Aerobic Physical Training Attenuates Oxidative Stress in the Spinal Cord of Adult Rats Induced by Binge-like Ethanol Intake
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspects and Experimental Animals
2.2. Exposure Protocol and Experimental Groups
2.2.1. Physical Training Protocol
2.2.2. Drinking Protocol
2.3. Euthanasia and Spinal Cord Collection
2.4. Oxidative Biochemistry Analyses
2.4.1. Protein Concentration Assay
2.4.2. Measurement of Trolox Equivalent Antioxidant Capacity (TEAC)
2.4.3. Measurement of Reduced Glutathione (GSH)
2.4.4. Determination of Thiobarbituric Acid Reactive Substances (TBARS)
2.5. Morphometric Analysis
2.6. Statistical Analyses
3. Results
3.1. Body Weight Gain Was Not Influenced by Binge-like EtOH Intake and Physical Training
3.2. Regular Physical Training Attenuated EtOH-Induced Oxidative Stress in the Spinal Cord of Rats
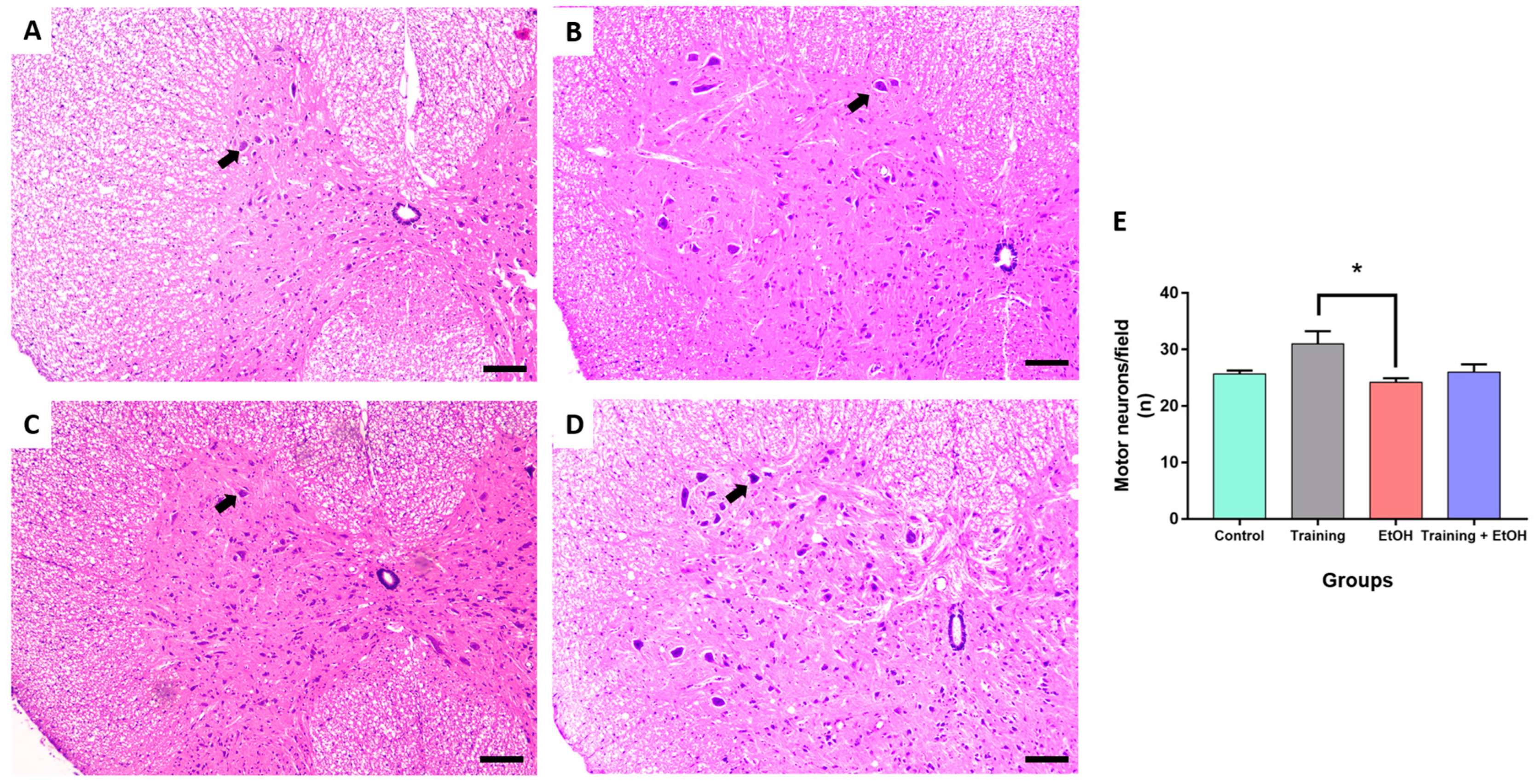
3.3. Regular Physical Training Did Not Prevent MN Density Reduction in the Cervical Segment Induced by Repeated Binge-like EtOH Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bitew, M.S.; Zewde, M.F.; Wubetu, M.; Alemu, A.A. Consumption of alcohol and binge drinking among pregnant women in Addis Ababa, Ethiopia: Prevalence and determinant factors. PLoS ONE 2020, 15, e0243784. [Google Scholar] [CrossRef] [PubMed]
- Donroe, J.H.; Edelman, E.J. Alcohol Use. Ann. Intern. Med. 2022, 175, ITC145–ITC160. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/alcohol (accessed on 28 February 2023).
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/alcohol/data-stats.htm#:~:text=According%20to%20the%20Behavioral%20Risk,drink%20heavily%20also%20binge%20drink (accessed on 28 February 2023).
- Schuckit, M.A. Alcohol-use disorders. Lancet 2009, 373, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Breese, G.R.; Overstreet, D.H.; Knapp, D.J. Conceptual framework for the etiology of alcoholism: A “kindling”/stress hypothesis. Psychopharmacology 2005, 178, 367–380. [Google Scholar] [CrossRef]
- Molina, P.E.; Nelson, S. Binge Drinking’s Effects on the Body. Alcohol Res. 2018, 39, 99–109. [Google Scholar] [PubMed]
- Clarren, S.K.; Bowden, D.M. Fetal alcohol syndrome: A new primate model for binge drinking and its relevance to human ethanol teratogenesis. J. Pediatr. 1982, 101, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health. 2006, 29, 245–254. [Google Scholar] [PubMed]
- Harrison, N.L.; Skelly, M.J.; Grosserode, E.K.; Lowes, D.C.; Zeric, T.; Phister, S.; Salling, M.C. Effects of acute alcohol on excitability in the CNS. Neuropharmacology 2017, 122, 36–45. [Google Scholar] [CrossRef]
- Peng, B.; Yang, Q.; Joshi, R.B.; Liu, Y.; Akbar, M.; Song, B.J.; Zhou, S.; Wang, X. Role of Alcohol Drinking in Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 2316. [Google Scholar] [CrossRef]
