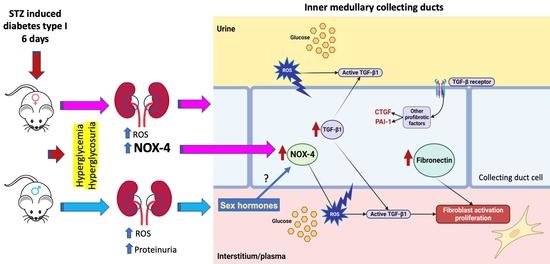
Increased Renal Medullary NOX-4 in Female but Not Male Mice during the Early Phase of Type 1 Diabetes: Potential Role of ROS in Upregulation of TGF-β1 and Fibronectin in Collecting Duct Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Samples
2.2. Blood Glucose Measurements
2.3. Sodium Measurement from Plasma and Urine
2.4. Blood Creatinine
2.5. Blood Pressure Measurements
2.6. Saline Challenge
2.7. Assays for Reactive Oxygen Species in Medullary Tissues
2.8. Fibronectin, TGF-β1 and NOX-4 Transcripts Quantitation by Real Time qRT-PCR
2.9. Immunoblotting Analyses
2.10. Primary Cultures of Inner Medullary Collecting Duct (IMCD) Cells
2.11. Immunofluorescence in Kidney Slides and IMCD Cells
2.12. M-1 Cell Culture
2.13. Statistical Analyses
3. Results
3.1. Physiological Parameters in Male and Female STZ Mice
3.2. Expression of TGF-β1, Fibronectin and NOX-4 in Renal Medullary Tissues from Male and Female Mice after 6 Days of Streptozotocin (STZ)-Induced Type 1 Diabetes
3.3. Expression of TGF-β1, Fibronectin and NOX-4 in Primary Cultured Inner Medullary Collecting Duct Cells Exposed to Normal and High Glucose Conditions
3.4. Effect of NOX-4 Inhibition on the mRNA Levels of Fibronectin and TGF-β1 in M-1 Collecting Duct Cell Line Exposed to High Glucose Conditions
4. Discussion
5. Limitation of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez-Castelao, A.; Navarro-Gonzalez, J.F.; Gorriz, J.L.; de Alvaro, F. The Concept and the Epidemiology of Diabetic Nephropathy Have Changed in Recent Years. J. Clin. Med. 2015, 4, 1207–1216. [Google Scholar] [CrossRef]
- Wolf, G. New insights into the pathophysiology of diabetic nephropathy: From haemodynamics to molecular pathology. Eur J. Clin. Investig. 2004, 34, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Breyer, M.D.; Bottinger, E.; Brosius, F.C.; Coffman, T.M.; Fogo, A.; Harris, R.C.; Heilig, C.W.; Sharma, K.; AMDCC. Diabetic nephropathy: Of mice and men. Adv. Chronic Kidney D 2005, 12, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharm. 2015, 70, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, D.; Kang, X.; Zhou, R.; Sun, Y.; Lian, F.; Tong, X. Signaling Pathways Involved in Diabetic Renal Fibrosis. Front. Cell Dev. Biol. 2021, 9, 696542. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakamura, T.; Noble, N.A.; Ruoslahti, E.; Border, W.A. Expression of Transforming Growth-Factor-Beta Is Elevated in Human and Experimental Diabetic Nephropathy. Proc. Natl. Acad. Sci. USA 1993, 90, 1814–1818. [Google Scholar] [CrossRef]
- Kolm-Litty, V.; Sauer, U.; Nerlich, A.; Lehmann, R.; Schleicher, E.D. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J. Clin. Investig. 1998, 101, 160–169. [Google Scholar] [CrossRef]
