Integral Valorization of Grape Pomace for Antioxidant Pickering Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. BCNC Production
2.2.1. BC Biosynthesis
2.2.2. BC Hydrolysis
2.3. BCNC Physicochemical and thermal Characterization
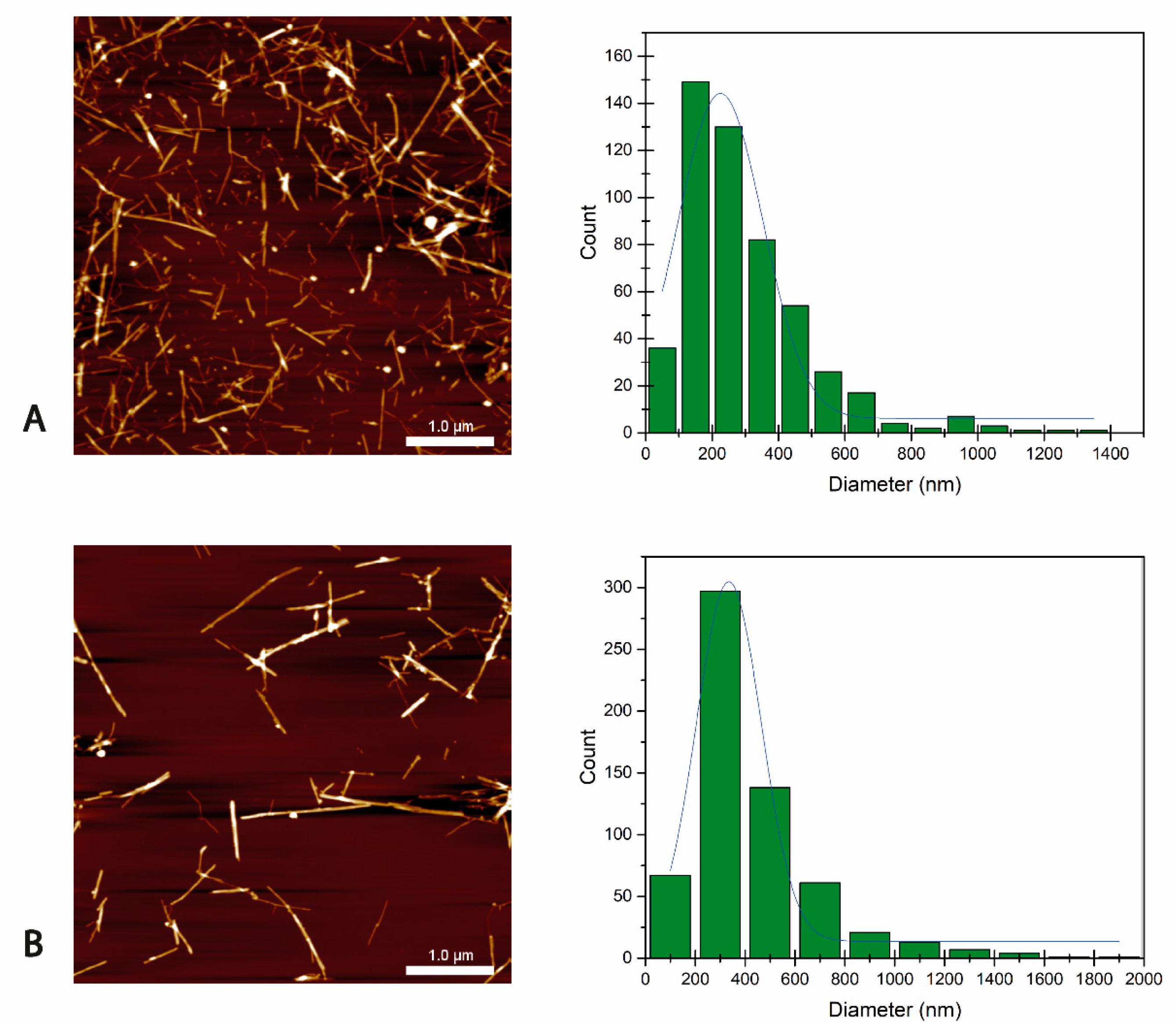
2.3.1. Atomic Force Microscopy (AFM)
2.3.2. X-ray Diffraction (XRD)
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. Elemental Analysis (EA)
2.4. GPPE Extraction and Characterization
2.4.1. Ultrasound-Assisted Polyphenol Extraction
2.4.2. Ultra-High-Performance Liquid Chromatography (UHPLC)
2.4.3. Mass Spectrometry (MS)
2.5. BCNC-GPPE Complex Preparation and Characterization
2.5.1. BCNC-GPPE Complex Preparation
2.5.2. Dynamic Light Scattering (DLS)
2.5.3. UV–Visible Spectrophotometry (UV-vis)—Antioxidant Activity Assay
2.6. Pickering Emulsions
2.6.1. Oxidative Stability
2.6.2. Physical Stability
2.6.3. Rheological Properties
2.7. Statistical Analysis
3. Results and Discussion
3.1. BCNC Production and Characterization
3.1.1. BCNC Particle Size Distribution
3.1.2. BCNC Characterization
3.2. GPPE Extraction and Characterization
3.3. BCNC-GPPE Complex Characterization
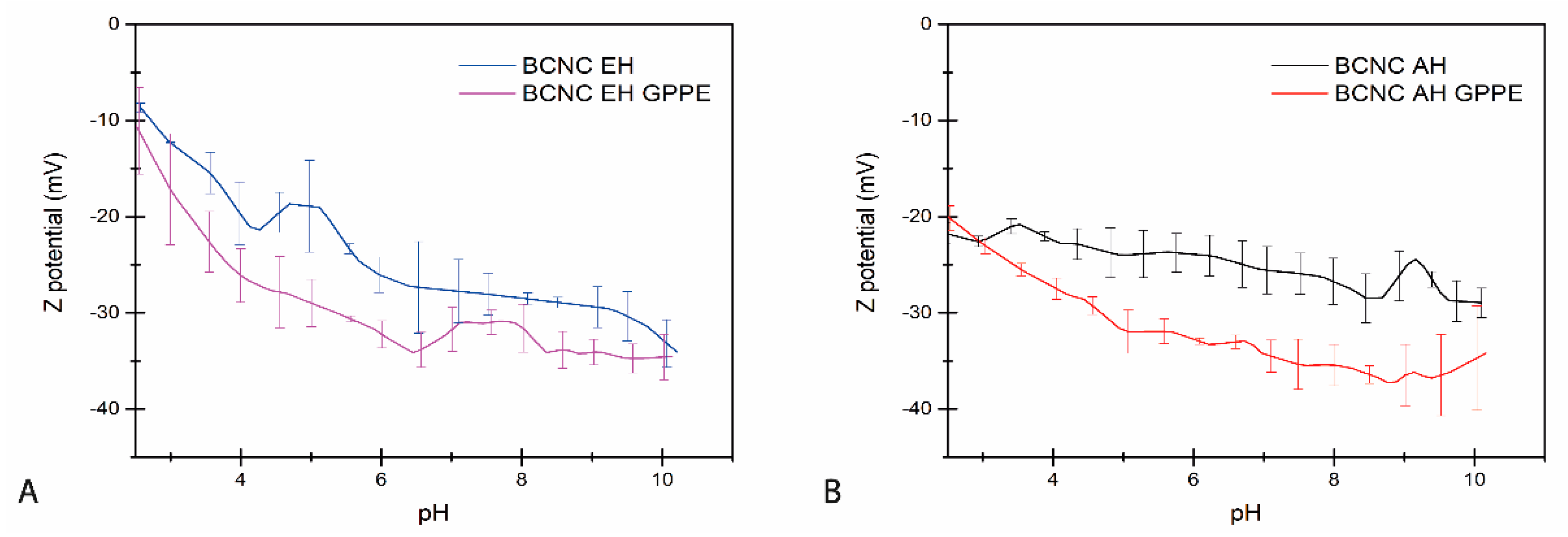
3.3.1. Z Potential
3.3.2. Antioxidant Activity Assays
3.4. Pickering Emulsion Characterization
3.4.1. Oxidative Stability
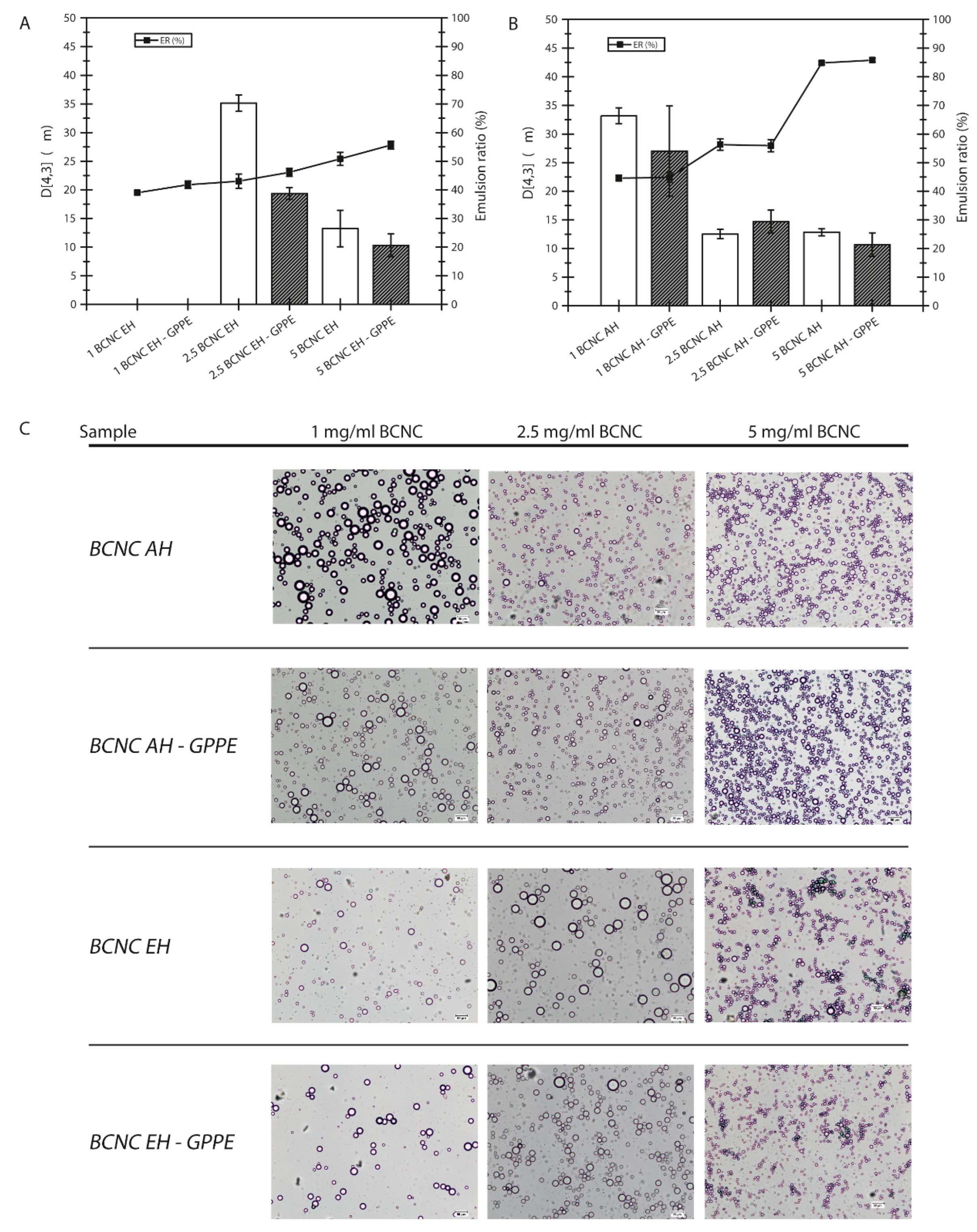
3.4.2. Physical Stability
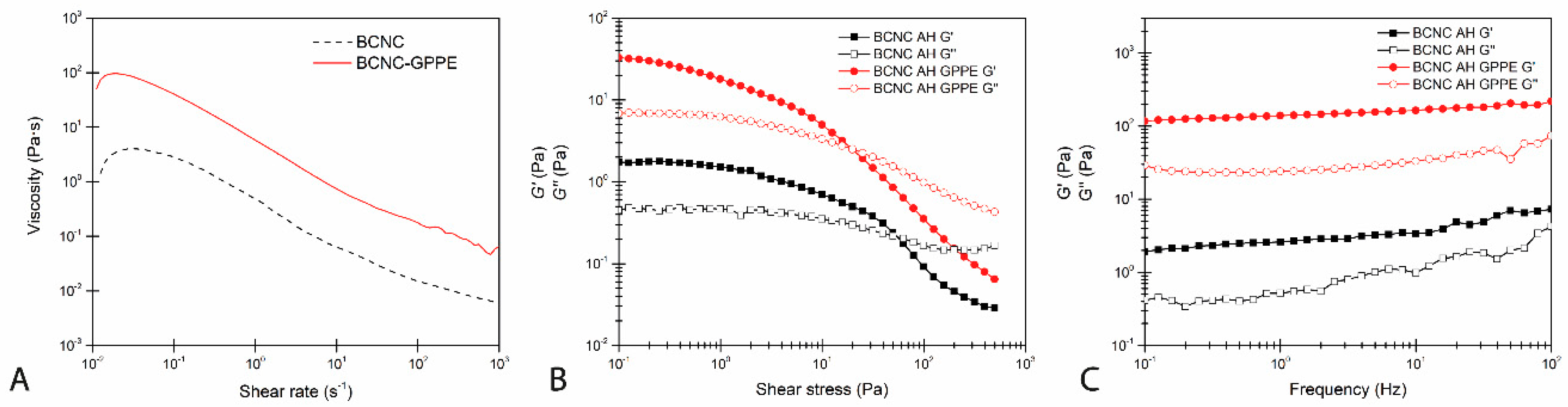
3.4.3. Rheological Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dickinson, E. Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocoll. 2017, 68, 219–231. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Wu, Y.; Zhang, X.; Qiu, D.; Pei, Y.; Li, Y.; Li, B.; Liu, S. Effect of surface charge density of bacterial cellulose nanofibrils on the rheology property of O/W Pickering emulsions. Food Hydrocoll. 2021, 120, 106944. [Google Scholar] [CrossRef]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent advances on cellulose nanocrystals for Pickering emulsions: Development and challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Nie, C.; Bu, X.; Ma, S.; Zhang, J.; Ma, Q.; Li, W.; Zhang, X.; Wu, H.; Hu, S.; Fan, G.; et al. Pickering emulsions synergistically stabilized by cellulose nanocrystals and peanut protein isolate. LWT 2022, 167, 113884. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; da Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; de Souza Filho, M.D.S.M.; Rosa, M.D.F. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef]
