Abstract
Depression has a multifactorial etiology comprising family history and unemployment. This review aims to summarize the evidence available for the antioxidant and anti-inflammatory effects of carotenoids in mood disorders. This review article’s methodologies were based on a search of the PubMed database for all linked published papers. Epidemiological studies indicate that a diet rich in vegetables, fruits, nuts, fish, and olive oil may prevent the development of depression. Antioxidant supplementation has been found to combat various stress-induced psychiatric disorders, including depression and anxiety. A growing body of evidence indicates that carotenoids have both antioxidant and anti-inflammatory. Studies also suggest that poor dietary intake, particularly low intakes of fruit and vegetables and high intakes of fast food and other convenience foods, may increase the risk of developing depression. Thus, dietary interventions have the potential to help mitigate the risk of mental health decline in both the general population and those with mood disorders. Considering that carotenoids have both antioxidant and anti-inflammatory effects, it is expected that they might exert a promising antidepressant effect. Nevertheless, further studies (including interventional and mechanistic studies) assessing the effect of carotenoids on preventing and alleviating depression symptoms are needed.
1. Introduction
Some of the most common noncommunicable illnesses are psychiatric disorders such as depression; together, these are responsible for 14.3% of global fatalities. It is estimated that 322 million individuals live with depression globally and that depression is involved in 50–70% of suicides [1]. The World Health Organization predicts depression will become the second most prevalent disorder after ischemic heart disease [2]. Depression is most commonly manifested as hopelessness, disturbances in sleep or appetite, and avoiding social activity, and it also acts as an independent predictor of the beginning of somatic illness [3]. Studies have shown that people with depression have a higher risk of developing lifestyle disorders such as cardiovascular disease, obesity, and diabetes, as well as cancer; it has also been associated with cognitive impairment [4,5,6,7,8]. However, while it is impossible to avoid some of the most common risk factors, such as family history, poverty, and unemployment [9,10], it is possible to modify others, such as a sedentary lifestyle [11], cigarette smoking [12] and alcohol consumption [13].
Depression has a multifactorial etiology comprising functional deficits in monoamine, such as serotonin, and disturbances in the brain’s hypothalamic–pituitary–adrenal (HPA) axis [14]. In addition, emotional disorders such as anxiety and depression are often associated with a reduction in the activity of antioxidant enzymes in the HPA axis. HPA axis hyperactivity is often observed in cases of depression; this correlates with an increase in glucocorticoid concentration, resulting in the development of oxidative stress and glutamatergic excitotoxicity and thus the death of nerve cells, especially in the hippocampus, the key region related to the regulation of mood [15,16,17,18,19,20,21]. Also, chronic psychological stress, known to contribute to the development of depression, causes structural changes in the hippocampus associated with the regulation of cognition [22].
It should be emphasized that although there are many theories of depression development, its accurate pathomechanism is still not fully known. Depression is widely believed to be strongly influenced by immuno-inflammatory mechanisms [23,24,25]. Patients with depression tend to demonstrate significantly elevated levels of various chemokines (CCL2, CXCL10), and pro-inflammatory cytokines, such as interleukin (IL)−1β, IL-6, and tumor necrosis factor (TNF) [26,27,28,29,30]. Some studies found patients with depression to have increased levels of C reactive protein (CRP), an important inflammatory marker [31,32]. Current literature indicates a strong two-way association between the development of inflammation and psychiatric disorders, including depression. It should also be borne in mind that depression is related to an exacerbation of behaviors associated with the development of inflammation, such as nutritional deficiencies/poor eating habits, and addiction to psychoactive substances, such as alcohol, drugs, or smoking [33]. In addition, there is evidence that commensal gut microorganisms, which comprise the gut microbiota, may play an important role in the etiopathogenesis of depression via the gut-brain axis [34]. The gut-brain axis is a two-way communication pathway between the central nervous system and the gut. This communication occurs via hormonal, neurological, and immunological signaling systems, as well as gut microbe metabolites, which trigger changes in neurotransmission, neuroinflammation, and behavior [35,36]. Disturbances in the composition, quality, and functioning of the intestinal microbiota (intestinal dysbiosis) are correlated with some neuropsychiatric disorders, especially depression. Numerous studies have investigated the potential impact of gut microbiota on the onset of depression [37,38,39,40,41,42,43,44,45].
This review aims to summarize the evidence available for the antioxidant and anti-inflammatory effects of carotenoids in mood disorders. This review article’s methodologies were based on a search of the PubMed database for all linked published papers. Various combinations of the keywords, among others, “carotenoids”, “nutrition”, “major depressive disorder”, “MDD”, “unipolar depression”, “mood disorders”, “diet”, “stress”, “oxidative stress”, “antioxidants”, “gut microbiota”, “microbiota”, and “neurodegenerative disorders” were used to make the selection. The World Health Organization and the Centers for Disease Control and Prevention provided the statistical data on the prevalence of mental disorders used in this research.
2. The Influence of Diet on the Development and Course of Mood Disorders
A number of studies indicate that an improperly-balanced diet is one of the elements associated with the development of depression and anxiety [46,47]. Recent years have seen a growth in interest in the relationship between nutrients and depression, particularly folic acid, vitamin D, and magnesium [48,49,50]. Mikkelsen et al. (2016) demonstrated a relationship between vitamin B deficiency, including B1, B3, B6, B9, B12, and depression [51,52]. Studies have also shown that a higher level of depression in adolescents is associated with irregular meals [53]. Research indicates that fat-soluble nutrients, such as vitamin E, protect against nerve damage, and low dietary intake is linked to mood changes and depression [54].
It is believed that carotenoid-cleaving enzymes, which take part in the metabolism of carotenoids, play a key role in depression. It should be pointed out that apocarotenoids are formed due to the oxidative breakdown of carotenoids catalyzed by carotenoid oxygenases. Apocarotenoids are, inter alia, retinal, retinol, retinoic acid, and abscisic acid. Some studies describe that retinoic acid, the active form of vitamin A, causes hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis and leads to the development of typical depressive behaviors. In addition, it has been shown that retinoic acid may cause suicide in some susceptible individuals [55,56].
A cross-sectional study of people of normal BMI by Nguyen et al. (2017) found significantly lower intake of β-carotene equivalent and vitamins C, E, B1, B3, B6, B9, and B5 in those with depressive symptoms [54]. It should be noted that vitamin C insufficiency has also been linked to an increased risk of depression symptoms [54]. Additionally, randomized, placebo-controlled clinical trials have found vitamin C to improve mood and lower the severity of depression in patients [57,58]. Interestingly, however, vitamin C supplementation appeared to have no effect on the depression score in these people [59]. A study conducted among elderly Japanese people showed that the consumption of carotene and vitamin C is associated with less severe depression symptoms [60]. Lower carotenoid concentrations may also reflect unhealthy eating patterns associated with overweight and obesity, which have been linked to an increased risk of depression by inflammation or dysregulation of the HPA axis [5,61,62].
Previous studies have shown that some dietary factors, such as fruit and vegetables, fish, dietary fiber, and some macro- and microelements, may play an important, protective role in the development of depression through their antioxidant and/or anti-inflammatory properties [63,64,65,66]. It is important to note that carotenoids are also known for their antioxidant activity and anti-inflammatory properties [67,68]. Additionally, research shows that depression leads to the development of diseases such as cardiovascular diseases, insulin resistance, metabolic syndrome, and obesity [69,70,71]. These data support the hypothesis that inflammation and oxidative stress may be involved in the pathophysiology of this disorder. Considering that carotenoids have both antioxidant and anti-inflammatory effects, it is expected that they may exert an antidepressant effect.
3. The Role of Stress in Unipolar Mood Disorder Pathology
The results of animal studies indicate that psychological stress can increase the level of lipid peroxidation, a significant source of the cell damage caused by reactive oxygen species (ROS) [72], and impair antioxidant protection in the plasma [73,74]. Due to its high oxygen consumption and relatively weak antioxidant defense, the brain is particularly susceptible to oxidative damage, which may increase the likelihood of developing depressive episodes. Therefore, oxidative stress, caused by an imbalance between antioxidants and prooxidants, may play a key role in the remission and chronic course of depressive disorder [75,76].
Milaneschi et al. (2012) report that antioxidants have a beneficial effect on markers of inflammation; their data, obtained in the InCHIANTI prospective population study among seniors in Tuscany, Italy, indicates that inflammatory markers, namely serum levels of the interleukin-1 receptor antagonist (IL-1ra), partially mediate the relationship between carotenoid levels and the development of depressive mood after six-year follow-up [77]. In addition, increased serum carotenoid was associated with a lower risk of developing depression, and this effect was found to be a little distorted by IL-1ra [74]. This relationship between IL-1ra and depression was also confirmed in a meta-analysis by Howren et al. (2009) [78]. Interestingly, studies in an animal model of depression indicated that nuclear factor kappa B is a major mediator, linking stress-induced increases in IL-1β with compromised hippocampal neurogenesis and depressive behavior [79]. The discovery that inflammation mediated the link between plasma carotenoids and depression may indicate that they share the same molecular mechanism. However, carotenoid levels may potentially influence symptoms of depression through various other molecular and behavioral mechanisms. Patients with major depressive disorder (MDD) have been found to demonstrate higher serum levels of nitric oxide (NO) and reactive oxygen species (ROS) than patients without [79,80]. However, the mechanism underlying the relationship between depression symptoms and oxidative stress is not yet understood. So far, studies have shown that different types of depression can be associated with both an overactive (melancholic depression) and an underactive (atypical depression) HPA axis [18]. There is evidence that the HPA axis is associated with an increase in ROS formation; this mechanism has also been shown to involve granulocytes, with significantly higher numbers being observed in depressed patients than in healthy subjects [81,82].
Patients with depression have been found to have a significantly lower mean intake of α-carotene compared to healthy subjects [83]. In addition, depression has been associated with lowered antioxidant levels, as evidenced by low levels of carotenoids and antioxidant enzymes [76,77,84,85]. Black et al. (2016) found reduced levels of carotenoids such as zeaxanthin/lutein, β-cryptoxanthin, lycopene, α-carotene, and β-carotene to be associated with an increase in depression symptoms. Most importantly, this relationship persisted after controlling for diet quality; as carotenoids are only acquired through the dietary route, diet could be considered a significant confounder [3]. Beydoun et al. (2013) report that among the studied carotenoids, β-carotene, lutein, and zeaxanthin levels were inversely related to the incidence of depressive symptoms among US adults [85]. Interestingly, these studies suggest that common genetic factors may influence the relationship between low carotenoid levels and depression: the presence of SNPs associated with low β-cryptoxanthin levels may also influence the occurrence of depression [86].
Some authors also suggest that antidepressants have antioxidant capabilities and may work by lowering pro-inflammatory cytokine levels and ROS generation while increasing those of antioxidants such as superoxide dismutase (SOD) [87,88,89,90,91,92]. Classically-used antidepressants, i.e., selective serotonin reuptake inhibitors, demonstrate an antidepressant effect within two to four weeks of therapy; however, these drugs have common side effects, including cognitive, sexual, and sleep disturbances. Therefore, there is a need to develop new therapeutic strategies that exhibit fewer potential side effects. Interestingly, herbal preparations show promising effects in treating mood disorders without the side effects of synthetic drug use. Jiang et al. (2017) and Sharma et al. (2017) report that treatment with astaxanthin and a combination of lutein and zeaxanthin showed an antidepressant-like effect with the participation of the serotoninergic system [93,94]. Kim et al. indicate that β-carotene has antidepressant properties, which probably result from their influence on reducing the levels of the pro-inflammatory cytokine, i.e., IL-6 and TNF, and increasing the level of brain-derived neurotrophic factor (BDNF) in the brain [95].
