Stomatal Responses of Two Drought-Tolerant Barley Varieties with Different ROS Regulation Strategies under Drought Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Preparation
2.2. Water Deficit Drought Assay
2.3. Stomatal Assay
2.3.1. ABA Treatment
2.3.2. H2O2 Treatment
2.3.3. CaCl2 Treatment
2.4. Gas Exchange Measurement
2.5. WUE Measurement
2.6. ROS Measurement
2.7. Antioxidant Capacity Measurement
2.7.1. SOD Activity
2.7.2. POD Activity
2.7.3. CAT Activity
2.7.4. MDA Content
2.8. Transcriptome Analysis
2.9. Data Analysis
3. Results
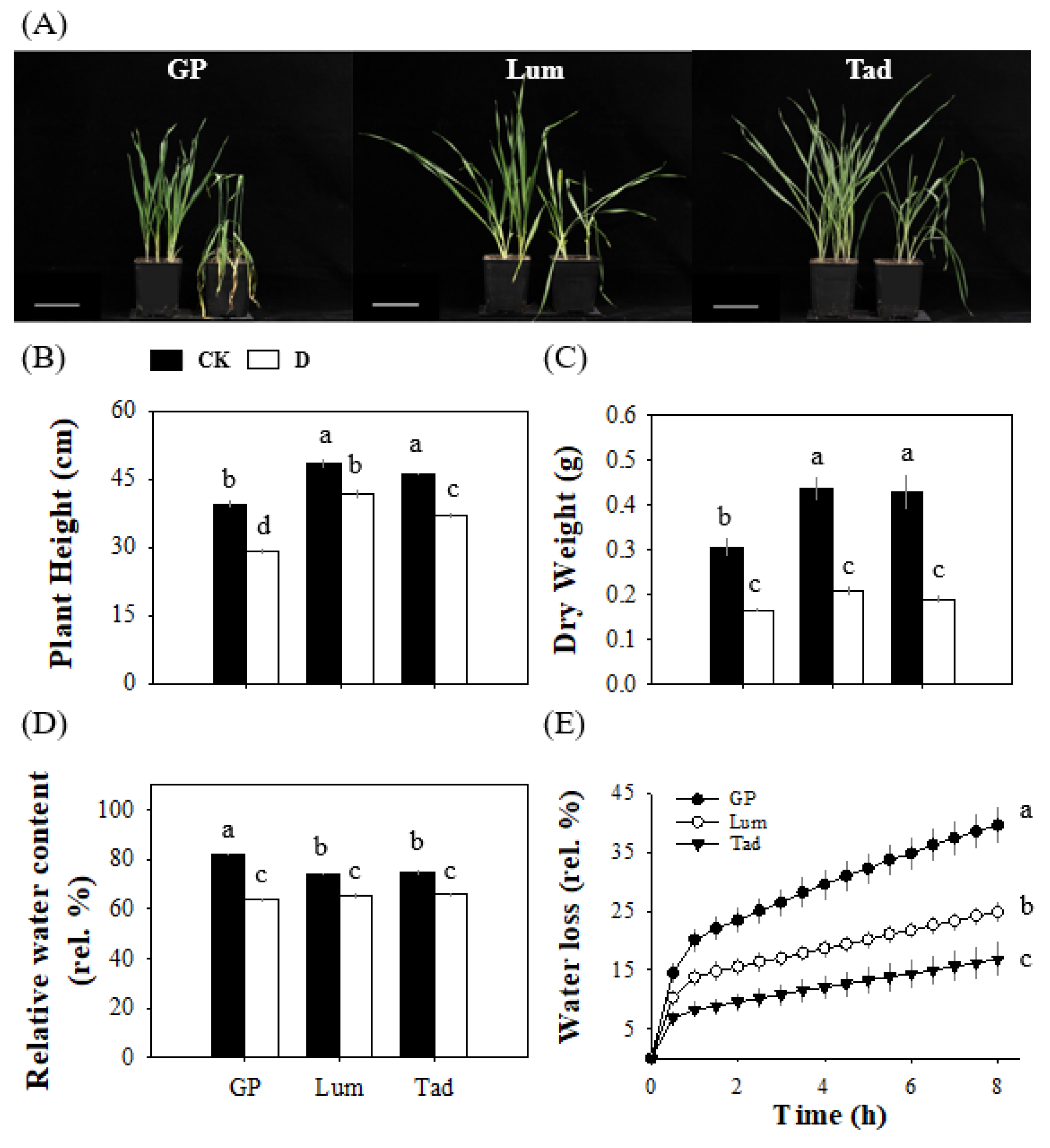
3.1. Lum Exhibits a Strong Tolerance to Long-Term, Progressive Drought Stress
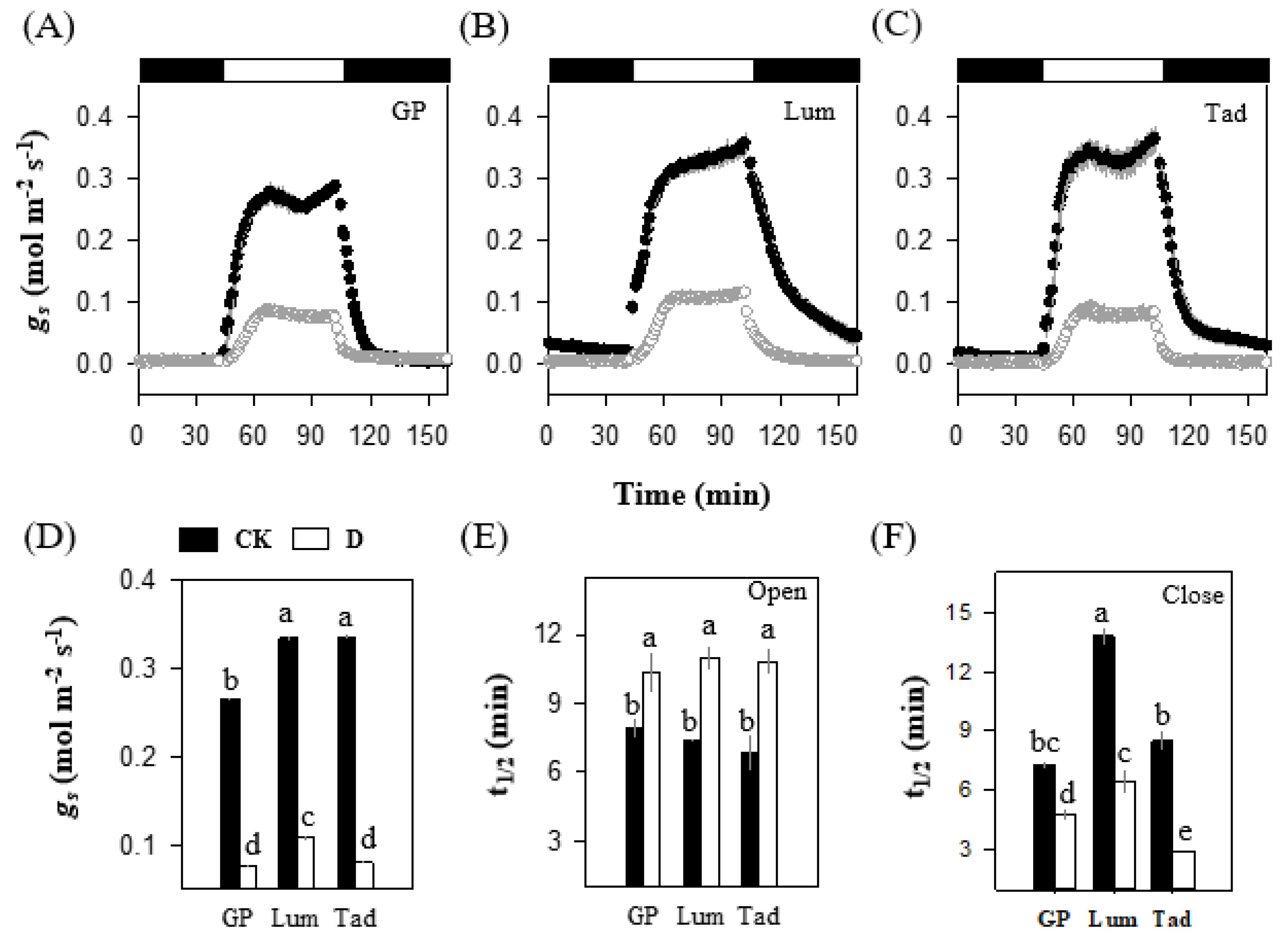
3.2. Lum Maintains Higher Stomatal Conductance for Photosynthesis under Drought Stress
3.3. Lum Exhibits a Sluggish Stomatal Closing under Drought Condition
3.4. ABA-Induced Stomatal Response Is Impaired in Lum
3.5. Transcriptome Analysis Revealed Changes in ROS-Associated Genes in the Drought-Tolerant Genotype Lum
3.6. ABA-Induced ROS Accumulation Was Impaired in Lum
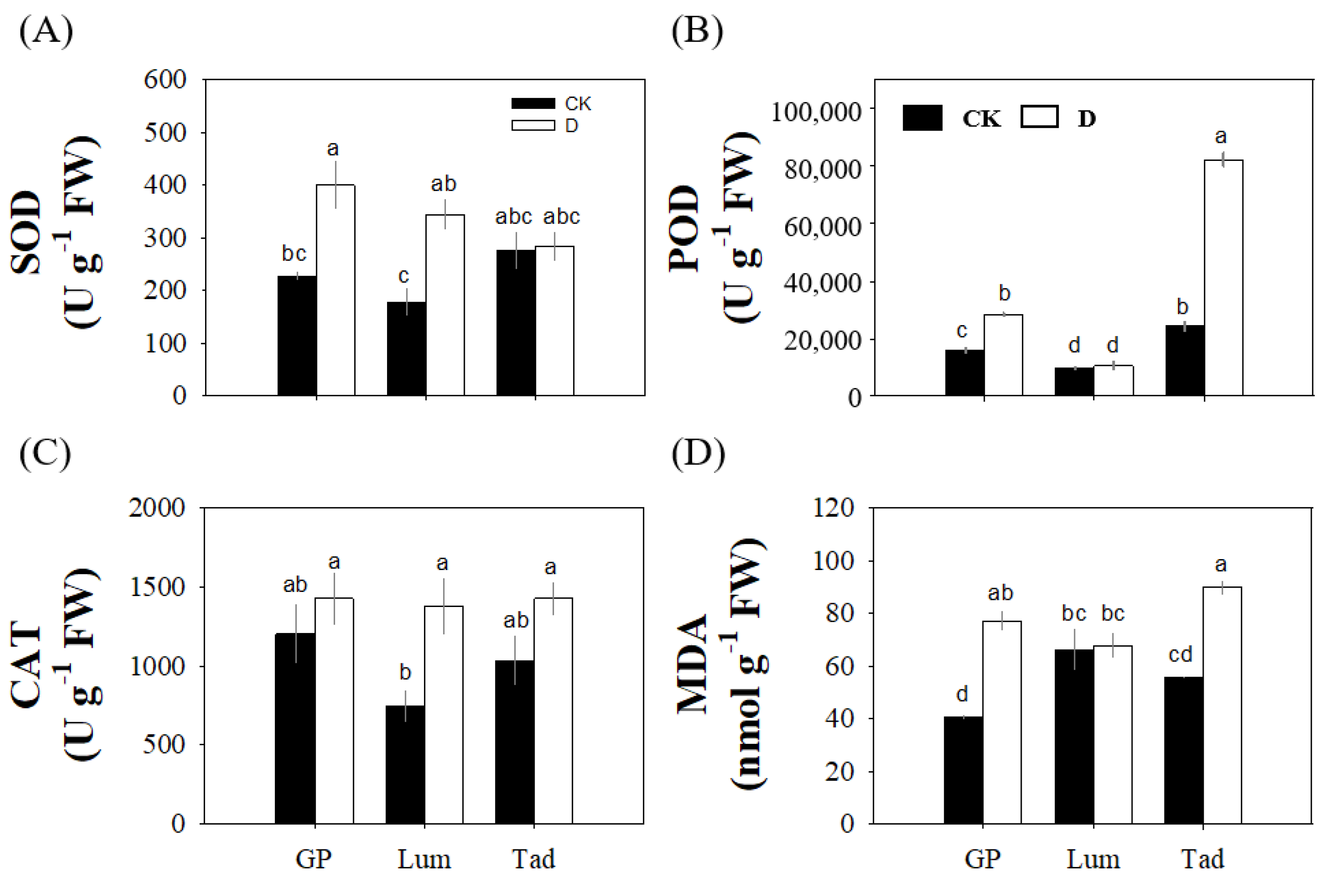
