Dietary Phenolic Compounds: Their Health Benefits and Association with the Gut Microbiota
Abstract
1. Introduction
2. Flavan-3-Ols
2.1. Dietary Source and Metabolism of Flavan-3-Ols
2.1.1. Tea
2.1.2. Cocoa
2.2. Health Benefits of Flavan-3-Ols
2.2.1. Tea
2.2.2. Cocoa
3. Condensed Tannins
3.1. Dietary Source and Metabolism of Tannins
Astringent Persimmon
3.2. Health Benefits of Tannins
Astringent Persimmon
4. Flavonols
4.1. Dietary Sources and Metabolism of Flavonols
4.1.1. Onions
4.1.2. Buckwheat
4.2. Health Benefits of Flavonols
4.2.1. Onions
4.2.2. Buckwheat
5. Isoflavones
5.1. Dietary Source and Metabolism of Isoflavones
Soybeans
5.2. Health Benefits of Isoflavones
Soybeans
6. Phenylpropanoids
6.1. Dietary Source and Metabolism of Phenylpropanoids
6.1.1. Coffee
6.1.2. Sesame
6.2. Health Benefits of Phenylpropanoids
6.2.1. Coffee
6.2.2. Sesame
7. Stilbenoids
7.1. Dietary Source and Metabolism of Stilbenoids
Grapes and Wine
7.2. Health Benefits of Stilbenoids
Grapes and Wine
8. Curcuminoids
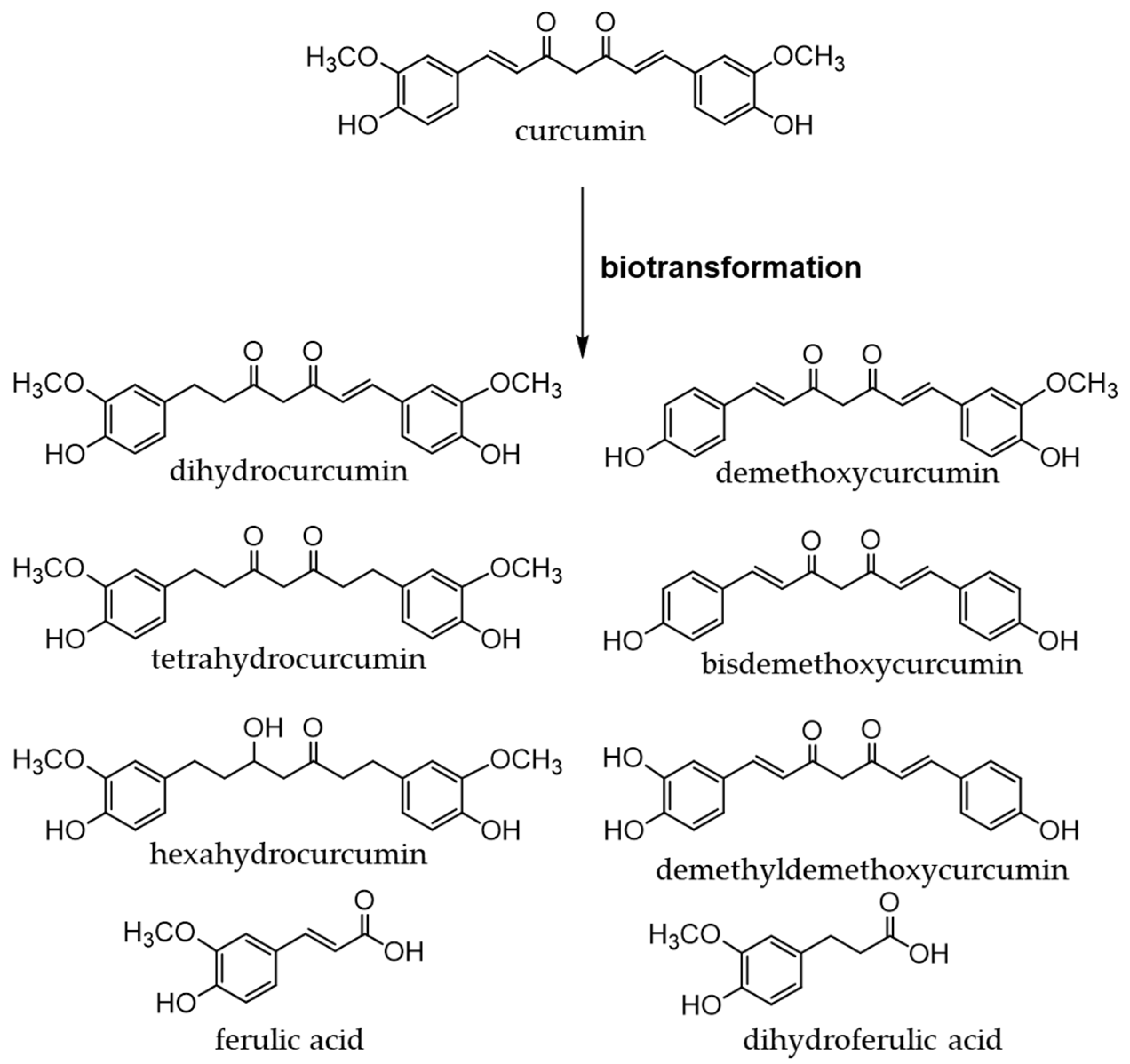
8.1. Dietary Source and Metabolism of Curcuminoids
Turmeric
8.2. Health Benefits of Curcuminoids
Turmeric
9. Other Phenolic Compounds: Dietary Sources, Metabolism, and Health Benefits
9.1. Protocatechuic Acid
9.2. Ellagic Acid
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otrisal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical Analysis of the Relationship Between Antioxidant Activity and the Structure of Flavonoid Compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Kabir, M.T.; Tewari, D.; Al Mamun, A.; Barreto, G.E.; Bungau, S.G.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Ashraf, G.M. Emerging Therapeutic Promise of Ketogenic Diet to Attenuate Neuropathological Alterations in Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 4961–4977. [Google Scholar] [CrossRef]
- Kumar, S.; Behl, T.; Sachdeva, M.; Sehgal, A.; Kumari, S.; Kumar, A.; Kaur, G.; Yadav, H.N.; Bungau, S. Implicating the Effect of Ketogenic Diet as a Preventive Measure to Obesity and Diabetes Mellitus. Life Sci. 2021, 264, 118661. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Upadhyay, T.; Singh, S.; Chigurupati, S.; Alsubayiel, A.M.; Mani, V.; Vargas-De-la-cruz, C.; Uivarosan, D.; Bustea, C.; Sava, C.; et al. Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis. Molecules 2021, 26, 6570. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Mehta, K.; Sehgal, A.; Singh, S.; Sharma, N.; Ahmadi, A.; Arora, S.; Bungau, S. Exploring the Role of Polyphenols in Rheumatoid Arthritis. Crit. Rev. Food Sci. Nutr. 2022, 62, 5372–5393. [Google Scholar] [CrossRef]
- Kabra, A.; Garg, R.; Brimson, J.; Živković, J.; Almawash, S.; Ayaz, M.; Nawaz, A.; Hassan, S.S.U.; Bungau, S. Mechanistic Insights into the Role of Plant Polyphenols and Their Nano-Formulations in the Management of Depression. Front. Pharm. 2022, 13, 1046599. [Google Scholar] [CrossRef]
- Behl, T.; Rana, T.; Alotaibi, G.H.; Shamsuzzaman, M.; Naqvi, M.; Sehgal, A.; Singh, S.; Sharma, N.; Almoshari, Y.; Abdellatif, A.A.H.; et al. Polyphenols Inhibiting MAPK Signalling Pathway Mediated Oxidative Stress and Inflammation in Depression. Biomed. Pharmacother. 2022, 146, 112545. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Antonietta Maselli, M.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Fisette, A.; Sergi, D.; Breton-Morin, A.; Descôteaux, S.; Martinoli, M.-G. New Insights on the Role of Bioactive Food Derivatives in Neurodegeneration and Neuroprotection. Curr. Pharm. Des. 2022, 28, 3068–3081. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Amruthanjali, T.; Singothu, S.; Singh, S.B.; Bhandari, V. Uncoupling Proteins as a Therapeutic Target for the Development of New Era Drugs against Neurodegenerative Disorder. Biomed. Pharmacother. 2022, 147, 112656. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, J.; Yang, Y.; Li, J.; Tu, H. Mitochondrial Sirtuins in Parkinson’s Disease. Neurochem. Res. 2022, 47, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Brito, R.; González-Montero, J.; Benedetti, V. Antioxidants in Human Disease: Potential Therapeutic Opportunities Clinical Pharmacology and Translational Medicine. Clin. Pharm. Transl. Med. 2017, 1, 44–53. [Google Scholar]
- Thanan, R.; Oikawa, S.; Hiraku, Y.; Ohnishi, S.; Ma, N.; Pinlaor, S.; Yongvanit, P.; Kawanishi, S.; Murata, M. Oxidative Stress and Its Significant Roles in Neurodegenerative Diseases and Cancer. Int. J. Mol. Sci. 2014, 16, 193–217. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kitabatake, M.; Ouji-Sageshima, N.; Yasui, S.; Mochida, N.; Nakano, R.; Kasahara, K.; Tomoda, K.; Yano, H.; Kayano, S.-i.; et al. Persimmon-Derived Tannin Has Bacteriostatic and Anti-Inflammatory Activity in a Murine Model of Mycobacterium Avium Complex (MAC) Disease. PLoS ONE 2017, 12, e0183489. [Google Scholar] [CrossRef]
- Kitabatake, M.; Matsumura, Y.; Ouji-Sageshima, N.; Nishioka, T.; Hara, A.; Kayano, S.-i.; Ito, T. Persimmon-Derived Tannin Ameliorates the Pathogenesis of Ulcerative Colitis in a Murine Model through Inhibition of the Inflammatory Response and Alteration of Microbiota. Sci. Rep. 2021, 11, 7286. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ito, T.; Yano, H.; Kita, E.; Mikasa, K.; Okada, M.; Furutani, A.; Murono, Y.; Shibata, M.; Nishii, Y.; et al. Antioxidant Potential in Non-Extractable Fractions of Dried Persimmon (Diospyros kaki Thunb.). Food Chem. 2016, 202, 99–103. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as Antioxidants: Determination of Radical-Scavenging Efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar]
- Nanjo, F.; Honda, M.; Okushio, K.; Matsumoto, N.; Ishigaki, F.; Ishigami, T.; Hara, Y. Effects of Dietary Tea Catechins on Alpha-Tocopherol Levels, Lipid Peroxidation, and Erythrocyte Deformability in Rats Fed on High Palm Oil and Perilla Oil Diets. Biol. Pharm. Bull. 1993, 16, 1156–1159. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am J Clin Nutr 2004, 79, 727–774. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Manach, C.; Faulks, R.M.; Kroon, P. A In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Blackwell Pub: Oxford, UK, 2006; ISBN 9781405125093. [Google Scholar]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.S.; Lee, J.Y.; Saitoh, S.I.; Miyake, K.; Kang, K.W.; Choi, Y.J.; Hwang, D.H. Suppression of MyD88- and TRIF-Dependent Signaling Pathways of Toll-like Receptor by (-)-Epigallocatechin-3-Gallate, a Polyphenol Component of Green Tea. Biochem. Pharm. 2006, 72, 850–859. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic Biochemical Mechanisms behind the Health Benefits of Polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef]
- Dias, T.R.; Tomás, G.; Teixeira, N.F.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. White Tea (Camellia sinensis (L.)): Antioxidant Properties And Beneficial Health Effects. Int. J. Food Sci. Nutr. Diet. 2013, 2, 19–26. [Google Scholar] [CrossRef]
- Hattori, M.; Kusumoto, I.T.; Namba, T.; Ishigami, T.; Hara, Y. Effect of Tea Polyphenols on Glucan Synthesis by Glucosyltransferase from Streptococcus Mutans. Chem. Pharm. Bull. 1990, 38, 717–720. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Hara, Y. Antioxidative Activity of Tea Leaf Catechins. Nippon Nogeikagaku Kaishi 1985, 59, 129–134. [Google Scholar] [CrossRef]
- Dias, T.R.; Alves, M.G.; Casal, S.; Oliveira, P.F.; Silva, B.M. Promising Potential of Dietary (Poly)Phenolic Compounds in the Prevention and Treatment of Diabetes Mellitus. Curr. Med. Chem. 2017, 24, 334–354. [Google Scholar] [CrossRef]
- Moderno, P.M.; Carvalho, M.; Silva, B.M. Recent Patents on Camellia Sinensis: Source of Health Promoting Compounds. Recent Pat. Food Nutr. Agric. 2009, 1, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Schiller, N.L.; Kahng, H.Y.; Oh, K.H. Cellular Responses and Proteomic Analysis of Escherichia Coli Exposed to Green Tea Polyphenols. Curr. Microbiol. 2007, 55, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of Tea Phenolics and Their Aromatic Fecal Bacterial Metabolites on Intestinal Microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Landau, J.M.; Lambert, J.D.; Yang, C.S. Green Tea. In Nutritional Oncology, 2nd, ed.; Academic Press: Cambridge, MA, USA, 2006; Chapter 35; pp. 597–606. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Moreno-Rojas, J.M.; Brindani, N.; del Rio, D.; Lean, M.E.J.; Hara, Y.; Crozier, A. Bioavailability of Black Tea Theaflavins: Absorption, Metabolism, and Colonic Catabolism. J. Agric. Food Chem. 2017, 65, 5365–5374. [Google Scholar] [CrossRef]
- Liu, Z.; de Bruijn, W.J.C.; Sanders, M.G.; Wang, S.; Bruins, M.E.; Vincken, J.P. Insights in the Recalcitrance of Theasinensin A to Human Gut Microbial Degradation. J. Agric. Food Chem. 2021, 69, 2477–2484. [Google Scholar] [CrossRef]
- Liu, Z.; de Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Microbial Metabolism of Theaflavin-3,3′-Digallate and Its Gut Microbiota Composition Modulatory Effects. J. Agric. Food Chem. 2021, 69, 232–245. [Google Scholar] [CrossRef]
- Mulder, T.P.J.; van Platerink, C.J.; Schuyl, P.J.W.; van Amelsvoort, J.M.M. Analysis of Theaflavins in Biological Fluids Using Liquid Chromatography-Electrospray Mass Spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2001, 760, 271–279. [Google Scholar] [CrossRef]
- Hashimoto, F.; Nonaka, G.; Nishioka, I. Tannins and Related Compounds. LXIX.: Isolation and Structure Elucidation of B, B’-Linked Bisflavanoids, Theasinensins D-G and Oolongtheanin from Oolong Tea. (2). Chem. Pharm. Bull. 1988, 36, 1676–1684. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef]
- Flores, M.E.J. Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors. Nutrients 2019, 11, 751. [Google Scholar] [CrossRef]
- Natsume, M.; Osakabe, N.; Yamagishi, M.; Takizawa, T.; Nakamura, T.; Miyatake, H.; Hatano, T.; Yoshida, T. Analyses of Polyphenols in Cacao Liquor, Cocoa, and Chocolate by Normal-Phase and Reversed-Phase HPLC. Biosci. Biotechnol. Biochem. 2000, 64, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Keen, C.L.; Hannum, S.M.; Gershwin, M.E. Cocoa and Chocolate: Composition, Bioavailability, and Health Implications. J. Med. Food 2000, 3, 77–105. [Google Scholar] [CrossRef]
- Gómez-Juaristi, M.; Sarria, B.; Martínez-López, S.; Clemente, L.B.; Mateos, R. Flavanol Bioavailability in Two Cocoa Products with Different Phenolic Content. A Comparative Study in Humans. Nutrients 2019, 11, 1441. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Mateus, L.Y.; Perez-Burillo, S.; Lerma-Aguilera, A.; Hinojosa-Nogueira, D.; Ruíz-Pérez, S.; Gosalbes, M.J.; Francino, M.P.; Rufián-Henares, J.Á.; Pastoriza De La Cueva, S. Effect of Roasting Conditions on Cocoa Bioactivity and Gut Microbiota Modulation. Food Funct. 2021, 12, 9680–9692. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Hodgson, J.M.; Proudfoot, J.M.; Mckinley, A.J.; Puddey, I.B.; Croft, K.D. Pure Dietary Flavonoids Quercetin and (−)-Epicatechin Augment Nitric Oxide Products and Reduce Endothelin-1 Acutely in Healthy Men. Am. J. Clin. Nutr. 2008, 88, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (−)-Epicatechin Mediates Beneficial Effects of Flavanol-Rich Cocoa on Vascular Function in Humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, J.I.; Borges, G.; Momma, T.Y.; Spencer, J.P.E.; Keen, C.L.; Crozier, A.; Schroeter, H. The Metabolome of [2-14C](−)-Epicatechin in Humans: Implications for the Assessment of Efficacy, Safety, and Mechanisms of Action of Polyphenolic Bioactives. Sci. Rep. 2016, 6, 29034. [Google Scholar] [CrossRef]
- Cremonini, E.; Wang, Z.; Bettaieb, A.; Adamo, A.M.; Daveri, E.; Mills, D.A.; Kalanetra, K.M.; Haj, F.G.; Karakas, S.; Oteiza, P.I. (−)-Epicatechin Protects the Intestinal Barrier from High Fat Diet-Induced Permeabilization: Implications for Steatosis and Insulin Resistance. Redox Biol. 2018, 14, 588–599. [Google Scholar] [CrossRef]
- Corral-Jara, K.F.; Nuthikattu, S.; Rutledge, J.; Villablanca, A.; Fong, R.; Heiss, C.; Ottaviani, J.I.; Milenkovic, D. Structurally Related (−)-Epicatechin Metabolites and Gut Microbiota Derived Metabolites Exert Genomic Modifications via VEGF Signaling Pathways in Brain Microvascular Endothelial Cells under Lipotoxic Conditions: Integrated Multi-Omic Study. J. Proteom. 2022, 263, 104603. [Google Scholar] [CrossRef]