In turn, Tsubi et al. [72] and Nouri et al. (2020) [96] found no correlation between the serum level of lycopene and depressive symptoms. Zhang et al. (2016) report that seven days of pretreatment with 60 mg/kg lycopene could reverse LPS-induced depressive behavior in mice based on the tail suspension test and the forced swim test [97]. In turn, a mechanistic study by Lin et al. (2014) found that three-day treatment with 10 mg/kg lycopene reverses the LPS-induced increase in serum TNF and IL-6 concentrations and IL-1β levels in the hippocampus [98]; in addition, pretreatment with 5, 10, or 20 M lycopene inhibited LPS-induced production of cyclooxygenase-2, inducible nitric oxide synthase, and IL-6 in primary cultured microglia via the activation of heme oxygenase-1 [98]. There is a possibility that lycopene supplementation may help maintain cellular homeostasis by restoring normal cell cytokines levels turn. These results suggest that inhibiting neuroinflammation may be a key factor in the antidepressant effects of lycopene.
4. Oxidative Stress and Antioxidants in the Course of Mood Disorders
Oxidative stress occurs as a result of an imbalance between the build-up of reactive oxygen species (ROS) or reactive nitrogen species (RNS) and their removal. ROS levels are believed to increase due to various environmental features such as tobacco smoke, ionizing, UV radiation, and by the initiation of cell receptors [99]. At least 5% of inhaled oxygen is converted to ROS, which naturally occurs as a byproduct of aerobic metabolism. In metabolic processes, cytochrome oxidase completely reduces most of the molecular oxygen to water in the mitochondria. Only partially reduced oxygen can react with long-chain molecules such as proteins, carbohydrates, lipids, and DNA. In higher organisms, RNS are produced by the oxidation of one of the terminal guanidonitrogen atoms of L-arginine [100] by nitric oxide synthase. NO can then be converted to various other forms of RNS [101].
The human body has a range of antioxidant defense mechanisms in place to protect against the potentially damaging effects of such active species., for example, by removing free radicals from the body. It is now known that oxidative stress, as well as ROS and RNS, negatively affect a number of cellular processes. When ROS exposure (or generation) increases or antioxidant levels fall, lipids, proteins, and DNA can be damaged, resulting in cell malfunction and even cell death [102]. Importantly, ROS participate in a number of physiological reactions of the body, such as the phagocytosis process [103]. Most importantly, the brain is particularly susceptible to oxidative stress because the level of aerobic respiration is high in the brain tissue. Additionally, brain tissue is rich in polyunsaturated fatty acids (PUFAs) that are susceptible to ROS damage [104].
A growing body of data indicates that ROS may also play an essential role in the pathophysiology of various neurological and psychiatric disorders, including mood disorders. Numerous studies have shown that individuals with neuropsychiatric disorders have higher levels of free radicals, lipid peroxides, pro-apoptotic markers, and altered antioxidant defense mechanisms [105,106,107]. A meta-analysis by Black et al. (2014) found oxidative stress to be elevated in people with MDD and/or depressive symptoms [108]. Importantly, oxidative stress is linked to various socio-demographic, health, and lifestyle variables, including socioeconomic status and smoking, which are also linked to depression [5,102,109,110,111,112,113]. Cigarette smoke has been demonstrated to decrease the levels of carotenoids and other antioxidants in human plasma [114]; it has been proposed that smoking may reduce carotenoid concentration by increasing metabolic rate, resulting in greater oxidative stress [115]. Today it is well known that antioxidants defend against the harmful effects of oxidative stress, which is believed to be associated with depression [116,117].
The antioxidant system consists of enzymatic antioxidants such as inter alia glutathione reductase, SOD, and catalase, as well as non-enzymatic forms such as vitamin C and E, N-acetylcysteine, reduced glutathione, flavonoids, and carotenoids. Carotenoids are natural antioxidants that can effectively prevent oxidative damage [67]. There is evidence that antioxidants exert a neuroprotective effect through their ability to repair the central nervous system and prevent oxidative stress-induced neurodegeneration.
The total antioxidant capacity of a diet has been shown to have an inverse relationship with depression, anxiety, and stress [118,119]. Some data suggest that people with depression consume lower levels of antioxidants in the form of fruit and vegetables compared to those without [120]. In addition, data suggest that patients with depression have lower plasma vitamin E and C levels than those without [88,121]. Vitamin E has been found to exert an antidepressant-like effect in depressed animal models, and this has been attributed to it supporting the enzymatic glutathione-based antioxidant defense system in the hippocampus and prefrontal cortex [122]. However, growing evidence suggests that antioxidant treatment has proven unsatisfactory and even damaging in some oxidation-related diseases such as cancer [123,124,125]. While we know that ROS plays a key role in defense against pathogens and intracellular signaling, the perception is that these compounds are harmful to cells. Likewise, antioxidants should not be regarded as purely beneficial agents. A clinical example of this is the finding that β-carotene supplementation in smokers leads to a significant increase in the incidence of lung cancer [126,127].
5. Carotenoids and Their Role in the Course of Depression
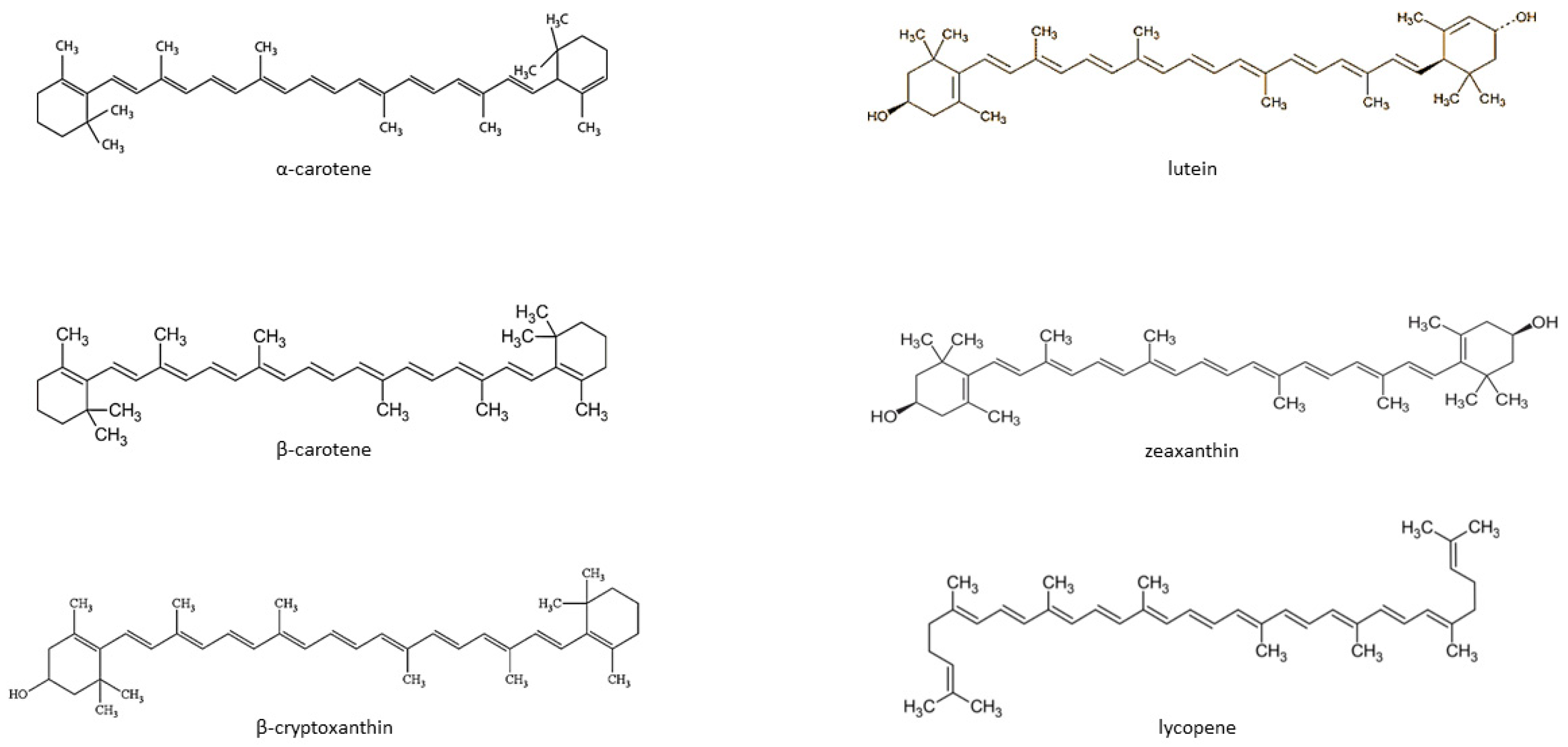
Carotenoids are fat-soluble color pigments that belong to the tetraterpene family, present in yellow-orange vegetables and fruits [128]. More than 700 carotenoids have been described, with the major forms being lycopene, β-carotene, ASTA, lutein, and zeaxanthin [129,130,131]. In nature, these pigments are found in many bacteria, fungi, and plants. The groups of carotenoids can also be divided into non-provitamin A and provitamin A (e.g., γ-carotene, β-carotene, α-carotene, and β-cryptoxanthin) [132]. The compounds can also be classified by the presence of specific functional groups: xanthophylls containing oxygen as a functional group (e.g., lutein, zeaxanthin), and carotenes containing only the parent hydrocarbon chain without any functional group (e.g., α-carotene, β-carotene, and lycopene) [132] (Figure 1).
Figure 1.
Chemical structure of some common carotenoids [133,134,135,136,137,138]. Source: Own elaboration based on the indicated data.
Carotenoids cannot be synthesized de novo by humans and can only be acquired through the dietary route. While around 700 carotenoids have been identified, only six are commonly found in the human diet and blood serum: α-carotene, β-carotene, lutein, zeaxanthin, lycopene, and β–cryptoxanthin. In addition, the typical human diet only includes about 40 carotenoids. Many studies show that providing the body with dietary carotenoids is associated with a reduced risk of developing lifestyle diseases, such as cancer, osteoporosis, diabetes, or cataracts, as well as certain infectious diseases, such as HIV infection [128,132,139]. The data also indicate that carotenoids may reduce the risk of developing CVD by lowering blood pressure and inflammatory markers and increasing insulin sensitivity in muscles, the liver, and adipose tissue. Interestingly, carotenoids could modulate the expression of specific genes involved in cell metabolism [140].
Carotenoids have many beneficial effects. They are mainly known for their antioxidant properties as major scavengers of ROS, including single molecular oxygen and peroxide radicals [132]. Recent epidemiological studies show that higher blood α-carotene and lycopene levels are linked to a lower risk of lung cancer, even among smokers [141]. Interestingly, an ever-increasing body of literature indicates that carotenoids may be effective in the treatment of a variety of cancers, e.g., neuroblastoma [142], cervical cancer [143], and prostate cancer [144]. A large number of existing in vitro and in vivo studies have revealed that carotenoids influence a variety of processes related to the body’s immune-inflammatory response. It has been demonstrated that these compounds influence both the cellular (lymphocyte proliferation, phagocytosis, and NK cell cytotoxicity) and humoral mechanisms of immunity (synthesis and secretion of cytokines) [145,146]. It was found that β-carotene can inhibit the upregulation of heme oxygenase 1 expression in human skin fibroblasts (FEK4) exposed to UV-A [128,147]. In turn, β-carotene has been shown to be less effective in preventing lipid peroxidation [147].
There is a growing body of evidence that the antioxidant and anti-inflammatory properties of carotenoids may promote efficient cognitive function [148,149,150,151,152] by increasing neuronal efficiency or stabilizing the lipid-protein bonds in neuronal membranes. Other neuroprotective mechanisms of carotenoids include enhancement of communication between clefts and modulation of the functional properties of synaptic membranes [151,152,153]. It should be stressed that some studies indicate that higher intake of β-carotene may be related to lower prevalence of depression, anxiety, and stress [104]. Epidemiological studies investigating the relationship between diet, carotenoids, and cognitive maintenance have reported that low levels of carotenoids may play a role in cognitive impairment [154,155]. Prohan et al. (2014) found depressed university male students to have a lower β-carotene intake compared to controls [156,157,158]. Increased β-carotene intake may also relieve depression and anxiety symptoms in cases of low blood antioxidant levels. Antioxidant supplementation has been found to resist stress-induced psychiatric disorders such as depression and anxiety [157].
