Combined Intake of Fish Oil and D-Fagomine Prevents High-Fat High-Sucrose Diet-Induced Prediabetes by Modulating Lipotoxicity and Protein Carbonylation in the Kidney
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design and Sample Collection
2.2. Biochemical and Antioxidant System Evaluation in Blood
2.3. Renal Fat Deposition and Fatty Acid Analysis in Plasma and Kidney
2.4. Lipid Peroxidation Levels in Kidney
2.5. Extraction and Fluorescent Labeling of Renal and Plasma Protein Carbonyls
2.6. Total and Specific Protein Carbonylation Relative Quantification
2.7. Identification of Carbonylated Proteins by NanoLC–ESI–IT–MS/MS
2.8. Gene Ontology (GO) and KEGG Pathway Enrichment Analysis of Carbonylated Proteins Identified in Kidney
2.9. Statistical Analysis
2.10. Materials and Reagents
3. Results and Discussion
3.1. Effects of the High-Fat and High-Sucrose Diet and the Supplementation with Fish Oil and D-Fagomine on Biometrical, Biochemical and General Oxidative Status
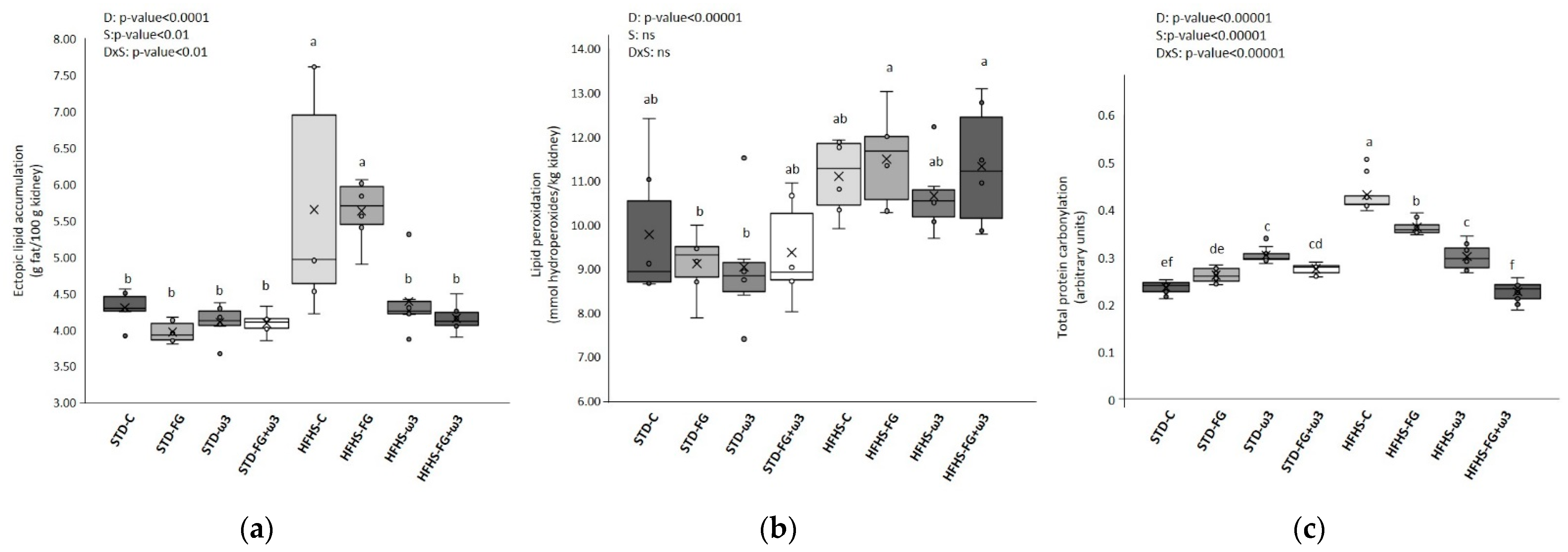
3.1.1. Long-Term High-Fat and High-Sucrose Diet Feeding Induces Prediabetes and Increases Oxidative Stress and Lipotoxicity in Kidney
3.1.2. Effect of Fish Oil and D-Fagomine on Prediabetes, Oxidative Stress, and Lipotoxicity Induced by the High-Fat and High-Sucrose Diet
3.2. Effects of the High-Fat and High-Sucrose Diet and the Supplementation with Fish Oil and D-Fagomine on Lipid Profiles
3.2.1. Modulation of Lipid Profiles in Kidneys by the High-Fat and High-Sucrose Diet Intake
3.2.2. Modulation of Total Lipid Profiles in Kidneys by the Effect of Supplementation with Fish Oil and D-Fagomine
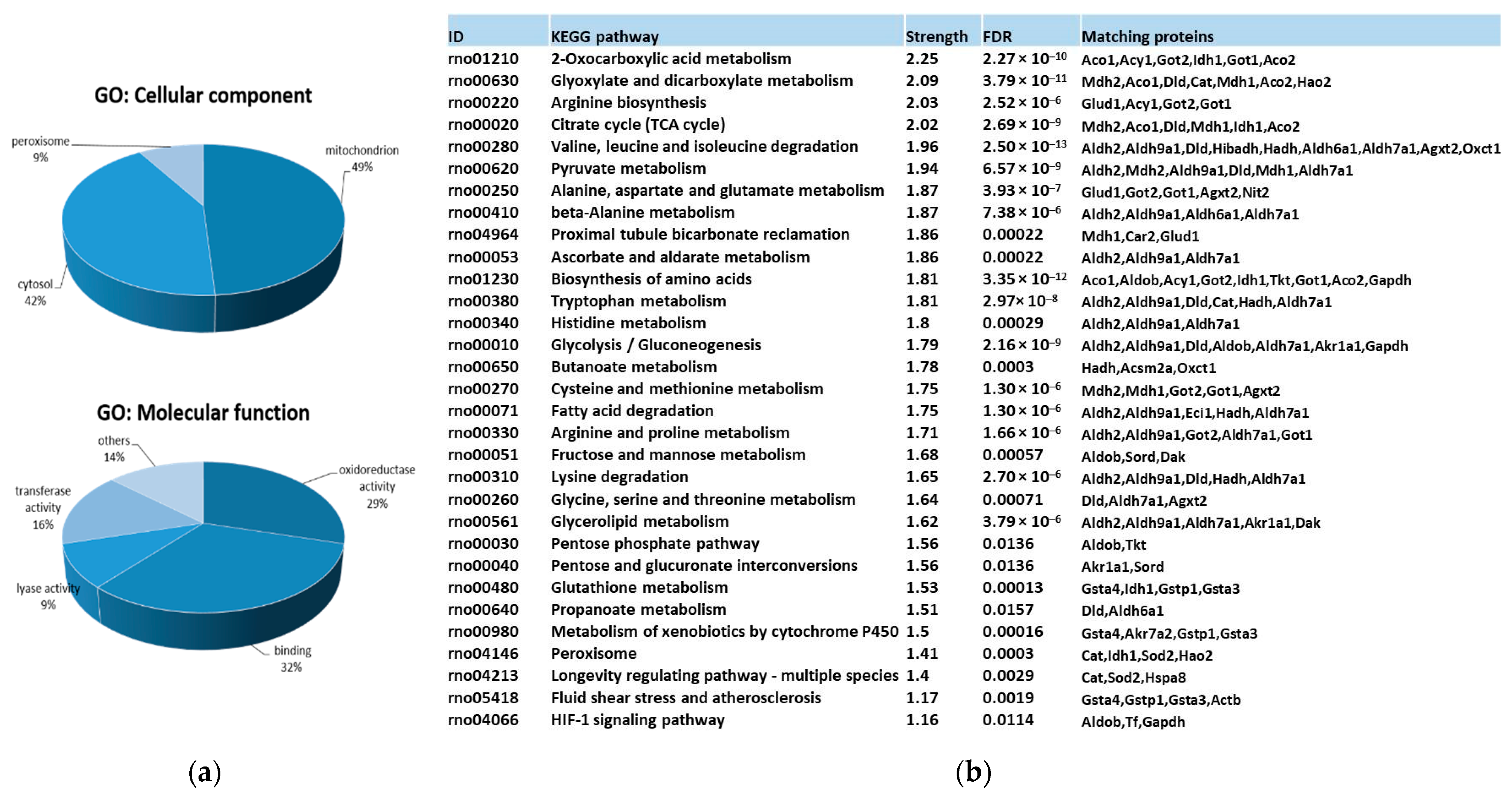
3.3. Identification and Functional Enrichment Analysis of Carbonylated Proteins in the Kidney
3.4. Quantitative Changes Induced on Renal Carbonylome by High-Fat and High-Sucrose Diet and the Effect of Fish Oil and D-Fagomine Supplementations
3.4.1. Quantitative Changes Induced on Renal Carbonylome by High-Fat and High-Sucrose Diet
3.4.2. Quantitative Changes Induced on Renal Carbonylome by Fish Oil and D-Fagomine Supplementation
3.5. Pathways Modulated by Diet and Supplements through Carbonylome Changes in the Kidney
3.5.1. Pathways Modulated by High-Fat and High-Sucrose Diet through Carbonylome Changes in Kidney
3.5.2. Pathways Modulated by Fish Oil and D-Fagomine Supplementation through Carbonylome Changes in Kidney
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.T.; Sung, C.C.; Hung, K.C.; Wu, C.C.; Lo, L.; Lu, K.C. Insulin resistance in patients with chronic kidney disease. J. Biomed. Biotechnol. 2012, 2012, 691369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spoto, B.; Pisano, A.; Zoccali, C. Insulin resistance in chronic kidney disease: A systematic review. Am. J. Physiol. Physiol. 2016, 311, F1087–F1108. [Google Scholar] [CrossRef] [Green Version]
- Forbes, J.M.; Coughlan, M.T.; Cooper, M.E. Oxidative Stress as a Major Culprit in Kidney Disease in Diabetes. Diabetes 2008, 57, 1446–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Mol, M.; Degani, G.; Coppa, C.; Baron, G.; Popolo, L.; Carini, M.; Aldini, G.; Vistoli, G.; Altomare, A. Advanced lipoxidation end products (ALEs) as RAGE binders: Mass spectrometric and computational studies to explain the reasons why. Redox Biol. 2019, 23, 101083. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Moghaddam, A.E.; Gartlan, K.H.; Kong, L.; Sattentau, Q.J. Reactive Carbonyls Are a Major Th2-Inducing Damage-Associated Molecular Pattern Generated by Oxidative Stress. J. Immunol. 2011, 187, 1626–1633. [Google Scholar] [CrossRef] [Green Version]
- Uchidan, K. Redox-derived damage-associated molecular patterns: Ligand function of lipid peroxidation adducts. Redox Biol. 2013, 1, 94–96. [Google Scholar] [CrossRef] [Green Version]
- Yazici, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 277–304. ISBN 978-3-319-48382-5. [Google Scholar]
- Opazo-Ríos, L.; Mas, S.; Marín-Royo, G.; Mezzano, S.; Gómez-Guerrero, C.; Moreno, J.A.; Egido, J. Lipotoxicity and diabetic nephropathy: Novel mechanistic insights and therapeutic opportunities. Int. J. Mol. Sci. 2020, 21, 2632. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.; Yang, Y.; Kim, B.M.; Kim, J.; Son, M.; Kim, D.; Yu, H.S.; Im, D.; Chung, H.Y.; Chung, K.W. Activation of PAR2 promotes high-fat diet-induced renal injury by inducing oxidative stress and inflammation. Biochim. Biophys. Acta—Mol. Basis Dis. 2022, 1868, 166474. [Google Scholar] [CrossRef]
- Jang, S.-A.; Namkoong, S.; Lee, S.R.; Lee, J.W.; Park, Y.; So, G.; Kim, S.H.; Kim, M.-J.; Jang, K.-H.; Avolio, A.P.; et al. Multi-tissue lipotoxicity caused by high-fat diet feeding is attenuated by the supplementation of Korean red ginseng in mice. Mol. Cell. Toxicol. 2020, 16, 39–50. [Google Scholar] [CrossRef]
- Szczuko, M.; Kaczkan, M.; Drozd, A.; Maciejewska, D.; Palma, J.; Owczarzak, A.; Marczuk, N.; Rutkowski, P.; Małgorzewicz, S. Comparison of fatty acid profiles in a group of female patients with chronic kidney diseases (CKD) and metabolic syndrome (MetS)–similar trends of changes, different pathophysiology. Int. J. Mol. Sci. 2019, 20, 1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muramatsu, H.; Akimoto, N.; Hashimoto, M.; Sugibayashi, K.; Katakura, M. Influence of polyunsaturated fatty acid intake on kidney functions of rats with chronic renal failure. Mar. Drugs 2021, 19, 692. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W.; et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Takabatake, Y.; Takahashi, A.; Kimura, T.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.; Matsui, I.; Matsusaka, T.; et al. High-Fat Diet–Induced Lysosomal Dysfunction and Impaired Autophagic Flux Contribute to Lipotoxicity in the Kidney. J. Am. Soc. Nephrol. 2017, 28, 1534–1551. [Google Scholar] [CrossRef] [Green Version]
