Antioxidant Activities of Natural Compounds from Caribbean Plants to Enhance Diabetic Wound Healing
Abstract
1. Introduction
2. Effect of Oxidative Stress on Different Mechanisms Altered in Diabetic Wounds
2.1. Oxidative Stress and MMPs
2.2. Oxidative Stress and AGEs
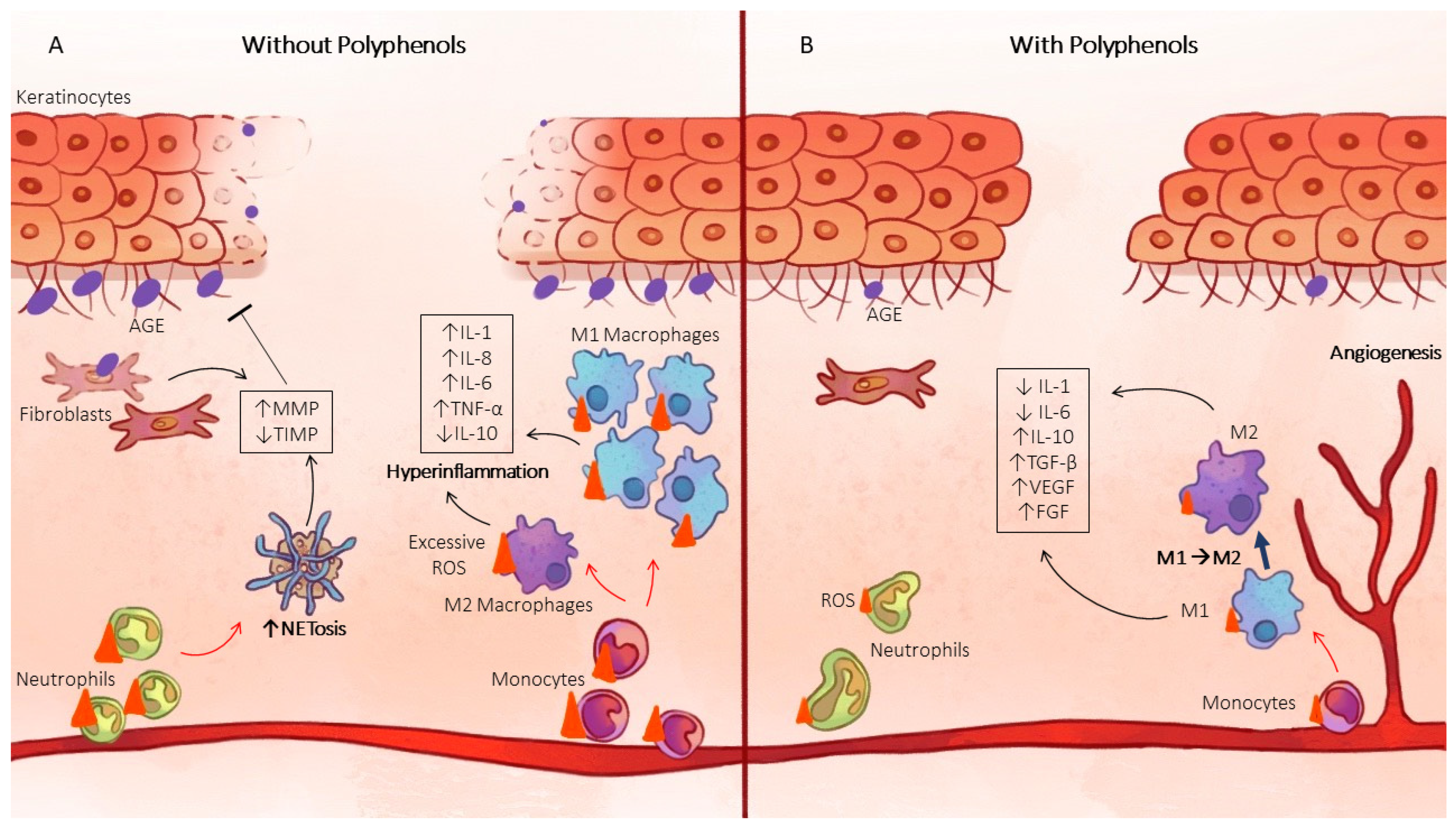
2.3. Oxidative Stress and Inflammation
2.3.1. Macrophage Polarization
2.3.2. NLRP3 Inflammasome
2.3.3. NETosis
2.4. Oxidative Stress and Nrf2
3. Improvement in Wound Healing by Plant Extracts
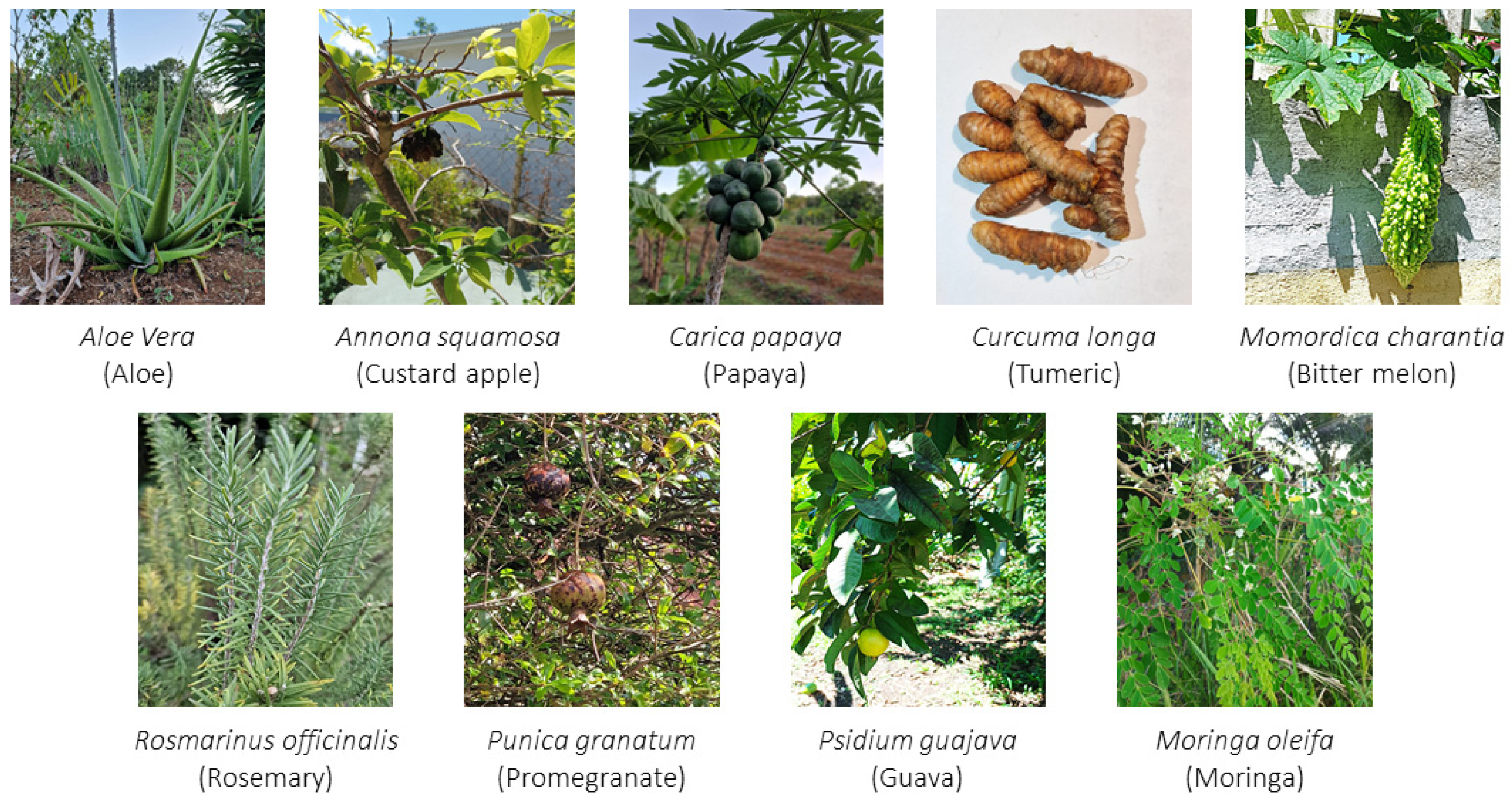
3.1. Selected Plants
3.1.1. Aloe vera (L.) Burm.f., 1768
3.1.2. Annona squamosa L., 1753
3.1.3. Carica papaya L., 1753
3.1.4. Curcuma longa L., 1753
3.1.5. Momordica charantia L., 1753
3.1.6. Moringa oleifera Lam., 1785
3.1.7. Psidium guajava L., 1753
3.1.8. Punica granatum L., 1753
3.1.9. Rosmarinus officinalis L., 1753
3.2. Compounds with Antioxidant and Healing Properties from Selected Caribean Plants
3.2.1. Quercetin
3.2.2. Kaempferol
3.2.3. Luteolin
3.2.4. Curcumin
3.2.5. Punicalagin
4. Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fédération Internationale du Diabète. Atlas du Diabète de la FID, 10th ed.; Fédération Internationale du Diabète: Brussels, Belgium, 2021. [Google Scholar]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Fui, L.W.; Lok, M.P.W.; Govindasamy, V.; Yong, T.K.; Lek, T.K.; Das, A.K. Understanding the Multifaceted Mechanisms of Diabetic Wound Healing and Therapeutic Application of Stem Cells Conditioned Medium in the Healing Process. J. Tissue Eng. Regen. Med. 2019, 13, 2218–2233. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ahmad, J. Role of Growth Factors and Cytokines in Diabetic Foot Ulcer Healing: A Detailed Review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes Primes Neutrophils to Undergo NETosis Which Severely Impairs Wound Healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M.; Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Bannon, P.; Wood, S.; Restivo, T.; Campbell, L.; Hardman, M.J.; Mace, K.A. Diabetes Induces Stable Intrinsic Changes to Myeloid Cells That Contribute to Chronic Inflammation during Wound Healing in Mice. Dis. Model. Mech. 2013, 6, 1434–1447. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, A.; Tokura, Y. Attempts to Accelerate Wound Healing. J. Dermatol. Sci. 2014, 76, 169–172. [Google Scholar] [CrossRef]
- Ferrante, C.J.; Leibovich, S.J. Regulation of Macrophage Polarization and Wound Healing. Adv. Wound Care 2012, 1, 10–16. [Google Scholar] [CrossRef]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Dardenne, C. Mécanismes de Dérégulation de la Polarisation des Macrophages et de la Résolution de L’inflammation au Cours de la Cicatrisation de Plaies Cutanées Chez des Souris Diabétiques de Type 2: Restauration par Application Topique D’aspirine. Ph.D. Thesis, Université Paul Sabatier—Toulouse III, Toulouse, France, 2015. [Google Scholar]
- Mirza, R.E.; Fang, M.M.; Weinheimer-Haus, E.M.; Ennis, W.J.; Koh, T.J. Sustained Inflammasome Activity in Macrophages Impairs Wound Healing in Type 2 Diabetic Humans and Mice. Diabetes 2014, 63, 1103–1114. [Google Scholar] [CrossRef]
- Fadini, G.P.; Menegazzo, L.; Rigato, M.; Scattolini, V.; Poncina, N.; Bruttocao, A.; Ciciliot, S.; Mammano, F.; Ciubotaru, C.D.; Brocco, E.; et al. NETosis Delays Diabetic Wound Healing in Mice and Humans. Diabetes 2016, 65, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Wagner, D.D. Peptidylarginine Deiminase 4: A Nuclear Button Triggering Neutrophil Extracellular Traps in Inflammatory Diseases and Aging. FASEB J. 2018, 32, 6358–6370. [Google Scholar] [CrossRef]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Roy, S. Redox Signals in Wound Healing. Biochim. Biophys. Acta BBA—Gen. Subj. 2008, 1780, 1348–1361. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative Stress in Normal and Impaired Wound Repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.; Ahswin, H.; Smart, N.; Bayon, Y.; Wohlert, S.; Hunt, J. Reactive Oxygen Species (ROS)—A Family of Fate Deciding Molecules Pivotal in Constructive Inflammation and Wound Healing. Eur. Cell. Mater. 2012, 24, 249–265. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative Stress and Diabetes: Antioxidative Strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative Stress and Stress-Activated Signaling Pathways: A Unifying Hypothesis of Type 2 Diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.-P.; Colette, C. Activation of Oxidative Stress by Acute Glucose Fluctuations Compared With Sustained Chronic Hyperglycemia in Patients With Type 2 Diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef]
