Flowers of Allium cepa L. as Nutraceuticals: Phenolic Composition and Anti-Obesity and Antioxidant Effects in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Plant Material and Soxhlet Extraction
2.3. Analysis of Phenolic Compounds
2.4. In Vitro Determination of Enzyme Inhibitory Effects
2.4.1. Inhibition of Pancreatic Lipase Assay
2.4.2. Inhibition of α-Glucosidase Assay
2.5. In Vitro Antioxidant Activity Assays
2.5.1. Determination of Folin–Ciocalteu Reducing Capacity
2.5.2. DPPH Scavenging Activity
2.5.3. Superoxide Radical Scavenging Activity Assay
2.5.4. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.5.5. Oxygen Radical Antioxidant Capacity (ORAC) Assay
2.6. C. elegans Assays
2.6.1. Strains and Maintenance Conditions
2.6.2. Assessment of Acute Toxicity
2.6.3. Analysis of Body Fat Accumulation in C. elegans Obesity Model
2.6.4. Evaluation of Resistance to Oxidative Stress
2.6.5. Endogenous Antioxidant Enzymes
2.7. Statistical Analysis
3. Results and Discussion
3.1. Polyphenolic Composition of A. cepa Flowers
3.2. In Vitro Inhibition of α-Glucosidase and Pancreatic Lipase
3.3. In Vitro Antioxidant Activity
3.4. C. elegans Assays
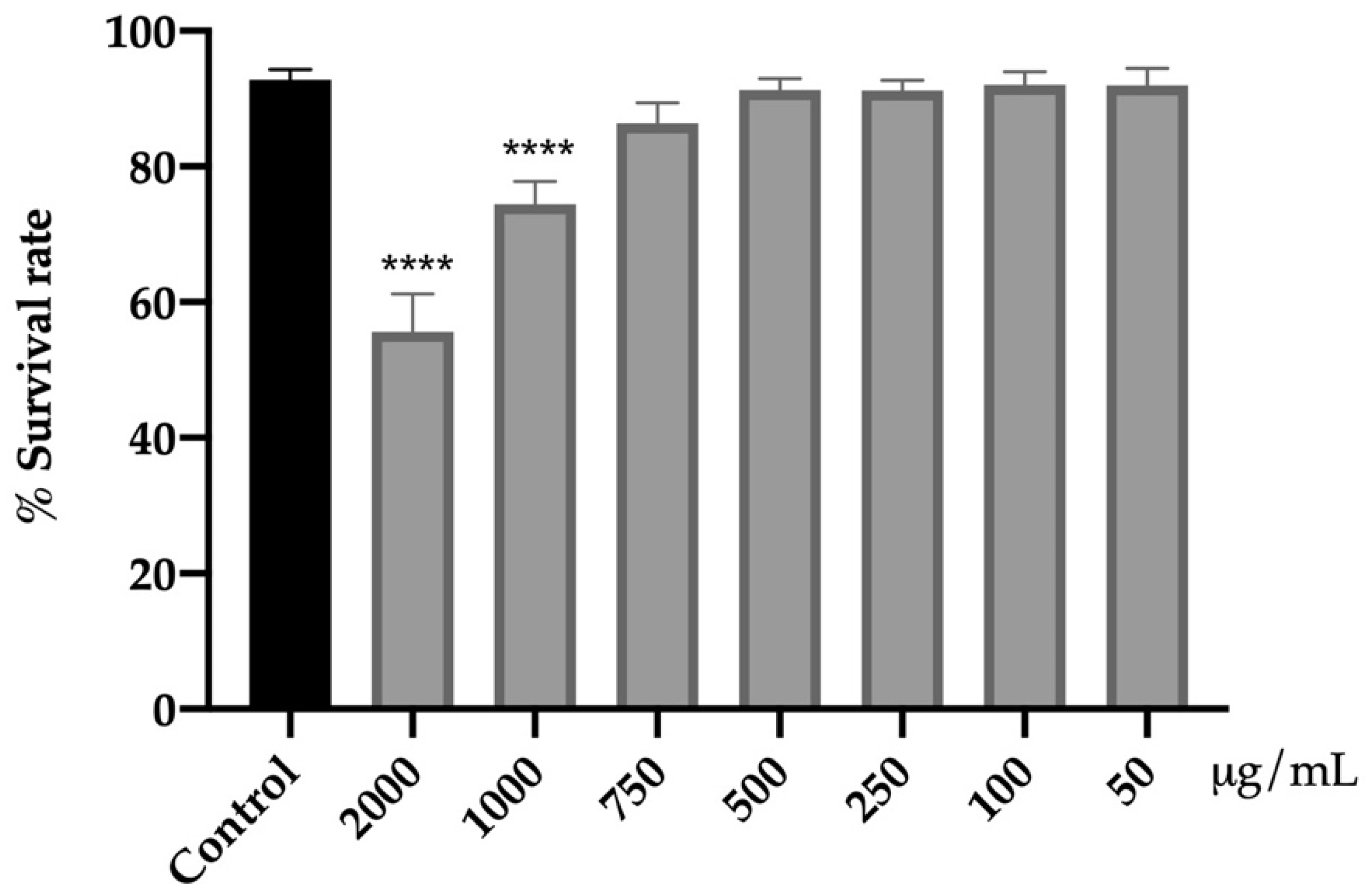
3.4.1. Assessment of Acute Toxicity of Fresh Flowers
3.4.2. A. cepa Flower Extract Decreased Fat Accumulation
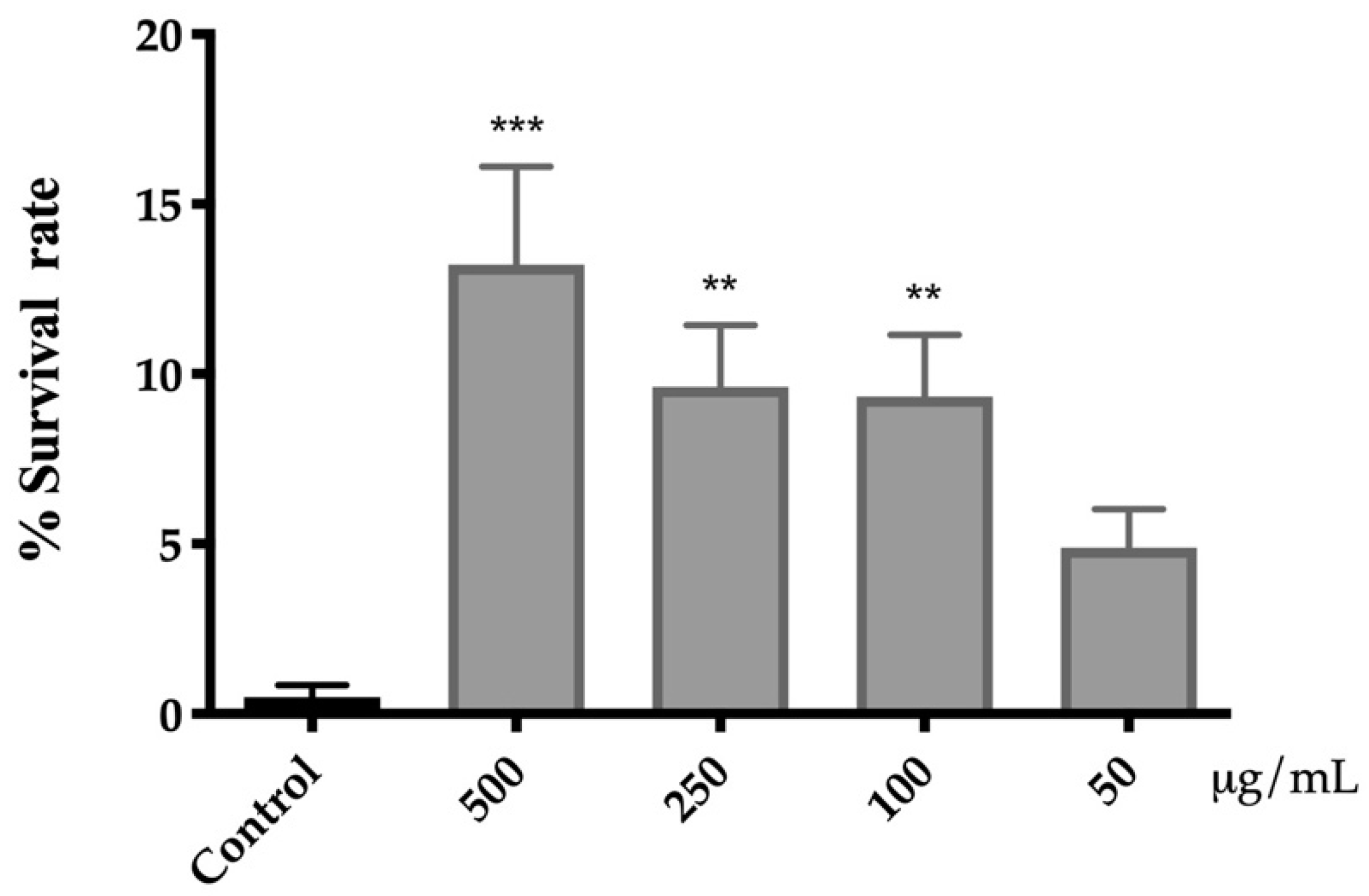
3.4.3. Onion Flower Extract Attenuates the Oxidative Stress Toxicity Induced by Juglone
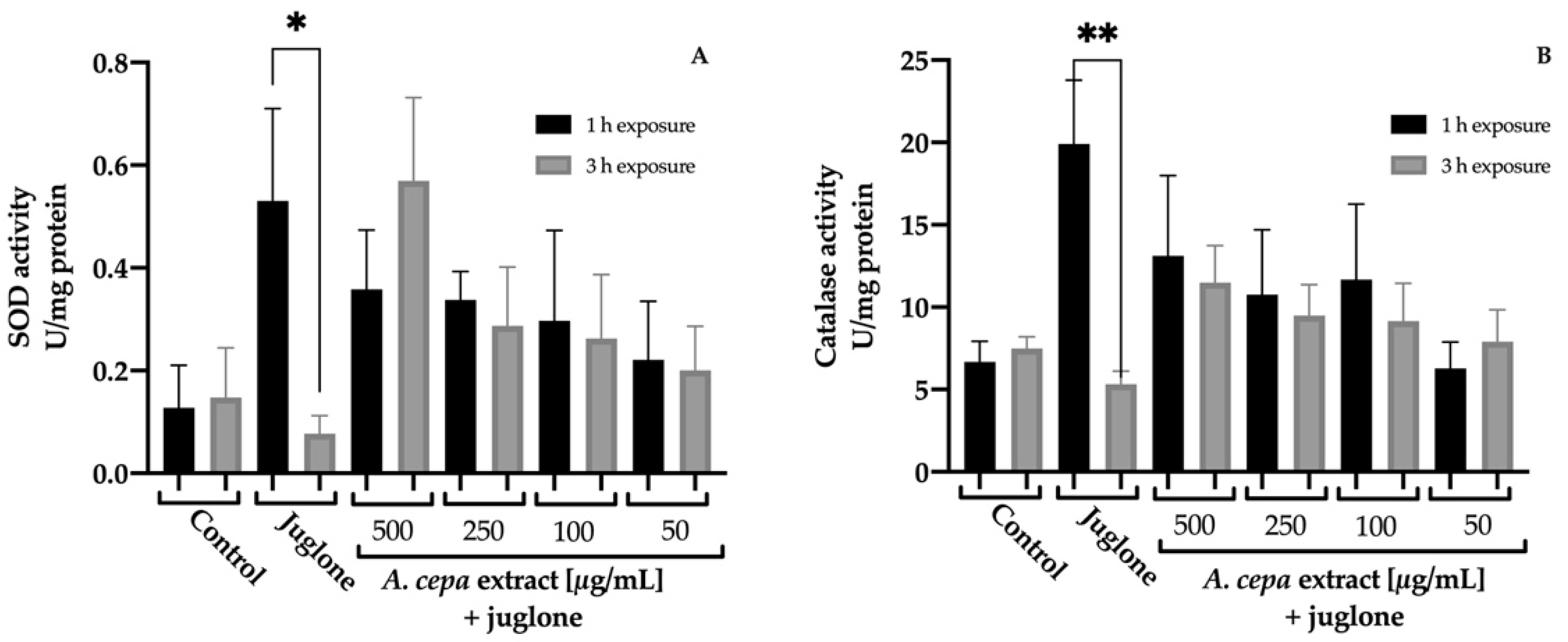
3.4.4. Impact of the Extract on Endogenous Antioxidant Enzyme Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO European Regional Obesity Report 2022; World Health Organization: Copenhagen, Denmark, 2022. [Google Scholar]
- Marseglia, L.; Manti, S.; Angelo, G.D.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2015, 16, 378–400. [Google Scholar] [CrossRef] [PubMed]
- Payab, M.; Aletaha, A.; Aminjan, H.H. Effect of the herbal medicines in obesity and metabolic syndrome: A systematic review and meta—Analysis of clinical trials. Phyther. Res. 2019, 34, 526–545. [Google Scholar] [CrossRef] [PubMed]
- Wallner, M.; Sandner, G.; Ko, A.; Weghuber, J. Functional foods—Dietary or herbal products on obesity: Application of selected bioactive compounds to target lipid metabolism. Curr. Opin. Food Sci. 2020, 34, 9–20. [Google Scholar] [CrossRef]
