Supplement Treatment with NAC and Omega-3 Polyunsaturated Fatty Acids during Pregnancy Partially Prevents Schizophrenia-Related Outcomes in the Poly I:C Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
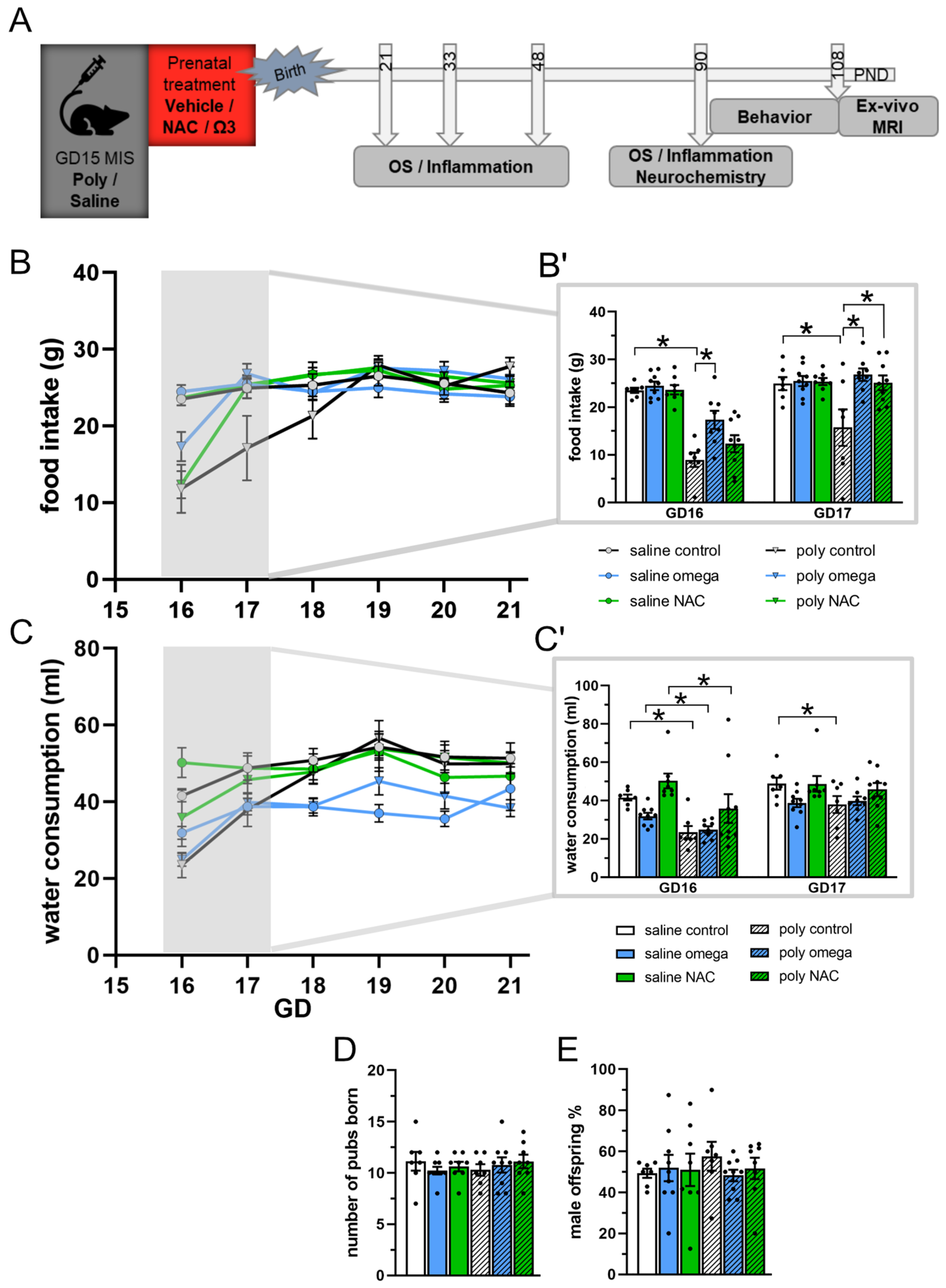
2.2. Experimental Design
2.3. Supplementary Treatment during Pregnancy
2.4. Behavioral Testing
2.5. Post-Mortem Analysis
2.6. Enzyme Activity
2.7. Western Blot
2.8. HPLC
2.9. Ex Vivo MRI
2.10. Statistical Analysis
3. Results
3.1. Maternal State
3.2. Oxidative and Inflammatory Parameters during Development
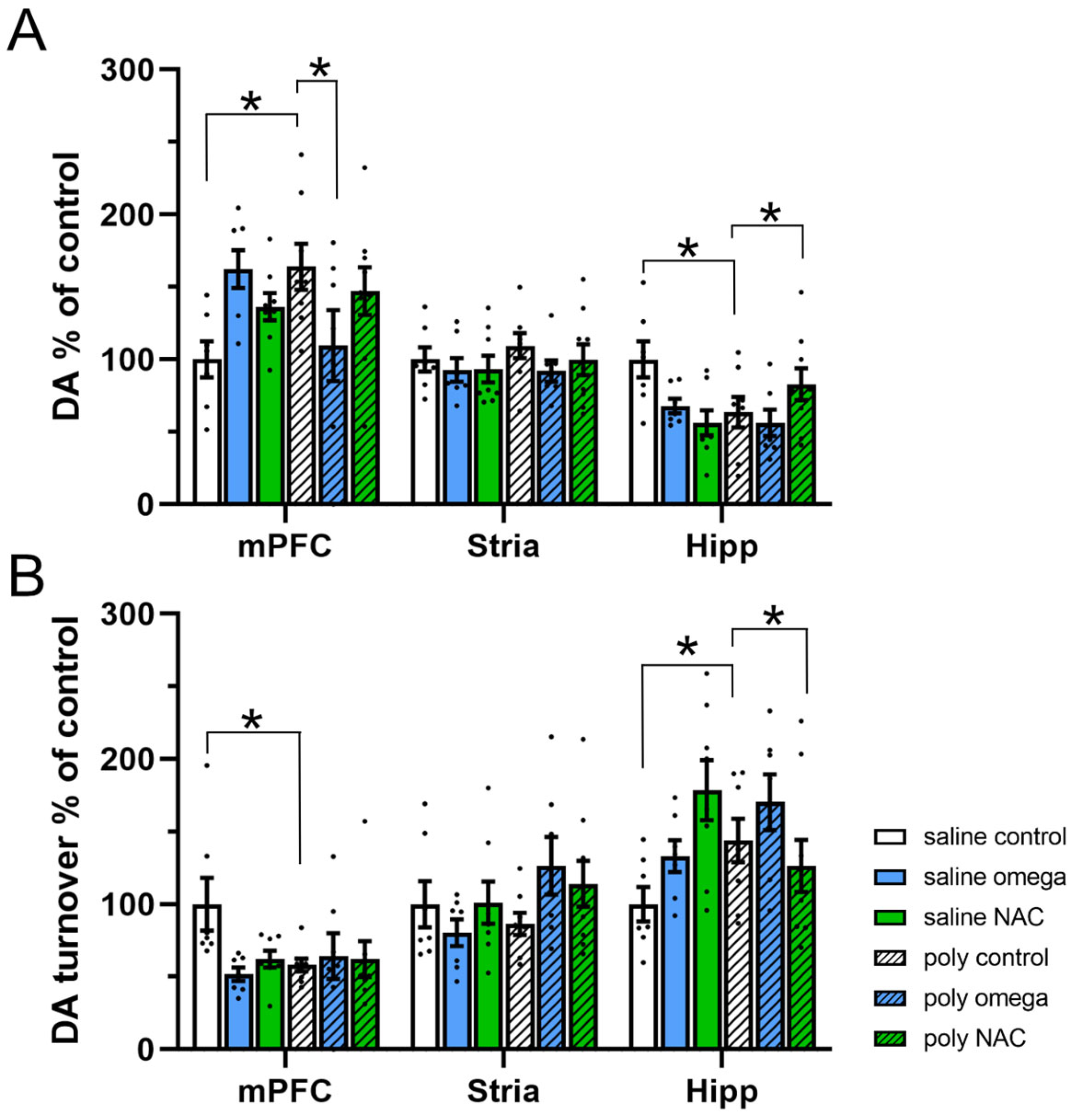
3.3. Dopamine System in Adulthood
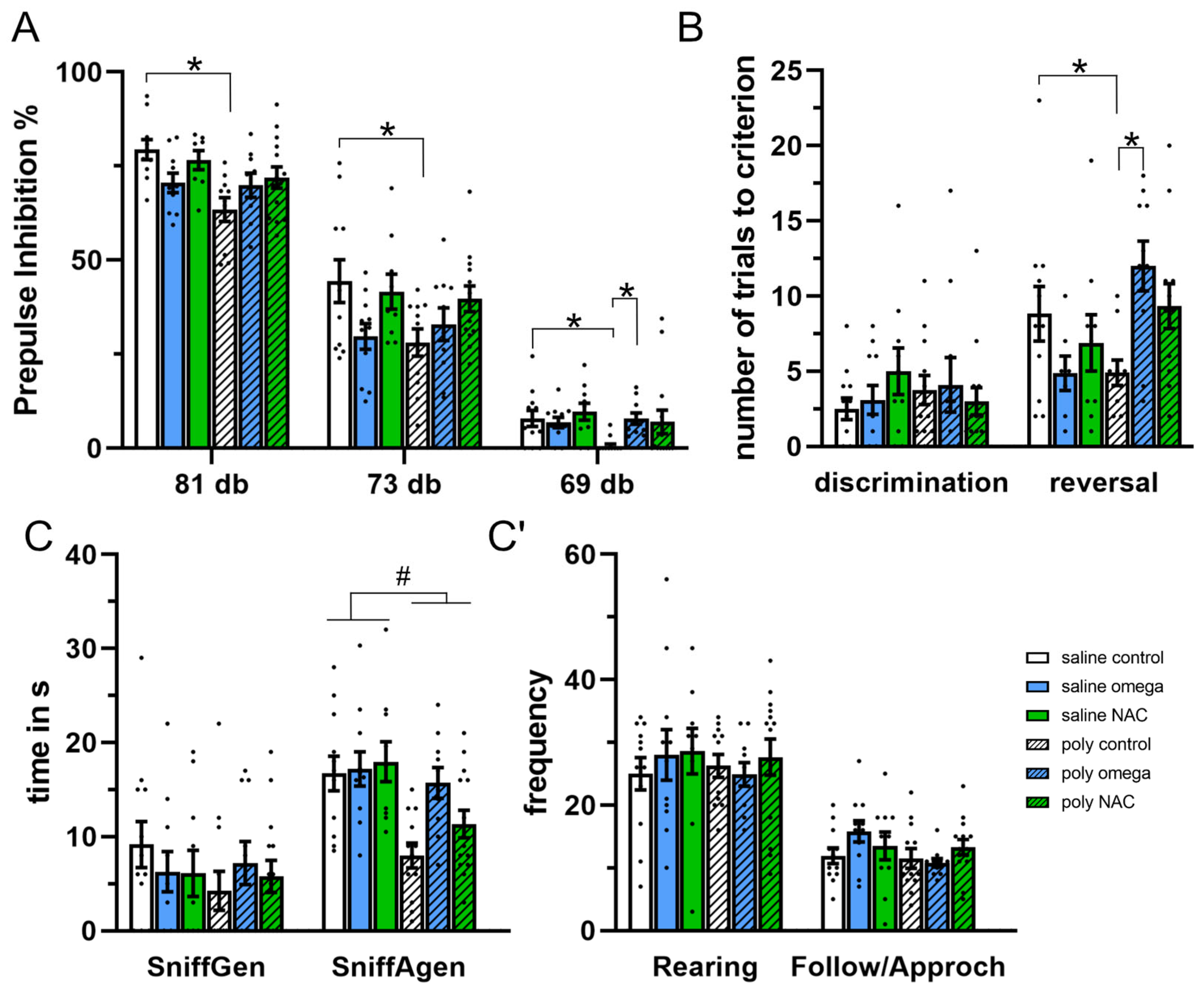
3.4. Behaviors in Adulthood
3.5. Brain Structure in Adulthood
4. Discussion
4.1. Supplementary Treatment Affected the Inflammatory Response in Both the Dams and Their Offspring
4.2. Prenatal Supplementary Treatment Partially Prevented Deregulation in the Collective Enzyme Anti-Oxidant Defense System in the Developing Offspring
4.3. Prenatal Supplementary Treatment Partially Prevented Schizophrenia-Related Outcomes in The Adult Offspring
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahn, R.S.; Sommer, I.E. The Neurobiology and Treatment of First-Episode Schizophrenia. Mol. Psychiatry 2015, 20, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, J.L.; Giedd, J.N.; Gogtay, N. Neurodevelopmental Model of Schizophrenia: Update 2012. Mol. Psychiatry 2012, 17, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Cuenod, M.; Steullet, P.; Cabungcal, J.H.; Dwir, D.; Khadimallah, I.; Klauser, P.; Conus, P.; Do, K.Q. Caught in Vicious Circles: A Perspective on Dynamic Feed-Forward Loops Driving Oxidative Stress in Schizophrenia. Mol. Psychiatry 2022, 27, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Fraguas, D.; Díaz-Caneja, C.M.; Ayora, M.; Hernández-Álvarez, F.; Rodríguez-Quiroga, A.; Recio, S.; Leza, J.C.; Arango, C. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2019, 45, 742–751. [Google Scholar] [CrossRef]
- Leza, J.C.; Bueno, B.; Bioque, M.; Arango, C.; Parellada, M.; Do, K.; O’Donnell, P.; Bernardo, M. Inflammation in Schizophrenia: A Question of Balance. Neurosci. Biobehav. Rev. 2015, 55, 612–626. [Google Scholar] [CrossRef]
- Meyer, U.; Schwarz, M.J.; Müller, N. Inflammatory Processes in Schizophrenia: A Promising Neuroimmunological Target for the Treatment of Negative/Cognitive Symptoms and Beyond. Pharmacol. Ther. 2011, 132, 96–110. [Google Scholar] [CrossRef]
