías, A.; Yanez-Gascon, M.J.; Selma, M.V.; Azorin-Ortuno, M.; Toti, S.; Tomas-Barberan, F.; Dolara, P.; Espin, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef]
- Lipińska, L.; Klewicka, E.; Sójka, M. The structure, occurrence and biological activity of ellagitannins: A general review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Bialonska, D.; Kasimsetty, S.G.; Khan, S.I.; Ferreira, D. Urolithins, intestinal microbial metabolites of Pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J. Agric. Food. Chem. 2009, 57, 10181–10186. [Google Scholar] [CrossRef]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agr. Food. Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Djedjibegovic, J.; Marjanovic, A.; Panieri, E.; Saso, L. Ellagic Acid-Derived Urolithins as Modulators of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 5194508. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.; González-Sarrías, A.; Larrosa, M.; Tomás-Barberán, F.; Espín, J.C.; García-Conesa, M.T. Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-α-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol. Nutr. Food Res. 2012, 56, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Toney, A.; Albusharif, M.; Works, D.; Polenz, L.; Schlange, S.; Chaidez, V.; Ramer-Tait, A.E.; Chung, S. Differential Effects of Whole Red Raspberry Polyphenols and Their Gut Metabolite Urolithin A on Neuroinflammation in BV-2 Microglia. Int. J. Environ. Res. Public Health 2020, 18, 68. [Google Scholar] [CrossRef]
- Kanjanasirirat, P.; Suksatu, A.; Manopwisedjaroen, S.; Munyoo, B.; Tuchinda, P.; Jearawuttanakul, K.; Seemakhan, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Rangkasenee, N.; et al. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents. Sci. Rep. 2020, 10, 19963. [Google Scholar] [CrossRef]
- Jayatunga, D.; Hone, E.; Khaira, H.; Lunelli, T.; Singh, H.; Guillemin, G.J.; Fernando, B.; Garg, M.L.; Verdile, G.; Martins, R.N. Therapeutic Potential of Mitophagy-Inducing Microflora Metabolite, Urolithin A for Alzheimer’s Disease. Nutrients 2021, 13, 3744. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.Y.; Ren, Y.S.; Zhou, J.C.; Zhou, Y.X.; Huang, W.Z.; Piao, X.H.; Yang, Z.Y.; Wang, S.M.; Ge, Y.W. Identification of ellagic acid and urolithins as natural inhibitors of Aβ25-35-induced neurotoxicity and the mechanism predication using network pharmacology analysis and molecular docking. Front. Nutr. 2022, 9, 966276. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Qiu, J.; Wang, L.; Zhuo, J.; Wang, B.; Sun, D.; Yu, S.; Lou, H. Urolithin A protects dopaminergic neurons in experimental models of Parkinson’s disease by promoting mitochondrial biogenesis through the SIRT1/PGC-1α signaling pathway. Food Funct. 2022, 13, 375–385. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, Y.; Zhuo, J.; Zhang, L.; Liu, J.; Wang, B.; Sun, D.; Yu, S.; Lou, H. Urolithin A promotes mitophagy and suppresses NLRP3 inflammasome activation in lipopolysaccharide-induced BV2 microglial cells and MPTP-induced Parkinson’s disease model. Neuropharmacology 2022, 207, 108963. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, N.; Nahar, P.P.; Ma, H.; Eid, A.; Wei, Z.; Meschwitz, S.; Zawia, N.H.; Slitt, A.L.; Seeram, N.P. Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutr. Neurosci. 2019, 22, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Jourdes, M.; Kurpik, M.; Szulc, M.; Szaefer, H.; Chmielarz, P.; Kreiner, G.; Krajka-Kuźniak, V.; Mikołajczak, P.Ł.; Teissedre, P.L.; et al. Neuroprotective Effects of Pomegranate Juice against Parkinson’s Disease and Presence of Ellagitannins-Derived Metabolite-Urolithin A-In the Brain. Int. J. Mol. Sci. 2019, 21, 202. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yuan, C.; Wang, G.; Luo, J.; Ma, H.; Xu, L.; Mu, Y.; Li, Y.; Seeram, N.P.; Huang, X.; et al. Urolithins Attenuate LPS-Induced Neuroinflammation in BV2Microglia via MAPK, Akt, and NF-κB Signaling Pathways. J. Agric. Food Chem. 2018, 66, 571–580. [Google Scholar] [CrossRef]
- Bobowska, A.; Granica, S.; Filipek, A.; Melzig, M.F.; Moeslinger, T.; Zentek, J.; Kruk, A.; Piwowarski, J.P. Comparative studies of urolithins and their phase II metabolites on macrophage and neutrophil functions. Eur. J. Nutr. 2021, 60, 1957–1972. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Vemula, P.K.; Haribabu, B.; Jala, V.R. Microbial Metabolite Urolithin B Inhibits Recombinant Human Monoamine Oxidase A Enzyme. Metabolites 2020, 10, 258. [Google Scholar] [CrossRef]
- Masella, R.; Santangelo, C.; D’Archivio, M.; Li Volti, G.; Giovannini, C.; Galvano, F. Protocatechuic acid and human disease prevention: Biological activities and molecular mechanisms. Curr. Med. Chem. 2012, 19, 2901–2917. [Google Scholar] [CrossRef]
- Li, J.; Deng, Y.; Yuan, C.; Pan, L.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Antioxidant and quinone reductase-inducing constituents of black chokeberry (Aronia melanocarpa) fruits. J. Agric. Food Chem. 2012, 60, 11551–11559. [Google Scholar] [CrossRef]
- Gureev, A.; Popov, V.N.; Starkov, A.A. Crosstalk between the mTOR and Nrf2/ARE signaling pathways as a target in the improvement of long-term potentiation. Exp. Neurol. 2020, 328, 113285. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q.; Liu, H.; Song, Z.; Chen, W. Phytochemical compounds targeting on Nrf2 for chemoprevention in colorectal cancer. Eur. J. Pharmacol. 2020, 887, 173588. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.; Crupi, R.; Di Paola, R.; Ontario, M.L.; Bella, R.; Calabrese, E.J.; Crea, R.; Cuzzocrea, S.; Calabrese, V. Inflammasomes, hormesis, and antioxidants in neuroinflammation: Role of NRLP3 in Alzheimer disease. J. Neurosci. Res. 2017, 95, 1360–1372. [Google Scholar] [CrossRef]
- Andreux, P.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; D’Amico, D.; Shankland, E.; Bhayana, S.; Garcia, J.M.; Aebischer, P.; Rinsch, C.; Singh, A.; Marcinek, D.J. Effect of Urolithin A Supplementation on Muscle Endurance and and Mitochondrial Health in Older Adults: A Randomized Clinical Trial. JAMA Netw. Open. 2022, 5, e2144279. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell. Rep. Med. 2022, 3, 100633. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Aguilar Peralta, G.; Arévalo Gardoqui, J.; Llamas Macías, F.J.; Navarro Ceja, V.H.; Mendoza Cisneros, S.A.; Martínez Macías, C.G. Clinical and capillaroscopic evaluation in the treatment of chronic venous insufficiency with Ruscus aculeatus, hesperidin methylchalcone and ascorbic acid in venous insufficiency treatment of ambulatory patients. Int. Angiol. 2007, 26, 378–384. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Najmanová, I.; Pourová, J.; Vopršalová, M.; Pilařová, V.; Semecký, V.; Nováková, L.; Mladěnka, P. Flavonoid metabolite 3-(3-hydroxyphenyl)propionic acid formed by human microflora decreases arterial blood pressure in rats. Mol. Nutr. Food Res. 2016, 60, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Pourová, J.; Najmanová, I.; Vopršalová, M.; Migkos, T.; Pilařová, V.; Applová, L.; Nováková, L.; Mladěnka, P. Two flavonoid metabolites, 3,4-dihydroxyphenylacetic acid and 4-methylcatechol, relax arteries ex vivo and decrease blood pressure in vivo. Vascul. Pharmacol. 2018, 111, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Khanna, D. Green tea catechins: Defensive role in cardiovascular disorders. Chin. J. Nat. Med. 2013, 11, 345–353. [Google Scholar] [CrossRef]
