Food Peptides for the Nutricosmetic Industry
Abstract
:1. Introduction
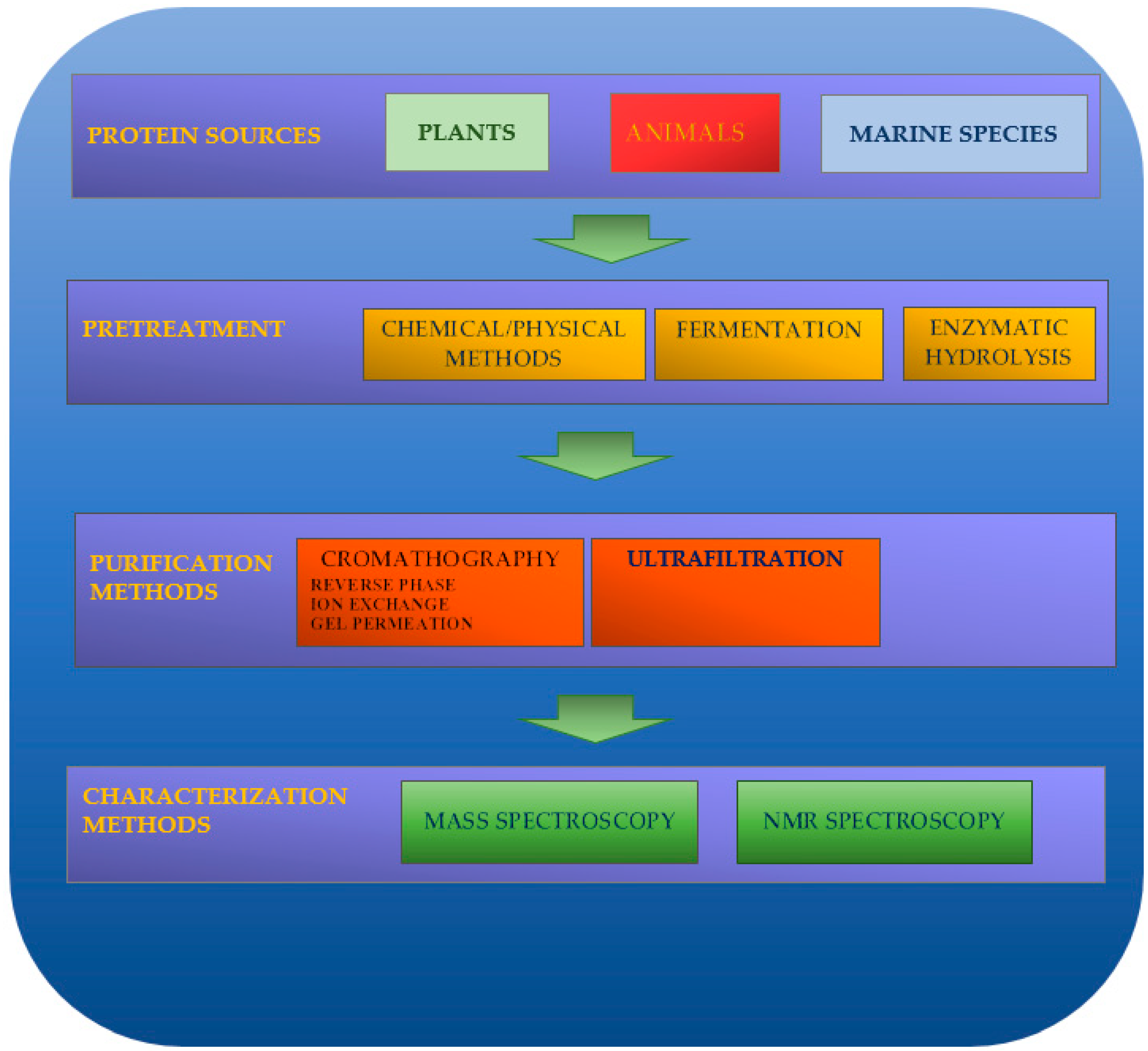
2. Production Methods for Natural Biopeptides
2.1. Pretreatment
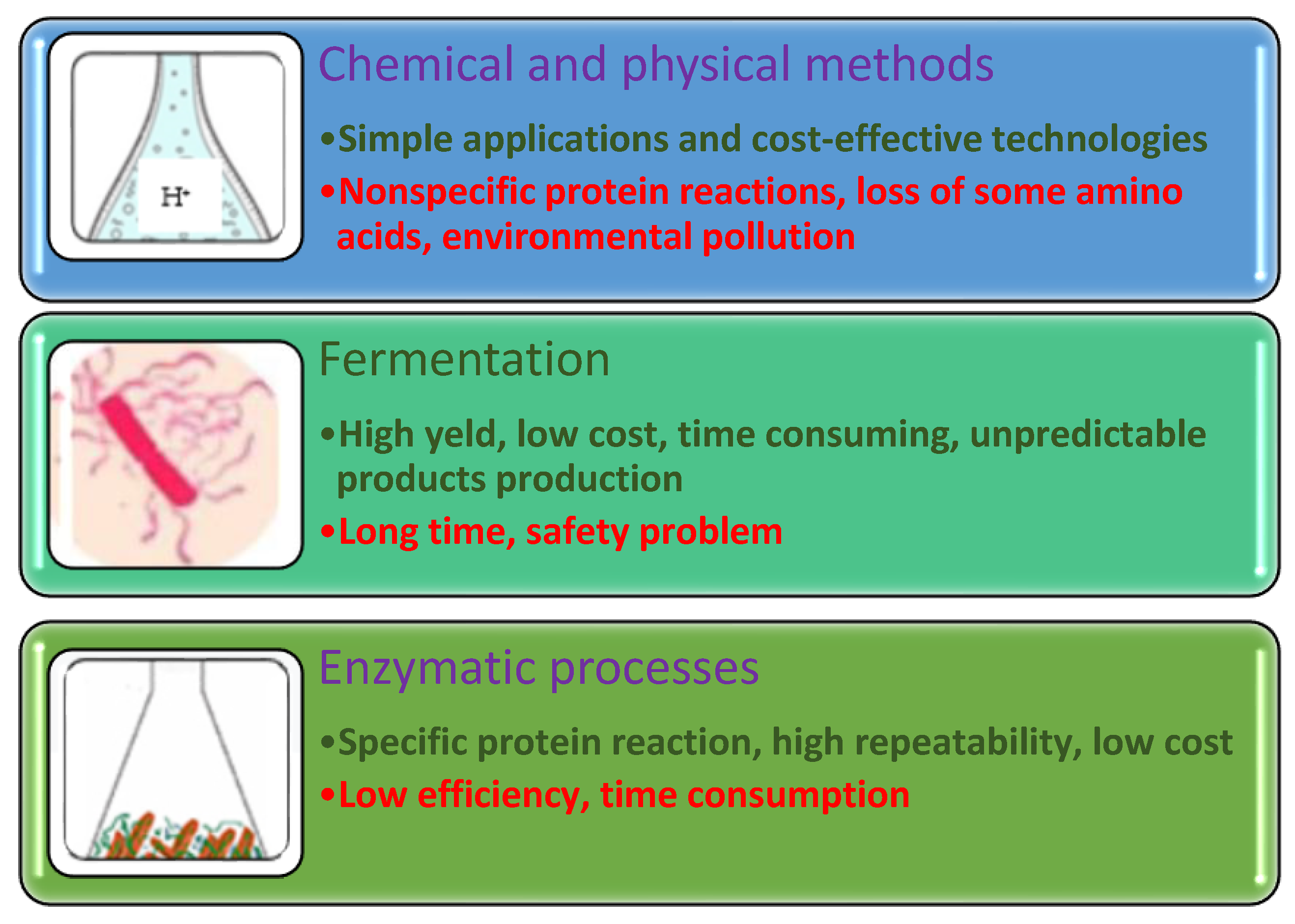
2.1.1. Chemical Processes
2.1.2. Physical Methods
2.1.3. Fermentation
2.1.4. Enzymatic Processes
2.2. Purification Technologies
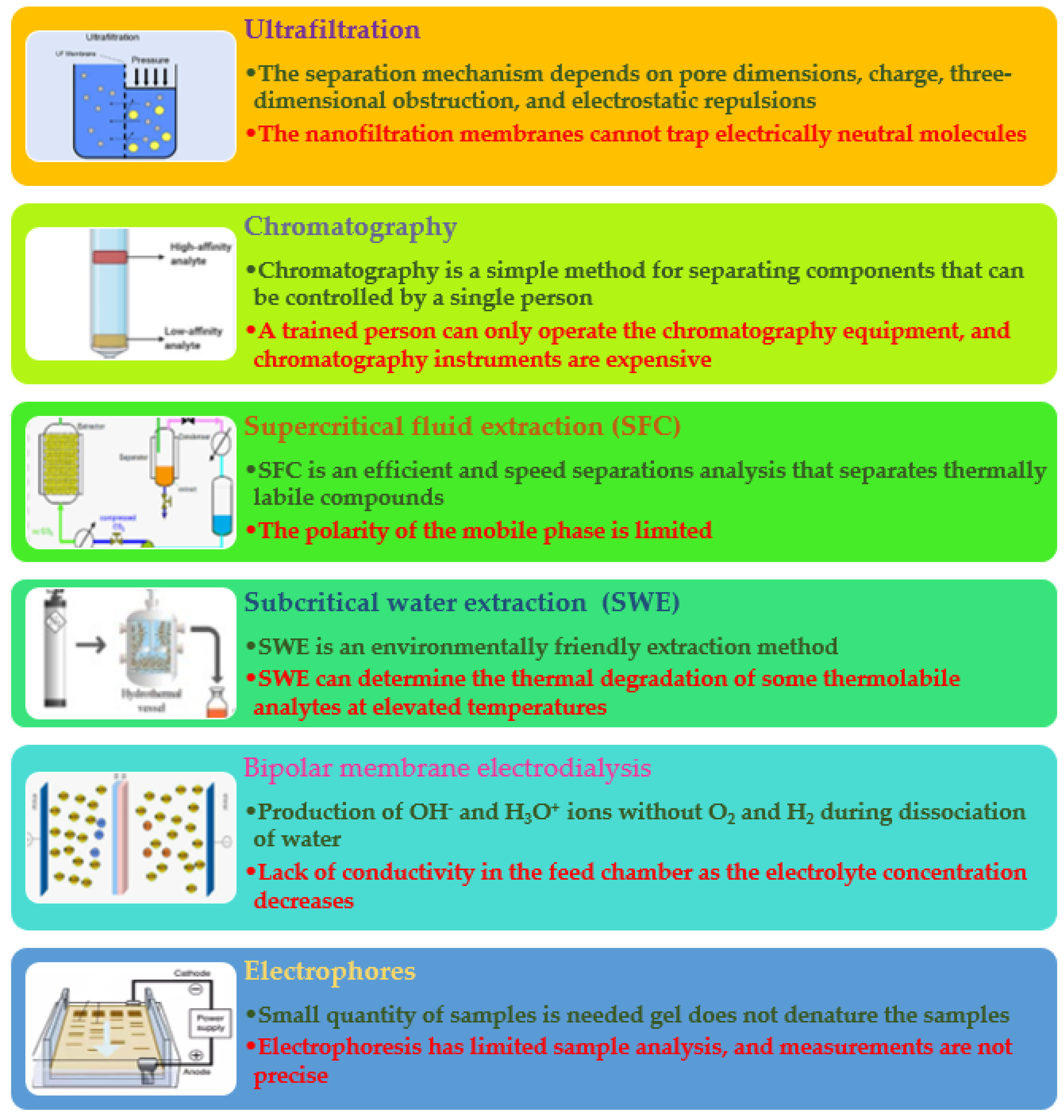
2.2.1. Ultrafiltration
2.2.2. Chromatography
2.2.3. Supercritical Fluid Extraction
2.2.4. Subcritical Water Extraction (Pressurized Hot Water or Hot-Compressed Water)
2.2.5. Bipolar Membrane Electrodialysis
2.2.6. Electrophores
2.3. Identification of Peptide Sequences
2.3.1. Mass Spectroscopy
2.3.2. NMR
3. The Human Skin
4. Biopeptides’ Potential in Cosmeceutical Applications
4.1. Biopeptides with Anti-Aging Properties
4.1.1. Biopeptides That Decrease Collagenase Activity
4.1.2. Biopeptides That Decrease Hyaluronidase Activity
4.1.3. Biopeptides That Decrease Tyrosinase Action
4.1.4. Biopeptides That Decrease Elastase Action
4.2. Biopeptides with Antioxidant Properties Derived from Foods
Methods Used to Evaluate the Antioxidant Potential
4.3. Peptides with Antimicrobial Activity
4.4. Peptides with Anti-Inflammatory Activity
5. Peptide Delivery Systems
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cosmetic Ingredient Review. Safety Assessment of Soy Proteins and Peptides as Used in Cosmetics. Available online: https://www.cir-safety.org/sites/default/files/soypep092015final.pdf (accessed on 30 April 2020).
- Ferreira, M.S.; Magalhães, M.C.; Sousa-Lobo, J.M.; Almeida, I.F. Trending Anti-Aging Peptides. Cosmetics 2020, 7, 91. [Google Scholar] [CrossRef]
- Tkaczewska, J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings—A review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Bioactive Peptides Market Analysis. Available online: https://www.coherentmarketinsights.com/market-insight/bioactive-peptide-market-3018 (accessed on 1 September 2022).
- European Commission. Commission Decision (EU) 2022/677 of 29 April 2022 establishing a glossary of common ingredient names for use in the labelling of cosmetic products. Off. J. Eur. Union 2022, 127, 1–448. [Google Scholar]
- European Commission. Commission Decision (EU) 2019/701 of 5 April 2019 establishing a glossary of common ingredient names for use in the labelling of cosmetic products. Off. J. Eur. Union 2019, 121, 1–370. [Google Scholar]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Zaky, A.A.; El-Aty, A.A.; Ma, A.; Jia, Y. An overview on antioxidant peptides from rice bran proteins: Extraction, identification, and applications. Crit. Rev. Food Sci. Nutr. 2020, 62, 1350–1362. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Tadesse, S.A.; Emire, S.A. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon 2020, 6, e04765. [Google Scholar] [CrossRef]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- Cermeño, M.; Kleekayai, T.; Amigo-Benavent, M.; Harnedy-Rothwell, P.; FitzGerald, R.J. Current knowledge on the extraction, purification, identification, and validation of bioactive peptides from seaweed. Electrophoresis 2020, 41, 1694–1717. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, O.; Aliakbarlu, J. Effects of ultrasound and ohmic heating pretreatments on hydrolysis, antioxidant and antibacterial activities of whey protein concentrate and its fractions. Food Sci. Technol. 2020, 131, 109913. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel technologies for the production of bioactive peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uluko, H.; Zhang, S.; Liu, L.; Tsakama, M.; Lu, J.; Lv, J. Effects of thermal, microwave, and ultrasound pretreatments on antioxidative capacity of enzymatic milk protein concentrate hydrolysates. J. Funct. Foods 2015, 18, 1138–1146. [Google Scholar] [CrossRef]
- Naderi, N.; House, J.D.; Pouliot, Y.; Doyen, A. Effects of high hydrostatic pressure processing on hen egg compounds and egg products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 707–720. [Google Scholar] [CrossRef] [Green Version]
- Guan, H.; Diao, X.; Jiang, F.; Han, J.; Kong, B. The enzymatic hydrolysis of soy protein isolate by Corolase PP under high hydrostatic pressure and its effect on bioactivity and characteristics of hydrolysates. Food Chem. 2018, 245, 89–96. [Google Scholar] [CrossRef]
- Saleh, A.S.; Wang, P.; Wang, N.; Yang, S.; Xiao, Z. Technologies for enhancement of bioactive components and potential health benefits of cereal and cereal-based foods: Research advances and application challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 207–227. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. Purification and identification of antioxidant peptides from fermented fish sauce (Budu). J. Aquat. Food Prod. Technol. 2019, 28, 14–24. [Google Scholar] [CrossRef]
- Nong, N.T.P.; Hsu, J.-L. Characteristics of Food Protein-Derived Antidiabetic Bioactive Peptides: A Literature Update. Int. J. Mol. Sci. 2021, 22, 9508. [Google Scholar] [CrossRef]
- Nong, N.T.P.; Chen, Y.-K.; Shih, W.-L.; Hsu, J.-L. Characterization of Novel Dipeptidyl Peptidase-IV Inhibitory Peptides from Soft-Shelled Turtle Yolk Hydrolysate Using Orthogonal Bioassay-Guided Fractionations Coupled with In Vitro and In Silico Study. Pharmaceuticals 2020, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; Montone, A.M.I.; Aita, S.E.; Capparelli, R.; Cerrato, A.; Cuomo, P.; Laganà, A.; Montone, C.M.; Piovesana, S.; Capriotti, A.L. Production and characterization of medium-sized and short antioxidant peptides from soy flour-simulated gastrointestinal hydrolysate. Antioxidants 2021, 10, 734. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.T.; Benjakul, S. Bitterness of fish protein hydrolysate and its debittering prospects. J. Food Biochem. 2019, 43, e12978. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, C.; Chen, Y.; Zheng, B. Purification and Characterization of Antioxidant Peptides of Pseudosciaena crocea Protein Hydrolysates. Molecules 2017, 22, 57. [Google Scholar] [CrossRef] [Green Version]
- Szymczak, M.; Felisiak, K.; Szymczak, B. Characteristics of herring marinated in reused brines after microfiltration. J. Food Sci. Technol. 2018, 55, 4395–4440. [Google Scholar] [CrossRef] [Green Version]
- Saidi, S.; Saoudi, M.; Amar, R. Valorisation of tuna processing waste biomass: Isolation, purification and characterisation of four novel antioxidant peptides from tuna by-product hydrolysate. Environ. Sci. Pollut. Res. Int. 2018, 25, 17383–17392. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Chen, F.-A.; Wang, L.-F.; Hsu, J.-L. Discovery and study of novel antihypertensive peptides derived from Cassia obtusifolia seeds. J. Agric. Food Chem. 2019, 67, 7810–7820. [Google Scholar] [CrossRef]
- Yu, X.; Lim, C.Y.X.; Dong, B.; Hadinoto, K. Development of magnetic solid phase extraction platform for the purification of bioactive γ-glutamyl peptides from garlic (Allium sativum). LWT 2020, 127, 109410. [Google Scholar] [CrossRef]
- Hara, T.; Huang, Y.; Ito, A.; Kawakami, T.; Hojo, H.; Murata, M. Trifluoroethanol-containing RP-HPLC mobile phases for the separation of transmembrane peptides human glycophorin-A, integrin alpha-1, and p24: Analysis and prevention of potential side reactions due to formic acid. J. Pept. Sci. 2015, 21, 61–70. [Google Scholar] [CrossRef]