- Hong, S.W.; Isono, M.; Chen, S.; Iglesias-De La Cruz, M.C.; Han, D.C.; Ziyadeh, F.N. Increased glomerular and tubular expression of transforming growth factor-beta1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse. Am. J. Pathol. 2001, 158, 1653–1663. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Hohn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Sedeek, M.; Callera, G.; Montezano, A.; Gutsol, A.; Heitz, F.; Szyndralewiez, C.; Page, P.; Kennedy, C.R.J.; Burns, K.D.; Touyz, R.M.; et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: Implications in type 2 diabetic nephropathy. Am. J. Physiol.-Ren. 2010, 299, F1348–F1358. [Google Scholar] [CrossRef]
- Iwano, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. Curr. Opin. Nephrol. Hypertens. 2004, 13, 279–284. [Google Scholar] [CrossRef]
- Miao, X.J.; Bi, T.T.; Tang, J.M.; Lv, R.; Gui, D.K.; Yang, X.F. Regulatory mechanism of TGF-beta 1/SGK1 pathway in tubulointerstitial fibrosis of diabetic nephropathy. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10482–10488. [Google Scholar]
- Chen, Q.Q.; Su, Y.; Ju, Y.H.; Ma, K.K.; Li, W.Z.; Li, W.P. Astragalosides IV protected the renal tubular epithelial cells from free fatty acids-induced injury by reducing oxidative stress and apoptosis. Biomed. Pharm. 2018, 108, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.C.; Xu, X.X.; Tang, C.Y.; Gao, P.; Chen, X.H.; Xiong, X.F.; Yang, M.; Yang, S.K.; Zhu, X.J.; Yuan, S.G.; et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Lin, Y.H.; Hsieh, H.H.; Lin, J.J.; Peng, S.L. Sex Differences in the Effect of Diabetes on Cerebral Glucose Metabolism. Biomedicines 2021, 9, 1661. [Google Scholar] [CrossRef]
- Kim, B.; Kim, Y.Y.; Nguyen, P.T.T.; Nam, H.; Suh, J.G. Sex differences in glucose metabolism of streptozotocin-induced diabetes inbred mice (C57BL/6J). Appl. Biol. Chem. 2020, 63, 59. [Google Scholar] [CrossRef]
- McGuinness, O.P.; Ayala, J.E.; Laughlin, M.R.; Wasserman, D.H. NIH experiment in centralized mouse phenotyping: The Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E849–E855. [Google Scholar] [CrossRef] [PubMed]
- Stoos, B.A.; Narayfejestoth, A.; Carretero, O.A.; Ito, S.; Fejestoth, G. Characterization of a Mouse Cortical Collecting Duct Cell-Line. Kidney Int. 1991, 39, 1168–1175. [Google Scholar] [CrossRef][Green Version]
- Gonzalez, A.A.; Liu, L.; Lara, L.S.; Bourgeois, C.R.; Ibaceta-Gonzalez, C.; Salinas-Parra, N.; Gogulamudi, V.R.; Seth, D.M.; Prieto, M.C. PKC-alpha-dependent augmentation of cAMP and CREB phosphorylation mediates the angiotensin II stimulation of renin in the collecting duct. Am. J. Physiol. Ren. Physiol. 2015, 309, F880–F888. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Martinez, C.; Nguyen, Q.M.; Kassan, M.; Gonzalez, A.A. (Pro)renin Receptor-Dependent Induction of Profibrotic Factors Is Mediated by COX-2/EP4/NOX-4/Smad Pathway in Collecting Duct Cells. Front. Pharm. 2019, 10, 803. [Google Scholar] [CrossRef]
- Guerrero, A.; Visniauskas, B.; Cárdenas, P.; Figueroa, S.M.; Vivanco, J.; Salinas-Parra, N.; Araos, P.; Nguyen, Q.M.; Kassan, M.; Amador, C.A.; et al. α-Ketoglutarate Upregulates Collecting Duct (Pro)renin Receptor Expression, Tubular Angiotensin II Formation, and Na+ Reabsorption During High Glucose Conditions. Front. Cardiovasc. Med. 2021, 8, 644797. [Google Scholar] [CrossRef]