- Li, B.Y.; Li, H.Y.; Zhou, D.D.; Huang, S.Y.; Luo, M.; Gan, R.Y.; Mao, Q.Q.; Saimaiti, A.; Shang, A.; Li, H. bin Effects of Different Green Tea Extracts on Chronic Alcohol Induced-Fatty Liver Disease by Ameliorating Oxidative Stress and Inflammation in Mice. Oxid. Med. Cell Longev. 2021, 2021, 5188205. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Zhang, N.; Zhou, J.; Mehmood, A.; Raka, R.N.; Zhou, F.; Zhao, L. The Beneficial Effects of Natural Extracts and Bioactive Compounds on the Gut-Liver Axis: A Promising Intervention for Alcoholic Liver Disease. Antioxidants 2022, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Mao, Q.; Zhou, D.; Luo, M.; Gan, R.; Li, H.; Huang, S.; Saimaiti, A.; Shang, A.; Li, H. Effects of Tea against Alcoholic Fatty Liver Disease by Modulating Gut Microbiota in Chronic Alcohol-Exposed Mice. Foods 2021, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Ushiroda, C.; Mizushima, K.; Inoue, R.; Yasukawa, Z.; Abe, A.; Takagi, T.; Gastroenterology, M. Epigallocatechin-3-Gallate (EGCG) Attenuates Non-Alcoholic Fatty Liver Disease via Modulating the Interaction between Gut Microbiota and Bile Acids. J. Clin. Biochem. Nutr 2020, 67, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.P.; Suk, K.T.; Kim, D.J. Significance of Gut Microbiota in Alcoholic and Non-Alcoholic Fatty Liver Diseases. World J. Gastroenterol. 2021, 27, 6161–6179. [Google Scholar] [CrossRef] [PubMed]
- He, L.H.; Yao, D.H.; Wang, L.Y.; Zhang, L.; Bai, X.L. Gut Microbiome-Mediated Alteration of Immunity, Inflammation, and Metabolism Involved in the Regulation of Non-Alcoholic Fatty Liver Disease. Front. Microbiol. 2021, 12, 761836. [Google Scholar] [CrossRef]
- Zhou, K. Strategies to Promote Abundance of Akkermansia Muciniphila, an Emerging Probiotics in the Gut, Evidence from Dietary Intervention Studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia Muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Dryden, G.W.; Lam, A.; Beatty, K.; Qazzaz, H.H.; McClain, C.J. A Pilot Study to Evaluate the Safety and Efficacy of an Oral Dose of (−)-Epigallocatechin-3-Gallate–Rich Polyphenon E in Patients with Mild to Moderate Ulcerative Colitis. Inflamm. Bowel. Dis. 2013, 19, 1904–1912. [Google Scholar] [CrossRef]
- Vasconcelos, P.C.D.P.; Seito, L.N.; di Stasi, L.C.; Akiko Hiruma-Lima, C.; Pellizzon, C.H. Epicatechin Used in the Treatment of Intestinal Inflammatory Disease: An Analysis by Experimental Models. Evid.-Based Complement. Altern. Med. 2012, 2012, 508902. [Google Scholar] [CrossRef]
- Brückner, M.; Westphal, S.; Domschke, W.; Kucharzik, T.; Lügering, A. Green Tea Polyphenol Epigallocatechin-3-Gallate Shows Therapeutic Antioxidative Effects in a Murine Model of Colitis. J. Crohns Colitis 2012, 6, 226–235. [Google Scholar] [CrossRef]
- Rodríguez-Ramiro, I.; Martín, M.Á.; Ramos, S.; Bravo, L.; Goya, L. Comparative Effects of Dietary Flavanols on Antioxidant Defenses and Their Response to Oxidant-Induced Stress on Caco2 Cells. Eur. J. Nutr. 2011, 50, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Cecarini, V.; Cuccioloni, M.; Zheng, Y.; Bonfili, L.; Gong, C.; Angeletti, M.; Mena, P.; del Rio, D.; Eleuteri, A.M. Flavan-3-Ol Microbial Metabolites Modulate Proteolysis in Neuronal Cells Reducing Amyloid-Beta (1-42) Levels. Mol. Nutr. Food Res. 2021, 65, 2100380. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yang, Y.; Zhu, S.; Lu, Y.; Zhu, L.; Wang, Y.; Wang, X. Inhibition of Aβ Aggregates in Alzheimer’s Disease by Epigallocatechin and Epicatechin-3-Gallate from Green Tea. Bioorg. Chem. 2020, 105, 104382. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, M.; Yao, W.; Du, K.; He, M.; Jin, X.; Jiao, L.; Ma, G.; Wei, B.; Wei, M. Epigallocatechin-3-Gallate Attenuates Microglial Inflammation and Neurotoxicity by Suppressing the Activation of Canonical and Noncanonical Inflammasome via TLR4/NF-ΚB Pathway. Mol. Nutr. Food Res. 2019, 63, 1801230. [Google Scholar] [CrossRef]
- Yamamoto, N.; Shibata, M.; Ishikuro, R.; Tanida, M.; Taniguchi, Y.; Ikeda-Matsuo, Y.; Sobue, K. Epigallocatechin Gallate Induces Extracellular Degradation of Amyloid β-Protein by Increasing Neprilysin Secretion from Astrocytes through Activation of ERK and PI3K Pathways. Neuroscience 2017, 362, 70–78. [Google Scholar] [CrossRef]
- Cheng-Chung Wei, J.; Huang, H.C.; Chen, W.J.; Huang, C.N.; Peng, C.H.; Lin, C.L. Epigallocatechin Gallate Attenuates Amyloid β-Induced Inflammation and Neurotoxicity in EOC 13.31 Microglia. Eur. J. Pharm. 2016, 770, 16–24. [Google Scholar] [CrossRef]
- Bao, J.; Liu, W.; Zhou, H.Y.; Gui, Y.R.; Yang, Y.H.; Wu, M.J.; Xiao, Y.F.; Shang, J.T.; Long, G.F.; Shu, X.J. Epigallocatechin-3-Gallate Alleviates Cognitive Deficits in APP/PS1 Mice. Curr. Med. Sci. 2020, 40, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Langley, M.; Kanthasamy, A.G.; Reddy, M.B. Epigallocatechin Gallate Has a Neurorescue Effect in a Mouse Model of Parkinson Disease. J. Nutr. 2017, 147, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.M. Epigallocatechin Gallate for Parkinson’s Disease. Clin. Exp. Pharm. Physiol. 2022, 49, 1029–1041. [Google Scholar] [CrossRef]
- Kim, S.R.; Seong, K.J.; Kim, W.J.; Jung, J.Y. Epigallocatechin Gallate Protects against Hypoxia-Induced Inflammation in Microglia via NF-ΚB Suppression and Nrf-2/HO-1 Activation. Int. J. Mol. Sci. 2022, 23, 4004. [Google Scholar] [CrossRef]
- Jang, S.; Sun, J.; Chen, P.; Lakshman, S.; Molokin, A.; Harnly, J.M.; Vinyard, B.T.; Urban, J.F.; Davis, C.D.; Solano-Aguilar, G. Flavanol-Enriched Cocoa Powder Alters the Intestinal Microbiota, Tissue and Fluid Metabolite Profiles, and Intestinal Gene Expression in Pigs. J. Nutr. 2016, 146, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Prebiotic Evaluation of Cocoa-Derived Flavanols in Healthy Humans by Using a Randomized, Controlled, Double-Blind, Crossover Intervention Study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Pérez-Cano, F.J.; Massot-Cladera, M.; Franch, À.; Castellote, C.; Castell, M. The Effects of Cocoa on the Immune System. Front. Pharm. 2013, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Pérez-Berezo, T.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Cocoa Modulatory Effect on Rat Faecal Microbiota and Colonic Crosstalk. Arch. Biochem. Biophys. 2012, 527, 105–112. [Google Scholar] [CrossRef]
- Álvarez-Cilleros, D.; Ramos, S.; López-Oliva, M.E.; Escrivá, F.; Álvarez, C.; Fernández-Millán, E.; Martín, M.Á. Cocoa Diet Modulates Gut Microbiota Composition and Improves Intestinal Health in Zucker Diabetic Rats. Food Res. Int. 2020, 132, 109058. [Google Scholar] [CrossRef]
- Rodríguez-Ramiro, I.; Ramos, S.; López-Oliva, E.; Agis-Torres, A.; Bravo, L.; Goya, L.; Martín, M.A. Cocoa Polyphenols Prevent Inflammation in the Colon of Azoxymethane-Treated Rats and in TNF-α-Stimulated Caco-2 Cells. Br. J. Nutr. 2013, 110, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Bresson, J.-L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on the Substantiation of a Health Claim Related to Cocoa Flavanols and Maintenance of Normal Endothelium-dependent Vasodilation Pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2809. [Google Scholar] [CrossRef]
- Sesso, H.D.; Manson, J.E.; Aragaki, A.K.; Rist, P.M.; Johnson, L.G.; Friedenberg, G.; Copeland, T.; Clar, A.; Mora, S.; Moorthy, M.V.; et al. Effect of Cocoa Flavanol Supplementation for the Prevention of Cardiovascular Disease Events: The COcoa Supplement and Multivitamin Outcomes Study (COSMOS) Randomized Clinical Trial. Am. J. Clin. Nutr. 2022, 115, 1490–1500. [Google Scholar] [CrossRef]
- Dubner, L.; Wang, J.; Ho, L.; Ward, L.; Pasinetti, G.M. Recommendations for Development of New Standardized Forms of Cocoa Breeds and Cocoa Extract Processing for the Prevention of Alzheimer’s Disease: Role of Cocoa in Promotion of Cognitive Resilience and Healthy Brain Aging. J. Alzheimer’s Dis. 2015, 48, 879–889. [Google Scholar] [CrossRef]
- Wang, J.; Varghese, M.; Ono, K.; Yamada, M.; Levine, S.; Tzavaras, N.; Gong, B.; Hurst, W.J.; Blitzer, R.D.; Pasinetti, G.M. Cocoa Extracts Reduce Oligomerization of Amyloid-β: Implications for Cognitive Improvement in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 41, 643–650. [Google Scholar] [CrossRef]
- Cimini, A.; Gentile, R.; D’Angelo, B.; Benedetti, E.; Cristiano, L.; Avantaggiati, M.L.; Giordano, A.; Ferri, C.; Desideri, G. Cocoa Powder Triggers Neuroprotective and Preventive Effects in a Human Alzheimer’s Disease Model by Modulating BDNF Signaling Pathway. J. Cell Biochem. 2013, 114, 2209–2220. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Zyzelewicz, D.; Budryn, G.; Luzak, B. Bioavailability and Metabolism of Selected Cocoa Bioactive Compounds: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1947–1985. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Ito, S. The Chemical Structure of Kaki-Tannin from Immature Fruit of the Persimmon (Diospyros kaki, L.). Agric. Biol. Chem. 1978, 42, 1637–1643. [Google Scholar]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current Knowledge of Food Sources, Intake, Bioavailability and Biological Effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kadowaki, A.; Ozaki, N.; Takenaka, M.; Ono, H.; Yokoyama, S.I.; Gato, N. Bile Acid-Binding Ability of Kaki-Tannin from Young Fruits of Persimmon (Diospyros kaki) In Vitro and In Vivo. Phytother. Res. 2011, 25, 624–628. [Google Scholar] [CrossRef]
- Nishida, S.; Katsumi, N.; Matsumoto, K. Prevention of the Rise in Plasma Cholesterol and Glucose Levels by Kaki-tannin and Characterization of Its Bile Acid Binding Capacity. J. Sci. Food Agric. 2021, 101, 2117–2124. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Du, J.; Deng, X.; Li, C. Persimmon Tannin Decreased the Glycemic Response through Decreasing the Digestibility of Starch and Inhibiting α-Amylase, α-Glucosidase, and Intestinal Glucose Uptake. J. Agric. Food Chem. 2018, 66, 1629–1637. [Google Scholar] [CrossRef]
- Zhu, W.; Lin, K.; Li, K.; Deng, X.; Li, C. Reshaped Fecal Gut Microbiota Composition by the Intake of High Molecular Weight Persimmon Tannin in Normal and High-Cholesterol Diet-Fed Rats. Food Funct. 2018, 9, 541–551. [Google Scholar] [CrossRef]
- Gorinstein, S.; Bartnikowska, E.; Kulasek, G.; Zemser, M.; Trakhtenberg, S. Dietary Persimmon Improves Lipid Metabolism in Rats Fed Diets Containing Cholesterol. J. Nutr. 1998, 128, 2023–2027. [Google Scholar] [CrossRef]
- Gorinstein, S.; Kulasek, G.W.; Bartnikowska, E.; Leontowicz, M.; Zemser, M.; Morawiec, M.; Trakhtenberg, S. The Effects of Diets, Supplemented with Either Whole Persimmon or Phenol-Free Persimmon, on Rats Fed Cholesterol. Food Chem. 2000, 70, 303–308. [Google Scholar] [CrossRef]
- Suzuki, T.; Moriguchi, Y.; Ozaki, Y.; Kometani, T.; Fukuda, M. Effects of Kaki-Tannin on Reducing Serum LDL Cholesterol Levels in Volunteers with Borderline and Mild Hyper-LDL Cholestrolemia—A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial. Jpn Pharm. 2022, 50, 237–246. [Google Scholar]
- Shabbir, U.; Rubab, M.; Daliri, E.B.M.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid Metabolism: The Interaction of Metabolites and Gut Microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A Source of Unique Dietary Flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Patil, B.S.; Yoo, K.S. Antioxidants of 15 Onions with White, Yellow, and Red Colors and Their Relationship with Pungency, Anthocyanin, and Quercetin. LWT Food Sci. Technol. 2015, 63, 108–114. [Google Scholar] [CrossRef]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Cools, K.; Terry, L.A.; Esteban, R.M. Characterization of Industrial Onion Wastes (Allium Cepa, L.): Dietary Fibre and Bioactive Compounds. Plant Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar] [CrossRef]
- Sharma, K.; Asnin, L.; Ko, E.Y.; Lee, E.T.; Park, S.W. Phytochemical Composition of Onion during Long-Term Storage. Acta Agric. Scand B Soil Plant Sci. 2015, 65, 150–160. [Google Scholar] [CrossRef]
- Cattivelli, A.; Conte, A.; Martini, S.; Tagliazucchi, D. Influence of Cooking Methods on Onion Phenolic Compounds Bioaccessibility. Foods 2021, 10, 1023. [Google Scholar] [CrossRef]
- Sinkovič, L.; Kokalj Sinkovič, D.; Meglič, V. Milling Fractions Composition of Common (Fagopyrum Esculentum Moench) and Tartary (Fagopyrum tataricum (L.) Gaertn.) Buckwheat. Food Chem. 2021, 365, 130459. [Google Scholar] [CrossRef]
- Sytar, O.; Biel, W.; Smetanska, I.; Brestic, M. Bioactive Compounds and Their Biofunctional Properties of Different Buckwheat Germplasms for Food Processing. In Buckwheat Germplasm in the World; Academic Press: London, UK, 2018. [Google Scholar] [CrossRef]