- Do, K.Q.; Cabungcal, J.H.; Frank, A.; Steullet, P.; Cuenod, M. Redox Dysregulation, Neurodevelopment, and Schizophrenia. Curr. Opin. Neurobiol. 2009, 19, 220–230. [Google Scholar] [CrossRef]
- Steullet, P.; Cabungcal, J.-H.; Coyle, J.; Didriksen, M.; Gill, K.; Grace, A.A.; Hensch, T.K.; LaMantia, A.-S.; Lindemann, L.; Maynard, T.M.; et al. Oxidative Stress-Driven Parvalbumin Interneuron Impairment as a Common Mechanism in Models of Schizophrenia. Mol. Psychiatry 2017, 22, 936–943. [Google Scholar] [CrossRef]
- Bošković, M.; Vovk, T.; Koprivšek, J.; Plesničar, B.K.; Grabnar, I. Vitamin E and Essential Polyunsaturated Fatty Acids Supplementation in Schizophrenia Patients Treated with Haloperidol. Nutr. Neurosci. 2016, 19, 156–161. [Google Scholar] [CrossRef]
- Sepehrmanesh, Z.; Heidary, M.; Akasheh, N.; Akbari, H.; Heidary, M. Therapeutic Effect of Adjunctive N-Acetyl Cysteine (NAC) on Symptoms of Chronic Schizophrenia: A Double-Blind, Randomized Clinical Trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 82, 289–296. [Google Scholar] [CrossRef]
- Reddy, R.; Reddy, R. Antioxidant Therapeutics for Schizophrenia. Antioxid. Redox Signal. 2011, 15, 2047–2055. [Google Scholar] [CrossRef]
- Chen, A.T.; Chibnall, J.T.; Nasrallah, H.A. A Meta-Analysis of Placebo-Controlled Trials of Omega-3 Fatty Acid Augmentation in Schizophrenia: Possible Stage-Specific Effects. Ann. Clin. Psychiatry 2015, 27, 289–296. [Google Scholar]
- Goh, K.K.; Chen, C.Y.A.; Chen, C.H.; Lu, M.L. Effects of Omega-3 Polyunsaturated Fatty Acids Supplements on Psychopathology and Metabolic Parameters in Schizophrenia: A Meta-Analysis of Randomized Controlled Trials. J. Psychopharmacol. 2021, 35, 221–235. [Google Scholar] [CrossRef]
- Yolland, C.O.B.; Hanratty, D.; Neill, E.; Rossell, S.L.; Berk, M.; Dean, O.M.; Castle, D.J.; Tan, E.J.; Phillipou, A.; Harris, A.W.F.; et al. Meta-Analysis of Randomised Controlled Trials with N-Acetylcysteine in the Treatment of Schizophrenia. Aust. N. Z. J. Psychiatry 2020, 54, 453–466. [Google Scholar] [CrossRef]
- Magalhães, P.V.S.; Dean, O.; Andreazza, A.C.; Berk, M.; Kapczinski, F. Antioxidant Treatments for Schizophrenia. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef]
- Hamazaki, K.; Hamazaki, T.; Inadera, H. Abnormalities in the Fatty Acid Composition of the Postmortem Entorhinal Cortex of Patients with Schizophrenia, Bipolar Disorder, and Major Depressive Disorder. Psychiatry Res. 2013, 210, 346–350. [Google Scholar] [CrossRef]