- Brewer, R.D.; Swahn, M.H. Binge drinking and violence. JAMA 2005, 294, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Herring, R.; Berridge, V.; Thom, B. Binge drinking: An exploration of a confused concept. J. Epidemiol. Community Health 2008, 62, 476–479. [Google Scholar] [CrossRef]
- Krieger, H.; Young, C.M.; Anthenien, A.M.; Neighbors, C. The Epidemiology of Binge Drinking Among College-Age Individuals in the United States. Alcohol Res. 2018, 39, 23–30. [Google Scholar]
- Guinle, M.I.B.; Sinha, R. The Role of Stress, Trauma, and Negative Affect in Alcohol Misuse and Alcohol Use Disorder in Women. Alcohol Res. 2020, 40, 05. [Google Scholar] [CrossRef]
- Kuntsche, E.; Kuntsche, S.; Thrul, J.; Gmel, G. Binge drinking: Health impact, prevalence, correlates and interventions. Psychol. Health 2017, 32, 976–1017. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Lueras, J.M.; Nagel, B.J. Effects of Binge Drinking on the Developing Brain. Alcohol Res. 2018, 39, 87–96. [Google Scholar] [PubMed]
- Dejong, K.; Olyaei, A.; Lo, J.O. Alcohol Use in Pregnancy. Clin. Obstet. Gynecol. 2019, 62, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Lamarão-Vieira, K.; Pamplona-Santos, D.; Nascimento, P.C.; Corrêa, M.G.; Bittencourt, L.O.; Dos Santos, S.M.; Cartágenes, S.C.; Fernandes, L.M.P.; Monteiro, M.C.; Maia, C.S.F.; et al. Physical Exercise Attenuates Oxidative Stress and Morphofunctional Cerebellar Damages Induced by the Ethanol Binge Drinking Paradigm from Adolescence to Adulthood in Rats. Oxid. Med. Cell. Longev. 2019, 2019, 6802424. [Google Scholar] [CrossRef] [PubMed]
- Currie, C.L.; Sanders, J.L.; Swanepoel, L.M.; Davies, C.M. Maternal adverse childhood experiences are associated with binge drinking during pregnancy in a dose-dependent pattern: Findings from the All Our Families cohort. Child. Abuse Negl. 2020, 101, 104348. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.O.; Aragão, W.A.B.; Bittencourt, L.O.; Fernandes, L.P.M.; Balbinot, K.M.; Alves-Junior, S.M.; Pinheiro, J.J.V.; Maia, C.D.S.F.; Crespo-Lopez, M.E.; Lima, R.R. Ethanol binge drinking during pregnancy and its effects on salivary glands of offspring rats: Oxidative stress, morphometric changes and salivary function impairments. BioMed Pharmacother. 2021, 133, 110979. [Google Scholar] [CrossRef]
- Fagundes, N.C.; Fernandes, L.M.; Paraense, R.S.; de Farias-Junior, P.M.; Teixeira, F.B.; Alves-Junior, S.M.; Pinheiro, J.J.; Crespo-López, M.E.; Maia, C.S.; Lima, R.R. Binge Drinking of Ethanol during Adolescence Induces Oxidative Damage and Morphological Changes in Salivary Glands of Female Rats. Oxid. Med. Cell. Longev. 2016, 2016, 7323627. [Google Scholar] [CrossRef]
