Expression of the H2O2 Biosensor roGFP-Tpx1.C169S in Fission and Budding Yeasts and Jurkat Cells to Compare Intracellular H2O2 Levels, Transmembrane Gradients, and Response to Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Plasmids Used in This Study
2.2. Fission and Budding Yeasts Growth Media and Genetic Manipulations
2.3. Growth Media for Jurkat Cells, and Selection of a Stable Cell Line Expressing roGFP2-Tpx1.C169S
2.4. Growth of Strains Expressing roGFP2-Tpx1.C169S for Fluorescence Determination
2.5. Live-Cell Measurements of Basal and Induced Oxidation of roGFP2-Tpx1.C169S
2.6. Western Blot of roGFP2-Tpx1.C169S
2.7. Microscopy
2.8. Statistics
3. Results
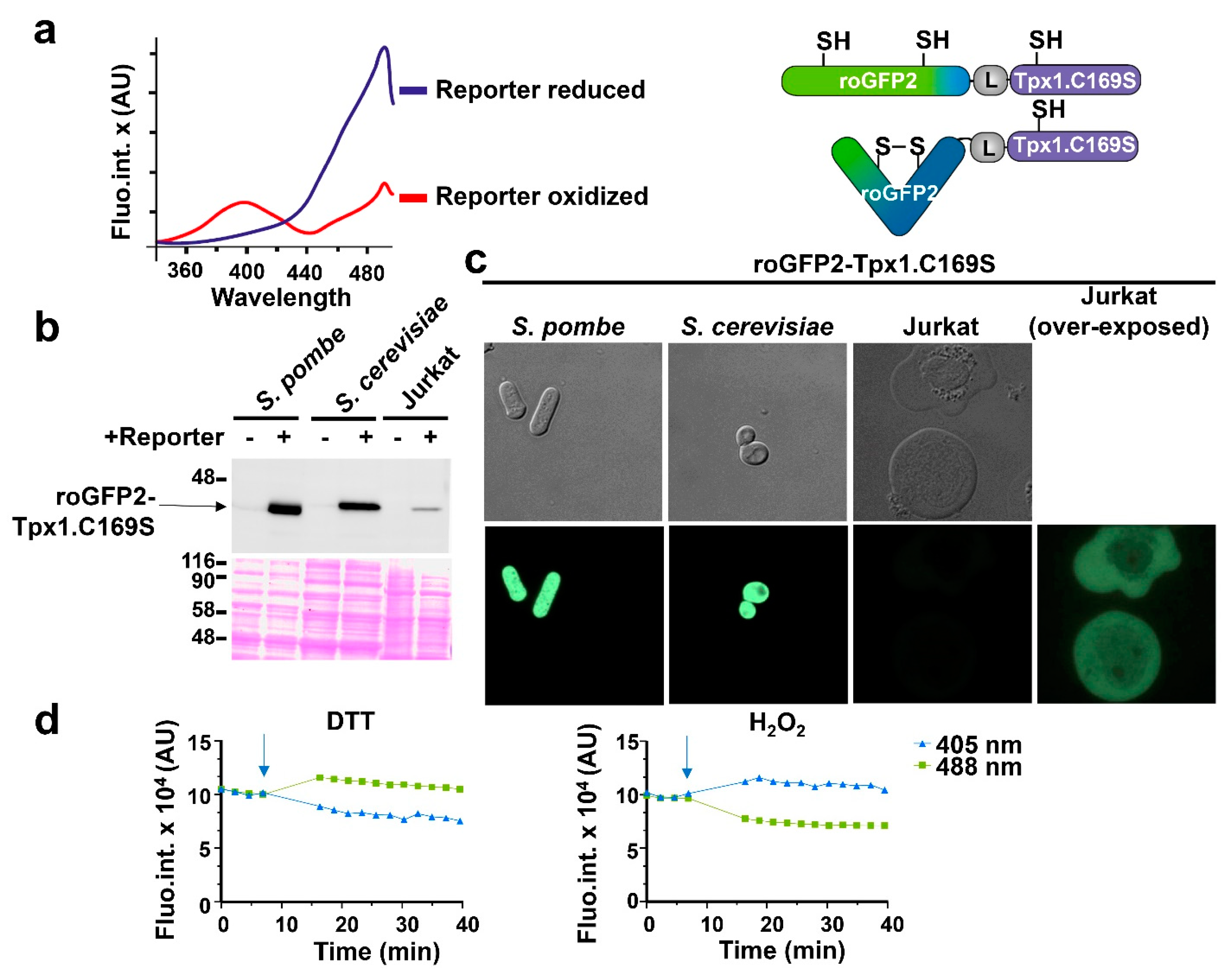
3.1. Expression of the H2O2 Biosensor roGFP2-Tpx1.C169S in Budding and Fission Yeast and in Jurkat Cells
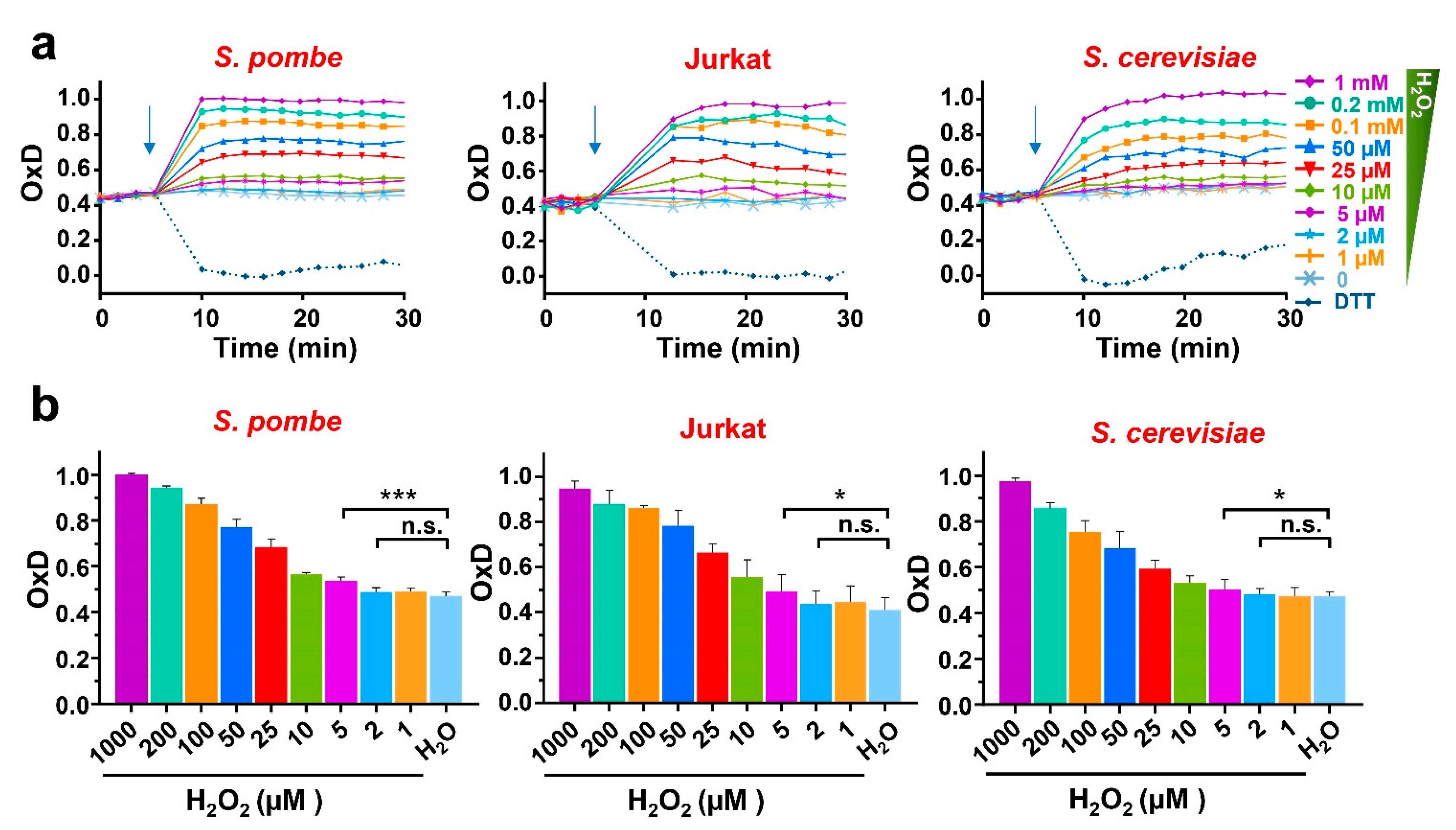
3.2. The Response to Extracellular Peroxides and the Steady-State Levels of H2O2 Are Very Similar in the Three Eukaryotic Models
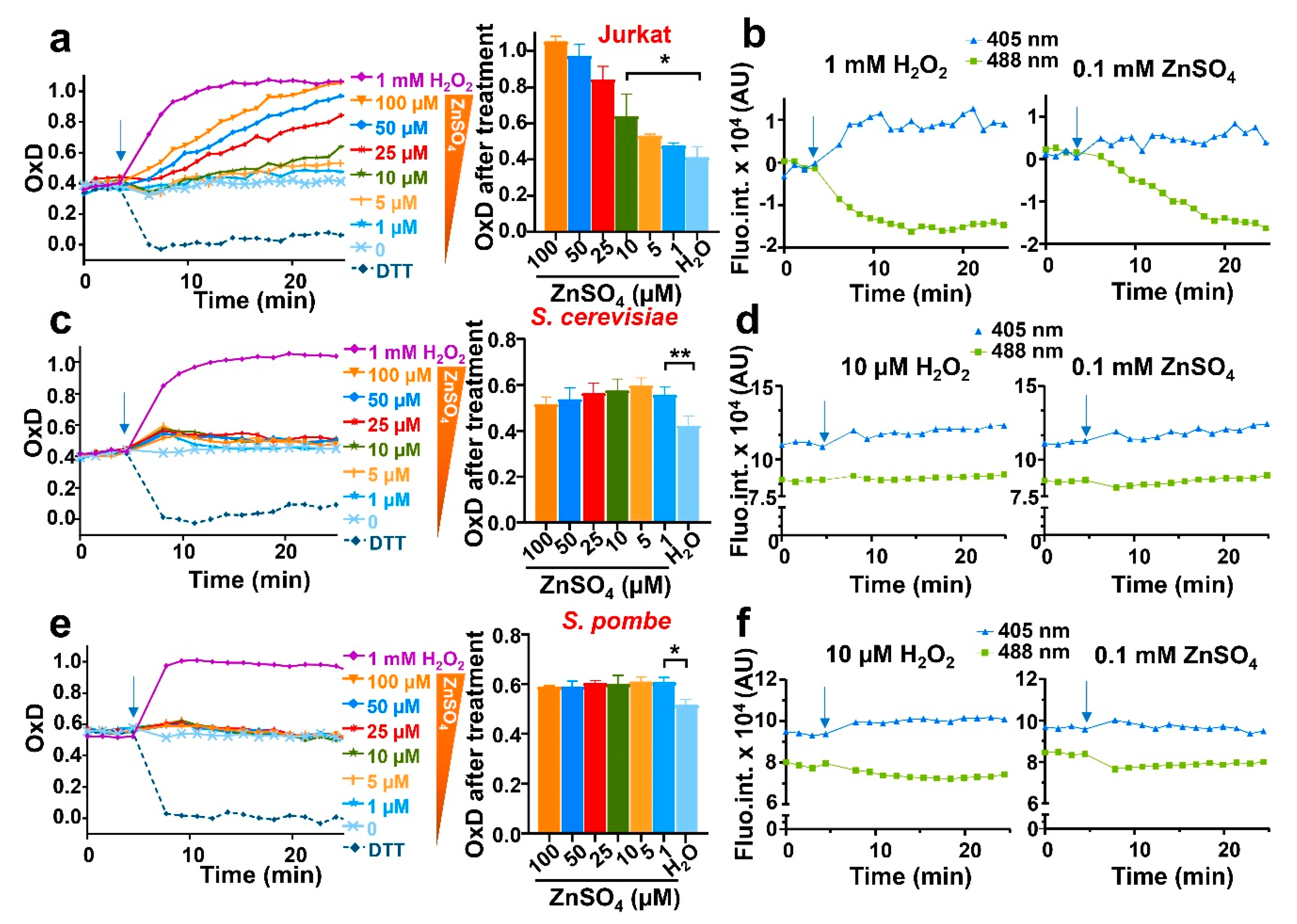
3.3. Addition of Extracellular Zinc Causes Intracellular Bursts of H2O2 in the Three Eukaryotic Models
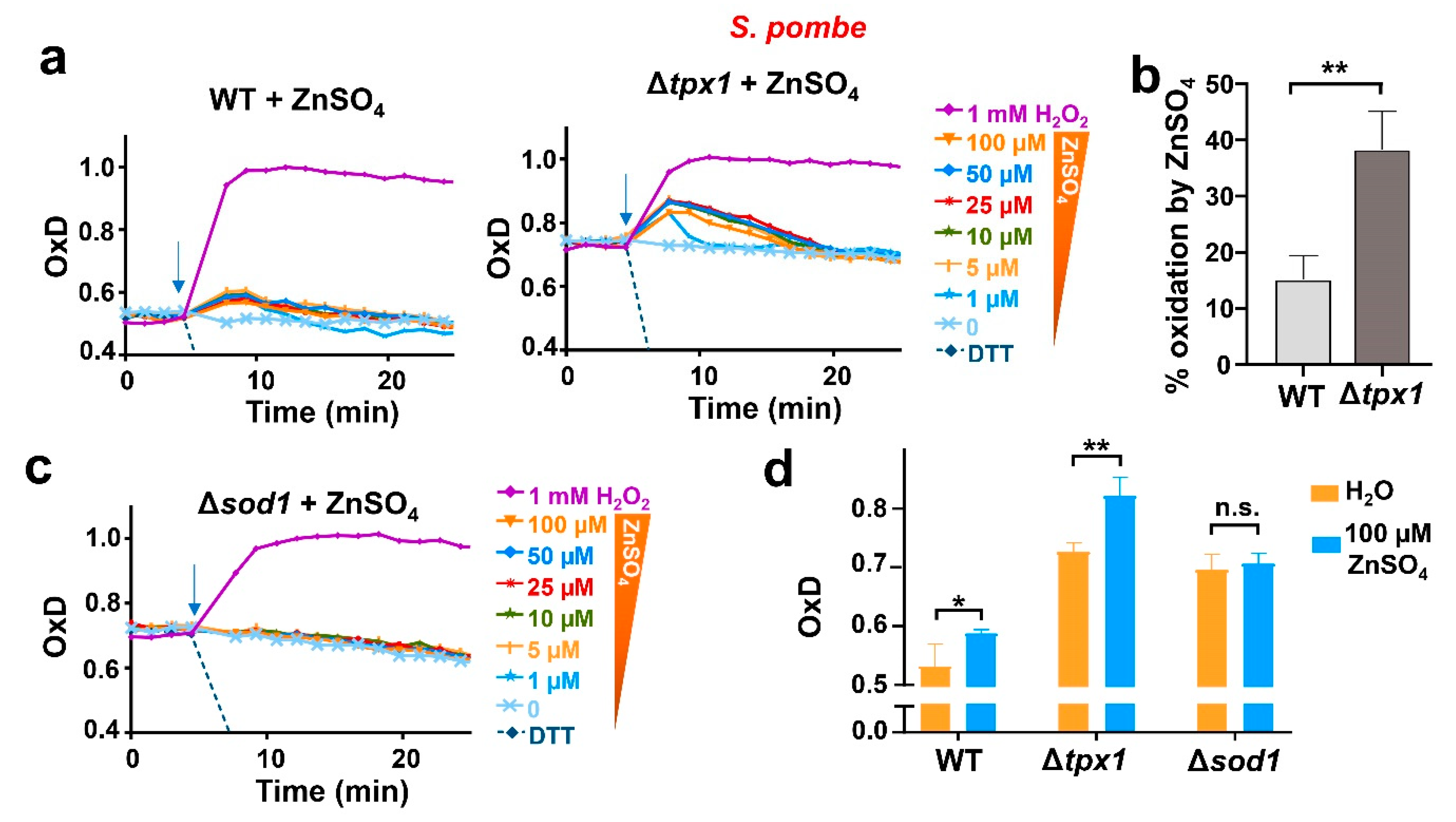
3.4. In Wild-Type Fission Yeast, Non-Toxic Zinc Causes a Sudden H2O2 Burst by Enhancing Cu/Zn Superoxide Dismutase Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- García-Santamarina, S.; Boronat, S.; Hidalgo, E. Reversible Cysteine Oxidation in Hydrogen Peroxide Sensing and Signal Transduction. Biochemistry 2014, 53, 2560–2580. [Google Scholar] [CrossRef] [PubMed]