- Biesalski, H.-K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine Bioactives as Functional Food Ingredients: Potential to Reduce the Incidence of Chronic Diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2003, 23, e20–e30. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, S.; Méndez, L.; Dasilva, G.; Torres, J.L.; Ramos-Romero, S.; Romeu, M.; Nogués, M.R.; Medina, I. Targeting hepatic protein carbonylation and oxidative stress occurring on diet-induced metabolic diseases through the supplementation with fish oils. Mar. Drugs 2018, 16, 353. [Google Scholar] [CrossRef] [Green Version]
- Taltavull, N.; Ras, R.; Mariné, S.; Romeu, M.; Giralt, M.; Méndez, L.; Medina, I.; Ramos-Romero, S.; Torres, J.L.; Nogués, M.R. Protective effects of fish oil on pre-diabetes: A lipidomic analysis of liver ceramides in rats. Food Funct. 2016, 7, 3981–3988. [Google Scholar] [CrossRef]
- Hereu, M.; Ramos-Romero, S.; Busquets, C.; Atienza, L.; Amézqueta, S.; Miralles-Pérez, B.; Nogués, M.R.; Méndez, L.; Medina, I.; Torres, J.L. Effects of combined d-fagomine and omega-3 PUFAs on gut microbiota subpopulations and diabetes risk factors in rats fed a high-fat diet. Sci. Rep. 2019, 9, 16628. [Google Scholar] [CrossRef] [Green Version]
- An, W.S.; Kim, H.J.; Cho, K.-H.; Vaziri, N.D. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am. J. Physiol. Physiol. 2009, 297, F895–F903. [Google Scholar] [CrossRef] [Green Version]
- Peake, J.M.; Gobe, G.C.; Fassett, R.G.; Coombes, J.S. The effects of dietary fish oil on inflammation, fibrosis and oxidative stress associated with obstructive renal injury in rats. Mol. Nutr. Food Res. 2011, 55, 400–410. [Google Scholar] [CrossRef]
- van Breda, S.G.J.; de Kok, T.M.C.M. Smart Combinations of Bioactive Compounds in Fruits and Vegetables May Guide New Strategies for Personalized Prevention of Chronic Diseases. Mol. Nutr. Food Res. 2018, 62, 1700597. [Google Scholar] [CrossRef] [PubMed]
- Méndez, L.; Muñoz, S.; Miralles-Pérez, B.; Nogués, M.R.; Ramos-Romero, S.; Torres, J.L.; Medina, I. Modulation of the Liver Protein Carbonylome by the Combined Effect of Marine Omega-3 PUFAs and Grape Polyphenols Supplementation in Rats Fed an Obesogenic High Fat and High Sucrose Diet. Mar. Drugs 2019, 18, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez, L.; Ciordia, S.; Fernández, M.S.; Juárez, S.; Ramos, A.; Pazos, M.; Gallardo, J.M.; Torres, J.L.; Nogués, M.R.; Medina, I. Changes in liver proteins of rats fed standard and high-fat and sucrose diets induced by fish omega-3 PUFAs and their combination with grape polyphenols according to quantitative proteomics. J. Nutr. Biochem. 2017, 41, 84–97. [Google Scholar] [CrossRef] [Green Version]
- Dasilva, G.; Pazos, M.; García-Egido, E.; Gallardo, J.M.; Ramos-Romero, S.; Torres, J.L.; Romeu, M.; Nogués, M.-R.; Medina, I. A lipidomic study on the regulation of inflammation and oxidative stress targeted by marine ω-3 PUFA and polyphenols in high-fat high-sucrose diets. J. Nutr. Biochem. 2017, 43, 53–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taltavull, N.; Miralles-Pérez, B.; Nogués, M.R.; Ramos-Romero, S.; Méndez, L.; Medina, I.; Torres, J.L.; Romeu, M. Effects of Fish Oil and Grape Seed Extract Combination on Hepatic Endogenous Antioxidants and Bioactive Lipids in Diet-Induced Early Stages of Insulin Resistance in Rats. Mar. Drugs 2020, 18, 318. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Hereu, M.; Molinar-Toribio, E.; Almajano, M.P.; Méndez, L.; Medina, I.; Taltavull, N.; Romeu, M.; Nogués, M.R.; Torres, J.L. Effects of the combination of ω-3 PUFAs and proanthocyanidins on the gut microbiota of healthy rats. Food Res. Int. 2017, 97, 364–371. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Molinar-Toribio, E.; Pérez-Jiménez, J.; Taltavull, N.; Dasilva, G.; Romeu, M.; Medina, I.; Torres, J.L. The combined action of omega-3 polyunsaturated fatty acids and grape proanthocyanidins on a rat model of diet-induced metabolic alterations. Food Funct. 2016, 7, 3516–3523. [Google Scholar] [CrossRef] [PubMed]
- Molinar-Toribio, E.; Fuguet, E.; Ramos-Romero, S.; Taltavull, N.; Méndez, L.; Nogués, M.R.R.; Medina, I.; Torres, J.L.; Pérez-Jiménez, J. A high-fat high-sucrose diet affects the long-term metabolic fate of grape proanthocyanidins in rats. Eur. J. Nutr. 2018, 57, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Molinar-Toribio, E.; Ramos-Romero, S.; Fuguet, E.; Taltavull, N.; Méndez, L.; Romeu, M.; Medina, I.; Torres, J.L.; Pérez-Jiménez, J. Influence of omega-3 PUFAs on the metabolism of proanthocyanidins in rats. Food Res. Int. 2017, 97, 133–140. [Google Scholar] [CrossRef]
- Amézqueta, S.; Ramos-Romero, S.; Martínez-Guimet, C.; Moreno, A.; Hereu, M.; Torres, J.L. Fate of d-Fagomine after Oral Administration to Rats. J. Agric. Food Chem. 2017, 65, 4414–4420. [Google Scholar] [CrossRef]
- Asano, N.; Oseki, K.; Tomioka, E.; Kizu, H.; Matsui, K. N-containing sugars fromMorus alba and their glycosidase inhibitory activities. Carbohydr. Res. 1994, 259, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.; Molinar-Toribio, E.; Calvo-Torras, M.Á.; Adelantado, C.; Juan, M.E.; Planas, J.M.; Cañas, X.; Lozano, C.; Pumarola, S.; Clapés, P.; et al. d-Fagomine lowers postprandial blood glucose and modulates bacterial adhesion. Br. J. Nutr. 2012, 107, 1739–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hereu, M.; Ramos-Romero, S.; García-González, N.; Amézqueta, S.; Torres, J.L. Eubiotic effect of buckwheat d-fagomine in healthy rats. J. Funct. Foods 2018, 50, 120–126. [Google Scholar] [CrossRef]
