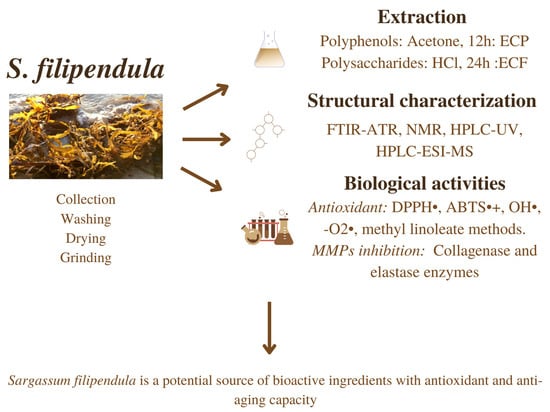
Sargassum filipendula, a Source of Bioactive Compounds with Antioxidant and Matrix Metalloproteinases Inhibition Activities In Vitro with Potential Dermocosmetic Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Biological Material and Extraction Process
2.2. Sugar Content Analysis of Fucoidan Extracts
2.2.1. Neutral Sugars Content
2.2.2. Acid Sugars Content
2.2.3. Sulfated Sugar Content
2.3. Antioxidant Properties of Fucoidan and Phlorotannins Extracts
2.3.1. Quantification of Total Phenolic Content
2.3.2. In-Vitro ABTS Radical Scavenging Method
2.3.3. DDPH Scavenging Activity Assay
2.3.4. Hydroxyl Radical Scavenging Activity Assay
- A: Sample with H2O2.
- B: Sample without H2O2.
- C: Control sample.
2.3.5. Superoxide Radical Scavenging Activity Assay
- A: Sample with pyrogallol.
- B: Sample without pyrogallol.
- C: Control sample.
2.3.6. Evaluation of the Lipid Peroxidation of Methyl Linoleate (MeLo) In Vitro
2.4. Determination of Matrix Metalloproteinases (MMPs) Inhibition Potential of Fucoidan and Phlorotannins Extracts
2.4.1. Anti-Collagenase Activity
- ANC: Absorbance of the negative control after incubation.
- ABE: Absorbance of the enzyme blank (buffer solution and enzyme).
- ASample: Absorbance of the sample after incubation.
2.4.2. Anti-Elastase Activity
2.5. Spectroscopic Characterization
2.5.1. Analysis of Monosaccharides by HPLC-UV
2.5.2. Analysis of Phlorotannins by HPLC-ESI-MS
2.6. Statistical Analysis
3. Results and Discussion
3.1. Yield Extraction and Proximate Composition of Fucoidan and Phlorotannin Extract
3.2. Spectroscopic Characterization of Fucoidan and Phlorotannin Extracts
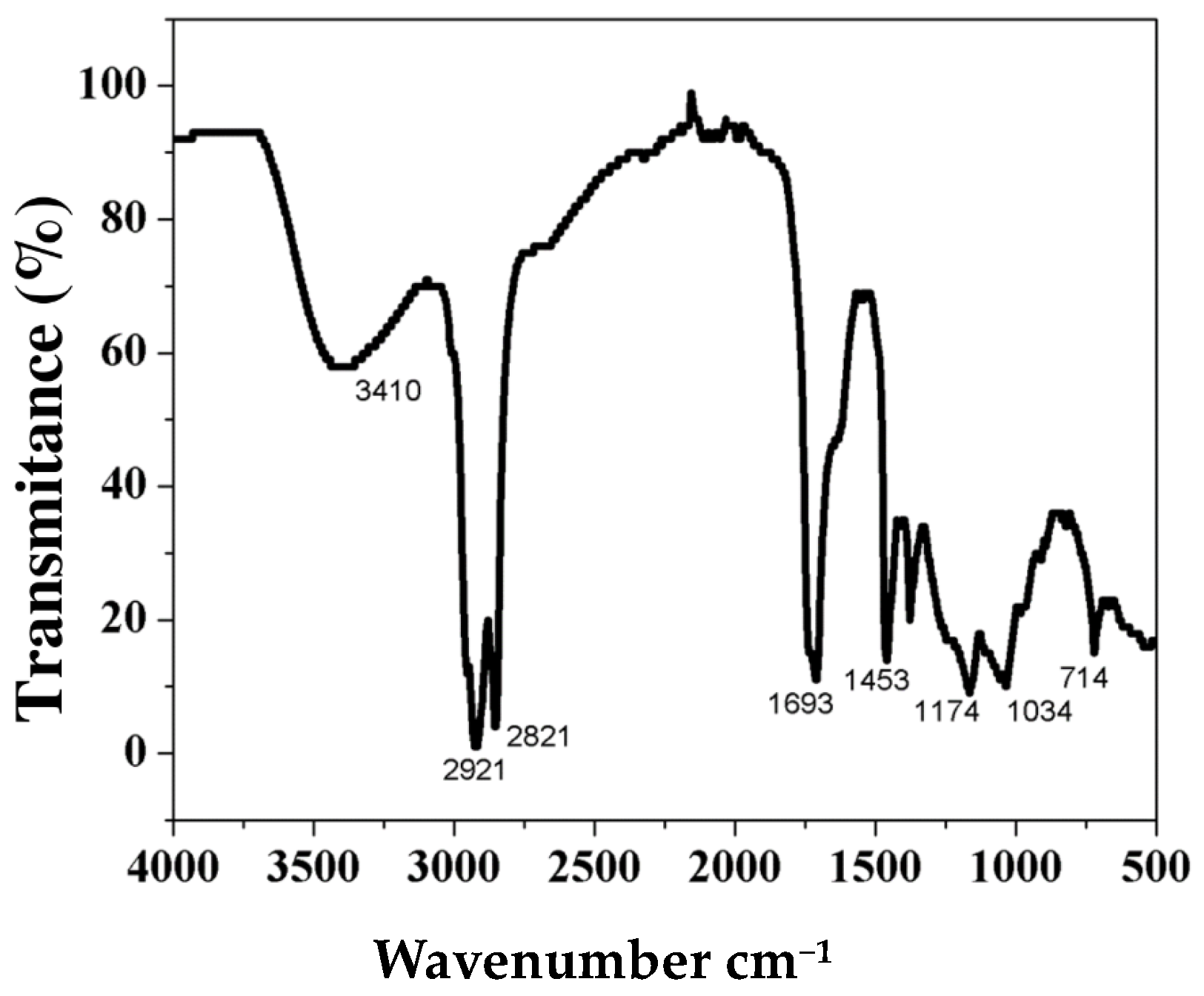
3.2.1. FTIR-ATR Characterization
3.2.2. NMR Characterization
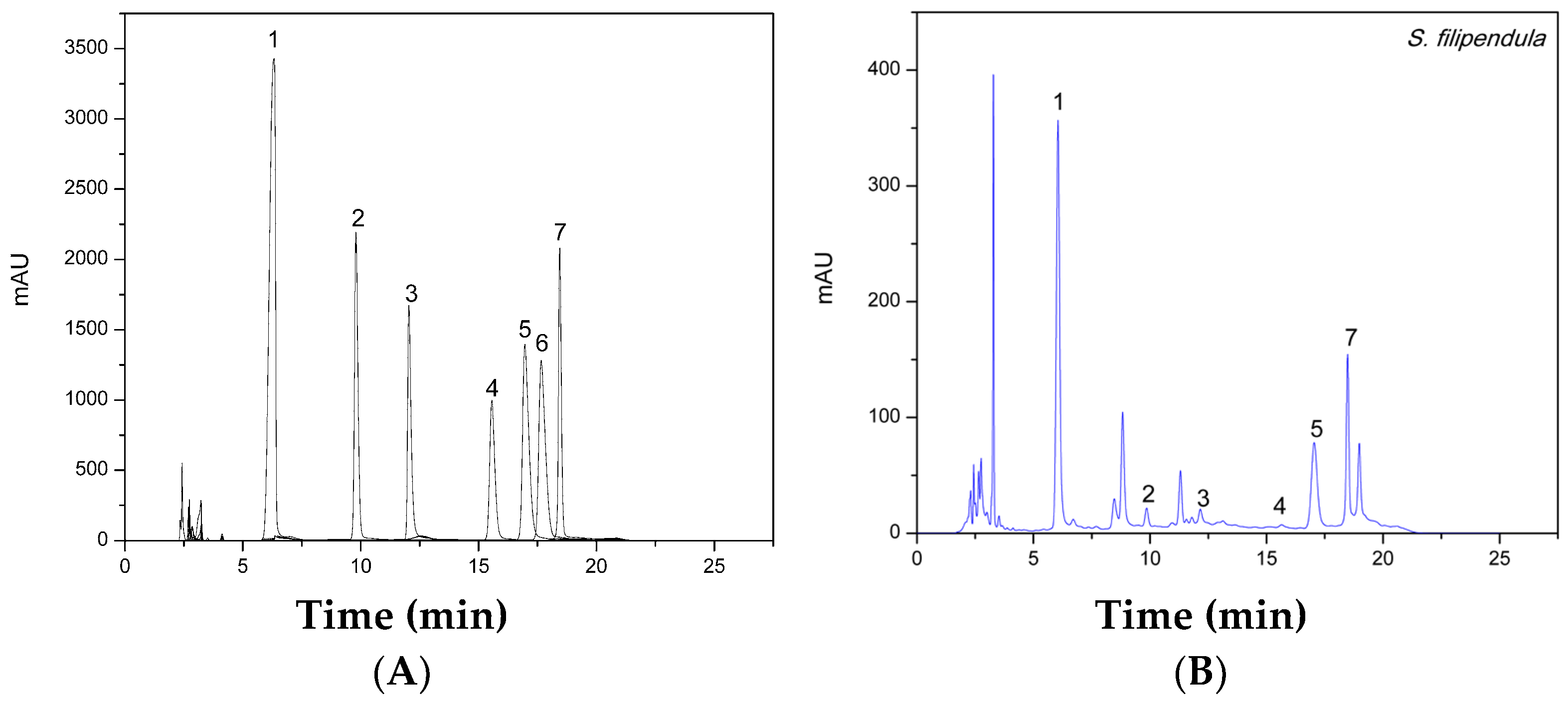
3.2.3. Analysis of Monosaccharides by HPLC-UV
3.2.4. Analysis of Phlorotannins by HPLC-ESI-MS
3.3. Antioxidant Activity of Fucoidan and Phlorotannins Extracts
3.4. Matrix Metalloproteinases (MMPs) Inhibition Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villamizar, G.E.Y.; Cervigón, F. Variability and sustainability of the Southern Subarea of the Caribbean Sea large marine ecosystem. Environ. Dev. 2017, 22, 30–41. [Google Scholar] [CrossRef]
- Rincón-Díaz, N.; Gavio, B. Diversidad de Macroalgas Marinas del Caribe Colombiano; Instituto de Investigaciones Marinas y Costeras: Santa Marta, Colombia, 2020. [Google Scholar]
- Bird, C. A Checklist of Benthic Marine Algae of the Tropical and Subtropical Western Atlantic: First Revision. Phycologia 1998, 37, 489–490. [Google Scholar] [CrossRef]