One particularly potent antioxidant among the carotenoids is lycopene, which can trap singlet oxygen and reduce mutagenesis. Some authors have also suggested that lycopene effectively reduces smoke-generated ROS and modulates redox-sensitive target cells [148]. Research on neurodegenerative and psychiatric disorders also suggests that lycopene may have neuroprotective effects on the central nervous system (CNS) [158,159,160,161,162,163]. It has been shown that long-term intake of lycopene reduces the risk of stroke in men and reduces neuronal apoptosis in the case of cerebral ischemia [162,163,164].
Unlike other carotenoids, xanthophylls such as lutein, astaxanthin, and zeaxanthin are orientated within cell membranes by free hydroxyl groups at each end [165]. Lutein is present in high amounts in green plants and leaves such as spinach, kale, and broccoli. It is also the predominant carotenoid in the primate brain: it was present at almost 10–20 times higher levels in the occipital cortex, prefrontal cortex, and cerebellum than its isomer, zeaxanthin [166]. Zeaxanthin and lutein play a number of roles in both plants and humans, including photoprotection and the maintenance of the structural and functional integrity of biological membranes [167]. Numerous studies have shown that zeaxanthin and lutein exhibit antioxidant properties, thereby protecting cells from potential free radical damage [168]. Lutein, zeaxanthin, and other carotenoids can enter the brain from the blood and accumulate in the retinal macula [169,170]. Lutein is known to accumulate in all cortexes and membranes of the brain.
Serum concentrations of β-carotene, β -cryptoxanthin, and α-carotene can be used as biomarkers to predict the concentration of carotenoids in the brain [148,169,170,171]. It has been documented that a low serum lutein level was associated with depression in Alzheimer’s disease patients [77,172]. Previous research has shown that lutein reduces very low and medium-density lipoprotein levels, as well as inflammation and oxidative stress; it also inhibits the progression of atherosclerosis in humans by lowering serum IL-10 concentration [173,174]. Zeaxanthin can effectively scavenge water- and lipid-soluble peroxide radicals.
It should be stressed that carotenoids, as antioxidants, play an important role in counterbalancing the age-related rise in oxidative stress. It has also been shown that with age, the central nervous system becomes increasingly vulnerable to the impact of free radicals; indeed, seniors are much more vulnerable to protein and lipid damage caused by free radicals, resulting in impaired mitochondrial activity and increased free radical production [174]. The inability to counter oxidative stress may result from progressive neuronal deficits but also from neurodegenerative diseases [175,176,177]. Depression has also been found to cause structural and functional changes in some areas of the brain, especially around the hippocampus [178].
Taking the above into account, carotenoids have the potential to play a protective role in depression through various mechanisms. First, pro-inflammatory cytokines such as IL-6 and TNF impair the expression of BDNF, leading to the onset of depression [179]. Additionally, it should be noted that some studies have shown that patients with depression have lower serum levels of BDNF [95,180]. Interestingly, it has been shown that β-carotene and zeaxanthin may reduce the mRNA expression of IL-6 and TNF [181,182]. What is important, carotenoids have been tested for mechanisms in very important drug targets such as MAO or BDNF, known to be closely related to depression through molecular docking studies for possible inhibitory activity. Recent BDNF and carotenoid docking results conducted by Park et al. (2021) indicate the possibility of allosteric activation of BDNF by carotenoids [183]. On this basis, the authors suggested that dietary carotenoids may be used in the treatment of depressive symptoms. Secondly, this organ is prone to oxidative stress due to high oxygen consumption and high levels of lipids in the brain. At the same time, it is believed that the development of depression is closely related to oxidation and an imbalance between pro- and antioxidants. Studies have shown that people with depression have elevated levels of 8-hydroxy-2’-deoxyguanosine, which is considered a marker of oxidative DNA damage [184]. These results indicate that depression appears to be closely related to oxidative stress. Today we know that carotenoids can effectively remove reactive oxygen species as well as other free radicals. Therefore, considering that carotenoids have both antioxidant and anti-inflammatory effects, it is expected that they may exert an antidepressant effect.
6. Dietary Carotenoids in Depression
Studies indicate that a varied diet rich in vegetables, fruits, and nuts, as well as the fish and olive oil characteristic of the Mediterranean diet, may protect against depression. A potential association has been reported between carotenoid intake and the risk of depression [83,95,120,156,185,186]. In addition, α- and β-carotene intake was found to be inversely associated with depressive symptoms in women in late midlife in the US [185], and an inverse association was noted between β-carotene intake and the risk of depression in a case-control study among Korean students [83].
Dietary cryptoxanthin intake was found to significantly influence the risk of depression in a cross-sectional study of American seniors; however, no relationship was observed for another four carotenoids used in the study or total carotenoid intake [120]. Research conducted by Ge et al. (2020) in US adults suggests that both carotenoids such as α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein with zeaxanthin, and total carotenoid consumption may be inversely proportional to the risk of developing depressive symptoms [186]. An important role in the etiology of depression may be played by the age-related reduction in total xanthophyll and carotenoid levels that takes place in the frontal lobes [187] (Table 1).

Table 1.
Localization of certain carotenoids in the brain. Source: Own elaboration based on the indicated data.
Indeed, xanthophylls have been found to account for 66–77% of total carotenoid levels in the human brain [120]. High dietary carotenoid consumption has been associated with less severe depression in one US study [188], as well as in two other cross-sectional studies in seniors [60,67]. However, it is important to mention that a similar analysis in Australia found no significant associations [189]. Similarly, a significant inverse relationship was found between dietary total carotenoids intake and depression in a cross-sectional analysis of 1274 US adults (30–64 y.o.) [188] and in 500 Japanese seniors (65–75 y.o.) [60]. Also, a similar observation was made for dietary β-cryptoxanthin intake among 278 US seniors (>60 y.o.) [120]. It should be noted that senior populations are more likely to demonstrate comorbidities that may influence the findings.
The role of β-cryptoxanthin and its preventive role in the epidemiology of depression is unknown, and there is not enough study on this topic. All carotenoids are believed to exert antioxidant properties that may protect neural tissue and thus prevent depression [190,191,192]; however, among the carotenoids, this effect is restricted to β-cryptoxanthin. In the human body, only the brain preferentially accumulates xanthophylls, particularly β-cryptoxanthin [187,188]. This is significant because structural and functional alterations in the limbic and cortical structures, particularly in the hippocampus, have been associated with the onset of depression [187]. In addition, xanthophylls degrade more slowly than nonpolar carotenoids and are concentrated within lipid bilayer membranes, particularly in the areas most prone to degradation [193].
Hence, higher dietary β-cryptoxanthin intake appears to lower the risk of depression, but prospective studies are needed to confirm this [127]. Interestingly, these results imply that a Mediterranean-style diet could not only reduce the risk of depression among adults but also could ameliorate the inflammation associated with depression in community-dwelling seniors [194]. Dietary interventions hence serve as a cost-effective approach for promoting healthy aging and reducing the incidence of age-related depression (Table 2).

Table 2.
Dietary sources of carotenoids [195,196]. Source: Own elaboration based on the indicated data.
Other studies have examined the effect of vitamin intake on depression with regard to sex or BMI. No such relationship was observed in underweight participants and overweight men; however, in overweight women, all tested vitamins, except for vitamin A1 (i.e., β-carotene), D, and B3, were associated with lower levels of depression, with vitamin B complex deficiency being most closely correlated with elevated depressive symptoms [197]. Another study [198] in seniors found vitamin E to inhibit the development of depressive symptoms in men; however, lycopene demonstrated 100 times greater singlet oxygen-quenching activity than vitamin E [199]. This is hardly surprising, as lycopene is believed to be the most efficient carotenoid antioxidant; more importantly, consumption has no toxic effects [72]. Indeed, tomatoes account for more than 85% of the dietary intake of lycopene among most people [186]. Such a Mediterranean-style diet may have a beneficial effect on the prevention of depressive symptoms; however, the relationship between tomato/lycopene and depression remains poorly studied [141], and no research has been carried out on the effects of a tomato-rich diet on depression in community-dwelling seniors. However, high dietary intake of various vegetables, especially tomato products, has been found to be independently associated with a reduced risk of depression in community-dwelling seniors (>70 y.o.) [200]. In addition, no relationship was observed between the consumption of vegetables and depressive symptoms. More studies have also reported a relationship between the consumption of tomato products and depression than those associated with dietary antioxidants, such as folic acid and vitamin E [141,200,201].
It needs to be highlighted that depression is the most common mental disorder in the course of neurodegenerative diseases, especially in the course of Alzheimer’s disease (AD). Increasing evidence indicates that depression may be a risk factor for the development of AD, and neurodegeneration in the course of AD may also predispose to the development of depression [202]. Importantly, these conditions are associated with dysfunction of certain centers in the brain, neurotransmission imbalance and dysregulation of the HPA axis, decreased BDNF levels, as well as disruption of the mechanisms that regulate neuroplasticity and cell survival [203]. In addition to BDNF, potential strong candidates for biomarkers implicated in the etiology of MDD contribute to cellular damage and impaired neuronal plasticity and neurotransmission in the prefrontal cortex and hippocampus, including IL-6, TNF, and CRP [204]. Interestingly, Lin et al. (2020) showed that in endothelial cells and monocytes, β-cryptoxanthin, lutein, and lycopene suppressed the fructose-induced expression of TNF and IL-1β [205]. On the other hand, β-carotene is associated with reduced levels of TNF and IL-6 and increased levels of BDNF in animal models [95]. Moreover, Nakamura et al. (2022) observed an inverse association between serum lutein and hs-CRP levels in non-smokers [206]. According to currently available clinical data, nutritional status seems to play an important role in the development and progression of neurodegenerative diseases. In patients with AD, the concentrations of β-carotene, lutein, lycopene, and zeaxanthin were found to be significantly lower compared to a control group [207]. Moreover, US middle-aged and older adults’ serum lutein+zeaxanthin was associated with a reduced risk of all-cause dementia [208]. In turn, astaxanthin-treated mice showed slower memory decline and decreased amyloid-beta (Aβ) and tau protein deposition [209].
Many chronic conditions, including mood disorders, are related to the composition and diversity of the gut microbiota. As dietary patterns influence intestinal microbiota, they could also influence health status. Numerous studies have shown that carotenoids can influence intestinal microbiota in various ways [210]. First, research has shown that carotenoids alter the expression of immunoglobulin A, which is a very important factor in bacterial colonization [211]. Studies in an animal model have shown that supplementation with astaxanthin-enriched yeast for 7 days increased the number of IgA antibody-secreting cells in mice [212,213]. In addition, lycopene has been shown to reduce oxidative stress in the intestines, which may have an effect on the number and diversity of microorganisms that form the gut microbiota [214]. Moreover, there is evidence that vitamin A deficiencies may lead to intestinal dysbiosis, increased susceptibility to infections, and/or damage to the gastrointestinal tract [215]. In a cystic fibrosis observational research, β-carotene intake was shown to be associated with a decrease in Bacteroides and an increase in the Firmicutes population [216]. In turn, research conducted by Schmidt et al. (2021) demonstrated that a carotenoid-rich diet during pregnancy supports intestinal microbiota diversity [217]. Wiese et al. (2019) noted dose-related improvement in gut microbiota profile with enhanced fractions of Bifidobacterium adolescentis and Bifidobacterium longum in middle-aged subjects with moderate obesity, to which lycopene was applied for 30 days [218]. It should be noted that β-carotene increases the number of intestinal bacteria that produce short-chain fatty acids and increases the synthesis of SCFAs [219]. Ramos et al. (2018) demonstrated that administering a β-carotene-rich oil to the caw rumen altered the amount of SCFA production (change favoring the production of propionate over acetate). It is well recognized that increasing the propionate-to-acetate ratio promotes a decrease in methane synthesis, which reduces the availability of hydrogen to Archaea and so avoids the formation of gut dysbiosis [220]. The study also shows that carotenoids protect the mucosa and epithelial gut barrier, as well as tight junction integrity [221]. Additionally, there is evidence that fucoxanthin, a marine carotenoid, affects the regulation of the intestinal microbiota and especially plays a key role in promoting the growth of Akkermansia muciniphila, which can repair intestinal mucosa through immune modulation [222,223,224]. It is worth emphasizing that, in contrast to the impact of carotenoids on the gut microbiota, little is known about the microbiota’s effect on carotenoid metabolites [211].