- Yasuda, T.; Masaki, K.; Kashiwagi, T. An Enzyme Degrading Rutin in Tartary Buckwheat Seeds. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 994–1000. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Zieliński, H. Buckwheat as a Functional Food and Its Effects on Health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.V; Regelson, W. Review of the Biology of Quercetin and Related Bioflavonoids. Food Chem. Toxic 1995, 33, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Grzelak-Błaszczyk, K.; Milala, J.; Kosmala, M.; Kołodziejczyk, K.; Sójka, M.; Czarnecki, A.; Klewicki, R.; Juśkiewicz, J.; Fotschki, B.; Jurgoński, A. Onion Quercetin Monoglycosides Alter Microbial Activity and Increase Antioxidant Capacity. J. Nutr. Biochem. 2018, 56, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Stehle, P.; et al. No Effects of Quercetin from Onion Skin Extract on Serum Leptin and Adiponectin Concentrations in Overweight-to-Obese Patients with (Pre-)Hypertension: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Eur. J. Nutr. 2017, 56, 2265–2275. [Google Scholar] [CrossRef]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Schalkwijk, C.; Kromhout, D.; Hollman, P.C. Supplementation of the Pure Flavonoids Epicatechin and Quercetin Affects Some Biomarkers of Endothelial Dysfunction and Inflammation in (Pre)Hypertensive Adults: A Randomized Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 1459–1463. [Google Scholar] [CrossRef]
- Lee, K.H.; Park, E.; Lee, H.J.; Kim, M.O.; Cha, Y.J.; Kim, J.M.; Lee, H.; Shin, M.J. Effects of Daily Quercetin-Rich Supplementation on Cardiometabolic Risks in Male Smokers. Nutr. Res. Pract. 2011, 5, 28–33. [Google Scholar] [CrossRef]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does Quercetin Improve Cardiovascular Risk Factors and Inflammatory Biomarkers in Women with Type 2 Diabetes: A Double-Blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar]
- Rezvan, N.; Moini, A.; Janani, L.; Mohammad, K.; Saedisomeolia, A.; Nourbakhsh, M.; Gorgani-Firuzjaee, S.; Mazaherioun, M.; Hosseinzadeh-Attar, M.J. Effects of Quercetin on Adiponectin-Mediated Insulin Sensitivity in Polycystic Ovary Syndrome: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Horm. Metab. Res. 2017, 49, 115–121. [Google Scholar] [CrossRef]
- Javadi, F.; Eghtesadi, S.; Ahmadzadeh, A.; Aryaeian, N.; Zabihiyeganeh, M.; Foroushani, A.R.; Jazayeri, S. The Effect of Quercetin on Plasma Oxidative Status, C-Reactive Protein and Blood Pressure in Women with Rheumatoid Arthritis. Int. J. Prev. Med. 2014, 5, 293–301. [Google Scholar]
- Mullen, W.; Rouanet, J.-M.; Auger, C.; Teissèdre, P.-L.; Caldwell, S.T.; Hartley, R.C.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. Bioavailability of [2-14C]Quercetin-4′-Glucoside in Rats. J. Agric. Food Chem. 2008, 56, 12127–12137. [Google Scholar] [CrossRef]
- Tang, Y.; Nakashima, S.; Saiki, S.; Myoi, Y.; Abe, N.; Kuwazuru, S.; Zhu, B.; Ashida, H.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic Acid Is a Predominant Biologically-Active Catabolite of Quercetin Glycosides. Food Res. Int. 2016, 89, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; et al. Antiglycative and Neuroprotective Activity of Colon-Derived Polyphenol Catabolites. Mol. Nutr. Food Res. 2011, 55, S35–S43. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, I.; Frøkiaer, J.; Nørregaard, R. Quercetin Attenuates Cyclooxygenase-2 Expression in Response to Acute Ureteral Obstruction. Am. J. Physiol. Ren. Physiol. 2015, 308, F1297–F1305. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, G.; Yang, Q.; Ye, J.; Cai, X.; Tsering, P.; Cheng, X.; Hu, C.; Zhang, S.; Cao, P. Gut Microbiota Drives the Attenuation of Dextran Sulphate Sodium-Induced Colitis by Huangqin Decoction. Oncotarget 2017, 8, 48863–48874. [Google Scholar] [CrossRef] [PubMed]
- Forney, L.A.; Lenard, N.R.; Stewart, L.K.; Henagan, T.M. Dietary Quercetin Attenuates Adipose Tissue Expansion and Inflammation and Alters Adipocyte Morphology in a Tissue-Specific Manner. Int. J. Mol. Sci. 2018, 19, 895. [Google Scholar] [CrossRef]
- Overman, A.; Chuang, C.C.; McIntosh, M. Quercetin Attenuates Inflammation in Human Macrophages and Adipocytes Exposed to Macrophage-Conditioned Media. Int. J. Obes. 2011, 35, 1165–1172. [Google Scholar] [CrossRef]
- Ju, S.; Ge, Y.; Li, P.; Tian, X.; Wang, H.; Zheng, X.; Ju, S. Dietary Quercetin Ameliorates Experimental Colitis in Mouse by Remodeling the Function of Colonic Macrophages via a Heme Oxygenase-1-Dependent Pathway. Cell Cycle 2018, 17, 53–63. [Google Scholar] [CrossRef]
- Shi, T.; Bian, X.; Yao, Z.; Wang, Y.; Gao, W.; Guo, C. Quercetin Improves Gut Dysbiosis in Antibiotic-Treated Mice. Food Funct. 2020, 11, 8003–8013. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.; Song, Y. Dietary Quercetin Increases Colonic Microbial Diversity and Attenuates Colitis Severity in Citrobacter Rodentium-Infected Mice. Front. Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef]
- Sato, S.; Mukai, Y. Modulation of Chronic Inflammation by Quercetin: The Beneficial Effects on Obesity. J. Inflamm. Res. 2020, 13, 421–431. [Google Scholar] [CrossRef]
- Jiménez-Aliaga, K.; Bermejo-Bescós, P.; Benedí, J.; Martín-Aragón, S. Quercetin and Rutin Exhibit Antiamyloidogenic and Fibril-Disaggregating Effects in Vitro and Potent Antioxidant Activity in APPswe Cells. Life Sci. 2011, 89, 939–945. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Mu, X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by A β 25-35 and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. Biomed. Res. Int. 2020, 2020, 8210578. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Luo, T.; Li, S.; Zhou, Y.; Shen, X.Y.; He, F.; Xu, J.; Wang, H.Q. Quercetin Protects against Okadaic Acid-Induced Injury via MAPK and PI3K/Akt/GSK3β Signaling Pathways in HT22 Hippocampal Neurons. PLoS ONE 2016, 11, e0152371. [Google Scholar] [CrossRef] [PubMed]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and Flavones as BACE-1 Inhibitors: Structure-Activity Relationship in Cell-Free, Cell-Based and in Silico Studies Reveal Novel Pharmacophore Features. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 819–825. [Google Scholar] [CrossRef]
- Nakagawa, T.; Ohta, K. Quercetin Regulates the Integrated Stress Response to Improve Memory. Int. J. Mol. Sci. 2019, 20, 2761. [Google Scholar] [CrossRef]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Molecular Mechanisms Underlying Protective Effects of Quercetin against Mitochondrial Dysfunction and Progressive Dopaminergic Neurodegeneration in Cell Culture and MitoPark Transgenic Mouse Models of Parkinson’s Disease. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef]
- El-Horany, H.E.; El-Latif, R.N.A.; ElBatsh, M.M.; Emam, M.N. Ameliorative Effect of Quercetin on Neurochemical and Behavioral Deficits in Rotenone Rat Model of Parkinson’s Disease: Modulating Autophagy (Quercetin on Experimental Parkinson’s Disease). J. Biochem. Mol. Toxicol. 2016, 30, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Raj, K.; Singh, S. Neuroprotective Effect of Quercetin in Combination with Piperine Against Rotenone- and Iron Supplement–Induced Parkinson’s Disease in Experimental Rats. Neurotox Res. 2020, 37, 198–209. [Google Scholar] [CrossRef]
- Singh, S.; Jamwal, S.; Kumar, P. Neuroprotective Potential of Quercetin in Combination with Piperine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Neurotoxicity. Neural Regen Res. 2017, 12, 1137–1144. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, Q.; Zhang, Y.; Yao, Z.; Song, P.; Wei, L.; Zhao, G.; Yan, Z. Effect of Tartary Buckwheat, Rutin, and Quercetin on Lipid Metabolism in Rats during High Dietary Fat Intake. Food Sci. Nutr. 2020, 8, 199–213. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Li, Y.; Lu, K.; Yin, R.; Ming, J. Phenolics Extracted from Tartary (Fagopyrum tartaricum, L. Gaerth) Buckwheat Bran Exhibit Antioxidant Activity, and an Antiproliferative Effect on Human Breast Cancer MDA-MB-231 Cells through the P38/MAP Kinase Pathway. Food Funct. 2017, 8, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Perk, A.A.; Shatynska-mytsyk, I.; Gerçek, Y.C.; Boztas, K.; Yazgan, M.; Fayyaz, S.; Farooqi, A.A. Rutin Mediated Targeting of Signaling Machinery in Cancer Cells. Cancer Cell Int. 2014, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Rajput, V.S.; Nagpal, P.; Kukrety, H.; Grover, S.; Grover, A. Dual Inhibition of SARS-CoV-2 Spike and Main Protease through a Repurposed Drug, Rutin. J. Biomol. Struct. Dyn. 2022, 40, 4987–4999. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Tabrez, S.; Ali, R.; Alqahtani, A.S.; Ahmed, M.Z.; Rub, A. Molecular Docking Analysis of Rutin Reveals Possible Inhibition of SARS-CoV-2 Vital Proteins. J. Tradit. Complement. Med. 2021, 11, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Kreft, I.; Germ, M.; Golob, A.; Vombergar, B.; Bonafaccia, F.; Luthar, Z. Impact of Rutin and Other Phenolic Substances on the Digestibility of Buckwheat Grain Metabolites. Int. J. Mol. Sci. 2022, 23, 3923. [Google Scholar] [CrossRef] [PubMed]
- Cirkovic Velickovic, T.D.; Stanic-Vucinic, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Wang, T.; Li, Z.; Gao, Y.; Cui, S.W.; Qiu, J. Comparison of Quercetin and Rutin Inhibitory Influence on Tartary Buckwheat Starch Digestion in Vitro and Their Differences in Binding Sites with the Digestive Enzyme. Food Chem. 2022, 367, 130762. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kishida, M. Digestibility of Proteins in Buckwheat Seed. Fagopyrum 1993, 13, 21–24. [Google Scholar]
- Zhang, C.; Zhang, R.; Li, Y.M.; Liang, N.; Zhao, Y.; Zhu, H.; He, Z.; Liu, J.; Hao, W.; Jiao, R.; et al. Cholesterol-Lowering Activity of Tartary Buckwheat Protein. J. Agric. Food Chem. 2017, 65, 1900–1906. [Google Scholar] [CrossRef]
- Bao, T.; Wang, Y.; Li, Y.T.; Gowd, V.; Niu, X.H.; Yang, H.Y.; Chen, L.S.; Chen, W.; Sun, C.D. Antioxidant and Antidiabetic Properties of Tartary Buckwheat Rice Flavonoids after in Vitro Digestion. J. Zhejiang Univ. Sci. B 2016, 17, 941–951. [Google Scholar] [CrossRef]
- Llanaj, E.; Ahanchi, N.S.; Dizdari, H.; Taneri, P.E.; Niehot, C.D.; Wehrli, F.; Khatami, F.; Raeisi-Dehkordi, H.; Kastrati, L.; Bano, A.; et al. Buckwheat and Cardiometabolic Health: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 1940. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Rizwan, B.; Khan, M.; Farooq, S. Health Benefits of Buckwheat (Fagopyrum Esculentum), Potential Remedy for Diseases, Rare to Cancer: A Mini Review. Infect. Disord. Drug. Targets 2021, 21, e170721189478. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Shinkaruk, S.; Carreau, C.; Flouriot, G.; Bennetau-Pelissero, C.; Potier, M. Comparative Effects of R- and S-Equol and Implication of Transactivation Functions (AF-1 and AF-2) in Estrogen Receptor-Induced Transcriptional Activity. Nutrients 2010, 2, 340–354. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of Pure Isoflavones in Healthy Humans and Analysis of Commercial Soy Isoflavone Supplements. J. Nutr. 2001, 131, 1362S–1375S. [Google Scholar] [CrossRef]
- Aguiar, C.L.; Baptista, A.S.; Alencar, S.M.; Haddad, R.; Eberlin, M.N. Analysis of Isoflavonoids from Leguminous Plant Extracts by RPHPLC/DAD and Electrospray Ionization Mass Spectrometry. Int. J. Food Sci. Nutr. 2007, 58, 116–124. [Google Scholar] [CrossRef]
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.C.; Morgan, M.R.A.; Williamson, G. Deglycosylation of Flavonoid and Isoflavonoid Glycosides by Human Small Intestine and Liver β-Glucosidase Activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.A.; Mclauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.A.; Williamson, G. Dietary Flavonoid and Isoflavone Glycosides Are Hydrolysed by the Lactase Site of Lactase Phlorizin Hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from the Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Clerici, C. Equol: History, Chemistry, and Formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef] [PubMed]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic Activity, Biological Effect and Bioavailability. Eur. J. Drug Metab. Pharm. 2013, 38, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Setchell, D.R.K.; Faughnan, M.S.; Avades, T.; Zimmer-Nechemias, L.; Brown, N.M.; Wolfe, B.E.; Brashear, W.T.; Desai, P.; Oldfield, M.F.; Botting, N.P.; et al. Comparing the Pharmacokinetics of Daidzein and Genistein with the Use of 13 C-Labeled Tracers in Premenopausal Women. Am. J. Clin. Nutr. 2003, 77, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-Equol, a Potent Ligand for Estrogen Receptor β, Is the Exclusive Enantiomeric Form of the Soy Isoflavone Metabolite Produced by Human Intestinal Bacterial Flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef]
- Jackson, R.L.; Greiwe, J.S.; Schwen, R.J. Emerging Evidence of the Health Benefits of S-Equol, an Estrogen Receptor β Agonist. Nutr. Rev. 2011, 69, 432–448. [Google Scholar] [CrossRef]