- Teixeira, F.B.; Santana, L.N.; Bezerra, F.R.; De Carvalho, S.; Fontes-Júnior, E.A.; Prediger, R.D.; Crespo-López, M.E.; Maia, C.S.; Lima, R.R. Chronic ethanol exposure during adolescence in rats induces motor impairments and cerebral cortex damage associated with oxidative stress. PLoS ONE 2014, 9, e101074. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.M.P.; Cartágenes, S.C.; Barros, M.A.; Carvalheiro, T.C.V.S.; Castro, N.C.F.; Schamne, M.G.; Lima, R.R.; Prediger, R.D.; Monteiro, M.C.; Fontes-Júnior, E.A.; et al. Repeated cycles of binge-like ethanol exposure induce immediate and delayed neurobehavioral changes and hippocampal dysfunction in adolescent female rats. Behav. Brain Res. 2018, 350, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Montesinos, J.; Marcos, M.; Torres, J.L.; Costa-Alba, P.; García-García, F.; Laso, F.J.; Guerri, C. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict. Biol. 2017, 22, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Frazão, D.R.; Maia, C.D.S.F.; Chemelo, V.D.S.; Monteiro, D.; Ferreira, R.O.; Bittencourt, L.O.; Balbinot, G.S.; Collares, F.M.; Rösing, C.K.; Martins, M.D.; et al. Ethanol binge drinking exposure affects alveolar bone quality and aggravates bone loss in experimentally-induced periodontitis. PLoS ONE 2020, 15, e0236161. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.M.; Mohn, C.E.; Burdet, B.; Zorrilla Zubilete, M.; Mandalunis, P.M.; Elverdin, J.C.; Fernández-Solari, J. Ethanol consumption enhances periodontal inflammatory markers in rats. Arch. Oral Biol. 2012, 57, 1211–1217. [Google Scholar] [CrossRef]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Hernández, J.A.; López-Sánchez, R.C.; Rendón-Ramírez, A. Lipids and Oxidative Stress Associated with Ethanol-Induced Neurological Damage. Oxidative Med. Cell. Longev. 2016, 2016, 1543809. [Google Scholar] [CrossRef]
- Shen, X.; Li, A.; Zhang, Y.; Dong, X.; Shan, T.; Wu, Y.; Jia, J.; Hu, Y. The effect of different intensities of treadmill exercise on cognitive function deficit following a severe controlled cortical impact in rats. Int. J. Mol. Sci. 2013, 14, 21598–21612. [Google Scholar] [CrossRef]
- Bernardi, C.; Tramontina, A.C.; Nardin, P.; Biasibetti, R.; Costa, A.P.; Vizueti, A.F.; Batassini, C.; Tortorelli, L.S.; Wartchow, K.M.; Dutra, M.F.; et al. Treadmill exercise induces hippocampal astroglial alterations in rats. Neural Plast. 2013, 2013, 709732. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Chinn, G.; Chou, M.; Kesslak, J.P.; Cotman, C.W. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience 2005, 133, 853–861. [Google Scholar] [CrossRef]