- Rovera, C.; Fiori, F.; Trabattoni, S.; Romano, D.; Farris, S. Enzymatic hydrolysis of bacterial cellulose for the production of nanocrystals for the food packaging industry. Nanomaterials 2020, 10, 735. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Karim, Z.; Afrin, S.; Husain, Q.; Danish, R. Necessity of enzymatic hydrolysis for production and functionalization of nanocelluloses. Crit. Rev. Biotechnol. 2017, 37, 355–370. [Google Scholar] [CrossRef]
- Bai, L.; Greca, L.G.; Xiang, W.; Lehtonen, J.; Huan, S.; Nugroho, R.W.N.; Tardy, B.L.; Rojas, O.J. Adsorption and Assembly of Cellulosic and Lignin Colloids at Oil/Water Interfaces. Langmuir 2019, 35, 571–588. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Song, H.; Li, J.; Feng, Y.; Shi, Z.; Wang, X.; Lin, Q. Synthesis of bacterial cellulose and bacterial cellulose nanocrystals for their applications in the stabilization of olive oil pickering emulsion. Food Hydrocoll. 2017, 72, 127–135. [Google Scholar] [CrossRef]
- Parajuli, S.; Ureña-Benavides, E.E. Fundamental aspects of nanocellulose stabilized Pickering emulsions and foams. Adv. Colloid Interface Sci. 2022, 299, 102530. [Google Scholar] [CrossRef]
- Paximada, P.; Koutinas, A.A.; Scholten, E.; Mandala, I.G. Effect of bacterial cellulose addition on physical properties of WPI emulsions. Comparison with common thickeners. Food Hydrocoll. 2016, 54, 245–254. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Berry, R.M.; Pelton, R.; Cranston, E.D. One-Pot Water-Based Hydrophobic Surface Modification of Cellulose Nanocrystals Using Plant Polyphenols. ACS Sustain. Chem. Eng. 2017, 5, 5018–5026. [Google Scholar] [CrossRef]
- Dai, T.; Li, T.; Li, R.; Zhou, H.; Liu, C.; Chen, J.; McClements, D.J. Utilization of plant-based protein-polyphenol complexes to form and stabilize emulsions: Pea proteins and grape seed proanthocyanidins. Food Chem. 2020, 329, 127219. [Google Scholar] [CrossRef]
- Tong, Q.; Yi, Z.; Ran, Y.; Chen, X.; Chen, G.; Li, X. Green Tea Polyphenol-Stabilized Gel-Like High Internal Phase Pickering Emulsions. ACS Sustain. Chem. Eng. 2021, 9, 4076–4090. [Google Scholar] [CrossRef]