- Stöcker, S.; Van Laer, K.; Mijuskovic, A.; Dick, T.P. The Conundrum of Hydrogen Peroxide Signaling and the Emerging Role of Peroxiredoxins as Redox Relay Hubs. Antioxid. Redox Signal. 2018, 28, 558–573. [Google Scholar] [CrossRef]
- Antunes, F.; Brito, P.M. Quantitative biology of hydrogen peroxide signaling. Redox Biol. 2017, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Weisenhorn, E.; MacDiarmid, C.W.; Andreini, C.; Bucci, M.; Taggart, J.; Banci, L.; Russell, J.; Coon, J.J.; Eide, D.J. The cellular economy of the Saccharomyces cerevisiae zinc proteome. Metallomics 2018, 10, 1755–1776. [Google Scholar] [CrossRef]
- Moreno, S.; Klar, A.; Nurse, P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991, 194, 795–823. [Google Scholar] [CrossRef]
- Boch, A.; Trampczynska, A.; Simm, C.; Taudte, N.; KrãMer, U.; Clemens, S. Loss of Zhf and the tightly regulated zinc-uptake system SpZrt1 in Schizosaccharomyces pombe reveals the delicacy of cellular zinc balance. FEMS Yeast Res. 2008, 8, 883–896. [Google Scholar] [CrossRef]
- Colomar-Carando, N.; Meseguer, A.; Company-Garrido, I.; Jutz, S.; Herrera-Fernández, V.; Olvera, A.; Kiefer, K.; Brander, C.; Steinberger, P.; Vicente, R. Zip6 Transporter Is an Essential Component of the Lymphocyte Activation Machinery. J. Immunol. 2019, 202, 441–450. [Google Scholar] [CrossRef]
- Pagani, M.A.; Casamayor, A.; Serrano, R.; Atrian, S.; Ariño, J. Disruption of iron homeostasis in Saccharomyces cerevisiae by high zinc levels: A genome-wide study. Mol. Microbiol. 2007, 65, 521–537. [Google Scholar] [CrossRef]
- Ryuko, S.; Ma, Y.; Ma, N.; Sakaue, M.; Kuno, T. Genome-wide screen reveals novel mechanisms for regulating cobalt uptake and detoxification in fission yeast. Mol. Genet. Genom. 2012, 287, 651–662. [Google Scholar] [CrossRef]
