Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles
Abstract
:Highlights
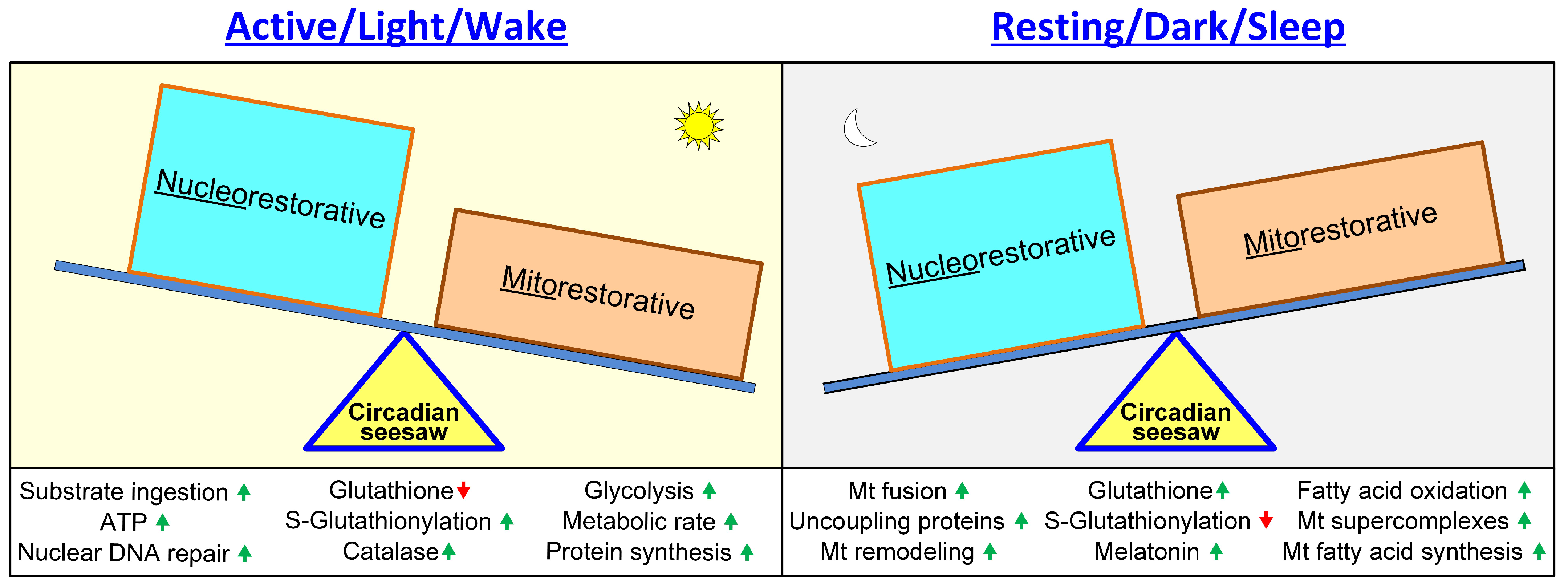
- Hypothesis: Redox, bioenergetic and temperature regulation is critical in maintaining cellular circadian rhythms; wakefulness is mainly “nucleorestorative” and sleep is mainly “mitorestorative”.
- Wakefulness: High metabolic rate induces oxidative stress and redox imbalance.
- Sleep: Fusion remodels mitochondria and the cellular redox balance is restored.
- Sleep: Mitochondria aid activation of rapid immune, inflammatory and heat shock responses.
- Sleep-wake cycling: Provides insights into the role of cysteine-mediated redox signaling, uncoupling and substrate cycles.
- Disorders of human development and aging: Perturbations of circadian tripartite-interactome signaling and mitochondrial-nuclear coregulation are implicated.
Abstract
1. Introduction
2. Protein Posttranslational Modifications, Redox Couples, and Circadian Rhythms
2.1. Introduction
2.2. Cellular and Mitochondrial S-Nitrosylation and Circadian Oscillations
2.3. Cellular S-Glutathionylation
2.4. Mitochondrial S-Glutathionylation and Circadian Oscillations
2.5. Redox Couples and Circadian Oscillations
3. Mitochondrial Energetics, Cellular Metabolism, and Circadian Rhythms
3.1. Introduction
3.2. Anion Carriers
3.3. Anion Carrier Mediators
3.4. Cellular and Mitochondrial Metabolic Circadian Rhythms—Futile Cycle Avoidance
4. Core Temperature, Heat Stress, Mitochondrial Oxidative Stress, and Circadian Rhythms
4.1. Introduction
4.2. Heat Shock Response
4.3. Mitochondrial Temperature and ROS Dependence
4.4. Redox/Temperature-Dependent Peripheral Clocks
5. Implications of the Hypothesis
5.1. Introduction
5.2. Reasons for Uncoupling
5.3. Sudden Infant Death Syndrome and Impaired Circadian Rhythm Development
5.4. Old Age and Associated Disease States
5.4.1. Impaired Redox–Bioenergetics–Temperature Regulation
5.4.2. Redox Cycling
5.4.3. Bioenergetics/Respiratory Cycling
5.4.4. Thermal Cycling
5.4.5. Circadian Rhythms
5.4.6. Ultradian Rhythms
5.5. Torpor and Hibernation
5.6. Hazards of Spaceflight and Space Radiation
6. Sleep Theories
6.1. Introduction
6.2. Energy Conservation and Free Radical Flux Theories
6.3. Protective and Restorative Aspects of the Sleep–Wake Cycle
6.3.1. Neuroprotective Aspects of Sleep
6.3.2. Nuclear DNA Repair during Wakefulness
6.3.3. Mitochondrial Fusion and Restoration during Sleep
6.3.4. Mitochondrial Respiratory Protection Provided by UCPs
6.4. Control of Protein Activity and Metabolic Futile Cycling Avoidance by Sleep–Wake Phases
7. Testing of the Hypothesis, and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-KGDH | α-ketoglutarate dehydrogenase |
| acetyl-CoA | acetyl coenzyme A |
| ADP | adenosine diphosphate |
| ANT | adenine nucleotide translocase |
| ATP | adenosine triphosphate |
| BCKDH | branched-chain keto acid dehydrogenase |
| CI–V | mitochondrial complexes I–V |
| DRP1 | dynamin-related protein 1 |
| ETC | electron transport chain |
| FAS | fatty-acid synthesis |
| GPX | glutathione peroxidase |
| GRX | glutaredoxin |
| GSH | glutathione |
| GSSG | glutathione disulfide |
| H2O2 | hydrogen peroxide |
| HIF-1 | hypoxia-inducible factor 1 |
| HSF1 | heat shock factor 1 |
| HSP | heat stress protein |
| mtDNA | mitochondrial DNA |
| mtFAS | mitochondrial fatty-acid synthesis |
| mTOR | mammalian target of rapamycin |
| mTORC1 | mTOR complex 1 |
| NAD+ | nicotinamide adenine dinucleotide |
| NADH | reduced NAD+ |
| NADP+ | nicotinamide adenine dinucleotide phosphate |
| NADPH | reduced NADP+ |
| •NO | nitric oxide |
| NOS | nitric oxide synthase |
| NREM | non-rapid eye movement |
| O2 | molecular oxygen |
| O2•− | superoxide radical |
| •OH | hydroxyl radical |
| ONOO− | peroxynitrite |
| PDH | pyruvate dehydrogenase |
| PRX | peroxiredoxin |
| PSSG | protein S-glutathionylation |
| REM | rapid eye movement |
| ROOR | peroxide functional group |
| ROS/RNS/RSS | reactive oxygen/nitrogen/sulfur species |
| RQ | respiratory quotient |
| SCN | suprachiasmatic nucleus |
| SIDS | sudden infant death syndrome |
| SIRT3 | sirtuin 3 |
| SOD | superoxide dismutase |
| SRX | sulfiredoxin |
| T3 | triiodothyronine |
| T4 | thyroxine |
| TCA cycle | tricarboxylic acid cycle |
| TRX | thioredoxin |
| TSH | thyroid-stimulating hormone |
| UCP | uncoupling protein |
References
- Rechtschaffen, A. Current perspectives on the function of sleep. Perspect. Biol. Med. 1998, 41, 359–390. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte, G.; Miguel-Puga, A.; Rodriguez, E.M.; Machado, S.; Manjarrez, E.; Arias-Carrion, O. Sleep deprivation and oxidative stress in animal models: A systematic review. Oxid. Med. Cell. Longev. 2015, 2015, 234952. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The free radical theory of aging. Antioxid. Redox Signal. 2003, 5, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Rubner, M. Das Problem der Lebensdaur und seine Beziehungen zu Wachstum und Ernärhung; De Gruyter Oldenbourg: Munich, Germany, 1908; p. 226. [Google Scholar]
- Pearl, R. The Rate of Living, Being an Account of Some Experimental Studies on the Biology of Life Duration; A.A. Knopf: New York, NY, USA, 1928; p. 185. [Google Scholar]
- Savage, V.M.; West, G.B. A quantitative, theoretical framework for understanding mammalian sleep. Proc. Natl. Acad. Sci. USA 2007, 104, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.H.; Swang, T.W.; Hamilton, I.M.; Best, J.A. State-dependent metabolic partitioning and energy conservation: A theoretical framework for understanding the function of sleep. PLoS ONE 2017, 12, e0185746. [Google Scholar] [CrossRef]
- Zitting, K.M.; Vujovic, N.; Yuan, R.K.; Isherwood, C.M.; Medina, J.E.; Wang, W.; Buxton, O.M.; Williams, J.S.; Czeisler, C.A.; Duffy, J.F. Human resting energy expenditure varies with circadian phase. Curr. Biol. 2018, 28, 3685–3690.e3683. [Google Scholar] [CrossRef]
- Stritesky Larssen, K.; Lyberg, T. Oxidative status—Age- and circadian variations?—A study in leukocytes/plasma. Neuro Endocrinol. Lett. 2006, 27, 445–452. [Google Scholar] [PubMed]
- Landsberg, L.; Young, J.B.; Leonard, W.R.; Linsenmeier, R.A.; Turek, F.W. Do the obese have lower body temperatures? A new look at a forgotten variable in energy balance. Trans. Am. Clin. Climatol. Assoc. 2009, 120, 287–295. [Google Scholar] [PubMed]
- Tan, C.L.; Knight, Z.A. Regulation of body temperature by the nervous system. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef]
- Du Bois, E.F. The basal metabolism in fever. J. Am. Med. Assoc. 1921, 77, 352–355. [Google Scholar]
- Refinetti, R.; Menaker, M. The circadian rhythm of body temperature. Physiol. Behav. 1992, 51, 613–637. [Google Scholar] [CrossRef]
- Ali, S.S.; Marcondes, M.C.; Bajova, H.; Dugan, L.L.; Conti, B. Metabolic depression and increased reactive oxygen species production by isolated mitochondria at moderately lower temperatures. J. Biol. Chem. 2010, 285, 32522–32528. [Google Scholar] [CrossRef] [PubMed]
- Milev, N.B.; Rhee, S.G.; Reddy, A.B. Cellular timekeeping: It’s redox o’clock. Cold Spring Harb. Perspect. Biol. 2018, 10, 1–31. [Google Scholar] [CrossRef]
- Mure, L.S.; Le, H.D.; Benegiamo, G.; Chang, M.W.; Rios, L.; Jillani, N.; Ngotho, M.; Kariuki, T.; Dkhissi-Benyahya, O.; Cooper, H.M.; et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 2018, 359, eaao0318. [Google Scholar] [CrossRef]