- Camacho, O.; Mattio, L.; Draisma, S.; Fredericq, S.; Diaz-Pulido, G. Morphological and molecular assessment of Sargassum (Fucales, Phaeophyceae) from Caribbean Colombia, including the proposal of Sargassum giganteum sp. nov., Sargassum schnetteri comb. nov. and Sargassum section Cladophyllum sect. nov. Syst. Biodivers 2015, 13, 105–130. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; June Chelyn, L.; Vimala, S.; Mohd Fairulnizal, M.N.; Brownlee, I.A.; Amin, I. Carotenoid composition and antioxidant potential of Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera. Heliyon 2020, 6, e04654. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification, and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chung, D.; Shin, I.-S.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Pei, P.; Zheng, C.; Cheong, K.L.; Huang, N. Unravelling property of polysaccharides from Sargassum sp. as an anti-wrinkle and skin whitening property. Int. J. Biol. Macromol. 2019, 140, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Kim, W.-J.; Kim, S.-M.; Kim, H.-G.; Oh, H.-R.; Lee, K.-B.; Lee, Y.-K.; Park, Y.-I. Purification and Anticoagulant Activity of a Fucoidan from Korean Undaria pinnatifida Sporophyll. Algae 2007, 22, 247–252. [Google Scholar] [CrossRef]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective substances derived from marine algae. Mar. Drugs. 2018, 16, 399. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.P.; Sidana, J. Phlorotannins. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing: Sawston, UK, 2013; pp. 181–204. [Google Scholar]
- Hermund, D.B. Antioxidant Properties of Seaweed-Derived Substances. In Bioactive Seaweeds for Food Applications: Natural Ingredients for Healthy Diets; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 201–221. [Google Scholar] [CrossRef]
- Barbosa, M.; Lopes, G.; Andrade, P.B.; Valentão, P. Bioprospecting of brown seaweeds for biotechnological applications: Phlorotannin actions in inflammation and allergy network. Trends Food Sci. Technol. 2019, 86, 153–171. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kisara, K.; Danielsson, S.; Lindstrom, M.E.; Gellerstedt, G. An improved methodology for the quantification of uronic acid units in xylans and other polysaccharides. Carbohydr. Res. 2007, 342, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Anand, J.; Sathuvan, M.; Babu, G.V.; Sakthivel, M.; Palani, P.; Nagaraj, S. Bioactive potential and composition analysis of sulfated polysaccharide from Acanthophora spicifera (Vahl) Borgeson. Int. J. Biol. Macromol. 2018, 111, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Hudz, N.; Yezerska, O.; Shanaida, M.; Sedláčková, V.H.; Wieczorek, P.P. Application of the Folin-Ciocalteu method to the evaluation of Salvia sclarea extracts. Pharmacia 2019, 66, 209–215. [Google Scholar] [CrossRef]