- Maux, S.L.; Nongonierma, A.B.; Murray, B.; Kelly, P.M.; FitzGerald, R.J. Identification of short peptide sequences in the nanofiltration permeate of a bioactive whey protein hydrolysate. Food Res. Int. 2015, 77, 534–539. [Google Scholar] [CrossRef]
- Karami, Z.; Peighambardoust, S.H.; Hesari, J.; Akbari-Adergani, B.; Andreu, D. Antioxidant, anticancer and ACE-inhibitory activities of bioactive peptides from wheat germ protein hydrolysates. Food Biosci. 2019, 32, 100450. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Al-u’datt, D.a.G.F.; Alhamad, M.N.; Tranchant, C.C.; Rababah, T.; Gammoh, S.; Althnaibat, R.M.; Daradkeh, M.G.; Kubow, S. Characterization and biological properties of peptides isolated from dried fermented cow milk products by RP-HPLC: Amino acid composition, antioxidant, antihypertensive, and antidiabetic properties. J. Food Sci. 2021, 86, 3046–3060. [Google Scholar] [CrossRef]
- Sompinit, K.; Lersiripong, S.; Reamtong, O.; Pattarayingsakul, W.; Patikarnmonthon, N.; Panbangred, W. In vitro study on novel bioactive peptides with antioxidant and antihypertensive properties from edible rhizomes. LWT 2020, 134, 110227. [Google Scholar] [CrossRef]
- Boersema, P.J.; Mohammed, S.; Heck, A.J.R. Hydrophilic interaction liquid chromatography (HILIC) in proteomics. Anal. Bioanal. Chem. 2008, 391, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Giacometti, J.; Buretić-Tomljanović, A. Peptidomics as a tool for characterizing bioactive milk peptides. Food Chem. 2017, 230, 91–98. [Google Scholar] [CrossRef]
- Kaprasob, R.; Khongdetch, J.; Laohakunjit, N.; Selamassakul, O.; Kaisangsri, N. Isolation and characterization, antioxidant, and antihypertensive activity of novel bioactive peptides derived from hydrolysis of King Boletus mushroom. LWT 2022, 160, 113287. [Google Scholar] [CrossRef]
- Safari, R.; Yaghoubzadeh, Z. Antioxidant activity of bioactive peptides extracted from sea cucumber (Holothuria leucospilata). Int. J. Pept. Res. Ther. 2020, 26, 2393–2398. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M.; Eun, J.-B.; Simal-Gandara, J. Influence of Enzymatic Hydrolysis and Molecular Weight Fractionation on the Antioxidant and Lipase/α-Amylase Inhibitory Activities In Vitro of Watermelon Seed Protein Hydrolysates. Molecules 2022, 27, 7897. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Wang, H.; Admassu, H.; Sulieman, A.A.; Wei, F.A. Health benefits of bioactive peptides produced from muscle proteins: Antioxidant, anti-cancer, and anti-diabetic activities. Process Biochem. 2022, 116, 116–125. [Google Scholar] [CrossRef]
- Olivera-Montenegro, L.; Bugarin, A.; Marzano, A.; Best, I.; Zabot, G.L.; Romero, H. Production of Protein Hydrolysate from Quinoa (Chenopodium quinoa Willd.): Economic and Experimental Evaluation of Two Pretreatments Using Supercritical Fluids’ Extraction and Conventional Solvent Extraction. Foods 2022, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Dubey, K.K.; Marathe, S.J.; Singhal, R. Supercritical fluid extraction of bioactives from fruit waste and its therapeutic potential. Food Biosci. 2023, 52, 102418. [Google Scholar] [CrossRef]
- Sun, J.; He, H.; Xie, B.J. Novel Antioxidant Peptides from Fermented Mushroom Ganoderma lucidum. J. Agric. Food Chem. 2004, 52, 6646–6652. [Google Scholar] [CrossRef]
- Lamoolphak, W.; Goto, M.; Sasaki, M.; Suphantharika, M.; Muangnapoh, C.; Prommuag, C.; Shotipruk, A. Hydrothermal decomposition of yeast cells for production of proteins and amino acids. J. Hazard. Mater. 2006, 137, 1643–1648. [Google Scholar] [CrossRef]
- Rivas-Vela, C.I.; Amaya-Llano, S.L.; Castaño-Tostado, E.; Castillo-Herrera, G.A. Protein Hydrolysis by Subcritical Water: A New Perspective on Obtaining Bioactive Peptides. Molecules 2021, 26, 6655. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoez, W.; Yoshida, H. Production of amino and organic acids from protein using sub-critical water technology. Int. J. Chem. React. Eng. 2013, 11, 369–384. [Google Scholar] [CrossRef]
- Rodiles-Lopez, J.O.; Arroyo-Maya, I.J.; Jaramillo-Flores, M.E.; Gutierrez-Lopez, G.F.; Hernandez-Arana, A.; Barbosa-Canovas, G.V.; Niranjan, K.; Hernandez-Sanchez, H. Effects of high hydrostatic pressure on the structure of bovine α-lactalbumin. J. Dairy Sci. 2010, 93, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Rogalinski, T.; Liu, K.; Albrecht, T.; Brunner, G. Hydrolysis kinetics of biopolymers in subcritical water. J. Supercrit. Fluids 2008, 46, 335–341. [Google Scholar] [CrossRef]
- Di Domenico Ziero, H.; Buller, L.S.; Mudhoo, A.; Ampese, L.C.; Mussatto, S.I.; Carneiro, T.F. An overview of subcritical and supercritical water treatment of different biomasses for protein and amino acids production and recovery. J. Environ. Chem. Eng. 2020, 8, 104406. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Gordon, T.A.; Villalobos-Carvajal, R.; Moreno-Osorio, L.; Salazar, F.N.; Pérez-Won, M.; Acuña, S. Effects of high hydrostatic pressure (HHP) on the protein structure and thermal stability of Sauvignon blanc wine. Food Chem. 2014, 155, 214–220. [Google Scholar] [CrossRef]
- Brunner, G. Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes. J. Supercrit. Fluids 2009, 47, 373–381. [Google Scholar] [CrossRef]
- Koh, B.B.; Lee, E.J.; Ramachandraiah, K.; Hong, G.P. Characterization of bovine serum albumin hydrolysates prepared by subcritical water processing. Food Chem. 2019, 278, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Melgosa, R.; Trigueros, E.; Sanz, M.T.; Cardeira, M.; Rodrigues, L.; Fernández, N.; Matias, A.A.; Bronze, M.R.; Marques, M.; Paiva, A.; et al. Supercritical CO2 and subcritical water technologies for the production of bioactive extracts from sardine (Sardina pilchardus) waste. J. Supercrit. Fluids 2020, 164, 104943. [Google Scholar] [CrossRef]
- Cho, Y.J.; Getachew, A.T.; Park, J.S.; Lim, C.T.; Lee, H.J.; Chun, B.S. Influence of temperature on decomposition reaction of compressed hot water to valorize Achatina fulica as a functional material. Food Bioprod. Process 2020, 122, 89–97. [Google Scholar] [CrossRef]
- Sereewatthanawut, I.; Prapintip, S.; Watchiraruji, K.; Goto, M.; Sasaki, M.; Shotipruk, A. Extraction of protein and amino acids from deoiled rice bran by subcritical water hydrolysis. Bioresour. Technol. 2008, 99, 555–561. [Google Scholar] [CrossRef]
- Watchararuji, K.; Goto, M.; Sasaki, M.; Shotipruk, A. Value-added subcritical water hydrolysate from rice bran and soybean meal. Bioresour. Technol. 2008, 99, 6207–6213. [Google Scholar] [CrossRef]
- Enteshari, M.; Martínez-Monteagudo, S.I. Subcritical hydrolysis of ice-cream wastewater: Modeling and functional properties of hydrolysate. Food Bioprod. Process 2018, 111, 104–113. [Google Scholar] [CrossRef]
- Park, J.S.; Jeong, Y.R.; Chun, B.S. Physiological activities and bioactive compound from laver (Pyropia yezoensis) hydrolysates by using subcritical water hydrolysis. J. Supercrit. Fluids 2019, 148, 130–136. [Google Scholar] [CrossRef]
- Espinoza, A.D.; Morawicki, R.O.; Hager, T. Hydrolysis of whey protein isolate using subcritical water. J. Food Sci. 2012, 77, 20–26. [Google Scholar] [CrossRef]
- Mikhaylin, V.; Nikonenko, G.; Pourcelly, G.; Bazinet, L. Hybrid bipolar membrane electrodialysis/ultrafiltration technology assisted by a pulsed electric field for casein production. Green Chem. 2016, 18, 307. [Google Scholar] [CrossRef]
- Siow, H.-L.; Gan, C.-Y. Extraction of antioxidative and antihypertensive bioactive peptides from Parkia speciosa seeds. Food Chem. 2013, 141, 3435–3442. [Google Scholar] [CrossRef]
