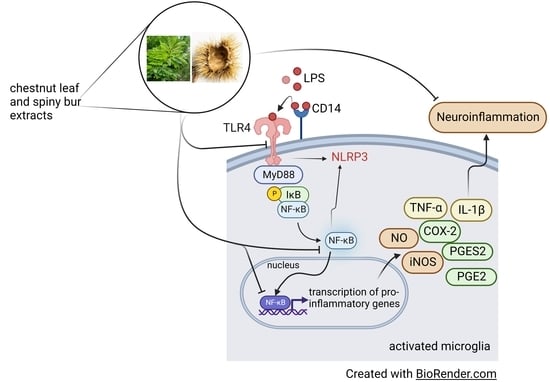
By-Product Extracts from Castanea sativa Counteract Hallmarks of Neuroinflammation in a Microglial Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Extracts Preparation
2.2. Materials
2.3. Cell Culture and Treatments
2.4. LPS Binding Assay
2.5. Surface TLR4 Expression
2.6. Western Blot Analysis
2.7. TNF-α ELISA
2.8. Nitrite Quantification
2.9. RT-PCR Analysis
2.10. Liquid/Liquid Partition and Medium Pressure Liquid Chromatography (MPLC) of Leaf Extract
2.11. NMR and UHPLC-MS Measurement
2.12. Statistical Analysis
3. Results
3.1. Effect of Chestnut Extracts on the Interaction between LPS and TLR4 in BV-2 Microglial Cells
3.2. Effect of Chestnut Extracts on the Level of TLR4 Exposed on the Surface of BV-2 Microglial Cells
3.3. Effect of Chestnut Extracts on mRNA Levels of TLR4 and CD14 in LPS-Stimulated BV-2 Microglial Cells
3.4. Effect of Chestnut Extracts on the NF-kB Signal Transduction Pathway in BV-2 Microglial Cells
3.5. Phytochemical Investigation of C. sativa Leaf Extracts
3.6. Evaluation of the Anti-Inflammatory Activity of the Fractions Obtained from Liquid/Liquid Partition of Chestnut Leaf Extract
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almeida, I.F.; Fernandes, E.; Lima, J.L.F.C.; Costa, P.C.; Bahia, M.F. Protective Effect of Castanea sativa and Quercus robur Leaf Extracts against Oxygen and Nitrogen Reactive Species. J. Photochem. Photobiol. B Biol. 2008, 91, 87–95. [Google Scholar] [CrossRef]
- Barreira, J.; Ferreira, I.; Oliveira, M.; Pereira, J. Antioxidant Activities of the Extracts from Chestnut Flower, Leaf, Skins and Fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Braga, N.; Rodrigues, F.; Oliveira, M.B. Castanea sativa by-Products: A Review on Added Value and Sustainable Application. Nat. Prod. Res. 2015, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Micucci, M.; Malaguti, M.; Budriesi, R.; Ioan, P.; Lenzi, M.; Fimognari, C.; Gallina Toschi, T.; Comandini, P.; Hrelia, S. Sweet Chestnut (Castanea sativa Mill.) Bark Extract: Cardiovascular Activity and Myocyte Protection against Oxidative Damage. Oxidative Med. Cell. Longev. 2013, 2013, 471790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; Lucia, A.D.; Colucci, G.; Cara, F.L.; Morana, A. Chestnut (Castanea sativa Mill.) Industrial Wastes as a Valued Bioresource for the Production of Active Ingredients. Process Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- de Vasconcelos, M.d.C.B.M.; Bennett, R.N.; Quideau, S.; Jacquet, R.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Evaluating the Potential of Chestnut (Castanea sativa Mill.) Fruit Pericarp and Integument as a Source of Tocopherols, Pigments and Polyphenols. Ind. Crops Prod. 2010, 31, 301–311. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant Potential of Chestnut (Castanea sativa L.) and Almond (Prunus dulcis L.) By-Products. Food Sci. Technol. Int. 2010, 16, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Cacciola, N.A.; Squillaci, G.; D’Apolito, M.; Petillo, O.; Veraldi, F.; La Cara, F.; Peluso, G.; Margarucci, S.; Morana, A. Castanea sativa Mill. Shells Aqueous Extract Exhibits Anticancer Properties Inducing Cytotoxic and Pro-Apoptotic Effects. Molecules 2019, 24, 3401. [Google Scholar] [CrossRef] [Green Version]