- Gobl, C.S.; Brannath, W.; Bozkurt, L.; Handisurya, A.; Anderwald, C.; Luger, A.; Krebs, M.; Kautzky-Willer, A.; Bischof, M.G. Sex-Specific Differences in Glycemic Control and Cardiovascular Risk Factors in Older Patients With Insulin-Treated Type 2 Diabetes Mellitus. Gend. Med. 2010, 7, 593–599. [Google Scholar] [CrossRef]
- Suys, B.E.; Katier, N.; Rooman, R.P.A.; Matthys, D.; De Beeck, L.O.; Du Caju, M.V.L.; De Wolf, D. Female children and adolescents with type 1 diabetes have more pronounced early echocardiographic signs of diabetic carcdiomyopathy. Diabetes Care 2004, 27, 1947–1953. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Worthington, A.; Huang, C.C.; Aranki, S.F.; Muehlschlegel, J.D. Sex Differences in the Prevalence of Diastolic Dysfunction in Cardiac Surgical Patients. J. Card. Surg. 2015, 30, 238–245. [Google Scholar] [CrossRef]
- Kim, K.H.; Rha, S.W.; Park, Y.; Byun, J.K.; Choi, B.G.; Na, J.O.; Park, C.G.; Seo, H.S. Sex Differences in the Prevalence of Diastolic Dysfunction in Peripheral Arterial Disease Patients Undergoing Percutaneous Transluminal Angioplasty. Arter. Throm. Vas. 2020, 40, 238–245. [Google Scholar]
- Islam, M.Z.; Van Dao, C.; Miyamoto, A.; Shiraishi, M. Rho-kinase and the nitric oxide pathway modulate basilar arterial reactivity to acetylcholine and angiotensin II in streptozotocin-induced diabetic mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Chandramouli, C.; Reichelt, M.E.; Curl, C.L.; Varma, U.; Bienvenu, L.A.; Koutsifeli, P.; Raaijmakers, A.J.A.; De Blasio, M.J.; Qin, C.X.; Jenkins, A.J.; et al. Diastolic dysfunction is more apparent in STZ-induced diabetic female mice, despite less pronounced hyperglycemia. Sci. Rep. 2018, 8, 2346. [Google Scholar] [CrossRef] [PubMed]
- Reverte, V.; Gogulamudi, V.R.; Rosales, C.B.; Musial, D.C.; Gonsalez, S.R.; Parra-Vitela, A.J.; Galeas-Pena, M.; Sure, V.N.; Visniauskas, B.; Lindsey, S.H.; et al. Urinary angiotensinogen increases in the absence of overt renal injury in high fat diet-induced type 2 diabetic mice. J. Diabetes Complicat. 2020, 34, 107448. [Google Scholar] [CrossRef] [PubMed]
- Gogulamudi, V.R.; Arita, D.Y.; Bourgeois, C.R.T.; Jorgensen, J.; He, J.; Wimley, W.C.; Satou, R.; Gonzalez, A.A.; Prieto, M.C. High glucose induces trafficking of prorenin receptor and stimulates profibrotic factors in the collecting duct. Sci. Rep. 2021, 11, 13815. [Google Scholar] [CrossRef] [PubMed]
- Saez, F.; Reverte, V.; Paliege, A.; Moreno, J.M.; Llinas, M.T.; Bachmann, S.; Salazar, F.J. Sex-dependent hypertension and renal changes in aged rats with altered renal development. Am. J. Physiol. Ren. Physiol. 2014, 307, F461–F470. [Google Scholar] [CrossRef]
- Byrd, C.A.; Rands, V.F.; Prieto, M.C.; Navar, L.G. Blood pressure independent sexual dimorphism in proteinuric response to high salt intake in Sprague-Dawley rats. Faseb J. 2010, 24, 1024. [Google Scholar] [CrossRef]