3.7. Different Antioxidant Activities of Three Varieties Reflect Different Response Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.; Rico-Medina, A.; Cano-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Simonneau, T.; Muller, B. The Physiological Basis of Drought Tolerance in Crop Plants: A Scenario-Dependent Probabilistic Approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condon, A.G. Drying times: Plant traits to improve crop water use efficiency and yield. J. Exp. Bot. 2020, 71, 2239–2252. [Google Scholar] [CrossRef] [PubMed]
- Schulte, D.; Close, T.J.; Graner, A.; Langridge, P.; Matsumoto, T.; Muehlbauer, G.; Sato, K.; Schulman, A.H.; Waugh, R.; Wise, R.P.; et al. The International Barley Sequencing Consortium-At the Threshold of Efficient Access to the Barley Genome. Plant Physiol. 2009, 149, 142–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Roychoudhury, A.; Saha, P.P.; Sengupta, D.N. Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul. 2010, 60, 51–59. [Google Scholar] [CrossRef]
- Kadam, N.N.; Tamilselvan, A.; Lawas, L.M.F.; Quinones, C.; Bahuguna, R.N.; Thomson, M.J.; Dingkuhn, M.; Muthurajan, R.; Struik, P.C.; Yin, X.Y.; et al. Genetic Control of Plasticity in Root Morphology and Anatomy of Rice in Response to Water Deficit. Plant Physiol. 2017, 174, 2302–2315. [Google Scholar] [CrossRef] [Green Version]