- Hamed, M.; Bougatef, H.; Karoud, W.; Krichen, F.; Haddar, A.; Bougatef, A.; Sila, A. Polysaccharides extracted from pistachio external hull: Characterization, antioxidant activity and potential application on meat as preservative. Ind. Crops Prod. 2020, 148, 112315. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Wen, C.; Zhang, J.; He, Y.; Ma, H.; Duan, Y. Purification, characterization, antioxidant and immunological activity of polysaccharide from Sagittaria sagittifolia L. Food Res. Int. 2020, 136, 109345. [Google Scholar] [CrossRef]
- Shang, X.L.; Liu, C.Y.; Dong, H.Y.; Peng, H.H.; Zhu, Z.Y. Extraction, purification, structural characterization, and antioxidant activity of polysaccharides from Wheat Bran. J. Mol. Struct. 2021, 1233, 130096. [Google Scholar] [CrossRef]
- Mejía-Giraldo, J.C.; Winkler, R.; Gallardo, C.; Sánchez-Zapata, A.M.; Puertas-Mejía, M.A. Photoprotective Potential of Baccharis antioquensis (Asteraceae) as Natural Sunscreen. Photochem. Photobiol. 2016, 92, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Giraldo, J.C.; Winkler, R.; Puertas-Mejía, M. Novel UV filters from Pentacalia pulchella extracts with photoprotective properties and antioxidant activity. Photochem. Photobiol. Sci. 2021, 20, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Fauziah, N.; Herdiman, H.; Afni, M.; Afifah, E.; Kusuma, H.S.W.; Nufus, H.; Arumwardana, S.; Rihibiha, D.D. Antioxidant and anti aging assays of Oryza sativa extracts, vanillin and coumaric acid. J. Nat. Remedies. 2016, 16, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Shanura Fernando, I.P.; Asanka Sanjeewa, K.K.; Samarakoon, K.W.; Kim, H.S.; Gunasekara, U.K.D.S.S.; Park, Y.J.; Abeytunga, D.T.U.; Lee, W.W.; Jeon, Y.J. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: Antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J. Appl. Phycol. 2018, 30, 3223–3232. [Google Scholar] [CrossRef]
- Siu, K.C.; Chen, X.; Wu, J.Y. Constituents actually responsible for the antioxidant activities of crude polysaccharides isolated from mushrooms. J. Funct. Foods 2014, 11, 548–556. [Google Scholar] [CrossRef]