- Fujisawa, G.; Okada, K.; Muto, S.; Fujita, N.; Itabashi, N.; Kusano, E.; Ishibashi, S. Spironolactone prevents early renal injury in streptozotocin-induced diabetic rats. Kidney Int. 2004, 66, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Noshahr, Z.S.; Salmani, H.; Khajavi Rad, A.; Sahebkar, A. Animal Models of Diabetes-Associated Renal Injury. J. Diabetes Res. 2020, 2020, 9416419. [Google Scholar] [CrossRef] [PubMed]
- Tay, Y.C.; Wang, Y.; Kairaitis, L.; Rangan, G.K.; Zhang, C.; Harris, D.C. Can murine diabetic nephropathy be separated from superimposed acute renal failure? Kidney Int. 2005, 68, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Bokenkamp, A. Proteinuria-take a closer look! Pediatr. Nephrol. 2020, 35, 533–541. [Google Scholar] [CrossRef] [PubMed]
- van Berkel, Y.; Ludwig, M.; van Wijk, J.A.E.; Bokenkamp, A. Proteinuria in Dent disease: A review of the literature. Pediatr. Nephrol. 2017, 32, 1851–1859. [Google Scholar] [CrossRef]
- Bjornstad, P.; Cherney, D.Z. Renal Hyperfiltration in Adolescents with Type 2 Diabetes: Physiology, Sex Differences, and Implications for Diabetic Kidney Disease. Curr. Diabetes Rep. 2018, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Piani, F.; Melena, I.; Tommerdahl, K.L.; Nokoff, N.; Nelson, R.G.; Pavkov, M.E.; van Raalte, D.H.; Cherney, D.Z.; Johnson, R.J.; Nadeau, K.J.; et al. Sex-related differences in diabetic kidney disease: A review on the mechanisms and potential therapeutic implications. J. Diabetes Complicat. 2021, 35, 107841. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Zhang, J.; Zhang, J.L.; Wu, Y.C.; Zhang, R.; Ren, H.H.; Cooper, M.E.; Liu, F. Sex Differences in Biopsy-Confirmed Diabetic Kidney Disease. Front. Endocrinol. 2021, 12, 670674. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Lyles, C.R.; Bent-Shaw, L.A.; Young, B.A.; Authors, P. Risk Factor, Age and Sex Differences in Chronic Kidney Disease Prevalence in a Diabetic Cohort: The Pathways Study. Am. J. Nephrol. 2012, 36, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Giandalia, A.; Giuffrida, A.E.; Gembillo, G.; Cucinotta, D.; Squadrito, G.; Santoro, D.; Russo, G.T. Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors. Int J. Mol. Sci. 2021, 22, 5808. [Google Scholar] [CrossRef] [PubMed]
- Piera-Velazquez, S.; Jimenez, S.A. Oxidative Stress Induced by Reactive Oxygen Species (ROS) and NADPH Oxidase 4 (NOX4) in the Pathogenesis of the Fibrotic Process in Systemic Sclerosis: A Promising Therapeutic Target. J. Clin. Med. 2021, 10, 4791. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.J.; Chew, G.T.; Watts, G.F. Therapeutic regulation of endothelial dysfunction in type 2 diabetes mellitus. Diab. Vasc. Dis. Res. 2007, 4, 89–102. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Yan, Y.; Liang, J.; Liang, Q.; Lu, Y.; Zhao, L.; Li, H. Preventative effects of resveratrol and estradiol on streptozotocin-induced diabetes in ovariectomized mice and the related mechanisms. PLoS ONE 2018, 13, e0204499. [Google Scholar] [CrossRef]