- Fernandes, I.D.A.A.; Maciel, G.M.; Ribeiro, V.R.; Rossetto, R.; Pedro, A.C.; Haminiuk, C.W.I. The role of bacterial cellulose loaded with plant phenolics in prevention of UV-induced skin damage. Carbohydr. Polym. Technol. Appl. 2021, 2, 100122. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Li, Z.; Hu, W.; Dong, J.; Azi, F.; Xu, X.; Tu, C.; Tang, S.; Dong, M. The use of bacterial cellulose from kombucha to produce curcumin loaded Pickering emulsion with improved stability and antioxidant properties. Food Sci. Hum. Wellness 2023, 12, 669–679. [Google Scholar] [CrossRef]
- Vukoja, J.; Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Formulation and stability of cellulose-based delivery systems of raspberry phenolics. Processes 2021, 9, 90. [Google Scholar] [CrossRef]
- Asante, B.; Sirviö, J.A.; Li, P.; Lavola, A.; Julkunen-Tiitto, R.; Haapala, A.; Liimatainen, H. Adsorption of bark derived polyphenols onto functionalized nanocellulose: Equilibrium modeling and kinetics. AIChE J. 2020, 66, e16823. [Google Scholar] [CrossRef]
- Yi, J.; Qiu, M.; Liu, N.; Tian, L.; Zhu, X.; Decker, E.A.; McClements, D.J. Inhibition of Lipid and Protein Oxidation in Whey-Protein-Stabilized Emulsions Using a Natural Antioxidant: Black Rice Anthocyanins. J. Agric. Food Chem. 2020, 68, 10149–10156. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Matei, C.; Deaconu, M.; Stanciuc, A.M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef] [PubMed]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.L. Characterization of polyphenols and antioxidant potential of white grape pomace byproducts (Vitis vinifera L.). J. Agric. Food Chem. 2013, 61, 11579–11587. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Castro, C.; Cleenwerck, I.; Trček, J.; Zuluaga, R.; de Vos, P.; Caro, G.; Aguirre, R.; Putaux, J.L.; Gañán, P. Gluconacetobacter medellinensis sp. nov., cellulose- and non-cellulose-producing acetic acid bacteria isolated from vinegar. Int. J. Syst. Evol. Microbiol. 2013, 63, 1119–1125. [Google Scholar] [CrossRef]
- Diaz-Ramirez, J.; Urbina, L.; Eceiza, A.; Retegi, A.; Gabilondo, N. Superabsorbent bacterial cellulose spheres biosynthesized from winery by-products as natural carriers for fertilizers. Int. J. Biol. Macromol. 2021, 191, 1212–1220. [Google Scholar] [CrossRef]
- George, J.; Ramana, K.V.; Bawa, A.S. Bacterial cellulose nanocrystals exhibiting high thermal stability and their polymer nanocomposites. Int. J. Biol. Macromol. 2011, 48, 50–57. [Google Scholar] [CrossRef]