- Borrelly, G.P.M.; Harrison, M.D.; Robinson, A.K.; Cox, S.G.; Robinson, N.J.; Whitehall, S.K. Surplus Zinc Is Handled by Zym1 Metallothionein and Zhf Endoplasmic Reticulum Transporter in Schizosaccharomyces pombe. J. Biol. Chem. 2002, 277, 30394–30400. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Bloss, T.; Vess, C.; Neumann, D.; Nies, D.H.; Zur Nieden, U. A Transporter in the Endoplasmic Reticulum of Schizosaccharomyces pombe Cells Mediates Zinc Storage and Differentially Affects Transition Metal Tolerance. J. Biol. Chem. 2002, 277, 18215–18221. [Google Scholar] [CrossRef] [PubMed]
- Eide, D.J. Transcription factors and transporters in zinc homeostasis: Lessons learned from fungi. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 88–110. [Google Scholar] [CrossRef] [PubMed]
- Dainty, S.J.; Kennedy, C.A.; Watt, S.; Bähler, J.; Whitehall, S.K. Response of Schizosaccharomyces pombe to Zinc Deficiency. Eukaryot. Cell 2008, 7, 454–464. [Google Scholar] [CrossRef]
- Palmiter, R.D. Protection against zinc toxicity by metallothionein and zinc transporter 1. Proc. Natl. Acad. Sci. USA 2004, 101, 4918–4923. [Google Scholar] [CrossRef]
- Tarhan, C.; Pekmez, M.; Karaer, S.; Arda, N.; Sarikaya, A.T. The effect of superoxide dismutase deficiency on zinc toxicity in Schizosaccharomyces pombe. J. Basic Microbiol. 2007, 47, 506–512. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Cao, C.-L.; Liu, Y.-L.; Wang, J.; Li, J.; Li, S.-Y.; Deng, Y. Identification of the Genetic Requirements for Zinc Tolerance and Toxicity in Saccharomyces cerevisiae. G3 Genes Genomes Genet. 2020, 10, 479–488. [Google Scholar] [CrossRef]