- Hereu, M.; Ramos-Romero, S.; Marín-Valls, R.; Amézqueta, S.; Miralles-Pérez, B.; Romeu, M.; Méndez, L.; Medina, I.; Torres, J.L. Combined Buckwheat d-Fagomine and Fish Omega-3 PUFAs Stabilize the Populations of Gut Prevotella and Bacteroides While Reducing Weight Gain in Rats. Nutrients 2019, 11, 2606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez, L.; Pazos, M.; Gallardo, J.M.; Torres, J.L.; Pérez-Jiménez, J.; Nogués, M.R.; Romeu, M.; Medina, I. Reduced protein oxidation in Wistar rats supplemented with marine ω3 PUFAs. Free Radic. Biol. Med. 2013, 55, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Dasilva, G.; Lois, S.; Méndez, L.; Miralles-Pérez, B.; Romeu, M.; Ramos-Romero, S.; Torres, J.L.; Medina, I. Fish Oil Improves Pathway-Oriented Profiling of Lipid Mediators for Maintaining Metabolic Homeostasis in Adipose Tissue of Prediabetic Rats. Front. Immunol. 2021, 12, 608875. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, G.; David, H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973, 19, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Drabkin, D.L.; Austin, J.H. Spectrophotometric Studies: II. preparations from washed blood cells.; nitric oxide hemoglobin and sulfhemoglobin. J. Biol. Chem. 1935, 112, 51–65. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Arc-Chagnaud, C.; Py, G.; Fovet, T.; Roumanille, R.; Demangel, R.; Pagano, A.F.; Delobel, P.; Blanc, S.; Jasmin, B.J.; Blottner, D.; et al. Evaluation of an Antioxidant and Anti-inflammatory Cocktail Against Human Hypoactivity-Induced Skeletal Muscle Deconditioning. Front. Physiol. 2020, 11, 71. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Méndez, L.; Barros, L.; Muñoz, S.; Medina, I. FTSC-Labeling Coupled with 2DE-LC–MS/MS Analysis of Complex Protein Mixtures for Identification and Relative Quantification of Tissue Carbonylome. In Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2259, pp. 227–246. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. Correction to ‘The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets’. Nucleic Acids Res. 2021, 49, 10800. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 11 March 2023).
- Ramos-Romero, S.; Hereu, M.; Atienza, L.; Casas, J.; Taltavull, N.; Romeu, M.; Amézqueta, S.; Dasilva, G.; Medina, I.; Torres, J.L. Functional Effects of the Buckwheat Iminosugar D-Fagomine on Rats with Diet-Induced Prediabetes. Mol. Nutr. Food Res. 2018, 62, 1800373. [Google Scholar] [CrossRef]
- Stefanović, V.; Nešić, V.; Stojimirović, B. Treatment of Insulin Resistance in Uremia. Int. J. Artif. Organs 2003, 26, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Simões, L.R.; Colpo, G.D.; Scaini, G.; Oses, J.P.; Generoso, J.S.; Prossin, A.R.; Kaddurah-Daouk, R.; Quevedo, J.; Barichello, T. Ketamine potentiates oxidative stress and influences behavior and inflammation in response to lipolysaccharide (LPS) exposure in early life. Neuroscience 2017, 353, 17–25. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.; Marly, C.; Spiazzi, S.; Bortolin, T.; Canever, L.; Petronilho, F.; Gonçalves, F.; Dal-pizzol, F.; Quevedo, J.; Zugno, A.I. Progress in Neuro-Psychopharmacology & Biological Psychiatry Different sub-anesthetic doses of ketamine increase oxidative stress in the brain of rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1003–1008. [Google Scholar] [CrossRef]
- Kamİloğlu, N.N.; Beytut, E.; Ketamin, A.; Peroksidasyon, L. Changes in Antioxidant Sytem, Lipid Peroxidation, Heart and Respiratory Rate and Rectal Temperature with Ketamine and Ketamine-Xylazine Anaestesia in Tuj Rams. Kafkas. Univ. Vet. Fak. Derg. 2009, 15, 205–210. [Google Scholar] [CrossRef]
- Mustafa, D.A.; Mohammed, B.H.; Radhi, M.M. Oxidative and anti-oxidative effect of anesthesia compounds (ketamine and xylazine) on the rabbit blood sample using electrochemical method. Rom. JouRnal Neurol. 2020, 19, 76–83. [Google Scholar] [CrossRef]
- Gokalp, E.; Gurgoze, S.; Altan, S. Effects of xylazine-ketamine, xylazine-propofol and xylazine- ketamine-propofol administration on free radical generation and blood gases in sheep. Indian J. Anim. Res. 2020, 54, 149–154. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frayn, K. Adipose tissue as a buffer for daily lipid flux. Diabetologia 2002, 45, 1201–1210. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Jin, Q.; Ren, F.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Li, P.; Zhan, Y. Potential therapeutic effects of natural compounds targeting autophagy to alleviate podocyte injury in glomerular diseases. Biomed. Pharmacother. 2022, 155, 113670. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, M.H.; Son, Y.K.; Kim, S.E.; Park, Y.; Rha, S.H.; An, W.S. Omega-3 fatty acid decreases oleic acid by decreasing SCD-1 expression in the liver and kidney of a cyclosporine-induced nephropathy rat model. Ren. Fail. 2019, 41, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Guillou, H.; Zadravec, D.; Martin, P.G.P.; Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid Res. 2010, 49, 186–199. [Google Scholar] [CrossRef]
- Paton, C.M.; Ntambi, J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E28–E37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karahashi, M.; Ishii, F.; Yamazaki, T.; Imai, K.; Mitsumoto, A.; Kawashima, Y.; Kudo, N. Up-Regulation of Stearoyl-CoA Desaturase 1 Increases Liver MUFA Content in Obese Zucker but Not Goto-Kakizaki Rats. Lipids 2013, 48, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Vinknes, K.J.; Elshorbagy, A.K.; Nurk, E.; Drevon, C.A.; Gjesdal, C.G.; Tell, G.S.; Nygård, O.; Vollset, S.E.; Refsum, H. Plasma stearoyl-CoA desaturase indices: Association with lifestyle, diet, and body composition. Obesity 2013, 21, E294–E302. [Google Scholar] [CrossRef]
- Rahman, S.M.; Dobrzyn, A.; Lee, S.-H.; Dobrzyn, P.; Miyazaki, M.; Ntambi, J.M. Stearoyl-CoA desaturase 1 deficiency increases insulin signaling and glycogen accumulation in brown adipose tissue. Am. J. Physiol. Metab. 2005, 288, E381–E387. [Google Scholar] [CrossRef] [Green Version]
- Svendsen, K.; Olsen, T.; Nordstrand Rusvik, T.C.; Ulven, S.M.; Holven, K.B.; Retterstøl, K.; Telle-Hansen, V.H. Fatty acid profile and estimated desaturase activities in whole blood are associated with metabolic health. Lipids Health Dis. 2020, 19, 102. [Google Scholar] [CrossRef]