- Arunkumar, K.; Raj, R.; Raja, R.; Carvalho, I.S. Brown seaweeds as a source of anti-hyaluronidase compounds. S. Afr. J. Bot. 2021, 139, 470–477. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, X.; Qi, H. Characterization and bioactivity of phlorotannin loaded protein-polysaccharide nanocomplexes. LWT 2021, 155, 112998. [Google Scholar] [CrossRef]
- Mahendran, S.; Maheswari, P.; Sasikala, V.; Rubika, J.J.; Pandiarajan, J. In vitro antioxidant study of polyphenol from red seaweeds dichotomously branched gracilaria Gracilaria edulis and robust sea moss Hypnea valentiae. Toxicol. Rep. 2021, 8, 1404–1411. [Google Scholar] [CrossRef]
- Kawamura-Konishi, Y.; Watanabe, N.; Saito, M.; Nakajima, N.; Sakaki, T.; Katayama, T.; Enomoto, T. Isolation of a new phlorotannin, a potent inhibitor of carbohydrate- hydrolyzing enzymes, from the brown alga Sargassum patens. J. Agric. Food Chem. 2012, 60, 5565–5570. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Yuan, Y.; Jing, C.; Cao, J.; Wang, Y.; Zhang, L.; Zhang, C.; Li, Y. Purification and characterization of a fucoidan from the brown algae Macrocystis pyrifera and the activity of enhancing salt-stress tolerance of wheat seedlings. Int. J. Biol. Macromol. 2021, 180, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; González-Muñoz, M.J.; Domínguez, H. Influence of molecular weight on the properties of Sargassum muticum fucoidan. Algal Res. 2018, 38, 101393. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Ben Salah, H.; Jardak, N.; Chaaben, R.; Jribi, I.; El Feki, A.; Rebai, T.; Jamoussi, K.; Allouche, N.; Blecker, C.; et al. Sulphated polysaccharide isolated from Sargassum vulgare: Characterization and hypolipidemic effects. Carbohydr. Polym. 2017, 170, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Wu, S.-J.; Yang, W.-N.; Kuan, A.-W.; Chen, C.-Y. Antioxidant activities of crude extracts of fucoidan extracted from Sargassum glaucescens by a compressional-puffing-hydrothermal extraction process. Food Chem. 2016, 197, 1121–1129. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Q.; Wang, Q.; He, Y.; Ren, D.; Liu, S.; Wu, L. Structural characterization and antitumor effects of fucoidans from brown algae Kjellmaniella crassifolia farmed in northern China. Int. J. Biol. Macromol. 2018, 119, 125–133. [Google Scholar] [CrossRef]
