Almond (Prunus dulcis) Bagasse as a Source of Bioactive Compounds with Antioxidant Properties: An In Vitro Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Almond Bagasse and Powder
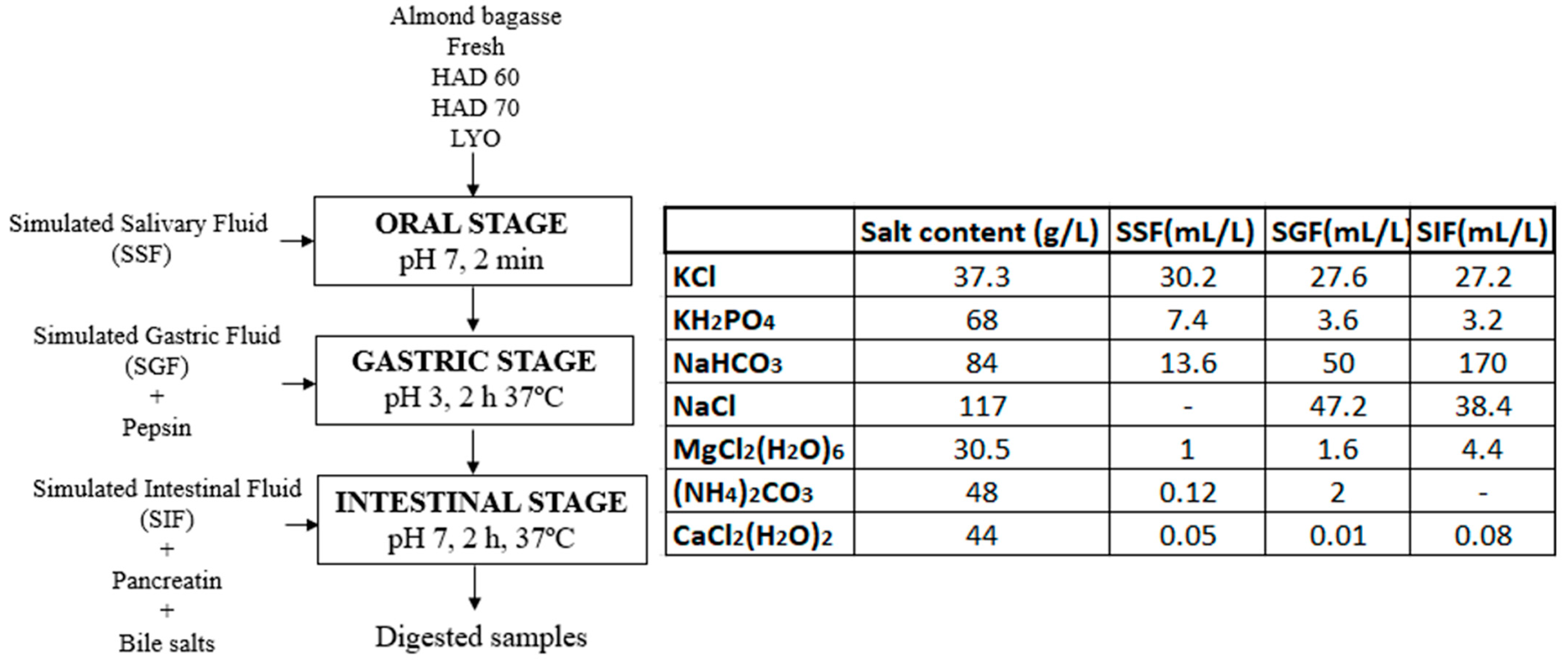
2.2. Simulation of Gastrointestinal Digestion In Vitro
2.3. In Vitro Colonic Fermentation
2.4. DNA Extraction, Sequencing, and Microbial Analysis
2.5. Determination of Antioxidant Compounds and Antiradical Capacity
2.5.1. Total Phenol Content
2.5.2. Antiradical Capacity by DPPH and ABTS Methods
2.5.3. Phenolic Compounds by HPLC Analysis