- Kim, S.W.; Jhon, M.; Kim, J.M.; Smesny, S.; Rice, S.; Berk, M.; Klier, C.M.; McGorry, P.D.; Schäfer, M.R.; Amminger, G.P. Relationship between Erythrocyte Fatty Acid Composition and Psychopathology in the Vienna Omega-3 Study. PLoS ONE 2016, 11, e0151417. [Google Scholar] [CrossRef]
- Sethom, M.M.; Fares, S.; Bouaziz, N.; Melki, W.; Jemaa, R.; Feki, M.; Hechmi, Z.; Kaabachi, N. Polyunsaturated Fatty Acids Deficits Are Associated with Psychotic State and Negative Symptoms in Patients with Schizophrenia. Prostaglandins. Leukot. Essent. Fatty Acids 2010, 83, 131–136. [Google Scholar] [CrossRef]
- Brown, A.S.; Derkits, E.J. Prenatal Infection and Schizophrenia: A Review of Epidemiologic and Translational Studies. Am. J. Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef]
- Miller, B.J.; Culpepper, N.; Rapaport, M.H.; Buckley, P. Prenatal Inflammation and Neurodevelopment in Schizophrenia: A Review of Human Studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 92–100. [Google Scholar] [CrossRef]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 Fatty Acid Addition during Pregnancy. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.F.; Shaaban, O.M.; Bediawy, M.A. N-Acetyl Cysteine for Treatment of Recurrent Unexplained Pregnancy Loss. Reprod. Biomed. Online 2008, 17, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Dugoua, J.-J.; Mills, E.; Perri, D.; Koren, G.K. Safety and efficacy of ginkgo (ginkgo biloba) during pregnancy and lactation. J. Popul. Ther. Clin. Pharmacol. 2006, 13, 277–284. [Google Scholar]
- Zuckerman, L.; Rehavi, M.; Nachman, R.; Weiner, I. Immune Activation during Pregnancy in Rats Leads to a Postpubertal Emergence of Disrupted Latent Inhibition, Dopaminergic Hyperfunction, and Altered Limbic Morphology in the Offspring: A Novel Neurodevelopmental Model of Schizophrenia. Neuropsychopharmacology 2003, 28, 1778–1789. [Google Scholar] [CrossRef]
- Hadar, R.; Soto-Montenegro, M.L.; Götz, T.; Wieske, F.; Sohr, R.; Desco, M.; Hamani, C.; Weiner, I.; Pascau, J.; Winter, C. Using a Maternal Immune Stimulation Model of Schizophrenia to Study Behavioral and Neurobiological Alterations over the Developmental Course. Schizophr. Res. 2015, 166, 238–247. [Google Scholar] [CrossRef]
- Garcia-Partida, J.A.; Torres-Sanchez, S.; MacDowell, K.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Soto-Montenegro, M.L.; Romero-Miguel, D.; Lamanna-Rama, N.; Leza, J.C.; et al. The Effects of Mango Leaf Extract during Adolescence and Adulthood in a Rat Model of Schizophrenia. Front. Pharmacol. 2022, 13, 2846. [Google Scholar] [CrossRef]