- Corral-Jara, K.; Nuthikattu, S.; Rutledge, J.; Villablanca, A.; Morand, C.; Schroeter, H.; Milenkovic, D. Integrated Multi-Omic Analyses of the Genomic Modifications by Gut Microbiome-Derived Metabolites of Epicatechin, 5-(4’-Hydroxyphenyl)-γ-Valerolactone, in TNFalpha-Stimulated Primary Human Brain Microvascular Endothelial Cells. Front. Neurosci. 2021, 15, 622640. [Google Scholar] [CrossRef]
- Corral-Jara, K.; Nuthikattu, S.; Rutledge, J.; Villablanca, A.; Fong, R.; Heiss, C.; Ottaviani, J.I.; Milenkovic, D. Structurally related (-)-epicatechin metabolites and gut microbiota derived metabolites exert genomic modifications via VEGF signaling pathways in brain microvascular endothelial cells under lipotoxic conditions: Integrated multi-omic study. J. Proteomics 2022, 263, 104603. [Google Scholar] [CrossRef]
- Lotito, S.; Zhang, W.J.; Yang, C.S.; Crozier, A.; Frei, B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free. Radic. Biol. Med. 2011, 51, 454–463. [Google Scholar] [CrossRef]
- Parvez, S.; Long, M.J.; Poganik, J.R.; Aye, Y. Redox signaling by reactive electrophiles and oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef]
- Na, H.-K.; Surh, Y.-J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef]
- Liu, C.; Boeren, S.; Rietjens, I.M.C.M. Intra- and Inter-individual Differences in the Human Intestinal Microbial Conversion of (-)-Epicatechin and Bioactivity of Its Major Colonic Metabolite 5-(3’,4’-Dihydroxy-Phenyl)-γ-Valerolactone in Regulating Nrf2-Mediated Gene Expression. Front. Nutr. 2022, 9, 910785. [Google Scholar] [CrossRef]
- De Rosso, V.; Morán Vieyra, F.E.; Mercadante, A.Z.; Borsarelli, C.D. Singlet oxygen quenching by anthocyanin’s flavylium cations. Free. Radic. Res. 2008, 42, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells--A Review. Int. J. Mol. Sci. 2015, 16, 1555–1574. [Google Scholar] [CrossRef]
- Minocha, T.; Birla, H.; Obaid, A.A.; Rai, V.; Sushma, P.; Shivamallu, C.; Moustafa, M.; Al-Shehri, M.; Al-Emam, A.; Tikhonova, M.A.; et al. Flavonoids as Promising Neuroprotectants and Their Therapeutic Potential against Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2022, 2022, 6038996. [Google Scholar] [CrossRef]
- Melrose, J.; Smith, M.M. Natural and Semi-Synthetic Flavonoid Anti-SARS-CoV-2 Agents for the Treatment of Long COVID-19 Disease and Neurodegenerative Disorders of Cognitive Decline. Front. Biosci. Elite 2022, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic Acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef]
- He, X.; Zhou, Y.Z.; Sheng, S.; Li, J.J.; Wang, G.Q.; Zhang, F. Ellagic Acid Protects Dopamine Neurons via Inhibition of NLRP3 Inflammasome Activation in Microglia. Oxid. Med. Cell. Longev. 2020, 2020, 2963540. [Google Scholar] [CrossRef] [PubMed]
- Nones, J.; Spohr, T.C.; Gomes, F.C. Hesperidin, a flavone glycoside, as mediator of neuronal survival. Neurochem. Res. 2011, 36, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Bandyopadhyay, J.; Chakraborty, S.; Basu, S. Multi-target screening mines hesperidin as a multi-potent inhibitor: Implication in Alzheimer’s disease therapeutics. Eur. J. Med. Chem. 2016, 121, 810–822. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rakshit, J.; Bandyopadhyay, J.; Basu, S. Multi-target inhibition ability of neohesperidin dictates its neuroprotective activity: Implication in Alzheimer’s disease therapeutics. Int. J. Biol. Macromol. 2021, 176, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, L.; Zhu, X.; Wu, W.; Wang, Y. Hesperidin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress in a mouse model of Alzheimer’s disease. Cell. Mol. Neurobiol. 2014, 34, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, Y.; Zhang, P.; Zhang, H.; Liu, X.; Zhang, Y. Neohesperidin Prevents Aβ25-35-Induced Apoptosis in Primary Cultured Hippocampal Neurons by Blocking the S-Nitrosylation of Protein-Disulphide Isomerase. Neurochem. Res. 2018, 43, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; An, Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Arch. Pharm. Res. 2018, 41, 655–663. [Google Scholar] [CrossRef]
- Mathew, B.; Suresh, J.; Mathew, G.E.; Parasuraman, R.; Abdulla, N. Plant secondary metabolites- potent inhibitors of monoamine oxidase isoforms. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14, 28–33. [Google Scholar] [CrossRef]
- Gidaro, M.; Astorino, C.; Petzer, A.; Carradori, S.; Alcaro, F.; Costa, G.; Artese, A.; Rafele, G.; Russo, F.M.; Petzer, J.P.; et al. Kaempferol as Selective Human MAO-A Inhibitor: Analytical Detection in Calabrian Red Wines, Biological and Molecular Modeling Studies. J. Agric. Food Chem. 2016, 64, 1394–1400. [Google Scholar] [CrossRef]
- Park, S.; Paudel, P.; Wagle, A.; Seong, S.H.; Kim, H.R.; Fauzi, F.M.; Jung, H.A.; Choi, J.S. Luteolin, a Potent Human Monoamine Oxidase-A Inhibitor and Dopamine D4 and Vasopressin V1A Receptor Antagonist. J. Agric. Food Chem. 2020, 68, 10719–10729. [Google Scholar] [CrossRef]
- Andersen, O.M.B.N.; Landau, A.M.; Pløen, G.G.; Jensen, A.M.G.; Monti, G.; Ulhøi, B.P.; Nyengaard, J.R.; Jacobsen, K.R.; Jørgensen, M.M.; Holm, I.E.; et al. A genetically modified minipig model for Alzheimer’s disease with SORL1 haploinsufficiency. Cell. Rep. Med. 2022, 3, 100740. [Google Scholar] [CrossRef]
- Dawson, T.; Golde, T.E.; Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1370–1379. [Google Scholar] [CrossRef]
- Hassan, H.; Elnagar, M.R.; Abdelrazik, E.; Mahdi, M.R.; Hamza, E.; Elattar, E.M.; ElNashar, E.M.; Alghamdi, M.A.; Al-Qahtani, Z.; Al-Khater, K.M.; et al. Neuroprotective effect of naringin against cerebellar changes in Alzheimer’s disease through modulation of autophagy, oxidative stress and tau expression: An experimental study. Front. Neuroanat. 2022, 16, 1012422. [Google Scholar] [CrossRef]
- Holm, I.E.; Alstrup, A.K.O.; Luo, Y. Genetically modified pig models for neurodegenerative disorders. J. Pathol. 2016, 238, 267–287. [Google Scholar] [CrossRef]
- Rockenstein, E.C.L.; Masliah, E. Transgenic animal models of neurodegenerative diseases and their application to treatment development. Adv. Drug. Deliv. Rev. 2007, 59, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Li, S.; Li, X.J.; Yang, W. New pathogenic insights from large animal models of neurodegenerative diseases. Protein Cell. 2022, 13, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, S.; Li, X.J. Genetically modified large animal models for investigating neurodegenerative diseases. Cell. Biosci. 2021, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, Y.K.; Ban, J.O.; Ha, T.Y.; Yun, Y.P.; Han, S.B.; Oh, K.W.; Hong, J.T. Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J. Nutr. 2009, 139, 1987–1993. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef]
- Rendeiro, C.; Spencer, J.P.; Vauzour, D.; Butler, L.T.; Ellis, J.A.; Williams, C.M. The impact of flavonoids on spatial memory in rodents: From behaviour to underlying hippocampal mechanisms. Genes. Nutr. 2009, 4, 251–270. [Google Scholar] [CrossRef]