- Bajaj, P.; Chowdhury, S.K.; Yucha, R.; Kelly, E.J.; Xiao, G. Emerging Kidney Models to Investigate Metabolism, Transport, and Toxicity of Drugs and Xenobiotics. Drug Metab. Dispos. 2018, 46, 1692–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, K.; Nichita, A.; Anoor, P.K.; Raju, S.; Singh, S.S.; Burgula, S. Understanding perspectives of signalling mechanisms regulating PEBP1 function. Cell Biochem. Funct. 2016, 34, 394–403. [Google Scholar] [CrossRef]
- Zhuo, W.-Q.; Wen, Y.; Luo, H.-J.; Luo, Z.-L.; Wang, L. Mechanisms of ferroptosis in chronic kidney disease. Front. Mol. Biosci. 2022, 9, 975582. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E.; Tyurina, Y.Y.; Zhao, J.; St. Croix, C.M.; Dar, H.H.; Mao, G.; Tyurin, V.A.; Anthonymuthu, T.S.; Kapralov, A.A.; Amoscato, A.A.; et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 2017, 171, 628–641.e26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez, L.; Pazos, M.; Giralt, M.; Nogués, M.R.; Pérez-Jiménez, J.; Torres, J.L.; Gallardo, J.M.; Medina, I. Targets of protein carbonylation in spontaneously hypertensive obese Koletsky rats and healthy Wistar counterparts: A potential role on metabolic disorders. J. Proteom. 2014, 106, 246–259. [Google Scholar] [CrossRef]
- Méndez, L.; Pazos, M.; Molinar-Toribio, E.; Sánchez-Martos, V.; Gallardo, J.M.; Rosa Nogués, M.; Torres, J.L.; Medina, I. Protein carbonylation associated to high-fat, high-sucrose diet and its metabolic effects. J. Nutr. Biochem. 2014, 25, 1243–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindner, H.A.; Täfler-Naumann, M.; Röhm, K.-H. N-acetylamino acid utilization by kidney aminoacylase-1. Biochimie 2008, 90, 773–780. [Google Scholar] [CrossRef]
- Maceyka, M.; Nava, V.E.; Milstien, S.; Spiegel, S. Aminoacylase 1 is a sphingosine kinase 1-interacting protein. FEBS Lett. 2004, 568, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Tyther, R.; Ahmeda, A.; Johns, E.; Sheehan, D. Protein carbonylation in kidney medulla of the spontaneously hypertensive rat. Proteom. —Clin. Appl. 2009, 3, 338–346. [Google Scholar] [CrossRef]
- Kieran, N.E.; Doran, P.P.; Connolly, S.B.; Greenan, M.-C.; Higgins, D.F.; Leonard, M.; Godson, C.; Taylor, C.T.; Henger, A.; Kretzler, M.; et al. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int. 2003, 64, 480–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Räisänen, S.R.; Lehenkari, P.; Tasanen, M.; Rahkila, P.; Härkönen, P.L.; Kalervo Väänänen, H. Carbonic anhydrase III protects cells from hydrogen peroxide-induced apoptosis. FASEB J. 1999, 13, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Mol, M.; Regazzoni, L.; Altomare, A.; Degani, G.; Carini, M.; Vistoli, G.; Aldini, G. Enzymatic and non-enzymatic detoxification of 4-hydroxynonenal: Methodological aspects and biological consequences. Free Radic. Biol. Med. 2017, 111, 328–344. [Google Scholar] [CrossRef]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, F.; Xu, R.; Awan, M.U.N.; Song, Y.; Han, Q.; Xia, X.; Zhang, J. PDIA3: Structure, functions and its potential role in viral infections. Biomed. Pharmacother. 2021, 143, 112110. [Google Scholar] [CrossRef] [PubMed]
- Ying, B.; Xu, W.; Nie, Y.; Li, Y. HSPA8 Is a New Biomarker of Triple Negative Breast Cancer Related to Prognosis and Immune Infiltration. Dis. Markers 2022, 2022, 8446857. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, P.; Pluznick, J.L. Acid-base regulation in the renal proximal tubules: Using novel pH sensors to maintain homeostasis. Am. J. Physiol. Physiol. 2018, 315, F1187–F1190. [Google Scholar] [CrossRef]
- Lakkis, J.I.; Weir, M.R. Obesity and Kidney Disease. Prog. Cardiovasc. Dis. 2018, 61, 157–167. [Google Scholar] [CrossRef]
- Baldini, N.; Avnet, S. The Effects of Systemic and Local Acidosis on Insulin Resistance and Signaling. Int. J. Mol. Sci. 2018, 20, 126. [Google Scholar] [CrossRef] [Green Version]
- Vanweert, F.; Schrauwen, P.; Phielix, E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr. Diabetes 2022, 12, 35. [Google Scholar] [CrossRef]
- Guo, L.; Chen, S.; Ou, L.; Li, S.; Ye, Z.-N.; Liu, H.-F. Disrupted Alpha-Ketoglutarate Homeostasis: Understanding Kidney Diseases from the View of Metabolism and Beyond. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Carnauba, R.; Baptistella, A.; Paschoal, V.; Hübscher, G. Diet-Induced Low-Grade Metabolic Acidosis and Clinical Outcomes: A Review. Nutrients 2017, 9, 538. [Google Scholar] [CrossRef] [Green Version]
- Corbet, C.; Draoui, N.; Polet, F.; Pinto, A.; Drozak, X.; Riant, O.; Feron, O. The SIRT1/HIF2α Axis Drives Reductive Glutamine Metabolism under Chronic Acidosis and Alters Tumor Response to Therapy. Cancer Res. 2014, 74, 5507–5519. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-Z.; Tao, S.-B.; Ma, L.; Fu, P. Roles of short-chain fatty acids in kidney diseases. Chin. Med. J. 2019, 132, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Felizardo, R.J.F.; Almeida, D.C.; Pereira, R.L.; Watanabe, I.K.M.; Doimo, N.T.S.; Ribeiro, W.R.; Cenedeze, M.A.; Hiyane, M.I.; Amano, M.T.; Braga, T.T.; et al. Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic- and GPR109a-mediated mechanisms. FASEB J. 2019, 33, 11894–11908. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| STD-C | STD-FG | STD-ω3 | STD-FG + ω3 | HFHS-C | HFHS-FG | HFHS-ω3 | HFHS-FG + ω3 | |
|---|---|---|---|---|---|---|---|---|
| Biometrical parameters | ||||||||
| Specific rate of body mass gain 2 (g/kg) *$ | 4.04 ab (0.76) | 3.21 b (0.23) | 3.97 ab (0.54) | 3.34 b (0.29) | 4.78 a (0.85) | 4.46 a (0.40) | 4.63 a (1.04) | 4.33 a (0.61) |
| BMI 3 (g/cm2) *$ | 0.83 ab (0.07) | 0.77 bc (0.03) | 0.80 abc (0.04) | 0.76 c (0.04) | 0.85 ab (0.04) | 0.83 ab (0.04) | 0.83 a (0.05) | 0.79 abc (0.04) |
| Perigonadal adipose tissue *$ | 8.99 bc (3.16) | 6.51 c (1.48) | 8.53 c (2.52) | 7.08 c (1.89) | 13.12 ab (3.92) | 10.78 abc (1.36) | 13.28 a (4.41) | 9.91 abc (2.15) |