Many chronic conditions, including depressive-like and anxiety-like mood disorders, are associated with the use of carotenoids. This meta-analysis [225] also found that alpha-carotene, beta-carotene, lycopene, lutein, and zeaxanthin, as well as total carotenoid intake, help decrease the risk of depressive-like symptoms. However, beta-cryptoxanthin levels did not approach statistical significance. Despite the observational studies presented, more longitudinal research or clinical trials should be done to identify optimal carotenoid consumption and plasma/serum levels to prevent or treat mental diseases characterized by significant emotional discomfort and dysfunction. Depression-like symptoms such as loss of pleasure and interest in everyday activities, which are frequently accompanied by headaches, dizziness, and other symptoms of different cerebrovascular disorders, can also be modified by carotenoids [226].
Carotenoids’ anti-PTSD-like action is poorly understood. The recent research [227] addressed this by adopting a single extended stress (SPS) strategy to develop PTSD-like behavioral effects in mice. The results showed that 12 days of lycopene treatment at 10 and 20 mg/kg concentrations, but not at 5 mg/kg dose, positively affected the PTSD-like phenotype induced by SPS, including an increase in freezing time in the contextual fear paradigm, a decrease in time and entries in the open arms in the elevated plus maze test, and a decrease in distance and time in the central area of the open field test, without affecting mouse locomotor activity. Lycopene (20 mg/kg, 12 days) supplementation also inhibited the SPS-induced reduction in brain-derived neurotrophic factor (BDNF) levels in mice’s hippocampus and prefrontal cortex. Overall, lycopene’s anti-PTSD-like effects may be associated with its anti-neuroinflammation and anti-oxidative stress activities.
Lycopene also has preventive and/or therapeutic benefits on the central nervous system (CNS) in several disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), cerebral ischemia, epilepsy, and depression. Lycopene also improves mouse cognition and memory skills in a variety of disease circumstances, including diabetes, colchicine exposure, a high-fat diet (HFD), and aging. Moreover, lycopene can protect against neurotoxicity caused by MSG, trimethyltin (TMT), methylmercury (MeHg), tert-butyl hydroperoxide (t-BHP), and cadmium (Cd). Lycopene administration has particular therapeutic effects in some situations, such as ethanol addiction and haloperidol-induced orofacial dyskinesia. In order to have a comprehensive understanding of the function of lycopene in the CNS, the authors outline and discuss lycopene’s pharmacological effects as well as its possible mechanisms in CNS disorder prevention and/or therapy [228].
The results of research on the spice crocin are promising [229]. It is a natural substance with anti-inflammatory and anti-oxidant effects. Unfortunately, little is known regarding crocin’s anti-inflammatory mechanisms in LPS-induced anxiety and depressive-like behaviors. This study aimed to emphasize crocin’s neuroprotective impact against LPS-induced anxiety and depressive-like behaviors in mice. These findings showed that crocin might be used to treat neuroinflammation and depressive-like behaviors caused by LPS. The result was found to be related to NLRP3 inflammasome and NF-B inhibition, which promoted M1 to M2 phenotypic conversion of microglia.
It should be noted at the end that carotenoids included in food may have differing levels of bioactivity and use for human health. Carotenoids’ stability can be modified by food production and storage.
Popular smoothies made from various fruits and vegetables provide vitamins, minerals, and bioactive components such as carotenoids and fiber to the diet. Thermal treatments increased the microbiological shelf-life of a fruit smoothie (mild and intensive). The color assessment was shown to be marginally better for smoothies following ultrasonic treatment rather than heat treatment. Although heat treatment lowered the concentration of carotenoids in foods, it was discovered that liberation, micellarization, and, therefore, bioaccessibility had a positive effect [230].
In another study [231], liquid chromatography coupled with a photodiode array detector was used to monitor changes in the number of carotenoids and their true retentions (% TR) throughout the manufacturing of pumpkin puree, as well as the stability of such compounds after 180 days of storage. Cooking resulted in greater losses than commercial sterilizing. During the preparation and storage of pumpkin puree, high losses of xanthophylls such as lutein and violaxanthin were observed. These losses demonstrate the low stability of these compounds. After processing, the major carotenoids, pro-vitamin A carotenes, namely α-Carotene and all-trans-β-carotene for C, had high retentions (>75%). The puree samples showed a slight degree of isomerization of β-carotene but with low concentrations of cis-isomers. The concentrations of these carotenoids did not change considerably after 180 days of storage.
Even though carotenoids are hydrophobic, their absorption from dietary items is often quite low. This review [232] summarizes current studies on fruits and vegetables that associate specific product characteristics, such as (micro)structural characteristics and the presence or addition of lipids, to carotenoid bioaccessibility. Lipids are naturally present in specific fruits and vegetables, although in small levels. Additionally, they are frequently used in manufacturing fruit and vegetable-derived products (for example, low-fat emulsions), where they play an essential role in satiety control, mouth feel, and taste. Since carotenoids are lipophilic micronutrients, the oil phase acts as a hydrophobic domain in which carotenoids may be dissolved. Therefore, its presence is essential for carotenoids to be incorporated into micelles and then taken up by humans.
Interesting research [233] focused on the characterization of tomato peel extracts. The presence of lycopene and β-carotene was indicated by the chromatographic profile. According to the in silico results, heat treatment of lycopene molecule pairs at temperatures over 100 °C may reduce their biological activity. In addition, the thermal degradation of total carotenoids, β-carotene, and lycopene was studied at temperatures ranging from 100–145 °C, which revealed that the enriched peanut and cotton oils with extract had longer induction time values, indicating a protection factor of tomato-derived carotenoids. The findings emphasized the thermostability of carotenoids in vegetable oils, as well as the possibility of using tomato peel as a valuable source of biologically active compounds with antioxidative properties. The study provides cumulative information for selecting industrial processing parameters from the perspective of preserving bioactives and obtaining the desired quality end products.
7. Conclusions
In conclusion, poor dietary habits, i.e., low fruit and vegetable intake and high fast food/convenience food intake, may increase the risk of depression. Carotenoids have the potential to play a protective role in the development of depression through various mechanisms. The most pertinent point is that pro-inflammatory cytokines such as IL-6 and TNF impair the expression of BDNF, which leads to the onset of depression. Recent BDNF and carotenoid docking results indicate the possibility of allosteric activation of BDNF by carotenoids. On this basis, it is suggested that dietary carotenoids may be used in the treatment of depressive symptoms. Another interesting point to consider is the fact that the development of depression is closely related to oxidation and an imbalance between pro- and antioxidants. It is well known that carotenoids can effectively remove reactive oxygen species as well as other free radicals. Therefore, considering that carotenoids have both antioxidant and anti-inflammatory effects, they are expected to exert a promising antidepressant effect. Nevertheless, further studies (including interventional and mechanistic studies) assessing the effect of carotenoids on preventing and alleviating depression symptoms are needed.
Author Contributions
Conceptualization, P.R. and E.K.; writing-original draft preparation, P.R. and E.K.; writing-review and editing, P.R. and E.K.; visualization, E.K.; supervision, P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BDNF | brain-derived neurotrophic factor |
| BMI | body mass index |
| CRP | C reactive protein |
| HPA | hypothalamic–pituitary–adrenal axis |
| IL | interleukin |
| IL-1ra | interleukin-1 receptor antagonist |
| LPS | lipopolysaccharide |
| MDD | major depressive disorder |
| NO | nitric oxide |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TNF | tumor necrosis factor |
References
- Bachmann, S. Epidemiology of suicide and the psychiatric perspective. Int. J. Environ. Res. Public Health 2018, 6, 1425. [Google Scholar] [CrossRef]
- Reddy, M.S. Depression: The disorder and the burden. Indian J. Psychol. Med. 2010, 32, 1–2. [Google Scholar] [CrossRef]
- Black, C.N.; Penninx, B.W.; Bot, M.; Odegaard, A.O.; Gross, M.D.; Matthews, K.A.; Jacobs, D.R., Jr. Oxidative stress, anti-oxidants and the cross-sectional and longitudinal association with depressive symptoms: Results from the CARDIA study. Transl. Psychiatry 2016, 23, 6, e743. [Google Scholar] [CrossRef]
- Nicholson, A.; Kuper, H.; Hemingway, H. Depression as an aetiologic and prognostic factor in coronary heart disease: A meta-analysis of 6362 events among 146,538 participants in 54 observational studies. Eur. Heart J. 2006, 27, 2763–2774. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Mezuk, B.; Eaton, W.W.; Albrecht, S.; Golden, S.H. Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care 2008, 31, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Alexopoulos, G.S.; Lopez, O.L.; Williamson, J.D.; Yaffe, K. Depressive 5-3 the cardiovascular health study. Arch. Gen. Psychiatry. 2006, 63, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Neale, M.C.; Kendler, K.S. Genetic epidemiology of major depression: Review and meta-analysis. Am. J. Psychiatry 2000, 157, 1552–1562. [Google Scholar] [CrossRef]
- Tozzi, F.; Prokopenko, I.; Perry, J.D.; Kennedy, J.L.; McCarthy, A.D.; Holsboer, F.; Berrettini, W.; Middleton, L.T.; Chilcoat, H.D.; Muglia, P. Family history of depression is associated with younger age of onset in patients with recurrent depression. Psychol. Med. 2008, 38, 641–649. [Google Scholar] [CrossRef]
- Zhai, L.; Zhang, Y.; Zhang, D. Sedentary behaviour and the risk of depression: A meta-analysis. Br. J. Sports Med. 2015, 49, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Boden, J.M.; Fergusson, D.M.; Horwood, L.J. Cigarette smoking and depression: Tests of causal linkages using a longitudinal birth cohort. Br. J. Psychiatry. 2010, 196, 440–446. [Google Scholar] [CrossRef]
- Paljarvi, T.; Koskenvuo, M.; Poikolainen, K.; Kauhanen, J.; Sillanmaki, L.; Makela, P. Binge drinking and depressive symptoms: A 5-year population-based cohort study. Addiction 2009, 104, 1168–1178. [Google Scholar] [CrossRef]
- Kumar, P.V.N.; Elango, P.; Asmathulla, S.; Kavimani, S. Lycopene treatment transposed antidepressant-like action in rats provoked to chronic mild stress. Biomed. Pharmacol. J. 2019, 12, 981–988. [Google Scholar] [CrossRef]
- Lee, A.L.; Ogle, W.O.; Sapolsky, R.M. Stress and depression: Possible links to neuron death in the hippocampus. Bipolar Disord. 2002, 4, 117–128. [Google Scholar] [CrossRef]
- Freitas, A.E.; Egea, J.; Buendía, I.; Navarro, E.; Rada, P.; Cuadrado, A.; Rodrigues, A.L.S.; López, M.G. Agmatine induces Nrf2 and protects against corticosterone effects in hippocampal neuronal cell line. Mol. Neurobiol. 2015, 51, 1504–1519. [Google Scholar] [CrossRef]
- Myers, B.; McKlveen, J.M.; Herman, J.P. Glucocorticoid actions on synapses, circuits, and behavior: Implications for the energetics of stress. Front. Neuroendocrinol. 2014, 35, 180–196. [Google Scholar] [CrossRef]