- Vauzour, D. Effect of flavonoids on learning, memory and neurocognitive performance: Relevance and potential implications for Alzheimer’s disease pathophysiology. J. Sci. Food Agric. 2014, 94, 1042–1056. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, J.Z.; Li, Y.; Li, K.; Chen, H.X.; Zhang, Y.Z.; Li, Y.F. Anxiolytic effects of flavonoids in animal models of posttraumatic stress disorder. Evid. Based Complement. Altern. Med. 2012, 2012, 623753. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, X.; Zhang, R.; Zhao, C. Flavonoids with Potential Anti-Amyloidogenic Effects as Therapeutic Drugs for Treating Alzheimer’s Disease. J. Alzheimers Dis. 2021, 84, 505–533. [Google Scholar] [CrossRef]
- Gandhi, G.; Neta, M.T.S.L.; Sathiyabama, R.G.; Quintans, J.S.S.; de Oliveira ESilva, A.M.; Araújo, A.A.S.; Narain, N.; Júnior, L.J.Q.; Gurgel, R.Q. Flavonoids as Th1/Th2 cytokines immunomodulators: A systematic review of studies on animal models. Phytomedicine 2018, 44, 74–84. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Nan, Y.; Wang, X.; Chen, Y.; Wang, S. (-)-Epigallocatechin-3-gallate ameliorates memory impairment and rescues the abnormal synaptic protein levels in the frontal cortex and hippocampus in a mouse model of Alzheimer’s disease. Neuroreport 2017, 28, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Nan, S.; Wang, P.; Zhang, Y.; Fan, J. Epigallocatechin-3-Gallate Provides Protection Against Alzheimer’s Disease-Induced Learning and Memory Impairments in Rats. Drug. Des. Devel. Ther. 2021, 15, 2013–2024. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Q.; Chen, J.Y.; OuYang, D.; Lu, J.H. The pharmacological activity of epigallocatechin-3-gallate (EGCG) on Alzheimer’s disease animal model: A systematic review. Phytomedicine 2020, 79, 153316. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.Y.; Ouyang, D.; Lu, J.H. Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 493. [Google Scholar] [CrossRef]
- Fakhri, S.; Abdian, S.; Zarneshan, S.N.; Akkol, E.K.; Farzaei, M.H.; Sobarzo-Sánchez, E. Targeting Mitochondria by Plant Secondary Metabolites: A Promising Strategy in Combating Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 12570. [Google Scholar] [CrossRef] [PubMed]
- Lorzadeh, E.; Ramezani-Jolfaie, N.; Mohammadi, M.; Khoshbakht, Y.; Salehi-Abargouei, A. The effect of hesperidin supplementation on inflammatory markers in human adults: A systematic review and meta-analysis of randomized controlled clinical trials. Chem. Biol. Interact. 2019, 307, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Ali, S.; Scapagnini, G.; Costagliola, C. Effects of Flavonoid Supplementation on Common Eye Disorders: A Systematic Review and Meta-Analysis of Clinical Trials. Front. Nutr. 2021, 8, 651441. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, F.; Haidari, F.; Hedayati, M.; Zakerkish, M.; Ahmadi, K. Blood pressure lowering and anti-inflammatory effects of hesperidin in type 2 diabetes; a randomized double-blind controlled clinical trial. Phytother. Res. 2018, 32, 1073–1079. [Google Scholar] [CrossRef]
- Giannini, I.; Amato, A.; Basso, L.; Tricomi, N.; Marranci, M.; Pecorella, G.; Tafuri, S.; Pennisi, D.; Altomare, D.F. Flavonoids mixture (diosmin, troxerutin, hesperidin) in the treatment of acute hemorrhoidal disease: A prospective, randomized, triple-blind, controlled trial. Tech. Coloproctol. 2015, 19, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Martínez Noguera, F.; Alcaraz, P.E.; Carlos Vivas, J.; Chung, L.H.; Marín Cascales, E.; Marín Pagán, C. 8 weeks of 2S-Hesperidin supplementation improves muscle mass and reduces fat in amateur competitive cyclists: Randomized controlled trial. Food Funct. 2021, 12, 3872–3882. [Google Scholar] [CrossRef] [PubMed]
- van Iersel, L.; Stevens, Y.R.; Conchillo, J.M.; Troost, F.J. The effect of citrus flavonoid extract supplementation on anaerobic capacity in moderately trained athletes: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.; Huang, H.Y.; Liao, Y.H. The efficacy and safety of an antiaging topical serum containing hesperetin and sodium cyclic lysophosphatidic acid: A single-center clinical trial. J. Cosmet. Dermatol. 2021, 20, 3960–3967. [Google Scholar] [CrossRef]
- Joshi, S.; Dhingra, A.K.; Chopra, B.; Guarve, K.; Bhateja, D. Therapeutic Potential and Clinical Evidence of Hesperidin as Neuroprotective Agent. Cent. Nerv. Syst. Agents Med. Chem. 2022, 22, 5–14. [Google Scholar] [CrossRef]
- Cieuta-Walti, C.; Cuenca-Royo, A.; Langohr, K.; Rakic, C.; López-Vílchez, M.Á.; Lirio, J.; González-Lamuño Leguina, D.; González, T.B.; García, J.G.; Roure, M.R.; et al. Safety and preliminary efficacy on cognitive performance and adaptive functionality of epigallocatechin gallate (EGCG) in children with Down syndrome. A randomized phase Ib clinical trial (PERSEUS study). Genet. Med. 2022, 24, 2004–2013. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, W.; Zhao, X.; Li, X.; Zhou, Z.; Zheng, M.; Meng, X.; Kong, L.; Zhang, S.; He, D.; et al. Efficacy of Epigallocatechin-3-Gallate in Preventing Dermatitis in Patients With Breast Cancer Receiving Postoperative Radiotherapy: A Double-Blind, Placebo-Controlled, Phase 2 Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 779–786. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, L.; Lin, F.; Lei, J.; Zhou, M.; Xu, A. Topical epigallocatechin-3-gallate in the treatment of vitiligo. Australas. J. Dermatol. 2021, 62, e404–e407. [Google Scholar] [CrossRef]
- Ud-Din, S.; Wilgus, T.A.; McGeorge, D.D.; Bayat, A. Pre-Emptive Priming of Human Skin Improves Cutaneous Scarring and Is Superior to Immediate and Delayed Topical Anti-Scarring Treatment Post-Wounding: A Double-Blind Randomised Placebo-Controlled Clinical Trial. Pharmaceutics 2021, 13, 510. [Google Scholar] [CrossRef]
- Ud-Din, S.; Foden, P.; Mazhari, M.; Al-Habba, S.; Baguneid, M.; Bulfone-Paus, S.; McGeorge, D.; Bayat, A. A Double-Blind, Randomized Trial Shows the Role of Zonal Priming and Direct Topical Application of Epigallocatechin-3-Gallate in the Modulation of Cutaneous Scarring in Human Skin. J. Investig. Dermatol. 2019, 139, 1680–1690.e16. [Google Scholar] [CrossRef]
- Din, U.; Sian, T.S.; Deane, C.S.; Smith, K.; Gates, A.; Lund, J.N.; Williams, J.P.; Rueda, R.; Pereira, S.L.; Atherton, P.J.; et al. Green Tea Extract Concurrent with an Oral Nutritional Supplement Acutely Enhances Muscle Microvascular Blood Flow without Altering Leg Glucose Uptake in Healthy Older Adults. Nutrients 2021, 13, 3895. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, R.; Yamamoto, M.; Kumazoe, M.; Fujimura, Y.; Yonekura, M.; Shimamoto, Y.; Nakasone, A.; Kondo, S.; Hattori, H.; Haseda, A.; et al. The combined effect of green tea and α-glucosyl hesperidin in preventing obesity: A randomized placebo-controlled clinical trial. Sci. Rep. 2021, 11, 19067. [Google Scholar] [CrossRef]
- Bazyar, H.; Hosseini, S.A.; Saradar, S.; Mombaini, D.; Allivand, M.; Labibzadeh, M.; Alipour, M. Effects of epigallocatechin-3-gallate of Camellia sinensis leaves on blood pressure, lipid profile, atherogenic index of plasma and some inflammatory and antioxidant markers in type 2 diabetes mellitus patients: A clinical trial. J. Complement. Integr. Med. 2020, 18, 405–411. [Google Scholar] [CrossRef] [PubMed]