- Boccia, F.; Punzo, G. Nutraceuticals: Some remarks by a choice experiment on food, health and new technologies. Food Res. Int. 2020, 130, 108888. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Caligiuri, S.P.B.; Brown, D.; Pierce, G.N. Clinical trials using functional foods provide unique challenges. J. Funct. Foods 2018, 45, 233–238. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, K.; Wang, Q.; Zhong, G. Caenorhabditis elegans as an emerging model in food and nutrition research: Importance of standardizing base diet. Crit. Rev. Food Sci. Nutr. 2022, 1–19. [Google Scholar] [CrossRef]
- Pires, E.D.O.; Di Gioia, F.; Rouphael, Y.; Ferreira, I.C.F.R.; Caleja, C.; Barros, L.; Petropoulos, S.A. The Compositional Aspects of Edible Flowers as an Emerging Horticultural Product. Molecules 2021, 26, 6940. [Google Scholar] [CrossRef]
- Rodrigues, H.; Cielo, D.P.; Silveira, A.A.S.; Marchesan, T.A.; Galmarini, M.V.; Richards, N.S.P.S. Eating flowers? Exploring attitudes and consumers’ representation of edible flowers. Food Res. Int. 2017, 100, 227–234. [Google Scholar] [CrossRef]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological Properties and Bioactive Components of Allium cepa L.: Focus on Potential Benefits in the Treatment of Obesity and Related Comorbidities. Molecules 2019, 24, 119. [Google Scholar] [CrossRef]
- Sargin, S.A. Plants used against obesity in Turkish folk medicine: A review. J. Ethnopharmacol. 2021, 270, 113841. [Google Scholar] [CrossRef]
- Jeong, S.; Chae, J.; Lee, G.; Shin, G.; Kwon, Y.; Shin, D.Y.; Lee, J.H. Effect of Steamed Onion (ONIRO) Consumption on Body Fat and Metabolic Profiles in Overweight Subjects: A 12-Week Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Am. Coll. Nutr. 2019, 39, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb. f.: An underexploited and highly disseminated species. Ind. Crop. Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, Y.M.; Kim, H.; Kim, J.; Jang, D.S.; Kim, J.H.; Kim, J.S. Anti-obesity effect of Morus bombycis root extract: Anti-lipase activity and lipolytic effect. J. Ethnopharmacol. 2010, 130, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Les, F.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Bioactive and functional properties of sour cherry juice (Prunus cerasus). Food Funct. 2016, 7, 4675–4682. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- López, V.; Akerreta, S.; Casanova, E.; García-mina, J.; Cavero, R.; Calvo, M. Screening of Spanish Medicinal Plants for Antioxidant and Antifungal Activities. Pharm. Biol. 2008, 46, 602–609. [Google Scholar] [CrossRef]