- Torrence, M.E.; MacArthur, M.R.; Hosios, A.M.; Valvezan, A.J.; Asara, J.M.; Mitchell, J.R.; Manning, B.D. The mTORC1-mediated activation of ATF4 promotes protein and glutathione synthesis downstream of growth signals. elife 2021, 10, e63326. [Google Scholar] [CrossRef]
- Plano, S.A.; Baidanoff, F.M.; Trebucq, L.L.; Suarez, S.A.; Doctorovich, F.; Golombek, D.A.; Chiesa, J.J. Redox and antioxidant modulation of circadian rhythms: Effects of nitroxyl, N-acetylcysteine and glutathione. Molecules 2021, 26, 2514. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, D.F.; Hulbert, A.J.; Brand, M.D. Characteristics of mitochondrial proton leak and control of oxidative phosphorylation in the major oxygen-consuming tissues of the rat. Biochim. Biophys. Acta 1994, 1188, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Singh, R.B.; Niaz, M.A.; Cornelissen, G.; Otsuka, K.; Siegelová, J.; Fišer, B.; Halberg, F. Circadian rhythmicity of circulating vitamin concentrations. Scripta Med. 2001, 74, 93–96. [Google Scholar]
- Blanco, R.A.; Ziegler, T.R.; Carlson, B.A.; Cheng, P.Y.; Park, Y.; Cotsonis, G.A.; Accardi, C.J.; Jones, D.P. Diurnal variation in glutathione and cysteine redox states in human plasma. Am. J. Clin. Nutr. 2007, 86, 1016–1023. [Google Scholar] [CrossRef]
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian rhythm connections to oxidative stress: Implications for human health. Antioxid. Redox Signal. 2013, 19, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J. The mouse thermoregulatory system: Its impact on translating biomedical data to humans. Physiol. Behav. 2017, 179, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Jezek, P.; Holendova, B.; Garlid, K.D.; Jaburek, M. Mitochondrial uncoupling proteins: Subtle regulators of cellular redox signaling. Antioxid. Redox Signal. 2018, 29, 667–714. [Google Scholar] [CrossRef]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Wang, Y.; Song, L.; Liu, M.; Ge, R.; Zhou, Q.; Liu, W.; Li, R.; Qie, J.; Zhen, B.; Wang, Y.; et al. A proteomics landscape of circadian clock in mouse liver. Nat. Commun. 2018, 9, 1553. [Google Scholar] [CrossRef]
- Neufeld-Cohen, A.; Robles, M.S.; Aviram, R.; Manella, G.; Adamovich, Y.; Ladeuix, B.; Nir, D.; Rousso-Noori, L.; Kuperman, Y.; Golik, M.; et al. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc. Natl. Acad. Sci. USA 2016, 113, E1673–E1682. [Google Scholar] [CrossRef]
- Robles, M.S.; Humphrey, S.J.; Mann, M. Phosphorylation is a central mechanism for circadian control of metabolism and physiology. Cell Metab. 2017, 25, 118–127. [Google Scholar] [CrossRef]
- Hebert, A.S.; Dittenhafer-Reed, K.E.; Yu, W.; Bailey, D.J.; Selen, E.S.; Boersma, M.D.; Carson, J.J.; Tonelli, M.; Balloon, A.J.; Higbee, A.J.; et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell 2013, 49, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Lushchak, V.I.; Cooper, A.J. A comparison of reversible versus irreversible protein glutathionylation. Adv. Cancer Res. 2014, 122, 177–198. [Google Scholar] [CrossRef]
- Putker, M.; O’Neill, J.S. Reciprocal control of the circadian clock and cellular redox state—A critical appraisal. Mol. Cells 2016, 39, 6–19. [Google Scholar] [CrossRef]
- Grek, C.L.; Zhang, J.; Manevich, Y.; Townsend, D.M.; Tew, K.D. Causes and consequences of cysteine S-glutathionylation. J. Biol. Chem. 2013, 288, 26497–26504. [Google Scholar] [CrossRef] [PubMed]
- Canals, S.; Casarejos, M.J.; de Bernardo, S.; Rodriguez-Martin, E.; Mena, M.A. Nitric oxide triggers the toxicity due to glutathione depletion in midbrain cultures through 12-lipoxygenase. J. Biol. Chem. 2003, 278, 21542–21549. [Google Scholar] [CrossRef]
- Foster, M.W.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol. Med. 2009, 15, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Piantadosi, C.A. Regulation of mitochondrial processes by protein S-nitrosylation. Biochim. Biophys. Acta 2012, 1820, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Doulias, P.T.; Tenopoulou, M.; Greene, J.L.; Raju, K.; Ischiropoulos, H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci. Signal. 2013, 6, rs1. [Google Scholar] [CrossRef]
- Cespuglio, R.; Amrouni, D.; Meiller, A.; Buguet, A.; Gautier-Sauvigne, S. Nitric oxide in the regulation of the sleep-wake states. Sleep Med. Rev. 2012, 16, 265–279. [Google Scholar] [CrossRef]
- Golombek, D.A.; Agostino, P.V.; Plano, S.A.; Ferreyra, G.A. Signaling in the mammalian circadian clock: The NO/cGMP pathway. Neurochem. Int. 2004, 45, 929–936. [Google Scholar] [CrossRef]
- Rodrigo, G.C.; Herbert, K.E. Regulation of vascular function and blood pressure by circadian variation in redox signalling. Free Radic. Biol. Med. 2018, 119, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Eriksson, S.; Wang, L. Oxidative stress induced S-glutathionylation and proteolytic degradation of mitochondrial thymidine kinase. J. Biol. Chem. 2012, 287, 24304–24312. [Google Scholar] [CrossRef]
- Townsend, D.M.; Manevich, Y.; He, L.; Hutchens, S.; Pazoles, C.J.; Tew, K.D. Novel role for glutathione S-transferase π: Regulator of protein S-glutathionylation following oxidative and nitrosative stress. J. Biol. Chem. 2009, 284, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Fratelli, M.; Demol, H.; Puype, M.; Casagrande, S.; Eberini, I.; Salmona, M.; Bonetto, V.; Mengozzi, M.; Duffieux, F.; Miclet, E.; et al. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 3505–3510. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Gill, R.; Young, A. Protein S-glutathionylation and the regulation of cellular functions. In Oxidative Stress: Eustress and Distress; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 217–246. [Google Scholar]
- Townsend, D.M. S-Glutathionylation: Indicator of cell stress and regulator of the unfolded protein response. Mol. Interv. 2007, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Kodali, V.K.; Gaffrey, M.J.; Guo, J.; Chu, R.K.; Camp, D.G.; Smith, R.D.; Thrall, B.D.; Qian, W.J. Quantitative profiling of protein S-glutathionylation reveals redox-dependent regulation of macrophage function during nanoparticle-induced oxidative stress. ACS Nano 2016, 10, 524–538. [Google Scholar] [CrossRef]
- Lind, C.; Gerdes, R.; Hamnell, Y.; Schuppe-Koistinen, I.; von Lowenhielm, H.B.; Holmgren, A.; Cotgreave, I.A. Identification of S-glutathionylated cellular proteins during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis. Arch. Biochem. Biophys. 2002, 406, 229–240. [Google Scholar] [CrossRef]
- Campbell, M.D.; Duan, J.; Samuelson, A.T.; Gaffrey, M.J.; Merrihew, G.E.; Egertson, J.D.; Wang, L.; Bammler, T.K.; Moore, R.J.; White, C.C.; et al. Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic. Biol. Med. 2019, 134, 268–281. [Google Scholar] [CrossRef]
- Mailloux, R.J. An update on mitochondrial reactive oxygen species production. Antioxidants 2020, 9, 472. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Goncalves, R.L.; Hey-Mogensen, M.; Yadava, N.; Bunik, V.I.; Brand, M.D. The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. J. Biol. Chem. 2014, 289, 8312–8325. [Google Scholar] [CrossRef] [PubMed]