- Wei, W.; Liu, Q.; Tan, Y.; Liu, L.; Li, X.; Cai, L. Oxidative Stress, Diabetes, and Diabetic Complications. Hemoglobin 2009, 33, 370–377. [Google Scholar] [CrossRef]
- Wlaschek, M.; Scharffetter-Kochanek, K. Oxidative Stress in Chronic Venous Leg Ulcers. Wound Repair Regen. 2005, 13, 452–461. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, e8852759. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, A.; Liu, B.; Huang, W.; Jiang, Z.; Bai, X.; Hu, L.; Zheng, S.; Guo, S.; Wu, J.; et al. Natural Biologics Accelerate Healing of Diabetic Foot Ulcers by Regulating Oxidative Stress. Front. Biosci.-Landmark 2022, 27, 285. [Google Scholar] [CrossRef]
- Dhall, S.; Do, D.C.; Garcia, M.; Kim, J.; Mirebrahim, S.H.; Lyubovitsky, J.; Lonardi, S.; Nothnagel, E.A.; Schiller, N.; Martins-Green, M. Generating and Reversing Chronic Wounds in Diabetic Mice by Manipulating Wound Redox Parameters. J. Diabetes Res. 2014, 2014, 562625. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Shin, M.-S.; Hwang, G.S.; Yamabe, N.; Yoo, J.-E.; Kang, K.S.; Kim, J.-C.; Lee, J.G.; Ham, J.; Lee, H.L. Beneficial Effects of Deoxyshikonin on Delayed Wound Healing in Diabetic Mice. Int. J. Mol. Sci. 2018, 19, 3660. [Google Scholar] [CrossRef]
- Yan, J.; Tie, G.; Wang, S.; Tutto, A.; DeMarco, N.; Khair, L.; Fazzio, T.G.; Messina, L.M. Diabetes Impairs Wound Healing by Dnmt1-Dependent Dysregulation of Hematopoietic Stem Cells Differentiation towards Macrophages. Nat. Commun. 2018, 9, 33. [Google Scholar] [CrossRef]
- Khanna, S.; Biswas, S.; Shang, Y.; Collard, E.; Azad, A.; Kauh, C.; Bhasker, V.; Gordillo, G.M.; Sen, C.K.; Roy, S. Macrophage Dysfunction Impairs Resolution of Inflammation in the Wounds of Diabetic Mice. PLoS ONE 2010, 5, e9539. [Google Scholar] [CrossRef]
- Lan, C.-C.E.; Wu, C.-S.; Huang, S.-M.; Wu, I.-H.; Chen, G.-S. High-Glucose Environment Enhanced Oxidative Stress and Increased Interleukin-8 Secretion from Keratinocytes: New Insights into Impaired Diabetic Wound Healing. Diabetes 2013, 62, 2530–2538. [Google Scholar] [CrossRef]
- Pustovrh, M.C.; Jawerbaum, A.; Capobianco, E.; White, V.; Martínez, N.; López-Costa, J.J.; González, E. Oxidative Stress Promotes the Increase of Matrix Metalloproteinases-2 and -9 Activities in the Feto-Placental Unit of Diabetic Rats. Free Radic. Res. 2005, 39, 1285–1293. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Meng, X.P.; Ramasamy, S.; Harrison, D.G.; Galis, Z.S. Reactive Oxygen Species Produced by Macrophage-Derived Foam Cells Regulate the Activity of Vascular Matrix Metalloproteinases in Vitro. Implications for Atherosclerotic Plaque Stability. J. Clin. Investig. 1996, 98, 2572–2579. [Google Scholar] [CrossRef]
- Loo, A.E.K.; Wong, Y.T.; Ho, R.; Wasser, M.; Du, T.; Ng, W.T.; Halliwell, B. Effects of Hydrogen Peroxide on Wound Healing in Mice in Relation to Oxidative Damage. PLoS ONE 2012, 7, e49215. [Google Scholar] [CrossRef]
- Kunkemoeller, B.; Kyriakides, T.R. Redox Signaling in Diabetic Wound Healing Regulates Extracellular Matrix Deposition. Antioxid. Redox Signal. 2017, 27, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, L.; Xiong, Y.; Panayi, A.C.; Abududilibaier, A.; Hu, Y.; Yu, C.; Zhou, W.; Sun, Y.; Liu, M.; et al. Antioxidant Therapy and Antioxidant-Related Bionanomaterials in Diabetic Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 707479. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.I.; Nguyen, T.T.; Peng, Z.; Chang, M. Targeting MMP-9 in Diabetic Foot Ulcers. Pharmaceuticals 2019, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Choma, P. Développement d’un Pansement Intelligent par Détection de Metalloprotéases. Ph.D. Thesis, IMT-Mines Alès Ecole Mines, Alès, France, 2019. [Google Scholar]