- Dineley, K.E.; Richards, L.L.; Votyakova, T.V.; Reynolds, I.J. Zinc causes loss of membrane potential and elevates reactive oxygen species in rat brain mitochondria. Mitochondrion 2005, 5, 55–65. [Google Scholar] [CrossRef]
- Medvedeva, Y.V.; Weiss, J.H. Intramitochondrial Zn2+ accumulation via the Ca2+ uniporter contributes to acute ischemic neurodegeneration. Neurobiol. Dis. 2014, 68, 137–144. [Google Scholar] [CrossRef]
- Rudolf, E.; Rudolf, K.; Cervinka, M. Zinc induced apoptosis in HEP-2 cancer cells: The role of oxidative stress and mitochondria. Biofactors 2005, 23, 107–120. [Google Scholar] [CrossRef]
- Wu, W.; Bromberg, P.A.; Samet, J.M. Zinc ions as effectors of environmental oxidative lung injury. Free. Radic. Biol. Med. 2013, 65, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hou, X.; Fang, H.; Yang, X. Boronate-Based Fluorescent Probes as a Prominent Tool for H2O2 Sensing and Recognition. Curr. Med. Chem. 2022, 29, 2476–2489. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Ng, K.K.-H.; Hu, J.J.; Ye, S.; Yang, D. Small-Molecule-Based Fluorescent Sensors for Selective Detection of Reactive Oxygen Species in Biological Systems. Annu. Rev. Biochem. 2019, 88, 605–633. [Google Scholar] [CrossRef] [PubMed]
- Hanson, G.T.; Aggeler, R.; Oglesbee, D.; Cannon, M.; Capaldi, R.A.; Tsien, R.Y.; Remington, S.J. Investigating Mitochondrial Redox Potential with Redox-sensitive Green Fluorescent Protein Indicators. J. Biol. Chem. 2004, 279, 13044–13053. [Google Scholar] [CrossRef] [PubMed]