- Hirschenson, J.; Mailloux, R.J. The glutathionylation agent disulfiram augments superoxide/hydrogen peroxide production when liver mitochondria are oxidizing ubiquinone pool-linked and branched chain amino acid substrates. Free Radic. Biol. Med. 2021, 172, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wohlhueter, R.M.; Harper, A.E. Coinduction of rat liver branched chain α-keto acid dehydrogenase activities. J. Biol. Chem. 1970, 245, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Clugston, G.A.; Garlick, P.J. The response of protein and energy metabolism to food intake in lean and obese man. Hum. Nutr. Clin. Nutr. 1982, 36C, 57–70. [Google Scholar] [PubMed]
- Jouffe, C.; Cretenet, G.; Symul, L.; Martin, E.; Atger, F.; Naef, F.; Gachon, F. The circadian clock coordinates ribosome biogenesis. PLoS Biol. 2013, 11, e1001455. [Google Scholar] [CrossRef]
- Young, A.; Gill, R.; Mailloux, R.J. Protein S-glutathionylation: The linchpin for the transmission of regulatory information on redox buffering capacity in mitochondria. Chem. Biol. Interact. 2019, 299, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, C.S.; Almeida, A.S.; Martel, C.; Brenner, C.; Alves, P.M.; Vieira, H.L. Glutathionylation of adenine nucleotide translocase induced by carbon monoxide prevents mitochondrial membrane permeabilization and apoptosis. J. Biol. Chem. 2010, 285, 17077–17088. [Google Scholar] [CrossRef]
- Jeon, D.; Park, H.J.; Kim, H.S. Protein S-glutathionylation induced by hypoxia increases hypoxia-inducible factor-1α in human colon cancer cells. Biochem. Biophys. Res. Commun. 2018, 495, 212–216. [Google Scholar] [CrossRef]
- Adamovich, Y.; Ladeuix, B.; Sobel, J.; Manella, G.; Neufeld-Cohen, A.; Assadi, M.H.; Golik, M.; Kuperman, Y.; Tarasiuk, A.; Koeners, M.P.; et al. Oxygen and carbon dioxide rhythms are circadian clock controlled and differentially directed by behavioral signals. Cell Metab. 2019, 29, 1092–1103. [Google Scholar] [CrossRef]
- Pfleger, J.; He, M.; Abdellatif, M. Mitochondrial complex II is a source of the reserve respiratory capacity that is regulated by metabolic sensors and promotes cell survival. Cell. Death Dis. 2015, 6, e1835. [Google Scholar] [CrossRef]
- Bose, H.S.; Marshall, B.; Debnath, D.K.; Perry, E.W.; Whittal, R.M. Electron transport chain complex II regulates steroid metabolism. iScience 2020, 23, 101295. [Google Scholar] [CrossRef] [PubMed]
- Nowak, N.; Gaisl, T.; Miladinovic, D.; Marcinkevics, R.; Osswald, M.; Bauer, S.; Buhmann, J.; Zenobi, R.; Sinues, P.; Brown, S.A.; et al. Rapid and reversible control of human metabolism by individual sleep states. Cell Rep. 2021, 37, 109903. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.; Shurubor, Y.; Valsecchi, F.; Manfredi, G.; Galkin, A. Differential susceptibility of mitochondrial complex II to inhibition by oxaloacetate in brain and heart. Biochim. Biophys. Acta 2016, 1857, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.H. The energy allocation function of sleep: A unifying theory of sleep, torpor, and continuous wakefulness. Neurosci. Biobehav. Rev. 2014, 47, 122–153. [Google Scholar] [CrossRef]
- Wang, T.A.; Yu, Y.V.; Govindaiah, G.; Ye, X.; Artinian, L.; Coleman, T.P.; Sweedler, J.V.; Cox, C.L.; Gillette, M.U. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science 2012, 337, 839–842. [Google Scholar] [CrossRef]
- Khazim, K.; Giustarini, D.; Rossi, R.; Verkaik, D.; Cornell, J.E.; Cunningham, S.E.; Mohammad, M.; Trochta, K.; Lorenzo, C.; Folli, F.; et al. Glutathione redox potential is low and glutathionylated and cysteinylated hemoglobin levels are elevated in maintenance hemodialysis patients. Transl. Res. 2013, 162, 16–25. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C. Reductive stress of sulfur-containing amino acids within proteins and implication of tandem protein-lipid damage. Int. J. Mol. Sci. 2021, 22, 12863. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Garcia, J.; Han, D.; Sancheti, H.; Yap, L.P.; Kaplowitz, N.; Cadenas, E. Regulation of Mitochondrial Glutathione Redox Status and Protein Glutathionylation by Respiratory Substrates. J. Biol. Chem. 2010, 285, 39646–39654. [Google Scholar] [CrossRef]
- Mullarky, E.; Cantley, L.C. Diverting glycolysis to combat oxidative stress. In Innovative Medicine: Basic Research and Development; Nakao, K., Minato, N., Uemoto, S., Eds.; Springer Open: Tokyo, Japan, 2015; pp. 3–23. [Google Scholar]
- O’Neill, J.S.; Reddy, A.B. Circadian clocks in human red blood cells. Nature 2011, 469, 498–503. [Google Scholar] [CrossRef]
- Rey, G.; Valekunja, U.K.; Feeney, K.A.; Wulund, L.; Milev, N.B.; Stangherlin, A.; Ansel-Bollepalli, L.; Velagapudi, V.; O’Neill, J.S.; Reddy, A.B. The pentose phosphate pathway regulates the circadian clock. Cell Metab. 2016, 24, 462–473. [Google Scholar] [CrossRef]
- Taylor, E.R.; Hurrell, F.; Shannon, R.J.; Lin, T.K.; Hirst, J.; Murphy, M.P. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J. Biol. Chem. 2003, 278, 19603–19610. [Google Scholar] [CrossRef]
- Xiong, Y.; Uys, J.D.; Tew, K.D.; Townsend, D.M. S-Glutathionylation: From molecular mechanisms to health outcomes. Antioxid. Redox Signal. 2011, 15, 233–270. [Google Scholar] [CrossRef]
- Gill, R.M.; O’Brien, M.; Young, A.; Gardiner, D.; Mailloux, R.J. Protein S-glutathionylation lowers superoxide/hydrogen peroxide release from skeletal muscle mitochondria through modification of complex I and inhibition of pyruvate uptake. PLoS ONE 2018, 13, e0192801. [Google Scholar] [CrossRef]
- Chen, Y.R.; Chen, C.L.; Pfeiffer, D.R.; Zweier, J.L. Mitochondrial complex II in the post-ischemic heart: Oxidative injury and the role of protein S-glutathionylation. J. Biol. Chem. 2007, 282, 32640–32654. [Google Scholar] [CrossRef]
- Wielgus-Serafinska, E.; Plewka, A.; Kaminski, M. Circadian variation of mitochondrial succinic dehydrogenase and microsomal cytochrome P-450 dependent monooxygenase activity in the liver of sexually immature and mature rats. J. Physiol. Pharmacol. 1993, 44, 55–63. [Google Scholar]
- Thiriveedi, V.R.; Mattam, U.; Pattabhi, P.; Bisoyi, V.; Talari, N.K.; Krishnamoorthy, T.; Sepuri, N.B.V. Glutathionylated and Fe-S cluster containing hMIA40 (CHCHD4) regulates ROS and mitochondrial complex III and IV activities of the electron transport chain. Redox Biol. 2020, 37, 101725. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Andrews, J.L.; McDearmon, E.L.; Campbell, K.S.; Barber, B.K.; Miller, B.H.; Walker, J.R.; Hogenesch, J.B.; Takahashi, J.S.; Esser, K.A. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol. Genomics 2007, 31, 86–95. [Google Scholar] [CrossRef]
- Sandbichler, A.M.; Jansen, B.; Peer, B.A.; Paulitsch, M.; Pelster, B.; Egg, M. Metabolic plasticity enables circadian adaptation to acute hypoxia in zebrafish cells. Cell. Physiol. Biochem. 2018, 46, 1159–1174. [Google Scholar] [CrossRef]
- O’Brien, M.; Chalker, J.; Slade, L.; Gardiner, D.; Mailloux, R.J. Protein S-glutathionylation alters superoxide/hydrogen peroxide emission from pyruvate dehydrogenase complex. Free Radic. Biol. Med. 2017, 106, 302–314. [Google Scholar] [CrossRef]
- Cho, D.H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-Nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 2009, 324, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Grimm, A.; Dallmann, R.; Oettinghaus, B.; Restelli, L.M.; Witzig, M.; Ishihara, N.; Mihara, K.; Ripperger, J.A.; Albrecht, U.; et al. Circadian control of DRP1 activity regulates mitochondrial dynamics and bioenergetics. Cell Metab. 2018, 27, 657–666.e655. [Google Scholar] [CrossRef]