- Rodríguez-Chávez, J.L.; Coballase-Urrutia, E.; Nieto-Camacho, A.; Delgado-Lamas, G. Antioxidant capacity of “mexican arnica” heterotheca inuloides cass natural products and some derivatives: Their anti-inflammatory evaluation and effect on C. elegans life span. Oxid. Med. Cell. Longev. 2015, 2015, 843237. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing / Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovéz, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. WormBook 1999, 2, 51–67. [Google Scholar] [CrossRef]
- Donkin, S.G.; Williams, P.L. Influence of developmental stage, salts and food presence on various end points using Caenorhabditis Elegans for aquatic toxicity testing. Enviromental Toxicol. Chem. 1995, 14, 2139–2147. [Google Scholar] [CrossRef]
- Martorell, P.; Llopis, S.; Gonza, N.; Monto, F.; Ortiz, P.; Genove, S. Caenorhabditis elegans as a Model To Study the E ff ectiveness and Metabolic Targets of Dietary Supplements Used for Obesity Treatment: The Speci fi c Case of a Conjugated Linoleic Acid Mixture (Tonalin). J. Agric. Food Chem. 2012, 60, 11071–11079. [Google Scholar] [CrossRef] [PubMed]
- Escorcia, W.; Ruter, D.L.; Nhan, J.; Curran, S.P. Quantification of lipid abundance and evaluation of lipid distribution in Caenorhabditis elegans by nile red and oil red O staining. J. Vis. Exp. 2018, 133, e57352. [Google Scholar] [CrossRef]
- Surco-Laos, F.; Dueñas, M.; González-Manzano, S.; Cabello, J.; Santos-Buelga, C.; González-Paramás, A.M. Influence of catechins and their methylated metabolites on lifespan and resistance to oxidative and thermal stress of Caenorhabditis elegans and epicatechin uptake. Food Res. Int. 2012, 46, 514–521. [Google Scholar] [CrossRef]
- Veiga, A.A.; Irioda, A.C.; Mogharbel, B.F.; Bonatto, S.J.R.; Souza, L.M. Quercetin-Rich Extracts from Onions (Allium cepa) Play Potent Cytotoxicity on Adrenocortical Carcinoma Cell Lines, and Quercetin Induces Important Anticancer Properties. Pharmaceuticals 2022, 15, 754. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, F.; Li, H.; Li, H.; Wu, D.; Geng, F. Recent Advances in Bioactive Compounds, Health Functions, and Safety Concerns of Onion (Allium cepa L.). Front. Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Carvalho, A.M.; Ferreira, I.C.F.R.; Santos-Buelga, C. Characterization of phenolic compounds in flowers of wild medicinal plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef]
- Moliner, C.; Barros, L.; Dias, M.I.; Reigada, I.; Ferreira, I.C.F.R.; López, V.; Langa, E.; Rincón, C.G. Viola cornuta and Viola x wittrockiana: Phenolic compounds, antioxidant and neuroprotective activities on Caenorhabditis elegans. J. Food Drug Anal. 2019, 27, 849–859. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Allium cepa L., Bulbus; Committee on Herbal Medicinal Products: London, UK, 2012. [Google Scholar]
- Kim, H.Y. Effects of onion (Allium cepa) skin extract on pancreatic lipase and body weight-related parameters. FOOD Sci. Biotechnol. 2007, 16, 434–438. [Google Scholar]
- Oboh, G.; Ademiluyi, A.O.; Agunloye, O.M.; Ademosun, A.O.; Ogunsakin, B.G. Inhibitory Effect of Garlic, Purple Onion, and White Onion on Key Enzymes Linked with Type 2 Diabetes and Hypertension. J. Diet. Suppl. 2019, 16, 105–118. [Google Scholar] [CrossRef]
- Nile, A.; Nile, S.H.; Kim, D.H.; Keum, Y.S.; Seok, P.G.; Sharma, K. Valorization of onion solid waste and their flavonols for assessment of cytotoxicity, enzyme inhibitory and antioxidant activities. FOOD Chem. Toxicol. 2018, 119, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Stoica, F.; Aprodu, I.; Enachi, E.; Stanciuc, N.; Condurache, N.N.; Duta, D.E.; Bahrim, G.E.; Rapeanu, G. Bioactive’s Characterization, Biological Activities, and In Silico Studies of Red Onion (Allium cepa L.) Skin Extracts. Plants 2021, 10, 2330. [Google Scholar] [CrossRef] [PubMed]
- Slanc, P.; Doljak, B.; Kreft, S.; Lunder, M.; Janes, D.; Strukelj, B. Screening of Selected Food and Medicinal Plant Extracts for Pancreatic Lipase Inhibition. Phyther. Res. 2009, 23, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Trisat, K.; Wong-on, M.; Lapphanichayakool, P.; Tiyaboonchai, W.; Limpeanchob, N. Vegetable Juices and Fibers Reduce Lipid Digestion or Absorption by Inhibiting Pancreatic Lipase, Cholesterol Solubility and Bile Acid Binding. Int. J. Veg. Sci. 2017, 23, 260–269. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status Aurelia. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. A thorough study of reactivity of various compound classes towards the Folin-Ciocalteu. J. Agric. Food Chem. 2014, 58, 8139–8144. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, J.; Jiang, Y.; Lu, B.; Hu, Y.; Zhou, F.; Mao, S.; Shen, C. Phenolic Compounds and Antioxidant Capacities of 10 Common Edible Flowers from China. J. Food Sci. 2014, 79, 517–525. [Google Scholar] [CrossRef]
- Nuutila, A.M.; Puupponen-pimia, R.; Aarni, M. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food 2003, 81, 485–493. [Google Scholar] [CrossRef]
- Santas, J.; Almajano, M.P.; Carbó, R. Antimicrobial and antioxidant activity of crude onion (Allium cepa, L.) extracts. Int. J. Food Sci. Technol. 2010, 45, 403–409. [Google Scholar] [CrossRef]
- Ye, C.; Dai, D.; Hu, W. Antimicrobial and antioxidant activities of the essential oil from onion (Allium cepa L.). Food Control 2013, 30, 48–53. [Google Scholar] [CrossRef]
- Kim, M.; Jo, S.; Jang, H.; Lee, M.S.; Kwon, Y. Antioxidant Activity and α -Glucosidase Inhibitory Potential of Onion (Allium cepa L.) Extracts. Food Sci. Biotechnol. 2010, 19, 159–164. [Google Scholar] [CrossRef]
- Amaral, D.R.; Da Rocha Oliveira, F.E.; Oliveira, D.F.; Campos, V.P. Purification of two substances from bulbs of onion (Allium cepa L.) with nematicidal activity against Meloidogyne exigua Goeldi. Nematology 2003, 5, 859–864. [Google Scholar] [CrossRef]
- Hoi, I.C.; Hin, S.S.; Ark, I.P. Nematicidal activity of onion (Allium cepa) oil and its components against the pine wood nematode (Bursaphelenchus xylophilus). Nematology 2007, 9, 231–235. [Google Scholar]
- Aranaz, P.; Navarro-Herrera, D.; Zabala, M.; Romo-Hualde, A.; López-Yoldi, M.; Vizmanos, J.L.; Milagro, F.I.; González-Navarro, C.J. Phenolic compounds reduce the fat content in caenorhabditis elegans by affecting lipogenesis, lipolysis, and different stress responses. Pharmaceuticals 2020, 13, 355. [Google Scholar] [CrossRef]
- Farias-Pereira, R.; Savarese, J.; Yue, Y.; Lee, S.; Park, Y. Fat-lowering effects of isorhamnetin are via NHR-49-dependent pathway in Caenorhabditis elegans. Curr. Res. Food Sci. 2020, 2, 70–76. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Kim, K.H.; Park, Y. Piceatannol Reduces Fat Accumulation in Caenorhabditis elegans. J. Med. Food 2017, 20, 887–894. [Google Scholar] [CrossRef]