- de Morais Junior, A.; Schincaglia, R.M.; Passarelli, M.; Pimentel, G.D.; Mota, J.F. Acute Epigallocatechin-3-Gallate Supplementation Alters Postprandial Lipids after a Fast-Food Meal in Healthy Young Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2020, 12, 2533. [Google Scholar] [CrossRef]
- de la Torre, R.; de Sola, S.; Farré, M.; Xicota, L.; Cuenca-Royo, A.; Rodriguez, J.; León, A.; Langohr, K.; Gomis-González, M.; Hernandez, G.; et al. A phase 1, randomized double-blind, placebo controlled trial to evaluate safety and efficacy of epigallocatechin-3-gallate and cognitive training in adults with Fragile X syndrome. Clin. Nutr. 2020, 39, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Kohri, T.; Matsumoto, N.; Yamakawa, M.; Suzuki, M.; Nanjo, F.; Hara, Y.; Oku, N. Metabolic fate of (-)-[4-(3)H]epigallocatechin gallate in rats after oral administration. J. Agric. Food Chem. 2001, 49, 4102–4112. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-gamma-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef]
- Nation, D.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Takagaki, A.; Nanjo, F. Metabolism of (-)-epigallocatechin gallate by rat intestinal flora. J. Agric. Food Chem. 2010, 58, 1313–1321. [Google Scholar] [CrossRef]
- van’t Slot, G.; Humpf, H.U. Degradation and metabolism of catechin, epigallocatechin-3-gallate (EGCG), and related compounds by the intestinal microbiota in the pig cecum model. J. Agric. Food Chem. 2009, 57, 8041–8048. [Google Scholar] [CrossRef]
- Nehlig, A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br. J. Clin. Pharmacol. 2013, 75, 716–727. [Google Scholar] [CrossRef]
- Wang, J.; Varghese, M.; Ono, K.; Yamada, M.; Levine Tzavaras, N.; Gong, B.; Hurst, W.; Blitzer, R.; Pasinetti, G.M. Cocoa extracts reduce oligomerization of amyloid-beta: Implications for cognitive improvement in Alzheimer’s disease. J. Alzheimers. Dis. 2014, 41, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.; Pavlova, M.A.; Klosterhalfen, S.; Enck, P. Chocolate and the brain: Neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci. Biobehav. Rev. 2013, 37, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Khalifa, K.; Bergland, A.K.; Soennesyn, H.; Oppedal, K.; Holteng, L.B.A.; Oesterhus, R.; Nakling, A.; Jarholm, J.A.; de Lucia, C.; et al. A Randomised Placebo-Controlled Study of Purified Anthocyanins on Cognition in Individuals at Increased Risk for Dementia. Am. J. Geriatr. Psychiatry 2023, 31, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, K.; Bergland, A.K.; Soennesyn, H.; Oppedal, K.; Oesterhus, R.; Dalen, I.; Larsen, A.I.; Fladby, T.; Brooker, H.; Wesnes, K.A.; et al. Effects of Purified Anthocyanins in People at Risk for Dementia: Study Protocol for a Phase II Randomized Controlled Trial. Front. Neurol. 2020, 11, 916. [Google Scholar] [CrossRef]
- Arisi, T.; Gorski, F.; Eibel, B.; Barbosa, E.; Boll, L.; Waclawovsky, G.; Lehnen, A.M. Dietary intake of anthocyanins improves arterial stiffness, but not endothelial function, in volunteers with excess weight: A randomized clinical trial. Phytother. Res. 2022, 37, 798–808. [Google Scholar] [CrossRef]
- Kent, K.; Yousefi, M.; do Rosario, V.A.; Fitzgerald, Z.; Broyd, S.; Visentin, D.; Roodenrys, S.; Walton, K.; Charlton, K.E. Anthocyanin intake is associated with improved memory in older adults with mild cognitive impairment. Nutr. Res. 2022, 104, 36–43. [Google Scholar] [CrossRef]
- Krikorian, R.; Skelton, M.R.; Summer, S.S.; Shidler, M.D.; Sullivan, P.G. Blueberry Supplementation in Midlife for Dementia Risk Reduction. Nutrients 2022, 14, 1619. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Iglesias, D.E.; Kang, J.; Wang, Z.; Gray, R.; Mastaloudis, A.; Kay, C.D.; Hester, S.N.; Wood, S.M.; et al. A randomized placebo-controlled cross-over study on the effects of anthocyanins on inflammatory and metabolic responses to a high-fat meal in healthy subjects. Redox Biol. 2022, 51, 102273. [Google Scholar] [CrossRef]
- Emamat, H.; Zahedmehr, A.; Asadian, S.; Nasrollahzadeh, J. The effect of barberry (Berberis integerrima) on lipid profile and systemic inflammation in subjects with cardiovascular risk factors: A randomized controlled trial. BMC Complement. Med. Ther. 2022, 22, 59. [Google Scholar] [CrossRef]
- Tian, Z.; Li, K.; Fan, D.; Zhao, Y.; Gao, X.; Ma, X.; Xu, L.; Shi, Y.; Ya, F.; Zou, J.; et al. Dose-dependent effects of anthocyanin supplementation on platelet function in subjects with dyslipidemia: A randomized clinical trial. EBioMedicine 2021, 70, 103533. [Google Scholar] [CrossRef] [PubMed]
- Ahles, S.; Joris, P.J.; Plat, J. Effects of Berry Anthocyanins on Cognitive Performance, Vascular Function and Cardiometabolic Risk Markers: A Systematic Review of Randomized Placebo-Controlled Intervention Studies in Humans. Int. J. Mol. Sci. 2021, 22, 6482. [Google Scholar] [CrossRef] [PubMed]
- Rosli, H.; Shahar, S.; Rajab, N.F.; Che Din, N.; Haron, H. The effects of polyphenols-rich tropical fruit juice on cognitive function and metabolomics profile—A randomized controlled trial in middle-aged women. Nutr. Neurosci. 2022, 25, 1577–1593. [Google Scholar] [CrossRef] [PubMed]
- do Rosario, V.; Fitzgerald, Z.; Broyd, S.; Paterson, A.; Roodenrys, S.; Thomas, S.; Bliokas, V.; Potter, J.; Walton, K.; Weston-Green, K.; et al. Food anthocyanins decrease concentrations of TNF-α in older adults with mild cognitive impairment: A randomized, controlled, double blind clinical trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 950–960. [Google Scholar] [CrossRef] [PubMed]
- do Rosario, V.; Chang, C.; Spencer, J.; Alahakone, T.; Roodenrys, S.; Francois, M.; Weston-Green, K.; Hölzel, N.; Nichols, D.S.; Kent, K.; et al. Anthocyanins attenuate vascular and inflammatory responses to a high fat high energy meal challenge in overweight older adults: A cross-over, randomized, double-blind clinical trial. Clin. Nutr. 2021, 40, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Watanabe, H.; Tanaka, A.; Nishihira, J.; Murayama, N. Effect of quercetin glycosides on cognitive functions and cerebral blood flow: A randomized, double-blind, and placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8700–8712. [Google Scholar] [PubMed]
- Gonzales, M.; Garbarino, V.R.; Marques Zilli, E.; Petersen, R.C.; Kirkland, J.L.; Tchkonia, T.; Musi, N.; Seshadri, S.; Craft, S.; Orr, M.E. Senolytic Therapy to Modulate the Progression of Alzheimer’s Disease (SToMP-AD): A Pilot Clinical Trial. J. Prev. Alzheimers Dis. 2022, 9, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Purohit, P.; Roy, P.K. Neuroprotective Drug Discovery From Phytochemicals and Metabolites for CNS Viral Infection: A Systems Biology Approach With Clinical and Imaging Validation. Front. Neurosci. 2022, 16, 917867. [Google Scholar] [CrossRef]
- Otsuka, Y.; Miyamoto, N.; Nagai, A.; Izumo, T.; Nakai, M.; Fukuda, M.; Arimitsu, T.; Yamada, Y.; Hashimoto, T. Effects of Quercetin Glycoside Supplementation Combined With Low-Intensity Resistance Training on Muscle Quantity and Stiffness: A Randomized, Controlled Trial. Front. Nutr. 2022, 9, 912217. [Google Scholar] [CrossRef]
- Sgrò, P.; Ceci, R.; Lista, M.; Patrizio, F.; Sabatini, S.; Felici, F.; Sacchetti, M.; Bazzucchi, I.; Duranti, G.; Di Luigi, L. Quercetin Modulates IGF-I and IGF-II Levels After Eccentric Exercise-Induced Muscle-Damage: A Placebo-Controlled Study. Front. Endocrinol. 2021, 12, 745959. [Google Scholar] [CrossRef]