| Adiposity index (%) *$ | 1.67 bc (0.44) | 1.33 c (0.28) | 1.65 bc (0.38) | 1.44 c (0.33) | 2.37 a (0.75) | 1.95 abc (0.27) | 2.32 ab (0.69) | 1.82 abc (0.33) |
| Biochemical determinations | ||||||||
| Plasma Insulin (ng/mL) * | 0.56 cd (0.32) | 0.34 d (0.08) | 0.65 bcd (0.19) | 0.43 d (0.20) | 1.81 a (0.82) | 1.36 abc (0.67) | 1.46 abc (0.72) | 1.41 abc (0.57) |
| Blood Glucose (mg/mL) * | 63.00 b (4.84) | 61.75 b (3.85) | 63.44 ab (4.10) | 63.33 ab (6.24) | 70.78 a (4.99) | 69.86 a (2.97) | 71.33 a (5.32) | 69.00 ab (3.71) |
| Triglycerides (mmol/L) ns | 0.69 (0.20) | 0.61 (0.14) | 0.56 (0.10) | 0.53 (0.15) | 0.52 (0.26) | 0.57 (0.07) | 0.59 (0.23) | 0.42 (0.13) |
| Cholesterol (mmol/L) *$# | 3.61 a (0.38) | 3.30 a (0.23) | 3.23 a (0.69) | 3.24 a (0.55) | 2.90 b (0.50) | 3.15 ab (0.52) | 2.55 b (0.42) | 2.60 b (0.29) |
| HDL-C (mmol/L) * | 1.15 a (0.12) | 1.08 a (0.08) | 1.06 a (0.18) | 1.07 a (0.11) | 0.94 b (0.16) | 0.94 b (0.07) | 0.85 b (0.12) | 0.93 b (0.07) |
| % fat in plasma * | 4.10 ab (0.87) | 4.62 ab (1.08) | 3.94 ab (1.54) | 5.28 a (1.20) | 4.00 ab (1.60) | 3.43 ab (1.44) | 3.31 ab (1.03) | 2.87 b (1.37) |
| Hemoglobin (g/dL) ns | 17.3 (1.24) | 16.86 (1.48) | 16.72 (1.99) | 16.32 (0.44) | 16.69 (0.77) | 16.76 (1.22) | 16.23 (1.37) | 16.19 (0.66) |
| Hematocrit (%) ns | 41.52 (3.12) | 39.6 (3.26) | 39.13 (4.56) | 38.55 (1.33) | 39.2 (1.82) | 38.59 (2.63) | 37.84 (3.06) | 37.81 (1.85) |
| Plasma Urea (ng/dL) * | 189.75 (11.58) | 230.94 (20.71) | 199.50 (94.42) | 214.21 (14.72) | 206.03 (40.54) | 262.01 (129.97) | 226.34 (37.05) | 155.37 (32.00) |
| Oxidative status | ||||||||
| Albumin carbonylation index $# | 0.41 ab (0.04) | 0.33 ab (0.05) | 0.39 ab (0.11) | 0.33 ab (0.05) | 0.43 a (0.11) | 0.42 ab (0.08) | 0.32 b (0.04) | 0.32 b (0.05) |
| Lipid peroxidation (mmol hydroperoxides/mL plasma) # | 0.13 (0.03) | 0.14 (0.03) | 0.13 (0.02) | 0.16 (0.02) | 0.21 (0.06) | 0.17 (0.04) | 0.14 (0.02) | 0.14 (0.02) |
| ORAC (µmol TE -Trolox equivalents-/mL) $ | 18.33 a (3.76) | 17.13 a (6.82) | 19.12 a (2.64) | 21.49 b (1.68) | 17.41 a (4.71) | 18.41 a (5.73) | 21.18 ab (6.12) | 21.08 ab (7.62) |
| GSSG/GSH in plasma *$ | 3.32 a (0.48) | 4.2 b (0.79) | 1.98 c (0.64) | 2.28 c (0.27) | 2.96 a (0.85) | 3.19 a (1.57) | 2.72 a (0.53) | 3.22 a (1.00) |
| GSSG/GSH in erythrocyte *$# | 0.60 a (0.25) | 0.78 ab (0.45) | 1.07 b (0.34) | 1.40 b (0.78) | 1.48 b (1.09) | 1.21 bc (0.34) | 1.56 b (0.87) | 0.29 c (0.11) |
| STD-C | STD-FG | STD-ω3 | STD-FG + ω3 | HFHS-C | HFHS-FG | HFHS-ω3 | HFHS-FG + ω3 | |
|---|---|---|---|---|---|---|---|---|
| Fatty acid | ||||||||
| 14:0 *$ | 0.71 abc (0.28) | 0.81 abc (0.19) | 0.60 bc (0.05) | 0.38 c (0.08) | 0.83 ab (0.16) | 1.05 a (0.41) | 0.90 ab (0.12) | 0.81 abc (0.18) |
| 16:0 *$# | 21.96 bc (0.21) | 21.77 bc (0.70) | 22.23 b (0.27) | 21.21 c (0.97) | 21.98 bc (0.28) | 22.28 b (0.72) | 23.40 a (0.43) | 22.49 ab (0.47) |
| 18:0 *$# | 11.01 bc (3.04) | 10.42 c (1.20) | 12.89 ab (1.47) | 14.84 b (0.66) | 15.08 a (0.28) | 14.99 a (0.81) | 14.98 a (0.79) | 15.27 a (0.54) |
| 20:0 *# | 0.15 cd (0.01) | 0.13 d (0.01) | 0.16 bcd (0.01) | 0.19 abc (0.01) | 0.19 ab (0.01) | 0.22 a (0.03) | 0.18 abc (0.00) | 0.19 abc (0.02) |
| 15:0 *$ | 0.28 bc (0.02) | 0.32 b (0.04) | 0.24 c (0.02) | 0.30 bc (0.04) | 0.42 a (0.02) | 0.49 a (0.04) | 0.40 a (0.03) | 0.47 a (0.03) |
| 17:0 *$ | 0.42 c (0.08) | 0.51 bc (0.04) | 0.44 c (0.04) | 0.56 ab (0.06) | 0.61 a (0.03) | 0.64 a (0.04) | 0.59 a (0.01) | 0.65 a (0.04) |
| SFAs *$# | 34.55 de (2.74) | 33.96 e (1.92) | 36.7 cd (1.16) | 37.48 bc (0.61) | 39.16 ab (1.15) | 39.67 ab (0.54) | 40.54 a (0.26) | 39.88 a (0.48) |
| 16:1ω7 *$ | 2.38 a (1.12) | 2.12 a (0.49) | 1.77 ab (0.96) | 0.93 b (0.32) | 1.04 b (0.24) | 1.03 b (0.27) | 1.00 b (0.29) | 0.86 b (0.10) |
| 18:1ω7 *$ | 3.72 a (0.57) | 3.77 a (0.24) | 3.29 ab (0.06) | 3.05 bc (0.29) | 2.93 bc (0.15) | 2.97 c (0.20) | 2.78 bc (0.09) | 2.71 c (0.17) |
| 18:1ω9 $# | 12.66 a (4.95) | 12.66 a (1.66) | 9.44 ab (2.94) | 6.56 b (0.90) | 9.83 ab (0.90) | 11.66 a (2.21) | 10.61 ab (1.50) | 10.47 ab (1.27) |
| MUFAs $# | 18.95 a (1.07) | 18.77 a (1.03) | 14.73 ab (2.71) | 10.69 b (1.10) | 14.03 ab (0.25) | 15.93 ab (0.95) | 14.65 ab (1.10) | 14.28 ab (1.71) |
| LA 18:2ω6 *$# | 20.21 ab (4.75) | 23.56 a (3.00) | 17.30 bc (2.22) | 15.74 c (2.05) | 9.26 d (0.85) | 9.03 d (0.99) | 10.72 d (0.28) | 11.09 d (0.77) |
| 20:2ω6 * | 0.37 a (0.04) | 0.36 a (0.02) | 0.37 a (0.06) | 0.42 a (0.04) | 0.16 b (0.01) | 0.15 b (0.02) | 0.14 b (0.01) | 0.14 b (0.02) |
| 20:3ω6 *$ | 0.61 c (0.18) | 0.57 c (0.07) | 0.76 bc (0.13) | 0.90 ab (0.06) | 0.97 a (0.12) | 0.93 ab (0.08) | 1.02 a (0.05) | 1.07 a (0.10) |
| ARA 20:4ω6 *$# | 21.46 cd (4.71) | 18.83 d (3.10) | 24.71 bcd (1.97) | 29.23 ab (2.20) | 31.67 a (1.50) | 29.86 ab (3.11) | 27.52 abc (0.86) | 28.03 abc (1.29) |
| 22:4ω6 *$# | 0.85 a (0.23) | 0.81 a (0.06) | 0.69 ab (0.15) | 0.84 a (0.15) | 0.76 a (0.08) | 0.77 a (0.11) | 0.46 c (0.01) | 0.51 bc (0.06) |
| 22:5ω6 $ | 0.23 a (0.01) | 0.26 a (0.03) | 0.21 ab (0.10) | 0.16 ab (0.08) | 0.27 a (0.04) | 0.22 ab (0.02) | 0.10 ab (0.01) | 0.05 b (0.06) |
| ω6 *# | 43.80 abc (2.76) | 44.49 ab (2.16) | 44.1 ab (2.34) | 47.18 a (3.19) | 43.05 bc (1.40) | 40.96 bc (2.27) | 40.01 c (1.03) | 40.83 bc (0.83) |
| ALA 18:3ω3 *$# | 0.55 ab (0.25) | 0.69 a (0.14) | 0.34 bc (0.03) | 0.26 cd (0.09) | 0.11 d (0.04) | 0.15 d (0.02) | 0.11 d (0.04) | 0.12 cd (0.03) |
| 18:4ω3 *$# | 0.23 b (0.03) | 0.32 a (0.05) | 0.17 bc (0.09) | 0.21 b (0.03) | 0.13 c (0.02) | 0.12 c (0.02) | 0.10 c (0.00) | 0.12 c (0.03) |
| EPA 20:5ω3 *$# | 0.18 cd (0.16) | 0.11 d (0.04) | 0.44 b (0.07) | 0.40 bc (0.08) | 0.35 bc (0.12) | 0.28 bcd (0.04) | 0.93 a (0.13) | 1.02 a (0.11) |
| DPA 22:5ω3 *$# | 0.52 d (0.13) | 0.49 d (0.03) | 0.81 b (0.12) | 0.95 a (0.07) | 0.57 cd (0.08) | 0.48 d (0.03) | 0.70 bc (0.09) | 0.70 bc (0.08) |