2.6. Statistical Analysis
3. Results and Discussion
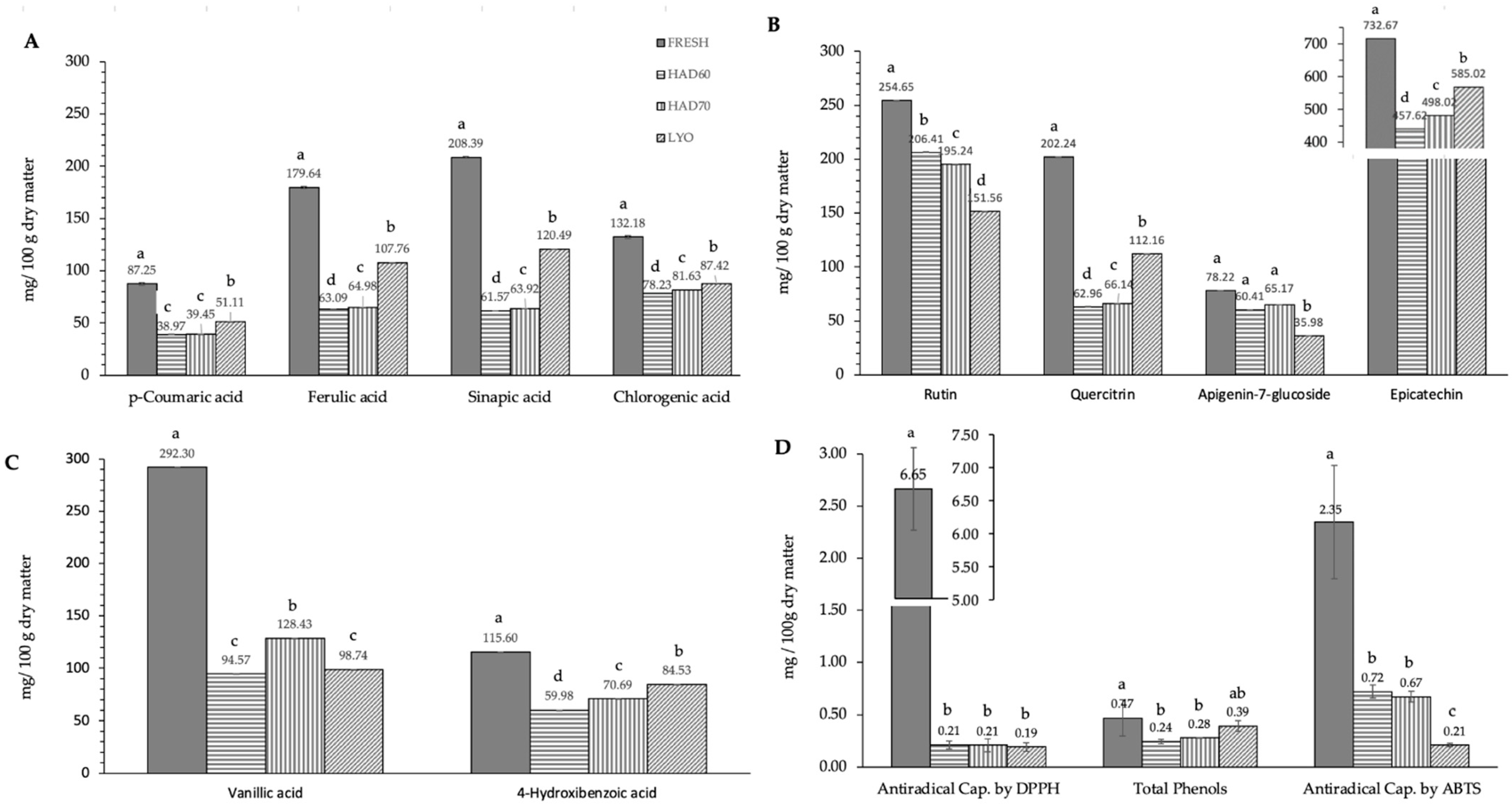
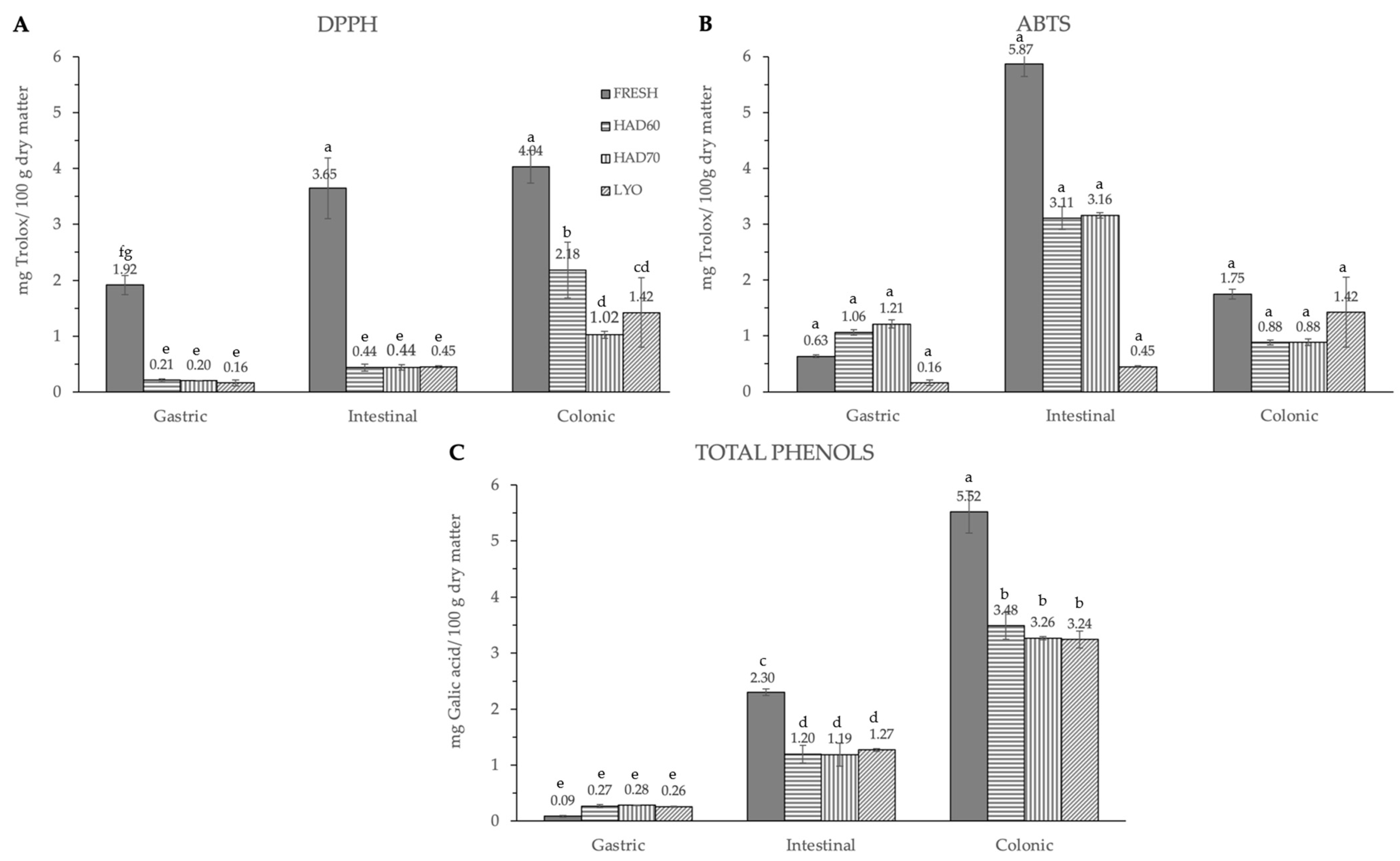
3.1. Effect of Processing on Antiradical Capacity and Phenol Content
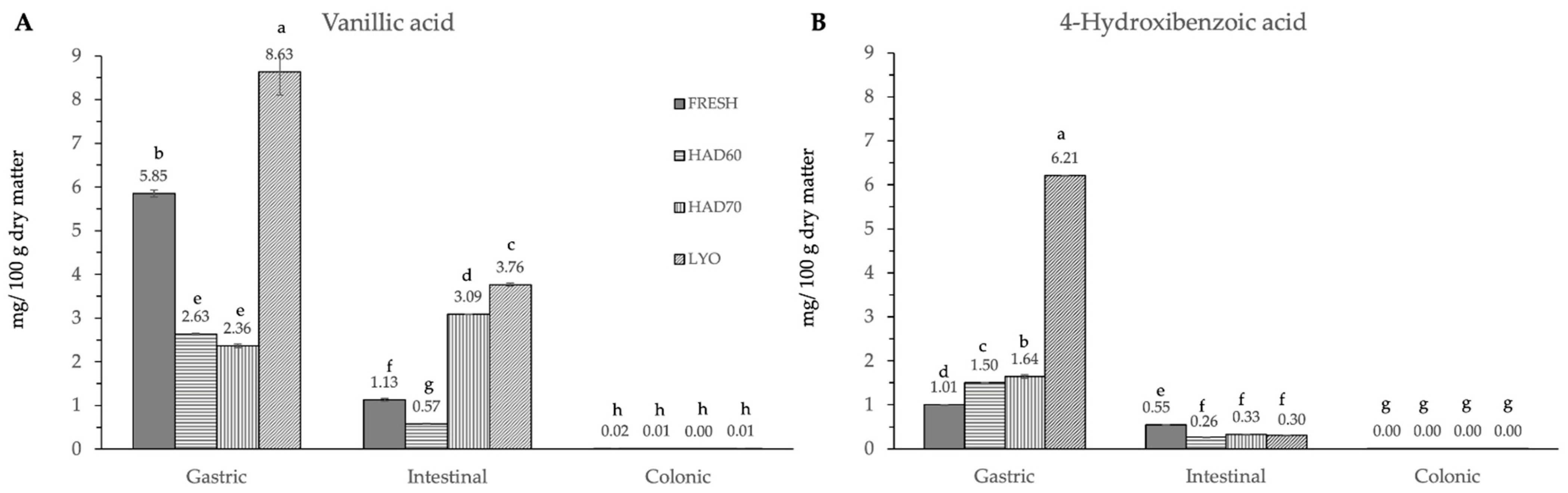
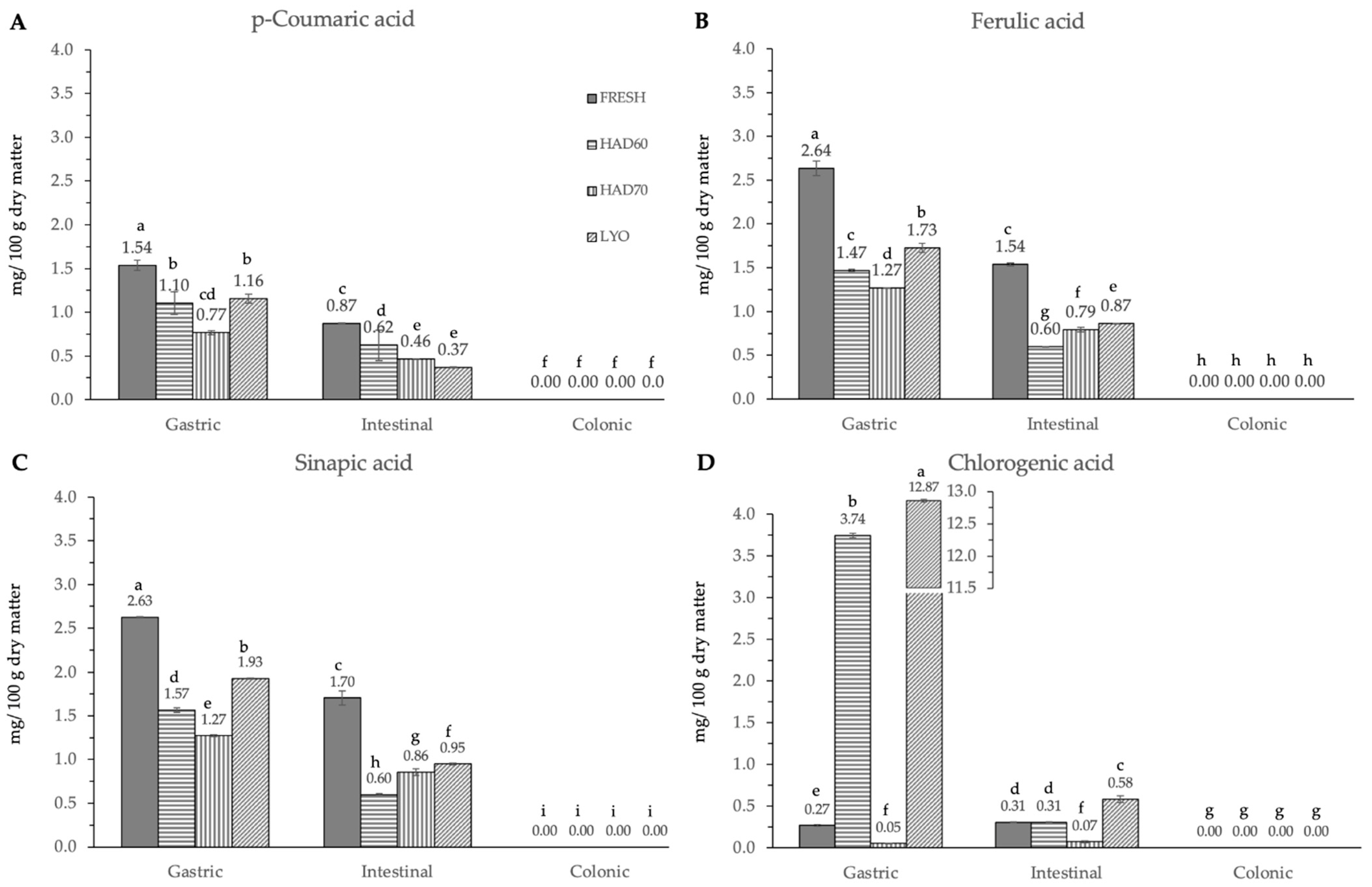
3.2. In Vitro Gastrointestinal Digestion
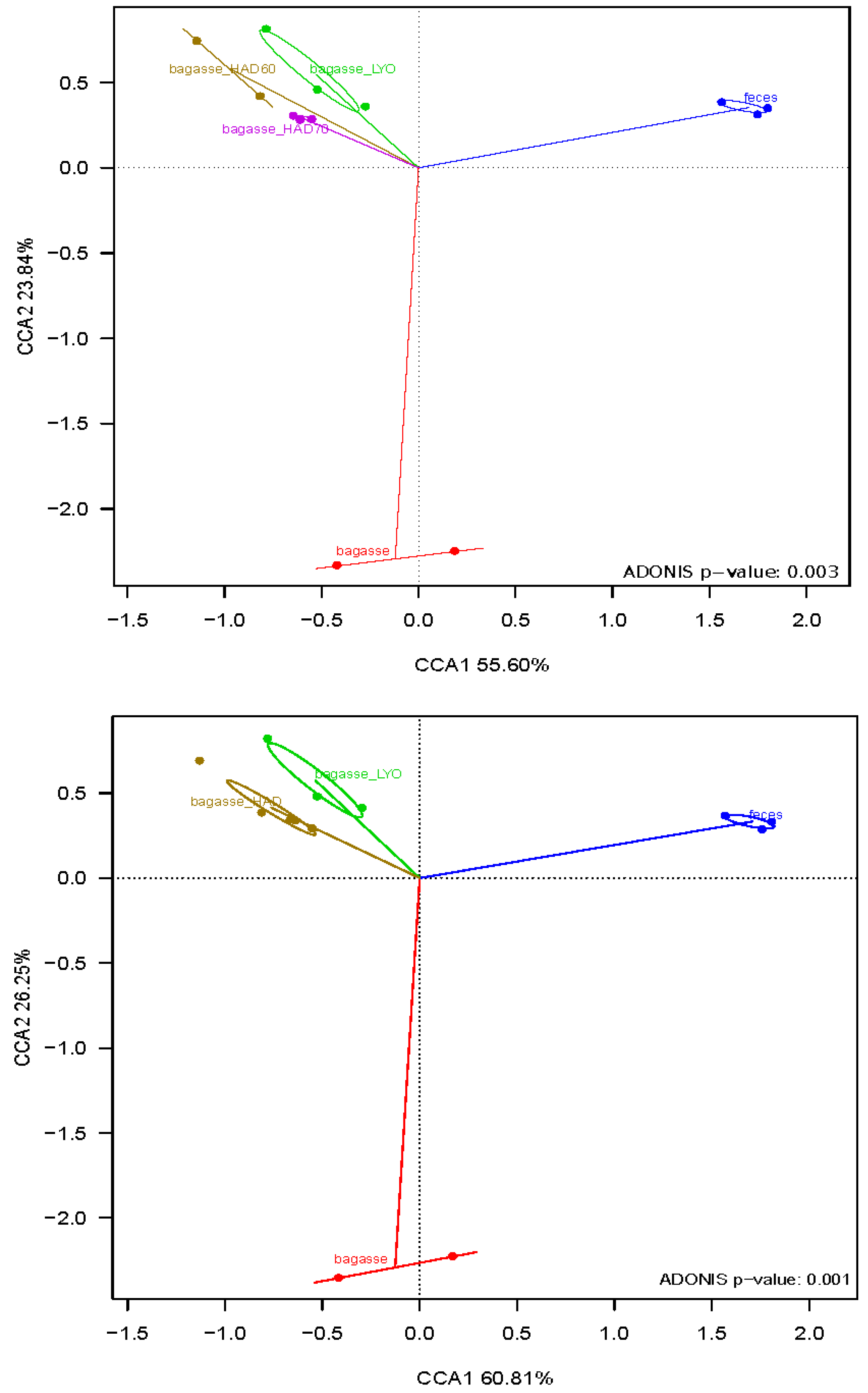
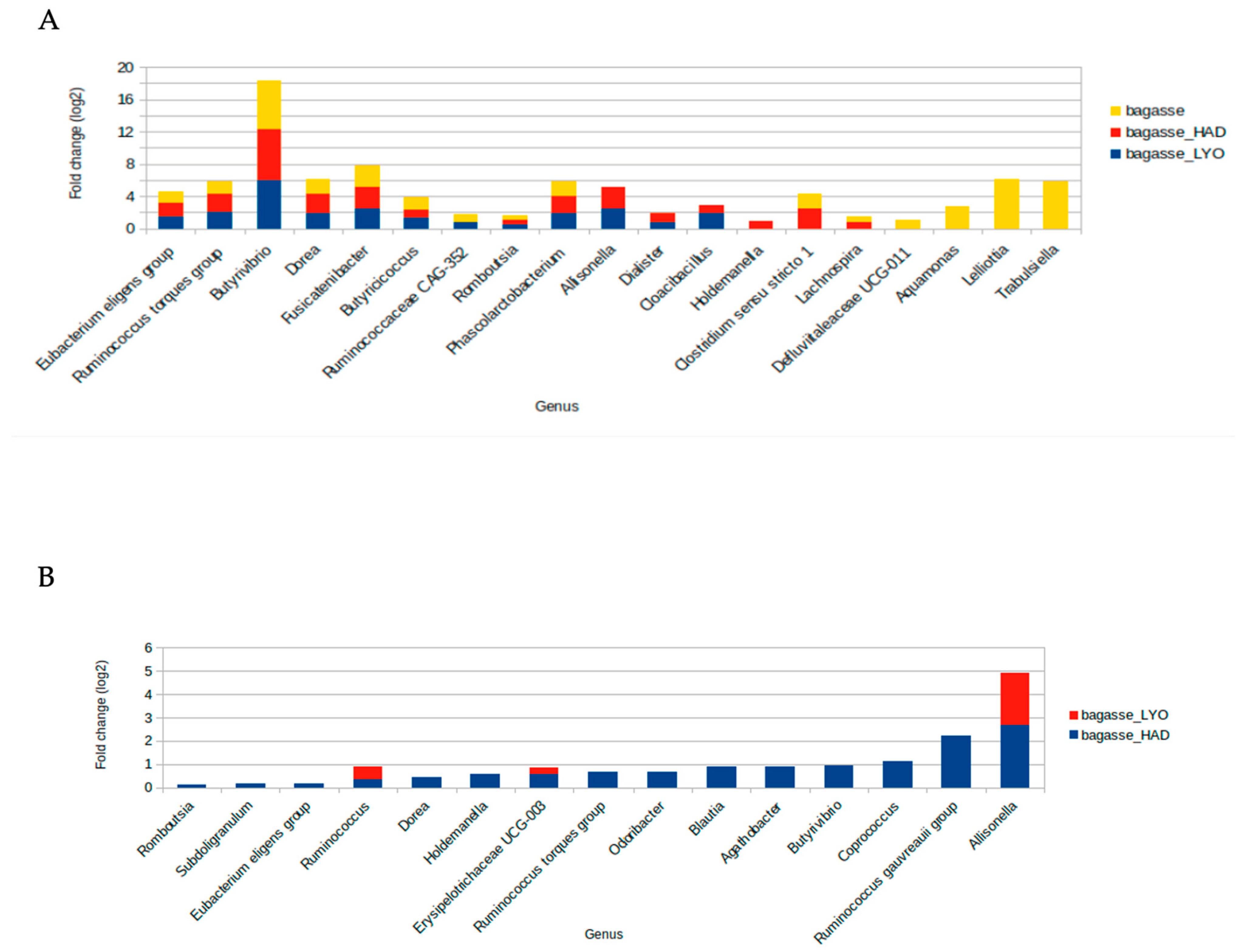
3.3. Fermentative Microbiota Analysis
4. Discussion
4.1. Effect of Processing on Antiradical Capacity and Phenol Content
4.2. In Vitro Gastrointestinal Digestion and Colonic Fermentation