- Gutscher, M.; Sobotta, M.C.; Wabnitz, G.H.; Ballikaya, S.; Meyer, A.J.; Samstag, Y.; Dick, T.P. Proximity-based Protein Thiol Oxidation by H2O2-scavenging Peroxidases. J. Biol. Chem. 2009, 284, 31532–31540. [Google Scholar] [CrossRef]
- Morgan, B.; Van Laer, K.E.; Owusu, T.N.; Ezeriņa, D.; Pastor-Flores, D.; Amponsah, P.S.; Tursch, A.; Dick, T.P. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat. Chem. Biol. 2016, 12, 437–443. [Google Scholar] [CrossRef]
- Carmona, M.; de Cubas, L.; Bautista, E.; Moral-Blanch, M.; Medraño-Fernández, I.; Sitia, R.; Boronat, S.; Ayté, J.; Hidalgo, E. Monitoring cytosolic H2O2 fluctuations arising from altered plasma membrane gradients or from mitochondrial activity. Nat. Commun. 2019, 10, 4526. [Google Scholar] [CrossRef]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar] [CrossRef]
- Pak, V.V.; Ezeriņa, D.; Lyublinskaya, O.G.; Pedre, B.; Tyurin-Kuzmin, P.A.; Mishina, N.M.; Thauvin, M.; Young, D.; Wahni, K.; Martinez Gache, S.A.; et al. Ultrasensitive Genetically Encoded Indicator for Hydrogen Peroxide Identifies Roles for the Oxidant in Cell Migration and Mitochondrial Function. Cell Metab. 2020, 31, 642–653.e6. [Google Scholar] [CrossRef]
- Domènech, A.; Ayté, J.; Antunes, F.; Hidalgo, E. Using in vivo oxidation status of one- and two-component redox relays to determine H2O2 levels linked to signaling and toxicity. BMC Biol. 2018, 16, 61. [Google Scholar] [CrossRef]
- Jara, M.; Vivancos, A.P.; Calvo, I.A.; Moldon, A.; Sanso, M.; Hidalgo, E. The peroxiredoxin Tpx1 is essential as a H2O2 scavenger during aerobic growth in fission yeast. Mol. Biol. Cell. 2007, 18, 2288–2295. [Google Scholar] [CrossRef]