- Cacciola, N.A.; Cerrato, A.; Capriotti, A.L.; Cavaliere, C.; D’Apolito, M.; Montone, C.M.; Piovesana, S.; Squillaci, G.; Peluso, G.; Laganà, A. Untargeted Characterization of Chestnut (Castanea sativa Mill.) Shell Polyphenol Extract: A Valued Bioresource for Prostate Cancer Cell Growth Inhibition. Molecules 2020, 25, 2730. [Google Scholar] [CrossRef]
- Lenzi, M.; Malaguti, M.; Cocchi, V.; Hrelia, S.; Hrelia, P. Castanea sativa Mill. Bark Extract Exhibits Chemopreventive Properties Triggering Extrinsic Apoptotic Pathway in Jurkat Cells. BMC Complement. Altern. Med. 2017, 17, 251. [Google Scholar] [CrossRef]
- Cerulli, A.; Napolitano, A.; Hošek, J.; Masullo, M.; Pizza, C.; Piacente, S. Antioxidant and In Vitro Preliminary Anti-Inflammatory Activity of Castanea sativa (Italian Cultivar “Marrone Di Roccadaspide” PGI) Burs, Leaves, and Chestnuts Extracts and Their Metabolite Profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants 2021, 10, 278. [Google Scholar] [CrossRef]
- Fei, W.; Xuan, Y.; Jian, X.; Yue, W.; Yuejun, Y.; Yu, J.; Huifang, X.; Yuancai, L.; Yifu, Y.; Xiangwei, Z. One New Phenolic Compound from Castanea mollissima Shells and Its Suppression of HepatomaCell Proliferation and Inflammation by Inhibiting NF-ΚB Pathway. Int. J. Mol. Sci. 2019, 20, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiocchio, I.; Prata, C.; Mandrone, M.; Ricciardiello, F.; Marrazzo, P.; Tomasi, P.; Angeloni, C.; Fiorentini, D.; Malaguti, M.; Poli, F.; et al. Leaves and Spiny Burs of Castanea sativa from an Experimental Chestnut Grove: Metabolomic Analysis and Anti-Neuroinflammatory Activity. Metabolites 2020, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Julien, J.-P.; Rivest, S. Innate Immunity: The Missing Link in Neuroprotection and Neurodegeneration? Nat. Rev. Neurosci. 2002, 3, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Hong, J.-S. Microglia and Inflammation-Mediated Neurodegeneration: Multiple Triggers with a Common Mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Sun, Y.; Peng, G. Neuroinflammation as a Potential Therapeutic Target in Alzheimer’s Disease. CIA 2022, 17, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s Disease: Its Role in Neuronal Death and Implications for Therapeutic Intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.-G.; Mohney, R.P.; Wilson, B.; Jeohn, G.-H.; Liu, B.; Hong, J.-S. Regional Difference in Susceptibility to Lipopolysaccharide-Induced Neurotoxicity in the Rat Brain: Role of Microglia. J. Neurosci. 2000, 20, 6309–6316. [Google Scholar] [CrossRef]
- Gangloff, S.C.; Zähringer, U.; Blondin, C.; Guenounou, M.; Silver, J.; Goyert, S.M. Influence of CD14 on Ligand Interactions between Lipopolysaccharide and Its Receptor Complex. J. Immunol. 2005, 175, 3940–3945. [Google Scholar] [CrossRef] [Green Version]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Liu, B.; Wang, K.; Gao, H.-M.; Mandavilli, B.; Wang, J.-Y.; Hong, J.-S. Molecular Consequences of Activated Microglia in the Brain: Overactivation Induces Apoptosis. J. Neurochem. 2001, 77, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Zusso, M.; Stokes, L.; Moro, S.; Giusti, P. Editorial: Neuroinflammation and Its Resolution: From Molecular Mechanisms to Therapeutic Perspectives. Front. Pharmacol. 