- Zhao, M.; Garcia-Vaquero, M.; Przyborska, J.; Sivagnanam, S.P.; Tiwari, B. The development of analytical methods for the purity determination of fucoidan extracted from brown seaweed species. Int. J. Biol. Macromol. 2021, 173, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Bilan, I.M.; Grachev, A.A.; Ustuzhanina, E.N.; Shashkov, A.S.; Nifantiev, E.N.; Usov, I.A. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Jin, W.; Tang, H.; Zhang, J.; Wei, B.; Sun, J.; Zhang, W.; Zhang, F.; Wang, H.; Linhardt, R.J.; Zhong, W. Structural analysis of a novel sulfated galacto-fuco-xylo-glucurono-mannan from Sargassum fusiforme and its anti-lung cancer activity. Int. J. Biol. Macromol. 2020, 149, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.-Y.-Y.; Wang, J.-Q.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Kalinovsky, A.I.; Miansong, Z.; Changheng, L.; Malyarenko, O.; Zueva, A.O.; Zvyagintseva, T.N.; Ermakova, S.P. Structure, enzymatic transformation, anticancer activity of fucoidan and sulphated fucooligosaccharides from Sargassum horneri. Carbohydr. Polym. 2017, 175, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Cong, Q.; Chen, H.; Liao, W.; Xiao, F.; Wang, P.; Qin, Y.; Dong, Q.; Ding, K. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2016, 136, 899–907. [Google Scholar] [CrossRef]
- Li, J.; Gu, F.; Cai, C.; Hu, M.; Fan, L.; Hao, J.; Yu, G. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2020, 143, 806–813. [Google Scholar] [CrossRef]
- Agrawal, P.K. NMR Spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Zhang, R.; Yuen, A.K.; Magnusson, M.; Wright, J.T.; de Nys, R.; Masters, A.F.; Maschmeyer, T. A comparative assessment of the activity and structure of phlorotannins from the brown seaweed Carpophyllum flexuosum. Algal Res. 2018, 29, 130–141. [Google Scholar] [CrossRef]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef]
- Vissers, A.M.; Caligiani, A.; Sforza, S.; Vincken, J.-P.; Gruppen, H. Phlorotannin Composition of Laminaria digitata. Phytochem. Anal. 2017, 28, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Imbs, T.I.; Silchenko, A.S.; Fedoreev, S.A.; Isakov, V.V.; Ermakova, S.P.; Zvyagintseva, T.N. Fucoidanase inhibitory activity of phlorotannins from brown algae. Algal Res. 2018, 32, 54–59. [Google Scholar] [CrossRef]
- Costa, L.S.; Telles, C.B.S.; Oliveira, R.M.; Nobre, L.T.D.B.; Dantas-Santos, N.; Camara, R.B.G.; Costa, M.S.S.P.; Almeida-Lima, J.; Melo-Silveira, R.F.; Albuquerque, I.R.L.; et al. Heterofucan from Sargassum filipendula Induces Apoptosis in HeLa Cells. Mar. Drugs 2011, 9, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B.S.; Dantas-Santos, N.; Camara, R.B.G.; Cordeiro, S.L.; Costa, M.S.S.P.; Almeida-Lima, J.; Melo-Silveira, R.F.; Oliveira, R.M.; et al. Antioxidant and Antiproliferative Activities of Heterofucans from the Seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phasanasophon, K.; Kim, S.M. Antioxidant and Cosmeceutical Activities of Agarum cribrosum Phlorotannin Extracted by Ultrasound Treatment. Nat. Prod. Commun. 2018, 13, 565–570. [Google Scholar] [CrossRef]