- Rovera, C.; Ghaani, M.; Santo, N.; Trabattoni, S.; Olsson, R.T.; Romano, D.; Farris, S. Enzymatic Hydrolysis in the Green Production of Bacterial Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2018, 6, 7725–7734. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Abad-García, B.; Berrueta, L.A.; Garmón-Lobato, S.; Gallo, B.; Vicente, F. A general analytical strategy for the characterization of phenolic compounds in fruit juices by high-performance liquid chromatography with diode array detection coupled to electrospray ionization and triple quadrupole mass spectrometry. J. Chromatogr. A 2009, 1216, 5398–5415. [Google Scholar] [CrossRef] [PubMed]
- Natolino, A.; Da Porto, C. Kinetic models for conventional and ultrasound assistant extraction of polyphenols from defatted fresh and distilled grape marc and its main components skins and seeds. Chem. Eng. Res. Des. 2020, 156, 1–12. [Google Scholar] [CrossRef]
- Ventura-Aguilar, R.I.; Bautista-Baños, S.; Flores-García, G.; Zavaleta-Avejar, L. Impact of chitosan based edible coatings functionalized with natural compounds on Colletotrichum fragariae development and the quality of strawberries. Food Chem. 2018, 262, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Liang, D.; Zou, Y.; Li, P.; Ma, F. Phenolic compounds and antioxidant activity in red-fleshed apples. J. Funct. Foods 2015, 18, 1086–1094. [Google Scholar] [CrossRef]
- Diñeiro García, Y.; Valles, B.S.; Picinelli Lobo, A. Phenolic and antioxidant composition of by-products from the cider industry: Apple pomace. Food Chem. 2009, 117, 731–738. [Google Scholar] [CrossRef]
- Costa, M.; Freiría-Gándara, J.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Effects of droplet size on the interfacial concentrations of antioxidants in fish and olive oil-in-water emulsions and nanoemulsions and on their oxidative stability. J. Colloid Interface Sci. 2020, 562, 352–362. [Google Scholar] [CrossRef]
- Lisete-Torres, P.; Losada-Barreiro, S.; Albuquerque, H.; Sánchez-Paz, V.; Paiva-Martins, F.; Bravo-Díaz, C. Distribution of hydroxytyrosol and hydroxytyrosol acetate in olive oil emulsions and their antioxidant efficiency. J. Agric. Food Chem. 2012, 60, 7318–7325. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef]