4.3. Fermentative Microbiota Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoehnel, A.; Zannini, E.; Arendt, E.K. Targeted Formulation of Plant-Based Protein-Foods: Supporting the Food System’s Transformation in the Context of Human Health, Environmental Sustainability and Consumer Trends. Trends Food Sci. Technol. 2022, 128, 238–252. [Google Scholar] [CrossRef]
- Shori, A.B.; Aljohani, G.S.; Al-zahrani, A.J.; Al-sulbi, O.S.; Baba, A.S. Viability of Probiotics and Antioxidant Activity of Cashew Milk-Based Yogurt Fermented with Selected Strains of Probiotic Lactobacillus spp. LWT 2022, 153, 112482. [Google Scholar] [CrossRef]
- Giacalone, D.; Clausen, M.P.; Jaeger, S.R. Understanding Barriers to Consumption of Plant-Based Foods and Beverages: Insights from Sensory and Consumer Science. Curr. Opin. Food Sci. 2022, 48, 100919. [Google Scholar] [CrossRef]
- Yada, S.; Huang, G.; Lapsley, K. Natural Variability in the Nutrient Composition of California-Grown Almonds. J. Food Compos. Anal. 2013, 30, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Atalar, I.; Gul, O.; Saricaoglu, F.T.; Besir, A.; Gul, L.B.; Yazici, F. Influence of Thermosonication (TS) Process on the Quality Parameters of High Pressure Homogenized Hazelnut Milk from Hazelnut Oil by-Products. J. Food Sci. Technol. 2019, 56, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The Importance of Almond (Prunus Amygdalus L.) and Its by-Products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Turning Agri-Food Cooperative Vegetable Residues into Functional Powdered Ingredients for the Food Industry. Sustainability 2020, 12, 1284. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, 1900952. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Bas-Bellver, C.; Andrés, C.; Seguí, L.; Barrera, C.; Jiménez-Hernández, N.; Artacho, A.; Betoret, N.; Gosalbes, M.J. Valorization of Persimmon and Blueberry Byproducts to Obtain Functional Powders: In Vitro Digestion and Fermentation by Gut Microbiota. J. Agric. Food Chem. 2020, 68, 8080–8090. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- CRAN—Package Vegan. Available online: https://cran.microsoft.com/snapshot/2020-04-03/web/packages/vegan/index.html (accessed on 5 May 2023).