2020, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Slivka, P.F.; Shridhar, M.; Lee, G.; Sammond, D.W.; Hutchinson, M.R.; Martinko, A.J.; Buchanan, M.M.; Sholar, P.W.; Kearney, J.J.; Harrison, J.A.; et al. A Peptide Antagonist of the TLR4-MD2 Interaction. ChemBioChem 2009, 10, 645–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeloni, S.; Freschi, M.; Marrazzo, P.; Hrelia, S.; Beghelli, D.; Juan-García, A.; Juan, C.; Caprioli, G.; Sagratini, G.; Angeloni, C. Antioxidant and Anti-Inflammatory Profiles of Spent Coffee Ground Extracts for the Treatment of Neurodegeneration. Oxidative Med. Cell. Longev. 2021, 2021, 6620913. [Google Scholar] [CrossRef] [PubMed]
- Zeka, K.; Marrazzo, P.; Micucci, M.; Ruparelia, K.C.; Arroo, R.R.J.; Macchiarelli, G.; Annarita Nottola, S.; Continenza, M.A.; Chiarini, A.; Angeloni, C.; et al. Activity of Antioxidants from Crocus sativus L. Petals: Potential Preventive Effects towards Cardiovascular System. Antioxidants 2020, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, M.; Cocchi, V.; Novaković, A.; Karaman, M.; Sakač, M.; Mandić, A.; Pojić, M.; Barbalace, M.C.; Angeloni, C.; Hrelia, P.; et al. Meripilus Giganteus Ethanolic Extract Exhibits Pro-Apoptotic and Anti-Proliferative Effects in Leukemic Cell Lines. BMC Complement. Altern. Med. 2018, 18, 300. [Google Scholar] [CrossRef]
- Kopitar-Jerala, N. Innate Immune Response in Brain, NF-Kappa B Signaling and Cystatins. Front. Mol. Neurosci. 2015, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 Signal Transduction Pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [Green Version]
- Knothe, G.; Kenar, J.A. Determination of the Fatty Acid Profile by 1H-NMR Spectroscopy. Eur. J. Lipid Sci. Technol. 2004, 106, 88–96. [Google Scholar] [CrossRef]
- Brasseur, T.; Angenot, L. Four Acylated Flavonol Glycosides from Leaves of Strychnos Variabilis. Phytochemistry 1987, 26, 3331–3334. [Google Scholar] [CrossRef]
- Brasseur, T.; Angenot, L. Six Flavonol Glycosides from Leaves of Strychnos Variabilis. Phytochemistry 1988, 27, 1487–1490. [Google Scholar] [CrossRef]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) Burs Extracts and Functional Compounds: UHPLC-UV-HRMS Profiling, Antioxidant Activity, and Inhibitory Effects on Phytopathogenic Fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, W.; Rowe, D.; Xie, W.; Ortiz, I.; He, Y.; Appel, S.H. Microglial Activation and Dopaminergic Cell Injury: An In Vitro Model Relevant to Parkinson’s Disease. J. Neurosci. 2001, 21, 8447–8455. [Google Scholar] [CrossRef] [Green Version]
- González-Scarano, F.; Baltuch, G. Microglia as Mediators of Inflammatory and Degenerative Diseases. Annu. Rev. Neurosci. 1999, 22, 219–240. [Google Scholar] [CrossRef]
- Lehnardt, S. Innate Immunity and Neuroinflammation in the CNS: The Role of Microglia in Toll-like Receptor-Mediated Neuronal Injury. Glia 2009, 58, 253–263. [Google Scholar] [CrossRef]
- Wurfel, M.M.; Wright, S.D. Lipopolysaccharide-Binding Protein and Soluble CD14 Transfer Lipopolysaccharide to Phospholipid Bilayers: Preferential Interaction with Particular Classes of Lipid. J. Immunol. 1997, 158, 3925–3934. [Google Scholar] [CrossRef]