- Jia, Y.; Zhai, X.; Fu, W.; Liu, Y.; Li, F.; Zhong, C. Surfactant-free emulsions stabilized by tempo-oxidized bacterial cellulose. Carbohydr. Polym. 2016, 151, 907–915. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.H. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.; De Souza, L.; Carminatti, C.; Recouvreux, D. Production with a High Yield of Bacterial Cellulose Nanocrystals by Enzymatic Hydrolysis. Int. J. Nanosci. 2020, 19, 1950015. [Google Scholar] [CrossRef]
- Wen, C.; Yuan, Q.; Liang, H.; Vriesekoop, F. Preparation and stabilization of d -limonene Pickering emulsions by cellulose nanocrystals. Carbohydr. Polym. 2014, 112, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Revol, J.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspensión. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Urbina, L.; Corcuera, M.Á.; Eceiza, A.; Retegi, A. Stiff all-bacterial cellulose nanopaper with enhanced mechanical and barrier properties. Mater. Lett. 2019, 246, 67–70. [Google Scholar] [CrossRef]
- Martelli-tosi, M.; Torricillas, S.; Martins, M.A.; Benedito, O.; De Assis, G.; Tapia-blácido, D.R. Using Commercial Enzymes to Produce Cellulose Nanofibers from Soybean Straw. J. Nanomater. 2016, 2016, 8106814. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Gong, J.; Kuang, Y.; Mo, L.; Song, T. Cellulose nanocrystals (CNCs) with different crystalline allomorph for oil in water Pickering emulsions. Carbohydr. Polym. 2018, 183, 303–310. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, S.; Bei, W.; Zahi, M.R.; Yuan, Q.; Liang, H. Preparation and antimicrobial activity of oregano essential oil Pickering emulsion stabilized by cellulose nanocrystals. Int. J. Biol. Macromol. 2018, 112, 7–13. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Martínez-sanz, M.; Lopez-rubio, A.; Lagaron, J.M. Optimization of the nanofabrication by acid hydrolysis of bacterial cellulose nanowhiskers. Carbohydr. Polym. 2011, 85, 228–236. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of Sulfate Groups from Sulfuric Acid Hydrolysis on the Thermal Degradation Behavior of Bacterial Cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Elejalde, E.; Lopez-de-armentia, I.; Ram, D.; Murillo, R.; Mar, R. Study of Unpicked Grapes Valorization: A Natural Source of Polyphenolic Compounds and Evaluation of Their Antioxidant Capacity. Resources 2022, 11, 33. [Google Scholar] [CrossRef]