- Lyublinskaya, O.; Antunes, F. Measuring intracellular concentration of hydrogen peroxide with the use of genetically encoded H2O2 biosensor HyPer. Redox Biol. 2019, 24, 101200. [Google Scholar] [CrossRef]
- Akama-Garren, E.H.; Joshi, N.S.; Tammela, T.; Chang, G.P.; Wagner, B.L.; Lee, D.-Y.; Rideout, W.M., 3rd; Papagiannakopoulos, T.; Xue, W.; Jacks, T. A Modular Assembly Platform for Rapid Generation of DNA Constructs. Sci. Rep. 2016, 6, 16836. [Google Scholar] [CrossRef]
- Leupold, U. Genetical Methods for Schizosaccharomyces pombe. Methods Cell Physiol. 1970, 4, 169–177. [Google Scholar] [CrossRef]
- Calvo, I.A.; Boronat, S.; Domènech, A.; García-Santamarina, S.; Ayté, J.; Hidalgo, E. Dissection of a Redox Relay: H2O2-Dependent Activation of the Transcription Factor Pap1 through the Peroxidatic Tpx1-Thioredoxin Cycle. Cell Rep. 2013, 5, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Bahler, J.; Wu, J.Q.; Longtine, M.S.; Shah, N.G.; McKenzie, A., III; Steever, A.B.; Wach, A.; Philippsen, P.; Pringle, J.R. Heter-ologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 1998, 14, 943–951. [Google Scholar] [CrossRef]
- Carrillo-Garcia, J.; Herrera-Fernández, V.; Serra, S.A.; Rubio-Moscardo, F.; Vogel-Gonzalez, M.; Doñate-Macian, P.; Hevia, C.F.; Pujades, C.; Valverde, M.A. The mechanosensitive Piezo1 channel controls endosome trafficking for an efficient cytokinetic abscission. Sci. Adv. 2021, 7, eabi7785. [Google Scholar] [CrossRef] [PubMed]