- Quinones, C.; Mattes, N.; Faronilo, J.; Sudhir-Yadav; Jagadish, K.S.V. Drought Stress Reduces Grain Yield by Altering Floral Meristem Development and Sink Size under Dry-Seeded Rice Cultivation. Crop Sci. 2017, 57, 2098–2108. [Google Scholar] [CrossRef]
- Todaka, D.; Zhao, Y.; Yoshida, T.; Kudo, M.; Kidokoro, S.; Mizoi, J.; Kodaira, K.S.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; et al. Temporal and spatial changes in gene expression, metabolite accumulation and phytohormone content in rice seedlings grown under drought stress conditions. Plant J. 2017, 90, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.Q.; Kong, Z.S.; Omo-Ikerodah, E.; Xu, W.Y.; Li, Q.; Xue, Y.B. Calcineurin B-like interacting protein kinase OsCIPK23 functions in pollination and drought stress responses in rice (Oryza sativa L.). J. Genet. Genom. 2008, 35, 531–543. [Google Scholar] [CrossRef]
- Blum, A. Crop responses to drought and the interpretation of adaptation. Plant Growth Regul. 1996, 20, 135–148. [Google Scholar] [CrossRef]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Allen, G.J.; Hugouvieux, V.; Kwak, J.M.; Waner, D. Guard cell signal transduction. Annu. Rev. Plant Phys. 2001, 52, 627–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blatt, M.R.; Armstrong, F. K+ Channels of Stomatal Guard-Cells—Abscisic-Acid-Evoked Control of the Outward Rectifier Mediated by Cytoplasmic Ph. Planta 1993, 191, 330–341. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Chen, Z.H.; Zhang, B.; Hills, A.; Blatt, M.R. PYR/PYL/RCAR Abscisic Acid Receptors Regulate K+ and Cl− Channels through Reactive Oxygen Species-Mediated Activation of Ca2+ Channels at the Plasma Membrane of Intact Arabidopsis Guard Cells. Plant Physiol. 2013, 163, 566–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.Z.; Blatt, M.R.; Chen, Z.H. Ion Transport at the Plant Plasma Membrane; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.F.; et al. Abscisic Acid Inhibits Type 2C Protein Phosphatases via the PYR/PYL Family of START Proteins. Science 2009, 324, 1068–1071. Available online: https://www.science.org/doi/10.1126/science.1173041 (accessed on 1 February 2023).