- Farzi, M.A.; Sadigh-Eteghad, S.; Ebrahimi, K.; Talebi, M. Exercise Improves Recognition Memory and Acetylcholinesterase Activity in the Beta Amyloid-Induced Rat Model of Alzheimer’s Disease. Ann. Neurosci. 2019, 25, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Hueston, C.M.; Cryan, J.F.; Nolan, Y.M. Adolescent social isolation stress unmasks the combined effects of adolescent exercise and adult inflammation on hippocampal neurogenesis and behavior. Neuroscience 2017, 365, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Gomes da Silva, S.; Simões, P.S.; Mortara, R.A.; Scorza, F.A.; Cavalheiro, E.A.; da Graça Naffah-Mazzacoratti, M.; Arida, R.M. Exercise-induced hippocampal anti-inflammatory response in aged rats. J. Neuroinflammation 2013, 10, 61. [Google Scholar] [CrossRef]
- Louzada, R.A.; Bouviere, J.; Matta, L.P.; Werneck-de-Castro, J.P.; Dupuy, C.; Carvalho, D.P.; Fortunato, R.S. Redox Signaling in Widespread Health Benefits of Exercise. Antioxid. Redox Signal. 2020, 33, 745–760. [Google Scholar] [CrossRef]
- de Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simões, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef]
- Pamplona-Santos, D.; Lamarão-Vieira, K.; Nascimento, P.C.; Bittencourt, L.O.; Corrêa, M.G.; Dos Santos, S.M.; Cartágenes, S.C.; Fernandes, L.M.P.; Monteiro, M.C.; Maia, C.S.F.; et al. Aerobic Physical Exercise as a Neuroprotector Strategy for Ethanol Binge-Drinking Effects in the Hippocampus and Systemic Redox Status in Rats. Oxid. Med. Cell. Longev. 2019, 2019, 2415243. [Google Scholar] [CrossRef]
- Kramer, A.F.; Hahn, S.; Cohen, N.J.; Banich, M.T.; McAuley, E.; Harrison, C.R.; Chason, J.; Vakil, E.; Bardell, L.; Boileau, R.A.; et al. Ageing, fitness and neurocognitive function. Nature 1999, 29, 418–419. [Google Scholar] [CrossRef]
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Erol, A.; Karpyak, V.M. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015, 156, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Arida, R.M.; Scorza, F.A.; de Lacerda, A.F.; Gomes da Silva, S.; Cavalheiro, E.A. Physical training in developing rats does not influence the kindling development in the adult life. Physiol. Behav. 2007, 16, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, L.Y.; de Oliveira, I.G.; Cartágenes, S.C.; Fernandes, L.M.P.; Dos Santos, S.M.; Ferreira, W.A.S.; Mello Junior, F.A.R.; Bittencourt, L.O.; Paiva, E.B.C.; Burbano, R.M.R.; et al. Repeated Cycles of Binge-Like Ethanol Exposure Induces Neurobehavioral Changes During Short- and Long-Term Withdrawal in Adolescent Female Rats. Oxid. Med. Cell. Longev. 2022, 2022, 7207755. [Google Scholar] [CrossRef]