- Ding, M.; Feng, N.; Tang, D.; Feng, J.; Li, Z.; Jia, M.; Liu, Z.; Gu, X.; Wang, Y.; Fu, F.; et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1α pathway. J. Pineal Res. 2018, 65, e12491. [Google Scholar] [CrossRef] [PubMed]
- Lillig, C.H.; Berndt, C.; Vergnolle, O.; Lonn, M.E.; Hudemann, C.; Bill, E.; Holmgren, A. Characterization of human glutaredoxin 2 as iron-sulfur protein: A possible role as redox sensor. Proc. Natl. Acad. Sci. USA 2005, 102, 8168–8173. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Jin, X.; Willmore, W.G. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol. 2014, 2, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Kil, I.S.; Ryu, K.W.; Lee, S.K.; Kim, J.Y.; Chu, S.Y.; Kim, J.H.; Park, S.; Rhee, S.G. Circadian oscillation of sulfiredoxin in the mitochondria. Mol. Cell 2015, 59, 651–663. [Google Scholar] [CrossRef]
- Reinke, H.; Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Harper, M.E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 2011, 51, 1106–1115. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Seifert, E.L.; Bouillaud, F.; Aguer, C.; Collins, S.; Harper, M.E. Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. Biol. Chem. 2011, 286, 21865–21875. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Fu, A.; Robson-Doucette, C.; Allister, E.M.; Wheeler, M.B.; Screaton, R.; Harper, M.E. Glutathionylation state of uncoupling protein-2 and the control of glucose-stimulated insulin secretion. J. Biol. Chem. 2012, 287, 39673–39685. [Google Scholar] [CrossRef]
- Seshadri, N.; Jonasson, M.E.; Hunt, K.L.; Xiang, B.; Cooper, S.; Wheeler, M.B.; Dolinsky, V.W.; Doucette, C.A. Uncoupling protein 2 regulates daily rhythms of insulin secretion capacity in MIN6 cells and isolated islets from male mice. Mol. Metab. 2017, 6, 760–769. [Google Scholar] [CrossRef]
- Yasumoto, Y.; Stoiljkovic, M.; Kim, J.D.; Sestan-Pesa, M.; Gao, X.B.; Diano, S.; Horvath, T.L. Ucp2-dependent microglia-neuronal coupling controls ventral hippocampal circuit function and anxiety-like behavior. Mol. Psychiatry 2021, 26, 2740–2752. [Google Scholar] [CrossRef]
- Skulachev, V.P. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophys. 1996, 29, 169–202. [Google Scholar] [CrossRef]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef]
- Gerencser, A.A.; Chinopoulos, C.; Birket, M.J.; Jastroch, M.; Vitelli, C.; Nicholls, D.G.; Brand, M.D. Quantitative measurement of mitochondrial membrane potential in cultured cells: Calcium-induced de- and hyperpolarization of neuronal mitochondria. J. Physiol. 2012, 590, 2845–2871. [Google Scholar] [CrossRef]
- de Goede, P.; Wefers, J.; Brombacher, E.C.; Schrauwen, P.; Kalsbeek, A. Circadian rhythms in mitochondrial respiration. J. Mol. Endocrinol. 2018, 60, R115–R130. [Google Scholar] [CrossRef]
- Porter, R.K.; Brand, M.D. Body mass dependence of H+ leak in mitochondria and its relevance to metabolic rate. Nature 1993, 362, 628–630. [Google Scholar] [CrossRef]
- Brand, M.D.; Pakay, J.L.; Ocloo, A.; Kokoszka, J.; Wallace, D.C.; Brookes, P.S.; Cornwall, E.J. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem. J. 2005, 392, 353–362. [Google Scholar] [CrossRef]
- Dummler, K.; Muller, S.; Seitz, H.J. Regulation of adenine nucleotide translocase and glycerol 3-phosphate dehydrogenase expression by thyroid hormones in different rat tissues. Biochem. J. 1996, 317 Pt 3, 913–918. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochim. Biophys. Acta 2007, 1767, 1032–1040. [Google Scholar] [CrossRef]
- Solmonson, A.; Mills, E.M. Uncoupling proteins and the molecular mechanisms of thyroid thermogenesis. Endocrinology 2016, 157, 455–462. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Samec, S. Uncoupling proteins: Their roles in adaptive thermogenesis and substrate metabolism reconsidered. Br. J. Nutr. 2001, 86, 123–139. [Google Scholar] [CrossRef]
- Ricquier, D. Uncoupling protein-2 (UCP2): Molecular and genetic studies. Int. J. Obes. Relat. Metab. Disord. 1999, 23, S38–S42. [Google Scholar] [CrossRef]
- He, M.; Ma, Y.; Wang, R.; Zhang, J.; Jing, L.; Li, P.A. Deletion of mitochondrial uncoupling protein 2 exacerbates mitochondrial damage in mice subjected to cerebral ischemia and reperfusion injury under both normo- and hyperglycemic conditions. Int. J. Biol. Sci. 2020, 16, 2788–2802. [Google Scholar] [CrossRef]
- Richard, D.; Rivest, R.; Huang, Q.; Bouillaud, F.; Sanchis, D.; Champigny, O.; Ricquier, D. Distribution of the uncoupling protein 2 mRNA in the mouse brain. J. Comp. Neurol. 1998, 397, 549–560. [Google Scholar] [CrossRef]
- Rose, G.; Crocco, P.; De Rango, F.; Montesanto, A.; Passarino, G. Further support to the uncoupling-to-survive theory: The genetic variation of human UCP genes is associated with longevity. PLoS ONE 2011, 6, e29650. [Google Scholar] [CrossRef]
- Pecqueur, C.; Alves-Guerra, C.; Ricquier, D.; Bouillaud, F. UCP2, a metabolic sensor coupling glucose oxidation to mitochondrial metabolism? IUBMB Life 2009, 61, 762–767. [Google Scholar] [CrossRef]
- Pecqueur, C.; Bui, T.; Gelly, C.; Hauchard, J.; Barbot, C.; Bouillaud, F.; Ricquier, D.; Miroux, B.; Thompson, C.B. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J. 2008, 22, 9–18. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Murphy, M.P.; Echtay, K.S.; Blaikie, F.H.; Asin-Cayuela, J.; Cocheme, H.M.; Green, K.; Buckingham, J.A.; Taylor, E.R.; Hurrell, F.; Hughes, G.; et al. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation: Studies using a mitochondria-targeted spin trap derived from alpha-phenyl-N-tert-butylnitrone. J. Biol. Chem. 2003, 278, 48534–48545. [Google Scholar] [CrossRef]
- Beck, V.; Jaburek, M.; Demina, T.; Rupprecht, A.; Porter, R.K.; Jezek, P.; Pohl, E.E. Polyunsaturated fatty acids activate human uncoupling proteins 1 and 2 in planar lipid bilayers. FASEB J. 2007, 21, 1137–1144. [Google Scholar] [CrossRef]
- Gooley, J.J. Circadian regulation of lipid metabolism. Proc. Nutr. Soc. 2016, 75, 440–450. [Google Scholar] [CrossRef]
- Dickmeis, T. Glucocorticoids and the circadian clock. J. Endocrinol. 2009, 200, 3–22. [Google Scholar] [CrossRef]
- Harper, M.E.; Brand, M.D. The quantitative contributions of mitochondrial proton leak and ATP turnover reactions to the changed respiration rates of hepatocytes from rats of different thyroid status. J. Biol. Chem. 1993, 268, 14850–14860. [Google Scholar] [CrossRef]
- Harper, M.E.; Seifert, E.L. Thyroid hormone effects on mitochondrial energetics. Thyroid 2008, 18, 145–156. [Google Scholar] [CrossRef]
- Ikegami, K.; Refetoff, S.; Van Cauter, E.; Yoshimura, T. Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 2019, 15, 590–600. [Google Scholar] [CrossRef]
- Vancamp, P.; Demeneix, B.A. Is the observed decrease in body temperature during industrialization due to thyroid hormone-dependent thermoregulation disruption? Front. Endocrinol. 2020, 11, 470. [Google Scholar] [CrossRef]
- Russell, W.; Harrison, R.F.; Smith, N.; Darzy, K.; Shalet, S.; Weetman, A.P.; Ross, R.J. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J. Clin. Endocrinol. Metab. 2008, 93, 2300–2306. [Google Scholar] [CrossRef]
- Grivas, T.B.; Savvidou, O.D. Melatonin the “light of night” in human biology and adolescent idiopathic scoliosis. Scoliosis 2007, 2, 6. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef]