- Leyva-Soto, A.; Chavez-Santoscoy, A.R.; Porras, O.; Hidalgo-Ledesma, M.; Serrano-Medina, A.; Ramírez-Rodríguez, A.A.; Castillo-Martinez, A.N. Epicatechin and quercetin exhibit in vitro antioxidant effect, improve biochemical parameters related to metabolic syndrome, and decrease cellular genotoxicity in humans. Food Res. Int. 2021, 142, 110101. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.; Milajerdi, A.; Dadgostar, E.; Kolahdooz, F.; Chamani, M.; Amirani, E.; Mirzaei, H.; Asemi, Z. The Effects of Quercetin Supplementation on Blood Pressures and Endothelial Function Among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2019, 25, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Tamtaji, O.R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Dadgostar, E.; Asemi, Z. The effects of quercetin supplementation on lipid profiles and inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1855–1868. [Google Scholar] [CrossRef]
- Ou, Q.; Zheng, Z.; Zhao, Y.; Lin, W. Impact of quercetin on systemic levels of inflammation: A meta-analysis of randomised controlled human trials. Int. J. Food Sci. Nutr. 2020, 71, 152–163. [Google Scholar] [CrossRef]
- Vahdat-Lasemi, F.; Aghaee-Bakhtiari, S.H.; Tasbandi, A.; Jaafari, M.R.; Sahebkar, A. Targeting interleukin-β by plant-derived natural products: Implications for the treatment of atherosclerotic cardiovascular disease. Phytother. Res. 2021, 35, 5596–5622. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Zhang, Q.; Liu, J.; Li, R.; Wang, D.; Peng, W.; Wu, C. Suppression of apoptosis in vascular endothelial cell, the promising way for natural medicines to treat atherosclerosis. Pharmacol. Res. 2021, 168, 105599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Luo, M.; Shang, A.; Mao, Q.Q.; Li, B.Y.; Gan, R.Y.; Li, H.B. Antioxidant Food Components for the Prevention and Treatment of Cardiovascular Diseases: Effects, Mechanisms, and Clinical Studies. Oxid. Med. Cell. Longev. 2021, 2021, 6627355. [Google Scholar] [CrossRef]
- Dehghani, F.; Jandaghi, S.S.S.H.; Janani, L.; Sarebanhassanabadi, M.; Emamat, H.; Vafa, M. Effects of quercetin supplementation on inflammatory factors and quality of life in post-myocardial infarction patients: A double blind, placebo-controlled, randomized clinical trial. Phytother. Res. 2021, 35, 2085–2098. [Google Scholar] [CrossRef]
- Vaez, S.; Parivr, K.; Amidi, F.; Rudbari, N.H.; Moini, A.; Amini, N. Quercetin and polycystic ovary syndrome; inflammation, hormonal parameters and pregnancy outcome: A randomized clinical trial. Am. J. Reprod. Immunol. 2022, 89, e13644. [Google Scholar] [CrossRef]
- Hipólito-Reis, M.; Neto, A.C.; Neves, D. Impact of curcumin, quercetin, or resveratrol on the pathophysiology of endometriosis: A systematic review. Phytother. Res. 2022, 36, 2416–2433. [Google Scholar] [CrossRef]
- Shohan, M.; Nashibi, R.; Mahmoudian-Sani, M.R.; Abolnezhadian, F.; Ghafourian, M.; Alavi, S.M.; Sharhani, A.; Khodadadi, A. The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: A randomized controlled trial. Eur. J. Pharmacol. 2022, 914, 174615. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.; Wang, P.; Lee, R.P.; Trang, A.; Husari, G.; Yang, J.; Grojean, E.M.; Ly, A.; Hsu, M.; Heber, D.; et al. Prospective randomized trial evaluating blood and prostate tissue concentrations of green tea polyphenols and quercetin in men with prostate cancer. Food Funct. 2020, 11, 4114–4122. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Barreto, T.A.; Martinez, F.J.; Comstock, A.T.; Sajjan, U.S. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open. Respir. Res. 2020, 7, e000392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Peng, X.; Li, S.; Zhang, N.; Wang, Y.; Wei, H. Isolation and identification of quercetin degrading bacteria from human fecal microbes. PLoS ONE 2014, 9, e90531. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, Z.; Zhang, N.; Liu, L.; Li, S.; Wei, H. In vitro catabolism of quercetin by human fecal bacteria and the antioxidant capacity of its catabolites. Food Nutr. Res. 2014, 15, 58. [Google Scholar] [CrossRef]
- Jaganath, I.; Mullen, W.; Lean, M.E.; Edwards, C.A.; Crozier, A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free. Radic. Biol. Med. 2009, 47, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Peng, X.; Zhang, N.; Liu, L.; Wang, Y.; Ou, S. Cytotoxicity comparison of quercetin and its metabolites from in vitro fermentation of several gut bacteria. Food Funct. 2014, 5, 2152–2156. [Google Scholar] [CrossRef]
- Blaut, M.; Schoefer, L.; Braune, A. Transformation of flavonoids by intestinal microorganisms. Int. J. Vitam. Nutr. Res. 2003, 73, 79–87. [Google Scholar] [CrossRef]
- Jain, J.; Hasan, W.; Biswas, P.; Yadav, R.S.; Jat, D. Neuroprotective effect of quercetin against rotenone-induced neuroinflammation and alterations in mice behavior. J. Biochem. Mol. Toxicol. 2022, 36, e23165. [Google Scholar] [CrossRef]
- Madiha, S.; Batool, Z.; Tabassum, S.; Liaquat, L.; Sadir, S.; Shahzad, S.; Naqvi, F.; Saleem, S.; Yousuf, S.; Nawaz, A.; et al. Quercetin exhibits potent antioxidant activity, restores motor and non-motor deficits induced by rotenone toxicity. PLoS ONE 2021, 16, e0258928. [Google Scholar] [CrossRef]
- Paula, P.; Angelica Maria, S.G.; Luis, C.H.; Gloria Patricia, C.G. Preventive Effect of Quercetin in a Triple Transgenic Alzheimer’s Disease Mice Model. Molecules 2019, 24, 2287. [Google Scholar] [CrossRef] [PubMed]
- Ossola, B.; Kääriäinen, T.M.; Männistö, P. The multiple faces of quercetin in neuroprotection. Expert Opin. Drug. Saf. 2009, 8, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Zou, L.; Cao, H.; Ni, X.; Xiao, J. Dietary proanthocyanidins on gastrointestinal health and the interactions with gut microbiota. Crit. Rev. Food Sci. Nutr. 2022, 3, 1–24. [Google Scholar]
- Ou, K.; Sarnoski, P.; Schneider, K.R.; Song, K.; Khoo, C.; Gu, L. Microbial catabolism of procyanidins by human gut microbiota. Mol. Nutr. Food Res. 2014, 58, 2196–2205. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Cecarini, V.; Cuccioloni, M.; Zheng, Y.; Bonfili, L.; Gong, C.; Angeletti, M.; Mena, P.; Del Rio, D.; Eleuteri, A.M. Flavan-3-ol Microbial Metabolites Modulate Proteolysis in Neuronal Cells Reducing Amyloid-beta (1-42) Levels. Mol. Nutr. Food Res. 2021, 65, e2100380. [Google Scholar] [CrossRef]
- Ruotolo, R.; Minato, I.; La Vitola, P.; Artioli, L.; Curti, C.; Franceschi, V.; Brindani, N.; Amidani, D.; Colombo, L.; Salmona, M.; et al. Flavonoid-Derived Human Phenyl-γ-Valerolactone Metabolites Selectively Detoxify Amyloid-β Oligomers and Prevent Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2020, 64, e1900890. [Google Scholar] [CrossRef]