- Kim, S.; Jin, C.; Kim, C.; Yoo, Y.; Choi, S.; Kim, G.; Yoon, H.; Park, H.; Choi, Y. Isorhamnetin Alleviates Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglia by Inactivating NF-ΚB, Blocking the TLR4 Pathway and Reducing ROS Generation. Int. J. Mol. Med. 2018, 43, 682–692. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Sapkota, K.; Kim, S.; Kim, H.; Kim, S. Kaempferol Acts through Mitogen-Activated Protein Kinases and Protein Kinase B/AKT to Elicit Protection in a Model of Neuroinflammation in BV2 Microglial Cells: Kaempferol Inhibits LPS-Induced Neuroinflammation. Br. J. Pharmacol. 2011, 164, 1008–1025. [Google Scholar] [CrossRef] [Green Version]
- Bakır, T.; Sönmezoğlu, İ.; İmer, F.; Apak, R. Antioxidant/Prooxidant Effects of α-Tocopherol, Quercetin and Isorhamnetin on Linoleic Acid Peroxidation Induced by Cu(II) and H2O2. Int. J. Food Sci. Nutr. 2014, 65, 226–234. [Google Scholar] [CrossRef]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A ROS-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broom, L.; Marinova-Mutafchieva, L.; Sadeghian, M.; Davis, J.B.; Medhurst, A.D.; Dexter, D.T. Neuroprotection by the Selective INOS Inhibitor GW274150 in a Model of Parkinson Disease. Free. Radic. Biol. Med. 2011, 50, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Egger, T.; Schuligoi, R.; Wintersperger, A.; Amann, R.; Malle, E.; Sattler, W. Vitamin E (Alpha-Tocopherol) Attenuates Cyclo-Oxygenase 2 Transcription and Synthesis in Immortalized Murine BV-2 Microglia. Biochem. J. 2003, 370, 459–467. [Google Scholar] [CrossRef]
- Cerulli, A.; Napolitano, A.; Masullo, M.; Hošek, J.; Pizza, C.; Piacente, S. Chestnut Shells (Italian Cultivar “Marrone Di Roccadaspide” PGI): Antioxidant Activity and Chemical Investigation with in Depth LC-HRMS/MSn Rationalization of Tannins. Food Res. Int. 2020, 129, 108787. [Google Scholar] [CrossRef]
- Kang, H. Inhibition of Lipopolysaccharide-Induced Neuroinflammatory Events in Bv-2 Microglia by Chestnut Peel Extract. Trop. J. Pharm. Res. 2014, 13, 1615. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.H.; Shim, Y.Y.; Lee, H.I.; Lee, S.; Reaney, M.J.T.; Chung, M.J. Astragalin and Isoquercitrin Isolated from Aster Scaber Suppress LPS-Induced Neuroinflammatory Responses in Microglia and Mice. Foods 2022, 11, 1505. [Google Scholar] [CrossRef]
- Vella, F.M.; Laratta, B.; La Cara, F.; Morana, A. Recovery of Bioactive Molecules from Chestnut (Castanea sativa Mill.) by-Products through Extraction by Different Solvents. Nat. Prod. Res. 2018, 32, 1022–1032. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Liu, M.; Cheng, X.; Li, W.-H.; Zhang, S.-S.; Wang, Y.-H.; Du, G.-H. Myricitrin Blocks Activation of NF-ΚB and MAPK Signaling Pathways to Protect Nigrostriatum Neuron in LPS-Stimulated Mice. J. Neuroimmunol. 2019, 337, 577049. [Google Scholar] [CrossRef] [Green Version]
- Grochowski, D.M.; Locatelli, M.; Granica, S.; Cacciagrano, F.; Tomczyk, M. A Review on the Dietary Flavonoid Tiliroside: Dietary Flavonoid Tiliroside. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1395–1421. [Google Scholar] [CrossRef] [Green Version]