- Gero, D.; Szabo, C. Glucocorticoids suppress mitochondrial oxidant production via upregulation of uncoupling protein 2 in hyperglycemic endothelial cells. PLoS One 2016, 11, e0154813. [Google Scholar] [CrossRef]
- Hucklebridge, F.; Hussain, T.; Evans, P.; Clow, A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrino 2005, 30, 51–57. [Google Scholar] [CrossRef]
- Galea, A.M.; Brown, A.J. Special relationship between sterols and oxygen: Were sterols an adaptation to aerobic life? Free Radic. Biol. Med. 2009, 47, 880–889. [Google Scholar] [CrossRef]
- Lim, L.; Jackson-Lewis, V.; Wong, L.C.; Shui, G.H.; Goh, A.X.; Kesavapany, S.; Jenner, A.M.; Fivaz, M.; Przedborski, S.; Wenk, M.R. Lanosterol induces mitochondrial uncoupling and protects dopaminergic neurons from cell death in a model for Parkinson’s disease. Cell Death Differ. 2012, 19, 416–427. [Google Scholar] [CrossRef]
- Van Cauter, E.; Polonsky, K.S.; Scheen, A.J. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr. Rev. 1997, 18, 716–738. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Gubler, M.; Montani, J.P.; Seydoux, J.; Solinas, G. Substrate cycling between de novo lipogenesis and lipid oxidation: A thermogenic mechanism against skeletal muscle lipotoxicity and glucolipotoxicity. Int. J. Obes. Relat. Metab. Disord. 2004, 28 (Suppl. 4), S29–S37. [Google Scholar] [CrossRef]
- Hiltunen, J.K.; Autio, K.J.; Schonauer, M.S.; Kursu, V.A.; Dieckmann, C.L.; Kastaniotis, A.J. Mitochondrial fatty acid synthesis and respiration. Biochim. Biophys. Acta 2010, 1797, 1195–1202. [Google Scholar] [CrossRef]
- Aviram, R.; Manella, G.; Kopelman, N.; Neufeld-Cohen, A.; Zwighaft, Z.; Elimelech, M.; Adamovich, Y.; Golik, M.; Wang, C.; Han, X.; et al. Lipidomics analyses reveal temporal and spatial lipid organization and uncover daily oscillations in intracellular organelles. Mol. Cell 2016, 62, 636–648. [Google Scholar] [CrossRef]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef]
- Lange, T.; Dimitrov, S.; Born, J. Effects of sleep and circadian rhythm on the human immune system. Ann. N. Y. Acad. Sci. 2010, 1193, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Raud, B.; McGuire, P.J.; Jones, R.G.; Sparwasser, T.; Berod, L. Fatty acid metabolism in CD8+ T cell memory: Challenging current concepts. Immunol. Rev. 2018, 283, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.E.; Chandel, N.S. Futility sustains memory T cells. Immunity 2014, 41, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, S.M.; Solmonson, A.; Rusin, S.F.; Maschek, J.A.; Bensard, C.L.; Fogarty, S.; Jeong, M.Y.; Lettlova, S.; Berg, J.A.; Morgan, J.T.; et al. Mitochondrial fatty acid synthesis coordinates oxidative metabolism in mammalian mitochondria. elife 2020, 9, e58041. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Badran, M.; Laher, I. Diabetes and alpha lipoic Acid. Front. Pharmacol. 2011, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J. Protein S-glutathionylation reactions as a global inhibitor of cell metabolism for the desensitization of hydrogen peroxide signals. Redox Biol. 2020, 32, 101472. [Google Scholar] [CrossRef] [PubMed]
- Chretien, D.; Benit, P.; Ha, H.H.; Keipert, S.; El-Khoury, R.; Chang, Y.T.; Jastroch, M.; Jacobs, H.T.; Rustin, P.; Rak, M. Mitochondria are physiologically maintained at close to 50 degrees C. PLoS Biol. 2018, 16, e2003992. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.B. The role of oxygen and the Goldilocks range in the development of cataracts induced by space radiation in US astronauts. Exp. Eye Res. 2022, 223, 109192. [Google Scholar] [CrossRef]
- Scialo, F.; Sriram, A.; Stefanatos, R.; Spriggs, R.V.; Loh, S.H.Y.; Martins, L.M.; Sanz, A. Mitochondrial complex I derived ROS regulate stress adaptation in Drosophila melanogaster. Redox Biol. 2020, 32, 101450. [Google Scholar] [CrossRef]
- Sottile, M.L.; Nadin, S.B. Heat shock proteins and DNA repair mechanisms: An updated overview. Cell Stress Chaperones 2018, 23, 303–315. [Google Scholar] [CrossRef]
- Zou, J.; Guo, Y.; Guettouche, T.; Smith, D.F.; Voellmy, R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF. Cell 1998, 94, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, D.; Hasegawa, T.; Hirose, S.I.; Sakurai, Y.; Ito, M.; Takamatsu, N. Circadian transcription factor HSF1 regulates differential HSP70 gene transcription during the arousal-torpor cycle in mammalian hibernation. Sci. Rep. 2019, 9, 832. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Yabunaka, N.; Fujisawa, H.; Watanabe, I.; Agishi, Y. Effect of thermal stress on glutathione metabolism in human erythrocytes. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 68, 87–91. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperthermia 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Hendriks, K.D.W.; Bruggenwirth, I.M.A.; Maassen, H.; Gerding, A.; Bakker, B.; Porte, R.J.; Henning, R.H.; Leuvenink, H.G.D. Renal temperature reduction progressively favors mitochondrial ROS production over respiration in hypothermic kidney preservation. J. Transl. Med. 2019, 17, 265. [Google Scholar] [CrossRef]
- Jarmuszkiewicz, W.; Woyda-Ploszczyca, A.; Koziel, A.; Majerczak, J.; Zoladz, J.A. Temperature controls oxidative phosphorylation and reactive oxygen species production through uncoupling in rat skeletal muscle mitochondria. Free Radic. Biol. Med. 2015, 83, 12–20. [Google Scholar] [CrossRef]
- Jones, D.P.; Sies, H. The redox code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef]
- Machado, F.S.M.; Zhang, Z.; Su, Y.; de Goede, P.; Jansen, R.; Foppen, E.; Coimbra, C.C.; Kalsbeek, A. Time-of-day effects on metabolic and clock-related adjustments to cold. Front. Endocrinol. 2018, 9, 199. [Google Scholar] [CrossRef]
- Brand, M.D. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp. Gerontol. 2000, 35, 811–820. [Google Scholar] [CrossRef]
- Herrero, A.; Barja, G. H2O2 production of heart mitochondria and aging rate are slower in canaries and parakeets than in mice: Sites of free radical generation and mechanisms involved. Mech. Ageing Dev. 1998, 103, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Talbot, D.A.; Selman, C.; Snart, S.; McLaren, J.S.; Redman, P.; Krol, E.; Jackson, D.M.; Johnson, M.S.; Brand, M.D. Uncoupled and surviving: Individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 2004, 3, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Cortassa, S.; O’Rourke, B.; Aon, M.A. Redox-optimized ROS balance and the relationship between mitochondrial respiration and ROS. Biochim. Biophys. Acta 2014, 1837, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Arrigo, T.; Barberi, I.; Reiter, R.J.; Gitto, E. Oxidative stress-mediated aging during the fetal and perinatal periods. Oxid. Med. Cell. Longev. 2014, 2014, 358375. [Google Scholar] [CrossRef] [PubMed]
- Lodemore, M.; Petersen, S.A.; Wailoo, M.P. Development of night time temperature rhythms over the first six months of life. Arch. Dis. Child 1991, 66, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Brauner, P.; Kopecky, P.; Flachs, P.; Ruffer, J.; Sebron, V.; Plavka, R.; Vitkova, I.; Vorlicek, J.; Kopecky, J. Induction of uncoupling protein 3 gene expression in skeletal muscle of preterm newborns. Pediatr. Res. 2003, 53, 691–697. [Google Scholar] [CrossRef]