- Heiss, C.; Istas, G.; Feliciano, R.P.; Weber, T.; Wang, B.; Favari, C.; Mena, P.; Del Rio, D.; Rodriguez-Mateos, A. Daily consumption of cranberry improves endothelial function in healthy adults: A double blind randomized controlled trial. Food Funct. 2022, 13, 3812–3838. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Spencer, J.P.; Schroeter, H.; Rechner, A.R. Bioavailability of flavonoids and potential bioactive forms in vivo. Drug. Metabol. Drug. Interact. 2000, 17, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Carregosa, D.; Domenech-Coca, C.; Savi, M.; Figueira, I.; Brindani, N.; Jang, S.; Lakshman, S.; Molokin, A.; Urban, J.F., Jr.; et al. 5-(Hydroxyphenyl)-γ-Valerolactone-Sulfate, a Key Microbial Metabolite of Flavan-3-ols, Is Able to Reach the Brain: Evidence from Different in Silico, In Vitro and In Vivo Experimental Models. Nutrients 2019, 11, 2678. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 2564. [Google Scholar] [CrossRef] [PubMed]
- Billowria, K.; Ali, R.; Rangra, N.K.; Kumar, R.; Chawla, P.A. Bioactive Flavonoids: A Comprehensive Review on Pharmacokinetics and Analytical Aspects. Crit. Rev. Anal. Chem. 2022, 5, 1–15. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, W.; Lin, C.; Zhang, L. A Comprehensive Review on Beneficial Effects of Catechins on Secondary Mitochondrial Diseases. Int. J. Mol. Sci. 2022, 23, 11569. [Google Scholar] [CrossRef]
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Khan, M.I.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022, 46, e14189. [Google Scholar] [CrossRef]
- Rapisarda, P.; Amenta, M.; Ballistreri, G.; Fabroni, S.; Timpanaro, N. Distribution, Antioxidant Capacity, Bioavailability and Biological Properties of Anthocyanin Pigments in Blood Oranges and Other Citrus Species. Molecules 2022, 27, 8675. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Wdowiak, K.; Walkowiak, J.; Pietrzak, R.; Bazan-Woźniak, A.; Cielecka-Piontek, J. Bioavailability of Hesperidin and Its Aglycone Hesperetin-Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)-Mini-Review. Nutrients 2022, 14, 2647. [Google Scholar] [CrossRef]
- Hamsalakshmi Alex, A.; Arehally Marappa, M.; Joghee, S.; Chidambaram, S.B. Therapeutic benefits of flavonoids against neuroinflammation: A systematic review. Inflammopharmacology 2022, 30, 111–136. [Google Scholar] [CrossRef]
- Spencer, J. Metabolism of tea flavonoids in the gastrointestinal tract. J. Nutr. 2003, 133, 3255S–3261S. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, X.; Hu, X.; Chen, F.; Ma, C. Health benefits of proanthocyanidins linking with gastrointestinal modulation: An updated review. Food Chem. 2023, 404 Pt A, 134596. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, X.; Yan, J.; Sun, C.; Zhao, X.; Wang, X. Potential roles of gut microbes in biotransformation of natural products: An overview. Front. Microbiol. 2022, 13, 956378. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Álvarez, J.; Nguyen, W.; Sivapatham, R.; Rane, A.; Andersen, J.K. Urolithin A reduces amyloid-beta load and improves cognitive deficits uncorrelated with plaque burden in a mouse model of Alzheimer’s disease. Geroscience 2023, 45, 1095–1113. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wan, H.; Wan, H. Urolithin A induces protective autophagy to alleviate inflammation, oxidative stress, and endoplasmic reticulum stress in pediatric pneumonia. Allergol. Immunopathol. 2022, 50, 147–153. [Google Scholar] [CrossRef]
- Chen, P.; Guo, Z.; Chen, F.; Wu, Y.; Zhou, B. Recent Advances and Perspectives on the Health Benefits of Urolithin B, A Bioactive Natural Product Derived from Ellagitannins. Front. Pharmacol. 2022, 13, 917266. [Google Scholar] [CrossRef]
- Al Khalaf, A.; Abdulrahman, A.O.; Kaleem, M.; Nur, S.M.; Asseri, A.H.; Choudhry, H.; Khan, M.I. Comparative Analysis of the Impact of Urolithins on the Composition of the Gut Microbiota in Normal-Diet Fed Rats. Nutrients 2021, 13, 3885. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Dunngalvin, G.; Kern, T.; Blanco-Bose, W.; Auwerx, J.; Aebischer, P.; Rinsch, C. Direct supplementation with Urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur. J. Clin. Nutr. 2022, 76, 297–308. [Google Scholar] [CrossRef]
- Hasani, A.; Ebrahimzadeh, S.; Hemmati, F.; Khabbaz, A.; Hasani, A.; Gholizadeh, P. The role of Akkermansia muciniphila in obesity, diabetes and atherosclerosis. J. Med. Microbiol. 2021, 70, 001435. [Google Scholar] [CrossRef]
- Zhou, K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Beltrán, D.; Frutos, M.D.; Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Metabolism of different dietary phenolic compounds by the urolithin-producing human-gut bacteria Gordonibacter urolithinfaciens and Ellagibacter isourolithinifaciens. Food Funct. 2020, 11, 7012–7022. [Google Scholar] [CrossRef] [PubMed]
- Keranmu, A.; Pan, L.B.; Yu, H.; Fu, J.; Liu, Y.F.; Amuti, S.; Han, P.; Ma, S.R.; Xu, H.; Zhang, Z.W.; et al. The potential biological effects of quercetin based on pharmacokinetics and multi-targeted mechanism in vivo. J. Asian Nat. Prod. Res. 2022, 24, 403–431. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Hong, W.; Qian, D.; Peng, F.; Li, H.; Liang, C.; Du, M.; Gu, J.; Mai, J.; Bai, B.; et al. Quercetin modulates the gut microbiota as well as the metabolome in a rat model of osteoarthritis. Bioengineered 2021, 12, 6240–6250. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Sekhon, P.K.; Ambat, A.; Nelson, J.; Jose, D.; Bhat, G.J.; Scaria, J. Screening of Human Gut Bacterial Culture Collection Identifies Species That Biotransform Quercetin into Metabolites with Anticancer Properties. Int. J. Mol. Sci. 2021, 22, 7045. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, F.; Xing, Z.; Chen, J.; Peng, C.; Li, D. Beneficial effects of natural flavonoids on neuroinflammation. Front. Immunol. 2022, 13, 1006434. [Google Scholar] [CrossRef]
- Payne, A.; Nahashon, S.; Taka, E.; Adinew, G.M.; Soliman, K.F.A. Epigallocatechin-3-Gallate (EGCG): New Therapeutic Perspectives for Neuroprotection, Aging, and Neuroinflammation for the Modern Age. Biomolecules 2022, 12, 371. [Google Scholar] [CrossRef]
- Burberry, A.; Wells, M.F.; Limone, F.; Couto, A.; Smith, K.S.; Keaney, J.; Gillet, G.; van Gastel, N.; Wang, J.Y.; Pietilainen, O.; et al. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 2020, 582, 89–94. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Shabbir, U.; Tyagi, A.; Elahi, F.; Aloo, S.O.; Oh, D.H. The Potential Role of Polyphenols in Oxidative Stress and Inflammation Induced by Gut Microbiota in Alzheimer’s Disease. Antioxidants 2021, 10, 1370. [Google Scholar] [CrossRef] [PubMed]
- Caballero, V.; Estévez, M.; Tomás-Barberán, F.A.; Morcuende, D.; Martín, I.; Delgado, J. Biodegradation of Punicalagin into Ellagic Acid by Selected Probiotic Bacteria: A Study of the Underlying Mechanisms by MS-Based Proteomics. J. Agric. Food Chem. 2022, 70, 16273–16285. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, J.; Chen, L.; Guo, Q.; Yang, B.; Zhang, W.; Kang, W. Anticancer Effects and Mechanisms of Action of Plumbagin: Review of Research Advances. Biomed. Res. Int. 2020, 2020, 6940953. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Deng, L.; Wu, H.; Liu, Z.; Lu, W.; He, Y. Untargeted Metabolomics Profiling Reveals Beneficial Changes in Milk of Sows Supplemented with Fermented Compound Chinese Medicine Feed Additive. Animals 2022, 12, 2879. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Li, X.; Zhang, Z.; Sun, L.; Wang, Y.; He, Z.; Liu, Z.; Zhu, L.; Fu, L. Kaempferol suppression of acute colitis is regulated by the efflux transporters BCRP and MRP2. Eur. J. Pharm. Sci. 2022, 179, 106303. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Ye, F.; Tan, Y.; Peng, M.; Yuan, F.; Liu, Z.; Zhou, D.; Yang, K.; Liu, W.; Guo, R.; et al. Metabolomics and proteomics analyses revealed mechanistic insights on the antimicrobial activity of epigallocatechin gallate against Streptococcus suis. Front. Cell. Infect. Microbiol. 2022, 12, 973282. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, J.; Wang, J.; Wang, Y.; Ma, F.; Zhai, R.; Li, P. Kaempferol inhibits the growth of Helicobacter pylori in a manner distinct from antibiotics. J. Food Biochem. 2022, 46, e14210. [Google Scholar] [CrossRef]