| DHA 22:6ω3 *$# | 1.22 b (0.32) | 1.19 b (0.17) | 2.57 a (0.30) | 2.71 a (0.26) | 2.61 a (0.25) | 2.44 a (0.23) | 2.97 a (0.20) | 3.04 a (0.24) |
| ω3 *$ | 2.69 e (0.32) | 2.77 de (0.13) | 4.32 ab (0.53) | 4.53 a (0.28) | 3.74 bc (0.22) | 3.43 ed (0.15) | 4.77 a (0.41) | 4.95 a (0.53) |
| PUFAs *$# | 46.49 b (2.91) | 47.26 b (1.72) | 48.48 ab (1.08) | 51.82 a (1.02) | 46.79 b (1.28) | 44.39 b (2.61) | 44.8 b (1.73) | 45.86 b (1.24) |
| ω6/ω3 *$# | 16:1 a [14:1, 18:1] | 16:1 a [16:1, 17:1] | 10:1 bc [9:1, 12:1] | 10:1 b [10:1, 13:1] | 12:1 b [12:1, 12:1] | 12:1 b [12:1, 12:1] | 8:1 cd [8:1, 11:1] | 8:1 d [8:1, 11:1] |
| EPA/DHA *$# | 1:10 bc [1:3, 1:17] | 1:12 bc [1:9, 1:15] | 1:6 b [1:4, 1:7] | 1:7 c [1:4, 1:10] | 1:8 bc [1:5, 1:14] | 1:9 bc [1:7, 1:11] | 1:3 a [1:2, 1:4] | 1:3 a [1:3, 1:4] |
| EPA/ARA *$# | 1:181 cd [1:48, 1:280] | 1:189 d [1:142, 1:220] | 1:59 b [1:43, 1:75] | 1:76 bc [1:57, 1:101] | 1:100 bcd [1:56, 1:171] | 1:110 bcd [1:80, 1:139] | 1:30 a [1:23, 1:38] | 1:28 a [1:22, 1:38] |
| DHA/ARA *$ | 1:18 d [1:15, 1:22] | 1:16 cd [1:14, 1:17] | 1:10 a [1:8, 1:11] | 1:11 ab [1:10, 1:14] | 1:12 bc [1:11, 1:15] | 1:12 bc [1:12, 1:13] | 1:10 a [1:9, 1:13] | 1:10 a [1:9, 1:13] |
| STD-C | STD-FG | STD-ω3 | STD-FG + ω3 | HFHS-C | HFHS-FG | HFHS- ω3 | HFHS-FG + ω3 | |
|---|---|---|---|---|---|---|---|---|
| Fatty acid | ||||||||
| 14:0 * | 0.66 b (0.04) | 0.62 b (0.06) | 0.67 b (0.09) | 0.63 b (0.11) | 1.46 a (0.20) | 1.49 a (0.13) | 1.65 a (0.29) | 1.51 a (0.22) |
| 16:0 * | 20.80 b (0.59) | 20.45 b (0.43) | 21.72 ab (1.04) | 21.03 b (1.17) | 23.81 ab (1.18) | 23.59 ab (0.57) | 22.44 ab (4.73) | 24.97 a (2.72) |
| 18:0 * | 7.98 c (0.54) | 8.61 abc (0.92) | 8.36 bc (0.48) | 8.13 bc (2.18) | 9.84 abc (0.84) | 10.31 a (0.78) | 9.90 ab (0.73) | 9.97 ab (0.78) |
| 15:0 * | 0.38 b (0.05) | 0.40 b (0.03) | 0.35 b (0.04) | 0.37 b (0.08) | 0.60 a (0.07) | 0.66 a (0.05) | 0.63 a (0.06) | 0.69 a (0.03) |
| 17:0 *$# | 0.52 cd (0.07) | 0.70 a (0.09) | 0.48 d (0.04) | 0.57 bcd (0.06) | 0.60 abc (0.03) | 0.63 ab (0.03) | 0.61 abc (0.05) | 0.65 ab (0.04) |
| SFAs * | 30.33 c (0.50) | 30.79 c (0.97) | 31.58 bc (0.82) | 30.73 c (1.92) | 36.30 a (1.48) | 36.68 a (0.76) | 35.23 ab (4.64) | 37.79 a (2.38) |
| 16:1ω7 * | 1.42 a (0.25) | 1.13 a (0.12) | 1.48 a (0.18) | 1.40 a (0.34) | 1.87 a (0.70) | 1.67 a (0.38) | 2.70 a (2.40) | 1.37 a (0.36) |
| 18:1ω7 * | 2.55 a (0.20) | 2.72 a (0.35) | 2.47 a (0.16) | 2.63 a (0.43) | 1.85 b (0.28) | 1.64 b (0.23) | 1.70 b (0.33) | 1.63 b (0.21) |
| 18:1ω9 * | 6.79 b (0.82) | 6.23 b (0.78) | 6.95 b (0.83) | 6.25 b (0.51) | 15.49 a (1.71) | 14.95 a (0.92) | 15.75 a (2.53) | 14.40 a (2.29) |
| 24:1ω9 *$ | 0.22 a (0.02) | 0.19 a (0.05) | 0.20 a (0.03) | 0.26 a (0.10) | 0.27 a (0.05) | 0.20 a (0.05) | 0.27 a (0.05) | 0.27 a (0.03) |
| MUFAs * | 11.29 b (1.06) | 10.47 b (1.04) | 11.28 b (1.01) | 10.80 b (1.07) | 19.69 a (2.42) | 18.69 a (1.29) | 20.66 a (4.77) | 17.85 a (2.25) |
| LA 18:2ω6 *$ | 20.80 a (2.49) | 20.62 a (1.94) | 22.46 a (2.60) | 21.85 a (1.56) | 11.36 b (0.97) | 10.66 b (0.96) | 12.42 b (0.96) | 12.37 b (0.42) |
| 18:3ω6 * | 0.55 a (0.08) | 0.54 a (0.05) | 0.41 abc (0.07) | 0.49 ab (0.17) | 0.35 bc (0.04) | 0.36 bc (0.07) | 0.33 c (0.04) | 0.32 c (0.07) |
| 20:3ω6 *$ | 0.26 d (0.04) | 0.30 d (0.02) | 0.39 cd (0.06) | 0.38 cd (0.11) | 0.61 ab (0.17) | 0.52 bc (0.08) | 0.73 a (0.10) | 0.74 a (0.09) |
| ARA 20:4ω6 *$ | 32.33 ab (2.76) | 33.16 a (2.46) | 27.26 bcd (3.81) | 29.41 abc (2.46) | 26.74 cd (3.72) | 27.78 abcd (1.58) | 22.86 d (2.67) | 23.63 d (3.64) |
| ω6 *$ | 53.95 ab (0.54) | 54.62 a (0.76) | 50.52 b (1.53) | 52.13 ab (1.45) | 39.07 c (2.85) | 39.32 c (1.01) | 36.34 c (1.91) | 37.06 c (3.79) |
| ALA 18:3ω3 * | 0.47 a (0.11) | 0.42 abc (0.06) | 0.45 ab (0.08) | 0.45 ab (0.12) | 0.31 bcd (0.07) | 0.27 d (0.03) | 0.29 cd (0.07) | 0.25 d (0.06) |
| EPA 20:5ω3 $# | 0.84 bcd (0.15) | 0.82 cd (0.37) | 1.29 abc (0.23) | 1.36 ab (0.42) | 0.69 d (0.14) | 0.61 d (0.14) | 1.74 a (0.41) | 1.76 a (0.27) |
| DPA 22:5ω3 $# | 0.80 b (0.10) | 0.71 b (0.11) | 1.35 a (0.07) | 0.90 b (0.18) | 0.65 b (0.10) | 1.01 ab (0.61) | 0.85 b (0.11) | 0.81 b (0.09) |
| DHA 22:6ω3 *$ | 2.32 c (0.26) | 2.16 c (0.25) | 3.53 b (0.49) | 3.63 b (0.54) | 3.29 b (0.40) | 3.42 b (0.39) | 4.88 a (0.49) | 4.48 a (0.56) |
| ω3 *$ | 4.43 de (0.33) | 4.12 e (0.56) | 6.62 b (0.47) | 6.34 bc (0.74) | 4.94 de (0.32) | 5.31 cd (0.55) | 7.77 a (0.82) | 7.30 ab (0.65) |
| PUFAs * | 58.38 a (0.81) | 58.74 a (0.97) | 57.15 a (1.62) | 58.47 a (1.20) | 44.01 b (2.94) | 44.64 b (1.24) | 44.11 b (1.76) | 44.36 b (4.25) |
| ω6/ω3 *$# | 12:1 a [11:1, 13:1] | 13:1 a [11:1, 15:1] | 8:1 b [7:1, 8:1] | 8:1 b [7:1, 10:1] | 8:1 b [7:1, 9:1] | 7:1 b [6:1, 8:1] | 5:1 c [4:1, 5:1] | 5:1 c [5:1, 6:1] |
| EPA/DHA *$# | 1:3 ab [1:2, 1:3] | 1:3 a [1:1, 1:5] | 1:3 ab [1:2, 1:3] | 1:3 a [1:2, 1:4] | 1:5 ab [1:3, 1:7] | 1:6 b [1:4, 1:9] | 1:3 ab [1:2, 1:4] | 1:3 a [1:2, 1:3] |
| EPA/ARA *$# | 1:38 b [1:32, 1:45] | 1:54 b [1:41, 1:85] | 1:22 b [1:15, 1:30] | 1:24 b [1:13, 1:34] | 1:41 b [1:25, 1:61] | 1:48 b [1:35, 1:73] | 1:14 a [1:9, 1:21] | 1:14 a [1:10, 1:17] |
| DHA/ARA *$ | 1:14 c [1:13, 1:15] | 1:15 c [1:15, 1:18] | 1:8 b [1:6, 1:9] | 1:8 b [1:7, 1:11] | 1:8 b [1:7, 1:9] | 1:8 b [1:8, 1:9] | 1:5 a [1:4, 1:5] | 1:5 a [1:5, 1:6] |
| STD-C | STD-FG | STD-ω3 | STD-FG + ω3 | HFHS-C | HFHS-FG | HFHS- ω3 | HFHS-FG + ω3 | |
|---|---|---|---|---|---|---|---|---|
| Desaturase and elongases activities in kidney | ||||||||
| Elongase-6 (18:0/16:0) *$# | 0.50 b (0.14) | 0.48 b (0.06) | 0.58 ab (0.07) | 0.70 a (0.06) | 0.69 a (0.02) | 0.67 a (0.06) | 0.64 a (0.02) | 0.68 a (0.03) |
| SCD-16 = [16:1ω7/16:0] *$ | 0.11 a (0.05) | 0.10 a (0.02) | 0.08 ab (0.02) | 0.04 b (0.03) | 0.05 b (0.01) | 0.05 b (0.01) | 0.04 b (0.01) | 0.04 b (0.01) |
| SCD-18 = [18:1ω9/18:0] *$# | 1.36 a (0.92) | 1.28 ab (0.27) | 0.74 abc (0.35) | 0.45 c (0.07) | 0.66 bc (0.08) | 0.79 abc (0.19) | 0.71 abc (0.07) | 0.69 abc (0.11) |