- Liang, Y.; Yang, C.; Lin, Y.; Parviz, Y.; Sun, K.; Wang, W.; Ren, M.; Yan, L. Matrix Metalloproteinase 9 Induces Keratinocyte Apoptosis through FasL/Fas Pathway in Diabetic Wound. Apoptosis 2019, 24, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.K.; Melendez, J.A. Mitochondrial Redox Control of Matrix Metalloproteinases. Free Radic. Biol. Med. 2004, 37, 768–784. [Google Scholar] [CrossRef]
- Belkhiri, A.; Richards, C.; Whaley, M.; McQueen, S.A.; Orr, F.W. Increased Expression of Activated Matrix Metalloproteinase-2 by Human Endothelial Cells after Sublethal H2O2 Exposure. Lab. Investig. J. Tech. Methods Pathol. 1997, 77, 533–539. [Google Scholar]
- Hsieh, H.-L.; Lin, C.-C.; Hsiao, L.-D.; Yang, C.-M. High Glucose Induces Reactive Oxygen Species-Dependent Matrix Metalloproteinase-9 Expression and Cell Migration in Brain Astrocytes. Mol. Neurobiol. 2013, 48, 601–614. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Shan, Y. Role of Oxidative Stress in Epigenetic Modification of MMP-9 Promoter in the Development of Diabetic Retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 955–962. [Google Scholar] [CrossRef]
- Nam, H.; Kim, M.-M. Eugenol with Antioxidant Activity Inhibits MMP-9 Related to Metastasis in Human Fibrosarcoma Cells. Food Chem. Toxicol. 2013, 55, 106–112. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ding, D.; Wolter, W.R.; Pérez, R.L.; Champion, M.M.; Mahasenan, K.V.; Hesek, D.; Lee, M.; Schroeder, V.A.; Jones, J.I.; et al. Validation of Matrix Metalloproteinase-9 (MMP-9) as a Novel Target for Treatment of Diabetic Foot Ulcers in Humans and Discovery of a Potent and Selective Small-Molecule MMP-9 Inhibitor That Accelerates Healing. J. Med. Chem. 2018, 61, 8825–8837. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced Glycoxidation and Lipoxidation End Products (AGEs and ALEs): An Overview of Their Mechanisms of Formation. Free Radic. Res. 2013, 47 (Suppl. 1), 3–27. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Urabe, K.; Moroi, Y.; Koga, T.; Nagai, R.; Horiuchi, S.; Furue, M. Migration of Keratinocytes Is Impaired on Glycated Collagen I. Wound Repair Regen. 2005, 13, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Yang, C.; Chen, L.-H.; Ren, M.; Lao, G.; Yan, L. Impairment of Human Keratinocyte Mobility and Proliferation by Advanced Glycation End Products-Modified BSA. Arch. Dermatol. Res. 2011, 303, 339–350. [Google Scholar] [CrossRef]
- Guimarães, E.L.M.; Empsen, C.; Geerts, A.; van Grunsven, L.A. Advanced Glycation End Products Induce Production of Reactive Oxygen Species via the Activation of NADPH Oxidase in Murine Hepatic Stellate Cells. J. Hepatol. 2010, 52, 389–397. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The Multiligand Receptor RAGE as a Progression Factor Amplifying Immune and Inflammatory Responses. J. Clin. Investig. 2001, 108, 949–955. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of Advanced Glycation End Products in Cellular Signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef]
- Peppa, M.; Stavroulakis, P.; Raptis, S.A. Advanced Glycoxidation Products and Impaired Diabetic Wound Healing. Wound Repair Regen. 2009, 17, 461–472. [Google Scholar] [CrossRef]
- Shaikh-Kader, A.; Houreld, N.N.; Rajendran, N.K.; Abrahamse, H. The Link between Advanced Glycation End Products and Apoptosis in Delayed Wound Healing. Cell Biochem. Funct. 2019, 37, 432–442. [Google Scholar] [CrossRef]
- Miyata, T.; Wada, Y.; Cai, Z.; Iida, Y.; Horie, K.; Yasuda, Y.; Maeda, K.; Kurokawa, K.; De Strihou, C.V.Y. Implication of an Increased Oxidative Stress in the Formation of Advanced Glycation End Products in Patients with End-Stage Renal Failure. Kidney Int. 1997, 51, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, C.; Graves, D.T. Abnormal Cell Responses and Role of TNF-α in Impaired Diabetic Wound Healing. BioMed Res. Int. 2013, 2013, 754802. [Google Scholar] [CrossRef]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive Oxygen Species (ROS) in Macrophage Activation and Function in Diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef]