- Agyei, D.; Pan, S.; Acquah, C.; Bekhit, A.E.D.A.; Danquah, M.K. Structure-informed detection, and quantification of peptides in food and biological fluids. J. Food Biochem. 2019, 43, e12482. [Google Scholar] [CrossRef] [Green Version]
- Kašička, V. Recent developments in capillary and microchip electroseparations of peptides (2017–mid 2019). Electrophoresis 2020, 41, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Kašička, V. Recent developments in capillary and microchip electroseparations of peptides (2019–mid 2021). Electrophoresis 2022, 43, 82–108. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Sun, N.; Dong, L.; Gao, Y.; Lin, S. Production of Bioactive Peptides from Sea Cucumber and Its Potential Health Benefits: A Comprehensive Review. J. Agric. Food Chem. 2022, 70, 7607–7625. [Google Scholar] [CrossRef]
- Ho, C.S.; Lam, C.; Chan, M.; Cheung, R.; Law, L.; Lit, L.; Ng, K.; Suen, M.; Tai, H. Electrospray ionisation mass spectrometry: Principles and clinical applications. Clin. Biochem. Rev. 2003, 24, 3. [Google Scholar] [PubMed]
- Worsztynowicz, P.; Białas, W.; Grajek, W. Integrated approach for obtaining bioactive peptides from whey proteins hydrolysed using a new proteolytic lactic acid bacteria. Food Chem. 2020, 312, 126035. [Google Scholar] [CrossRef]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Hempseed Peptides Exert Hypocholesterolemic Effects with a Statin-Like Mechanism. J. Agric. Food Chem. 2017, 65, 8829–8838. [Google Scholar] [CrossRef] [PubMed]
- Soboleva, A.; Schmidt, R.; Vikhnina, M.; Grishina, T.; Frolov, A. Maillard Proteomics: Opening new pages. Int. J. Mol. Sci. 2017, 18, 2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala-Niño, A.; Rodríguez-Serrano, G.M.; González-Olivares, L.G.; Contreras-López, E.; Regal-López, P.; Cepeda-Saez, A. Sequence Identification of bioactive peptides from amaranth seed proteins (Amaranthus hypochondriacus spp.). Molecules 2019, 24, 3033. [Google Scholar] [CrossRef] [Green Version]
- Smolikova, G.; Gorbach, D.; Lukasheva, E.; Mavropolo-Stolyarenko, G.; Bilova, T.; Soboleva, A.; Tsarev, A.; Romanovskaya, E.; Podolskaya, E.; Zhukov, V.; et al. Bringing New Methods to the Seed Proteomics Platform: Challenges and Perspectives. Int. J. Mol. Sci. 2020, 21, 9162. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Gulseren, I. Identification of Novel Proteins from Black Cumin Seed Meals Based on 2D Gel Electrophoresis and MALDI-TOF/TOF-MS Analysis. Plant Foods Hum. Nutr. 2019, 74, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Acién-Fernándeza, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal. Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ashour, M.L.; Singab, A.N.B.; Wink, M. A Comprehensive Review of Bioactive Peptides from Marine Fungi and Their Biological Significance. Mar. Drugs 2019, 17, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, J.S.; Wanat, K.; Seykora, J. Skin: Basic Structure and Function; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780123864567. [Google Scholar]
- Bonté, F.; Girard, D.; Archambault, J.C.; Desmoulière, A. Skin Changes during Ageing. Subcell Biochem. 2019, 91, 249–280. [Google Scholar]
- Jensen, J.-M.; Förl, M.; Winoto-Morbach, S.; Seite, S.; Schunck, M.; Proksch, E.; Schütze, S. Acid and neutral sphingomyelinase, ceramide synthase, and acid ceramidase activities in cutaneous aging. Exp. Dermatol. 2005, 14, 609–618. [Google Scholar] [CrossRef]
- Kaya, G.; Tran, C.; Sorg, O.; Hotz, R.; Grand, D.; Carraux, P.; Didierjean, L.; Stamenkovic, I.; Saurat, J.-H. Hyaluronate Fragments Reverse Skin Atrophy by a CD44-Dependent Mechanism. PLoS Med. 2006, 3, e493. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C.; William, J. Cunliffe Scientific Awards. Characteristics and pathomechanisms of endogenously aged skin. Dermatology 2007, 214, 352–360. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Lovell, C.R.; Smolenski, K.A.; Duance, V.C.; Light, N.D.; Young, S.; Dyson, M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987, 117, 419–428. [Google Scholar] [CrossRef]
- Autio, P.; Risteli, J.; Haukipuro, K.; Risteli, L.; Oikarinen, A. Collagen synthesis in human skin in vivo: Modulation by aging, ultraviolet B irradiation and localization. Photodermatol. Photoimmunol. Photomed. 1994, 10, 212–216. [Google Scholar] [PubMed]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Ski. Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Law, M.H.; Medland, S.E.; Zhu, G.; Yazar, S.; Viñuela, A.; Wallace, L.; Shekar, S.N.; Duffy, D.L.; Bataille, V.; Glass, D.; et al. Genome-wide association shows that pigmentation genes play a role in skin aging. J. Investig. Dermatol. 2017, 137, 1887–1894. [Google Scholar] [CrossRef] [Green Version]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.B.; Hay, R.J.; Griffiths, C.E.M. Age-Associated Skin Conditions and Diseases: Current Perspectives and Future Options. Gerontologist 2016, 56, S230–S242. [Google Scholar] [CrossRef] [Green Version]
- Kurban, R.S.; Kurban, A.K. Common skin disorders of aging: Diagnosis and treatment. Geriatrics 1993, 48, 30–31, 35–36, 39–42. [Google Scholar]
- Aguilar-Toalá, J.; Santiago-López, L.; Peres, C.; Garcia, H.; Vallejo-Cordoba, B.; González-Córdova, A.; Hernández-Mendoza, A. Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J. Dairy Sci. 2017, 100, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Amigo, L.; Hernández-Ledesma, B. Current Evidence on the Bioavailability of Food Bioactive Peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, M.; Shrestha, N.; Mahmoudi, Z.; Khademi, Z.; Ghasempour, A.; Dehghan, H.; Talebi, S.F.; Toolabi, M.; Préat, V.; Chen, B.; et al. Multifunctional Self-Assembled Peptide Hydrogels for Biomedical Applications. Polymers 2023, 15, 1160. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Fogliano, V. Food matrix interaction and bioavailability of bioactive peptides: Two faces of the same coin? J. Funct. Foods 2017, 35, 9–12. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y. Bioactive peptides and protein hydrolysates may pass into the systemic circulation: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Ying, X.; Agyei, D.; Udenigwe, C.; Adhikari, B.; Wang, B. Manufacturing of Plant-Based Bioactive Peptides Using Enzymatic Methods to Meet Health and Sustainability Targets of the Sustainable Development Goals. Front. Sustain. Food Syst. 2021, 5, 769028. [Google Scholar] [CrossRef]
- Manzanares, P.; Gandía, M.; Garrigues, S.; Marcos, J.F. Improving Health-Promoting Effects of Food-Derived Bioactive Peptides through Rational Design and Oral Delivery Strategies. Nutrients 2019, 11, 2545. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interfac. Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef]
- Patil, P.J.; Usman, M.; Zhang, C.N.; Mehmood, A.; Zhou, M.C.; Teng, C.; Li, X.T. An updated review on food-derived bioactive peptides: Focus on the regulatory requirements, safety, and bioavailability. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1732–1776. [Google Scholar] [CrossRef]
- Apone, F.; Barbulova, A.; Colucci, M.G. Plant and Microalgae Derived Peptides Are Advantageously Employed as Bioactive Compounds in Cosmetics. Front. Plant Sci. 2019, 10, 756. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Jacome, T.P.; Alcantara-Quintana, L.E.; Montalvo-Gonzalez, E.; Chacon-Lopez, A.; Kalixto-Sanchez, M.A.; Rivera, M.D.; Lopez-Garcia, U.M.; Tovar-Perez, E.G. Skin-protective properties of peptide extracts produced from white sorghum grain kafirins. Ind. Crops Prod. 2021, 167, 113551. [Google Scholar] [CrossRef]