- Lean, M.E.; James, W.P.; Jennings, G.; Trayhurn, P. Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin. Sci. 1986, 71, 291–297. [Google Scholar] [CrossRef]
- Piccione, G.; Caola, G.; Refinetti, R. Daily and estrous rhythmicity of body temperature in domestic cattle. BMC Physiol. 2003, 3, 7. [Google Scholar] [CrossRef]
- Bach, V.; Libert, J.P. Hyperthermia and heat stress as risk factors for sudden infant death syndrome: A narrative review. Front. Pediatr. 2022, 10, 816136. [Google Scholar] [CrossRef]
- Yates, J. Perspective: The long-term effects of light exposure on establishment of newborn circadian rhythm. J. Clin. Sleep Med. 2018, 14, 1829–1830. [Google Scholar] [CrossRef]
- Harrington, C.T.; Hafid, N.A.; Waters, K.A. Butyrylcholinesterase is a potential biomarker for Sudden Infant Death Syndrome. EBioMedicine 2022, 80, 104041. [Google Scholar] [CrossRef]
- Haynes, R.L.; Frelinger, A.L., 3rd; Giles, E.K.; Goldstein, R.D.; Tran, H.; Kozakewich, H.P.; Haas, E.A.; Gerrits, A.J.; Mena, O.J.; Trachtenberg, F.L.; et al. High serum serotonin in sudden infant death syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 7695–7700. [Google Scholar] [CrossRef]
- Shekhawat, P.S.; Matern, D.; Strauss, A.W. Fetal fatty acid oxidation disorders, their effect on maternal health and neonatal outcome: Impact of expanded newborn screening on their diagnosis and management. Pediatr. Res. 2005, 57, 78R–86R. [Google Scholar] [CrossRef]
- Maher, P. The effects of stress and aging on glutathione metabolism. Ageing Res. Rev. 2005, 4, 288–314. [Google Scholar] [CrossRef]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Millyard, A.; Layden, J.D.; Pyne, D.B.; Edwards, A.M.; Bloxham, S.R. Impairments to thermoregulation in the elderly during heat exposure events. Gerontol. Geriatr. Med. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Waalen, J.; Buxbaum, J.N. Is older colder or colder older? The association of age with body temperature in 18,630 individuals. J. Gerontol. Biol. Sci. Med. Sci. 2011, 66, 487–492. [Google Scholar] [CrossRef]
- Eggenberger, P.; Burgisser, M.; Rossi, R.M.; Annaheim, S. Body temperature is associated with cognitive performance in older adults with and without mild cognitive impairment: A cross-sectional analysis. Front. Aging Neurosci. 2021, 13, 585904. [Google Scholar] [CrossRef]
- Choromanska, B.; Mysliwiec, P.; Luba, M.; Wojskowicz, P.; Mysliwiec, H.; Choromanska, K.; Dadan, J.; Zalewska, A.; Maciejczyk, M. The impact of hypertension and metabolic syndrome on nitrosative stress and glutathione metabolism in patients with morbid obesity. Oxid. Med. Cell. Longev. 2020, 2020, 1057570. [Google Scholar] [CrossRef]
- Sabens Liedhegner, E.A.; Gao, X.H.; Mieyal, J.J. Mechanisms of altered redox regulation in neurodegenerative diseases—Focus on S-glutathionylation. Antioxid. Redox Signal. 2012, 16, 543–566. [Google Scholar] [CrossRef]
- Cha, S.J.; Kim, H.; Choi, H.J.; Lee, S.; Kim, K. Protein glutathionylation in the pathogenesis of neurodegenerative diseases. Oxid. Med. Cell. Longev. 2017, 2017, 2818565. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Rosa, G.; Greco, A.V.; Manco, M.; Vega, N.; Hesselink, M.K.; Castagneto, M.; Schrauwen, P.; Vidal, H. Decreased uncoupling protein expression and intramyocytic triglyceride depletion in formerly obese subjects. Obes. Res. 2003, 11, 632–640. [Google Scholar] [CrossRef]
- Shinozaki, S.; Chang, K.; Sakai, M.; Shimizu, N.; Yamada, M.; Tanaka, T.; Nakazawa, H.; Ichinose, F.; Yamada, Y.; Ishigami, A.; et al. Inflammatory stimuli induce inhibitory S-nitrosylation of the deacetylase SIRT1 to increase acetylation and activation of p53 and p65. Sci. Signal 2014, 7, ra106. [Google Scholar] [CrossRef]
- Hood, S.; Amir, S. Neurodegeneration and the circadian clock. Front Aging Neurosci 2017, 9, 170. [Google Scholar] [CrossRef]
- Vrettou, S.; Wirth, B. S-glutathionylation and S-nitrosylation in mitochondria: Focus on homeostasis and neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 15849. [Google Scholar] [CrossRef] [PubMed]
- Karhu, T.; Myllymaa, S.; Nikkonen, S.; Mazzotti, D.R.; Toyras, J.; Leppanen, T. Longer and deeper desaturations are associated with the worsening of mild sleep apnea: The Sleep Heart Health Study. Front. Neurosci. 2021, 15, 657126. [Google Scholar] [CrossRef]
- Lacedonia, D.; Carpagnano, G.E.; Crisetti, E.; Cotugno, G.; Palladino, G.P.; Patricelli, G.; Sabato, R.; Foschino Barbaro, M.P. Mitochondrial DNA alteration in obstructive sleep apnea. Respir. Res. 2015, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial uncoupling: A key controller of biological processes in physiology and diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.S.; Morris, E.M.; Wheatley, J.L.; Archer, A.E.; McCoin, C.S.; White, K.S.; Wilson, D.R.; Meers, G.M.; Koch, L.G.; Britton, S.L.; et al. Deficiency in the heat stress response could underlie susceptibility to metabolic disease. Diabetes 2016, 65, 3341–3351. [Google Scholar] [CrossRef] [PubMed]
- Koziel, A.; Sobieraj, I.; Jarmuszkiewicz, W. Increased activity of mitochondrial uncoupling protein 2 improves stress resistance in cultured endothelial cells exposed in vitro to high glucose levels. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H147–H156. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, A.; Cassell, T.; Smith, P.; Lu, D.; Nam, H.W.; Lane, A.N.; Zhao, Y. UCP2 overexpression redirects glucose into anabolic metabolic pathways. Proteomics 2019, 19, e1800353. [Google Scholar] [CrossRef]
- Twig, G.; Shirihai, O.S. The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta. Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Leak, R.K. Heat shock proteins in neurodegenerative disorders and aging. J. Cell. Commun. Signal. 2014, 8, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318. [Google Scholar] [CrossRef]
- Sulli, G.; Lam, M.T.Y.; Panda, S. Interplay between circadian clock and cancer: New frontiers for cancer treatment. Trends Cancer 2019, 5, 475–494. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The circadian syndrome: Is the metabolic syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef]
- Aguilar-Lopez, B.A.; Moreno-Altamirano, M.M.B.; Dockrell, H.M.; Duchen, M.R.; Sanchez-Garcia, F.J. Mitochondria: An integrative hub coordinating circadian rhythms, metabolism, the microbiome, and immunity. Front. Cell Dev. Biol. 2020, 8, 51. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Neri, A.; Somma, G.; Coppeta, L.; Iavicoli, I.; Bergamaschi, A.; Magrini, A. Incidence of metabolic syndrome among night-shift healthcare workers. Occup. Environ. Med. 2010, 67, 54–57. [Google Scholar] [CrossRef]
- Walker, W.H., 2nd; Borniger, J.C. Molecular mechanisms of cancer-induced sleep disruption. Int. J. Mol. Sci. 2019, 20, 2780. [Google Scholar] [CrossRef]
- Bevinakoppamath, S.; Ramachandra, S.C.; Yadav, A.K.; Basavaraj, V.; Vishwanath, P.; Prashant, A. Understanding the emerging link between circadian rhythm, Nrf2 pathway, and breast cancer to overcome drug resistance. Front. Pharmacol. 2021, 12, 719631. [Google Scholar] [CrossRef]