- Rocha, E.G.; Santiago, L.F.; Freire, M.A.M.; Gomes-Leal, W.; Lent, R.; Houzel, J.C.; Franca, J.G.; Pereira, A., Jr.; Picanço-Diniz, C.W. Callosal axon arbors in the limb representations of the somatosensory cortex (SI) in the agouti (Dasyprocta primnolopha). J. Comp. Neurol. 2007, 500, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Eiró-Quirino, L.; Lima, W.F.; Aragão, W.A.B.; Bittencourt, L.O.; Mendes, P.F.S.; Fernandes, R.M.; Rodrigues, C.A.; Dionízio, A.; Buzalaf, M.A.R.; Monteiro, M.C.; et al. Exposure to tolerable concentrations of aluminum triggers systemic and local oxidative stress and global proteomic modulation in the spinal cord of rats. Chemosphere 2023, 313, 137296. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Percario, S.; Vital, A.C.C.; Jablonka, F. Dosagem do malondialdeido. Newslab 1994, 2, 46–50. [Google Scholar]
- Ferrucci, M.; Lazzeri, G.; Flaibani, M.; Biagioni, F.; Cantini, F.; Madonna, M.; Bucci, D.; Limanaqi, F.; Soldani, P.; Fornai, F. In search for a gold-standard procedure to count motor neurons in the spinal cord. Histol. Histopathol. 2018, 33, 1021–1046. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1998. [Google Scholar]
- Gois, A.M.; Mendonça, D.M.F.; Freire, M.A.M.; Santos, J.R. In vitro and in vivo models of Amyotrophic Lateral Sclerosis: An updated review. Brain Res. Bull. 2020, 159, 32–43. [Google Scholar] [CrossRef]
- Bican, O.; Minagar, A.; Pruitt, A.A. The spinal cord: A review of functional neuroanatomy. Neurol. Clin. 2013, 31, 1–18. [Google Scholar] [CrossRef]
- Ganau, M.; Zewude, R.; Fehlings, M.G. Functional anatomy of the spinal cord. In Degenerative Cervical Myelopathy and Radiculopathy: Treatment Approaches and Options, 1st ed.; Kaiser, M.G., Shaffrey, C.I., Fehling, M.G., Eds.; Springer Nature: Cham, Switzerland, 2019; Volume 1, pp. 3–12. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA council approves definition of binge drinking. NIAAA Newsl. 2004, 3, 3. [Google Scholar]
- Kamal, H.; Tan, G.C.; Ibrahim, S.F.; Shaikh, M.F.; Mohamed, I.N.; Mohamed, R.M.P.; Hamid, A.A.; Ugusman, A.; Kumar, J. Alcohol Use Disorder, Neurodegeneration, Alzheimer’s and Parkinson’s Disease: Interplay Between Oxidative Stress, Neuroimmune Response and Excitotoxicity. Front. Cell. Neurosci. 2020, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Han, J.; Lee, C.; Yoon, M.; Jung, Y. Pathophysiological Aspects of Alcohol Metabolism in the Liver. Int. J. Mol. Sci. 2021, 22, 5717. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, H.F.J. Alcohol and Human Health: What Is the Evidence? Annu. Rev. Food Sci. Technol. 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Hernández, S.; Velázquez-Fernández, J.B.; Díaz-Chávez, J.; López-Sánchez, R.C.; Hernández, J.A.; Rendón-Ramírez, A. Alcoholism: Common and Oxidative Damage Biomarkers. J. Clin. Toxicol. 2014, 0. [Google Scholar] [CrossRef]