- Islam, M.M.; Munemasa, S.; Hossain, M.A.; Nakamura, Y.; Mori, I.C.; Murata, Y. Roles of AtTPC1, Vacuolar Two Pore Channel 1, in Arabidopsis Stomatal Closure. Plant Cell Physiol. 2010, 51, 302–311. [Google Scholar] [CrossRef]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Munemasa, S.; Oda, K.; Watanabe-Sugimoto, M.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol. 2007, 143, 1398–1407. [Google Scholar] [CrossRef] [Green Version]
- Murata, Y.; Pei, Z.M.; Mori, I.C.; Schroeder, J. Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 2001, 13, 2513–2523. [Google Scholar] [CrossRef] [Green Version]
- Pei, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klusener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef]
- Keenan, T.F.; Hollinger, D.Y.; Bohrer, G.; Dragoni, D.; Munger, J.W.; Schmid, H.P.; Richardson, A.D. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 2013, 499, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Jezek, M.; Blatt, M.R. The Membrane Transport System of the Guard Cell and Its Integration for Stomatal Dynamics. Plant Physiol. 2017, 174, 487–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebetzke, G.J.; Condon, A.G.; Richards, R.A.; Farquhar, G.D. Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Sci. 2002, 42, 739–745. [Google Scholar] [CrossRef]
- Masle, J.; Gilmore, S.R.; Farquhar, G.D. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 2005, 436, 866–870. [Google Scholar] [CrossRef]
- Eisenach, C.; Chen, Z.H.; Grefen, C.; Blatt, M.R. The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K plus channel activity with vegetative growth. Plant J. 2012, 69, 241–251. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Hills, A.; Blatt, M.R. Systems Analysis of Guard Cell Membrane Transport for Enhanced Stomatal Dynamics and Water Use Efficiency. Plant Physiol. 2014, 164, 1593–1599. [Google Scholar] [CrossRef] [Green Version]
- Antunes, W.C.; Provart, N.J.; Williams, T.C.R.; Loureiro, M.E. Changes in stomatal function and water use efficiency in potato plants with altered sucrolytic activity. Plant Cell Environ. 2012, 35, 747–759. [Google Scholar] [CrossRef]
- Wang, Y.; Noguchi, K.; Ono, N.; Inoue, S.; Terashima, I.; Kinoshita, T. Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proc. Natl. Acad. Sci. USA 2014, 111, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.T.; Pearcy, R.W. Stomatal versus biochemical limitations to dynamic photosynthetic performance in four tropical rainforest shrub species. Oecologia 2000, 122, 479–486. [Google Scholar] [CrossRef]
- Allen, M.T.; Pearcy, R.W. Stomatal behavior and photosynthetic performance under dynamic light regimes in a seasonally dry tropical rain forest. Oecologia 2000, 122, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Kramer, D.M.; Raines, C.A. Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Curr. Opin. Biotechnol. 2012, 23, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Morales, A.; Harbinson, J.; Heuvelink, E.; Prinzenberg, A.E.; Marcelis, L.F.M. Metabolic and diffusional limitations of photosynthesis in fluctuating irradiance in Arabidopsis thaliana. Sci. Rep. 2016, 6, 31252. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [Green Version]
- Papanatsiou, M.; Petersen, J.; Henderson, L.; Wang, Y.; Christie, J.M.; Blatt, M.R. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 2019, 363, 1456–1459. [Google Scholar] [CrossRef] [Green Version]
- Lawson, T.; Matthews, J. Guard Cell Metabolism and Stomatal Function. Annu. Rev. Plant Biol. 2020, 71, 273–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Noctor, G. Redox signaling in plants. Antioxid. Redox Signal 2013, 18, 2087–2090. [Google Scholar] [CrossRef] [PubMed]