- Spiers, J.G.; Chen, H.J.C.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamicpituitary- adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2015, 9, 456. [Google Scholar] [CrossRef]
- Stanić, D.; Plećaš-Solarović, B.; Petrović, J.; Bogavac-Stanojević, N.; Sopić, M.; Kotur-Stevuljević, J.; Ignjatović, S.; Pešić, V. Hydrogen peroxide-induced oxidative damage in peripheral blood lymphocytes from rats chronically treated with corticosterone: The protective effect of oxytocin treatment. Chem. Biol. Interact. 2016, 256, 134–141. [Google Scholar] [CrossRef]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch. Eur. J. Physiol. 2010, 460, 525–542. [Google Scholar] [CrossRef]
- Freitas, A.E.; Egea, J.; Buendia, I.; Gómez-Rangel, V.; Parada, E.; Navarro, E.; Casas, A.I.; Wojnicz, A.; Ortiz, J.A.; Cuadrado, A.; et al. Agmatine, by improving neuroplasticity markers and inducing Nrf2, prevents corticosterone-induced depressive-like behavior in mice. Mol. Neurobiol. 2016, 53, 3030–3045. [Google Scholar] [CrossRef]
- Czéh, B.; Lucassen, P.J. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur. Arch. Psychiatry Clin. Neurosci. 2007, 257, 250–260. [Google Scholar] [CrossRef]
- Hong, H.; Kim, B.S.; Im, H.-I. Pathophysiological role of neuroinflammation in neurodegenerative diseases and psychiatric disorders. Int. Neurourol. J. 2016, 20, S2. [Google Scholar] [CrossRef]
- Müller, N. The role of anti-inflammatory treatment in psychiatric disorders. Psychiatr. Danub. 2013, 25, 292–298. [Google Scholar]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflammation 2013, 10, 43. [Google Scholar] [CrossRef]
- Eyre, H.A.; Air, T.; Pradhan, A. A meta-analysis of chemokines in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 68, 1–8. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K. A metaanalysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef]
- Verduijn, J.; Milaneschi, Y.; Schoevers, R.A.; van Hemert, A.M.; Beekman, A.T. Pathophysiology of major depressive disorder: Mechanisms involved in etiology are not associated with clinical progression. Transl. Psychiatry 2015, 5, e649. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, Y.L.; Tian, L.P.; Lai, J.B.; Hu, C.C.; Zhang, P.; Chen, J.K.; Hu, J.B.; Huang, M.L.; Wei, N. Circulating T lymphocyte subsets, cytokines, and immune checkpoint inhibitors in patients with bipolar II or major depression: A preliminary study. Sci. Rep. 2017, 7, 40530. [Google Scholar] [CrossRef]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nordestgaard, B.G. Elevated C-reactive protein, depression, somatic diseases, and all-cause mortality: A mendelian randomization study. Biol. Psychiatry 2014, 76, 249–257. [Google Scholar] [CrossRef]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nordestgaard, B.G. Elevated C-reactive protein associated with late- and very-late-onset schizophrenia in the general population: A prospective study. Schizophr. Bull. 2014, 40, 1117–1127. [Google Scholar] [CrossRef]
- Mac Giollabhui, N.; Ng, T.H.; Ellman, L.M.; Alloy, L.B. The longitudinal associations of inflammatory biomarkers and depression revisited: Systematic review, meta-analysis, and meta-regression. Mol. Psychiatry 2020, 26, 3302–3314. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Ren, L.; Du, H.; Fei, C.; Qian, C.; Li, B.; Zhang, R.; Liu, H.; Li, Z.; et al. High-fiber diet ameliorates gut microbiota, serum metabolism and emotional mood in type 2 diabetes patients. Front. Cell. Infect. Microbiol. 2023, 13, 1069954. [Google Scholar] [CrossRef]
- Carlessi, A.S.; Borba, L.A.; Zugno, A.I.; Quevedo, J.; Réus, G.Z. Gut microbiota-brain axis in depression: The role of neuroinflammation. Eur. J. Neurosci. 2021, 53, 222–235. [Google Scholar] [CrossRef]
- Cruz-Pereira, J.S.; Rea, K.; Nolan, Y.M.; O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Depression’s unholy trinity: Dysregulated stress, immunity, and the microbiome. Annu. Rev. Psychol. 2020, 71, 49–78. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Gui, X.; Shi, X.; Bao, Z.; Han, H.; Li, M.D. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol. Psychiatry 2020, 25, 2759–2772. [Google Scholar] [CrossRef]
- Leclercq, S.; Le Roy, T.; Furgiuele, S.; Coste, V.; Bindels, L.B.; Leyrolle, Q.; Neyrinck, A.M.; Quoilin, C.; Amadieu, C.; Petit, G.; et al. Gut microbiota-induced changes in β-hydroxybutyrate metabolism are linked to altered sociability and depression in alcohol use disorder. Cell. Rep. 2020, 33, 108238. [Google Scholar] [CrossRef]
- Bharwani, A.; West, C.; Champagne-Jorgensen, K.; McVey Neufeld, K.A.; Ruberto, J.; Kunze, W.A.; Bienenstock, J.; Forsythe, P. The vagus nerve is necessary for the rapid and widespread neuronal activation in the brain following oral administration of psychoactive bacteria. Neuropharmacology 2020, 170, 108067. [Google Scholar] [CrossRef]
- Abildgaard, A.; Kern, T.; Pedersen, O.; Hansen, T.; Lund, S.; Wegener, G. A diet-induced gut microbiota component and related plasma metabolites are associated with depressive-like behaviour in rats. Eur. Neuropsychopharmacol. 2021, 43, 10–21. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yang, R.; Wang, W.; Qi, L.; Huang, T. Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J. Neuroinflamm. 2020, 17, 88. [Google Scholar] [CrossRef]
- Chen, M.; Xie, C.R.; Shi, Y.Z.; Tang, T.C.; Zheng, H. Gut microbiota and major depressive disorder: A bidirectional Mendelian randomization. J. Affect. Disord. 2022, 316, 187–193. [Google Scholar] [CrossRef]
- McGuinness, A.J.; Davis, J.A.; Dawson, S.L.; Loughman, A.; Collier, F.; O’Hely, M.; Simpson, C.A.; Green, J.; Marx, W.; Hair, C.; et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 2022, 27, 1920–1935. [Google Scholar] [CrossRef]
- Winter, G.; Hart, R.A.; Charlesworth, R.P.G.; Sharpley, C.F. Gut microbiome and depression: What we know and what we need to know. Rev. Neurosci. 2018, 29, 629–643. [Google Scholar] [CrossRef]
- Bodnar, L.; Wisner, K. Nutrition and depression: Implications for improving mental health among childbearing-aged women. Biol. Psychiatry 2005, 58, 679–685. [Google Scholar] [CrossRef]
- Murphy, J.; Wehler, C.; Pagano, M.; Little, M.; Kleinman, R.; Jellinek, M. Relationship between hunger and psychosocial functioning in lowincome American children. J. Am. Acad. Child Adolesc. Psychiatry 1998, 37, 163–170. [Google Scholar] [CrossRef]
- Petridou, E.T.; Kousoulis, A.A.; Michelakos, T.; Papathoma, P.; Dessypris, N.; Papadopoulos, F.C.; Stefanadis, C. Folate and B12 serum levels in association with depression in the aged: A systematic review and meta-analysis. Aging Ment. 2016, 20, 965–973. [Google Scholar] [CrossRef]
- Li, B.; Lv, J.; Wang, W.; Zhang, D. Dietary magnesium and calcium intake and risk of depression in the general population: A meta-analysis. Aust. N. Z. J. Psychiatry. 2017, 51, 219–229. [Google Scholar] [CrossRef]
- Anglin, R.E.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Apostolopoulos, V. The effects of vitamin B in depression. Curr. Med. Chem. 2016, 23, 4317–4337. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Stojanovska, L.; Prakash, M.; Apostolopoulos, V. The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas 2017, 96, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, J.A.; Sherwood, N.; Perry, C.; Neumark-Sztainer, D.; Story, M. Depressive symptoms and adolescent eating and health behaviors: A multifaceted view in a population-based sample. Prev. Med. 2004, 38, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Tsujiguchi, H.; Kambayashi, Y.; Hara, A.; Miyagi, S.; Yamada, Y.; Nakamura, H.; Shimizu, Y.; Hori, D.; Suzuki, F.; et al. Relationship between vitamin intake and depressive symptoms in elderly Japanese individuals: Differences with gender and body mass index. Nutrients 2017, 3, 1319. [Google Scholar] [CrossRef]
- Bremner, J.D.; Shearer, K.D.; McCaffery, P.J. Retinoic acid and affective disorders: The evidence for an association. J. Clin. Psychiatry 2012, 73, 37–50. [Google Scholar] [CrossRef]
- Ludot, M.; Mouchabac, S.; Ferreri, F. Inter-relationships between isotretinoin treatment and psychiatric disorders: Depression, bipolar disorder, anxiety, psychosis and suicide risks. World J. Psychiatry 2015, 5, 222–227. [Google Scholar] [CrossRef]
- Brody, S. High-dose ascorbic acid increases intercourse frequency and improves mood: A randomized controlled clinical trial. Biol. Psychiatry 2002, 52, 371–374. [Google Scholar] [CrossRef]
- Khajehnasiri, F.; Mortazavi, S.B.; Allameh, A.; Akhondzadeh, S. Effect of omega-3 and ascorbic acid on inflammation markers in depressed shift workers in Shahid Tondgoyan Oil Refinery, Iran: A randomized double-blind placebo-controlled study. J. Clin. Biochem. Nutr. 2013, 53, 36–40. [Google Scholar] [CrossRef]
- Mazloom, Z.; Ekramzadeh, M.; Hejazi, N. Efficacy of supplementary vitamins c and e on anxiety, depression and stress in type 2 diabetic patients: A randomized, single-blind, placebo-controlled trial. Pak. J. Biol. Sci. 2013, 16, 1597–1600. [Google Scholar] [CrossRef]
- Oishi, J.; Doi, H.; Kawakami, N. Nutrition and depressive symptoms in community-dwelling elderly persons in Japan. Acta Med. Okayama 2009, 63, 9–17. [Google Scholar] [CrossRef]
- Vogelzangs, N.; Kritchevsky, S.B.; Beekman, A.T.; Newman, A.B.; Satterfield, S.; Simonsick, E.M.; Yaffe, K.; Harris, T.B.; Penninx, B.W. Depressive symptoms and change in abdominal obesity in older persons. Arch. Gen. Psychiatry 2008, 65, 1386–1389. [Google Scholar] [CrossRef]
- Miller, G.E.; Freedland, K.E.; Carney, R.M.; Stetler, C.A.; Banks, W.A. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav. Immun. 2003, 17, 276–285. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Zhang, D. Fish consumption and risk of depression: A meta-analysis. J. Epidemiol. Community Health 2016, 70, 299–304. [Google Scholar] [CrossRef]
- Liu, X.; Yan, Y.; Li, F.; Zhang, D. Fruit and vegetable consumption and the risk of depression: A meta-analysis. Nutrition 2016, 32, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, S.; Song, X.; Li, Z.; Zhang, D. Exploration of the association between dietary fiber intake and depressive symptoms in adults. Nutrition 2018, 54, 48–53. [Google Scholar] [CrossRef]
- Sun, C.; Wang, R.; Li, Z.; Zhang, D. Dietary magnesium intake and risk of depression. J. Affect. Disord. 2019, 246, 627–632. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Rubin, L.P.; Ross, A.C.; Stephensen, C.B.; Bohn, T.; Tanumihardjo, S.A. Metabolic effects of inflammation on vitamin A and carotenoids in humans and animal models. Adv. Nutr. 2017, 8, 197–212. [Google Scholar] [CrossRef]
- Ali, S.; Stone, M.A.; Peters, J.L.; Davies, M.J.; Khunti, K. The prevalence of comorbid depression in adults with Type 2 diabetes: A systematic review and meta-analysis. Diabet. Med. 2006, 23, 1165–1173. [Google Scholar] [CrossRef]
- González, H.M.; Tarraf, W. Comorbid cardiovascular disease and major depression among ethnic and racial groups in the United States. Int. Psychogeriatr. 2013, 25, 833–841. [Google Scholar] [CrossRef]
- Euesden, J.; Danese, A.; Lewis, C.M.; Maughan, B. A bidirectional relationship between depression and the autoimmune disorders—New perspectives from the National Child Development Study. PLoS ONE 2017, 12, 0173015. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, H.; Shimoi, K.; Kinae, N.; Oguni, I.; Hori, R.; Kobayashi, F. Depressive symptoms are independently correlated with lipid peroxidation in a female population: Comparison with vitamins and carotenoids. J. Psychosom. Res. 2004, 56, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Moller, P.; Wallin, H.; Knudsen, L.E. Oxidative stress associated with exercise, psychological stress and life-style factors. Chem. Biol. Interact. 1996, 102, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Cernak, I.; Savic, V.; Kotur, J.; Prokic, V.; Kuljic, B.; Grbovic, D.; Veljovic, M. Alterations in magnesium and oxidative status during chronic emotional stress. Magnes. Res. 2000, 13, 29–36. [Google Scholar] [PubMed]