- Boonchum, W.; Peerapornpisal, Y.; Kanjanapothi, D.; Pekkoh, J.; Pumas, C.; Jamjai, U.; Amornlerdpison, D.; Noiraksar, T.; Vacharapiyasophon, P. Antioxidant activity of some seaweed from the Gulf of Thailand. Int. J. Agric. Biol. 2011, 13, 95–99. [Google Scholar]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Dore, C.M.P.G.; Alves, M.G.d.C.F.; Will, L.S.E.P.; Costa, T.G.; Sabry, D.A.; de Souza Rêgo, L.A.R.; Accardo, C.M.; Rocha, H.A.; Filgueira, L.G.A.; Leite, E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Ashayerizadeh, O.; Dastar, B.; Pourashouri, P. Study of antioxidant and antibacterial activities of depolymerized fucoidans extracted from Sargassum tenerrimum. Int. J. Biol. Macromol. 2019, 151, 1259–1266. [Google Scholar] [CrossRef]
- Costa, L.; Fidelis, G.P.; Cordeiro, S.; Oliveira, R.; Sabry, D.; Câmara, R.; Nobre, L.; Costa, M.; Almeida-Lima, J.; Farias, E.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M.; Gomaa, M. Industrial optimization of fucoidan extraction from Sargassum sp. and its potential antioxidant and emulsifying activities. Food Hydrocoll. 2016, 54, 77–88. [Google Scholar] [CrossRef]
- Athukorala, Y.; Kim, K.-N.; Jeon, Y.-J. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem. Toxicol. 2006, 44, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Martins, A.; Silva, J.; Alves, C.; Pinteus, S.; Alves, J.; Teodoro, F.; Ribeiro, H.M.; Gonçalves, L.; Petrovski, Ž.; et al. Highlighting the Biological Potential of the Brown Seaweed Fucus spiralis for Skin Applications. Antioxidants 2020, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.V.; Manivasagan, P.; Kim, S.-K. Potential matrix metalloproteinase inhibitors from edible marine algae: A review. Environ. Toxicol. Pharmacol. 2014, 37, 1090–1100. [Google Scholar] [CrossRef]

| Sample 1 | Yield (%) | Fucose (% w/w) | Uronic Acids (% w/w) | Sulfated Sugars (% w/w) |
|---|---|---|---|---|
| CEF | 1.8 ± 0.1 | 63.0 ± 1.1 a | 8.60 ± 1.5 a | 43.6 ± 1.8 a |
| HiP1 | 17.1 ± 0.1 * | 66.3 ± 1.9 a | 16.2 ± 0.21 b | 43.5 ± 1.6 a |
| CF | - | 46.9 ± 0.6 b | 0.24 ± 0.058 c | 38.2 ± 2.0 b |
| CEP | 0.7 ± 0.1 | - | - | - |
| Residue | Atom | Chemical Shift (ppm) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
A: (1→3) α-l-Fucp(OSO3−) | C | 99.21 | 68.53 | 73.34 | 80.62 | 66.72 | 16.48 |
| H | 5.26 | 3.96 | 4.22 | 4.63 | 4.38 | 1.17 | |
B: (1→4) β-d-Glcp | C | 103.41 | 72.68 | ND | 84.52 | ND | 61.25 |