- Velagapudi, R.; Aderogba, M.; Olajide, O.A. Tiliroside, a Dietary Glycosidic Flavonoid, Inhibits TRAF-6/NF-ΚB/P38-Mediated Neuroinflammation in Activated BV2 Microglia. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 3311–3319. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.S.; Reed, S.C.; Simpson, M.J.A.; Millington, K.J. The Health Benefits of Omega-3 Polyunsaturated Fatty Acids: A Review of the Evidence. J. Hum. Nutr. Diet 2004, 17, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Choque, B.; Catheline, D.; Rioux, V.; Legrand, P. Linoleic Acid: Between Doubts and Certainties. Biochimie 2014, 96, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, J.D.; Valdés, A.; Gallego, R.; Suárez-Montenegro, Z.J.; Alarcón, M.; Ibañez, E.; Alvarez-Rivera, G.; Cifuentes, A. Blood–Brain Barrier Permeability Study of Potential Neuroprotective Compounds Recovered from Plants and Agri-Food by-Products. Front. Nutr. 2022, 9, 924596. [Google Scholar] [CrossRef] [PubMed]
- AL-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers 2021, 13, 3934. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [Green Version]














| Target | Sequence (5′–3′) | ID RefSeq |
|---|---|---|
| GAPDH * | Forward ACCACAGTCCATGCCATCAC | NM_008084 |
| Reverse TCCACCACCCTGTTGCTGTA | ||
| TLR4 | Forward GATCAGAAACTCAGCAAAGTC | NM_021297 |
| Reverse TGTTTCAATTTCACACCTGG | ||
| CD14 | Forward GCC AAA TTG GTC GAA CAA GC | NM_009841 |
| Reverse CCA TGG TCG GTA GAT TCT GAA AGT | ||
| NLRP3 | Forward GATGCTGGAATTAGACAACTG | NM_145827 |
| Reverse GTACATTTCACCCAACTGTAG | ||
| PGES2 | Forward GAAGGACTGAGATCAAATTCTC | NM_008964 |
| Reverse ATGACAGAGGAGTCATTGAG | ||
| IL-1β | Forward GTTCCCATTAGACAACTGCACTACAG | NM_008361 |
| Reverse GTCGTTGCTTGGTTCTCCTTGTA | ||
| TNF-α | Forward CCCCAAAGGGATGAGAAGTTC | NM_013693 |
| Reverse CCTCCACTTGGTGGTTTGCT | ||
| iNOS | Forward CCTCCTCCACCCTACCAAGT | NM_010927 |
| Reverse CACCCAAAGTGCTTCAGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrazzo, P.; Mandrone, M.; Chiocchio, I.; Zambonin, L.; Barbalace, M.C.; Zalambani, C.; Angeloni, C.; Malaguti, M.; Prata, C.; Poli, F.; et al. By-Product Extracts from Castanea sativa Counteract Hallmarks of Neuroinflammation in a Microglial Model. Antioxidants 2023, 12, 808. https://doi.org/10.3390/antiox12040808
Marrazzo P, Mandrone M, Chiocchio I, Zambonin L, Barbalace MC, Zalambani C, Angeloni C, Malaguti M, Prata C, Poli F, et al. By-Product Extracts from Castanea sativa Counteract Hallmarks of Neuroinflammation in a Microglial Model. Antioxidants. 2023; 12(4):808. https://doi.org/10.3390/antiox12040808
Chicago/Turabian StyleMarrazzo, Pasquale, Manuela Mandrone, Ilaria Chiocchio, Laura Zambonin, Maria Cristina Barbalace, Chiara Zalambani, Cristina Angeloni, Marco Malaguti, Cecilia Prata, Ferruccio Poli, and et al. 2023. "By-Product Extracts from Castanea sativa Counteract Hallmarks of Neuroinflammation in a Microglial Model" Antioxidants 12, no. 4: 808. https://doi.org/10.3390/antiox12040808
APA StyleMarrazzo, P., Mandrone, M., Chiocchio, I., Zambonin, L., Barbalace, M. C., Zalambani, C., Angeloni, C., Malaguti, M., Prata, C., Poli, F., Fiorentini, D., & Hrelia, S. (2023). By-Product Extracts from Castanea sativa Counteract Hallmarks of Neuroinflammation in a Microglial Model. Antioxidants, 12(4), 808. https://doi.org/10.3390/antiox12040808