| Δ4D = [22:6ω3/22:5ω3] *# | 2.35 b (0.21) | 2.39 b (0.21) | 3.22 b (0.28) | 2.86 b (0.33) | 4.58 a (0.84) | 5.07 a (0.63) | 4.33 a (0.46) | 4.33 a (0.11) |
| Δ5D = [20:4ω6/20:3ω6] *$ | 35.24 a (4.52) | 33.09 ab (3.03) | 32.52 ab (3.21) | 32.39 ab (1.97) | 32.65 ab (1.03) | 32.61 ab (4.67) | 27.37 b (2.22) | 26.78 b (2.99) |
| Δ6D = [20:3ω6/18:2ω6] *$# | 0.03 cd (0.02) | 0.02 d (0.01) | 0.04 bc (0.00) | 0.05 b (0.01) | 0.10 a (0.01) | 0.09 a (0.01) | 0.09 a (0.03) | 0.09 a (0.02) |
| Δ5D + Δ6D = [20:5ω3/18:3ω3] *$# | 0.73 d (0.39) | 0.51 d (0.16) | 1.43 bc (0.33) | 1.63 bc (0.48) | 3.17 b (0.55) | 4.25 bc (0.42) | 8.78 a (1.97) | 9.45 a (2.28) |
| Desaturase and elongases activities in plasma | ||||||||
| Elongase-6 (18:0/16:0) ns | 0.38 (0.03) | 0.42 (0.05) | 0.39 (0.04) | 0.39 (0.11) | 0.41 (0.04) | 0.44 (0.04) | 0.47 (0.15) | 0.41 (0.07) |
| SCD-16 = [16:1ω7/16:0] ns | 0.07 (0.01) | 0.06 (0.01) | 0.07 (0.01) | 0.07 (0.01) | 0.08 (0.03) | 0.07 (0.01) | 0.16 (0.21) | 0.06 (0.02) |
| SCD-18 = [18:1ω9/18:0] * | 0.86 b (0.16) | 0.73 b (0.15) | 0.84 b (0.14) | 0.88 b (0.50) | 1.60 a (0.33) | 1.46 a (0.19) | 1.61 a (0.36) | 1.47 a (0.34) |
| Δ4D = [22:6ω3/22:5ω3] *$# | 2.93 c (0.40) | 3.10 c (0.57) | 2.63 c (0.45) | 4.13 bc (0.86) | 5.15 ab (1.18) | 4.08 bc (1.47) | 5.81 a (0.93) | 5.57 ab (0.74) |
| Δ5D = [20:4ω6/20:3ω6] *$ | 123.48 a (14.14) | 111.62 ab (10.90) | 72.67 cd (20.84) | 83.94 bc (26.77) | 47.94 de (18.18) | 54.77 cde (12.04) | 32.06 e (6.18) | 32.10 e (5.02) |
| Δ6D = [20:3ω6/18:2ω6] *$ | 0.01 b (0.00) | 0.01 b (0.00) | 0.02 b (0.00) | 0.02 b (0.00) | 0.05 a (0.01) | 0.05 a (0.01) | 0.06 a (0.01) | 0.06 a (0.01) |
| Δ5D + Δ6D = [20:5ω3/18:3ω3] *$# | 1.85 b (0.53) | 1.97 b (0.78) | 2.95 b (0.77) | 3.09 b (0.90) | 2.30 b (0.50) | 2.27 b (0.40) | 6.14 a (1.58) | 7.32 a (2.06) |
| Spot Nº | Protein ID | Gene Name | Avg. Mass | UniProtKB Code |
|---|---|---|---|---|
| 1 | Phosphatidylethanolamine-binding protein 1 OS = Rattus norvegicus OX = 10,116 GN = Pebp1 PE = 1 SV = 3 | Pebp1 | 20,801 | P31044|PEBP1_RAT |
| 2 | Superoxide dismutase [Mn] mitochondrial OS = Rattus norvegicus OX = 10116 GN = Sod2 PE = 1 SV = 2 | Sod2 | 24,674 | P07895|SODM_RAT |
| 3 | Glutathione S-transferase alpha-3 OS = Rattus norvegicus OX = 10116 GN = Gsta3 PE = 1 SV = 3 | Gsta3 | 25,319 | P04904|GSTA3_RAT |
| Glutathione S-transferase P OS = Rattus norvegicus OX = 10116 GN = Gstp1 PE = 1 SV = 2 | Gstp1 | 23,439 | P04906|GSTP1_RAT | |
| Glutathione S-transferase alpha-4 OS = Rattus norvegicus OX = 10116 GN = Gsta4 PE = 1 SV = 2 | Gsta4 | 25,510 | P14942|GSTA4_RAT | |
| 4 | Peroxisomal trans-2-enoyl-CoA reductase OS = Rattus norvegicus OX = 10116 GN = Pecr PE = 2 SV = 1 | Pecr | 32,433 | Q9WVK3|PECR_RAT |
| 5 | Triosephosphate isomerase OS = Rattus norvegicus OX = 10116 GN = Tpi1 PE = 1 SV = 2 | Tpi1 | 26,849 | P48500|TPIS_RAT |
| Electron transfer flavoprotein subunit beta OS = Rattus norvegicus OX = 10116 GN = Etfb PE = 1 SV = 3 | Etfb | 27,687 | Q68FU3|ETFB_RAT | |
| 6 | Enoyl-CoA delta isomerase 1 mitochondrial OS = Rattus norvegicus OX = 10116 GN = Eci1 PE = 1 SV = 1 | Eci1 | 32,254 | P23965|ECI1_RAT |
| 7 | Carbonic anhydrase 2 OS = Rattus norvegicus OX = 10116 GN = Ca2 PE = 1 SV = 2 | Ca2 | 29,114 | P27139|CAH2_RAT |
| 8 | Omega-amidase NIT2 OS = Rattus norvegicus OX = 10116 GN = Nit2 PE = 1 SV = 1 | Nit2 | 30,701 | Q497B0|NIT2_RAT |
| 9 | 3-hydroxyisobutyrate dehydrogenase mitochondrial OS = Rattus norvegicus OX = 10116 GN = Hibadh PE = 1 SV = 3 | Hibadh | 35,303 | P29266|3HIDH_RAT |
| 10 | Malate dehydrogenase cytoplasmic OS = Rattus norvegicus OX = 10116 GN = Mdh1 PE = 1 SV = 3 | Mdh1 | 36,483 | O88989|MDHC_RAT |
| 11 | Actin cytoplasmic 1 OS = Rattus norvegicus OX = 10116 GN = Actb PE = 1 SV = 1 | Actb | 41,737 | P60711|ACTB_RAT |
| 12 | Heat shock cognate 71 kDa protein OS = Rattus norvegicus OX = 10116 GN = Hspa8 PE = 1 SV = 1 | Hspa8 | 70,871 | P63018|HSP7C_RAT |
| 13 | Fructose-bisphosphate aldolase B OS = Rattus norvegicus OX = 10116 GN = Aldob PE = 1 SV = 2 | Aldob | 39,618 | P00884|ALDOB_RAT |
| 14 | Aspartate aminotransferase mitochondrial OS = Rattus norvegicus OX = 10116 GN = Got2 PE = 1 SV = 2 | Got2 | 47,314 | P00507|AATM_RAT |
| 15 | Malate dehydrogenase mitochondrial OS = Rattus norvegicus OX = 10116 GN = Mdh2 PE = 1 SV = 2 | Mdh2 | 35,684 | P04636|MDHM_RAT |
| 16 | Glyceraldehyde-3-phosphate dehydrogenase OS = Rattus norvegicus OX = 10116 GN = Gapdh PE = 1 SV = 3 | Gapdh | 35,828 | P04797|G3P_RAT |
| 17 | Hydroxyacyl-coenzyme A dehydrogenase mitochondrial OS = Rattus norvegicus OX = 10116 GN = Hadh PE = 2 SV = 1 | Hadh | 34,448 | Q9WVK7|HCDH_RAT |
| 18 | Hydroxyacid oxidase 2 OS = Rattus norvegicus OX = 10116 GN = Hao2 PE = 1 SV = 2 | Hao2 | 39,201 | Q07523|HAOX2_RAT |
| 19 | Aflatoxin B1 aldehyde reductase member 2 OS = Rattus norvegicus OX = 10116 GN = Akr7a2 PE = 1 SV = 2 | Akr7a2 | 40,675 | Q8CG45|ARK72_RAT |
| 20 | Aldo-keto reductase family 1 member A1 OS = Rattus norvegicus OX = 10116 GN = Akr1a1 PE = 1 SV = 2 | Akr1a1 | 36,506 | P51635|AK1A1_RAT |
| 21 | Sorbitol dehydrogenase OS = Rattus norvegicus OX = 10116 GN = Sord PE = 1 SV = 4 | Sord | 38,235 | P27867|DHSO_RAT |
| 22 | Aspartate aminotransferase cytoplasmic OS = Rattus norvegicus OX = 10116 GN = Got1 PE = 1 SV = 3 | Got1 | 46,429 | P13221|AATC_RAT |
| 23 | Isocitrate dehydrogenase [NADP] cytoplasmic OS = Rattus norvegicus OX = 10116 GN = Idh1 PE = 1 SV = 1 | Idh1 | 46,734 | P41562|IDHC_RAT |
| 24 | Aminoacylase-1A OS = Rattus norvegicus OX = 10116 GN = Acy1a PE = 1 SV = 1 | Acy1a | 45,804 | Q6AYS7|ACY1A_RAT |
| 25 | Aldehyde dehydrogenase mitochondrial OS = Rattus norvegicus OX = 10116 GN = Aldh2 PE = 1 SV = 1 | Aldh2 | 56,488 | P11884|ALDH2_RAT |
| 4-trimethylaminobutyraldehyde dehydrogenase OS = Rattus norvegicus OX = 10116 GN = Aldh9a1 PE = 1 SV = 1 | Aldh9a1 | 53,653 | Q9JLJ3|AL9A1_RAT | |