- Aitcheson, S.M.; Frentiu, F.D.; Hurn, S.E.; Edwards, K.; Murray, R.Z. Skin Wound Healing: Normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules 2021, 26, 4917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Choksi, S.; Chen, K.; Pobezinskaya, Y.; Linnoila, I.; Liu, Z.-G. ROS Play a Critical Role in the Differentiation of Alternatively Activated Macrophages and the Occurrence of Tumor-Associated Macrophages. Cell Res. 2013, 23, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Mirza, R.; Koh, T.J. Dysregulation of Monocyte/Macrophage Phenotype in Wounds of Diabetic Mice. Cytokine 2011, 56, 256–264. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Yi, J.; Zhao, Z.; Ye, R. Hyperglycemia Modulates M1/M2 Macrophage Polarization via Reactive Oxygen Species Overproduction in Ligature-Induced Periodontitis. J. Periodontal Res. 2021, 56, 991–1005. [Google Scholar] [CrossRef]
- Pérez, S.; Rius-Pérez, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Covarrubias, A.; Byles, V.; Horng, T. ROS Sets the Stage for Macrophage Differentiation. Cell Res. 2013, 23, 984–985. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Jo, E.-K.; Kim, J.K.; Shin, D.-M.; Sasakawa, C. Molecular Mechanisms Regulating NLRP3 Inflammasome Activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.-L. Redox Regulation of NLRP3 Inflammasomes: ROS as Trigger or Effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Chen, H.; Chai, Y. Advanced Glycation End Products (AGEs) Induce Apoptosis of Fibroblasts by Activation of NLRP3 Inflammasome via Reactive Oxygen Species (ROS) Signaling Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7499–7508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dai, J.; Li, L.; Chen, H.; Chai, Y. NLRP3 Inflammasome Expression and Signaling in Human Diabetic Wounds and in High Glucose Induced Macrophages. J. Diabetes Res. 2017, 2017, e5281358. [Google Scholar] [CrossRef]
- Ding, Y.; Ding, X.; Zhang, H.; Li, S.; Yang, P.; Tan, Q. Relevance of NLRP3 Inflammasome-Related Pathways in the Pathology of Diabetic Wound Healing and Possible Therapeutic Targets. Oxid. Med. Cell. Longev. 2022, 2022, e9687925. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochem. Mosc. 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Karima, M.; Kantarci, A.; Ohira, T.; Hasturk, H.; Jones, V.L.; Nam, B.-H.; Malabanan, A.; Trackman, P.C.; Badwey, J.A.; Van Dyke, T.E. Enhanced Superoxide Release and Elevated Protein Kinase C Activity in Neutrophils from Diabetic Patients: Association with Periodontitis. J. Leukoc. Biol. 2005, 78, 862–870. [Google Scholar] [CrossRef]
- Dovi, J.V.; He, L.-K.; DiPietro, L.A. Accelerated Wound Closure in Neutrophil-Depleted Mice. J. Leukoc. Biol. 2003, 73, 448–455. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Yin, Y.; Mai, Y.; Wang, D.; Zhang, X. Hyperglycemia Induces Neutrophil Extracellular Traps Formation Through an NADPH Oxidase-Dependent Pathway in Diabetic Retinopathy. Front. Immunol. 2019, 9, 3076. [Google Scholar] [CrossRef]
- Yang, C.; Chen, L.; Chen, W.; Li, N.; Chen, M.; Li, X.; Zheng, X.; Zhao, Y.; Wu, Y.; Xian, M.; et al. Hydrogen Sulfide Primes Diabetic Wound to Close through Inhibition of NETosis. Mol. Cell. Endocrinol. 2019, 480, 74–82. [Google Scholar] [CrossRef]
- Lee, M.K.S.; Sreejit, G.; Nagareddy, P.R.; Murphy, A.J. Attack of the NETs! NETosis Primes IL-1β-Mediated Inflammation in Diabetic Foot Ulcers. Clin. Sci. 2020, 134, 1399–1401. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, P.; Gao, M.; Yu, T.; Shi, Y.; Zhang, M.; Yao, M.; Liu, Y.; Zhang, X. NLRP3 Activation Induced by Neutrophil Extracellular Traps Sustains Inflammatory Response in the Diabetic Wound. Clin. Sci. 2019, 133, 565–582. [Google Scholar] [CrossRef]