- Salat-Canela, C.; Carmona, M.; Martín-García, R.; Pérez, P.; Ayté, J.; Hidalgo, E. Stress-dependent inhibition of polarized cell growth through unbalancing the GEF/GAP regulation of Cdc42. Cell Rep. 2021, 37, 109951. [Google Scholar] [CrossRef]
- Corral-Ramos, C.; Barrios, R.; Ayte, J.; Hidalgo, E. TOR and MAP kinase pathways synergistically regulate autophagy in re-sponse to nutrient depletion in fission yeast. Autophagy 2021, 18, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Liochev, S.I.; Fridovich, I. Cross-compartment protection by SOD1. Free. Radic. Biol. Med. 2005, 38, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. Int. Ed. Engl. 2021, 60, 9215–9246. [Google Scholar] [CrossRef] [PubMed]
- Peggion, C.; Scalcon, V.; MassimBetqeeno, M.L.; Nies, K.; Lopreiato, R.; Rigobello, M.P.; Bertoli, A. SOD1 in ALS: Taking Stock in Pathogenic Mechanisms and the Role of Glial and Muscle Cells. Antioxidants 2022, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Cicardi, M.E.; Marrone, L.; Azzouz, M.; Trotti, D. Proteostatic imbalance and protein spreading in amyotrophic lateral sclerosis. EMBO J. 2021, 40, e106389. [Google Scholar] [CrossRef] [PubMed]
- Marguerat, S.; Schmidt, A.; Codlin, S.; Chen, W.; Aebersold, R.; Bähler, J. Quantitative Analysis of Fission Yeast Transcriptomes and Proteomes in Proliferating and Quiescent Cells. Cell 2012, 151, 671–683. [Google Scholar] [CrossRef]
- de Cubas, L.; Pak, V.; Belousov, V.; Ayté, J.; Hidalgo, E. The Mitochondria-to-Cytosol H2O2 Gradient Is Caused by Peroxiredoxin-Dependent Cytosolic Scavenging. Antioxidants 2021, 10, 731. [Google Scholar] [CrossRef]