- Vaahtera, L.; Brosche, M.; Wrzaczek, M.; Kangasjarvi, J. Specificity in ROS signaling and transcript signatures. Antioxid. Redox Signal 2014, 21, 1422–1441. [Google Scholar] [CrossRef] [Green Version]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Gadjev, I.; Vanderauwera, S.; Gechev, T.S.; Laloi, C.; Minkov, I.N.; Shulaev, V.; Apel, K.; Inze, D.; Mittler, R.; Van Breusegem, F. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 2006, 141, 436–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenabeele, S.; Van Der Kelen, K.; Dat, J.; Gadjev, I.; Boonefaes, T.; Morsa, S.; Rottiers, P.; Slooten, L.; Van Montagu, M.; Zabeau, M.; et al. A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proc. Natl. Acad. Sci. USA 2003, 100, 16113–16118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenabeele, S.; Vanderauwera, S.; Vuylsteke, M.; Rombauts, S.; Langebartels, C.; Seidlitz, H.K.; Zabeau, M.; Van Montagu, M.; Inze, D.; Van Breusegem, F. Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J. 2004, 39, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Vanderauwera, S.; Zimmermann, P.; Rombauts, S.; Vandenabeele, S.; Langebartels, C.; Gruissem, W.; Inze, D.; Van Breusegem, F. Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005, 139, 806–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Choi, H.; Suh, S.; Doo, I.S.; Oh, K.Y.; Choi, E.J.; Taylor, A.T.S.; Low, P.S.; Lee, Y. Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 1999, 121, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desikan, R.; Last, K.; Harrett-Williams, R.; Tagliavia, C.; Harter, K.; Hooley, R.; Hancock, J.T.; Neill, S.J. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 2006, 47, 907–916. [Google Scholar] [CrossRef]
- Mori, I.C.; Pinontoan, R.; Kawano, T.; Muto, S. Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol. 2001, 42, 1383–1388. [Google Scholar] [CrossRef]
- Kolla, V.A.; Vavasseur, A.; Raghavendra, A.S. Hydrogen peroxide production is an early event during bicarbonate induced stomatal closure in abaxial epidermis of Arabidopsis. Planta 2007, 225, 1421–1429. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005, 8, 397–403. [Google Scholar] [CrossRef]
- Berry, J.A.; Downton, W. Environmental Regulation of Photosynthesis. In Photosynthesis: Development, Carbon Metabolism, and Plant Productivity; Academic Press: New York, NY, USA, 1982; Volume II, pp. 263–343. [Google Scholar] [CrossRef]
- Ramanjulu, S.; Sreenivasalu, N.; Giridhara Kumar, S.; Sudhakar, C. Photosynthetic Characteristics in Mulberry during Water Stress and Rewatering. Photosynthetica 1998, 35, 259–263. [Google Scholar] [CrossRef]
- Ramanjulu, S.; Sreenivasulu, N.; Sudhakar, C. Effect of Water Stress on Photosynthesis in Two Mulberry Genotypes with Different drought Tolerance. Photosynthetica 1998, 35, 279–283. [Google Scholar] [CrossRef]
- Wu, F.B.; Zhang, G.P.; Dominy, P. Four barley genotypes respond differently to cadmium: Lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Willmer, C.; Fricker, M. Stomata; Springer: Berlin, Germany; Dordrecht, The Netherlands, 1996. [Google Scholar] [CrossRef]
- Flexas, J.; Diaz-Espejo, A.; Gago, J.; Galle, A.; Galmes, J.; Gulias, J.; Medrano, H. Photosynthetic limitations in Mediterranean plants: A review. Environ. Exp. Bot. 2014, 103, 12–23. [Google Scholar] [CrossRef]
- Peterhansel, C.; Blume, C.; Offermann, S. Photorespiratory bypasses: How can they work? J. Exp. Bot. 2013, 64, 709–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef] [Green Version]