- Wei, X.J.; Wu, J.; Ni, Y.D.; Lu, L.Z.; Zhao, R.Q. Antioxidant Effect of a Phytoestrogen Equol on Cultured Muscle Cells of Embryonic Broilers. Vitr. Cell Dev. Biol. Anim. 2011, 47, 735–741. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.H. The Antioxidant Activity of Daidzein Metabolites, O-Desmethylangolensin and Equol, in HepG2 Cells. Mol. Med. Rep. 2014, 9, 328–332. [Google Scholar] [CrossRef]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef]
- Harland, J.I.; Haffner, T.A. Systematic Review, Meta-Analysis and Regression of Randomised Controlled Trials Reporting an Association between an Intake of circa 25 g Soya Protein per Day and Blood Cholesterol. Atherosclerosis 2008, 200, 13–27. [Google Scholar] [CrossRef]
- Wei, P.; Liu, M.; Chen, Y.; Chen, D.-C.; De-Cai, C. Systematic Review of Soy Isoflavone Supplements on Osteoporosis in Women. Asian Pac. J. Trop Med. 2012, 5, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Wei-Jie, Y. Effects of Soy Protein Containing Isoflavones in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Clin. Nutr. 2016, 35, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Xu, B. An Insight into the Health Benefits of Fermented Soy Products. Food Chem. 2019, 271, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and Their Human Metabolites Show Distinct Agonistic and Antagonistic Properties on Estrogen Receptor α (ERα) and ERβ in Human Cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Kabir, M.T. Emerging Signal Regulating Potential of Genistein against Alzheimer’s Disease: A Promising Molecule of Interest. Front. Cell Dev. Biol. 2019, 7, 197. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Wang, D.; Zhang, L.; Lv, J.; Jiang, N.; Fan, B.; Liu, X.; Wang, F. Neuroprotective Effects of Soy Isoflavones on Scopolamine-Induced Amnesia in Mice. Nutrients 2018, 10, 853. [Google Scholar] [CrossRef]
- Ye, S.; Wang, T.T.; Cai, B.; Wang, Y.; Li, J.; Zhan, J.X.; Shen, G.M. Genistein Protects Hippocampal Neurons against Injury by Regulating Calcium/Calmodulin Dependent Protein Kinase IV Protein Levels in Alzheimer’s Disease Model Rats. Neural Regen. Res. 2017, 12, 1479–1484. [Google Scholar] [CrossRef]
- Arbabi, E.; Hamidi, G.; Talaei, S.A.; Salami, M. Estrogen Agonist Genistein Differentially Influences the Cognitive and Motor Disorders in an Ovariectomized Animal Model of Parkinsonism. Iran J. Basic. Med. Sci. 2016, 19, 1285–1290. [Google Scholar] [CrossRef]
- Razeghi Jahromi, S.; Rafi Arrefhosseini, S.; Ghaemi, A.; Alizadeh, A.; Sabetghadam, F.; Togha, M.; Jahromi, R.S. Effect of Oral Genistein Administration in Early and Late Phases of Allergic Encephalomyelitis. Iran J. Basic Med. Sci. 2014, 17, 509–515. [Google Scholar]
- Franca Adriana, S.; Oliveira Leandro, S. Coffee and Its By-Products as Sources of Bioactive Compounds; Massey, J.L., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2016; ISBN 978-1-63484-714-8. [Google Scholar]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic Acids and the Acyl-Quinic Acids: Discovery, Biosynthesis, Bioavailability and Bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef]
- Perrone, D.; Farah, A.; Donangelo, C.M.; de Paulis, T.; Martin, P.R. Comprehensive Analysis of Major and Minor Chlorogenic Acids and Lactones in Economically Relevant Brazilian Coffee Cultivars. Food Chem. 2008, 106, 859–867. [Google Scholar] [CrossRef]
- Clifford, M.N. Chemical and Physical Aspects of Green Coffee and Coffee Products. In Coffee: Botany, Biochemistry and Production of Beans and Beverage; Croom Helm: London, UK, 1985; Chapter 13; pp. 305–374. ISBN 978-1-4615-6659-5. [Google Scholar]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of Chlorogenic Acids Following Acute Ingestion of Coffee by Humans with an Ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic Acid and Caffeic Acid Are Absorbed in Humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef]
- Langyan, S.; Yadava, P.; Sharma, S.; Gupta, N.C.; Bansal, R.; Yadav, R.; Kalia, S.; Kumar, A. Food and Nutraceutical Functions of Sesame Oil: An Underutilized Crop for Nutritional and Health Benefits. Food Chem. 2022, 389, 132990. [Google Scholar] [CrossRef]
- Pathak, N.; Rai, A.K.; Kumari, R.; Bhat, K. v. Value Addition in Sesame: A Perspective on Bioactive Components for Enhancing Utility and Profitability. Pharm. Rev. 2014, 8, 147–155. [Google Scholar] [CrossRef]
- Andargie, M.; Vinas, M.; Rathgeb, A.; Möller, E.; Karlovsky, P. Lignans of Sesame (Sesamum indicum, L.): A Comprehensive Review. Molecules 2021, 26, 883. [Google Scholar] [CrossRef]
- Wan, Y.; Li, H.; Fu, G.; Chen, X.; Chen, F.; Xie, M. The Relationship of Antioxidant Components and Antioxidant Activity of Sesame Seed Oil. J. Sci. Food Agric. 2015, 95, 2571–2578. [Google Scholar] [CrossRef]
- Nakai, M.; Harada, M.; Nakahara, K.; Akimoto, K.; Shibata, H.; Miki, W.; Kiso, Y. Novel Antioxidative Metabolites in Rat Liver with Ingested Sesamin. J. Agric. Food Chem. 2003, 51, 1666–1670. [Google Scholar] [CrossRef]
- Kang, M.-H.; Naito, M.; Tsujihara, N.; Osawa, T. Nutrient Metabolism Sesamolin Inhibits Lipid Peroxidation in Rat Liver and Kidney 1. J. Nutr. 1998, 128, 1018–1022. [Google Scholar] [CrossRef]
- Cha, J.W.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Zheng, J.; Kim, S.M.; Hyun, C.L.; Ahn, Y.S.; Hyun, J.W. The Polyphenol Chlorogenic Acid Attenuates UVB-Mediated Oxidative Stress in Human HaCaT Keratinocytes. Biomol. Ther. 2014, 22, 136–142. [Google Scholar] [CrossRef]
- Zang, L.-Y.; Cosma, G.; Gardner, H.; Castranova, V.; Vallyathan, V. Effect of Chlorogenic Acid on Hydroxyl Radical. Mol. Cell. Biochem. 2003, 247, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K.; Ueda, A.; Sawa, Y.; Shibata, H. Antioxidant Activity of Polyphenolics in Diets Rate Constants of Reactions of Chlorogenic Acid and Caffeic Acid with Reactive Species of Oxygen and Nitrogen. Biochim. Biophys. Acta 1997, 1335, 335–342. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zheng, S.; Sheng, Y.; Miao, T.; Xu, J.; Xu, W.; Huang, K.; Zhao, C. Chlorogenic Acid Ameliorates Obesity by Preventing Energy Balance Shift in High-Fat Diet Induced Obese Mice. J. Sci. Food Agric. 2021, 101, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic Acid-Induced Gut Microbiota Improves Metabolic Endotoxemia. Front. Endocrinol. 2021, 12, 762691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lam, K.L.; Hu, J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. Chlorogenic Acid Alleviates Obesity and Modulates Gut Microbiota in High-Fat-Fed Mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef]
- Huxley, R.; Man Ying Lee, C.; Barzi, F.; Timmermeister, L.; Czernichow, S.; Perkovic, V.; Grobbee, D.E.; Batty, D.; Woodward, M. Coffee, Decaffeinated Coffee, and Tea Consumption in Relation to Incident Type 2 Diabetes Mellitus. Arch. Intern. Med. 2009, 169, 2053–2063. [Google Scholar] [CrossRef]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. In Vitro Colonic Metabolism of Coffee and Chlorogenic Acid Results in Selective Changes in Human Faecal Microbiota Growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef]
- Sales, A.L.; de Paula, J.; Mellinger Silva, C.; Cruz, A.; Lemos Miguel, M.A.; Farah, A. Effects of Regular and Decaffeinated Roasted Coffee (Coffea arabica and Coffea canephora) Extracts and Bioactive Compounds on in Vitro Probiotic Bacterial Growth. Food Funct. 2020, 11, 1410–1424. [Google Scholar] [CrossRef]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaź, P. Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int. J. Mol. Sci. 2020, 22, 107. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Dilnashin, H.; Singh, R.; Singh, S.P. Neuroprotective Effect of Chlorogenic Acid on Mitochondrial Dysfunction-Mediated Apoptotic Death of Da Neurons in a Parkinsonian Mouse Model. Oxid. Med. Cell Longev. 2020, 2020, 6571484. [Google Scholar] [CrossRef]
- Miyazaki, I.; Isooka, N.; Wada, K.; Kikuoka, R.; Kitamura, Y.; Asanuma, M. Effects of Enteric Environmental Modification by Coffee Components on Neurodegeneration in Rotenone-Treated Mice. Cells 2019, 8, 221. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Kumar, G.; Gedda, M.R.; Tiwari, N.; Patnaik, R.; Singh, R.K.; Singh, S.P. Effect of Chlorogenic Acid Supplementation in MPTP-Intoxicated Mouse. Front. Pharm. 2018, 9, 757. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Meng, S.; Ma, T.; Wan, L.; Xu, S. Chlorogenic Acid Alleviates Aβ25-35-Induced Autophagy and Cognitive Impairment via the MTOR/TFEB Signaling Pathway. Drug. Des. Devel. 2020, 14, 1705–1716. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Moazzami, A.; Washi, S. Sesame Seed Lignans: Potent Physiological Modulators and Possible Ingredients in Functional Foods & Nutraceuticals. Recent Pat. Food Nutr. Agric. 2011, 3, 17–29. [Google Scholar] [CrossRef]
- Dalibalta, S.; Majdalawieh, A.F.; Manjikian, H. Health Benefits of Sesamin on Cardiovascular Disease and Its Associated Risk Factors. Saudi Pharm. J. 2020, 28, 1276–1289. [Google Scholar] [CrossRef]
- Wu, W.-H.; Kang, Y.-P.; Wang, N.-H.; Jou, H.-J.; Wang, T.-A. Sesame Ingestion Affects Sex Hormones, Antioxidant Status, and Blood Lipids in Postmenopausal Women. J. Nutr. 2006, 136, 1270–1275. [Google Scholar] [CrossRef]
- Oikawa, D.; Yamashita, S.; Takahashi, S.; Waki, T.; Kikuchi, K.; Abe, T.; Katayama, T.; Nakayama, T. (+)-Sesamin, a Sesame Lignan, is a Potent Inhibitor of Gut Bacterial Tryptophan Indole-Lyase That Is a Key Enzyme in Chronic Kidney Disease Pathogenesis. Biochem. Biophys. Res. Commun. 2022, 590, 158–162. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Sun, Y.; Su, D.; Sun, Y.; Hu, B.; Zeng, X. Purification and Fermentation in Vitro of Sesaminol Triglucoside from Sesame Cake by Human Intestinal Microbiota. J. Agric. Food Chem. 2013, 61, 1868–1877. [Google Scholar] [CrossRef]
- Wang, M.; Liu, P.; Kong, L.; Xu, N.; Lei, H. Promotive Effects of Sesamin on Proliferation and Adhesion of Intestinal Probiotics and Its Mechanism of Action. Food Chem. Toxicol. 2021, 149, 112049. [Google Scholar] [CrossRef]
- Yun, D.; Wang, Y.; Zhang, Y.; Jia, M.; Xie, T.; Zhao, Y.; Yang, C.; Chen, W.; Guo, R.; Liu, X.; et al. Sesamol Attenuates Scopolamine-Induced Cholinergic Disorders, Neuroinflammation, and Cognitive Deficits in Mice. J. Agric. Food Chem. 2022, 70, 13602–13614. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, T.; Xi, Y.; Li, L.; Mo, F.; Liu, X.; Liu, Z.; Gao, J.-M.; Yuan, T. Sesamol Attenuates Amyloid Peptide Accumulation and Cognitive Deficits in APP/PS1 Mice: The Mediating Role of the Gut–Brain Axis. J. Agric. Food Chem. 2021, 69, 12717–12729. [Google Scholar] [CrossRef]
- Ruankham, W.; Suwanjang, W.; Wongchitrat, P.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Sesamin and Sesamol Attenuate H2O2-Induced Oxidative Stress on Human Neuronal Cells via the SIRT1-SIRT3-FOXO3a Signaling Pathway. Nutr. Neurosci. 2021, 24, 90–101. [Google Scholar] [CrossRef]
- Kongtawelert, P.; Kaewmool, C.; Phitak, T.; Phimphilai, M.; Pothacharoen, P.; Shwe, T.H. Sesamin Protects against Neurotoxicity via Inhibition of Microglial Activation under High Glucose Circumstances through Modulating P38 and JNK Signaling Pathways. Sci. Rep. 2022, 12, 11296. [Google Scholar] [CrossRef]
- Keowkase, R.; Shoomarom, N.; Bunargin, W.; Sitthithaworn, W.; Weerapreeyakul, N. Sesamin and Sesamolin Reduce Amyloid-β Toxicity in a Transgenic Caenorhabditis Elegans. Biomed. Pharmacother. 2018, 107, 656–664. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A Major Update of the Phenol-Explorer Database to Incorporate Data on the Effects of Food Processing on Polyphenol Content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Gitea, M.A.; Bungau, S.G.; Gitea, D.; Pasca, B.M.; Purza, A.L.; Radu, A.-F. Evaluation of the Phytochemistry–Therapeutic Activity Relationship for Grape Seeds Oil. Life 2023, 13, 178. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Francioso, A.; Mastromarino, P.; Masci, A.; d’Erme, M.; Mosca, L. Chemistry, Stability and Bioavailability of Resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and Bioavailability of Trans-Resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Wu, B.; Basu, S.; Meng, S.; Wang, X.; Zhang, S.; Hu, M. Regioselective Sulfation and Glucuronidation of Phenolics: Insights into the Structural Basis of Conjugation. Curr. Drug Metab. 2011, 12, 900–916. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, S.E. In Vivo and in Vitro Metabolism of Trans-Resveratrol by Human Gut Microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- El-Mohsen, M.A.; Bayele, H.; Kuhnle, G.; Gibson, G.; Debnam, E.; Srai, S.K.; Rice-Evans, C.; Spencer, J.P.E. Distribution of [H]Trans-Resveratrol in Rat Tissues Following Oral Administration. Br. J. Nutr. 2006, 96, 62. [Google Scholar] [CrossRef]
- Renaud, J.; Martinoli, M.-G. Resveratrol as a Protective Molecule for Neuroinflammation: A Review of Mechanisms. Curr. Pharm. Biotechnol. 2014, 15, 318–329. [Google Scholar] [CrossRef]