- Zhmak, M.N.; Utkin, Y.N.; Andreeva, T.V.; Kudryavtsev, D.S.; Kryudova, E.V.; Tsetlin, V.I.; Shelukhina, I.V.E. Peptide Inhibitors of Nicotinic Acetylcholine Receptor. US Patent US 20,150,361,137 A1, 17 December 2015. [Google Scholar]
- Gruber, J.V.; Smith, D.; Bouldin, L. Personal Care Composition Containing Yeast Extract and Hexapeptide. U.S. Patent Application No. 13/247,689, 12, 03, 2014. [Google Scholar]
- Hama, R.; Ulziibayar, A.; Reinhardt, J.W.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Developments in Biopolymer-Based Hydrogels for Tissue Engineering Applications. Biomolecules 2023, 13, 280. [Google Scholar] [CrossRef]
- Kisling, A.; Lust, R.M.; Katwa, L.C. What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci. 2019, 228, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen Based Materials in Cosmetic Applications: A Review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef]
- Kwatra, B. Collagen Supplementation: Therapy for Skin Disorders: A Review. World J. Pharm. Res. 2020, 9, 2504–2518. [Google Scholar]
- Zhao, X.; Zhang, X.; Liu, D. Collagen peptides and the related synthetic peptides: A review on improving skin health. J. Funct. Foods 2021, 86, 104680. [Google Scholar] [CrossRef]
- Ahmed, M.; Verma, A.K.; Patel, R. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustain. Chem. Pharm. 2020, 18, 100315. [Google Scholar] [CrossRef]
- Kim, D.-U.; Chung, H.-C.; Choi, J.; Sakai, Y.; Lee, B.-Y. Oral intake of low-molecular-weight collagen peptide improves hydration, elasticity, and wrinkling in human skin: A randomized, double-blind, placebo-controlled study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef] [Green Version]
- Addor, F.A.S.; Cotta Vieira, J.; Abreu Melo, C.S. Improvement of dermal parameters in aged skin after oral use of a nutrient supplement. Clin. Cosmet. Investig. Dermatol. 2018, 11, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Czajka, A.; Kania, E.M.; Genovese, L.; Corbo, A.; Merone, G.; Luci, C.; Sibilla, S. Daily oral supplementation with collagen peptides combined with vitamins and other bioactive compounds improves skin elasticity and has a beneficial effect on joint and general wellbeing. Nutr. Res. 2018, 57, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Maia Campos, P.M.; Melo, M.O.; Siqueira César, F.C. Topical application and oral supplementation of peptides in the improvement of skin viscoelasticity and density. J. Cosmet. Dermatol. 2019, 18, 1693–1699. [Google Scholar] [CrossRef]
- Song, H.; Zhang, S.; Zhang, L.; Li, B. Effect of Orally Administered Collagen Peptides from Bovine Bone on Skin Aging in Chronologically Aged Mice. Nutrients 2017, 9, 1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazaki, M.; Ito, Y.; Yamada, M.; Goulas, S.; Teramoto, S.; Nakaya, M.-a.; Ohno, S.; Yamaguchi, K. Oral ingestion of collagen hydrolysate leads to the transportation of highly concentrated Gly-Pro-Hyp and its hydrolyzed form of Pro-Hyp into the bloodstream and skin. J. Agric. Food Chem. 2017, 65, 2315–2322. [Google Scholar] [CrossRef] [Green Version]
- Offengenden, M.; Chakrabarti, S.; Wu, J. Chicken collagen hydrolysates differentially mediate antiinflammatory activity and type I collagen synthesis on human dermal fibroblasts. Food Sci. Hum. Wellness 2018, 7, 138–147. [Google Scholar] [CrossRef]
- Xiao, Z.; Liang, P.; Chen, J.; Chen, M.F.; Gong, F.; Li, C.; Zhou, C.; Hong, P.; Yang, P.; Qian, Z.J. A Peptide YGDEY from Tilapia gelatin hydrolysates inhibits UVB-mediated skin photoaging by regulating MMP-1 and MMP-9 expression in HaCaT cells. Photochem Photobiol. 2019, 95, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Tkaczewska, J.; Bukowski, M.; Mak, P. Identification of antioxidant peptides in enzymatic hydrolysates of carp (Cyprinus carpio) skin gelatin. Molecules 2019, 24, 97. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, A.; Blanco, M.; Correa, B.; Perez-Martin, R.I.; Sotelo, C.G. Effect of Fish Collagen Hydrolysates on Type I Collagen mRNA Levels of Human Dermal Fibroblast Culture. Mar. Drugs 2018, 16, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Ma, X.; Che, S.; Wang, C.; Li, B. The effect of hydrolysis with neutrase on molecular weight, functional properties, and antioxidant activities of Alaska pollock protein isolate. J. Ocean. Univ. China 2018, 17, 1423–1431. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Zhang, J.; Su, T. Extraction and characterization of collagen hydrolysates from the skin of Rana chensinensis. 3 Biotech. 2018, 8, 181. [Google Scholar] [CrossRef]
- Montalvo, G.E.B.; Thomaz-Soccol, V.; Vandenberghe, L.P.S.; Carvalho, J.C.; Faulds, C.B.; Bertrand, E.; Prado, M.R.M.; Bonatto, S.J.R.; Soccol, C.R. Arthrospira maxima OF15 biomass cultivation at laboratory and pilot scale from sugarcane vinasse for potential biological new peptides production. Bioresour. Technol. 2019, 273, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Norzagaray-Valenzuela, C.D.; Valdez-Ortiz, A.; Shelton, L.M.; Jiménez-Edeza, M.; Rivera-López, J.; Valdez-Flores, M.A.; Germán-Báez, L.J. Residual biomasses and protein hydrolysates of three green microalgae species exhibit antioxidant and antiaging activity. J. Appl. Phycol. 2016, 29, 189–198. [Google Scholar] [CrossRef]
- Huang, G.; Chen, J. Preparation and applications of hyaluronic acid and its derivatives. Int. J. Biol. Macromol. 2019, 125, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Garg, C.; Khurana, P.; Garg, M. Molecular mechanisms of skin photoaging and plant inhibitors. Inter. J. Green Pharm. 2017, 11, 217–232. [Google Scholar]
- Saranraj, P.; Naidu, M.A. Hyaluronic acid production and its applications-a review. Int. J. Pharm. Biol. Arch. 2013, 4, 853–859. [Google Scholar]
- Cui, Y.; Hu, Y.-H.; Yu, F.; Zheng, J.; Chen, L.-S.; Chen, Q.-X.; Wang, Q. Inhibition kinetics and molecular simulation of p-substituted cinnamic acid derivatives on tyrosinase. Int. J. Biol. Macromol. 2017, 95, 1289–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Toalá, J.; Hernández-Mendoza, A.; González-Córdova, A.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef]
- Kubglomsong, S.; Theerakulkait, C.; Reed, R.L.; Yang, L.; Maier, C.S.; Stevens, J.F. Isolation and Identification of Tyrosinase-Inhibitory and Copper-Chelating Peptides from Hydrolyzed Rice-Bran-Derived Albumin. J. Agric. Food Chem. 2018, 66, 8346–8354. [Google Scholar] [CrossRef]
- Ochiai, A.; Tanaka, S.; Tanaka, T.; Taniguchi, M. Rice Bran Protein as a Potent Source of Antimelanogenic Peptides with Tyrosinase Inhibitory Activity. J. Nat. Prod. 2016, 79, 2545–2551. [Google Scholar] [CrossRef] [Green Version]
- Nakchum, L.; Kim, S.M. Preparation of squid skin collagen hydrolysate as an antihyaluronidase, antityrosinase, and antioxidant agent. Prep. Biochem. Biotechnol. 2016, 46, 123–130. [Google Scholar] [CrossRef]
- Karkouch, I.O.; Tabbene, D.; Gharbi, M.A.; Ben Mlouka, S.; Elkahoui, C.; Rihouey, L.; Coquet, P.; Cosette Jouenne, T.; Limam, F. Antioxidant, antityrosinase and antibiofilm activities of synthesized peptides derived from vicia faba protein hydrolysate: A powerful agents in cosmetic application. Ind. Crops Prod. 2017, 109, 310–319. [Google Scholar] [CrossRef]