- Moylan, S.; Maes, M.; Wray, N.R.; Berk, M. The neuroprogressive nature of major depressive disorder: Pathways to disease evolution and resistance, and therapeutic implications. Mol. Psychiatry 2013, 18, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Sarandol, A.; Sarandol, E.; Eker, S.S.; Erdinc, S.; Vatansever, E.; Kirli, S. Major depressive disorder is accompanied with oxidative stress: Short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum. Psychopharmacol. 2007, 22, 67–73. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Bandinelli, S.; Penninx, B.W.; Corsi, A.M.; Lauretani, F.; Vazzana, R.; Semba, R.D.; Guralnik, J.M.; Ferrucci, L. The relationship between plasma carotenoids and depressive symptoms in older persons. World J. Biol. Psychiatr. 2012, 13, 588–598. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Koo, J.W.; Russo, S.J.; Ferguson, D.; Nestler, E.J.; Duman, R.S. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. USA 2010, 107, 2669–2674. [Google Scholar] [CrossRef]
- Talarowska, M.; Gałecki, P.; Maes, M.; Orzechowska, A.; Chamielec, M.; Bartosz, G. Nitric oxide plasma concentration associated with cognitive impairment in patients with recurrent depressive disorder. Neurosci. Lett. 2012, 510, 127–131. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, M.; Zhou, J. Myeloid-derived suppressor cells in major depression patients suppress T-cell responses through the production of reactive oxygen species. Psychiatry Res. 2015, 228, 695–701. [Google Scholar] [CrossRef]
- Bekaroglu, M.; Deger, O.; Karahan, S.C.; Bilici, M.; Soylu, C.; Orem, A. Effects of antidepressant treatments on polymorphonuclear elastase levels in patients with depression. J. Affect. Disord. 2000, 59, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; You, J.S.; Chang, K.J. Dietary taurine intake, nutrients intake, dietary habits and life stress by depression in Korean female college students: A case-control study. J. Biomed. Sci. 2010, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Palta, P.; Samuel, L.J.; Miller, E.R.; Szanton, S.L. Depression and oxidative stress: Results from a meta-analysis of observational studies. Psychosom. Med. 2014, 76, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Beydoun, H.A.; Boueiz, A.; Shroff, M.R.; Zonderman, A.B. Antioxidant status and its association with elevated depressive symptoms among US adults: National Health and Nutrition Examination Surveys 2005–6. Br. J. Nutr. 2013, 109, 1714–1729. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Nalls, M.A.; Canas, J.A.; Evans, M.K.; Zonderman, A.B. Gene polymorphisms and gene scores linked to low serum carotenoid status and their associations with metabolic disturbance and depressive symptoms in African–American adults. Br. J. Nutr. 2014, 112, 992–1003. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, S.-J.; Han, C.; Patkar, A.A.; Masand, P.S.; Pae, C.-U. Oxidative/nitrosative stress and antidepressants: Targets for novel antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 224–235. [Google Scholar] [CrossRef]
- Khanzode, S.D.; Dakhale, G.N.; Khanzode, S.S.; Saoji, A.; Palasodkar, R. Oxidative damage and major depression: The potential antioxidant action of selective serotonin reuptake inhibitors. Redox Rep. 2003, 8, 365–370. [Google Scholar] [CrossRef]
- Galecki, P.; Szemraj, J.; Bienkiewicz, M.; Zboralski, K.; Galecka, E. Oxidative stress parameters after combined fluoxetine and acetylsalicylic acid therapy in depressive patients. Hum. Psychopharmacol. 2009, 24, 277–286. [Google Scholar] [CrossRef]
- Herken, H.; Gurel, A.; Selek, S.; Armutcu, F.; Ozen, M.E.; Bulut, M.; Kap, O.; Yumru, M.; Savas, H.A.; Akyol, O. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: Impact of antidepressant treatment. Arch. Med. Res. 2007, 38, 247–252. [Google Scholar] [CrossRef]
- Cumurcu, B.E.; Ozyurt, H.; Etikan, I.; Demir, S.; Karlidag, R. Total antioxidant capacity and total oxidant status in patients with major depression: Impact of antidepressant treatment. Psychiatry Clin. Neurosci. 2009, 63, 639–645. [Google Scholar] [CrossRef]
- Kotan, V.O.; Sarandol, E.; Kirhan, E.; Ozkaya, G.; Kirli, S. Effects of long-term antidepressant treatment on oxidative status in major depressive disorder: A 24-week follow-up study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhu, K.; Xu, Q.; Wang, G.; Zhang, J.; Cao, R.; Ye, J.; Yu, X. The antidepressant-like effect of trans-astaxanthin involves the serotonergic system. Oncotarget 2017, 8, 25552–25563. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Oommen, S.; Kumar, A. Antidepressant-like activity of lutein-zeaxanthin in mice: Evidence for involvement of monoaminergic and opioid systems. Int. J. Pharm. Technol. 2017, 9, 28551–28566. [Google Scholar] [CrossRef]
- Kim, N.-R.; Kim, H.-Y.; Kim, M.-H.; Kim, H.-M.; Jeong, H.-J. Improvement of depressive behavior by Sweetme Sweet Pumpkin™ and its active compound, β- carotene. Life Sci. 2016, 147, 39–45. [Google Scholar] [CrossRef]
- Nouri, M.; Nasr-Esfahani, M.H.; Tarrahi, M.J.; Amani, R. The effect of lycopene supplementation on mood status and quality of life in infertile men: A randomized, double-blind, placebo-controlled clinical trial. Int. J. Fertil. Steril. 2020, 14, 17–22. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, Y.; Zhou, X.; Pan, W.; Shi, Y.; Wang, M.; Zhang, X.; Qi, D.; Li, L.; Ma, K. Depression-like behaviors and heme oxygenase-1 are regulated by Lycopene in lipopolysaccharide-induced neuroinflammation. J. Neuroimmunol. 2016, 15, 298. [Google Scholar] [CrossRef]
- Lin, H.Y.; Huang, B.R.; Yeh, W.L.; Lee, C.H.; Huang, S.S.; Lai, C.H.; Lin, H.; Lu, D.Y. Antineuroinflammatory effects of lycopene via activation of adenosine monophosphate-activated protein kinase-α1/heme oxygenase-1 pathways. Neurobiol. Aging 2014, 35, 191–202. [Google Scholar] [CrossRef]
- Felicity, N.; Michael, B.; Olivia, D.; Ashley, I.B. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar] [CrossRef]
- Ferret, P.J.; Soum, E.; Negre, O.; Wollman, E.E.; Fradelizi, D. Protective effect of thioredoxin upon NO- mediated cell injury in THP1 monocytic human cells. Biochem. J. 2000, 346, 759–765. [Google Scholar] [CrossRef]
- Hughes, M.N. Relationships between nitric oxide, nitroxyl ion, nitrosonium cation and peroxynitrite. Biochim. Biophys. Acta 1999, 1411, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our current understanding of a universal biological process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Farhadnejad, H.; Neshatbini Tehrani, A.; Salehpour, A.; Hekmatdoost, A. Antioxidant vitamin intakes and risk of depression, anxiety and stress among female adolescents. Clin. Nutr. ESPEN 2020, 40, 257–262. [Google Scholar] [CrossRef]
- Boskovic, M.; Vovk, T.; Kores Plesnicar, B.; Grabnar, I. Oxidative stress in schizophrenia. Curr. Neuropharmacol. 2011, 9, 301–312. [Google Scholar] [CrossRef]
- Casademont, J.; Garrabou, G.; Miro, O.; Lopez, S.; Pons, A.; Bernardo, M.; Cardellach, F. Neuroleptic treatment effect on mitochondrial electron transport chain: Peripheral blood mononuclear cells analysis in psychotic patients. J. Clin. Psychopharmacol. 2007, 27, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Rezin, G.T.; Amboni, G.; Zugno, A.I.; Quevedo, J.; Streck, E.L. Mitochondrial dysfunction and psychiatric disorders. Neurochem. Res. 2009, 34, 1021–1029. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W.J.H. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2014, 51C, 164–175. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef]
- Janicki-Deverts, D.; Cohen, S.; Matthews, K.A.; Gross, M.D.; Jacobs, D.R.J. Socioeconomic status, antioxidant micronutrients, and correlates of oxidative damage: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom. Med. 2009, 71, 541–548. [Google Scholar] [CrossRef]
- Glassman, A.H.; Helzer, J.E.; Covey, L.S.; Cottler, L.B.; Stetner, F.; Tipp, J.E.; Johnson, J. Smoking, smoking cessation, and major depression. JAMA 1990, 264, 1546–1549. [Google Scholar] [CrossRef]
- Abu-Omar, K.; Rutten, A.; Lehtinen, V. Mental health and physical activity in the European Union. Soz. Praventivmed. 2004, 49, 301–309. [Google Scholar] [CrossRef]
- Sullivan, L.E.; Fiellin, D.A.; O’Connor, P.G. The prevalence and impact of alcohol problems in major depression: A systematic review. Am. J. Med. 2005, 118, 330–341. [Google Scholar] [CrossRef]
- Handelman, G.J.; Packer, L.; Cross, C.E. Destruction of tocopherols, carotenoids, and retinol in human plasma by cigarette smoke. Am. J. Clin. Nutr. 1996, 63, 559–565. [Google Scholar] [CrossRef]
- Alberg, A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology 2002, 180, 121–137. [Google Scholar] [CrossRef]
- Kodydková, J.; Vávrová, L.; Zeman, M.; Jirák, R.; Macásek, J.; Stanková, B.; Tvrzická, E.; Zák, A. Antioxidative enzymes and increased oxidative stress in depressive women. Clin. Biochem. 2009, 42, 1368–1374. [Google Scholar] [CrossRef]
- Maes, M.; Mihaylova, I.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Increased 8-hydroxy-deoxyguanosine, a marker of oxidative damage to DNA, in major depression and myalgic encephalomyelitis / chronic fatigue syndrome. Neuro. Endocrinol. Lett. 2009, 30, 15–22. [Google Scholar]
- Abshirini, M.; Siassi, F.; Koohdani, F.; Qorbani, M.; Mozaffari, H.; Aslani, Z.; Soleymani, M.; Entezarian, M.; Sotoudeh, G. Dietary total antioxidant capacity is inversely associated with depression, anxiety and some oxidative stress biomarkers in postmenopausal women: A cross-sectional study. Ann. Gen. Psychiatr. 2019, 18, 3. [Google Scholar] [CrossRef]
- Daneshzad, E.; Keshavarz, S.-A.; Qorbani, M.; Larijani, B.; Azadbakht, L. Dietary total antioxidant capacity and its association with sleep, stress, anxiety, and depression score: A cross-sectional study among diabetic women. Clin. Nutr. ESPEN 2020, 37, 187–194. [Google Scholar] [CrossRef]
- Payne, M.E.; Steck, S.E.; George, R.R.; Steffens, D.C. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J. Acad. Nutr. Diet. 2012, 112, 2022–2027. [Google Scholar] [CrossRef]
- Owen, A.; Batterham, M.; Probst, Y.; Grenyer, B.; Tapsell, L.C. Low plasma vitamin E levels in major depression: Diet or disease? Eur. J. Clin. Nutr. 2005, 59, 304. [Google Scholar] [CrossRef]
- Lobato, K.R.; Cardoso, C.C.; Binfare, R.W.; Budni, J.; Wagner, C.L.; Brocardo, P.S.; de Souza, L.F.; Brocardo, C.; Flesch, S.; Freitas, A.E.; et al. a-Tocopherol administration produces an antidepressant-like effect in predictive animal models of depression. Behav. Brain Res. 2010, 209, 249–259. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 3, CD007176. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for preventing gastrointestinal cancers. Cochrane Database Syst. Rev. 2008, 3, CD004183. [Google Scholar] [CrossRef]
- Cortés-Jofré, M.; Rueda, J.-R.; Corsini-Muñoz, G.; Fonseca-Cortés, C.; Caraballoso, M.; Bonfill Cosp, X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2012, 10, CD002141. [Google Scholar] [CrossRef]
- Satia, J.A.; Littman, A.; Slatore, C.G.; Galanko, J.A.; White, E. Long-term use of betacarotene, retinol, lycopene, and lutein supplements and lung cancer risk: Results from the VITamins and Lifestyle (VITAL) study. Am. J. Epidemiol. 2009, 169, 815–828. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of microparticles of carotenoids from natural and syn- thetic sources for applications in food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Nile, S.H.; Park, W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 5281235, Beta-Cryptoxanthin” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/beta-Cryptoxanthin (accessed on 26 January 2022).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 5281243, Lutein” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lutein (accessed on 26 January 2022).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 5280899, Zeaxanthin” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Zeaxanthin (accessed on 26 January 2022).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 5280489, Beta-Carotene” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/beta-Carotene (accessed on 26 January 2022).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 6419725, Alpha-Carotene” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Carotene (accessed on 26 January 2022).