- Aizpurua-olaizola, O.; Ormazabal, M.; Vallejo, A.; Olivares, M.; Navarro, P.; Etxebarria, N. Optimization of Supercritical Fluid Consecutive Extractions of Fatty Acids and Polyphenols from Vitis Vinifera Grape Wastes. J. Food Sci. 2015, 80, E101–E107. [Google Scholar] [CrossRef] [PubMed]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Rusjan, D.; Koro, Z. A Comparison of Extraction Methods for Selected Phenolic Compounds from Grape Berry Skins Using Liquid Chromatography and Spectrophotometry. Acta Chim. Slov. 2007, 54, 114–118. [Google Scholar]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From winery waste to bioactive compounds and new polymeric biocomposites: A contribution to the circular economy concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Maleti, E. Extraction Methods of Polyphenol From Grapes: Extractions of Grape Polyphenols. In Polyphenols in Plants; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Meirelles, A.A.D.; Costa, A.L.R.; Cunha, R.L. Cellulose nanocrystals from ultrasound process stabilizing O/W Pickering emulsion. Int. J. Biol. Macromol. 2020, 158, 75–84. [Google Scholar] [CrossRef]
- Chenglin, Y.; Yiqun, Y.; Ye, Z.; Na, L.; Xiaoya, L.; Jing, L.; Ming, J. Self-Assembly and Emulsification of Poly{[styrene-alt-maleic acid]-co-[styrene-alt-(N-3,4-dihydroxyphenylethyl-maleamic acid)]}. Langmuir 2012, 28, 9211–9222. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Wang, D.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 2015, 171, 388–396. [Google Scholar] [CrossRef]
- Hu, Z.; Patten, T.; Pelton, R.; Cranston, E.D. Synergistic Stabilization of Emulsions and Emulsion Gels with Water-Soluble Polymers and Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2015, 3, 1023–1031. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Navarro, P.; Vallejo, A.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Microencapsulation and storage stability of polyphenols from Vitis vinifera grape wastes. Food Chem. 2016, 190, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, M.; Ni, Y. Use of sulfated cellulose nanocrystals towards stability enhancement of gelatin-encapsulated tea polyphenols. Cellulose 2018, 25, 5157–5173. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, S.; Ma, M.; Wang, D.; Xu, Y. Encapsulation and delivery of curcumin in cellulose nanocrystals nanoparticles using pH-driven method. LWT 2022, 155, 112863. [Google Scholar] [CrossRef]