- Lin, H.; Peddada, S.D. Analysis of Compositions of Microbiomes with Bias Correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of Total Content of Phenolic Compounds and Their Antioxidant Activity in Vegetables—Evaluation of Spectrophotometric Methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de Diversos Métodos Químicos Para Determinar Actividad Antioxidante En Pulpa de Frutos. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Caprioli, G.; Nzekoue, F.K.; Giusti, F.; Vittori, S.; Sagratini, G. Optimization of an Extraction Method for the Simultaneous Quantification of Sixteen Polyphenols in Thirty-One Pulse Samples by Using HPLC-MS/MS Dynamic-MRM Triple Quadrupole. Food Chem. 2018, 266, 490–497. [Google Scholar] [CrossRef]
- Giusti, F.; Capuano, E.; Sagratini, G.; Pellegrini, N. A Comprehensive Investigation of the Behaviour of Phenolic Compounds in Legumes during Domestic Cooking and in Vitro Digestion. Food Chem. 2019, 285, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Tanleque-Alberto, F.; Juan-Borrás, M.; Escriche, I. Antioxidant Characteristics of Honey from Mozambique Based on Specific Flavonoids and Phenolic Acid Compounds. J. Food Compos. Anal. 2020, 86, 103377. [Google Scholar] [CrossRef]
- Cuvas-Limon, R.B.; Ferreira-Santos, P.; Cruz, M.; Teixeira, J.A.; Belmares, R.; Nobre, C. Effect of Gastrointestinal Digestion on the Bioaccessibility of Phenolic Compounds and Antioxidant Activity of Fermented Aloe Vera Juices. Antioxidants 2022, 11, 2479. [Google Scholar] [CrossRef]
- Salcedo, C.L.; López de Mishima, B.A.; Nazareno, M.A. Walnuts and Almonds as Model Systems of Foods Constituted by Oxidisable, pro-Oxidant and Antioxidant Factors. Food Res. Int. 2010, 43, 1187–1197. [Google Scholar] [CrossRef]
- Čolić, S.D.; Fotirić Akšić, M.M.; Lazarević, K.B.; Zec, G.N.; Gašić, U.M.; Dabić Zagorac, D.; Natić, M.M. Fatty Acid and Phenolic Profiles of Almond Grown in Serbia. Food Chem. 2017, 234, 455–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yıldırım, F.; San, B.; Polat, M.; Sesli, Y.; Üniversitesi, K.M. Variability of Phenolic Composition and Tocopherol Content of Some Commercial Almond Cultivars Economic Analysis of IPM in Apple Growing and Factors Affecting IPM Adoption View Project Red Currant View Project. Artic. J. Appl. Bot. Food Qual. 2016, 89, 163–170. [Google Scholar] [CrossRef]
- Banjanin, T.; Nikolic, D.; Uslu, N.; Gökmen, F.; Özcan, M.M.; Milatovic, D.; Zec, G.; Boškov, Đ.; Dursun, N. Physicochemical Properties, Fatty Acids, Phenolic Compounds, and Mineral Contents of 12 Serbia Regional and Commercial Almond Cultivars. J. Food Process. Preserv. 2021, 45, e15015. [Google Scholar] [CrossRef]
- Contreras, C.; Martín, M.E.; Martínez-Navarrete, N.; Chiralt, A. Effect of Vacuum Impregnation and Microwave Application on Structural Changes Which Occurred during Air-Drying of Apple. LWT Food Sci. Technol. 2005, 38, 471–477. [Google Scholar] [CrossRef]
- George, H.L.; Christoffersen, R.E. Differential Latency toward (–)-Epicatechin and Catechol Mediated by Avocado Mesocarp Polyphenol Oxidase (PPO). Postharvest Biol. Technol. 2016, 112, 31–38. [Google Scholar] [CrossRef]
- Del Caro, A.; Piga, A.; Vacca, V.; Agabbio, M. Changes of Flavonoids, Vitamin C and Antioxidant Capacity in Minimally Processed Citrus Segments and Juices during Storage. Food Chem. 2004, 84, 99–105. [Google Scholar] [CrossRef]