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 446925, Lycopene” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lycopene (accessed on 26 January 2022).
- Pechinskii, S.V.; Kuregyan, A.G. Molecular-biological problems of drug design and mechanism of drug action. Pharmaceut. Chem. J. 2014, 47, 509–513. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Lu, Q.Y. Overview of mechanisms of action of lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.A.; Lim, J.Y.; Kim, Y.; Jung, C.H.; Yoo, S.H.; Kim, Y. β-Carotene inhibits neuroblastoma cell invasion and metastasis in vitro and in vivo by decreasing level of hypoxia-inducible factor-1α. J. Nutr. Biochem. 2014, 25, 655–664. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, H.; Lin, C.; Che, J.; Tian, X.; Han, S.; Zhao, H.; Zhu, Y.; Mao, D. Associations between antioxidant vitamins and the risk of invasive cervical cancer in chinese women: A case–control study. Sci. Rep. 2015, 5, 13607. [Google Scholar] [CrossRef]
- Karppi, J.; Kurl, S.; Laukkanen, J.A.; Kauhanen, J. Serum betacarotene in relation to risk of prostate cancer: The kuopio ischaemic heart disease risk factor study. Nutr. Cancer 2012, 64, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef]
- Seyedzadeh, M.H.; Safari, Z.; Zare, A.; Gholizadeh Navashenaq, J.; Razavi, S.A.; Kardar, G.A. Study of curcumin immunomodulatory effects on reactive astrocyte cell function. Int. Immunopharmacol. 2014, 22, 230–235. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Honarvar, N.M.; Saedisomeolia, A.; Abdolahi, M.; Shayeganrad, A.; Sangsari, G.T.; Rad, B.H.; Muench, G. Molecular anti-inflammatory mechanisms of retinoids and carotenoids in Alzheimer’s disease: A review of current evidence. J. Mol. Neurosci. 2017, 61, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Davinelli, S.; Scapagnini, G.; Willcox, B.J.; Allsopp, R.C.; Willcox, D.C. Astaxanthin as a putative geroprotector: Molecular basis and focus on brain aging. Mar. Drugs 2020, 18, 351. [Google Scholar] [CrossRef]
- Sujak, A.; Gabrielska, J.; Grudzinski, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef]
- Gruszecki, W. Carotenoid orientation: Role in membrane stabilization. In Carotenoids in Health and Disease, 1st ed.; Norman, I.K., Susan, T.M., Helmut, S., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 170–183. [Google Scholar]
- Stahl, W.; Sies, H. Effects of carotenoids and retinoids on gap junctional communication. BioFactors 2001, 15, 95–98. [Google Scholar] [CrossRef]
- Akbaraly, N.T.; Faure, H.; Gourlet, V.; Favier, A.; Berr, C. Plasma carotenoid levels and cognitive performance in an elderly population: Results of the EVA study. J. Gerontol. Ser. A 2007, 62, 308–316. [Google Scholar] [CrossRef]
- Christensen, K.; Gleason, C.E.; Mares, J.A. Dietary carotenoids and cognitive function among US adults, NHANES 2011–2014. Nutr. Neurosci. 2020, 23, 554–562. [Google Scholar] [CrossRef]
- Prohan, M.; Amani, R.; Nematpour, S.; Jomehzadeh, N.; Haghighizadeh, M.H. Total antioxidant capacity of diet and serum, dietary antioxidant vitamins intake, and serum hs-CRP levels in relation to depression scales in university male students. Redox Rep. 2014, 19, 133–139. [Google Scholar] [CrossRef]
- Gautam, M.; Agrawal, M.; Gautam, M.; Sharma, P.; Gautam, A.S.; Gautam, S. Role of antioxidants in generalised anxiety disorder and depression. Indian J. Psychiatr. 2012, 54, 244. [Google Scholar] [CrossRef]
- Yu, L.; Wang, W.; Pang, W.; Xiao, Z.; Jiang, Y.; Hong, Y. Dietary lycopene supplementation improves cognitive performances in Tau transgenic mice expressing P301L mutation via inhibiting oxidative stress and Tau hyperphosphorylation. J. Alzheimers Dis. 2017, 57, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Li, L.; Chen, C.; Li, M.; Pei, L.; Chu, F.; Yang, J.; Yu, Z.; Wang, D.; Zhou, Z. Protective effects of lycopene against amyloid β-induced neurotoxicity in cultured rat cortical neurons. Neurosci. Lett. 2011, 21, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Mehrotra, A.; Kamboj, S.S. Lycopene prevents 3-nitropropionic acid-induced mitochondrial oxidative stress and dysfunctions in nervous system. Neurochem. Int. 2010, 57, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Prema, A.; Janakiraman, U.; Manivasagam, T.; Thenmozhi, A.J. Neuroprotective effect of lycopene against MPTP induced experimental Parkinson’s disease in mice. Neurosci. Lett. 2015, 10, 599. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Lei, L.; Zhang, Z.; Cheng, Y. Neuroprotective effects of lycopene pretreatment on transient global cerebral ischemia reperfusion in rats: The role of the Nrf2/HO-1 signaling pathway. Mol. Med. Rep. 2016, 13, 412–418. [Google Scholar] [CrossRef]
- Fujita, K.; Yoshimoto, N.; Kato, T.; Imada, H.; Matsumoto, G.; Inakuma, T.; Nagata, Y.; Miyachi, E. Lycopene inhibits ischemia/reperfusion-induced neuronal apoptosis in gerbil hippocampal tissue. Neurochem. Res. 2013, 38, 461–469. [Google Scholar] [CrossRef]
- Karppi, J.; Laukkanen, J.A.; Sivenius, J.; Ronkainen, K.; Kurl, S. Serum lycopene decreases the risk of stroke in men: A population-based follow-up study. Neurology 2012, 9, 1540–1547. [Google Scholar] [CrossRef]
- Roberts, R.L.; Green, J.; Lewis, B. Lutein and zeaxanthin in eye and skin health. Clin. Dermatol. 2009, 27, 195–201. [Google Scholar] [CrossRef]
- Vishwanathan, R.; Neuringer, M.; Snodderly, D.M.; Schalch, W.; Johnson, E.J. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr. Neurosci. 2013, 16, 21–29. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W. Zeaxanthin and Lutein: Photoprotectors, anti-inflammatories, and brain food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Johnson, E.J.; Vishwanathan, R.; Scott, T.M.; Schalch, W.; Wittwer, J.; Hausman, D.B.; Davey, A.; Johnson, M.A.; Green, R.C.; Gearing, M.; et al. Serum carotenoids as a biomarker for carotenoid concentrations in the brain. FASEB J. 2011, 25, 3442. [Google Scholar] [CrossRef]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Joffe, S.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between serum and brain carotenoids, -tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013, 951786. [Google Scholar] [CrossRef]
- Tanprasertsuk, M.J.; Mohn, P.E.S.; Matthan, P.N.R.; Lichtenstein, D.A.H.; Barger, P.K.; Vishwanathan, P.R.; Johnson, M.A.; Poon, P.L.W.; Johnson, E.J. Serum carotenoids, tocopherols, total n-3 polyunsaturated fatty acids, and n-6/n-3 polyunsaturated fatty acid ratio reflect brain concentrations in a cohort of centenarians. J. Gerontol. Ser. A 2019, 74, 306–314. [Google Scholar] [CrossRef]
- Wang, M.; Jiao, J.; Li, Z.; Liu, R.; Shi, Q.; Ma, L. Lutein supplementation reduces plasma lipid peroxidation and C-reactive protein in healthy nonsmokers. Atherosclerosis 2013, 227, 380–385. [Google Scholar] [CrossRef]
- Dwyer, J.H.; Navab, M.; Dwyer, K.M.; Hassan, K.; Sun, P.; Shircore, A.; Hama-Levy, S.; Hough, G.; Wang, X.; Drake, T.; et al. Oxygenated carotenoid lutein and progression of early atherosclerosis: The Los Angeles atherosclerosis study. Circulation 2001, 103, 2922–2927. [Google Scholar] [CrossRef]
- Kim, H. Inhibitory mechanism of lycopene on cytokine expression in experimental pancreatitis. Ann. NY Acad. Sci. 2011, 1229, 99–102. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Beal, M.F. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005, 58, 495–505. [Google Scholar] [CrossRef]
- Patten, D.A.; Germain, M.; Kelly, M.A.; Slack, R.S. Reactive oxygen species: Stuck in the middle of neurodegeneration. J. Alzheimers Dis. 2010, 20, 357–367. [Google Scholar] [CrossRef]
- Lorenzetti, V.; Allen, N.B.; Fornito, A.; Yücel, M. Structural brain abnormalities in major depressive disorder: A selective review of recent MRI studies. J. Affect. Disord. 2009, 117, 1–17. [Google Scholar] [CrossRef]
- Videbech, P.; Ravnkilde, B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am. J. Psychiatry 2004, 161, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Bocchio-Chiavetto, L.; Bagnardi, V.; Zanardini, R.; Molteni, R.; Gabriela Nielsen, M.; Placentino, A.; Giovannini, C.; Rillosi, L.; Ventriglia, M.; Riva, M.A.; et al. Serum and plasma BDNF levels in major depression: A replication study and meta-analyses. World J. Biol. Psychiatry 2010, 11, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; Bus, B.A.; Spinhoven, P.; Penninx, B.W.; Kenis, G.; Prickaerts, J.; Oude Voshaar, R.C.; Elzinga, B.M. Serum levels of brain-derived neurotrophic factor in major depressive disorder: State-trait issues, clinical features and pharmacological treatment. Mol. Psychiatry 2011, 16, 1088–1095. [Google Scholar] [CrossRef]
- Zhou, X.; Gan, T.; Fang, G.; Wang, S.; Mao, Y.; Ying, C. Zeaxanthin improved diabetes-induced anxiety and depression through inhibiting inflammation in hippocampus. Metab. Brain Dis. 2018, 33, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Jaiswal, V.; Lee, H.J. Dietary intake of flavonoids and carotenoids is associated with anti-depressive symptoms: Epidemiological study and in silico-mechanism analysis. Antioxidants 2021, 11, 53. [Google Scholar] [CrossRef]
- Forlenza, M.J.; Miller, G.E. Increased serum levels of 8-hydroxy-2’-deoxyguanosine in clinical depression. Psychosom. Med. 2006, 68, 1–7. [Google Scholar] [CrossRef]
- Li, D.; Li, Y. Associations of alpha-carotenoid and beta-carotenoid with depressive symptoms in late midlife women. J. Affect. Disord. 2019, 256, 424–430. [Google Scholar] [CrossRef]
- Ge, H.; Yang, T.; Sun, J.; Zhang, D. Associations between dietary carotenoid intakes and the risk of depressive symptoms. Food Nutr. Res. 2020, 28, 64. [Google Scholar] [CrossRef]