- Santi, M.; Ortega, M.G.; Peralta, M.A. A State-of-the-art Review and Prospective Therapeutic Applications of Prenyl Flavonoids as Chemosensitizers against Antifungal Multidrug Resistance in Candida albicans. Curr. Med. Chem. 2022, 29, 4251–4281. [Google Scholar]
- Waditzer, M.; Bucar, F. Flavonoids as Inhibitors of Bacterial Efflux Pumps. Molecules 2021, 26, 6904. [Google Scholar] [CrossRef]
- Palko-Łabuz, A.; Błaszczyk, M.; Środa-Pomianek, K.; Wesołowska, O. Isobavachalcone as an Active Membrane Perturbing Agent and Inhibitor of ABCB1 Multidrug Transporter. Molecules 2021, 26, 4637. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Wen, L.; Jiang, Y.; Yang, J.; Zhao, Y.; Tian, M.; Yang, B. Structure, bioactivity, and synthesis of methylated flavonoids. Ann. N. Y. Acad. Sci. 2017, 1398, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Bioavailability of anthocyanins. Drug. Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, S.; Yang, W.; Huang, Q.; Ho, C.T. The biological fate and bioefficacy of citrus flavonoids: Bioavailability, biotransformation, and delivery systems. Food Funct. 2021, 12, 3307–3323. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta Pol. Pharm. 2015, 72, 643–650. [Google Scholar]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological properties of protocatechuic Acid and its potential roles as complementary medicine. Evid. Based Complement. Alternat. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Adewuni, T.M.; Ademiluyi, A.O.; Olasehinde, T.A.; Ademosun, A.O. Phenolic Constituents and Inhibitory Effects of Hibiscus sabdariffa L. (Sorrel) Calyx on Cholinergic, Monoaminergic, and Purinergic Enzyme Activities. J. Diet. Suppl. 2018, 15, 610–922. [Google Scholar] [CrossRef]
- Haskell-Ramsay, C.; Schmitt, J.; Actis-Goretta, L. The Impact of Epicatechin on Human Cognition: The Role of Cerebral Blood Flow. Nutrients 2018, 10, 986. [Google Scholar] [CrossRef]
- Kazem, Y.; Mahmoud, M.H.; Essa, H.A.; Azmy, O.; Kandeel, W.A.; Al-Moghazy, M.; El-Attar, I.; Hasheesh, A.; Mehanna, N.S. Role of Bifidobacterium spp. intake in improving depressive mood and well-being and its link to kynurenine blood level: An interventional study. J. Complement. Integr. Med. 2021. [Google Scholar] [CrossRef]
- Peirce, J.; Alviña, K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019, 97, 1223–1241. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Ge, D.; Liu, T.Q.; Ma, X.H.; Cui, Z.F. Protocatechuic acid promotes cell proliferation and reduces basal apoptosis in cultured neural stem cells. Toxicol In Vitro 2009, 23, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimers. Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zivkovic, A.M. The Potential Utility of Prebiotics to Modulate Alzheimer’s Disease: A Review of the Evidence. Microorganisms 2021, 9, 2310. [Google Scholar] [CrossRef]
- Li, H.; Zheng, T.; Lian, F.; Xu, T.; Yin, W.; Jiang, Y. Anthocyanin-rich blueberry extracts and anthocyanin metabolite protocatechuic acid promote autophagy-lysosomal pathway and alleviate neurons damage in in vivo and in vitro models of Alzheimer’s disease. Nutrition 2022, 93, 111473. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef]
- Shabbir, U.; Arshad, M.S.; Sameen, A.; Oh, D.H. Crosstalk between Gut and Brain in Alzheimer’s Disease: The Role of Gut Microbiota Modulation Strategies. Nutrients 2021, 13, 690. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease-a Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef]
- Fitzgerald, E.; Murphy, S.; Martinson, H.A. Alpha-Synuclein Pathology and the Role of the Microbiota in Parkinson’s Disease. Front. Neurosci. 2019, 13, 369. [Google Scholar] [CrossRef]
- Johnson, A.; Ou, Z.A.; Gordon, R.; Saminathan, H. Environmental neurotoxicants and inflammasome activation in Parkinson’s disease—A focus on the gut-brain axis. Int J Biochem Cell Biol. 2022, 142, 106113. [Google Scholar] [CrossRef] [PubMed]
- Toh, T.; Chong, C.W.; Lim, S.Y.; Bowman, J.; Cirstea, M.; Lin, C.H.; Chen, C.C.; Appel-Cresswell, S.; Finlay, B.B.; Tan, A.H. Gut microbiome in Parkinson’s disease: New insights from meta-analysis. Park. Relat. Disord. 2021, 94, 1–9. [Google Scholar] [CrossRef]
- Yap, C.; Henders, A.K.; Alvares, G.A.; Wood, D.L.A.; Krause, L.; Tyson, G.W.; Restuadi, R.; Wallace, L.; McLaren, T.; Hansell, N.K.; et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell. 2021, 184, 5916–5931.e17. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Zhao, C. Treating autism spectrum disorder by intervening with gut microbiota. J. Med. Microbiol. 2021, 70, 001469. [Google Scholar] [CrossRef]
- Chernikova, M.; Flores, G.D.; Kilroy, E.; Labus, J.S.; Mayer, E.A.; Aziz-Zadeh, L. The Brain-Gut-Microbiome System: Pathways and Implications for Autism Spectrum Disorder. Nutrients 2021, 13, 4497. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liang, J.; Fu, N.; Han, Y.; Qin, J. A Ketogenic Diet and the Treatment of Autism Spectrum Disorder. Front. Pediatr. 2021, 9, 650624. [Google Scholar] [CrossRef]
- Evans, S.; Bassis, C.M.; Hein, R.; Assari, S.; Flowers, S.A.; Kelly, M.B.; Young, V.B.; Ellingrod, V.E.; McInnis, M.G. The gut microbiome composition associates with bipolar disorder and illness severity. J. Psychiatr. Res. 2017, 87, 23–29. [Google Scholar] [CrossRef]
- Flowers, S.; Ward, K.M.; Clark, C.T. The Gut Microbiome in Bipolar Disorder and Pharmacotherapy Management. Neuropsychobiology 2020, 79, 43–49. [Google Scholar] [CrossRef]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef]
- Butler, M.; Mörkl, S.; Sandhu, K.V.; Cryan, J.F.; Dinan, T.G. The Gut Microbiome and Mental Health: What Should We Tell Our Patients? Le microbiote Intestinal et la Santé Mentale: Que Devrions-Nous dire à nos Patients? Can. J. Psychiatry 2019, 64, 747–760. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2021, 100, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.G.M. The gut microbiota in anxiety and depression—A systematic review. Clin Psychol Rev. 2021, 83, 101943. [Google Scholar] [CrossRef] [PubMed]
- Del Toro-Barbosa, M.; Hurtado-Romero, A.; Garcia-Amezquita, L.E.; García-Cayuela, T. Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients 2020, 12, 3896. [Google Scholar] [CrossRef]
- Dahlin, M.; Prast-Nielsen, S. The gut microbiome and epilepsy. EBioMedicine 2019, 44, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, H.; Liu, X.; Zhang, J.; Liu, G. Crosstalk between the Ketogenic Diet and Epilepsy: From the Perspective of Gut Microbiota. Mediat. Inflamm. 2019, 2019, 9373060. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Lang, Y.; Shu, H.; Shao, J.; Cui, L. Microbiota-Gut-Brain Axis and Epilepsy: A Review on Mechanisms and Potential Therapeutics. Front. Immunol. 2021, 12, 742449. [Google Scholar] [CrossRef]