- Tapia-Rojas, C.; Mira, R.G.; Torres, A.K.; Jara, C.; Pérez, M.J.; Vergara, E.H.; Cerpa, W.; Quintanilla, R.A. Alcohol consumption during adolescence: A link between mitochondrial damage and ethanol brain intoxication. Birth Defects Res. 2017, 109, 1623–1639. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Pereira, M.C.; Santana, L.N.; Fernandes, R.M.; Teixeira, F.B.; Oliveira, G.B.; Fernandes, L.M.; Fontes-Júnior, E.A.; Prediger, R.D.; Crespo-López, M.E.; et al. Chronic ethanol exposure during adolescence through early adulthood in female rats induces emotional and memory deficits associated with morphological and molecular alterations in hippocampus. J. Psychopharmacol. 2015, 29, 712–724. [Google Scholar] [CrossRef]
- David, P.; Subramaniam, K. The Effects of Prenatal Alcohol Exposure on the Morphological Characteristics of Spinal Motoneurons. Birth Def. Res. (Part A) 2009, 85, 791–799. [Google Scholar] [CrossRef]
- Tsermpini, E.E.; Iljes, A.P.; Dolzan, V. Alcohol-Induced Oxidative Stress and the Role of Antioxidants in Alcohol Use Disorder: A Systematic Review. Antioxidants. Antioxidants 2022, 11, 1374. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Díaz-Soto, M.T.; Calderín-Miranda, J.M. Alcohol withdrawal syndrome: Result of oxidative stress and neuronal imbalance. State of the art. Rev. Biomédica 2020, 31, 95–107. [Google Scholar]
- Pingitore, A.; Lima, G.P.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.C.L.; Duarte, R.B.; Sousa, T.B.; Santos, J.R.; Freire, M.A.M.; Costa, M.S.M.O. Expression of the immediate-early gene egr-1 and substance P in the spinal cord following locomotor training in adult rats. Brain Res. 2010, 1345, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.-H.; Shih, C.-T.; Ching, C.-H.; Huang, J.-Y.; Jen, C.J.; Yu, L.; Kuo, Y.-M.; Wu, F.-S.; Chuang, J.-I. Treadmill exercise activates Nrf2 antioxidant system to protect the nigrostriatal dopaminergic neurons from MPP+ toxicity. Exp. Neurol. 2015, 263, 50–62. [Google Scholar] [CrossRef]
- Chew, C.; Sengelaub, D.R. Neuroprotective Effects of Exercise on the Morphology of Somatic Motoneurons Following the Death of Neighboring Motoneurons. Neurorehabilit. Neural Repair 2019, .33, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.C.; Ferreira, R.O.; Frazão, D.R.; de Souza Né, Y.G.; Mendes, P.F.S.; Marañón-Vásquez, G.; Royes, L.F.F.; Fagundes, N.C.F.; Maia, L.C.; Lima, R.R. The Relationship between Exercise and Salivary Oxidative Stress: A Systematic Review. Antioxidants 2022, 11, 1489. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J Sport. Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Chen, Y.; Singh, S.; Matsumoto, A.; Manna, S.K.; Abdelmegeed, M.A.; Golla, S.; Murphy, R.C.; Dong, H.; Song, B.J.; Gonzalez, F.J.; et al. Chronic Glutathione Depletion Confers Protection against Alcohol-induced Steatosis: Implication for Redox Activation of AMP-activated Protein Kinase Pathway. Sci. Rep. 2016, 6, 29743. [Google Scholar] [CrossRef]