- Chen, Y.M.; Shih, T.W.; Chiu, C.P.; Pan, T.M.; Tsai, T.Y. Effects of lactic acid bacteria-fermented soy milk on melanogenesis in B16F0 melanocytes. J. Funct. Foods 2013, 5, 395–405. [Google Scholar] [CrossRef]
- Qin, Z. Soluble elastin peptides in cardiovascular homeostasis: Foe or ally. Peptides 2015, 67, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Shen, Y.; Wang, Y.; Wang, X.; Li, D. Self-assembly of short elastin-like amphiphilic peptides: Effects of temperature, molecular hydrophobicity and charge distribution. Molecules 2019, 24, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiros, G.J.; Kusinsky, A.G.; Balana, M.E.; Hagelin, K. Triolein reduces MMP-1 upregulation in dermal fibroblasts generated by ROS production in UVB-irradiated keratinocytes. J. Dermatol. Sci. 2017, 85, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, G.; Zhou, F.; Zhang, J.; Zheng, L.; Zhao, M. Protective effect of bovine elastin peptides against photoaging in mice and identification of novel antiphotoaging peptides. J. Agric. Food Chem. 2018, 66, 10760–10768. [Google Scholar] [CrossRef] [PubMed]
- Le Page, A.; Khalil, A.; Vermette, P.; Frost, E.H.; Larbi, A.; Witkowski, J.M.; Fulop, T. The role of elastin-derived peptides in human physiology and diseases. Matrix Biol. 2019, 84, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Amakye, W.K.; Yang, L.; Yao, M.; Yuan, E.; Ren, R.; Ren, J. Skipjack (Katsuwonus pelamis) elastin hydrolysate-derived peptides attenuate UVA irradiation-induced cell damage in human HaCaT keratinocytes. Food Frontiers 2021, 2, 184–194. [Google Scholar] [CrossRef]
- Xiong, Y.; Peng, P.; Chen, S.J.; Chang, M.; Wang, Q.; Yin, S.N.; Ren, D.F. Preparation, identification, and molecular docking of novel elastase inhibitory peptide from walnut (Juglans regia L.) meal. Food Chem. Mol. Sci. 2022, 5, 100139. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Dini, I. Bio Discarded from Waste to Resource. Foods 2021, 10, 2652. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Bai, F.; Fang, Y.; Wang, J.; Gao, R. The anti-skin-aging effect of oral administration of gelatin from the swim bladder of Amur sturgeon (Acipenser schrenckii). Food Funct. 2019, 10, 3890–3897. [Google Scholar] [CrossRef]
- Dini, I. The Potential of Dietary Antioxidants. Antioxidants 2021, 10, 1752. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Laneri, S. Spices, Condiments, Extra Virgin Olive Oil and Aromas as Not Only Flavorings, but Precious Allies for Our Wellbeing. Antioxidants 2021, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; De Biasi, M.-G.; Mancusi, A. An Overview of the Potentialities of Antimicrobial Peptides Derived from Natural Sources. Antibiotics 2022, 11, 1483. [Google Scholar] [CrossRef]
- Stefanucci, A.; Mollica, A.; Macedonio, G.; Dall’Acqua, S.; Ahmed, A.A.; Novellino, E. Exogenous opioid peptides derived from food proteins and their possible uses as dietary supplements: A critical review. Food Rev. Int. 2016, 34, 70–86. [Google Scholar] [CrossRef]
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-Assisted Discovery of Antioxidant Peptides from Edible Marine Invertebrates: A Review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Ding, D.; Chi, C.; Wang, B.; Huo, J.C. Preparation, identification, and activity evaluation of eight antioxidant peptides from protein hydrolysate of hairtail (Trichiurus japonicas) muscle. Mar. Drugs 2019, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Ketnawa, S.; Wickramathilaka, M.; Liceaga, A.M. Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: Purification and identification. Food Chem. 2018, 254, 36–46. [Google Scholar] [CrossRef]
- Abd-Talib, N.; Yaji, E.L.A.; Wahab, N.S.A.; Razali, N.; Len, K.Y.T.; Roslan, J.; Saari, N.; Pa’ee, K.F. Bioactive peptides and its alternative processes: A review. Biotechnol. Bioprocess Eng. 2022, 27, 306–335. [Google Scholar] [CrossRef]
- Pouzo, L.B.; Descalzo, A.M.; Zaritzky, N.E.; Rossetti, L.; Pavan, E. Antioxidant status, lipid and color stability of aged beef from grazing steers supplemented with corn grain and increasing levels of flaxseed. Meat Sci. 2016, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tironi, V.A.; Anon, M.C. Amaranth proteins as a source of antioxidant peptides: Effect of proteolysis. Food Res. Int. 2010, 43, 315–322. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, M.; Lin, L.; Wang, J.; Sun-Waterhouse, D.; Dong, Y.; Zhuang, M.; Su, G. Identification of antioxidant peptides from defatted walnut meal hydrolysate with potential for improving learning and memory. Food Res. Int. 2015, 78, 216–233. [Google Scholar] [CrossRef]
- Eftekharzadeh, B.; Khodagholi, F.; Abdi, A.; Maghsoudi, N. Alginate protects NT2 neurons against H2O2-induced neurotoxicity. Carbohydr. Polym. 2010, 79, 1063–1072. [Google Scholar] [CrossRef]
- Cai, L.Y.; Wu, X.S.; Zhang, Y.H.; Li, X.X.; Ma, S.; Li, J.R. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods 2015, 16, 234. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Vásquez-Villanueva, R.; Marina, M.L.M.; Garcia, C. Identification by hydrophilic interaction and reversed-phase liquid chromatography-tandem mass spectrometry of peptides with antioxidant capacity in food residues. J. Chromatogr. A 2015, 1428, 185–192. [Google Scholar] [CrossRef]
- Song, R.; Wei, R.B.; Ruan, G.Q.; Luo, H.Y. Isolation and identification of antioxidant peptides from peptic hydrolysates of half-fin anchovy (Setipinna taty). LWT-Food Sci. Technol. 2015, 60, 221–229. [Google Scholar] [CrossRef]
- Yan, Q.J.; Huang, L.H.; Sun, Q.; Jiang, Z.Q.; Wu, X. Isolation, identification and synthesis of four novel antioxidant peptides from rice residue protein hydrolyzed by multiple proteases. Food Chem. 2015, 179, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Montone, A.M.I.; Capuano, F.; Mancusi, A.; Di Maro, O.; Peruzy, M.F.; Proroga, Y.T.R.; Cristiano, D. Exposure to Bacillus cereus in Water Buffalo Mozzarella Cheese. Foods 2020, 9, 1899. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Hernández-Ledesma, B.; Jeong, H.J.; Park, J.H.; de Lumen, B.O. Complementary roles in cancer prevention: Protease inhibitor makes the cancer preventive peptide lunasin bioavailable. PLoS ONE 2010, 5, e8890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pihlanto, A. Lactic fermentation and bioactive peptides. In Lactic Acid Bacteria R & R for Food, Health and Livestock Purposes; IntechOpen: Marcelino, Kongo, 2013. [Google Scholar]
- Rizzello, C.G.; Tagliazucchi, D.; Babini, E.; Rutella, G.S.; Saa, D.L.T.; Gianotti, A. Bioactive peptides from vegetable food matrices: Research trends and novel biotechnologies for synthesis and recovery. J. Funct. Foods 2016, 27, 549–569. [Google Scholar] [CrossRef]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.F.G.; Hrelia, S. Bioactive peptides in cereals and legumes: Agronomical, biochemical and clinical aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef] [Green Version]
- Aluko, R. Amino acids, peptides, and proteins as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 105–140. [Google Scholar]
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef] [Green Version]