- Nakazawa, D.; Shida, H.; Kusunoki, Y.; Miyoshi, A.; Nishio, S.; Tomaru, U.; Atsumi, T.; Ishizu, A. The Responses of Macrophages in Interaction with Neutrophils That Undergo NETosis. J. Autoimmun. 2016, 67, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Liu, Y.; Jiang, T.; Ren, S.; Chen, J.; Xiong, H.; Yuan, M.; Li, W.; Machens, H.-G.; et al. NRF2 Signalling Pathway: New Insights and Progress in the Field of Wound Healing. J. Cell. Mol. Med. 2021, 25, 5857–5868. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Çetinkaya, S.; Panieri, E.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Regulatory Role of Nrf2 Signaling Pathway in Wound Healing Process. Molecules 2021, 26, 2424. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.-L. The Keap1-Nrf2 Pathway: Mechanisms of Activation and Dysregulation in Cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Long, M.; Rojo de la Vega, M.; Wen, Q.; Bharara, M.; Jiang, T.; Zhang, R.; Zhou, S.; Wong, P.K.; Wondrak, G.T.; Zheng, H.; et al. An Essential Role of NRF2 in Diabetic Wound Healing. Diabetes 2016, 65, 780–793. [Google Scholar] [CrossRef]
- Jindam, A.; Yerra, V.G.; Kumar, A. Nrf2: A Promising Trove for Diabetic Wound Healing. Ann. Transl. Med. 2017, 5, 469. [Google Scholar] [CrossRef]
- Soares, M.A.; Cohen, O.D.; Low, Y.C.; Sartor, R.A.; Ellison, T.; Anil, U.; Anzai, L.; Chang, J.B.; Saadeh, P.B.; Rabbani, P.S.; et al. Restoration of Nrf2 Signaling Normalizes the Regenerative Niche. Diabetes 2016, 65, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, P.S.; Zhou, A.; Borab, Z.M.; Frezzo, J.A.; Srivastava, N.; More, H.T.; Rifkin, W.J.; David, J.A.; Berens, S.J.; Chen, R.; et al. Novel Lipoproteoplex Delivers Keap1 SiRNA Based Gene Therapy to Accelerate Diabetic Wound Healing. Biomaterials 2017, 132, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Grochot-Przeczek, A.; Lach, R.; Mis, J.; Skrzypek, K.; Gozdecka, M.; Sroczynska, P.; Dubiel, M.; Rutkowski, A.; Kozakowska, M.; Zagorska, A.; et al. Heme Oxygenase-1 Accelerates Cutaneous Wound Healing in Mice. PLoS ONE 2009, 4, e5803. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.S.; Al-Mulla, F. A Defect in Nrf2 Signaling Constitutes a Mechanism for Cellular Stress Hypersensitivity in a Genetic Rat Model of Type 2 Diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1119–E1129. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid.-Based Complement. Altern. Med. ECAM 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Firdous, S.M.; Sautya, D. Medicinal Plants with Wound Healing Potential. Bangladesh J. Pharmacol. 2018, 13, 41–52. [Google Scholar] [CrossRef]
- Budovsky, A.; Yarmolinsky, L.; Ben-Shabat, S. Effect of Medicinal Plants on Wound Healing. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2015, 23, 171–183. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Medicinal Plants and Their Effects on Diabetic Wound Healing. Vet. World 2019, 12, 653–663. [Google Scholar] [CrossRef]
- Ande, S.N.; Dhandar, K.M.; Bakal, R.L. Medicinal Herbs: Why to Include in Diabetic Foot Ulcer Therapy? A Review. Innov. Pharm. Pharmacother. 2022, 10, 8–13. [Google Scholar]
- Herman, A.; Herman, A.P. Herbal Products and Their Active Constituents for Diabetic Wound Healing—Preclinical and Clinical Studies: A Systematic Review. Pharmaceutics 2023, 15, 281. [Google Scholar] [CrossRef]
- Nayak, S.B.; Pinto Pereira, L.; Maharaj, D. Wound Healing Activity of Carica Papaya L. in Experimentally Induced Diabetic Rats. Indian J. Exp. Biol. 2007, 45, 739–743. [Google Scholar] [PubMed]
- Fournet, J. Flore Illustrée des Phanérogames de Guadeloupe et de Martinique/Jacques Fournet; Nouvelle Edition Revue et Augmentée; CIRAD, Centre de Coopération Internationale en Recherche Agronomique pour le Développement Gondwana éd: Montpellier, France, 2002; ISBN 978-2-87614-489-7. [Google Scholar]
- Chithra, P.; Sajithlal, G.B.; Chandrakasan, G. Influence of Aloe Vera on the Healing of Dermal Wounds in Diabetic Rats. J. Ethnopharmacol. 1998, 59, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kahramanoğlu, İ.; Chen, C.; Chen, J.; Wan, C. Chemical Constituents, Antimicrobial Activity, and Food Preservative Characteristics of Aloe Vera Gel. Agronomy 2019, 9, 831. [Google Scholar] [CrossRef]