- Tsikas, D. Handling of commercially available enzyme immunoassays for 8-iso-prostaglandin F2alpha (8-iso-PGF2alpha, iPF2alpha-III, 15-F2t-IsoP) in clinical research and science: Considerations from the analytical and review point of view. Clin. Chim. Acta 2004, 344, 215–217. [Google Scholar] [CrossRef]
- Sindona, C.; Schepici, G.; Contestabile, V.; Bramanti, P.; Mazzon, E. NOX2 Activation in COVID-19: Possible Implications for Neurodegenerative Diseases. Medicina 2021, 57, 604. [Google Scholar] [CrossRef]
- Hernandes, M.S.; D’Avila, J.C.; Trevelin, S.C.; Reis, P.A.; Kinjo, E.R.; Lopes, L.R.; Castro-Faria-Neto, H.C.; Cunha, F.Q.; Britto, L.R.; Bozza, F.A. The role of Nox2-derived ROS in the development of cognitive impairment after sepsis. J. Neuroinflammation 2014, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, A.M.; Goltash, S.; Lalonde, N.R.; Bui, T.V. Propriospinal Neurons: Essential Elements of Locomotor Control in the Intact and Possibly the Injured Spinal Cord. Front. Cell. Neurosci. 2019, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.R.; Graham, B.A.; Galea, M.P.; Callister, R.J. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology 2011, 60, 809–822. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.d.N.; da Silva, D.C.B.; Baia-da-Silva, D.C.; Mendes, P.F.S.; Ferreira, M.K.M.; Rocha, G.S.; Freire, M.A.M.; Fernandes, L.M.P.; Maia, C.d.S.F.; Gomes-Leal, W.; et al. Aerobic Physical Training Attenuates Oxidative Stress in the Spinal Cord of Adult Rats Induced by Binge-like Ethanol Intake. Antioxidants 2023, 12, 1051. https://doi.org/10.3390/antiox12051051
Rodrigues AdN, da Silva DCB, Baia-da-Silva DC, Mendes PFS, Ferreira MKM, Rocha GS, Freire MAM, Fernandes LMP, Maia CdSF, Gomes-Leal W, et al. Aerobic Physical Training Attenuates Oxidative Stress in the Spinal Cord of Adult Rats Induced by Binge-like Ethanol Intake. Antioxidants. 2023; 12(5):1051. https://doi.org/10.3390/antiox12051051
Chicago/Turabian StyleRodrigues, Amanda do Nascimento, Diane Cleydes Baia da Silva, Daiane Claydes Baia-da-Silva, Paulo Fernando Santos Mendes, Maria Karolina Martins Ferreira, Gabriel Sousa Rocha, Marco Aurelio M. Freire, Luanna Melo Pereira Fernandes, Cristiane do Socorro Ferraz Maia, Walace Gomes-Leal, and et al. 2023. "Aerobic Physical Training Attenuates Oxidative Stress in the Spinal Cord of Adult Rats Induced by Binge-like Ethanol Intake" Antioxidants 12, no. 5: 1051. https://doi.org/10.3390/antiox12051051
APA StyleRodrigues, A. d. N., da Silva, D. C. B., Baia-da-Silva, D. C., Mendes, P. F. S., Ferreira, M. K. M., Rocha, G. S., Freire, M. A. M., Fernandes, L. M. P., Maia, C. d. S. F., Gomes-Leal, W., & Lima, R. R. (2023). Aerobic Physical Training Attenuates Oxidative Stress in the Spinal Cord of Adult Rats Induced by Binge-like Ethanol Intake. Antioxidants, 12(5), 1051. https://doi.org/10.3390/antiox12051051