| 26 | Serum albumin OS = Rattus norvegicus OX = 10116 GN = Alb PE = 1 SV = 2 | Alb | 68,731 | P02770|ALBU_RAT |
| 27 | Protein disulfide-isomerase A3 OS = Rattus norvegicus OX = 10116 GN = Pdia3 PE = 1 SV = 2 | Pdia3 | 56,623 | P11598|PDIA3_RAT |
| Alpha-aminoadipic semialdehyde dehydrogenase OS = Rattus norvegicus OX = 10116 GN = Aldh7a1 PE = 1 SV = 2 | Aldh7a1 | 58,749 | Q64057|AL7A1_RAT | |
| 28 | Glutamate dehydrogenase 1 mitochondrial OS = Rattus norvegicus OX = 10116 GN = Glud1 PE = 1 SV = 2 | Glud1 | 61,416 | P10860|DHE3_RAT |
| 29 | Alanine--glyoxylate aminotransferase 2 mitochondrial OS = Rattus norvegicus OX = 10116 GN = Agxt2 PE = 1 SV = 2 | Agxt2 | 57,201 | Q64565|AG_RAT |
| 30 | Retinal dehydrogenase 1 OS = Rattus norvegicus OX = 10116 GN = Aldh1a1 PE = 1 SV = 3 | Aldh1a1 | 54,459 | P51647|AL1A1_RAT |
| 31 | Succinyl-CoA:3-ketoacid coenzyme A transferase 1 mitochondrial OS = Rattus norvegicus OX = 10116 GN = Oxct1 PE = 1 SV = 1 | Oxct1 | 56,204 | B2GV06|SCOT1_RAT |
| Dihydrolipoyl dehydrogenase mitochondrial OS = Rattus norvegicus OX = 10116 GN = Dld PE = 1 SV = 1 | Dld | 54,038 | Q6P6R2|DLDH_RAT | |
| Methylmalonate-semialdehyde dehydrogenase [acylating] mitochondrial OS = Rattus norvegicus OX = 10116 GN = Aldh6a1 PE = 1 SV = 1 | Aldh6a1 | 57,808 | Q02253|MMSA_RAT | |
| 32 | Catalase OS = Rattus norvegicus OX = 10116 GN = Cat PE = 1 SV = 3 | Cat | 59,757 | P04762|CATA_RAT |
| Triokinase/FMN cyclase OS = Rattus norvegicus OX = 10116 GN = Tkfc PE = 1 SV = 1 | Tkfc | 59,444 | Q4KLZ6|TKFC_RAT | |
| 33 | Acyl-coenzyme A synthetase ACSM2 mitochondrial OS = Rattus norvegicus OX = 10116 GN = Acsm2 PE = 2 SV = 2 | Acsm2 | 64,145 | O70490|ACSM2_RAT |
| Transketolase OS = Rattus norvegicus OX = 10116 GN = Tkt PE = 1 SV = 1 | Tkt | 67,644 | P50137|TKT_RAT | |
| 34 | Aconitate hydratase mitochondrial OS = Rattus norvegicus OX = 10116 GN = Aco2 PE = 1 SV = 2 | Aco2 | 85,433 | Q9ER34|ACON_RAT |
| 35 | Serotransferrin OS = Rattus norvegicus OX = 10116 GN = Tf PE = 1 SV = 3 | Tf | 76,395 | P12346|TRFE_RAT |
| 36 | Cytoplasmic aconitate hydratase OS = Rattus norvegicus OX = 10116 GN = Aco1 PE = 1 SV = 1 | Aco1 | 98,127 | Q63270|ACOC_RAT |
| ID | KEGG Pathway | Effect of HFHS | Effect of FG | Effect of ω3 | Effect of FG + ω3 |
|---|---|---|---|---|---|
| rno04964 | Proximal tubule bicarbonate reclamation | 6.10 × 10−3 | |||
| rno00910 | Nitrogen metabolism | 4.60 × 10−3 | |||
| rno01230 | Biosynthesis of amino acids | 3.61 × 10−8 | 0.00051 | 5.05 × 10−8 | 1.28 × 10−6 |
| rno00400 | Phenylalanine, tyrosine and tryptophan biosynthesis | ||||
| rno00360 | Phenylalanine metabolism | ||||
| rno00350 | Tyrosine metabolism | ||||
| rno00380 | Tryptophan metabolism | 3.20 × 10−7 | 0.0116 | 0.00083 | |
| rno00280 | Valine, leucine and isoleucine degradation | 8.40 × 10−9 | 0.00028 | 0.0222 | |
| rno00250 | Alanine, aspartate and glutamate metabolism | 0.0114 | 0.0062 | 0.0117 | |
| rno00410 | beta-Alanine metabolism | 1.90 × 10−4 | |||
| rno00260 | Glycine, serine and threonine metabolism | ||||
| rno00330 | Arginine and proline metabolism | 0.00077 | 0.0222 | ||
| rno00220 | Arginine biosynthesis | 8.47 × 10−5 | 0.003 | 0.0056 | |
| rno00270 | Cysteine and methionine metabolism | ||||
| rno00340 | Histidine metabolism | 0.0072 | |||
| rno00310 | Lysine degradation | 4.07 × 10−5 | 0.0258 | ||
| rno01200 | Carbon metabolism | 2.86 × 10−16 | 1.59 × 10−13 | 1.59 × 10−13 | 5.01× 10−13 |
| rno00010 | Glycolysis/Gluconeogenesis | 1.44 × 10−8 | 0.00034 | 0.00034 | 3.51 × 10−5 |
| rno00020 | Citrate cycle (TCA cycle) | 9.20 × 10−3 | 0.0066 | ||
| rno00620 | Pyruvate metabolism | 3.10 × 10−4 | |||
| rno01210 | 2-Oxocarboxylic acid metabolism | 6.91 × 10−5 | 0.0028 | 0.0055 | |
| rno00030 | Pentose phosphate pathway | 0.0092 | 0.0054 | 0.0099 | |
| rno00040 | Pentose and glucuronate interconversions | 9.20 × 10−3 | 0.0054 | 0.0099 | |
| rno00051 | Fructose and mannose metabolism | 0.00026 | 0.0001 | 7.81 × 10−5 | 0.00038 |
| rno00630 | Glyoxylate and dicarboxylate metabolism | 2.10 × 10−4 | 0.0066 | 0.0099 | |
| rno00071 | Fatty acid degradation | 2.04 × 10−5 | 0.00083 | ||
| rno00561 | Glycerolipid metabolism | 5.00 × 10−5 | 0.0174 | 1.50 × 10−3 | |
| rno00650 | Butanoate metabolism | 0.00016 | 0.0086 | ||
| rno00640 | Propanoate metabolism | 0.0099 | 0.0066 | ||
| rno00053 | Ascorbate and aldarate metabolism | 0.0061 | |||
| rno00480 | Glutathione metabolism | ||||
| rno00980 | Metabolism of xenobiotics by cytochrome P450 | ||||
| rno04146 | Peroxisome | 0.0028 | 0.0287 | 0.0263 | 2.80 × 10−3 |
| rno04213 | Longevity regulating pathway—multiple species | 0.0323 | 0.0281 | ||
| rno05418 | Fluid shear stress and atherosclerosis | ||||
| rno04066 | HIF-1 signaling pathway | 5.90 × 10−3 | 0.0465 | 0.0429 | 0.0055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez, L.; Muñoz, S.; Barros, L.; Miralles-Pérez, B.; Romeu, M.; Ramos-Romero, S.; Torres, J.L.; Medina, I. Combined Intake of Fish Oil and D-Fagomine Prevents High-Fat High-Sucrose Diet-Induced Prediabetes by Modulating Lipotoxicity and Protein Carbonylation in the Kidney. Antioxidants 2023, 12, 751. https://doi.org/10.3390/antiox12030751
Méndez L, Muñoz S, Barros L, Miralles-Pérez B, Romeu M, Ramos-Romero S, Torres JL, Medina I. Combined Intake of Fish Oil and D-Fagomine Prevents High-Fat High-Sucrose Diet-Induced Prediabetes by Modulating Lipotoxicity and Protein Carbonylation in the Kidney. Antioxidants. 2023; 12(3):751. https://doi.org/10.3390/antiox12030751
Chicago/Turabian StyleMéndez, Lucía, Silvia Muñoz, Lorena Barros, Bernat Miralles-Pérez, Marta Romeu, Sara Ramos-Romero, Josep Lluís Torres, and Isabel Medina. 2023. "Combined Intake of Fish Oil and D-Fagomine Prevents High-Fat High-Sucrose Diet-Induced Prediabetes by Modulating Lipotoxicity and Protein Carbonylation in the Kidney" Antioxidants 12, no. 3: 751. https://doi.org/10.3390/antiox12030751