- Huang, Q.; Xia, J. Influence of the gut microbiome on inflammatory and immune response after stroke. Neurol. Sci. 2021, 42, 4937–4951. [Google Scholar] [CrossRef]
- Huang, Q.; Di, L.; Yu, F.; Feng, X.; Liu, Z.; Wei, M.; Luo, Y.; Xia, J. Alterations in the gut microbiome with hemorrhagic transformation in experimental stroke. CNS Neurosci. Ther. 2022, 28, 77–91. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. The Role of Gut Microbiota in an Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 915. [Google Scholar] [CrossRef]
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef]
- Yamashiro, K.; Kurita, N.; Urabe, T.; Hattori, N. Role of the Gut Microbiota in Stroke Pathogenesis and Potential Therapeutic Implications. Ann. Nutr. Metab. 2021, 77 (Suppl. S2), 36–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, J.; Yang, F.; Li, S.; Ma, W.; Chang, X.; Yang, L. Neuroprotective Effects of Quercetin on Ischemic Stroke: A Literature Review. Front. Pharmacol. 2022, 13, 854249. [Google Scholar] [CrossRef] [PubMed]
- Sbrana, C.; Avio, L.; Giovannetti, M. Beneficial mycorrhizal symbionts affecting the production of health-promoting phytochemicals. Electrophoresis 2014, 35, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiome shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Li, D.; Sun, T.; Tong, Y.; Le, J.; Yao, Q.; Tao, J.; Liu, H.; Jiao, W.; Mei, Y.; Chen, J.; et al. Gut-microbiome-expressed 3β-hydroxysteroid dehydrogenase degrades estradiol and is linked to depression in premenopausal females. Cell Metab. 2023. [Google Scholar] [CrossRef] [PubMed]
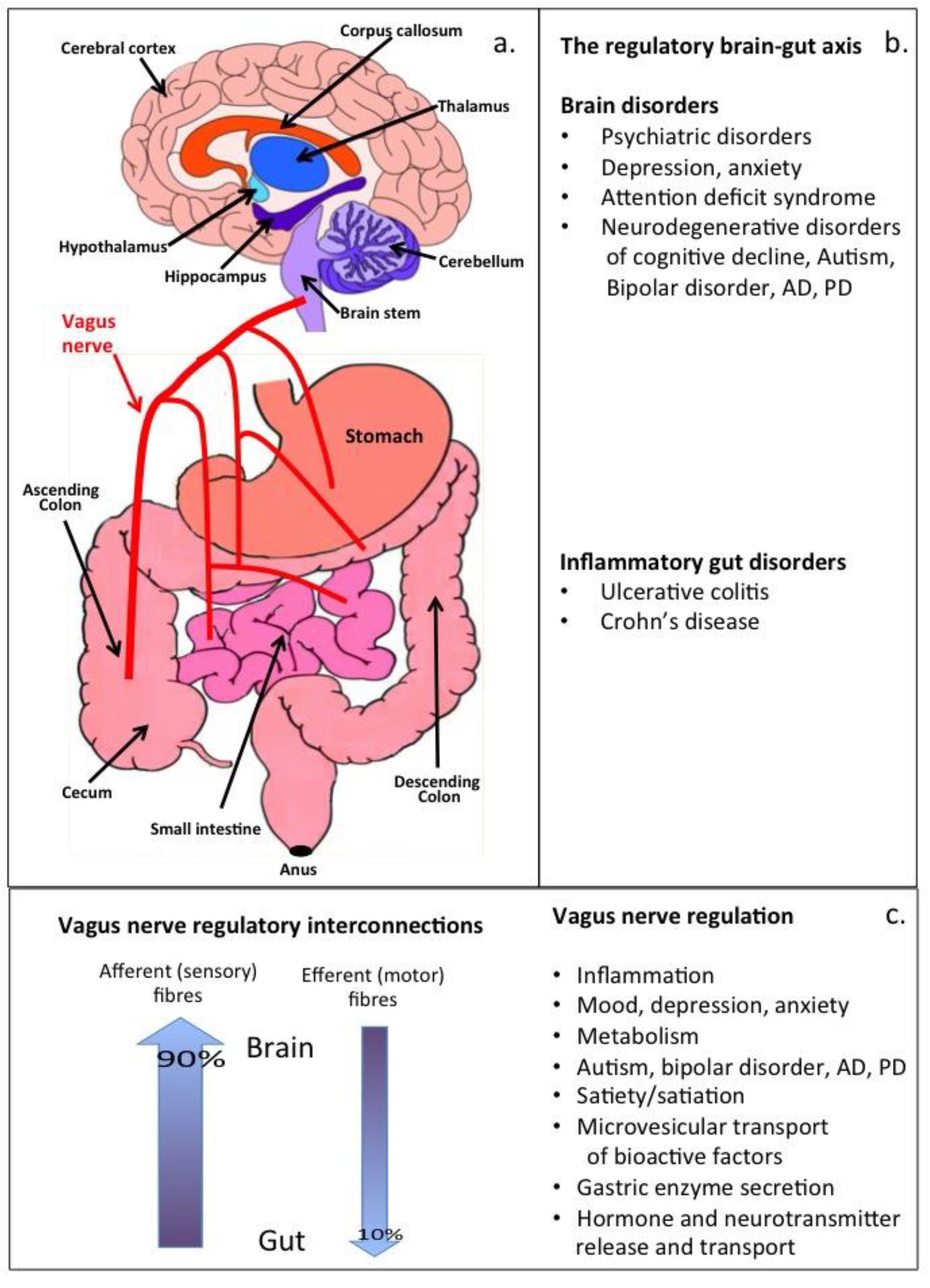
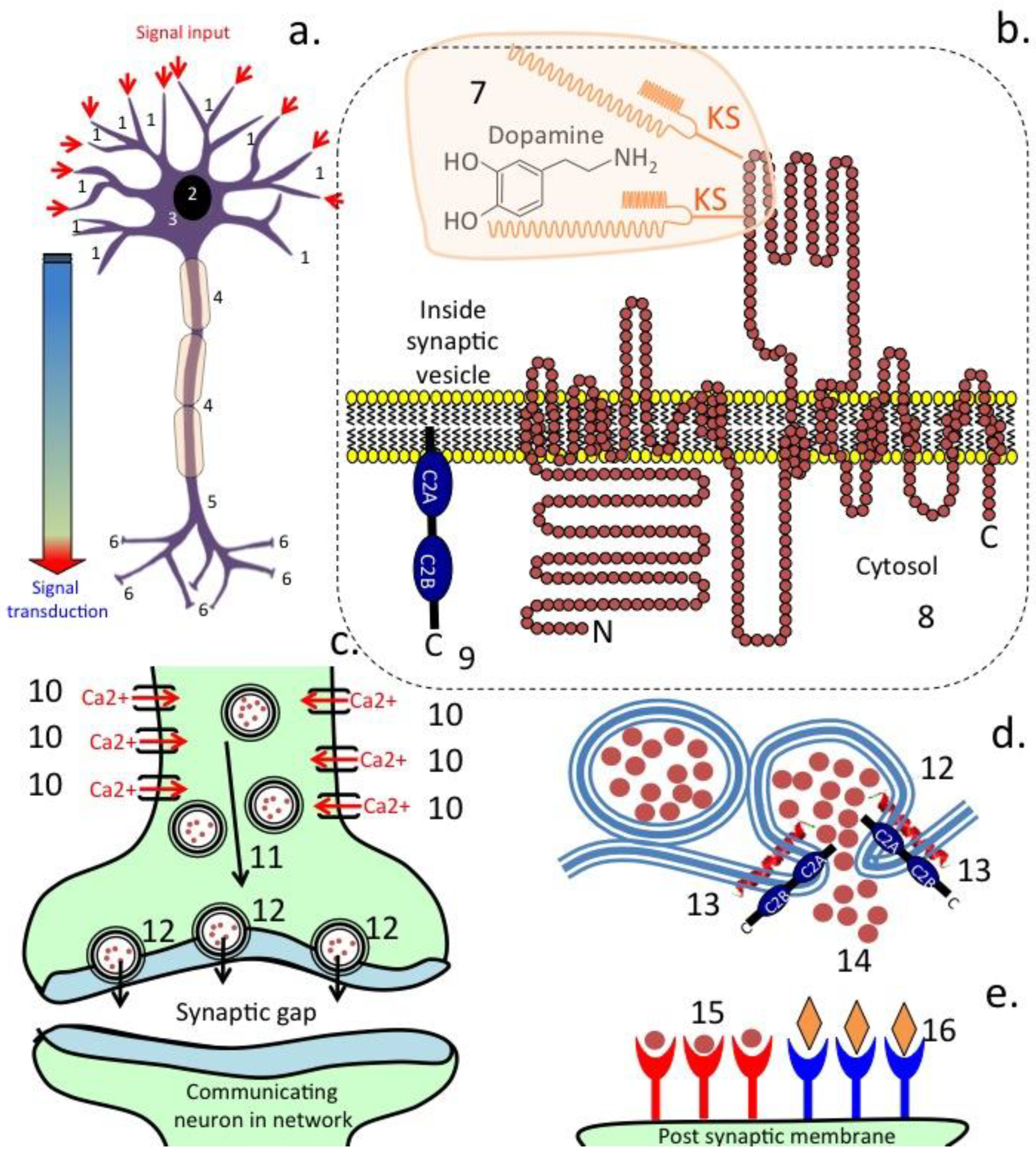
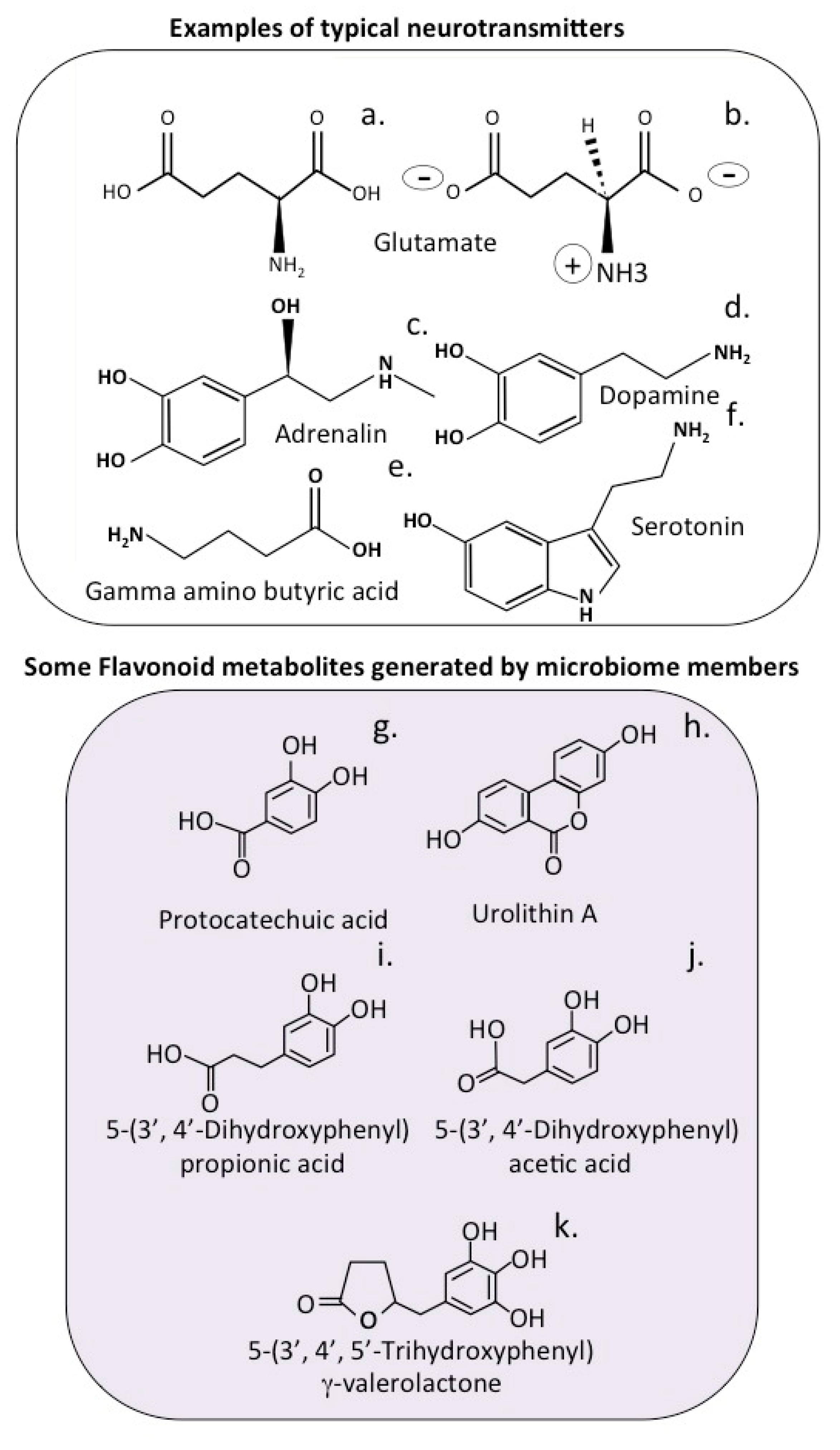
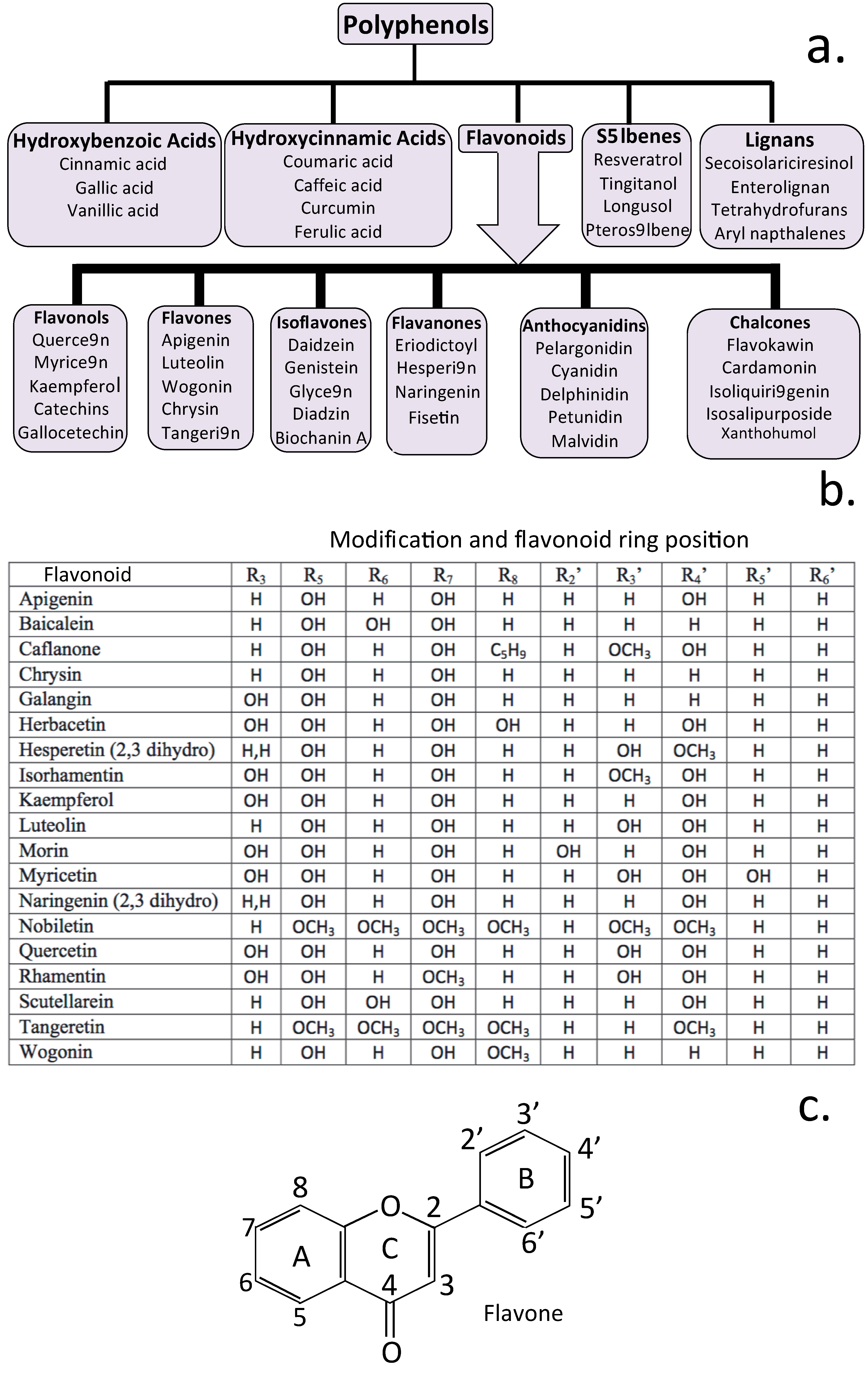
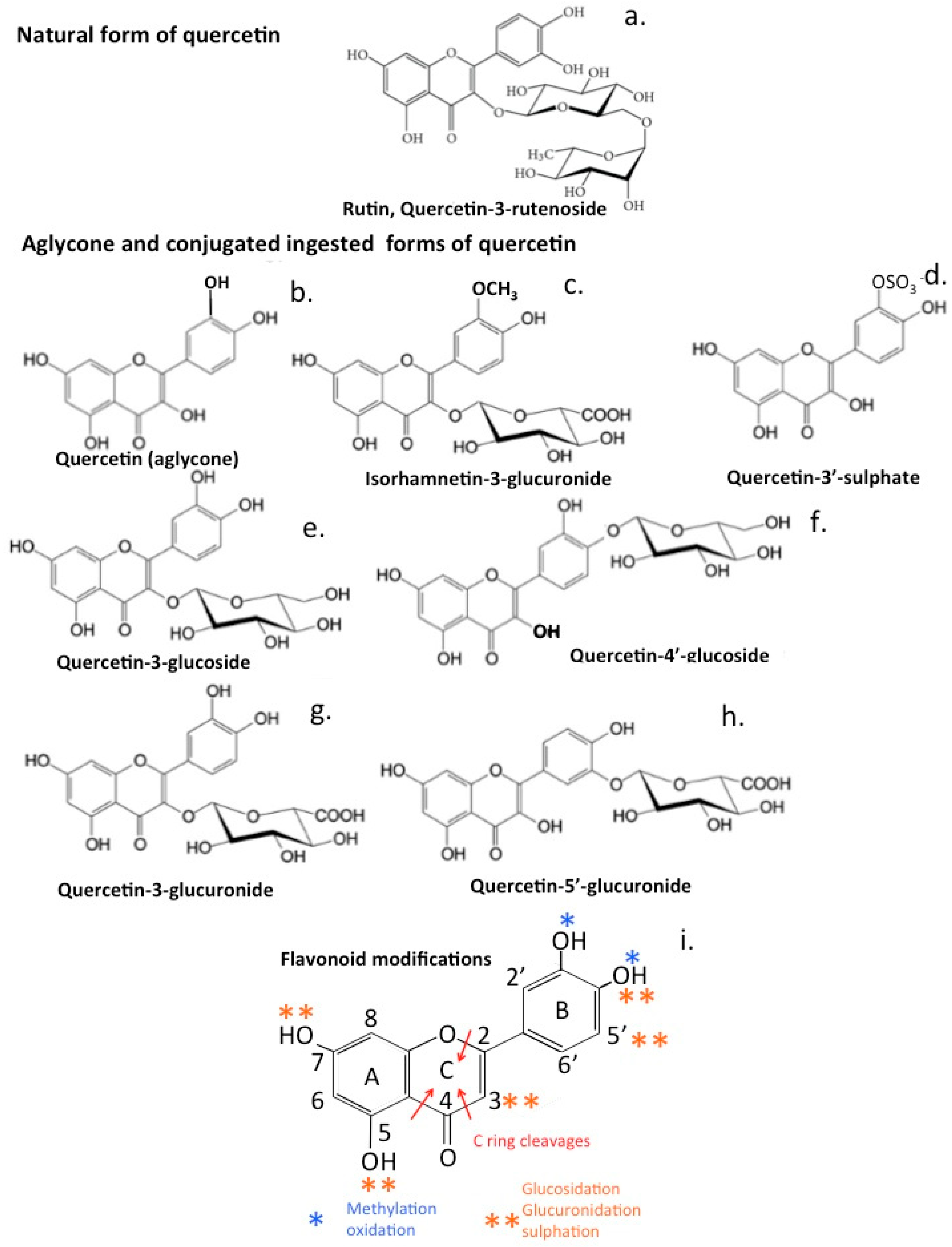
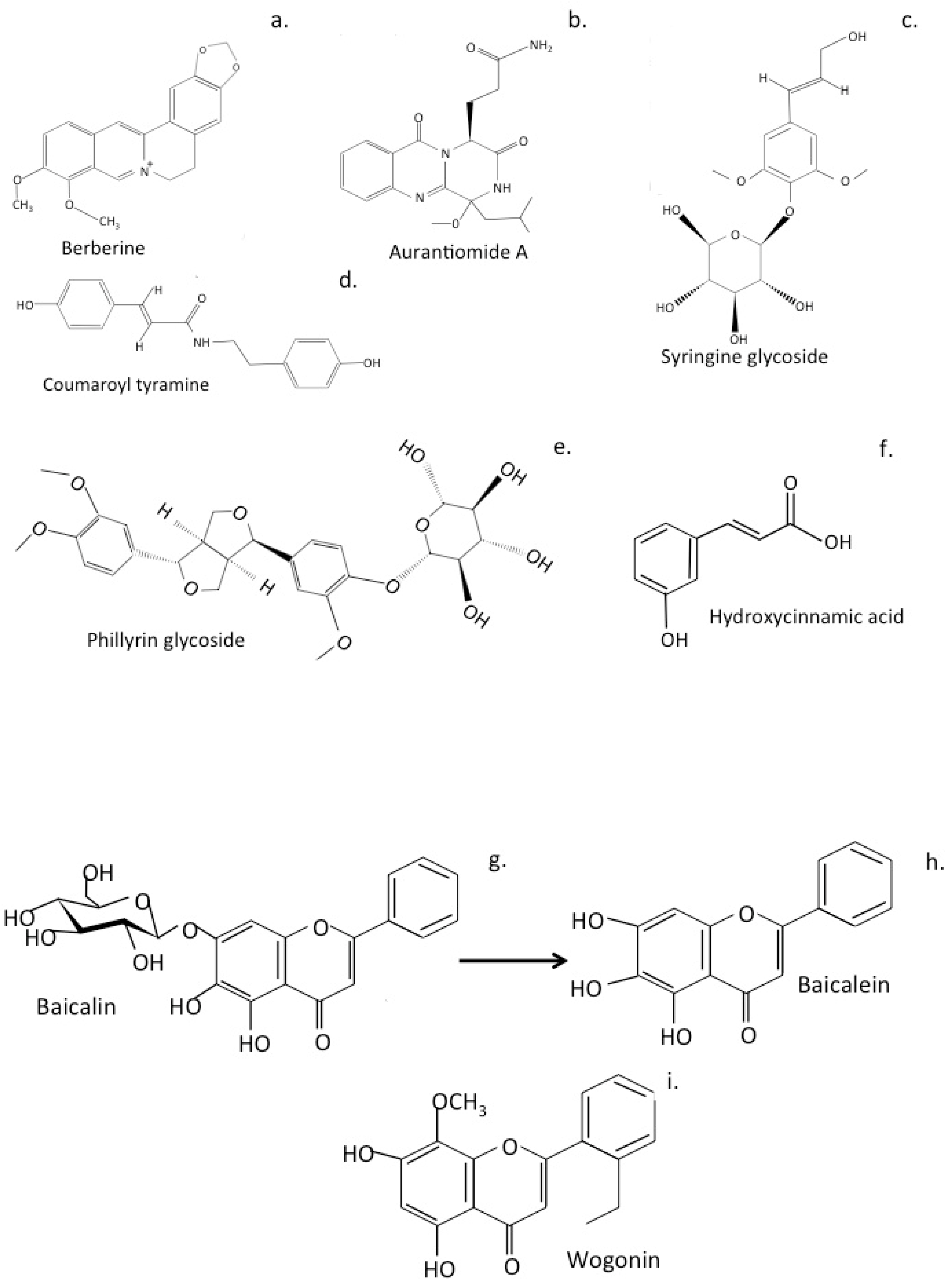
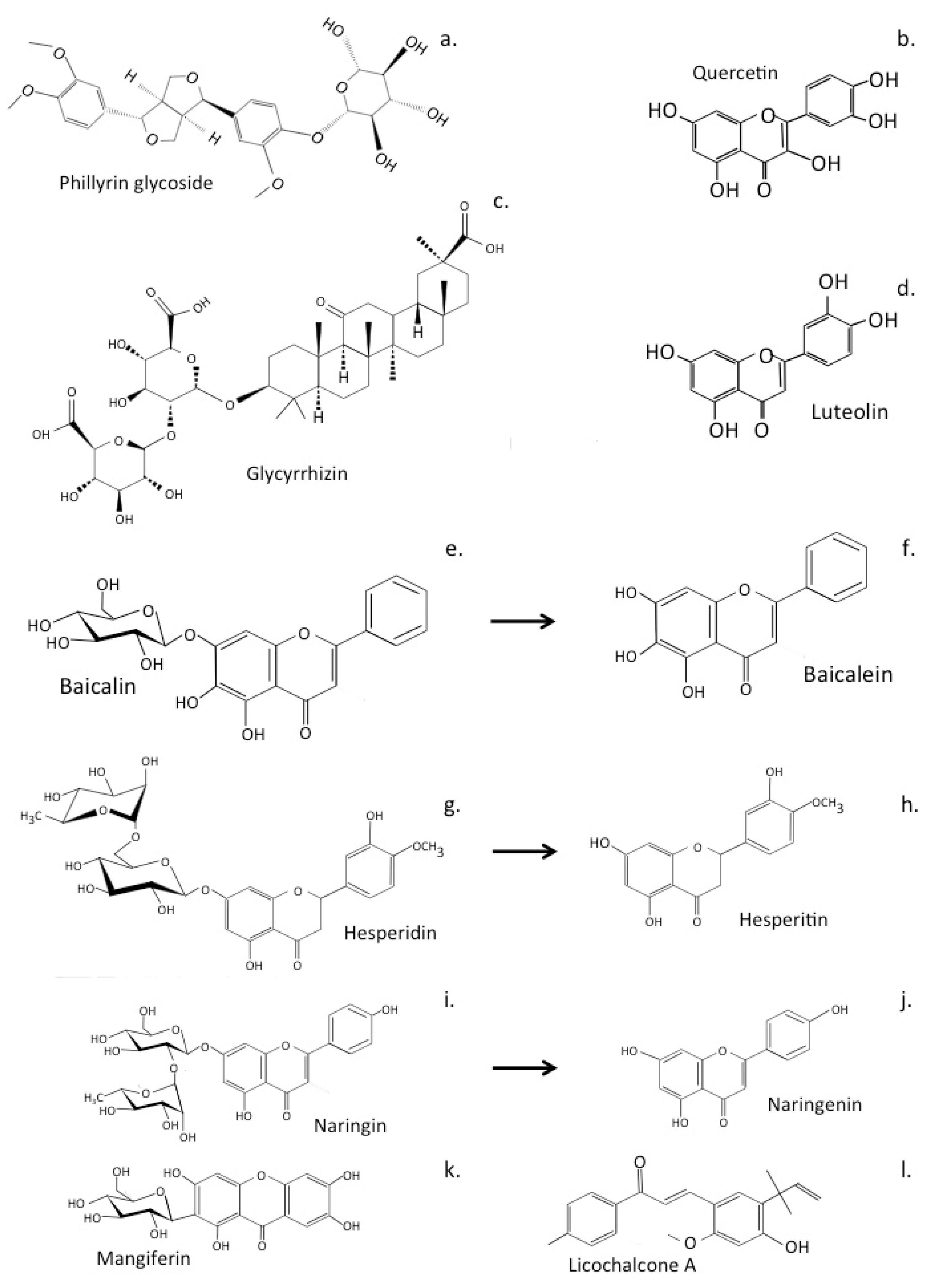
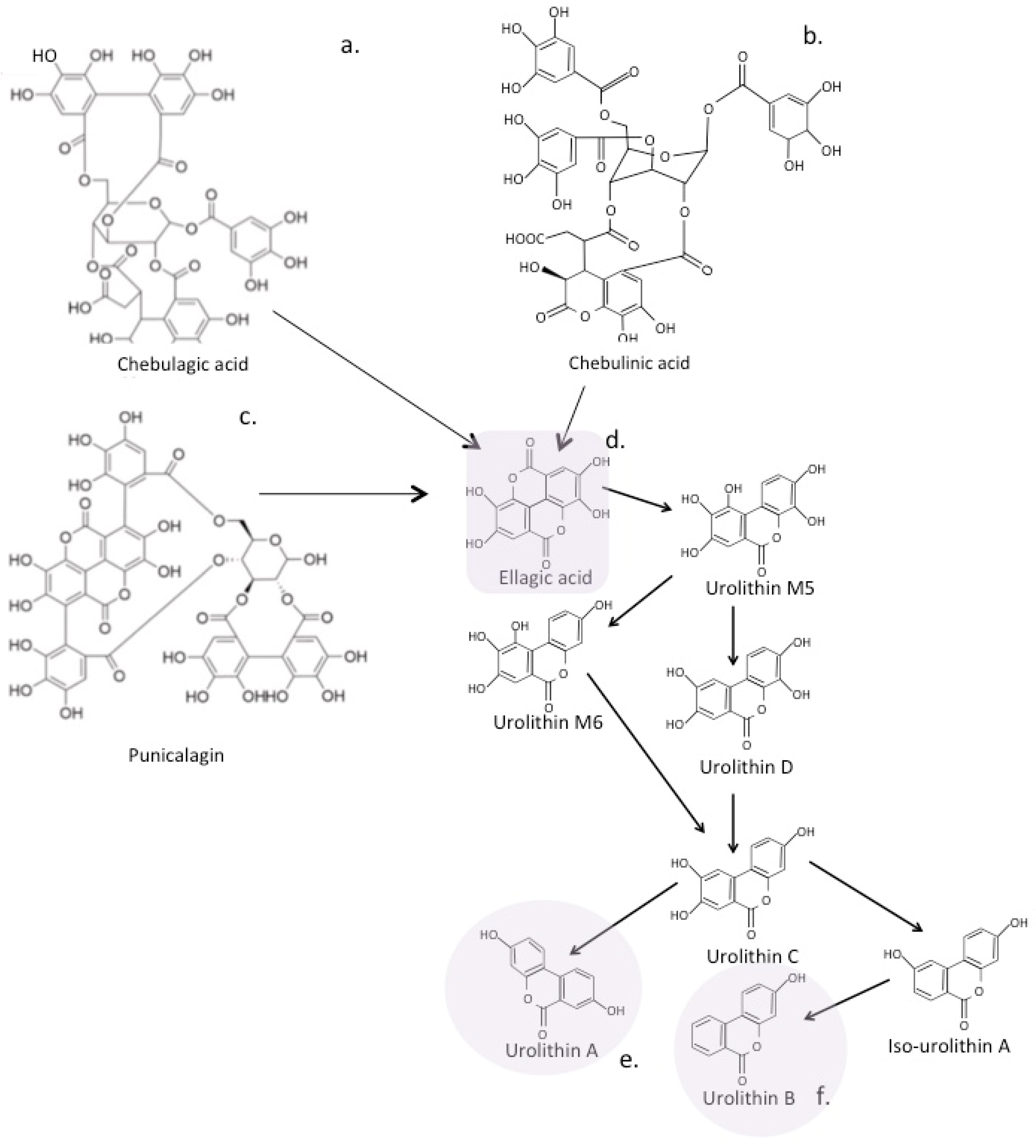

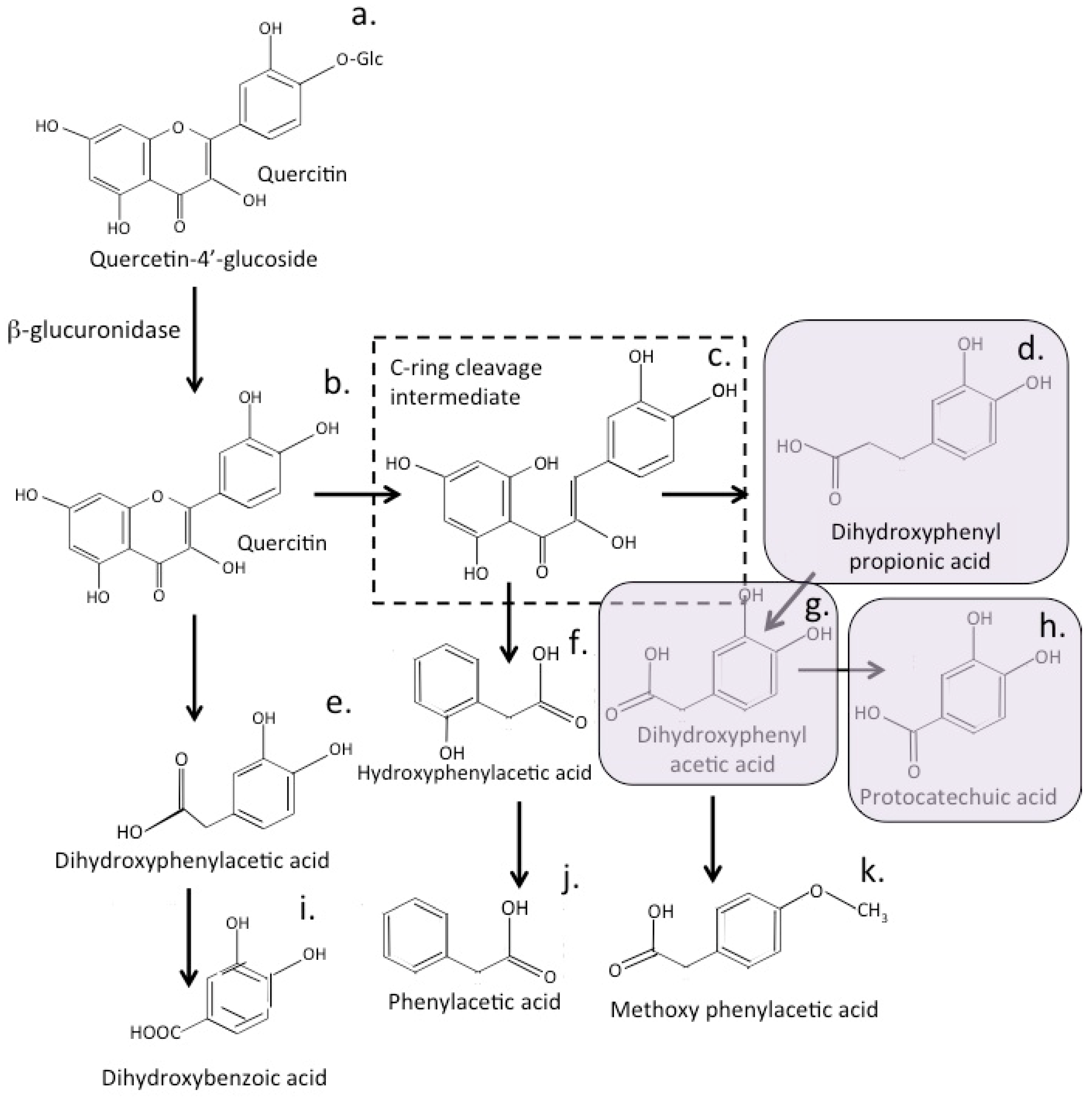
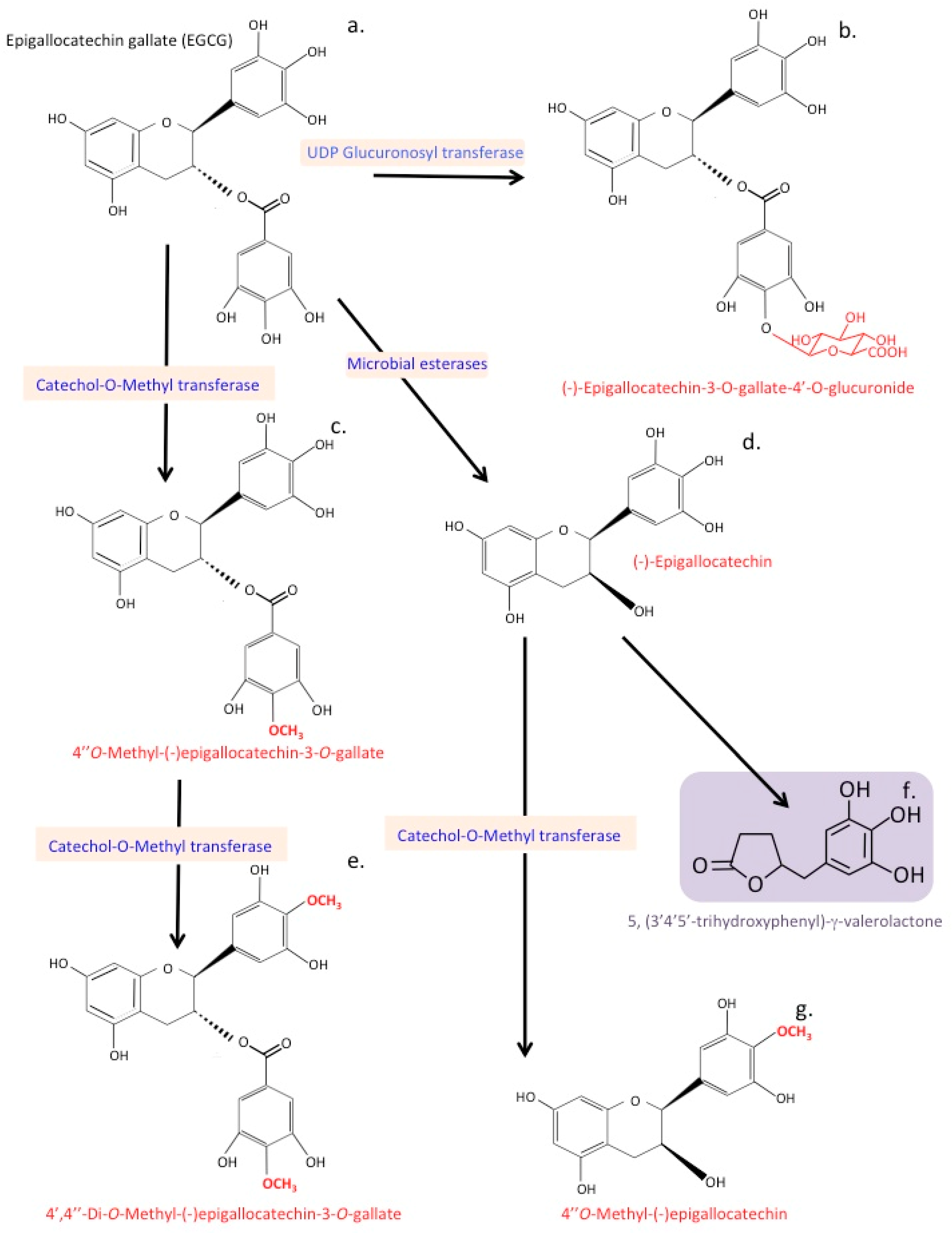
| Compound | Flavonoid Class | Reference |
|---|---|---|
| Quercetin | Flavonol | [119,120,121] |
| Myrcetin | [122] | |
| Kaempferol | [123,124] | |
| Catechin | [125] | |
| Gallocatechin | [126] | |
| Apigenin | Flavone | [127] |
| Luteolin | [128,129,130] | |
| Wogonin | [131,132] | |
| Chrysin | [133,134] | |
| Baicalin | [135] | |
| Diadzein | Isoflavone | [135] |
| Genistein | [136,137,138] | |
| Biochanin A | [139] | |
| Hesperidin | Flavonone | [139,140] |
| Hesperitin | [141,142,143] | |
| Naringenin | [144,145] | |
| Pelargonidin | Anthocyanidin | [146] |
| Cyanidin | [147] | |
| Delphinidin | [148] | |
| Petunidin | [149] | |
| Cardamonin | Chalcone | [150,151] |
| Xanthohumol | [152,153] | |
| Isoliquiritigenin | [154] |
| Neurological Disorder | How the Gut Microbiome Impacts the Disorder | Ref. |
|---|---|---|
| AD | Induction of Nrf2 expression by flavonoids is neuroprotective countering neuroinflammation. Flavonoids also have intrinsic antioxidant activity against generation of ROS by COX, LOX, MPO, XO. Many microbiome generated flavonoid metabolites retain or have enhanced or new bioactive properties not evident in the native flavonoid and greater bioavailability. Protocatechuic acid is present in the circulation at higher concentrations for significantly longer than native flavonoids and easily crosses the BBB, inhibits accumulation of β-amyloid plaques, hyperphosphorylation of tau protein in neurons and excessive generation of neuroinflammatory ROS, has potent antioxidant and anti-inflammatory properties, is neuroprotective, increases neuronal proliferation and inhibits apoptosis of neural stem cells. Urolithin A potently inhibits the pro-oxidant heme peroxidases MPO and LPO reducing tissue inflammation and significantly reduces phorbol myristate acetate stimulated ROS generated by neutrophils. Urolithin B is a MAO inhibitor and improves cognitive deficits. Urolithin M5 is a neuraminidase inhibitor, urolithin M6 is an inhibitor of LDH. Urolithins are neuroprotective, inhibit Aβ25-35-induced neurotoxicity and neurodegenerative MAO activity. Urolithin A promotes mitophagy and mitochondrial biogenic neuronal function. γ-valerolactones detoxify the effects of amyloid β oligomers. Some flavonoid metabolites have vasodilatory properties that improve cerebrovascular circulation and lower blood pressure. | [20,53,392,393,394,395,396,397,398,399] |
| PD | Anti-oxidant properties of flavonoids and metabolites and ability to induce Nrf2 expression is neuroprotective. Gut microbiome generated components may potentially regulate α-synuclein folding lowering the levels of misfolded α-synuclein deposition in pathological protein aggregates leading to neurotoxicity and a decline in neural function. Induction of mitochondrial biogenesis by flavonoid species promotes neuronal bioenergetics and viability. | [54,400,401,402] |
| Autism | Modulation of the gut microbiota to deliver high-fat low-carbohydrate ketogenic products has proven beneficial in countering the deficits in communication and social interaction evident in autism. | [403,404,405,406] |
| Bipolar disorder | Diets rich in n-3 fatty acids, folate, S-adenosylmethionine, N-acetyl cysteine and probiotic mediated effects offer promising interventions in the treatment of bipolar disorder | [84,407,408,409,410] |
| Depression and Anxiety | Symptoms of depression and anxiety have been shown to be linked to alterations in the microbiota and can be treated by probiotic dietary manipulation with certain food products known as psychobiotics. | [390,391,411,412,413] |
| Epilepsy | Manipulation of the gut microbiome to deliver a ketogenic high-fat low-carbohydrate diet mimics the fasting state of the body and is beneficial in treatment of drug-resistant epilepsy. | [55,414,415,416] |
| Stroke | Anti-oxidant, anti-thrombotic and vasodilatory properties of flavones and flavone metabolites may lower possibility of stroke and improve vascular repair processes. The anti-oxidant, anti-inflammatory, neuroprotective properties of quercetin may minimize the incidence of ischemic stroke. Promotion of endothelial cells by flavonoids improves vascular repair processes. | [417,418,419,420,421,422] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melrose, J. The Potential of Flavonoids and Flavonoid Metabolites in the Treatment of Neurodegenerative Pathology in Disorders of Cognitive Decline. Antioxidants 2023, 12, 663. https://doi.org/10.3390/antiox12030663
Melrose J. The Potential of Flavonoids and Flavonoid Metabolites in the Treatment of Neurodegenerative Pathology in Disorders of Cognitive Decline. Antioxidants. 2023; 12(3):663. https://doi.org/10.3390/antiox12030663
Chicago/Turabian StyleMelrose, James. 2023. "The Potential of Flavonoids and Flavonoid Metabolites in the Treatment of Neurodegenerative Pathology in Disorders of Cognitive Decline" Antioxidants 12, no. 3: 663. https://doi.org/10.3390/antiox12030663
APA StyleMelrose, J. (2023). The Potential of Flavonoids and Flavonoid Metabolites in the Treatment of Neurodegenerative Pathology in Disorders of Cognitive Decline. Antioxidants, 12(3), 663. https://doi.org/10.3390/antiox12030663