- Romero-Miguel, D.; Casquero-Veiga, M.; Macdowell, K.S.; Torres-Sanchez, S.; Garcia-Partida, J.A.; Lamanna-Rama, N.; Romero-Miranda, A.; Berrocoso, E.; Leza, J.C.; Desco, M.; et al. A Characterization of the Effects of Minocycline Treatment During Adolescence on Structural, Metabolic, and Oxidative Stress Parameters in a Maternal Immune Stimulation Model of Neurodevelopmental Brain Disorders. Int. J. Neuropsychopharmacol. 2021, 24, 734–748. [Google Scholar] [CrossRef]
- Casquero-Veiga, M.; Romero-Miguel, D.; MacDowell, K.S.; Torres-Sanchez, S.; Garcia-Partida, J.A.; Lamanna-Rama, N.; Gómez-Rangel, V.; Romero-Miranda, A.; Berrocoso, E.; Leza, J.C.; et al. Omega-3 Fatty Acids during Adolescence Prevent Schizophrenia-Related Behavioural Deficits: Neurophysiological Evidences from the Prenatal Viral Infection with PolyI:C. Eur. Neuropsychopharmacol. 2021, 46, 14–27. [Google Scholar] [CrossRef]
- Casquero-Veiga, M.; García-García, D.; MacDowell, K.S.; Pérez-Caballero, L.; Torres-Sánchez, S.; Fraguas, D.; Berrocoso, E.; Leza, J.C.; Arango, C.; Desco, M.; et al. Risperidone Administered during Adolescence Induced Metabolic, Anatomical and Inflammatory/Oxidative Changes in Adult Brain: A PET and MRI Study in the Maternal Immune Stimulation Animal Model. Eur. Neuropsychopharmacol. 2019, 29, 880–896. [Google Scholar] [CrossRef]
- Monte, A.S.; da Silva, F.E.R.; Lima, C.N.d.C.; Vasconcelos, G.S.; Gomes, N.S.; Miyajima, F.; Vasconcelos, S.M.M.; Gama, C.S.; Seeman, M.V.; de Lucena, D.F.; et al. Sex Influences in the Preventive Effects of N-Acetylcysteine in a Two-Hit Animal Model of Schizophrenia. J. Psychopharmacol. 2020, 34, 125–136. [Google Scholar] [CrossRef]
- Hadar, R.; Winter, R.; Edemann-Callesen, H.; Wieske, F.; Habelt, B.; Khadka, N.; Felgel-Farnholz, V.; Barroeta-Hlusicka, E.; Reis, J.; Tatarau, C.A.; et al. Prevention of Schizophrenia Deficits via Non-Invasive Adolescent Frontal Cortex Stimulation in Rats. Mol. Psychiatry 2020, 25, 896–905. [Google Scholar] [CrossRef]
- Hadar, R.; Bikovski, L.; Soto-Montenegro, M.L.; Schimke, J.; Maier, P.; Ewing, S.; Voget, M.; Wieske, F.; Götz, T.; Desco, M.; et al. Early Neuromodulation Prevents the Development of Brain and Behavioral Abnormalities in a Rodent Model of Schizophrenia. Mol. Psychiatry 2018, 23, 943–951. [Google Scholar] [CrossRef]
- Piontkewitz, Y.; Assaf, Y.; Weiner, I. Clozapine Administration in Adolescence Prevents Postpubertal Emergence of Brain Structural Pathology in an Animal Model of Schizophrenia. Biol. Psychiatry 2009, 66, 1038–1046. [Google Scholar] [CrossRef]