- El-Salam, M.A.; El-Shibiny, S. Bioactive peptides of buffalo, camel, goat, sheep, mare, and yak milks and milk products. Food Rev. Int. 2013, 29, 1–23. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.; Li-Chan, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Wu, R.B.; Wu, C.L.; Liu, D.; Yang, X.H.; Huang, J.F.; Zhang, J.; Liao, B.Q.; He, H.L.; Li, H. Overview of antioxidant peptides derived from marine resources: The Sources, characteristic, purification, and evaluation methods. Appl. Biochem. Biotechnol. 2015, 176, 1815–1833. [Google Scholar] [CrossRef] [PubMed]
- Nimalaratne, C.; Wu, J. Hen Egg as an Antioxidant Food Commodity: A Review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef] [Green Version]
- Mora, L.; Reig, M.; Toldrá, F. Bioactive peptides generated from meat industry by-products. Food Res. Int. 2014, 65, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Mu, T.H.; Sun, M.J. Purification and identification of antioxidant peptides from sweet potato protein hydrolysates by Alcalase. J. Funct. Foods 2014, 7, 191–200. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assay. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nagao, K. “Deepening” Insight on Skin Aging and Antimicrobial Immunity. Cell Metab. 2019, 29, 515–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Kadyan, S.; Ukhanov, V.; Cheng, J.; Nagpal, R.; Cui, L. Recent advances in the health benefits of pea protein (Pisum sativum): Bioactive peptides and the interaction with the gut microbiome. Curr. Opin. Food Sci. 2022, 48, 100944. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Kiatsurayanon, C.; Chieosilapatham, P.; Ogawa, H. Friends or Foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp. Dermatol. 2017, 26, 989–998. [Google Scholar] [CrossRef] [Green Version]
- Iinuma, K.; Noguchi, N.; Nakaminami, H.; Sasatsu, M.; Nishijima, S.; Tsuboi, I. Susceptibility of Propionibacterium acnes isolated from patients with acne vulgaris to zinc ascorbate and antibiotics. Clin. Cosmet. Investig. Dermatol. 2011, 4, 161–165. [Google Scholar]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef]
- Le, C.F.; Fang, C.M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, M.; Kameda, M.; Namae, T.; Ochiai, A.; Saitoh, E.; Tanaka, T. Identification and characterization of multifunctional cationic peptides derived from peptic hydrolysates of rice bran protein. J. Funct. Foods 2017, 34, 287–296. [Google Scholar] [CrossRef]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.-E.; Turgeon, S.L. Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- Kobbi, S.; Nedjar, N.; Chihib, N.; Balti, R.; Chevalier, M.; Silvain, A.; Chaabouni, S.; Dhulster, P.; Bougatef, A. Synthesis and antibacterial activity of new peptides from Alfalfa RuBisCO protein hydrolysates and mode of action via a membrane damage mechanism against Listeria innocua. Microb. Pathog. 2018, 115, 41–49. [Google Scholar] [CrossRef]
- Lueangsakulthai, J.; Jangpromma, N.; Temsiripong, T.; McKendrick, J.E.; Khunkitti, W.; Maddocks, S.E.; Klaynongsruang, S. A novel antibacterial peptide derived from Crocodylus siamensis haemoglobin hydrolysate induces membrane permeabilization causing iron dysregulation, oxidative stress and bacterial death. J. Appl. Microbiol. 2017, 123, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Zanutto-Elgui, M.R.; Vieira, J.C.S.; do Prado, D.Z.; Buzalaf, M.A.R.; de Magalhães Padilha, P.; de Oliveira, D.E.; Fleuri, L.F. Production of milk peptides with antimicrobial and antioxidant properties through fungal proteases. Food Chem. 2019, 278, 823–831. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Li, H.; Zhang, J.; An, J.; Liu, X. Antiinflammatory Function of Plant-Derived Bioactive Peptides: A Review. Foods 2022, 11, 2361. [Google Scholar] [CrossRef]
- Dragoș, D.; Petran, M.; Gradinaru, T.-C.; Gilca, M. Phytochemicals and Inflammation: Is Bitter Better? Plants 2022, 11, 2991. [Google Scholar] [CrossRef]
- Zlobin, A.; Bloodworth, J.C.; Osipo, C. Mitogen-activated protein kinase (MAPK) signaling. In Predictive Biomarkers in Oncology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 213–221. [Google Scholar]
- Alharbi, K.S.; Fuloria, N.K.; Fuloria, S.; Rahman, S.B.; Al-Malki, W.H.; Shaikh, M.A.J.; Thangavelu, L.; Singh, S.K.; Raju, V.S.R.; Jha, N.K. Nuclear factor-kappa B and its role in inflammatory lung disease. Chem. Biol. Interact. 2021, 345, 109568. [Google Scholar] [CrossRef]
- Xu, Z.; Shang, W.; Zhao, Z.; Zhang, B.; Liu, C.; Cai, H. Curcumin alleviates rheumatoid arthritis progression through the phosphatidylinositol 3-kinase/protein kinase B pathway: An in vitro and in vivo study. Bioengineered 2022, 13, 12899–12911. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Field, C.J.; Wu, J. Purification and identification of antiinflammatory peptides from spent hen muscle proteins hydrolysate. Food Chem. 2018, 253, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Meram, C.; Wu, J. Antiinflammatory effects of egg yolk livetins (α, β, and γ-livetin) fraction and its enzymatic hydrolysates in lipopolysaccharide-induced RAW 264.7 macrophages. Food Res. Int. 2017, 100, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Zhang, S.H.; Ren, M.; Zeng, X.F.; He, P.L.; Ma, X.; Qiao, S.Y. Leucine stimulates ASCT2 amino acid transporter expression in porcine jejunal epithelial cell line (IPEC-J2) through PI3K/Akt/mTOR and ERK signaling pathways. Amino Acids 2014, 46, 2633–2642. [Google Scholar] [CrossRef]
- Bonvini, A.; Rogero, M.M.; Coqueiro, A.Y.; Raizel, R.; Bella, L.M.; Fock, R.A.; Borelli, P.; Tirapegui, J. Effects of different branched-chain amino acids supplementation protocols on the inflammatory response of LPS-stimulated RAW 264.7 macrophages. Amino Acids 2021, 53, 597–607. [Google Scholar] [CrossRef]
- Singh, S.; Datta, A.; Schmidtchen, A.; Bhunia, A.; Malmsten, M. Tryptophan end-tagging for promoted lipopolysaccharide interactions and antiinflammatory effects. Sci. Rep. 2017, 7, 212. [Google Scholar] [CrossRef]
- Ding, L.; Wang, L.Y.; Yu, Z.P.; Ma, S.T.; Du, Z.Y.; Zhang, T.; Liu, J.B. Importance of terminal amino acid residues to the transport of oligopeptides across the Caco-2 cell monolayer. J. Agric. Food Chem. 2017, 65, 7705–7712. [Google Scholar] [CrossRef]
- Han, H.; Yin, J.; Wang, B.; Huang, X.G.; Yao, J.M.; Zheng, J.; Fan, W.J.; Li, T.J.; Yin, Y.L. Effects of dietary lysine restriction on inflammatory responses in piglets. Sci. Rep. 2018, 8, 2451. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.; Dou, X.J.; Li, J.W.; Yang, Y.; Xue, C.Y.; Wang, C.X.; Gao, N.; Shan, A. L-arginine ameliorates lipopolysaccharide-induced intestinal inflammation through inhibiting the TLR4/NF-κB and MAPK pathways and stimulating β-defensins expression in vivo and in vitro. J. Agric. Food Chem. 2020, 68, 2648–2663. [Google Scholar] [CrossRef]
- Tang, N.; Skibsted, L.H. Calcium binding to amino acids and small glycine peptides in aqueous solution: Toward peptide design for better calcium bioavailability. J. Agric. Food Chem. 2016, 64, 4376–4389. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Bradford, B.U.; Wheeler, M.D.; Stimpson, S.A.; Pink, H.M.; Brodie, T.A.; Schwab, J.H.; Thurman, R.G. Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: Role for glycine-gated chloride channel. Infect. Immun. 2001, 69, 5883–5891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Liu, J.; Chang, G.J.; Wang, Y.; Ma, N.; Roy, A.C.; Shen, X.Z. Glutamine supplementation attenuates the inflammation caused by LPS-induced acute lung injury in mice by regulating the TLR4/MAPK signaling pathway. Inflammation 2021, 44, 2180–2192. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.; Ryu, B.M.; Kim, S.K. Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal Spirulina maxima. J. Funct. Food 2013, 5, 1336–1346. [Google Scholar] [CrossRef]