- Widomska, J.; Zareba, M.; Subczynski, W.K. Can xanthophyll-membrane interactions explain their selective presence in the retina and brain? Foods 2016, 5, 7. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Fanelli-Kuczmarski, M.T.; Kitner-Triolo, M.H.; Beydoun, H.A.; Kaufman, J.S.; Mason, M.A.; Evans, M.K.; Zonderman, A.B. Dietary antioxidant intake and its association with cognitive function in an ethnically diverse sample of US adults. Psychosom. Med. 2015, 77, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.S.; Oldmeadow, C.; Hure, A.J.; McEvoy, M.; Hiles, S.A.; Boyle, M.; Attia, J. Inflammation mediates the association between fatty acid intake and depression in older men and women. Nutr. Res. 2016, 36, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Davinelli, S.; Drago, F.; De Lorenzo, A.; Oriani, G. Antioxidants as antidepressants: Fact or fiction? CNS Drugs 2012, 26, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Yager, S.; Forlenza, M.J.; Miller, G.E. Depression and oxidative damage to lipids. Psychoneuroendocrinology 2010, 35, 1356–1362. [Google Scholar] [CrossRef]
- Socaciu, C.; Jessel, R.; Diehl, H.A. Carotenoid incorporation into microsomes: Yields, stability and membrane dynamics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2000, 56, 2799–2809. [Google Scholar] [CrossRef]
- Alpert, J.E.; Mischoulon, D.; Nierenberg, A.A.; Fava, M. Nutrition and depression: Focus on folate. Nutrition 2000, 16, 544–546. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Bandinelli, S.; Penninx, B.W.; Vogelzangs, N.; Corsi, A.M.; Lauretani, F.; Kisialiou, A.; Vazzana, R.; Terracciano, A.; Guralnik, J.M.; et al. Depressive symptoms and inflammation increase in a prospective study of older adults: A protective effect of a healthy (Mediterranean- style) diet. Mol. Psychiatry 2010, 16, 589–590. [Google Scholar] [CrossRef]
- Xavier, A.A.; Pérez-Gálvez, A. Carotenoids as a source of antioxidants in the diet. Subcell. Biochem. 2016, 79, 359–375. [Google Scholar] [CrossRef]
- Bhosale, P.; Li, B.; Sharifzadeh, M.; Gellermann, W.; Frederick, J.M.; Tsuchida, K.; Bernstein, P.S. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry 2009, 48, 4798–4807. [Google Scholar] [CrossRef]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Yokoyama, T.; Ohya, Y.; Fukushima, W.; Saito, K.; Ohfuji, S.; Kiyohara, C.; Hirota, Y. Dietary folate and vitamins B12, B6, and B2 intake and the risk of postpartum depression in Japan: The Osaka Maternal and Child Health Study. J. Affect. Disord. 2006, 96, 133–138. [Google Scholar] [CrossRef]
- Maes, M.; De Vos, N.; Pioli, R.; Demedts, P.; Wauters, A.; Neels, H.; Christophe, A. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J. Affect. Disord. 2000, 58, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Guo, H.; Kakizaki, M.; Cui, Y.; Ohmori-Matsuda, K.; Guan, L.; Hozawa, A.; Kuriyama, S.; Tsuboya; Ohrui, T.; et al. A tomato-rich diet is related to depressive symptoms among an elderly population aged 70 years and over: A population-based, cross-sectional analysis. J. Affect. Disord. 2013, 144, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Atessahin, A.; Yilmaz, S.; Karahan, I.; Ceribasi, A.O.; Karaoglu, A. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology 2005, 212, 116–123. [Google Scholar] [CrossRef]
- Shibata, H.; Kumagai, S.; Watanabe, S.; Suzuki, T. Relationship of serum cholesterols and vitamin E to depressive status in the elderly. J. Epidemiol. 1999, 9, 261–267. [Google Scholar] [CrossRef]
- Diniz, B.S.; Butters, M.A.; Albert, S.M.; Dew, M.A.; Reynolds, C.F. Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry 2013, 202, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Galts, C.P.C.; Bettio, L.E.B.; Jewett, D.C.; Yang, C.C.; Brocardo, P.S.; Rodrigues, A.L.S.; Thacker, J.S.; Gil-Mohapel, J. Depression in neurodegenerative diseases: Common mechanisms and current treatment options. Neurosci. Biobehav. Rev. 2019, 102, 56–84. [Google Scholar] [CrossRef]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef]
- Lin, P.; Ren, Q.; Wang, Q.; Wu, J. Carotenoids inhibit fructose-induced inflammatory response in human endothelial cells and monocytes. Mediat. Inflamm. 2020, 2020, 5373562. [Google Scholar] [CrossRef]
- Nakamura, M.; Sugiura, M. Serum lutein and zeaxanthin are inversely associated with high-sensitivity C-reactive protein in non-smokers: The Mikkabi Study. Antioxidants 2022, 11, 259. [Google Scholar] [CrossRef]
- Mullan, K.; Cardwell, C.R.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Plasma antioxidant status in patients with Alzheimer’s disease and cognitively intact elderly: A meta-analysis of case-control studies. J. Alzheimers Dis. 2018, 62, 305–317. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Fanelli-Kuczmarski, M.T.; Weiss, J.; Hossain, S.; Canas, J.A.; Evans, M.K.; Zonderman, A.B. Association of serum antioxidant vitamins and carotenoids with incident Alzheimer disease and all-cause dementia among US adults. Neurology 2022, 98, e2150–e2162. [Google Scholar] [CrossRef] [PubMed]
- Balendra, V.; Singh, S.K. Therapeutic potential of astaxanthin and superoxide dismutase in Alzheimer’s disease. Open Biol. 2021, 11, 210013. [Google Scholar] [CrossRef]
- Eroglu, A.; Al’Abri, I.S.; Kopec, R.E.; Crook, N.; Bohn, T. Carotenoids and their health benefits as derived via their interactions with gut microbiota. Adv. Nutr. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, T.; Sugimoto, M.; Ikeda, S.; Kume, S. Effects of astaxanthin-enriched yeast on mucosal IgA induction in the jejunum and ileum of weanling mice. Anim. Sci. J. 2014, 85, 449–453. [Google Scholar] [CrossRef]
- Kabir, M.T.; Rahman, M.H.; Shah, M.; Jamiruddin, M.R.; Basak, D.; Al-Harrasi, A.; Bhatia, S.; Ashraf, G.M.; Najda, A.; El-Kott, A.F.; et al. Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomed. Pharmacother. 2022, 146, 112610. [Google Scholar] [CrossRef] [PubMed]
- Yucel, Y.; Tabur, S.; Gozeneli, O.; Kocarslan, S.; Seker, A.; Buyukaslan, H.; Şavik, E.; Aktumen, A.; Ozgonul, A.; Uzunkoy, A.; et al. The effects of lycopene on intestinal injury due to methotrexate in rats. Redox Rep. 2016, 21, 113–118. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192. [Google Scholar] [CrossRef]
- Li, L.; Krause, L.; Somerset, S. Associations between micronutrient intakes and gut microbiota in a group of adults with cystic fibrosis. Clin. Nutr. 2017, 36, 1097–1104. [Google Scholar] [CrossRef]
- Schmidt, K.M.; Haddad, E.N.; Sugino, K.Y.; Vevang, K.R.; Peterson, L.A.; Koratkar, R.; Gross, M.D.; Kerver, J.M.; Comstock, S.S. Dietary and plasma carotenoids are positively associated with alpha diversity in the fecal microbiota of pregnant women. J. Food Sci. 2021, 86, 602–613. [Google Scholar] [CrossRef]
- Wiese, M.; Bashmakov, Y.; Chalyk, N.; Nielsen, D.S.; Krych, L.; Kot, W.; Klochkov, V.; Pristensky, D.; Bandaletova, T.; Chernyshova, M.; et al. Prebiotic effect of lycopene and dark chocolate on gut microbiome with systemic changes in liver metabolism, skeletal muscles and skin in moderately obese persons. Biomed. Res. Int. 2019, 2019, 4625279. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dai, Z.; Shi, E.; Wan, P.; Chen, G.; Zhang, Z.; Xu, Y.; Gao, R.; Zeng, X.; Li, D. Study on the interaction between β-carotene and gut microflora using an in vitro fermentation model. Food Sci. Hum. Wellness 2023, 12, 1369–1378. [Google Scholar] [CrossRef]
- Ramos, A.F.O.; Terry, S.A.; Holman, D.B.; Breves, G.; Pereira, L.G.R.; Silva, A.G.M.; Chaves, A.V. Tucumã oil shifted ruminal fermentation, reducing methane production and altering the microbiome but decreased substrate digestibility within a RUSITEC fed a mixed hay–concentrate diet. Front. Microbiol. 2018, 26, 1647. [Google Scholar] [CrossRef] [PubMed]
- Stevens, Y.; Pinheiro, I.; Salden, B.; Duysburgh, C.; Bolca, S.; Degroote, J.; Majdeddin, M.; Van Noten, N.; Gleize, B.; Caris-Veyrat, C.; et al. Effect of a carotenoid-producing Bacillus strain on intestinal barrier integrity and systemic delivery of carotenoids: A randomised trial in animals and humans. J. Funct. Foods 2021, 80, 104445. [Google Scholar] [CrossRef]
- Liu, M.; Li, W.; Chen, Y.; Wan, X.; Wang, J. Fucoxanthin: A promising compound for human inflammation-related diseases. Life Sci. 2020, 255, 117850. [Google Scholar] [CrossRef]
- Guo, B.; Yang, B.; Pang, X.; Chen, T.; Chen, F.; Cheng, K.W. Fucoxanthin modulates cecal and fecal microbiota differently based on diet. Food Funct. 2019, 10, 5644–5655. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef]
- Yu, Q.; Xue, F.; Li, Z.; Li, X.; Ai, L.; Jin, M.; Xie, M.; Yu, Y. Dietary intake of carotenoids and risk of depressive symptoms: A systematic review and meta-analysis. Antioxidants 2022, 11, 2205. [Google Scholar] [CrossRef]
- Li, Z.; Hao, Y.; Han, Y.; Wu, S.; Zhu, D.; Liu, M.; Dong, Q.; Wang, X.; Guan, Y. Prevalence and associated physical symptoms of depressive and anxiety symptoms in neurology outpatient clinic. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1286–1287. [Google Scholar] [CrossRef]
- Li, F.; Xiang, H.; Lu, J.; Chen, Z.; Huang, C.; Yuan, X. Lycopene ameliorates PTSD-like behaviors in mice and rebalances the neuroinflammatory response and oxidative stress in the brain. Physiol. Behav. 2020, 224, 113026. [Google Scholar] [CrossRef]
- Chen, D.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019, 111, 791–801. [Google Scholar] [CrossRef]
- Zhang, L.; Previn, R.; Lu, L.; Liao, R.-F.; Jin, Y.; Wang, R.-K. Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res. Bull. 2018, 142, 352–359. [Google Scholar] [CrossRef]
- Buniowska, M.; Arrigoni, E.; Znamirowska, A.; Blesa, J.; Frígola, A.; Esteve, M.J. Liberation and micellarization of carotenoids from different smoothies after thermal and ultrasound treatments. Foods 2019, 8, 492. [Google Scholar] [CrossRef]
- Provesi, J.G.; Dias, C.O.; Amante, E.R. Changes in carotenoids during processing and storage of pumpkin puree. Food Chem. 2011, 128, 195–202. [Google Scholar] [CrossRef]
- Lemmens, L.; Colle, I.; Van Buggenhout, S.; Palmero, P.; Van Loey, A.; Hendrickx, M. Carotenoid bioaccessibility in fruit- and vegetable-based food products as affected by product (micro)structural characteristics and the presence of lipids: A review. Trends Food Sci. Technol. 2014, 38, 125–135. [Google Scholar] [CrossRef]
- Gheonea, I.; Aprodu, I.; Enachi, E.; Horincar, G.; Bolea, C.A.; Bahrim, G.E.; Râpeanu, G.; Stănciuc, N. Investigations on thermostability of carotenoids from tomato peels in oils using a kinetic approach. J. Food Process. Preserv. 2020, 44, e14303. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).