- Piontkewitz, Y.; Arad, M.; Weiner, I. Risperidone Administered during Asymptomatic Period of Adolescence Prevents the Emergence of Brain Structural Pathology and Behavioral Abnormalities in an Animal Model of Schizophrenia. Schizophr. Bull. 2011, 37, 1257–1269. [Google Scholar] [CrossRef]
- Felgel-Farnholz, V.; Hlusicka, E.B.; Edemann-Callesen, H.; Garthe, A.; Winter, C.; Hadar, R. Adolescent Raloxifene Treatment in Females Prevents Cognitive Deficits in a Neurodevelopmental Rodent Model of Schizophrenia. Behav. Brain Res. 2023, 441, 114276. [Google Scholar] [CrossRef]
- Fortier, M.E.; Kent, S.; Ashdown, H.; Poole, S.; Boksa, P.; Luheshi, G.N. The Viral Mimic, Polyinosinic:Polycytidylic Acid, Induces Fever in Rats via an Interleukin-1-Dependent Mechanism. Am. J. Physiol-Regul. Integr. Comp. Physiol. 2004, 287, 759–766. [Google Scholar] [CrossRef]
- Kristal, M.B. Effects of Lateral Hypothalamic Lesions on Placentophagia in Virgin, Primiparous, and Multiparous Rats. J. Comp. Physiol. Psychol. 1973, 84, 53–62. [Google Scholar] [CrossRef]
- Novais, A.R.B.; Guiramand, J.; Cohen-Solal, C.; Crouzin, N.; De Jesus Ferreira, M.C.; Vignes, M.; Barbanel, G.; Cambonie, G. N-Acetyl-Cysteine Prevents Pyramidal Cell Disarray and Reelin-Immunoreactive Neuron Deficiency in CA3 after Prenatal Immune Challenge in Rats. Pediatr. Res. 2013, 73, 750–755. [Google Scholar] [CrossRef]
- Lanté, F.; Meunier, J.; Guiramand, J.; Maurice, T.; Cavalier, M.; de Jesus Ferreira, M.C.; Aimar, R.; Cohen-Solal, C.; Vignes, M.; Barbanel, G. Neurodevelopmental Damage after Prenatal Infection: Role of Oxidative Stress in the Fetal Brain. Free Radic. Biol. Med. 2007, 42, 1231–1245. [Google Scholar] [CrossRef]
- Tuzun, F.; Kumral, A.; Dilek, M.; Ozbal, S.; Ergur, B.; Yesilirmak, D.C.; Duman, N.; Yılmaz, O.; Ozkan, H. Maternal Omega-3 Fatty Acid Supplementation Protects against Lipopolysaccharide-Induced White Matter Injury in the Neonatal Rat Brain. J. Matern.-Fetal Neonatal Med. 2012, 25, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Piontkewitz, Y.; Arad, M.; Weiner, I. Abnormal Trajectories of Neurodevelopment and Behavior Following in Utero Insult in the Rat. Biol. Psychiatry 2011, 70, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Sams-Dodd, F. Effect of Novel Antipsychotic Drugs on Phencyclidine-Induced Stereotyped Behaviour and Social Isolation in the Rat Social Interaction Test PhD Project View Project. Behav. Pharmacol. 1997, 8, 196–215. [Google Scholar] [PubMed]