| H | 4.42 | 3.63 | ND | 3.72 | ND | 3.74 | |
C: (1→3) α-l-Fucp | C | 99.49 | 68.50 | 73.32 | ND | 67.42 | 16.49 |
| H | 5.17 | 3.77 | 3.92 | ND | 4.27 | 1.09 | |
| Compound | Suggested Florotannin | Retention Time (min) | Molecular Formula | Molecular Mass (Daltons) | Protonated Parent Ion [M + H]+ |
|---|---|---|---|---|---|
| 1 | Eckol | 8.4 | C18H10O10 | 386.2 | 387.1489 |
| 2 | Bifuhalol | 20.9 | C12H10O7 | 266.2 | 267.2174 |
| 3 | Trifuhalol | 26.8 | C18H14O10 | 390.3 | 391.2858 |
| Sample 1 | TPC 2 (% w/w) | mmol CDH 3/kg MeLo | mmol MDA 4/kg MeLo | DPPH Radical Scavenging Capacity, Expressed as EC50 5 | ABTS Radical Scavenging Capacity, Expressed as EC50 |
|---|---|---|---|---|---|
| HiP1 | 1.38 ± 0.12 a | ND 6 | ND | 8.19 ± 0.033 a | 10.1 ± 0.26 a |
| CEF | 3.21 ± 0.03 b | 19.97 ± 2.97 a | 0.28 ± 0.01 a | 2.90 ± 0.01 b | 1.86 ± 0.026 b |
| CEP | 32.11 ± 0.03 c | 57.46 ± 2.36 a | 0.50 ± 0.16 a | 2.83 ± 0.01 b | 2.90 ± 0.02 c |
| BHT | - | 31.4 ± 0.74 c | 0.0769 ± 0.0064 b | 0.571 ± 0.050 c | 0.377 ± 0.010 d |
| AA | - | - | - | 0.212 ± 0.0019 d | 0.134 ± 0.057 e |
| MeLo | - | 155.3 ± 4.5 d | 9.85 ± 0.28 c | - | - |
| Sample 1 | Anti-Collagenase Capacity Expressed as IC50 (mg/mL) 2 | Anti-Elastase Capacity Expressed as IC50 (mg/mL) |
|---|---|---|
| HiP1 | 9.97 ± 0.16 a | ND 3 |
| CEF | 1.61 ± 0.00 b | 0.04 ± 0.02 a |
| CEP | 0.36 ± 0.01 c | 0.04 ± 0.01 a |
| EGCG | 0.17 ± 0.00 d | 0.04 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Pérez, Y.; Ríos-López, L.G.; Otero-Tejada, E.L.; Mejía-Giraldo, J.C.; Puertas-Mejía, M.Á. Sargassum filipendula, a Source of Bioactive Compounds with Antioxidant and Matrix Metalloproteinases Inhibition Activities In Vitro with Potential Dermocosmetic Application. Antioxidants 2023, 12, 876. https://doi.org/10.3390/antiox12040876
Luna-Pérez Y, Ríos-López LG, Otero-Tejada EL, Mejía-Giraldo JC, Puertas-Mejía MÁ. Sargassum filipendula, a Source of Bioactive Compounds with Antioxidant and Matrix Metalloproteinases Inhibition Activities In Vitro with Potential Dermocosmetic Application. Antioxidants. 2023; 12(4):876. https://doi.org/10.3390/antiox12040876
Chicago/Turabian StyleLuna-Pérez, Yonadys, Lady Giselle Ríos-López, Elver Luis Otero-Tejada, Juan Camilo Mejía-Giraldo, and Miguel Ángel Puertas-Mejía. 2023. "Sargassum filipendula, a Source of Bioactive Compounds with Antioxidant and Matrix Metalloproteinases Inhibition Activities In Vitro with Potential Dermocosmetic Application" Antioxidants 12, no. 4: 876. https://doi.org/10.3390/antiox12040876