- Koritala, B.S.C.; Porter, K.I.; Arshad, O.A.; Gajula, R.P.; Mitchell, H.D.; Arman, T.; Manjanatha, M.G.; Teeguarden, J.; Van Dongen, H.P.A.; McDermott, J.E.; et al. Night shift schedule causes circadian dysregulation of DNA repair genes and elevated DNA damage in humans. J. Pineal Res. 2021, 70, e12726. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.B.; Anghel, C.V.; Deng, D.S. Profound synchrony of age-specific incidence rates and tumor suppression for different cancer types as revealed by the multistage-senescence model of carcinogenesis. Aging 2021, 13, 23545–23578. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E. Diurnal and ultradian rhythms in human endocrine function: A minireview. Horm. Res. 1990, 34, 45–53. [Google Scholar] [CrossRef]
- Tocchetti, C.G.; Stanley, B.A.; Sivakumaran, V.; Bedja, D.; O’Rourke, B.; Paolocci, N.; Cortassa, S.; Aon, M.A. Impaired mitochondrial energy supply coupled to increased H2O2 emission under energy/redox stress leads to myocardial dysfunction during Type I diabetes. Clin. Sci. 2015, 129, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Harding, E.C.; Franks, N.P.; Wisden, W. The temperature dependence of sleep. Front. Neurosci. 2019, 13, 336. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Ozpinar, A.; Seyfried, T.N. Caprylic (octanoic) acid as a potential fatty acid chemotherapeutic for glioblastoma. Prostaglandins Leukot. Essent. Fatty Acids 2020, 159, 102142. [Google Scholar] [CrossRef]
- Carey, H.V.; Rhoads, C.A.; Aw, T.Y. Hibernation induces glutathione redox imbalance in ground squirrel intestine. J. Comp. Physiol. B 2003, 173, 269–276. [Google Scholar] [CrossRef]
- Barger, J.L.; Barnes, B.M.; Boyer, B.B. Regulation of UCP1 and UCP3 in Arctic ground squirrels and relation with mitochondrial proton leak. J. Appl. Physiol. 2006, 101, 339–347. [Google Scholar] [CrossRef]
- Boyer, B.B.; Barnes, B.M. Molecular and metabolic aspects of mammal hibernation. Bioscience 1999, 49, 713–724. [Google Scholar] [CrossRef]
- Brown, J.C.; Chung, D.J.; Belgrave, K.R.; Staples, J.F. Mitochondrial metabolic suppression and reactive oxygen species production in liver and skeletal muscle of hibernating thirteen-lined ground squirrels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R15–R28. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.J.; Storey, K.B. Peripheral circadian gene activity is altered during hibernation in the thirteen-lined ground squirrel. Cryobiology 2022, 107, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Turbill, C.; Bieber, C.; Ruf, T. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. Biol. Sci. 2011, 278, 3355–3363. [Google Scholar] [CrossRef] [PubMed]
- Strollo, F.; Gentile, S.; Strollo, G.; Mambro, A.; Vernikos, J. Recent progress in space physiology and aging. Front. Physiol. 2018, 9, 1551. [Google Scholar] [CrossRef]
- Richardson, R.B.; Harper, M.E. Mitochondrial stress controls the radiosensitivity of the oxygen effect: Implications for radiotherapy. Oncotarget 2016, 7, 21469–21483. [Google Scholar] [CrossRef]
- Scrima, R.; Cela, O.; Agriesti, F.; Piccoli, C.; Tataranni, T.; Pacelli, C.; Mazzoccoli, G.; Capitanio, N. Mitochondrial calcium drives clock gene-dependent activation of pyruvate dehydrogenase and of oxidative phosphorylation. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118815. [Google Scholar] [CrossRef]
- Bisserier, M.; Shanmughapriya, S.; Rai, A.K.; Gonzalez, C.; Brojakowska, A.; Garikipati, V.N.S.; Madesh, M.; Mills, P.J.; Walsh, K.; Arakelyan, A.; et al. Cell-free mitochondrial DNA as a potential biomarker for astronauts’ health. J. Am. Heart Assoc. 2021, 10, e022055. [Google Scholar] [CrossRef]
- da Silveira, W.A.; Fazelinia, H.; Rosenthal, S.B.; Laiakis, E.C.; Kim, M.S.; Meydan, C.; Kidane, Y.; Rathi, K.S.; Smith, S.M.; Stear, B.; et al. Comprehensive multi-omics analysis reveals mitochondrial stress as a central biological hub for spaceflight impact. Cell 2020, 183, 1185–1201.e1120. [Google Scholar] [CrossRef]
- Pecaut, M.J.; Mao, X.W.; Bellinger, D.L.; Jonscher, K.R.; Stodieck, L.S.; Ferguson, V.L.; Bateman, T.A.; Mohney, R.P.; Gridley, D.S. Is spaceflight-induced immune dysfunction linked to systemic changes in metabolism? PLoS ONE 2017, 12, e0174174. [Google Scholar] [CrossRef]
- Navarro, J.; Obrador, E.; Pellicer, J.A.; Aseni, M.; Vina, J.; Estrela, J.M. Blood glutathione as an index of radiation-induced oxidative stress in mice and humans. Free Radic. Biol. Med. 1997, 22, 1203–1209. [Google Scholar] [CrossRef]
- Stahn, A.C.; Werner, A.; Opatz, O.; Maggioni, M.A.; Steinach, M.; von Ahlefeld, V.W.; Moore, A.; Crucian, B.E.; Smith, S.M.; Zwart, S.R.; et al. Increased core body temperature in astronauts during long-duration space missions. Sci. Rep. 2017, 7, 16180. [Google Scholar] [CrossRef] [PubMed]
- Patten, D.A.; Ouellet, M.; Allan, D.S.; Germain, M.; Baird, S.D.; Harper, M.E.; Richardson, R.B. Mitochondrial adaptation in human mesenchymal stem cells following ionizing radiation. FASEB J. 2019, 33, 9263–9278. [Google Scholar] [CrossRef]
- Speakman, J.R.; Selman, C.; McLaren, J.S.; Harper, E.J. Living fast, dying when? The link between aging and energetics. J. Nutr. 2002, 132, 1583S–1597S. [Google Scholar] [CrossRef]
- Buttgereit, F.; Brand, M.D. A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J. 1995, 312 Pt 1, 163–167. [Google Scholar] [CrossRef]
- Petrushanko, I.Y.; Yakushev, S.; Mitkevich, V.A.; Kamanina, Y.V.; Ziganshin, R.H.; Meng, X.; Anashkina, A.A.; Makhro, A.; Lopina, O.D.; Gassmann, M.; et al. S-Glutathionylation of the Na,K-ATPase catalytic α subunit is a determinant of the enzyme redox sensitivity. J. Biol. Chem. 2012, 287, 32195–32205. [Google Scholar] [CrossRef]
- Bilen, M.; Benhammouda, S.; Slack, R.S.; Germain, M. The integrated stress response as a key pathway downstream of mitochondrial dysfunction. Curr. Opin. Physiol. 2022, 27, 100555. [Google Scholar] [CrossRef]
- Estrela, J.M.; Pallardo, F.V. Role of glutathione in the regulation of protein synthesis and degradation in eukaryotes. In Gluathione: Metabolism and Physiological Functions; Viña, J., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 177–186. [Google Scholar]
- Patten, D.A.; McGuirk, S.; Anilkumar, U.; Antoun, G.; Gandhi, K.; Parmar, G.; Iqbal, M.A.; Wong, J.; Richardson, R.B.; St-Pierre, J.; et al. Altered mitochondrial fusion drives defensive glutathione synthesis in cells able to switch to glycolytic ATP production. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118854. [Google Scholar] [CrossRef]
- Reimund, E. The free radical flux theory of sleep. Med. Hypotheses 1994, 43, 231–233. [Google Scholar] [CrossRef]
- Eugene, A.R.; Masiak, J. The neuroprotective aspects of sleep. MEDtube Sci. 2015, 3, 35–40. [Google Scholar]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Piéron, H. Le Problème Physiologique du Sommeil; Masson: Paris, France, 1912. [Google Scholar]
- Komoda, Y.; Honda, K.; Inoue, S. SPS-B, a physiological sleep regulator, from the brainstems of sleep-deprived rats, identified as oxidized glutathione. Chem. Pharm Bull. 1990, 38, 2057–2059. [Google Scholar] [CrossRef] [PubMed]
- Adolfsen, K.J.; Brynildsen, M.P. Futile cycling increases sensitivity toward oxidative stress in Escherichia coli. Metab. Eng. 2015, 29, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Reinke, H.; Altmeyer, M.; Gutierrez-Arcelus, M.; Hottiger, M.O.; Schibler, U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell 2010, 142, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, J.; Spickett, C.M.; Ploszaj, T.; Virag, L.; Robaszkiewicz, A. PARP1 promoter links cell cycle progression with adaptation to oxidative environment. Redox Biol. 2018, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Feillet, C.; van der Horst, G.T.; Levi, F.; Rand, D.A.; Delaunay, F. Coupling between the circadian clock and cell cycle oscillators: Implication for healthy cells and malignant growth. Front. Neurol. 2015, 6, 96. [Google Scholar] [CrossRef]