- Vidavalur, R.; Otani, H.; Singal, P.K.; Maulik, N. Significance of Wine and Resveratrol in Cardiovascular Disease: French Paradox Revisited. Exp. Clin. Cardiol. 2006, 11, 217–225. [Google Scholar]
- Renaud, S.; de Lorgeril, M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart Disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Song, X.; Liu, L.; Peng, S.; Liu, T.; Chen, Y.; Jia, R.; Zou, Y.; Li, L.; Zhao, X.; Liang, X.; et al. Resveratrol Regulates Intestinal Barrier Function in Cyclophosphamide-Induced Immunosuppressed Mice. J. Sci. Food Agric. 2022, 102, 1205–1215. [Google Scholar] [CrossRef]
- Yao, M.; Fei, Y.; Zhang, S.; Qiu, B.; Zhu, L.; Li, F.; Berglund, B.; Xiao, H.; Li, L. Gut Microbiota Composition in Relation to the Metabolism of Oral Administrated Resveratrol. Nutrients 2022, 14, 1013. [Google Scholar] [CrossRef]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-Induced Gut Microbiota Reduces Obesity in High-Fat Diet-Fed Mice. Int. J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Li, D.; Ke, W.; Chen, F.; Hu, X. Targeting the Gut Microbiota with Resveratrol: A Demonstration of Novel Evidence for the Management of Hepatic Steatosis. J. Nutr. Biochem. 2020, 81, 108363. [Google Scholar] [CrossRef]
- Chen, M.; Hou, P.; Zhou, M.; Ren, Q.; Wang, X.; Huang, L.; Hui, S.; Yi, L.; Mi, M. Resveratrol Attenuates High-Fat Diet-Induced Non-Alcoholic Steatohepatitis by Maintaining Gut Barrier Integrity and Inhibiting Gut Inflammation through Regulation of the Endocannabinoid System. Clin. Nutr. 2020, 39, 1264–1275. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Lv, H.; Pang, W.; Wang, J.; Ma, H.; Wang, S. Intestinal Pharmacokinetics of Resveratrol and Regulatory Effects of Resveratrol Metabolites on Gut Barrier and Gut Microbiota. Food Chem. 2021, 357, 129532. [Google Scholar] [CrossRef]
- Korsholm, A.S.; Kjær, T.N.; Ornstrup, M.J.; Pedersen, S.B. Comprehensive Metabolomic Analysis in Blood, Urine, Fat, and Muscle in Men with Metabolic Syndrome: A Randomized, Placebo-Controlled Clinical Trial on the Effects of Resveratrol after Four Months’ Treatment. Int. J. Mol. Sci. 2017, 18, 554. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; di Minno, G.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef]
- Golan, R.; Shelef, I.; Shemesh, E.; Henkin, Y.; Schwarzfuchs, D.; Gepner, Y.; Harman-Boehm, I.; Witkow, S.; Friger, M.; Chassidim, Y.; et al. Effects of Initiating Moderate Wine Intake on Abdominal Adipose Tissue in Adults with Type 2 Diabetes: A 2-Year Randomized Controlled Trial. Public Health Nutr. 2017, 20, 549–555. [Google Scholar] [CrossRef]
- Novakovic, R.; Rajkovic, J.; Gostimirovic, M.; Gojkovic-Bukarica, L.; Radunovic, N. Resveratrol and Reproductive Health. Life 2022, 12, 294. [Google Scholar] [CrossRef]
- Shivananda Nayak, B.; Dan Ramdath, D.; Marshall, J.R.; Isitor, G.; Xue, S.; Shi, J. Wound-Healing Properties of the Oils of Vitis Vinifera and Vaccinium Macrocarpon. Phytother. Res. 2011, 25, 1201–1208. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Zahran, E.M.; Selim, S.; Al-Sanea, M.M.; Ghoneim, M.M.; Maher, S.A.; Mostafa, Y.A.; Alsenani, F.; Elrehany, M.A.; Almuhayawi, M.S.; et al. Antioxidant and Wound Healing Potential of Vitis Vinifera Seeds Supported by Phytochemical Characterization and Docking Studies. Antioxidants 2022, 11, 881. [Google Scholar] [CrossRef]
- Niknami, E.; Sajjadi, S.-E.; Talebi, A.; Minaiyan, M. Protective Effect of Vitis Vinifera (Black Grape) Seed Extract and Oil on Acetic Acid-Induced Colitis in Rats. Int. J. Prev. Med. 2020, 11, 102. [Google Scholar] [CrossRef]
- Ismail, A.F.M.; Salem, A.A.M.; Eassawy, M.M.T. Hepatoprotective Effect of Grape Seed Oil against Carbon Tetrachloride Induced Oxidative Stress in Liver of γ-Irradiated Rat. J. Photochem. Photobiol. B 2016, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Kang, X.; Zeng, L.; Li, J.; Yang, Y.; Liu, D. The Protective Effects and Genetic Pathways of Thorn Grape Seeds Oil against High Glucose-Induced Apoptosis in Pancreatic β-Cells. BMC Complement. Altern Med. 2014, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Akhtar, N.; Khan, M.S.; Menaa, A.; Menaa, B.; Khan, B.A.; Menaa, F. Formulation and Evaluation on Human Skin of a Water-in-Oil Emulsion Containing Muscat Hamburg Black Grape Seed Extract. Int. J. Cosmet. Sci. 2015, 37, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Navaee, M.; Rakhshkhorshid, M. Comparing the Effect of Foot Massage with Grape Seed Oil and Sweet Almond Oil on Physiological Leg Edema in Primigravidae: A Randomized Clinical Trial. Evid. Based Complement. Altern. Med. 2020, 2020, 6835814. [Google Scholar] [CrossRef] [PubMed]
- Kaseb, F.; Biregani, A.N. Effects of Olive Oil and Grape Seed Oil on Lipid Profile and Blood Pressure in Patients with Hyperlipidemia: A Randomized Clinical Trial. Food Nutr. Sci. 2016, 07, 682–688. [Google Scholar] [CrossRef]
- Al-Edresi, S.; Alsalahat, I.; Freeman, S.; Aojula, H.; Penny, J. Resveratrol-Mediated Cleavage of Amyloid Β1–42 Peptide: Potential Relevance to Alzheimer’s Disease. Neurobiol. Aging 2020, 94, 24–33. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; Zhou, Y.; Wu, J.; Tan, Y.; Ma, X.; Zhao, Y.; Liu, X.; Zhao, Y. Resveratrol Abrogates Hypoxia-Induced Up-Regulation of Exosomal Amyloid-β Partially by Inhibiting CD147. Neurochem. Res. 2019, 44, 1113–1126. [Google Scholar] [CrossRef]
- Corpas, R.; Griñán-Ferré, C.; Rodríguez-Farré, E.; Pallàs, M.; Sanfeliu, C. Resveratrol Induces Brain Resilience Against Alzheimer Neurodegeneration Through Proteostasis Enhancement. Mol. Neurobiol. 2019, 56, 1502–1516. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.; Cheng, Y.C. Resveratrol Activation of AMPK-Dependent Pathways Is Neuroprotective in Human Neural Stem Cells against Amyloid-Beta-Induced Inflammation and Oxidative Stress. Neurochem. Int. 2018, 115, 1–10. [Google Scholar] [CrossRef]
- Xia, D.; Sui, R.; Zhang, Z. Administration of Resveratrol Improved Parkinson’s Disease-like Phenotype by Suppressing Apoptosis of Neurons via Modulating the MALAT1/MiR-129/SNCA Signaling Pathway. J. Cell Biochem. 2019, 120, 4942–4951. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, D.; Jiang, P.; Tang, X.; Lang, Q.; Yu, Q.; Zhang, S.; Che, Y.; Feng, X. Resveratrol Synergizes with Low Doses of L-DOPA to Improve MPTP-Induced Parkinson Disease in Mice. Behav. Brain Res. 2019, 367, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Zhang, J.L.; Duan, Y.L.; Zhang, Q.S.; Li, G.F.; Zheng, D.L. MicroRNA-214 Participates in the Neuroprotective Effect of Resveratrol via Inhibiting α-Synuclein Expression in MPTP-Induced Parkinson’s Disease Mouse. Biomed. Pharmacother. 2015, 74, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Naia, L.; Rosenstock, T.R.; Oliveira, A.M.; Oliveira-Sousa, S.I.; Caldeira, G.L.; Carmo, C.; Laço, M.N.; Hayden, M.R.; Oliveira, C.R.; Rego, A.C. Comparative Mitochondrial-Based Protective Effects of Resveratrol and Nicotinamide in Huntington’s Disease Models. Mol. Neurobiol. 2017, 54, 5385–5399. [Google Scholar] [CrossRef] [PubMed]
- Ghaiad, H.R.; Nooh, M.M.; El-Sawalhi, M.M.; Shaheen, A.A. Resveratrol Promotes Remyelination in Cuprizone Model of Multiple Sclerosis: Biochemical and Histological Study. Mol. Neurobiol. 2017, 54, 3219–3229. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Núñez, O.; Pallás, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol Improves Motoneuron Function and Extends Survival in SOD1G93A ALS Mice. Neurotherapeutics 2014, 11, 419–432. [Google Scholar] [CrossRef]
- Sato, F.; Martinez, N.E.; Shahid, M.; Rose, J.W.; Carlson, N.G.; Tsunoda, I. Resveratrol Exacerbates Both Autoimmune and Viral Models of Multiple Sclerosis. Am. J. Pathol. 2013, 183, 1390–1396. [Google Scholar] [CrossRef]
- Bresciani, L.; Favari, C.; Calani, L.; Francinelli, V.; Riva, A.; Petrangolini, G.; Allegrini, P.; Mena, P.; del Rio, D. The Effect of Formulation of Curcuminoids on Their Metabolism by Human Colonic Microbiota. Molecules 2020, 25, 940. [Google Scholar] [CrossRef]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative Stress and Dietary Phytochemicals: Role in Cancer Chemoprevention and Treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Zam, W. Gut Microbiota as a Prospective Therapeutic Target for Curcumin: A Review of Mutual Influence. J. Nutr. Metab. 2018, 2018, 1367984. [Google Scholar] [CrossRef]
- Tan, S.; Rupasinghe, T.W.T.; Tull, D.L.; Boughton, B.; Oliver, C.; McSweeny, C.; Gras, S.L.; Augustin, M.A. Degradation of Curcuminoids by in Vitro Pure Culture Fermentation. J. Agric. Food Chem. 2014, 62, 11005–11015. [Google Scholar] [CrossRef] [PubMed]
- Naeini, M.B.; Momtazi, A.A.; Jaafari, M.R.; Johnston, T.P.; Barreto, G.; Banach, M.; Sahebkar, A. Antitumor Effects of Curcumin: A Lipid Perspective. J. Cell Physiol. 2019, 234, 14743–14758. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Kishor Roy, N.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Eke-Okoro, U.J.; Raffa, R.B.; Pergolizzi, J.V.; Breve, F.; Taylor, R. Curcumin in Turmeric: Basic and Clinical Evidence for a Potential Role in Analgesia. J. Clin. Pharm. 2018, 43, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Kao, C.L.; Liu, C.M. The Cancer Prevention, Anti-Inflammatory and Anti-Oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef] [PubMed]
- Burge, K.; Gunasekaran, A.; Eckert, J.; Chaaban, H. Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection. Int. J. Mol. Sci. 2019, 20, 1912. [Google Scholar] [CrossRef]
- Carlos-Reyes, Á.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.A.; Astudillo-De La Vega, H.; Hernández De La Cruz, O.N.; López-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 79. [Google Scholar] [CrossRef]
- Bahrami, A.; Amerizadeh, F.; ShahidSales, S.; Khazaei, M.; Ghayour-Mobarhan, M.; Sadeghnia, H.R.; Maftouh, M.; Hassanian, S.M.; Avan, A. Therapeutic Potential of Targeting Wnt/β-Catenin Pathway in Treatment of Colorectal Cancer: Rational and Progress. J. Cell. Biochem. 2017, 118, 1979–1983. [Google Scholar] [CrossRef]
- Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V. Curcumin as a Clinically-Promising Anti-Cancer Agent: Pharmacokinetics and Drug Interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 953–972. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef]
- Koosirirat, C.; Linpisarn, S.; Changsom, D.; Chawansuntati, K.; Wipasa, J. Investigation of the Anti-Inflammatory Effect of Curcuma Longa in Helicobacter Pylori-Infected Patients. Int. Immunopharmacol. 2010, 10, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, F.; Cavallaro, L.G.; Nouvenne, A.; Stefani, N.; Cavestro, G.M.; Iori, V.; Maino, M.; Comparato, G.; Fanigliulo, L.; Morana, E.; et al. A Curcumin-Based 1-Week Triple Therapy for Eradication of Helicobacter Pylori Infection: Something to Learn from Failure? Helicobacter 2007, 12, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Zou, J.; Jiang, X.; Yang, J.; Cao, Z.; He, Y.; Feng, D. Curcumin Supplementation Ameliorates Bile Cholesterol Supersaturation in Hamsters by Modulating Gut Microbiota and Cholesterol Absorption. Nutrients 2022, 14, 1828. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.P.; Zhong, Y.B.; Kang, Z.P.; Huang, J.Q.; Fang, W.Y.; Wei, S.Y.; Long, J.; Li, S.S.; Zhao, H.M.; Liu, D.Y. Curcumin Regulates the Homeostasis of Th17/Treg and Improves the Composition of Gut Microbiota in Type 2 Diabetic Mice with Colitis. Phytother. Res. 2022, 36, 1708–1723. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xu, Y.; Geng, R.; Qiu, J.; He, X. Curcumin Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice Through Regulating Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, e2100943. [Google Scholar] [CrossRef]
- Liu, J.; Luo, W.; Chen, Q.; Chen, X.; Zhou, G.; Sun, H. Curcumin Sensitizes Response to Cytarabine in Acute Myeloid Leukemia by Regulating Intestinal Microbiota. Cancer Chemother. Pharm. 2022, 89, 243–253. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Rea, A.; Michel, S. Efficacy of a Curcumin Extract (CurcugenTM) on Gastrointestinal Symptoms and Intestinal Microbiota in Adults with Self-Reported Digestive Complaints: A Randomised, Double-Blind, Placebo-Controlled Study. BMC Complement. Med. 2021, 21, 40. [Google Scholar] [CrossRef]