- Farquhar, G.D.; Sharkey, T.D. Stomatal Conductance and Photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C(3) plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Blatt, M.R. Ca2+ signalling and control of guard-cell volume in stomatal movements. Curr. Opin. Plant Biol. 2000, 3, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Grabov, A.; Blatt, M.R. Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc. Natl. Acad. Sci. USA 1998, 95, 4778–4783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, D.W.; Hills, A.; Kohler, B.; Blatt, M.R. Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl. Acad. Sci. USA 2000, 97, 4967–4972. [Google Scholar] [CrossRef] [Green Version]
- Grabov, A.; Blatt, M.R. A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol. 1999, 119, 277–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, D.W.; Hills, A.; Blatt, M.R. Extracellular Ba2+ and voltage interact to gate Ca2+ channels at the plasma membrane of stomatal guard cells. FEBS Lett. 2001, 491, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Moller, I.M.; Sweetlove, L.J. ROS signalling—Specificity is required. Trends Plant Sci. 2010, 15, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.; Cheung, M.K.; Bright, J.; Henson, D.; Hancock, J.T.; Neill, S.J. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J. Exp. Bot. 2004, 55, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Cardenas, L.; McKenna, S.T.; Kunkel, J.G.; Hepler, P.K. NAD(P)H oscillates in pollen tubes and is correlated with tip growth. Plant Physiol. 2006, 142, 1460–1468. [Google Scholar] [CrossRef] [Green Version]
- Stone, J.R.; Yang, S.P. Hydrogen peroxide: A signaling messenger. Antioxid. Redox Sign 2006, 8, 243–270. [Google Scholar] [CrossRef] [Green Version]
- Baisak, R.; Rana, D.; Acharya, P.B.B.; Kar, M. Alterations in the Activities of Active Oxygen Scavenging Enzymes of Wheat Leaves Subjected to Water-Stress. Plant Cell Physiol. 1994, 35, 489–495. [Google Scholar] [CrossRef]
- Bowler, C.; Vanmontagu, M.; Inze, D. Superoxide Dismutase and Stress Tolerance. Annu. Rev. Plant Phys. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Foyer, C.H.; Descourvieres, P.; Kunert, K.J. Protection against Oxygen Radicals—An Important Defense-Mechanism Studied in Transgenic Plants. Plant Cell Environ. 1994, 17, 507–523. [Google Scholar] [CrossRef]
- Chaoui, A.; Mazhoudi, S.; Ghorbal, M.H.; ElFerjani, E. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci. 1997, 127, 139–147. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ferguson, J.N.; Pignon, C.P.; Wu, A.; Jin, Z.N.; Hammer, G.L.; Lobell, D.B. Water Use Efficiency as a Constraint and Target for Improving the Resilience and Productivity of C-3 and C-4 Crops. Annu. Rev. Plant Biol. 2019, 70, 781–808. [Google Scholar] [CrossRef]
- Cai, S.G.; Chen, G.; Wang, Y.Y.; Huang, Y.Q.; Marchant, D.B.; Wang, Y.Z.; Yang, Q.; Dai, F.; Hills, A.; Franks, P.J.; et al. Evolutionary Conservation of ABA Signaling for Stomatal Closure. Plant Physiol. 2017, 174, 732–747. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.H.; Chen, G.; Dai, F.; Wang, Y.Z.; Hills, A.; Ruan, Y.L.; Zhang, G.P.; Franks, P.J.; Nevo, E.; Blatt, M.R. Molecular Evolution of Grass Stomata. Trends Plant Sci. 2017, 22, 124–139. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 2002, 215, 1022–1030. [Google Scholar] [CrossRef]
- Asada, K. Molecular Mechanism of Production and Scavenging of Active Oxygen Species in Chloroplasts. Nippon. Nogeik Kaishi 1993, 67, 1255–1263. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; LopezDelgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant. 1997, 100, 241–254. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. ASCORBATE AND GLUTATHIONE: Keeping Active Oxygen Under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Slooten, L.; Vandenbranden, S.; Derycke, R.; Botterman, J.; Sybesma, C.; Vanmontagu, M.; Inze, D. Manganese Superoxide-Dismutase Can Reduce Cellular-Damage Mediated by Oxygen Radicals in Transgenic Plants. EMBO J. 1991, 10, 1723–1732. [Google Scholar] [CrossRef]