- He, R.; Liu, M.T.; Zou, Z.P.; Wang, M.J.; Wang, Z.G.; Ju, X.R.; Hao, G.F. Anti-inflammatory activity of peptides derived from millet bran in vitro and in vivo. Food Funct. 2022, 13, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Suttisuwan, R.; Phunpruch, S.; Saisavoey, T.; Sangtanoo, P.; Karnchanatat, A. Isolation and characterization of anti-inflammatory peptides derived from trypsin hydrolysis of microalgae protein (Synechococcus sp. VDW). Food Biotech. 2019, 33, 303–324. [Google Scholar] [CrossRef]
- Velliquette, R.A.; Fast, D.J.; Maly, E.R.; Alashi, A.M.; Aluko, R.E. Enzymatically derived sunflower protein hydrolysate and peptides inhibit NF-κB and promote monocyte differentiation to a dendritic cell phenotype. Food Chem. 2020, 319, 126563. [Google Scholar] [CrossRef]
- Saisavoey, T.; Sangtanoo, P.; Chanchao, C.; Reamtong, O.; Karnchanatat, A. Identification of novel anti-inflammatory peptides from bee pollen (Apis mellifera) hydrolysate in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Apic. Res. 2020, 60, 280–289. [Google Scholar] [CrossRef]
- Swain, S.; Mondal, D.; Beg, S.; Niranjan Patra, C.; Chandra Dinda, S.; Sruti, J.; Eswara Bhanoji Rao, M. Stabilization and delivery approaches for protein and peptide pharmaceuticals: An extensive review of patents. Recent Pat. Biotechnol. 2013, 7, 28–46. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, P.; Zou, Y.X.; Luo, Z.G.; Tamer, T.M. Co-encapsulation of Vitamin C and beta-Carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging trends of nanotechnology in advanced cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440. [Google Scholar] [CrossRef] [PubMed]
- Yasamineh, S.; Yasamineh, P.; Ghafouri Kalajahi, H.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Hossein Kheirkhah, A.; Taghizadeh, M.; Yazdani, Y.; et al. A State-of-the-Art Review on the Recent Advances of Niosomes as a Targeted Drug Delivery System. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of nanotechnology in cosmeceuticals: A review of recent advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef] [Green Version]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to Supercritical Fluids. Processes 2020, 8, 1022. [Google Scholar] [CrossRef]
- Sguizzato, M.; Pepe, A.; Baldisserotto, A.; Barbari, R.; Montesi, L.; Drechsler, M.; Mariani, P.; Cortesi, R. Niosomes for Topical Application of Antioxidant Molecules: Design and In Vitro Behavior. Gels 2023, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Agrawal, R. Nanotechnology: A new paradigm in cosmeceuticals. Recent Pat. Drug Deliv. Formul. 2007, 1, 171–182. [Google Scholar] [CrossRef]
- Mosallam, S.; Ragaie, M.H.; Moftah, N.H.; Elshafeey, A.H.; Abdelbary, A.A. Use of novasomes as a vesicular carrier for improving the topical delivery of terconazole: In vitro characterization, in vivo assessment and exploratory clinical experimentation. Int. J. Nanomed. 2021, 16, 119–132. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Saraf, G.J.S. Topical Delivery of Curcuma longa Extract Loaded Nanosized Ethosomes to Combat Facial Wrinkles Research Article. J. Pharm. Drug Deliv. Res. 2014, 3, 1. [Google Scholar]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef]
- Wu, P.-S.; Li, Y.-S.; Kuo, Y.-C.; Tsai, S.-J.J.; Lin, C.-C. Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol. Molecules 2019, 24, 600. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Kim, B. Synthesis and characterization of ethosomal carriers containing cosmetic ingredients for enhanced transdermal delivery of cosmetic ingredients. Korean J. Chem. Eng. 2018, 35, 792–797. [Google Scholar] [CrossRef]
- Yaghmur, A.; Mu, H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm. Sin. B 2021, 11, 871–885. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Decome, L.; De Méo, M.; Geffard, A.; Doucet, O.; Duménil, G.; Botta, A. Evaluation of photolyase (Photosome®) repair activity in human keratinocytes after a single dose of ultraviolet B irradiation using the comet assay. J. Photochem. Photobiol. B Biol. 2005, 79, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.B.; Mandawgade, S.D. Novel cosmetic delivery systems: An application update. Int. J. Cosmet. Sci. 2008, 30, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Zhang, P.-Y. Polymersomes in nanomedicine—A review. Curr. Nanosci. 2017, 13, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef] [Green Version]
- Bermudez, H.; Brannan, A.K.; Hammer, D.A.; Bates, F.S.; Discher, D.E. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules 2002, 35, 8203–8208. [Google Scholar] [CrossRef] [Green Version]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Allamraju, K.V. Green Hydrogels. Green Compos. 2021, 225–249. [Google Scholar] [CrossRef]
- Pramanik, B.; Ahmed, S. Peptide-Based Low Molecular Weight Photosensitive Supramolecular Gelators. Gels 2022, 8, 533. [Google Scholar] [CrossRef]
- Perry, S.L.; McClements, D.J. Recent Advances in Encapsulation, Protection, and Oral Delivery of Bioactive Proteins and Peptides using Colloidal Systems. Molecules 2020, 25, 1161. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2018, 19, e1800256. [Google Scholar] [CrossRef]
- Haddada, M.B.; Gerometta, E.; Chawech, R.; Sorres, J.; Bialecki, A.; Pesnel, S.; Morel, A.L. Assessment of antioxidant and dermoprotective activities of gold nanoparticles as safe cosmetic ingredient. Colloids Surf. B Biointerfaces 2020, 189, 110855. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jiménez, A.J.; Abad-Jordá, M.; Rubio-Moraga, A.; Ahraz, O.; Gómez-Gómez, L.; Niza, E. Biogenic Silver Nanoparticles from Iris tuberosa as Potential Preservative in Cosmetic Products. Molecules 2021, 26, 4696. [Google Scholar] [CrossRef]
- Lee, C.-C.; Lin, Y.-H.; Hou, W.-C.; Li, M.-H.; Chang, J.-W. Exposure to ZnO/TiO2 Nanoparticles Affects Health Outcomes in Cosmetics Salesclerks. Int. J. Environ. Res. Public Health 2020, 17, 6088. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, K.A.; Lane, M.E. Dermal and Transdermal Drug Delivery Systems. In Dermal Drug Delivery, 1st ed.; Ghosh, T.K., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–60. [Google Scholar]
- Gugleva, V.; Ivanova, N.; Sotirova, Y.; Andonova, V. Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies. Pharmaceuticals 2021, 14, 837. [Google Scholar] [CrossRef] [PubMed]
- Morais, R.P.; Hochheim, S.; de Oliveira, C.C.; Riegel-Vidotti, I.C.; Marino, C.E.B. Skin interaction, permeation, and toxicity of silica nanoparticles: Challenges and recent therapeutic and cosmetic advances. Int. J. Pharm. 2022, 614, 121439. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. New Insights on Unique Features and Role of Nanostructured Materials in Cosmetics. Cosmetics 2020, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Pentek, T.; Newenhouse, E.; O’Brien, B.; Singh Chauhan, A. Development of a Topical Resveratrol Formulation for Commercial Applications Using Dendrimer Nanotechnology. Molecules 2017, 22, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Luo, D.; Chen, D.; Tan, X.; Bai, X.; Liu, Z.; Yang, X.; Liu, W. Current Advances of Nanocarrier Technology-Based Active Cosmetic Ingredients for Beauty Applications. Clin. Cosmet. Investig. Dermatol. 2021, 14, 867–887. [Google Scholar] [CrossRef]