- Yousefzadeh, M.; Henpita, C.; Vyas, R.; Soto-Palma, C.; Robbins, P.; Niedernhofer, L. DNA damage-how and why we age? elife 2021, 10, e62852. [Google Scholar] [CrossRef]
- Kang, T.H.; Lindsey-Boltz, L.A.; Reardon, J.T.; Sancar, A. Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc. Natl. Acad. Sci. USA 2010, 107, 4890–4895. [Google Scholar] [CrossRef]
- Kang, T.H. Circadian rhythm of NER and ATR pathways. Biomolecules 2021, 11, 715. [Google Scholar] [CrossRef]
- Manzella, N.; Bracci, M.; Strafella, E.; Staffolani, S.; Ciarapica, V.; Copertaro, A.; Rapisarda, V.; Ledda, C.; Amati, M.; Valentino, M.; et al. Circadian modulation of 8-oxoguanine DNA damage repair. Sci. Rep. 2015, 5, 13752. [Google Scholar] [CrossRef]
- Richardson, R.B. Ionizing radiation and aging: Rejuvenating an old idea. Aging 2009, 1, 887–902. [Google Scholar] [CrossRef]
- Zada, D.; Sela, Y.; Matosevich, N.; Monsonego, A.; Lerer-Goldshtein, T.; Nir, Y.; Appelbaum, L. Parp1 promotes sleep, which enhances DNA repair in neurons. Mol. Cell 2021, 81, 4979–4993.e4977. [Google Scholar] [CrossRef]
- Rong, Z.; Tu, P.; Xu, P.; Sun, Y.; Yu, F.; Tu, N.; Guo, L.; Yang, Y. The mitochondrial response to DNA damage. Front. Cell Dev. Biol. 2021, 9, 669379. [Google Scholar] [CrossRef]
- Rabinovich-Nikitin, I.; Lieberman, B.; Martino, T.A.; Kirshenbaum, L.A. Circadian-regulated cell death in cardiovascular diseases. Circulation 2019, 139, 965–980. [Google Scholar] [CrossRef]
- Shutt, T.; Geoffrion, M.; Milne, R.; McBride, H.M. The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep. 2012, 13, 909–915. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Yang, C.; Pu, S.; Guo, Z.; Wu, Q.; Zhou, Z.; Zhao, H. Mitochondrial membrane remodeling. Front. Bioeng. Biotechnol. 2021, 9, 786806. [Google Scholar] [CrossRef]
- Sancar, A.; Lindsey-Boltz, L.A.; Kang, T.H.; Reardon, J.T.; Lee, J.H.; Ozturk, N. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 2010, 584, 2618–2625. [Google Scholar] [CrossRef]
- Kempf, A.; Song, S.M.; Talbot, C.B.; Miesenbock, G. A potassium channel beta-subunit couples mitochondrial electron transport to sleep. Nature 2019, 568, 230–234. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Andersen, M.L.; Alvarenga, T.A.; de-Oliveira, M.R.; Armani, F.; Ruiz, F.S.; Giglio, L.; Moreira, J.C.; Kapczinski, F.; Tufik, S. Impairment of the mitochondrial electron transport chain due to sleep deprivation in mice. J. Psychiatr. Res. 2010, 44, 775–780. [Google Scholar] [CrossRef]
- Cirelli, C.; Tononi, G. Uncoupling proteins and sleep deprivation. Arch. Ital. Biol. 2004, 142, 541–549. [Google Scholar]
- Bar-Yaacov, D.; Blumberg, A.; Mishmar, D. Mitochondrial-nuclear co-evolution and its effects on OXPHOS activity and regulation. Biochim. Biophys. Acta 2012, 1819, 1107–1111. [Google Scholar] [CrossRef]
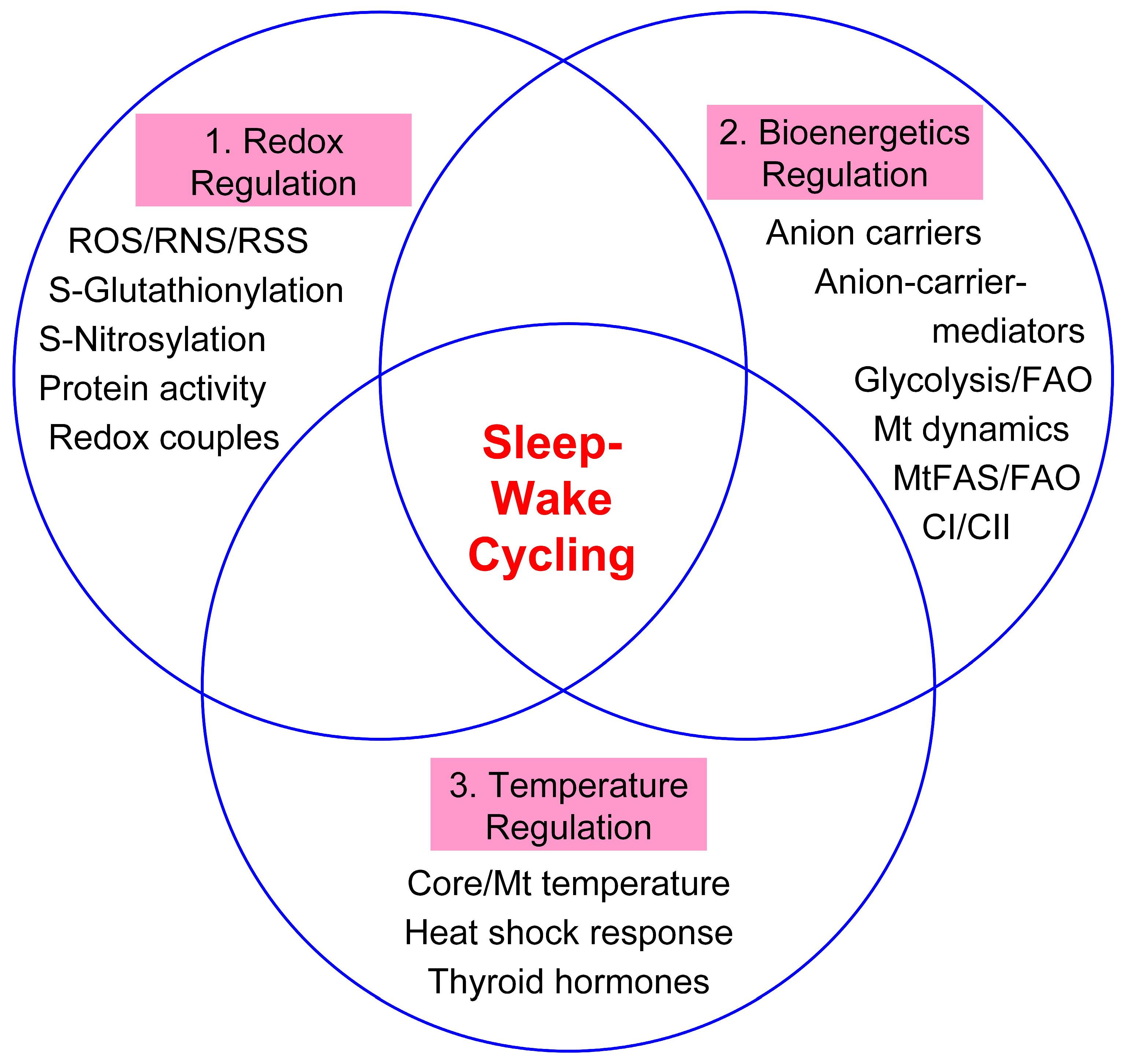
 or lowered
or lowered  for parameters such as glutathione (GSH), the oxidized form of GSH (GSSG), metabolic pathways, mitochondrial complexes I and II (CI & CII), NADPH:NADP+ agents of nicotinamide adenine dinucleotide phosphate, tricarboxylic acid (TCA) cycle and triiodothyronine (T3).
for parameters such as glutathione (GSH), the oxidized form of GSH (GSSG), metabolic pathways, mitochondrial complexes I and II (CI & CII), NADPH:NADP+ agents of nicotinamide adenine dinucleotide phosphate, tricarboxylic acid (TCA) cycle and triiodothyronine (T3).
 or lowered
or lowered  for parameters such as glutathione (GSH), the oxidized form of GSH (GSSG), metabolic pathways, mitochondrial complexes I and II (CI & CII), NADPH:NADP+ agents of nicotinamide adenine dinucleotide phosphate, tricarboxylic acid (TCA) cycle and triiodothyronine (T3).
for parameters such as glutathione (GSH), the oxidized form of GSH (GSSG), metabolic pathways, mitochondrial complexes I and II (CI & CII), NADPH:NADP+ agents of nicotinamide adenine dinucleotide phosphate, tricarboxylic acid (TCA) cycle and triiodothyronine (T3).
 or lowered
or lowered  .
.
 or lowered
or lowered  .
.
| Mitochondrial Proteins | Protein Function | Abbreviation [ROS Emitting] | a ROS Inhibiting (Anti-ROS) or Redox Sensor | b Redox Inhibited or [Activated] | c Circadian Regulated | References |
|---|---|---|---|---|---|---|
| Complex I (Ubiquinone oxidoreductase) | ETC enzyme, oxidizes NADH from TCA cycle | CI [most ETC ROS] | ✓ redox sensor | ✓ PSSG, ✓ SNO | ✓ | a [22], b [76], b [77], b [78], c [32] |
| Complex II (Succinate dehydrogenase) | ETC enzyme, oxidizes succinate in TCA cycle | CII [little ETC ROS] | ✓ PSSG [activated], ✓ SNO | ✓ | b [79], c [80] | |
| Complex III (Coenzyme Q) | ETC enzyme | CIII [some ETC ROS] | ✓ PSSG (via MIA40), ✓ SNO | ✓ (& via MIA40) | b [81], c [32] | |
| Complex IV (Cytochrome C oxidase) | ETC enzyme | CIV | ✓ PSSG (& via MIA40), ✓ SNO | ✓ (& via MIA40) | b [47], b [81], c [32] | |
| Complex V (ATP synthase) | ETC enzyme | CV | ✓ PSSG, ✓ SNO | ✓ | b [72], c [82], c [32] | |
| Adenine nucleotide translocase | ADP/ATP exchange | ANT | ✓ Reverses apoptosis process | ✓ PSSG [activated], ✓ SNO | ✓ based on ADP/ATP ratio | a,b [60], c [83] |
| α-ketoglutarate dehydrogenase | Lipoate TCA cycle catalysis of substrates | d, f α-KGDH or OGDH [ROS 2 x CI] | ✓ redox sensor | ✓ PSSG (10 sites), ALA, ✓ SNO | ✓ | b [84], c [32] |
| Branched-chain keto acid dehydrogenase | Rate-limiting enzyme catabolizing α-ketoacids | d, f BCKDH [ROS 8 x CI] | ✗ non-PSSG, ALA | ✓ | b [55], c [56] | |
| Dynamin-related protein-1 | Pro-fission protein | DRP1 | ✗ non-PSSG, ✓ SNO | ✓ | b [85], c [86], c [87] | |
| Glutaredoxin-2 | GSSG reducing enzyme using GSH as cofactor | GRX2 | ✓ anti-ROS, Fe-S cluster redox sensor | ✗ non-PSSG, reverses PSSG reactions | ✓ based on GSH:GSSG | a [88], a [48], c [24] |
| Peroxiredoxin III | Hydrogen peroxide-scavenging enzyme | e PRXIII | ✓ anti-ROS, redox sensor | ✓ S-sulfinylation | ✓ | b [89], c [16], c [90] |
| Pyruvate dehydrogenase | Lipoate enzyme in glycolysis, pyruvate to acetyl-CoA pathway | d, f PDH [ROS 4 x CI] | ✓ redox sensor | ✓ PSSG, ALA, ✓ SNO | ✓ | b [84], c [32], c [91] |
| Uncoupling proteins | Transports protons across the mitochondrial inner membrane | UCP2/3 | ✓ anti-ROS, redox sensor | ✓ PSSG [activated] | ✓ | a [92], b [93], b [94], c [95], c [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, R.B.; Mailloux, R.J. Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles. Antioxidants 2023, 12, 674. https://doi.org/10.3390/antiox12030674
Richardson RB, Mailloux RJ. Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles. Antioxidants. 2023; 12(3):674. https://doi.org/10.3390/antiox12030674
Chicago/Turabian StyleRichardson, Richard B., and Ryan J. Mailloux. 2023. "Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles" Antioxidants 12, no. 3: 674. https://doi.org/10.3390/antiox12030674
APA StyleRichardson, R. B., & Mailloux, R. J. (2023). Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles. Antioxidants, 12(3), 674. https://doi.org/10.3390/antiox12030674