- Liu, Z.J.; Li, Z.H.; Liu, L.; Tang, W.X.; Wang, Y.; Dong, M.R.; Xiao, C. Curcumin Attenuates Beta-Amyloid-Induced Neuroinflammation via Activation of Peroxisome Proliferator-Activated Receptor-Gamma Function in a Rat Model of Alzheimer’s Disease. Front Pharm. 2016, 7, 261. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin Inhibits Formation of Amyloid β Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid In Vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Sang, Q.; Liu, X.; Wang, L.; Qi, L.; Sun, W.; Wang, W.; Sun, Y.; Zhang, H. Curcumin Protects an SH-SY5Y Cell Model of Parkinson’s Disease against Toxic Injury by Regulating HSP90. Cell. Physiol. Biochem. 2018, 51, 681–691. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin Nanoparticles Attenuate Neurochemical and Neurobehavioral Deficits in Experimental Model of Huntington’s Disease. Neuromolecular Med. 2014, 16, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Chico, L.; Ienco, E.C.; Bisordi, C.; lo Gerfo, A.; Petrozzi, L.; Petrucci, A.; Mancuso, M.; Siciliano, G. Amyotrophic Lateral Sclerosis and Oxidative Stress: A Double-Blind Therapeutic Trial After Curcumin Supplementation. CNS Neurol Disord. Drug Targets 2018, 17, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Ramaholimihaso, T.; Bouazzaoui, F.; Kaladjian, A. Curcumin in Depression: Potential Mechanisms of Action and Current Evidence—A Narrative Review. Front. Psychiatry 2020, 11, 572533. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Curcumin for Depression: A Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharm. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New Progress in the Pharmacology of Protocatechuic Acid: A Compound Ingested in Daily Foods and Herbs Frequently and Heavily. Pharm. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Hung, M.Y.; Fu, T.Y.C.; Shih, P.H.; Lee, C.P.; Yen, G.C. Du-Zhong (Eucommia ulmoides Oliv.) Leaves Inhibits CCl4-Induced Hepatic Damage in Rats. Food Chem. Toxicol. 2006, 44, 1424–1431. [Google Scholar] [CrossRef]
- del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of Dietary Flavonoids and Phenolic Compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Masella, R.; Santangelo, C.; D’Archivio, M.; LiVolti, G.; Giovannini, C.; Galvano, F. Protocatechuic Acid and Human Disease Prevention: Biological Activities and Molecular Mechanisms. Curr. Med. Chem. 2012, 19, 2901–2917. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological Effects of Protocatechuic Acid and Its Therapeutic Potential in Neurodegenerative Diseases: Review on the Basis of in Vitro and in Vivo Studies in Rodents and Humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tian, R.; Liu, H.; Xue, H.; Zhang, R.; Han, S.; Ji, L.; Huang, W.; Zhan, J.; You, Y. Research Progress on Intervention Effect and Mechanism of Protocatechuic Acid on Nonalcoholic Fatty Liver Disease. Crit. Rev. Food Sci. Nutr. 2021, 62, 9053–9075. [Google Scholar] [CrossRef] [PubMed]
- Thakare, V.N.; Lakade, S.H.; Mahajan, M.P.; Kulkarni, Y.P.; Dhakane, V.D.; Harde, M.T.; Patel, B.M. Protocatechuic Acid Attenuates Chronic Unpredictable Mild Stress Induced-Behavioral and Biochemical Alterations in Mice. Eur. J. Pharm. 2021, 898, 173992. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, Ellagic Acid and Vascular Health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef] [PubMed]
- Kilic, I.; Yeşiloğlu, Y.; Bayrak, Y. Spectroscopic Studies on the Antioxidant Activity of Ellagic Acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 447–452. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltrán, D.; Luna, M.C.; Romo-Vaquero, M.; García-Villalba, R.; Mira, A.; Espín, J.C.; Tomás-Barberán, F.A. Isolation of Human Intestinal Bacteria Capable of Producing the Bioactive Metabolite Isourolithin a from Ellagic Acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, X.; Wang, Y.; Guo, Y.; Zhu, P.; Li, G.; Zhang, J.; Ma, Q.; Zhao, L. Dietary Ellagic Acid Ameliorated Clostridium Perfringens-Induced Subclinical Necrotic Enteritis in Broilers via Regulating Inflammation and Cecal Microbiota. J. Anim Sci. Biotechnol. 2022, 13, 47. [Google Scholar] [CrossRef]
- Wu, T.; Chu, X.; Cheng, Y.; Tang, S.; Zogona, D.; Pan, S.; Xu, X. Modulation of Gut Microbiota by Lactobacillus Casei Fermented Raspberry Juice In Vitro and In Vivo. Foods 2021, 10, 3055. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Romero, M.P.; Motilva, M.J. Stability and Metabolism of Arbutus Unedo Bioactive Compounds (Phenolics and Antioxidants) under in Vitro Digestion and Colonic Fermentation. Food Chem. 2016, 201, 120–130. [Google Scholar] [CrossRef]
- Kiasalari, Z.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Azadi-Ahmadabadi, E.; Esmaeil-Jamaat, E.; Fahanik-Babaei, J.; Fakour, M.; Fereidouni, F.; Ghasemi-Tarie, R.; Jalalzade-Ogvar, S.; et al. Ellagic Acid Ameliorates Neuroinflammation and Demyelination in Experimental Autoimmune Encephalomyelitis: Involvement of NLRP3 and Pyroptosis. J. Chem. Neuroanat. 2021, 111, 101891. [Google Scholar] [CrossRef]
- Farbood, Y.; Sarkaki, A.; Dianat, M.; Khodadadi, A.; Haddad, M.K.; Mashhadizadeh, S. Ellagic Acid Prevents Cognitive and Hippocampal Long-Term Potentiation Deficits and Brain Inflammation in Rat with Traumatic Brain Injury. Life Sci. 2015, 124, 120–127. [Google Scholar] [CrossRef] [PubMed]

















| Dietary Phenolic Compound Source/Compound | Disease | Study Results | Reference(s) |
|---|---|---|---|
| polyphenols | cardiovascular disease | database-linked survey of preclinical trials and clinical trials on polyphenols for the treatment of cardiovascular disease | Behl et al., 2020 [5] |
| polyphenols | rheumatoid Arthritis | efficacy of polyphenols to mitigate rheumatoid arthritis by inhibiting the MAPK signaling pathway | Behl et al., 2021 [6] |
| polyphenols | rheumatoid Arthritis | a review of preclinical and clinical data on various pathways involved in rheumatoid arthritis and polyphenols as therapeutic agents | Behl et al., 2022 [7] |
| plant polyphenols | depression | a review of the chemical, pharmacological, and neurological evidence for the potential of polyphenols in depression | Kabra et al., 2022 [8] |
| polyphenols | depression | a review of polyphenols that inhibit oxidative stress and inflammation through signaling pathways in depression | Behl et al., 2022 [9] |
| polyphenols carotenoids | eye disease | a review of the health benefits of polyphenols and carotenoids for the prevention and treatment of age-related eye diseases | Bungau et al., 2019 [10] |
| quercetin, EC | arteriosclerosis | augmentation of nitric oxide status and attenuation of endothelin-1 concentration in plasma of healthy men | Loke et al., 2008 [47] |
| cocoa/EC | cardiovascular disease | acute elevations in levels of circulating nitric oxide species, an enhanced flow-mediated vasodilation response of conduit arteries, and an augmented microcirculation | Schroeter et al., 2006 [48] |
| EC | brain endothelial dysfunction, neurodegenerative disorders | regulated protein expression and gene expression in brain endothelial cells | Corral-Jara et al., 2022 [51] |
| green tea extracts | alcoholic fatty liver disease | attenuation of triacylglycerol levels in serum and liver and aminotransferase activities in mice | Li et al., 2021 [52] |
| tea extracts | alcoholic fatty liver disease | prevention of liver steatosis, decrease in oxidative stress and inflammation, modulation of gut microbiota | Li et al., 2021 [54] |
| green tea | alcoholic fatty liver disease | amelioration of alcoholic liver disease by activation of Akkermansia muciniphila | Zhao et al., 2022 [53] |
| EGCG | non-alcoholic fatty liver disease | inhibited the increase in histological fatty deposits and triglyceride accumulation in the liver induced by high fat diet, improved intestinal dysbiosis, and involved in sirtuin genes | Naito et al., 2020 [55] |
| concord grape polyphenols | obesity | increase in the growth of Akkermansia muciniphila and decrease in the proportion of Firmicutes to Bacteroidetes | Roopchand et al., 2015 [59] |
| EGCG | ulcerative colitis | the active treatment remission rate was 53.3% (8 of 15) compared with 0% (0 of 4) for placebo | Dryden et al., 2013 [60] |
| EC | acute and chronic colitis | attenuation of COX-2 expression and increase in cell proliferation, repair of the epithelium by stimulating the expression of EGF | Vasconcelos et al., 2012 [61] |
| EGCG and piperine | ulcerative colitis | increased bioavailability, decreased colonic histological damage and MDA levels, and increased antioxidant enzyme activity | Brückner et al., 2012 [62] |
| EGC and ECG | Alzheimer’s disease | attenuation of amyloid-β aggregation, reduced ROS production, less neurotoxicity to neurons | Chen et al., 2020 [65] |
| EGCG | Alzheimer’s disease | negative regulation of microglial inflammation and neurotoxicity | Zhong et al., 2019 [66] |
| EGCG | Alzheimer’s disease | activated ERK-and PI3K-mediated pathways in astrocytes and accelerated amyloid-β degradation | Yamamoto et al., 2017 [67] |
| EGCG | Alzheimer’s disease | inhibition of neuroinflammatory response in microglia, protection from indirect neurotoxicity | Cheng-Chung Wei et al., 2016 [68] |
| EGCG | Alzheimer’s disease | attenuation of cognitive deficits in APP/PS1 mice | Bao et al., 2020 [69] |
| EGCG | Parkinson’s disease | modulation of the substantia nigra iron transport protein ferroportin, attenuation of oxidative stress, neuroprotective effects | Xu et al., 2017 [70] |
| EGCG | Parkinson’s disease | inhibition of substantia nigra neurodegeneration, neuroprotective effect | Sergi 2022 [71] |
| EGCG | hypoxia-induced neuroinflammation | protection of microglia by disabling the NF-κB pathway and activating the Nrf-2/HO-1 pathway | Kim et al., 2022 [72] |
| flavanol-enriched cocoa powder | amelioration of intestinal environment | enhanced the abundance of Lactobacillus and Bifidobacterium species, modulated markers of local gut immunity | Jang et al., 2016 [73] |
| cocoa flavanols | disorder of the intestinal environment | growth of select gut microflora in humans | Tzounis et al., 2011 [74] |
| cocoa | disorder of the intestinal environment | improved gut-associated lymphoid tissue function by modulating IgA secretion and gut microbiota | Pérez-Cano et al., 2013 [75] |
| cocoa | deterioration of the intestinal immune system | differential TLR patterns, attenuation of intestinal IgA secretion and IgA-coating bacteria | Massot-Cladera et al., 2012 [76] |
| cocoa | diabetes mellitus | amelioration of intestinal flora, barrier integrity, and the inflammatory status of the intestine | Álvarez-Cilleros et al., 2020 [77] |
| cocoa | inflammation-related colon carcinogenesis | attenuation of NF-κB, pro-inflammatory enzyme expression, and inducible NO synthase expression | Rodríguez-Ramiro et al., 2013 [78] |
| cocoa flavanols | coronary artery disease | maintenance of normal endothelium-dependent vasodilation | Agostoni C. et al., 2012 [79] |
| cocoa extract | cardiovascular disease among older adults | lowered risk of total cardiovascular events | Sesso et al., 2022 [80] |
| cocoa extract | Alzheimer’s disease | modification of the physical structure of amyloid-β oligomers | Dubner et al., 2015 [81] |
| cocoa extract | Alzheimer’s disease | attenuation of amyloid-β oligomerization | Wang et al., 2014 [82] |
| cocoa extract | Alzheimer’s disease | neuroprotection by activating the brain-derived neurotrophic factor survival pathway | Cimini et al., 2013 [83] |
| kaki tannin | metabolic syndrome | strong binding capacity for bile acids | Matsumoto et al., 2011 [87] |
| kaki tannin | hypercholesterolemia | cholesterol lowering effect and glucose metabolism amelioration by the ability of kaki tannin to bind bile acids | Nishida et al., 2021 [88] |
| kaki tannin | postprandial hyperglycemia | kaki tannins limited starch digestion and inhibited glucose uptake and transport, thereby alleviating postprandial hyperglycemia | Li et al., 2018 [89] |
| kaki tannin | disruption of intestinal flora | reshaped fecal gut microbiota | Zhu et al., 2018 [90] |
| kaki tannin | Mycobacterium avium complex (MAC) disease | bacteriostatic effect on MAC, attenuation of pulmonary granuloma formation, suppression of pro-inflammatory cytokine expression | Matsumura et al., 2017 [17] |
| kaki tannin | ulcerative colitis | decreased disease activity and colonic inflammation, changed microbiota composition and immune response | Kitabatake et al., 2021 [18] |
| dry persimmon | dyslipidemia | lipid-lowering and antioxidant properties | Gorinstein et al., 1998 [91], Gorinstein et al., 2000 [92] |
| kaki tannin | hyper-LDL cholesterolemia | attenuation of serum LDL cholesterol levels in humans | Suzuki et al., 2022 [93] |
| quercetin and isoflavones | osteoporosis | elucidation of metabolic pathways by intestinal microbiota, amelioration of bioavailability | Murota et al., 2018 [95] |
| quercetin/red onion | obesity and insulin resistance | adipose tissue remodeling | Forney et al., 2018 [118] |