- Ponrasu, T.; Suguna, L. Efficacy of Annona squamosa on Wound Healing in Streptozotocin-Induced Diabetic Rats. Int. Wound J. 2012, 9, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Varadharaj, V.; Kumba, U.; Krishnamurthy, V. Physicochemical, Phytochemical Screening and Profiling of Secondary Metabolites of Annona squamosa Leaf Extract. World J. Pharm. Res. 2012, 1, 1143–1164. [Google Scholar]
- Kumar, M.; Changan, S.; Tomar, M.; Prajapati, U.; Saurabh, V.; Hasan, M.; Sasi, M.; Maheshwari, C.; Singh, S.; Dhumal, S.; et al. Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Biological Activities. Biomolecules 2021, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bachheti, A.; Sharma, P.; Bachheti, R.K.; Husen, A. Phytochemistry, Pharmacological Activities, Nanoparticle Fabrication, Commercial Products and Waste Utilization of Carica Papaya L.: A Comprehensive Review. Curr. Res. Biotechnol. 2020, 2, 145–160. [Google Scholar] [CrossRef]
- Khadam, S.; Afzal, U.; Gul, H.; Hira, S.; Satti, M.; Yaqub, A.; Ajab, H.; Gulfraz, M. Phytochemical Screening and Bioactivity Assessment of Leaves and Fruits Extract of Carica Papaya. Pak. J. Pharm. Sci. 2019, 32, 1941–1948. [Google Scholar] [PubMed]
- Gayosso-García Sancho, L.E.; Yahia, E.M.; González-Aguilar, G.A. Identification and Quantification of Phenols, Carotenoids, and Vitamin C from Papaya (Carica Papaya L., Cv. Maradol) Fruit Determined by HPLC-DAD-MS/MS-ESI. Food Res. Int. 2011, 44, 1284–1291. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Mani, H.; Gaddipati, J.P.; Singh, A.K.; Seth, P.; Banaudha, K.K.; Patnaik, G.K.; Maheshwari, R.K. Curcumin Enhances Wound Healing in Streptozotocin Induced Diabetic Rats and Genetically Diabetic Mice. Wound Repair Regen. 1999, 7, 362–374. [Google Scholar] [CrossRef]
- Niranjan, A.; Prof, D. Chemical Constituents and Biological Activities of Turmeric (Curcuma longa L.)—A Review. J. Food Sci. Technol. 2008, 45, 109–116. [Google Scholar]
- Teoh, S.L.; Latiff, A.A.; Das, S. The Effect of Topical Extract of Momordica Charantia (Bitter Gourd) on Wound Healing in Nondiabetic Rats and in Rats with Diabetes Induced by Streptozotocin. Clin. Exp. Dermatol. 2009, 34, 815–822. [Google Scholar] [CrossRef]
- La Torre, V.; Guarniz, W.; Silva-Correa, C.; Razco, L.; Siche, R. Antimicrobial Activity and Chemical Composition of Momordica Charantia: A Review. Pharmacogn. J. 2020, 12, 213–222. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Chen, J.-C.; Wang, C.-F.; Qiu, M.-H. New Cucurbitane Triterpenoids and Steroidal Glycoside from Momordica Charantia. Molecules 2009, 14, 4804–4813. [Google Scholar] [CrossRef] [PubMed]
- Hussan, F.; Teoh, S.L.; Muhamad, N.; Mazlan, M.; Latiff, A.A. Momordica Charantia Ointment Accelerates Diabetic Wound Healing and Enhances Transforming Growth Factor-β Expression. J. Wound Care 2014, 23, 404–407. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Alhussaini, M.S.; Asad, M.; Joseph, B. Moringa Oleifera Leaf Extract Promotes Healing of Infected Wounds in Diabetic Rats: Evidence of Antimicrobial, Antioxidant and Proliferative Properties. Pharmaceuticals 2022, 15, 528. [Google Scholar] [CrossRef]
- Muhammad, A.A.; Pauzi, N.A.S.; Arulselvan, P.; Abas, F.; Fakurazi, S. In Vitro Wound Healing Potential and Identification of Bioactive Compounds from Moringa Oleifera Lam. BioMed Res. Int. 2013, 2013, e974580. [Google Scholar] [CrossRef]
- Muhammad, A.A.; Arulselvan, P.; Cheah, P.S.; Abas, F.; Fakurazi, S. Evaluation of Wound Healing Properties of Bioactive Aqueous Fraction from Moringa Oleifera Lam on Experimentally Induced Diabetic Animal Model. Drug Des. Dev. Ther. 2016, 10, 1715–1730. [Google Scholar] [CrossRef] [PubMed]
- Braham, F.; Carvalho, D.O.; Almeida, C.M.R.; Zaidi, F.; Magalhães, J.M.C.S.; Guido, L.F.; Gonçalves, M.P. Online HPLC-DPPH Screening Method for Evaluation of Radical Scavenging Phenols Extracted from Moringa Oleifera Leaves. S. Afr. J. Bot. 2020, 129, 146–154. [Google Scholar] [CrossRef]