- Guerrero-Rubio, M.A.; Hernández-García, S.; García-Carmona, F.; Gandía-Herrero, F. Flavonoids’ effects on Caenorhabditis elegans’ longevity, fat accumulation, stress resistance and gene modulation involve mTOR, SKN-1 and DAF-16. Antioxidants 2021, 10, 438. [Google Scholar] [CrossRef]
- Pluci, B.; Kuczy, P. The oxidative stress in allelopathy: Participation of prenyllipid antioxidants in the response to juglone in Chlamydomonas reinhardtii. Phytochemistry 2017, 144, 171–179. [Google Scholar] [CrossRef]
- Kampkötter, A.; Gombitang, C.; Ruben, N.; Zurawski, F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Mol. Toxicol. 2007, 81, 849–858. [Google Scholar] [CrossRef]
- Surco-Laos, F.; Cabello, J.; Gómez-Orte, E.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Dueñas, M. Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans. Food Funct. 2011, 2, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Protective properties of Salvia lavandulifolia Vahl. essential oil against oxidative stress-induced neuronal injury. Food Chem. Toxicol. 2015, 80, 154–162. [Google Scholar] [CrossRef]
- Zhu, A.; Zheng, F.; Zhang, W.; Li, L.; Li, Y.; Hu, H.; Wu, Y. Oxidation and Antioxidation of Natural Products in the Model Organism Caenorhabditis elegans. Antioxidants 2022, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Kim, S.; Jeong, S.; Jeong, U.; Jung, J.; Lee, S.; Lee, S. Antioxidant and Anti-Inflammatory Effects of Ethanol Extract from Whole Onion (Allium cepa L.) with Leaves. Agriculture 2022, 12, 963. [Google Scholar] [CrossRef]
- Lee, B.; Jung, J.; Kim, H. Assessment of red onion on antioxidant activity in rat. FOOD Chem. Toxicol. 2012, 50, 3912–3919. [Google Scholar] [CrossRef]
- Ogunmodede, O.S.; Saalu, L.C.; Babatunde, O.; Akunna, G.G. An Evaluation of the Hypoglycemic, Antioxidant and Hepatoprotective Potentials of Onion (Allium cepa L.) on Alloxan-induced Diabetic Rabbits. Int. J. Pharmacol. 2012, 8, 21–29. [Google Scholar] [CrossRef]


| Peak | Rt (min) | λmax (nm) | Molecular Ion [M-H]− (m/z) | MS2 (m/z) | Tentative Identification | Qauntification (mg/g of Extract) |
|---|---|---|---|---|---|---|
| 1 | 14.46 | 350 | 609 | 447 (72), 285 (100) | Kaempferol-O-dihexoside | 0.887 ± 0.001 |
| 2 | 16.44 | 346 | 609 | 429 (100), 285 (73) | Kaempferol-O-dihexoside | 0.52 ± 0.01 |