- Wages, P.A.; Silbajoris, R.; Speen, A.; Brighton, L.; Henriquez, A.; Tong, H.; Bromberg, P.A.; Simmons, S.O.; Samet, J.M. Role of H2O2 in the oxidative effects of zinc exposure in human airway epithelial cells. Redox Biol. 2014, 3, 47–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Cubas, L.; Mallor, J.; Herrera-Fernández, V.; Ayté, J.; Vicente, R.; Hidalgo, E. Expression of the H2O2 Biosensor roGFP-Tpx1.C169S in Fission and Budding Yeasts and Jurkat Cells to Compare Intracellular H2O2 Levels, Transmembrane Gradients, and Response to Metals. Antioxidants 2023, 12, 706. https://doi.org/10.3390/antiox12030706
de Cubas L, Mallor J, Herrera-Fernández V, Ayté J, Vicente R, Hidalgo E. Expression of the H2O2 Biosensor roGFP-Tpx1.C169S in Fission and Budding Yeasts and Jurkat Cells to Compare Intracellular H2O2 Levels, Transmembrane Gradients, and Response to Metals. Antioxidants. 2023; 12(3):706. https://doi.org/10.3390/antiox12030706
Chicago/Turabian Stylede Cubas, Laura, Jorge Mallor, Víctor Herrera-Fernández, José Ayté, Rubén Vicente, and Elena Hidalgo. 2023. "Expression of the H2O2 Biosensor roGFP-Tpx1.C169S in Fission and Budding Yeasts and Jurkat Cells to Compare Intracellular H2O2 Levels, Transmembrane Gradients, and Response to Metals" Antioxidants 12, no. 3: 706. https://doi.org/10.3390/antiox12030706
APA Stylede Cubas, L., Mallor, J., Herrera-Fernández, V., Ayté, J., Vicente, R., & Hidalgo, E. (2023). Expression of the H2O2 Biosensor roGFP-Tpx1.C169S in Fission and Budding Yeasts and Jurkat Cells to Compare Intracellular H2O2 Levels, Transmembrane Gradients, and Response to Metals. Antioxidants, 12(3), 706. https://doi.org/10.3390/antiox12030706