- Chiu, K.; Bellay, T. Micro-Dissection of Rat Brain for RNA or Protein Extraction from Specific Brain Region Related Papers Mult i-Elect Rode Array Recordings of Neuronal Avalanches in Organot Ypic Cult Ures. J. Vis. Exp. 2007, 269. [Google Scholar] [CrossRef]
- Winter, R.; Akinola, B.; Barroeta-Hlusicka, E.; Meister, S.; Pietzsch, J.; Winter, C.; Bernhardt, N. Comparison of Manual and Automated Ventricle Segmentation in the Maternal Immune Stimulation Rat Model of Schizophrenia. bioRxiv 2020. [Google Scholar] [CrossRef]
- Li, X.; Tian, X.; Lv, L.; Hei, G.; Huang, X.; Fan, X.; Zhang, J.; Zhang, J.; Pang, L.; Song, X. Microglia Activation in the Offspring of Prenatal Poly I: C Exposed Rats: A PET Imaging and Immunohistochemistry Study. Gen. Psychiatry 2018, 31. [Google Scholar] [CrossRef]
- Mattei, D.; Djodari-Irani, A.; Hadar, R.; Pelz, A.; de Cossío, L.F.; Goetz, T.; Matyash, M.; Kettenmann, H.; Winter, C.; Wolf, S.A. Minocycline Rescues Decrease in Neurogenesis, Increase in Microglia Cytokines and Deficits in Sensorimotor Gating in an Animal Model of Schizophrenia. Brain. Behav. Immun. 2014, 38, 175–184. [Google Scholar] [CrossRef]
- Van Den Eynde, K.; Missault, S.; Fransen, E.; Raeymaekers, L.; Willems, R.; Drinkenburg, W.; Timmermans, J.P.; Kumar-Singh, S.; Dedeurwaerdere, S. Hypolocomotive Behaviour Associated with Increased Microglia in a Prenatal Immune Activation Model with Relevance to Schizophrenia. Behav. Brain Res. 2014, 258, 179–186. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, Y.; Liu, Y.; Zhang, X.; Zhao, J. Minocycline Alleviates Behavioral Deficits and Inhibits Microglial Activation in the Offspring of Pregnant Mice after Administration of Polyriboinosinic–Polyribocytidilic Acid. Psychiatry Res. 2014, 219, 680–686. [Google Scholar] [CrossRef]
- Hadar, R.; Dong, L.; del-Valle-Anton, L.; Guneykaya, D.; Voget, M.; Edemann-Callesen, H.; Schweibold, R.; Djodari-Irani, A.; Goetz, T.; Ewing, S.; et al. Deep Brain Stimulation during Early Adolescence Prevents Microglial Alterations in a Model of Maternal Immune Activation. Brain. Behav. Immun. 2017, 63, 71–80. [Google Scholar] [CrossRef]
- Monji, A.; Kato, T.A.; Mizoguchi, Y.; Horikawa, H.; Seki, Y.; Kasai, M.; Yamauchi, Y.; Yamada, S.; Kanba, S. Neuroinflammation in Schizophrenia Especially Focused on the Role of Microglia. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2013, 42, 115–121. [Google Scholar] [CrossRef]