- Shen, K.P.; Lin, H.L.; Yen, H.W.; Hsieh, S.L.; An, L.M.; Wu, B.N. Eugenosedin-A improves glucose metabolism and inhibits MAPKs expression in streptozotocin/nicotinamide-induced diabetic rats. Kaohsiung J. Med. Sci. 2018, 34, 142–149. [Google Scholar] [CrossRef] [PubMed]










| Control Females | STZ Females | Control Males | STZ Males | |
|---|---|---|---|---|
| Hct (%) | 46 ± 6 | 48 ± 4 | 52 ± 5 | 48 ± 5 |
| plasma Na+ (mEq/L) | 147 ± 3 | 147 ± 4 | 145 ± 2 | 143 ± 4 |
| plasma K+ (mEq/L) | 3.3 ± 0.6 | 3.5 ± 0.3 | 3.4 ± 0.3 | 3.5 ± 0.3 |
| plasma Cl− (mEq/L) | 106 ± 5 | 107 ± 3 | 106 ± 7 | 108 ± 5 |
| Fasting blood glucose (mg/dL) | 98 ± 25 | 368 ± 60 ** | 102 ± 32 | 385 ± 35 ** |
| Serum creatinine (mg/dL) | 0.22 ± 0.02 | 0.23 ± 0.04 | 0.21 ± 0.02 | 0.24 ± 0.05 |
| Body weight (g) | 35 ± 5 | 28 ± 3 * | 34 ± 4 | 24 ± 5 * |
| Food intake (g) | 2.5 ± 0.1 | 3.1 ± 0.2 * | 2.3 ± 0.2 | 3.4 ± 0.3 * |
| Water intake (mL) | 5.1 ± 0.2 | 9.1 ± 0.8 * | 4.9 ± 0.7 | 12.4 ± 0.7 * |
| Urine output (mL) | 2.2 ± 0.1 | 6.1 ± 0.7 * | 2.6 ± 0.4 * | 5.7 ± 0.6 * |
| Systolic blood pressure (mm Hg) | 122 ± 5 | 129 ± 8 | 126 ± 5 | 125 ± 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado-Barragán, F.; Lazcano-Páez, G.; Larenas, P.E.; Aguirre-Delgadillo, M.; Olivares-Aravena, F.; Witto-Oyarce, D.; Núñez-Allimant, C.; Silva, K.; Nguyen, Q.M.; Cárdenas, P.; et al. Increased Renal Medullary NOX-4 in Female but Not Male Mice during the Early Phase of Type 1 Diabetes: Potential Role of ROS in Upregulation of TGF-β1 and Fibronectin in Collecting Duct Cells. Antioxidants 2023, 12, 729. https://doi.org/10.3390/antiox12030729
Casado-Barragán F, Lazcano-Páez G, Larenas PE, Aguirre-Delgadillo M, Olivares-Aravena F, Witto-Oyarce D, Núñez-Allimant C, Silva K, Nguyen QM, Cárdenas P, et al. Increased Renal Medullary NOX-4 in Female but Not Male Mice during the Early Phase of Type 1 Diabetes: Potential Role of ROS in Upregulation of TGF-β1 and Fibronectin in Collecting Duct Cells. Antioxidants. 2023; 12(3):729. https://doi.org/10.3390/antiox12030729
Chicago/Turabian StyleCasado-Barragán, Felipe, Geraldine Lazcano-Páez, Paulina E. Larenas, Monserrat Aguirre-Delgadillo, Fernanda Olivares-Aravena, Daniela Witto-Oyarce, Camila Núñez-Allimant, Katherin Silva, Quynh My Nguyen, Pilar Cárdenas, and et al. 2023. "Increased Renal Medullary NOX-4 in Female but Not Male Mice during the Early Phase of Type 1 Diabetes: Potential Role of ROS in Upregulation of TGF-β1 and Fibronectin in Collecting Duct Cells" Antioxidants 12, no. 3: 729. https://doi.org/10.3390/antiox12030729
APA StyleCasado-Barragán, F., Lazcano-Páez, G., Larenas, P. E., Aguirre-Delgadillo, M., Olivares-Aravena, F., Witto-Oyarce, D., Núñez-Allimant, C., Silva, K., Nguyen, Q. M., Cárdenas, P., Kassan, M., & Gonzalez, A. A. (2023). Increased Renal Medullary NOX-4 in Female but Not Male Mice during the Early Phase of Type 1 Diabetes: Potential Role of ROS in Upregulation of TGF-β1 and Fibronectin in Collecting Duct Cells. Antioxidants, 12(3), 729. https://doi.org/10.3390/antiox12030729