| quercetin/grape powder | obesity and insulin resistance | prevented macrophage inflammation and adipocyte macrophage-mediated insulin resistance | Overman et al., 2011 [119] |
| quercetin | kidney disease due to atheroembolism | attenuation of COX-2 induction by stress | Carlsen et al., 2015 [116] |
| quercetin | obesity-related diseases | antioxidant, anti-inflammatory, and antifibrotic effects on insulin resistance and atherosclerosis | Sato et al., 2020 [123] |
| quercetin | colitis | rebalanced the pro-inflammatory, anti-inflammatory, and bactericidal function of enteric macrophages | Ju et al., 2018 [120] |
| quercetin | disruption of intestinal flora | restoration of gut microbiota in mice after antibiotic treatment | Shi et al., 2020 [121] |
| quercetin | C. rodentium-induced colitis | modification of gut microbiota and suppression of proinflammatory cytokines in Citrobacter rodentium-induced colitis mice | Lin et al., 2019 [122] |
| quercetin and rutin | Alzheimer’s disease | anti-amyloidogenic and fibril-disaggregating effects | Jiménez-Aliaga et al., 2011 [124] |
| quercetin | Alzheimer’s disease | promotion of viability and proliferation of Alzheimer’s disease model cells, increase in expression of sirtuin 1/Nrf2/HO-1 and antioxidant-related enzymes | Yu et al., 2020 [125] |
| quercetin | Alzheimer’s disease | inhibition of tau protein hyperphosphorylation and oxidative stress, inhibition of PI3K/Akt/GSK3β, MAPK, and NF-κB p65 in a cell line of mouse hippocampal neurons | Jiang et al., 2016 [126] |
| quercetin | Alzheimer’s disease | inhibition of BACE-1 (Beta-site APP Cleaving Enzyme-1, β-secretase), attenuation of amyloid-β peptide levels | Shimmyo et al., 2008 [127] |
| quercetin | Alzheimer’s disease | targeted integrated stress response signaling, suppressed amyloid-β (Aβ) production and prevented cognitive impairment in a mouse model | Nakagawa et al., 2019 [128] |
| quercetin | Parkinson’s disease | activation of the PKD1–Akt cell survival signaling axis, neuroprotective signaling in a dopaminergic neuronal model | Ay et al., 2017 [129] |
| quercetin | Parkinson’s disease | significant attenuation of rotenone-induced behavioral impairment, augment of autophagy, attenuation of ER stress-induced apoptosis with attenuated oxidative stress | El-Horany et al., 2016 [130] |
| quercetin with piperine | Parkinson’s disease | attenuation of movement disorders and biochemical and neurotransmitter changes | Sharma et al., 2020 [131] |
| quercetin with piperine | Parkinson’s disease | significantly amelioration of MPTP-induced behavioral abnormalities in rats, reversal of the abnormal alterations of neurotransmitters in the striatum | Singh et al., 2017 [132] |
| buckwheat | Hypercholesterolemia, neurodegenerative disease, cancer, inflammation, diabetes, hypertension | buckwheat as a food and its effects on health | Giménez-Bastida et al., 2015 [104] |
| quercetin, rutin/buckwheat | dyslipidemia, metabolic syndromes, | quercetin reduced obesity due to high-fat diet, rutin, quercetin, and tartary buckwheat shaped specific structures of the intestinal microbiota | Peng et al., 2020 [133] |
| phenolic compounds/tartary buckwheat | human breast cancer | inhibitory ability of phenolic compounds on breast cancer cell proliferation | Li et al., 2017 [134] |
| rutin | cancer | regulation of molecular networks and signaling mechanisms in cancer cells by rutin | Perk et al., 2014 [135] |
| rutin | COVID-19 | conformational change upon binding of rutin and SARS-CoV-2 spike protein | Kumari et al., 2022 [136] Rahman et al., 2021 [137] |
| rutin, quercetin/buckwheat | postprandial rise in blood sugar, diabetes, hypercholesterolemia | the rutin and phenolic compounds contained in buckwheat inhibited the action of digestive enzymes, suppressing the sudden rise in postprandial blood sugar levels and lowering cholesterol | Kreft et al., 2022 [138] Cirkovic Velickovic et al., 2018 [139] Wang et al., 2022 [140] Ikeda et al., 1993 [141] Zhang et al., 2017 [142] Bao et al., 2016 [143] |
| buckwheat | cardiovascular disease, dyslipidemia | review and meta-analysis on buckwheat and cardiometabolic health | Llanaj et al., 2022 [144] |
| rutin | neurodegenerative disease | a review of the neuroprotective mechanisms of rutin | Enogieru et al., 2018 [145] |
| buckwheat | hypercholesterolemia, inflammation, neurodegenerative disease, cancer, diabetes, hypertension, celiac disease | health benefits of buckwheat, potential remedy for diseases | Noreen et al., 2021 [146] |
| isoflavone | a wide range of hormonal disorders | classification, structure, and occurrence, with their metabolism, biological, and health effects in humans and animals, and their utilization and potential risks | Křížová et al., 2019 [147] |
| isoflavone and metabolites | cardiovascular diseases, metabolic syndromes, osteoporosis, diabetes, brain-related diseases, etc. | the latest research trends that have shown substantial interest in the biological efficacy of isoflavones in humans and plants, and their related mechanisms | Kim 2021 [148] |
| isoflavones | some hormone-dependent diseases | effects of isoflavones on chemoprevention of breast cancer, prostate cancer, and cardiovascular osteoporosis and alleviation of osteoporosis and postmenopausal symptoms | Vitale et al., 2013 [156] |
| S-equol | vasomotor symptoms, osteoporosis, prostate cancer, cardiovascular disease | summary of studies demonstrating effects of isoflavone supplements on menopausal symptoms, bone, prostate cancer, and cardiovascular biomarkers | Jackson et al., 2011 [159] |
| isoflavone/soybeans | breast, thyroid, and uterus of postmenopausal women | a review of key studies related to soy, with a focus on clinical and epidemiological studies | Messina 2016 [162] |
| soy protein | blood cholesterol | attenuation of total and LDL cholesterol | Harland et al., 2008 [163] |
| soy isoflavones | osteoporosis | significant increase in bone density, decrease in urinary deoxypyridinoline, a marker of bone resorption | Wei et al., 2012 [164] |
| dietary soy | chronic kidney disease | significantly reduced serum creatinine, serum phosphorus, CRP, and proteinuria; no significant change was found in creatinine clearance and glomerular filtration rate | Jing et al., 2016 [165] |
| fermented soy products | diabetes mellitus, blood pressure, cardiac disorders, and cancer-related issues | attenuation of serum levels of total cholesterol, low-density lipoprotein (LDL), and triglycerides, maintenance of bone health and prevention of osteoporosis and maintenance of normal endothelial function | Jayachandran et al., 2019 [166] |
| genistein | Alzheimer’s disease | directly targeted amyloid-β and tau to regulate intracellular signaling pathways involved in neuronal death in the brain | Uddin et al., 2019 [168] |
| soy isoflavones | Alzheimer’s disease | neuroprotective effects on scopolamine-induced memory impairment, enhancement of cholinergic function, suppression of oxidative stress and activation of ERK/CREB/BDNF signaling | Lu et al., 2018 [169] |
| genistein | Alzheimer’s disease | regulated CAMK4 to regulate tau hyperphosphorylation | Ye et al., 2017 [170] |
| genistein | Parkinson’s disease | neuroprotective effect on dopaminergic neurons | Arbabi et al., 2016 [171] |
| genistein | early phases of allergic encephalomyelitis, multiple sclerosis | decreased cell cytotoxicity | Razeghi Jahromi et al., 2014 [172] |
| sesame | diabetes mellitus, hypercholesterolemia, osteoarthritis, some types of cancer | detailed research on sesame oil contents, health effects, nutraceuticals, oil quality, and value addition strategies | Langyan et al., 2022 [179] |
| sesame | free radical-related diseases | Nutraceutical, pharmacological, traditional, and industrial value of sesame seeds with respect to bioactive components that have high antioxidant activity | Pathak et al., 2014 [180] |
| chlorogenic acid | obesity and associated glucose intolerance | attenuation of food intake, elevation of body temperature, increase in heat dissipation and activation of brown adipose tissue | He et al., 2021 [188] |
| chlorogenic acid | obesity and obesity-related metabolic endotoxemia | suppression of body weight gain, attenuation of relative weight of fat, amelioration of intestinal barrier integrity, prevention of impaired glucose metabolism and endotoxemia, significant alteration of intestinal microbiota composition | Ye et al., 2021 [189] |
| chlorogenic acid | high-fat diet-induced obesity | attenuation of plasma lipids, alteration of adipose tissue-associated gene expression, reversal of gut microbiota dysbiosis | Wang et al., 2019 [190] |
| coffee | type 2 diabetes mellitus | attenuation of diabetes risk in humans | Huxley et al., 2009 [191] |
| coffee | disruption of intestinal flora | increase in the growth of Bifidobacterium spp and Clostridium coccoides-Eubacterium rectale group | Mills et al., 2015 [192] |
| coffee | disruption of intestinal flora | coffee consumption can selectively improve the growth of probiotic strains, thus exerting a prebiotic effect | Sales et al., 2020 [193] |
| chlorogenic acid | Parkinson’s disease | activation of Akt/ERK signaling in the mitochondrial intrinsic apoptotic pathway, neuroprotection against MPTP-induced toxicity in a Parkinson’s disease mouse model | Singh et al., 2020 [195] |
| caffeic acid, chlorogenic acid | Parkinson’s disease | protection of rotenone-induced neurodegeneration of both nigral dopaminergic and enteric neurons, upregulation of metallothionein | Miyazaki et al., 2019 [196] |
| chlorogenic acid | Parkinson’s disease | attenuation of oxidative stress and neuroinflammation in MPTP-poisoned mice | Singh et al., 2018 [197] |
| chlorogenic acid | Alzheimer’s disease | attenuation of cognitive deficits in APP/PS1 mice by activation of the mTOR/TFEB signaling pathway | Gao et al., 2020 [198] |
| sesamin | variety of cardiovascular diseases | attenuation of cardiovascular disease effects on RAS/MAPK, PI3K/AKT, ERK1/2, p38, p53, IL-6, TNFα, and NF-κB signaling networks | Dalibalta et al., 2020 [200] |
| sesame | climacteric disorder | amelioration of blood lipid, antioxidant, and sex hormone status | Wu et al., 2006 [201] |
| sesamin | chronic kidney disease | suppression of uremic toxin production by inhibition of bacterial L-tryptophan indole-lyase | Oikawa et al., 2022 [202] |
| sesamin | disruption of intestinal flora | increase in the adhesive index of probiotics, up-regulation of the adhesive protein (β-cadherin and E-cadherin) expression | Wang et al., 2021 [204] |
| sesamol | Alzheimer’s disease | attenuation of SCOP-induced cognitive dysfunction via balancing the cholinergic system and reducing neuroinflammation and oxidative stress | Yun et al., 2022 [205] |
| sesamol | Alzheimer’s disease | attenuation of Alzheimer’s disease-related cognitive impairment and neuroinflammatory response by mediating the gut microbe–SCFA–brain axis | Liu et al., 2021 [206] |
| sesamin, sesamol | Alzheimer’s disease, Parkinson’s disease, Huntington’s disease | activation of SIRT1/SIRT3/FOXO3a expression, inhibition of BAX (pro-apoptotic protein) and upregulation of BCL-2 (anti-apoptotic protein) | Ruankham et al., 2021 [207] |
| sesamin | diabetes-induced neurodegenerative diseases | attenuation of microglial activation by high glucose, reduction of inflammatory response and neurotoxicity | Kongtawelert et al., 2022 [208] |
| sesamin, sesamolin, sesamol | Alzheimer’s disease | sesamin protected against Aβ toxicity by reducing toxic Aβ oligomers, sesamin and sesamolin ameliorated amyloid-β-induced deficits in chemotactic behavior, anti-amyloid-β toxic activity and structure–activity relationship of sesame lignans | Keowkase et al., 2018 [209] |
| resveratrol | neuroinflammatory disease | prevention of self-destruction of nerve cells | Renaud et al., 2014 [218] |
| resveratrol/red wine | cardiovascular disease, lung cancer, prostate cancer | effect of red wine on cardiovascular morbidity and mortality | Vidavalur et al., 2006 [219] |
| red wine | coronary heart disease | inhibition of platelet reactivity by wine (alcohol) | Renaud et al., 1992 [220] |
| resveratrol | intestinal dysfunction | regulation of intestinal barrier function under immunosuppression | Song et al., 2022 [221] |
| resveratrol | colitis | activation of metabolism by intestinal microbiota, modification of intestinal microbiota | Yao et al., 2022 [222] |
| resveratrol | obesity | amelioration of intestinal flora, regulation of lipid metabolism, recovery of intestinal barrier function, amelioration of insulin sensitivity | Wang et al., 2020 [223] |
| resveratrol | NAFLD | amelioration of insulin resistance, amelioration of intestinal barrier function and intestinal microbiota composition, amelioration of lipid metabolism | Wang et al., 2020 [224] |