- Azevedo, Í.M.; Araújo-Filho, I.; Teixeira, M.M.A.; Moreira, M.D.F.D.C.; Medeiros, A.C. Wound Healing of Diabetic Rats Treated with Moringa Oleifera Extract. Acta Cirúrgica Bras. 2018, 33, 799–805. [Google Scholar] [CrossRef]
- Kumari, J.; Sangeetha, M.; Ali, S. Formulation and Evaluation of Herbal Gel from Tannin-Enriched Fraction of Psidium guajava Linn. Leaves for Diabetic Wound Healing. Int. J. Green Pharm. IJGP 2018, 12, S491–S496. [Google Scholar]
- Gutiérrez, R.M.P.; Mitchell, S.; Solis, R.V. Psidium Guajava: A Review of Its Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2008, 117, 1–27. [Google Scholar] [CrossRef]
- Yan, H.; Peng, K.; Wang, Q.; Gu, Z.; Lu, Y.; Zhao, J.; Xu, F. Effect of Pomegranate Peel Polyphenol Gel on Cutaneous Wound Healing in Alloxan-Induced Diabetic Rats. Chin. Med. J. 2013, 126, 1700–1706. [Google Scholar] [PubMed]
- Middha, S.K.; Usha, T.; Pande, V. HPLC Evaluation of Phenolic Profile, Nutritive Content, and Antioxidant Capacity of Extracts Obtained from Punica granatum Fruit Peel. Adv. Pharmacol. Pharm. Sci. 2013, 2013, e296236. [Google Scholar] [CrossRef]
- Abu-Al-Basal, M.A. Healing Potential of Rosmarinus Officinalis L. on Full-Thickness Excision Cutaneous Wounds in Alloxan-Induced-Diabetic BALB/c Mice. J. Ethnopharmacol. 2010, 131, 443–450. [Google Scholar] [CrossRef]
- Jena, J.; Gupta, A.K. Ricinus communis linn: A phytopharmacological review. Int. J. Pharm. Pharm. Sci. 2012, 4, 6. [Google Scholar]
- Vijayalakshmi, D.; Dhandapani, R.; Jayaveni, S.; Jithendra, P.S.; Rose, C.; Mandal, A.B. In Vitro Anti Inflammatory Activity of Aloe Vera by down Regulation of MMP-9 in Peripheral Blood Mononuclear Cells. J. Ethnopharmacol. 2012, 141, 542–546. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, X.; Yue, L.; Liu, L. Main Anthraquinone Components in Aloe Vera and Their Inhibitory Effects on the Formation of Advanced Glycation End-Products. J. Food Process. Preserv. 2017, 41, e13160. [Google Scholar] [CrossRef]
- Ashouri, F.; Beyranvand, F.; Beigi Boroujeni, N.; Tavafi, M.; Sheikhian, A.; Varzi, A.M.; Shahrokhi, S. Macrophage Polarization in Wound Healing: Role of Aloe Vera/Chitosan Nanohydrogel. Drug Deliv. Transl. Res. 2019, 9, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Budai, M.M.; Varga, A.; Milesz, S.; Tőzsér, J.; Benkő, S. Aloe Vera Downregulates LPS-Induced Inflammatory Cytokine Production and Expression of NLRP3 Inflammasome in Human Macrophages. Mol. Immunol. 2013, 56, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hua, H.; Zhu, H.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Aloe Polysaccharides Ameliorate Acute Colitis in Mice via Nrf2/HO-1 Signaling Pathway and Short-Chain Fatty Acids Metabolism. Int. J. Biol. Macromol. 2021, 185, 804–812. [Google Scholar] [CrossRef]
- Natalia, F.; Pinatih, N.I.; Praharsini, G.A.A. Administration of Sugar Apple Leaf Extract Cream (Annona squamosa L.) Increased Superoxide Dismutase (SOD) Activity and Decreased Skin Matrix Metalloproteinase-1 (MMP-1) Activity in Male White Rats (Rattus Norvegicus) Wistar Strain Exposed to Ultraviolet B Light. Int. J. Res. Rev. 2023, 10, 623–633. [Google Scholar] [CrossRef]
- Seo, S.A.; Ngo, H.T.T.; Hwang, E.; Park, B.; Yi, T.-H. Protective Effects of Carica Papaya Leaf against Skin Photodamage by Blocking Production of Matrix Metalloproteinases and Collagen Degradation in UVB-Irradiated Normal Human Dermal Fibroblasts. S. Afr. J. Bot. 2020, 131, 398–405. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Adeyemi, A.A.; Adenowo, A.F.; Akinsanya, M.A. Carica Papaya Linn. Fruit Extract Inhibited the Activities of Aldose Reductase and Sorbitol Dehydrogenase: Possible Mechanism for Amelioration of Diabetic Complications. Future J. Pharm. Sci. 2020, 6, 96. [Google Scholar] [CrossRef]
- Jang, S.; Chun, J.; Shin, E.M.; Kim, H.; Kim, Y.S. Inhibitory Effects of Curcuminoids from Curcuma longa on Matrix Metalloproteinase-1 Expression in Keratinocytes and Fibroblasts. J. Pharm. Investig. 2012, 42, 33–39. [Google Scholar] [CrossRef]