- García-Villalba, R.; Antonio Giménez-Bastida, J.; Cortés-Martín, A.; Ángeles Ávila-Gálvez, M.; Tomás-Barberán, F.A.; Selma, V.; Carlos Espín, J.; González-Sarrías, A.; García-Villalba, R.; Giménez-Bastida, J.A.; et al. Urolithins: A Comprehensive Update on Their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, 2101019. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A. Non-Extractable Polyphenols Produce Gut Microbiota Metabolites That Persist in Circulation and Show Anti-Inflammatory and Free Radical-Scavenging Effects. Trends Food Sci. Technol. 2017, 69, 281–288. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef] [PubMed]
- Kelly, W.J.; Leahy, S.C.; Altermann, E.; Yeoman, C.J.; Dunne, J.C.; Kong, Z.; Pacheco, D.M.; Li, D.; Noel, S.J.; Moon, C.D.; et al. The Glycobiome of the Rumen Bacterium Butyrivibrio Proteoclasticus B316T Highlights Adaptation to a Polysaccharide Rich Environment. PLoS ONE 2010, 5, e11942. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between Diet, Gut Microbiota Composition and Gut Metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takada, T.; Kurakawa, T.; Tsuji, H.; Nomoto, K. Fusicatenibacter Saccharivorans Gen. Nov., Sp. Nov., Isolated from HumanFaeces. Int. J. Syst. Evol. Microbiol. 2013, 63, 3691–3696. [Google Scholar] [CrossRef]
- Gerritsen, J.; Hornung, B.; Renckens, B.; van Hijum, S.A.F.T.; Martins dos Santos, V.A.P.; Rijkers, G.T.; Schaap, P.J.; de Vos, W.M.; Smidt, H. Genomic and Functional Analysis of Romboutsia Ilealis CRIBT Reveals Adaptation to the Small Intestine. PeerJ 2017, 2017, e3698. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Guo, X.; Zhang, J.; Zhang, M.; Ou, Z.; Peng, Y. Phascolarctobacterium Faecium Abundant Colonization in HumanGastrointestinal Tract. Exp. Ther. Med. 2017, 14, 3122–3126. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Perdigones, C.M.; Muñoz-Garach, A.; Álvarez-Bermúdez, M.D.; Moreno-Indias, I.; Tinahones, F.J. Gut Microbiota ofPatients with Type 2 Diabetes and Gastrointestinal Intolerance to Metformin Differs in Composition and Functionalityfrom Tolerant Patients. Biomed. Pharmacother. 2022, 145, 112448. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The Neuroactive Potential of the Human Gut Microbiota in Quality of Life and Depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Romaní-Pérez, M.; López-Almela, I.; Bullich-Vilarrubias, C.; Rueda-Ruzafa, L.; Gómez Del Pulgar, E.M.; Benítez-Páez, A.; Liebisch, G.; Lamas, J.A.; Sanz, Y. Holdemanella Biformis Improves Glucose Tolerance and Regulates GLP-1 Signaling in Obese Mice. FASEB J. 2021, 35, e21734. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, S.; Puchades, A.; Jiménez-Hernández, N.; Betoret, E.; Gosalbes, M.J.; Betoret, N. Almond (Prunus dulcis) Bagasse as a Source of Bioactive Compounds with Antioxidant Properties: An In Vitro Assessment. Antioxidants 2023, 12, 1229. https://doi.org/10.3390/antiox12061229
Duarte S, Puchades A, Jiménez-Hernández N, Betoret E, Gosalbes MJ, Betoret N. Almond (Prunus dulcis) Bagasse as a Source of Bioactive Compounds with Antioxidant Properties: An In Vitro Assessment. Antioxidants. 2023; 12(6):1229. https://doi.org/10.3390/antiox12061229
Chicago/Turabian StyleDuarte, Stevens, Almudena Puchades, Nuria Jiménez-Hernández, Ester Betoret, María José Gosalbes, and Noelia Betoret. 2023. "Almond (Prunus dulcis) Bagasse as a Source of Bioactive Compounds with Antioxidant Properties: An In Vitro Assessment" Antioxidants 12, no. 6: 1229. https://doi.org/10.3390/antiox12061229