| resveratrol | NAFLD | inhibition of high-fat diet-induced elevation in cannabinoid receptor type 1 (CB1) mRNA expression, inhibition of colonic CB2 mRNA levels, and maintenance of intestinal barrier integrity | Chen et al., 2020 [225] |
| resveratrol | metabolic and intestinal disease | upregulation of mRNA expression of tight junction and mucin-associated proteins, maintenance of intestinal barrier | Zhang et al., 2021 [226] |
| resveratrol | metabolic syndrome | regulation of intestinal bacterial composition and metabolism and alteration of steroid metabolism in middle-aged men | Korsholm et al., 2017 [227] |
| resveratrol | obesity | metabolic activation and amelioration of mitochondrial respiration to muscle fatty acid-derived substrates and caloric restriction-like effect in obese men | Timmers et al., 2011 [228] |
| resveratrol | cardiovascular disease and a variety of cancers | accumulation of resveratrol in epithelial cells along the aerodigestive tract and presence of potentially active resveratrol metabolites | Walle et al., 2004 [229] |
| red wine | coronary heart disease | changes in lipid profiles, attenuation of insulin resistance, and decrease in oxidative stress | Castaldo et al., 2019 [230] |
| wine | obesity | consuming moderate amounts of wine as part of a Mediterranean diet did not promote weight gain or abdominal obesity. | Golan et al., 2017 [231] |
| resveratrol | pregnancy-related complications | effects of resveratrol on embryogenesis and spermatogenesis mediated by several mechanisms | Novakovic et al., 2022 [232] |
| grape seed oil | wound | wound-healing properties of the oils of Vitis vinifera and Vaccinium macrocarpon in animal model | Shivananda Nayak et al., 2011 [233] Al-Warhi et al., 2022 [234] |
| grape seed oil | ulcerative colitis | oral administration of grape seed oil and grape seed extract showed anti-inflammatory effect and effect on ulcerative colitis | Niknami et al., 2020 [235] |
| grape seed oil | acute liver injury | grape seed oil suppressed inflammation and protected the liver against acute liver injury caused by oxidative stress | Ismail et al., 2016 [236] |
| grape seed oil | diabetes mellitus | seed oil of Vitis davidii Foex. protected pancreatic β-cells from anti-glucose-induced apoptosis and maintained insulin secretion | Lai et al., 2014 [237] |
| grape seed oil | erythema of the skin | the application of a cream milky lotion containing grape seed oil was found to ameliorate the skin’s moisture content, sebum content, and erythema | Sharif et al., 2015 [238] |
| grape seed oil | physiological leg edema in primigravidae | physiological edema in pregnancy was suppressed with foot massage using grape seed oil | Navaee et al., 2020 [239] |
| grape seed oil | hyperlipidemia | blood triglycerides were suppressed by oral administration of grapeseed oil for 6 weeks | Kaseb et al., 2016 [240] |
| resveratrol | Alzheimer’s disease | significant attenuation of cytotoxicity of amyloid-β1-42 peptide against SH-SY5Y human neuroblastoma cells, neuroprotective effect | Al-Edresi et al., 2020 [241] |
| resveratrol | hypoxia, Alzheimer’s disease | prevention of hypoxia-induced upregulation of total amyloid and exosomal amyloid-β by inhibiting CD147 | Xie et al., 2019 [242] |
| resveratrol | Alzheimer’s disease | upregulation of the SIRT1 pathway, induction of cognitive enhancement and neuroprotection against amyloid and tau pathologies | Corpas et al., 2019 [243] |
| resveratrol | Alzheimer’s disease | activation of AMPK-dependent signaling by resveratrol rescued amyloid-β-mediated neurotoxicity in hNSCs. | Chiang et al., 2018 [244] |
| resveratrol | Parkinson’s disease | regulation of the MALAT1/miR-129/SNCA signaling pathway | Xia et al., 2019 [245] |
| resveratrol | Parkinson’s disease | attenuation of MPTP-induced loss of dopaminergic neurons, attenuation of astroglial activation in the nigrostriatal pathway, attenuation of motor dysfunction in MPTP-treated mice | Liu et al., 2019 [246] |
| resveratrol | Parkinson’s disease | neuroprotective effects of regulation of α-synuclein expression upon loss of miR-214 in Parkinson’s disease | Wang et al., 2015 [247] |
| resveratrol | Huntington’s disease | improved motor coordination and learning, enhanced expression of mitochondrial-encoded electron transport chain genes in YAC128 mice | Naia et al., 2017 [248] |
| resveratrol | multiple sclerosis | promoted remyelination effect of resveratrol | Ghaiad et al., 2017 [249] |
| resveratrol | amyotrophic lateral sclerosis (ALS) | increase in mitochondrial biogenesis in the SOD1(G93A) spinal cord, increase in expression and activation of Sirtuin 1 and AMPK in the ventral spinal cord | Mancuso et al., 2014 [250] |
| curcumin | cancer | potential of curcumin to influence lipogenic pathways that regulate human cancer cell metabolism | Naeini et al., 2019 [257] |
| curcumin | various chronic diseases including various types of cancers, diabetes, obesity, cardiovascular, pulmonary, neurological, and autoimmune diseases | Anti-inflammatory activity through the suppression of numerous cells signaling pathways including NF-κB, STAT3, Nrf2, ROS, and COX-2, | Kunnumakkara et al., 2017 [258] |
| curcumin | cancer | inhibition of activation of Toll-like receptor 4 (TLR4) signaling pathway associated with inflammatory response and cancer progression | Chen et al., 2018 [260] |
| curcumin | intestinal inflammatory diseases, such as Crohn’s disease, ulcerative colitis, and necrotizing enterocolitis | improved intestinal barrier function, regulated the gut microbiota, exhibited antioxidant and anti-inflammatory effects | Burge et al., 2019 [261] |
| curcumin | cancer | potent antitumor activity by reversing epigenetic changes associated with oncogene activation and tumor suppressor gene inactivation | Carlos-Reyes et al., 2019 [262] |
| curcumin | colorectal adenoma | regulation of the Wnt/β-catenin pathway associated with colorectal cancer | Bahrami et al., 2017 [263] |
| curcumin | colorectal cancer | disruption of tumor growth signaling such as COX-2 enzyme expression, attenuation of NF-kB signaling, suppression of EGFR phosphorylation, inhibition of angiogenesis, and apoptosis of malignant cells | Adiwidjaja et al., 2017 [264] |
| curcumin | ulcerative colitis | reduced recurrence rates and maintained remission in patients with quiescent ulcerative colitis | Hanai et al., 2006 [265] |
| curcumin | Helicobacter pylori-infected gastritis | although treatment of H. pylori-infected patients with curcumin did not alter levels of inflammatory cytokine mRNA expression and had limited anti-bactericidal effect, it improved common symptoms in the patients | Koosirirat et al., 2010 [266] |
| curcumin | Helicobacter pylori-infected gastritis | significant amelioration of dyspeptic symptoms and attenuation of serologic signs of gastric inflammation were observed in H. pylori-positive patients with functional dyspepsia despite the lack of eradication of H. pylori | Mario et al., 2007 [267] |
| curcumin | gallstone disease | defense against biliary cholesterol supersaturation by modulating intestinal microbiota and inhibiting intestinal cholesterol absorption | Hong et al., 2022 [268] |
| curcumin | ulcerative colitis complicated by diabetes mellitus | effectively alleviated colitis in mice with type 2 diabetes by restoring Th17/Treg homeostasis and improving gut microbiota composition | Xiao et al., 2022 [269] |
| curcumin | intestinal inflammatory diseases | enhancement of the intestinal barrier, attenuation of intestinal apoptosis by suppressing the caspase-3 pathway, reduction in intestinal inflammation by inhibiting the MAPK/NFκB/STAT3 pathway, and amelioration of gut bacteria involved in colitis | Guo et al., 2022 [270] |
| curcumin | acute myeloid leukemia | promoted responses to cytarabine through modulation of the microbiota, highlighting the importance of enhancing gut integrity in chemoresistance therapy | Liu et al., 2022 [271] |
| curcumin | irritable bowel syndrome | significant improvement in gastrointestinal symptom rating scale and stress scale indicators | Lopresti et al., 2021 [272] |
| curcumin | Alzheimer’s disease | effects of curcumin-activated PPARγ on anti-neuroinflammatory and neuroprotective effects in Alzheimer’s disease | Liu et al., 2016 [273] |
| curcumin | Alzheimer’s disease | blocked amyloid-β aggregation and fibril formation in vitro and in vivo by directly binding curcumin to small beta-amyloid species | Yang et al., 2005 [274] |
| curcumin | Parkinson’s disease | effective inhibition of the toxic effects of MPP+ on SH-SY5Y cells, greatly attenuating the adverse effects of MPP+ on dopaminergic neurons via upregulation of HSP90 | Sang et al., 2018 [275] |
| curcumin/encapsulated | Huntington’s disease | amelioration of mitochondrial dysfunction and significant enhancement in neuromotor coordination | Sandhir et al., 2014 [276] |
| curcumin | amyotrophic lateral sclerosis (ALS) | amelioration of aerobic metabolism and oxidative damage, and slowed disease progression | Chico et al., 2018 [277] |
| curcumin | major depressive disorder | potency to modulate neurotransmitter levels, inflammatory pathways, excitotoxicity, neuroplasticity, hypothalamic–pituitary–adrenal disorders, insulin resistance, oxidative and nitrosative stress, and the endocannabinoid system | Ramaholimihaso et al., 2020 [278] |
| protocatechuic acid | cancer, hyperlipidemia, diabetes | potential to agent of antioxidant, antibacterial, anticancer, antihyperlipidemic, antidiabetic, and anti-inflammatory | Kakkar et al., 2014 [280] |
| protocatechuic acid | neurodegenerative disease, tumors, osteoporosis, liver disease, kidney disease, metabolic syndrome | regulation of oxidative stress and inflammatory responses via multiple signaling pathways | Song et al., 2020 [281] |
| protocatechuic acid/Du-Zhong | chronic hepatotoxicity | attenuation of liver lesions incidence | Hung et al., 2006 [282] |
| protocatechuic acid | Alzheimer’s disease, Parkinson’s disease | inhibition of β-amyloid plaque accumulation and tau hyperphosphorylation in brain tissue | Krzysztoforska et al., 2019 [286] |
| protocatechuic acid | NAFLD | regulation of glucose and lipid metabolism, oxidative stress, inflammation, gut microbiota, and metabolites, increase in energy expenditure of brown adipose tissue | Gao et al., 2021 [287] |
| protocatechuic acid | depression | maintained brain-derived neurotrophic factor levels and modulated oxidative stress responses, cytokine systems, and antioxidant defense systems in mice | Thakare et al., 2021 [288] |
| ellagic acid | inflammatory disease, neurodegenerative diseases | discovery of a novel bacterial strain capable of converting ellagic acid to isourolithin A with anti-inflammatory, anti-carcinogenic, cardioprotective, and neuroprotective properties | Selma et al., 2017 [292] |
| ellagic acid | subclinical necrotic enteritis of broiler caused by Clostridium perfringens | regulation of jejunal inflammatory signaling pathways TLR/NF-κB and JAK3/STAT6, alleviation of jejunal oxidative stress, inhibition of intestinal barrier damage, prevention of systemic inflammatory response | Tang et al., 2022 [293] |
| ellagic acid | multiple sclerosis | attenuation of astrogliosis, astrocyte activation, demyelination, neuroinflammation, and axonal damage via NLRP3 inflammasome and pyroptotic pathway | Kiasalari et al., 2021 [295] |
| ellagic acid | cognitive impairments, long-term potentiation deficits | significant prevention of traumatic brain injury-induced memory impairment and hippocampal long-term potentiation impairment | Farbood et al., 2015 [296] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumura, Y.; Kitabatake, M.; Kayano, S.-i.; Ito, T. Dietary Phenolic Compounds: Their Health Benefits and Association with the Gut Microbiota. Antioxidants 2023, 12, 880. https://doi.org/10.3390/antiox12040880
Matsumura Y, Kitabatake M, Kayano S-i, Ito T. Dietary Phenolic Compounds: Their Health Benefits and Association with the Gut Microbiota. Antioxidants. 2023; 12(4):880. https://doi.org/10.3390/antiox12040880
Chicago/Turabian StyleMatsumura, Yoko, Masahiro Kitabatake, Shin-ichi Kayano, and Toshihiro Ito. 2023. "Dietary Phenolic Compounds: Their Health Benefits and Association with the Gut Microbiota" Antioxidants 12, no. 4: 880. https://doi.org/10.3390/antiox12040880
APA StyleMatsumura, Y., Kitabatake, M., Kayano, S.-i., & Ito, T. (2023). Dietary Phenolic Compounds: Their Health Benefits and Association with the Gut Microbiota. Antioxidants, 12(4), 880. https://doi.org/10.3390/antiox12040880