- Mustilli, A.C.; Merlot, S.; Vavasseur, A.; Fenzi, F.; Giraudat, J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 2002, 14, 3089–3099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edel, K.H.; Kudla, J. Integration of calcium and ABA signaling. Curr. Opin. Plant Biol. 2016, 33, 83–91. [Google Scholar] [CrossRef]
- Hauser, F.; Li, Z.X.; Waadt, R.; Schroeder, J.I. SnapShot: Abscisic Acid Signaling. Cell 2017, 171, 1708. [Google Scholar] [CrossRef] [PubMed]
- Sirichandra, C.; Gu, D.; Hu, H.C.; Davanture, M.; Lee, S.; Djaoui, M.; Valot, B.; Zivy, M.; Leung, J.; Merlot, S.; et al. Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 2009, 583, 2982–2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Song, C.P. Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol. 2008, 178, 703–718. [Google Scholar] [CrossRef] [PubMed]






| Group | AGI Code | Gene ID | Fold Change (Drought vs. Control) | ||
|---|---|---|---|---|---|
| GP | Lum | Tad | |||
| Alternative oxidase (AOX) | At4g22260.1 | HORVU2Hr1G122660 | 0.31 | 1.00 | −0.49 |
| Ascorbate peroxidase (APX) | At4g09010.1 | HORVU2Hr1G101730 | 0.27 | −0.93 | 1.22 |
| At3g09640.1 | HORVU5Hr1G097270 | −0.36 | 1.89 | −2.37 | |
| At4g32320.1 | HORVU6Hr1G009500 | −2.00 | - | −5.21 | |
| At4g35970.1 | HORVU7Hr1G083550 | 0.88 | 3.24 | 0.47 | |
| Catalase (CAT) | At1g20630.1 | HORVU6Hr1G008640 | −0.37 | 0.41 | −1.50 |
| At4g35090.1 | HORVU6Hr1G008730 | −0.44 | 0.15 | −1.50 | |
| Dehydroascorbate reductase (DHAR) | At5g16710.1 | HORVU5Hr1G045850 | −1.24 | 0.42 | −2.42 |
| At5g16710.1 | HORVU7Hr1G038770 | 0.62 | −0.98 | 1.22 | |
| Ferritin | At5g01600.1 | HORVU5Hr1G047730 | 0.44 | 2.91 | 0.10 |
| Glutathione peroxidase (GPX) | At2g31570.1 | HORVU6Hr1G063830 | −0.17 | 0.46 | −0.19 |
| At1g63460.1 | HORVU2Hr1G096960 | −1.25 | 0.05 | −0.36 | |
| Monodehydroascorbate reductase (MDAR) | At5g03630.1 | HORVU1Hr1G013740 | −0.09 | −1.18 | 0.82 |
| At3g09940.1 | HORVU2Hr1G023170 | 0.14 | −0.77 | 0.86 | |
| At5g03630.1 | HORVU7Hr1G101500 | 0.12 | −0.58 | 0.51 | |
| NADPH oxidase (RBOH) | At1g09090.2 | HORVU3Hr1G037600 | 1.05 | 1.18 | 0.38 |
| At5g47910.1 | HORVU3Hr1G069780 | 0.37 | 1.01 | −0.32 | |
| At4g25090.1 | HORVU5Hr1G078630 | −1.91 | 2.29 | −1.19 | |
| Peroxiredoxin (PRX) | At3g11630.1 | HORVU2Hr1G073760 | 0.92 | −0.50 | 0.94 |
| At3g26060.1 | HORVU7Hr1G033500 | 0.85 | 0.00 | 1.09 | |
| At3g52960.1 | HORVU6Hr1G034620 | 0.73 | −0.29 | 1.06 | |
| Superoxide dismutases (SOD) | At1g08830.1 | HORVU2Hr1G021110 | 0.85 | 1.25 | 0.74 |
| At2g28190.1 | HORVU7Hr1G060130 | 0.69 | −0.13 | 1.15 | |
| At5g51100.1 | HORVU7Hr1G008390 | 0.11 | −0.09 | −1.39 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Li, Y.; Chen, R.; Rui, M.; Wang, Y. Stomatal Responses of Two Drought-Tolerant Barley Varieties with Different ROS Regulation Strategies under Drought Conditions. Antioxidants 2023, 12, 790. https://doi.org/10.3390/antiox12040790
Lv X, Li Y, Chen R, Rui M, Wang Y. Stomatal Responses of Two Drought-Tolerant Barley Varieties with Different ROS Regulation Strategies under Drought Conditions. Antioxidants. 2023; 12(4):790. https://doi.org/10.3390/antiox12040790
Chicago/Turabian StyleLv, Xiachen, Yihong Li, Rongjia Chen, Mengmeng Rui, and Yizhou Wang. 2023. "Stomatal Responses of Two Drought-Tolerant Barley Varieties with Different ROS Regulation Strategies under Drought Conditions" Antioxidants 12, no. 4: 790. https://doi.org/10.3390/antiox12040790
APA StyleLv, X., Li, Y., Chen, R., Rui, M., & Wang, Y. (2023). Stomatal Responses of Two Drought-Tolerant Barley Varieties with Different ROS Regulation Strategies under Drought Conditions. Antioxidants, 12(4), 790. https://doi.org/10.3390/antiox12040790