- Zheng, L.; Ding, Z.; Zhang, M.; Sun, J. Microencapsulation of bayberry polyphenols by ethyl cellulose: Preparation and characterization. J. Food Eng. 2011, 104, 89–95. [Google Scholar] [CrossRef]
- Wang, J.; Wang, A.; Zang, X.; Tan, L.; Ge, Y.; Lin, X.; Xu, B.; Jin, Z.; Ma, W. Physical and oxidative stability of functional avocado oil high internal phase emulsions collaborative formulated using citrus nano fi bers and tannic acid. Food Hydrocoll. 2018, 82, 248–257. [Google Scholar] [CrossRef]
- Mao, X.; Gu, C.; Chen, D.; Yu, B.; He, J. Oxidative stress-induced diseases and tea polyphenols. Oncotarget 2017, 8, 81649–81661. [Google Scholar] [CrossRef]
- Gallardo, M.; Lluı, J.; Medina, I.; Pazos, M. Activity of grape polyphenols as inhibitors of the oxidation of fish lipids and frozen fish muscle. Food Chem. 2005, 92, 547–557. [Google Scholar]
- Noon, J.; Mills, T.B.; Norton, I.T. The use of natural antioxidants to combat lipid oxidation in O/W emulsions. J. Food Eng. 2020, 281, 110006. [Google Scholar] [CrossRef]
- Ean, M.A.R.M.A.C.L.; Ardner, P.E.G.; Uthie, G.A.G.D.; Okota, T.A.Y.; Rozier, A.L.A.N.C. Ellagitannins, Flavonoids, and Other Phenolics in Red Raspberries and Their Contribution to Antioxidant Capacity and Vasorelaxation Properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar]
- Di Mattia, C.D.; Sacchetti, G.; Mastrocola, D.; Pittia, P. Effect of phenolic antioxidants on the dispersion state and chemical stability of olive oil O/W emulsions. Food Res. Int. 2009, 42, 1163–1170. [Google Scholar] [CrossRef]
- Abdelazim, A.A.; Mahmoud, A. Oxidative stability of vegetable oils as affected by sesame extracts during accelerated oxidative storage. J. Food Sci. Technol. 2013, 50, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Wang, S.; Ma, L.; Yu, Y.; Dai, H.; Zhang, Y. Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. 2020, 238, 116180. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Du, G.; Li, C.; Zhang, H.; Long, Y.; Ni, Y. Preparation of cellulose nanocrystals from asparagus (Asparagus officinalis L.) and their applications to palm oil/water Pickering emulsion. Carbohydr. Polym. 2016, 151, 1–8. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Gao, X.; Xu, Z.; Liu, G.; Wu, J. Polyphenols as a versatile component in tissue engineering. Acta Biomater. 2021, 119, 57–74. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Li, Y.; Li, B.; Pei, Y.; Liu, S. Effects of the interaction between bacterial cellulose and soy protein isolate on the oil-water interface on the digestion of the Pickering emulsions. Food Hydrocoll. 2022, 126, 107480. [Google Scholar] [CrossRef]
- Sun, C.; Gunasekaran, S.; Richards, M.P. Effect of xanthan gum on physicochemical properties of whey protein isolate stabilized oil-in-water emulsions. Food Hydrocoll. 2007, 21, 555–564. [Google Scholar] [CrossRef]


| BCNC:GPPE | Samples | t1/2 (Days) | r2 |
|---|---|---|---|
| 0:1 | light | 43.5 | 0.96 |
| dark | 64.8 | 0.97 | |
| dark + 4 °C | 97.9 | 0.97 | |
| 1:1 | AH light | 46.8 | 0.99 |
| AH dark | 45.9 | 0.98 | |
| AH dark + 4 °C | 121.6 | 0.80 | |
| EH light | 43.6 | 0.99 | |
| EH dark | 55.5 | 0.99 | |
| EH dark + 4 °C | 96.3 | 0.96 | |
| 2.5:1 | AH light | 46.2 | 0.97 |
| AH dark | 68.0 | 0.92 | |
| AH dark + 4 °C | 150.7 | 0.80 | |
| EH light | 42.3 | 0.95 | |
| EH dark | 67.3 | 0.80 | |
| EH dark + 4 °C | 106.6 | 0.86 | |
| 5:1 | AH light | 41.0 | 0.90 |
| AH dark | 111.8 | 0.93 | |
| AH dark + 4 °C | 256.7 | 0.90 | |
| EH light | 37.3 | 0.92 | |
| EH dark | 68.6 | 0.78 | |
| EH dark + 4 °C | 130.8 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Ramirez, J.; Basasoro, S.; González, K.; Eceiza, A.; Retegi, A.; Gabilondo, N. Integral Valorization of Grape Pomace for Antioxidant Pickering Emulsions. Antioxidants 2023, 12, 1064. https://doi.org/10.3390/antiox12051064
Diaz-Ramirez J, Basasoro S, González K, Eceiza A, Retegi A, Gabilondo N. Integral Valorization of Grape Pomace for Antioxidant Pickering Emulsions. Antioxidants. 2023; 12(5):1064. https://doi.org/10.3390/antiox12051064
Chicago/Turabian StyleDiaz-Ramirez, Julen, Senda Basasoro, Kizkitza González, Arantxa Eceiza, Aloña Retegi, and Nagore Gabilondo. 2023. "Integral Valorization of Grape Pomace for Antioxidant Pickering Emulsions" Antioxidants 12, no. 5: 1064. https://doi.org/10.3390/antiox12051064
APA StyleDiaz-Ramirez, J., Basasoro, S., González, K., Eceiza, A., Retegi, A., & Gabilondo, N. (2023). Integral Valorization of Grape Pomace for Antioxidant Pickering Emulsions. Antioxidants, 12(5), 1064. https://doi.org/10.3390/antiox12051064