| 3 | 18.38 | 341 | 593 | 285 (100) | Kaempferol-3-O-rutinoside | 0.276 ± 0.001 |
| 4 | 21.27 | 343 | 609 | 285 (100) | Kaempferol-O-dihexoside | 0.447 ± 0.001 |
| 5 | 22.39 | 347 | 447 | 285 (100) | Kaempferol-3-O-glucoside | 1.12 ± 0.01 |
| 6 | 23.34 | 353 | 477 | 315 (100) | Isorhamnetin-3-O-glucoside | 0.93 ± 0.01 |
| 7 | 24.23 | 317 | 623 | 477 (10), 315 (100) | Isorhamnetin-O-coumaroylhexoside | 0.333 ± 0.004 |
| Total phenolic compounds | 4.50 ± 0.01 |
| Sample | α-Glucosidase IC50 (μg/mL) | Lipase IC50 (μg/mL) |
|---|---|---|
| A. cepa flower extract | 412.1 ± 0.4 | 677.1 ± 68.4 |
| Acarbose | 297.2 ± 15.8 | - |
| Orlistat | - | 27.7 ± 13.3 |
| Assay | DPPH IC50 (μg/mL) | O2− IC50 (μg/mL) | Folin-Ciocalteau mg PE/g Extract | FRAP mmol Fe2+/g Extract | ORAC μmol TE/mg Extract |
|---|---|---|---|---|---|
| A. cepa flower extract | 471 ± 46 | 229 ± 39 | 17 ± 2 | 6 ± 2 | 1 ± 0.1 |
| Ascorbic acid | 1.5 ± 0.1 | - | - | - | - |
| Trolox | - | 28 ± 1 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moliner, C.; Núñez, S.; Cásedas, G.; Valero, M.S.; Dias, M.I.; Barros, L.; López, V.; Gómez-Rincón, C. Flowers of Allium cepa L. as Nutraceuticals: Phenolic Composition and Anti-Obesity and Antioxidant Effects in Caenorhabditis elegans. Antioxidants 2023, 12, 720. https://doi.org/10.3390/antiox12030720
Moliner C, Núñez S, Cásedas G, Valero MS, Dias MI, Barros L, López V, Gómez-Rincón C. Flowers of Allium cepa L. as Nutraceuticals: Phenolic Composition and Anti-Obesity and Antioxidant Effects in Caenorhabditis elegans. Antioxidants. 2023; 12(3):720. https://doi.org/10.3390/antiox12030720
Chicago/Turabian StyleMoliner, Cristina, Sonia Núñez, Guillermo Cásedas, Marta Sofía Valero, Maria Inês Dias, Lillian Barros, Víctor López, and Carlota Gómez-Rincón. 2023. "Flowers of Allium cepa L. as Nutraceuticals: Phenolic Composition and Anti-Obesity and Antioxidant Effects in Caenorhabditis elegans" Antioxidants 12, no. 3: 720. https://doi.org/10.3390/antiox12030720
APA StyleMoliner, C., Núñez, S., Cásedas, G., Valero, M. S., Dias, M. I., Barros, L., López, V., & Gómez-Rincón, C. (2023). Flowers of Allium cepa L. as Nutraceuticals: Phenolic Composition and Anti-Obesity and Antioxidant Effects in Caenorhabditis elegans. Antioxidants, 12(3), 720. https://doi.org/10.3390/antiox12030720