- Bland, S.T.; Beckley, J.T.; Young, S.; Tsang, V.; Watkins, L.R.; Maier, S.F.; Bilbo, S.D. Enduring Consequences of Early-Life Infection on Glial and Neural Cell Genesis within Cognitive Regions of the Brain. Brain. Behav. Immun. 2010, 24, 329–338. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Schwarz, J.M. Early-Life Programming of Later-Life Brain and Behavior: A Critical Role for the Immune System. Front. Behav. Neurosci. 2009, 3, 14. [Google Scholar] [CrossRef]
- Catale, C.; Gironda, S.; Iacono, L.L.; Carola, V. Microglial Function in the Effects of Early-Life Stress on Brain and Behavioral Development. J. Clin. Med. 2020, 9, 468. [Google Scholar] [CrossRef]
- Edemann-Callesen, H.; Winter, C.; Hadar, R. Using Cortical Non-Invasive Neuromodulation as a Potential Preventive Treatment in Schizophrenia - A Review. Brain Stimul. 2021, 14, 643–651. [Google Scholar] [CrossRef]
- Sakurai, T.; Gamo, N.J.; Hikida, T.; Kim, S.H.; Murai, T.; Tomoda, T.; Sawa, A. Converging Models of Schizophrenia-Network Alterations of Prefrontal Cortex Underlying Cognitive Impairments. Prog. Neurobiol. 2015, 134, 178. [Google Scholar] [CrossRef]
- Wang, L.; Mamah, D.; Harms, M.P.; Karnik, M.; Price, J.L.; Gado, M.H.; Thompson, P.A.; Barch, D.M.; Miller, M.I.; Csernansky, J.G. Progressive Deformation of Deep Brain Nuclei and Hippocampal-Amygdala Formation in Schizophrenia. Biol. Psychiatry 2008, 64, 1060–1068. [Google Scholar] [CrossRef]
- Fornito, A.; Harrison, B.J.; Goodby, E.; Dean, A.; Ooi, C.; Nathan, P.J.; Lennox, B.R.; Jones, P.B.; Suckling, J.; Bullmore, E.T. Functional Dysconnectivity of Corticostriatal Circuitry as a Risk Phenotype for Psychosis. JAMA Psychiatry 2013, 70, 1143–1151. [Google Scholar] [CrossRef]
- Rosenbaum, B. Prospective Investigations of the Prodromal State of Schizophrenia: Review of Studies. Acta Psychiatr. Scand. 2006, 113, 247–272. [Google Scholar] [CrossRef]
- Ribeiro, B.M.M.; do Carmo, M.R.S.; Freire, R.S.; Rocha, N.F.M.; Borella, V.C.M.; de Menezes, A.T.; Monte, A.S.; Gomes, P.X.L.; de Sousa, F.C.F.; Vale, M.L.; et al. Evidences for a Progressive Microglial Activation and Increase in INOS Expression in Rats Submitted to a Neurodevelopmental Model of Schizophrenia: Reversal by Clozapine. Schizophr. Res. 2013, 151, 12–19. [Google Scholar] [CrossRef]
- Sharma, G.; Shin, E.J.; Sharma, N.; Nah, S.Y.; Mai, H.N.; Nguyen, B.T.; Jeong, J.H.; Lei, X.G.; Kim, H.C. Glutathione Peroxidase-1 and Neuromodulation: Novel Potentials of an Old Enzyme. Food Chem. Toxicol. 2021, 148, 111945. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.K.; Reddy, R.D.; Van Kammen, D.P. Oxidative Damage and Schizophrenia: An Overview of the Evidence and Its Therapeutic Implications. CNS Drugs 2001, 15, 287–310. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.M.; Thome, J.; Martin, D.; Nara, K.; Zwerina, S.; Tatschner, T.; Weijers, H.G.; Koutsilieri, E. Cu, Zn- and Mn-Superoxide Dismutase Levels in Brains of Patients with Schizophrenic Psychosis. J. Neural Transm. 2004, 111, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Mander, P.K.; Jekabsone, A.; Brown, G.C. Microglia Proliferation Is Regulated by Hydrogen Peroxide from NADPH Oxidase. J. Immunol. 2006, 176, 1046–1052. [Google Scholar] [CrossRef]
- Davis, K.L.; Kahn, R.S.; Ko, G.; Davidson, M. Dopamine in Schizophrenia: A Review and Reconceptualization. Am. J. Psychiatry 1991, 148, 1474–1486. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The Dopamine Hypothesis of Schizophrenia: Version III--the Final Common Pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edemann-Callesen, H.; Bernhardt, N.; Hlusicka, E.B.; Hintz, F.; Habelt, B.; Winter, R.; Neubert, I.; Pelz, M.; Filla, A.; Soto-Montenegro, M.L.; et al. Supplement Treatment with NAC and Omega-3 Polyunsaturated Fatty Acids during Pregnancy Partially Prevents Schizophrenia-Related Outcomes in the Poly I:C Rat Model. Antioxidants 2023, 12, 1068. https://doi.org/10.3390/antiox12051068
Edemann-Callesen H, Bernhardt N, Hlusicka EB, Hintz F, Habelt B, Winter R, Neubert I, Pelz M, Filla A, Soto-Montenegro ML, et al. Supplement Treatment with NAC and Omega-3 Polyunsaturated Fatty Acids during Pregnancy Partially Prevents Schizophrenia-Related Outcomes in the Poly I:C Rat Model. Antioxidants. 2023; 12(5):1068. https://doi.org/10.3390/antiox12051068
Chicago/Turabian StyleEdemann-Callesen, Henriette, Nadine Bernhardt, Elizabeth Barroeta Hlusicka, Franziska Hintz, Bettina Habelt, Rebecca Winter, Isabell Neubert, Meike Pelz, Alexandra Filla, Maria Luisa Soto-Montenegro, and et al. 2023. "Supplement Treatment with NAC and Omega-3 Polyunsaturated Fatty Acids during Pregnancy Partially Prevents Schizophrenia-Related Outcomes in the Poly I:C Rat Model" Antioxidants 12, no. 5: 1068. https://doi.org/10.3390/antiox12051068
APA StyleEdemann-Callesen, H., Bernhardt, N., Hlusicka, E. B., Hintz, F., Habelt, B., Winter, R., Neubert, I., Pelz, M., Filla, A., Soto-Montenegro, M. L., Winter, C., & Hadar, R. (2023). Supplement Treatment with NAC and Omega-3 Polyunsaturated Fatty Acids during Pregnancy Partially Prevents Schizophrenia-Related Outcomes in the Poly I:C Rat Model. Antioxidants, 12(5), 1068. https://doi.org/10.3390/antiox12051068