- Eckert, R.W.; Wiemann, S.; Keck, C.M. Improved dermal and transdermal delivery of curcumin with smartfilms and nanocrystals. Molecules 2021, 26, 1633. [Google Scholar] [CrossRef]
- Wadhawan, J.; Parmar, P.K.; Bansal, A.K. Nanocrystals for improved topical delivery of medium soluble drug: A case study of acyclovir. J. Drug Deliv. Sci. Technol. 2021, 65, 102662. [Google Scholar] [CrossRef]
- Kazemzadeh, H.; Mozafari, M. Fullerene-based delivery systems. Drug Discov. Today 2019, 24, 898–905. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133. [Google Scholar] [CrossRef] [Green Version]
- Miljkovic, S.; Jeftic, B.; Stankovic, I.; Stojiljkovic, N.; Koruga, D. Mechanisms of skin moisturization with hyperharmonized hydroxyl modified fullerene substance. J. Cosmet. Dermatol. 2021, 20, 3018–3025. [Google Scholar] [CrossRef]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Rubin Pedrazzo, A.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Torne, S.J.; Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded nanosponges. Drug Deliv. 2010, 17, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Cavalli, R.; Martina, K.; Biasizzo, M.; Vitillo, J.; Bordiga, S.; Vavia, P.; Ansari, K. Cyclodextrin nanosponges as effective gas carriers. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 189–194. [Google Scholar] [CrossRef]
- Mortazavi, S.M.; Moghimi, H.R. Skin permeability, a dismissed necessity for anti-wrinkle peptide performance. Int. J. Cosmet. Sci. 2022, 44, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Engelskirchen, S.; Maurer, R.; Levy, T.; Berghaus, R.; Auweter, H.; Glatter, O. Highly concentrated emulsified microemulsions as solvent-free plant protection formulations. J. Colloid Interface Sci. 2012, 388, 151–161. [Google Scholar] [CrossRef]
- Yaghmur, A.; de Campo, L.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Emulsified microemulsions and oil-containing liquid crystalline phases. Langmuir 2005, 21, 569–577. [Google Scholar] [CrossRef]
- Dini, I. Contribution of Nanoscience Research in Antioxidants Delivery Used in Nutricosmetic Sector. Antioxidants 2022, 11, 563. [Google Scholar] [CrossRef]
- Sabri, F.; Raphael, W.; Berthomier, K.; Fradette, L.; Tavares, J.R.; Virgilio, N. One-Step Processing of Highly Viscous Multiple Pickering Emulsions. J. Colloid Interface Sci. 2020, 560, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Debeli, D.K.; Gan, L.; Deng, J.; Hu, L.; Shan, G. Polyether-modified siloxane stabilized dispersion system on the physical stability and control release of double (W/O/W) emulsions. Food Chem. 2020, 332, 127381. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Gao, J.; Lu, J.; Ma, C.; Lv, J.; Adhikari, B.; Wang, B. Preparation and drying of water-in-oil-in-water (W/O/W) double emulsion to encapsulate soy peptides. Food Res. Int. 2021, 141, 110148. [Google Scholar] [CrossRef] [PubMed]
- Giroux, H.J.; Shea, R.; Sabik, H.; Fustier, P.; Robitaille, G.; Britten, M. Effect of oil phase properties on peptide release from water-in-oil-in-water emulsions in gastrointestinal conditions. LWT 2019, 109, 429–435. [Google Scholar] [CrossRef]
| Peptides | Source | Skin-Aging Effect | Biblio |
|---|---|---|---|
| Type I collagen-derived collagen peptide Chicken collagen | Pig collagen | Enhancement of skin collagen content by changing the ratio of type I and type III collagen. No effect on skin moisturizing. | [118] |
| High tripeptide-containing collagen hydrolysate (HTC-col) has high tripeptides comprising the Gly-X-Y sequence. | Porcine skin | Anti-photoaging action. Skin dryness improvement. | [119] |
| Chicken-derived collagen peptide | Chicken collagen | Anti-inflammatory. Antioxidant. Collagen I synthesis. Improve cell proliferation on human skin fibroblasts | [120] |
| YGDEY (Tyr-Gly-Asp-Glu-Tyr) from. | Tilapia collagen hydrolysate | Prevention of ultraviolet (UVB)-induced damage to cells Inhibition of UVB-mediated photoaging of the skin. Improvement of the glutathione and superoxide dismutase expression. Enhancement of type I procollagen. Reduction of the ROS in keratinocytes. Prevention of DNA oxidative damage. Inhibition of the collagenase and gelatinase expression. | [121] |
| Ala-Tyr dipeptide | Carp skin hydrolysate | Antioxidant activity | [122] |
| Hydrolyzed collagen | Prionace glauca | Stimulation of the collagen type I mRNA by fibroblasts. mRNA production improvement. | [123] |
| Hydrolyzed collagen with neutrase | Alaska pollock | Antioxidant activity | [124] |
| Hydrolyzed collagen with pepsin under acidic conditions | Rana chensinensis | Antioxidant activity | [125] |
| Hydrolyzed collagen with pepsin, subtilisin A, and both enzymes | Arthrospira maxima (spirulina) | Peptides obtained from PHS showed the highest collagenase inhibition activity | [126] |
| Peptides | Tetraselmis suecica Dunaliella tertiolecta, and Nannochloropsis | Decrease in hyaluronidase enzyme | [127] |
| Peptides | Source | Activity | Biblio |
|---|---|---|---|
| Skin collagen peptides (3–10 kDa fraction) | Todarodes pacificus | Copper-chelation | [135] |
| Albumin peptide obtained using papain | Rice bran | Tyrosinase inhibition, copper-chelation | [133] |
| HGGEGGRPY, LQPSHY, and HPTSEVY | Rice | Tyrosinase inhibition | [134] |
| Peptides | Faba bean (Vicia faba) | Tyrosinase inhibition | [136] |
| Water and ethanol extracts from soy milk fermented with lactic acid bacteria strains, | Soy milk | Tyrosinase inhibition | [137] |
| Peptides | Source | Activity | Biblio |
|---|---|---|---|
| TITLDVEPSDTIDGVK ILVLQSNQIR ISGLIYEETR MALSSLPR ISAILPSR LPDAALNR IGNGGELPR QVHPDTGISK EAESSLTGGNGCAK | Saccharina longicruris | Staphylococcus aureus | [191] |
| MDN ELAAAC LRDDF GNAPGAVA ALRMSG RDRFL | Alfalfa RuBisCo | Listeria innocua | [192] |
| QAIIHNEKVQAHGKKVL | Crocodylus siamensis | Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae and Pseudomonas aeruginosa. | [193,194] |
| Cationic peptides | Rice bran | Propionibacterium acnes JCM 6473 | [190] |
| Peptides generated by Aspergillus oryzae, Aspergillus flavipes proteases | Bovine milk | Listeria monocytogenes Staphylococcus aureus Salmonella enterica Enteritidis Escherichia coli Pseudomonas aeruginosa | [191] |
| Peptides | Source | Activity | Biblio |
|---|---|---|---|
| LDAVNR (686 Da) and MMLDF (655 Da) [35] | Spirulina | IL-8 produced by endothelial cells EA.hy926 | [212] |
| FLWGKSY | Spent hen muscle | IL-6 | [200] |
| VLER, WVGK, VVRP, VLLF, VALVR, LFGK, FGPK | Millet bran | TNF-α, IL-1β, PGE2 | [213] |
| DQWL | Whey | IL-1β, COX-2, and TNF-α, and the secretion of IL-1β and TNF-α proteins in LPS-induced RAW 264.7 | [214] |
| YFVP, SGRDP, MVWGP, TGSYTEGWS | Sunflower | IL-1β | [215]. |
| KLRSRNLLHPT, TNGRHSAKKH | Bee pollen | COX-2, IL-6, iNOS, TNF-α | [216] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, I.; Mancusi, A. Food Peptides for the Nutricosmetic Industry. Antioxidants 2023, 12, 788. https://doi.org/10.3390/antiox12040788
Dini I, Mancusi A. Food Peptides for the Nutricosmetic Industry. Antioxidants. 2023; 12(4):788. https://doi.org/10.3390/antiox12040788
Chicago/Turabian StyleDini, Irene, and Andrea Mancusi. 2023. "Food Peptides for the Nutricosmetic Industry" Antioxidants 12, no. 4: 788. https://doi.org/10.3390/antiox12040788
APA StyleDini, I., & Mancusi, A. (2023). Food Peptides for the Nutricosmetic Industry. Antioxidants, 12(4), 788. https://doi.org/10.3390/antiox12040788