- Sajithlal, G.B.; Chithra, P.; Chandrakasan, G. Effect of Curcumin on the Advanced Glycation and Cross-Linking of Collagen in Diabetic Rats. Biochem. Pharmacol. 1998, 56, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Afiat, B.; Hendi, A.; Andromeda, A.; Alexander, K.; Almahitta Cintami, P.; Nur, A. Topical Administration of Curcuma longa L. Extract Gel Increases M2 Macrophage Polarization and Collagen Density in Skin Excision. J. Appl. Pharm. Sci. 2020, 11, 95–100. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, J.; Liu, N.; Wang, L.; Gao, Q.; Wu, Y.; Zhao, Q.; Liu, P.; Wang, S.; Liu, Y.; et al. Curcumin Induces M2 Macrophage Polarization by Secretion IL-4 and/or IL-13. J. Mol. Cell. Cardiol. 2015, 85, 131–139. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Han, W.; Yang, M.; Wen, L.; Wang, K.; Jiang, P. Curcumin Relieves Depressive-like Behaviors via Inhibition of the NLRP3 Inflammasome and Kynurenine Pathway in Rats Suffering from Chronic Unpredictable Mild Stress. Int. Immunopharmacol. 2019, 67, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Vinayak, M. Curcumin Modulates Glycolytic Metabolism and Inflammatory Cytokines via Nrf 2 in Dalton’s Lymphoma Ascites Cells In Vivo. Anticancer Agents Med. Chem. 2018, 18, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Pitchakarn, P.; Ogawa, K.; Suzuki, S.; Takahashi, S.; Asamoto, M.; Chewonarin, T.; Limtrakul, P.; Shirai, T. Momordica Charantia Leaf Extract Suppresses Rat Prostate Cancer Progression in Vitro and in Vivo. Cancer Sci. 2010, 101, 2234–2240. [Google Scholar] [CrossRef]
- Aljohi, A.; Matou-Nasri, S.; Ahmed, N. Antiglycation and Antioxidant Properties of Momordica charantia. PLoS ONE 2016, 11, e0159985. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.L.; Shivanagoudra, S.R.; Perera, W.H.; Kim, D.M.; Wu, C.S.; Sun, Y.; Jayaprakasha, G.K.; Patil, B.S. Bitter Melon Extracts and Cucurbitane-Type Triterpenoid Glycosides Antagonize Lipopolysaccharide-Induced Inflammation via Suppression of NLRP3 Inflammasome. J. Funct. Foods 2021, 86, 104720. [Google Scholar] [CrossRef]
- Nerurkar, P.V.; Orias, D.; Soares, N.; Kumar, M.; Nerurkar, V.R. Momordica Charantia (Bitter Melon) Modulates Adipose Tissue Inflammasome Gene Expression and Adipose-Gut Inflammatory Cross Talk in High-Fat Diet (HFD)-Fed Mice. J. Nutr. Biochem. 2019, 68, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Guo, P.; Jin, Y.; Li, M.; Hu, X.; Wang, W.; Wei, X.; Qi, S. Momordica Charantia Polysaccharide Ameliorates D-Galactose-Induced Aging through the Nrf2/β-Catenin Signaling Pathway. Metab. Brain Dis. 2022, 38, 1067–1077. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, Q.; Chang, X.; Yang, M.; He, J.; Tian, Y.; Sheng, J. Anti-Photoaging Effects of Flexible Nanoliposomes Encapsulated Moringa Oleifera Lam. Isothiocyanate in UVB-Induced Cell Damage in HaCaT Cells. Drug Deliv. 2022, 29, 871–881. [Google Scholar] [CrossRef]
- Adeniran, O.I.; Mogale, M.A. Inhibitory Effect and Cross-Link Breaking Activity of Moringa Oleifera Leaf Crude Extracts on Fructose-Derived Advanced Glycation End-Products. S. Afr. J. Bot. 2021, 139, 122–129. [Google Scholar] [CrossRef]
- Soliman, M.M.; Al-Osaimi, S.H.; HassanMohamed, E.; Aldhahrani, A.; Alkhedaide, A.; Althobaiti, F.; Mohamed, W.A. Protective Impacts of Moringa Oleifera Leaf Extract against Methotrexate-Induced Oxidative Stress and Apoptosis on Mouse Spleen. Evid. Based Complement. Altern. Med. 2020, 2020, 6738474. [Google Scholar] [CrossRef]
- Kim, C.G.; Chang, S.N.; Park, S.M.; Hwang, B.S.; Kang, S.-A.; Kim, K.S.; Park, J.G. Moringa Oleifera Mitigates Ethanol-Induced Oxidative Stress, Fatty Degeneration and Hepatic Steatosis by Promoting Nrf2 in Mice. Phytomedicine 2022, 100, 154037. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-C.; Peng, C.-H.; Chen, K.-C.; Hsieh, C.-L.; Peng, R.Y. The Aqueous Soluble Polyphenolic Fraction of Psidium guajava Leaves Exhibits Potent Anti-Angiogenesis and Anti-Migration Actions on DU145 Cells. Evid. Based Complement. Altern. Med. 2011, 2011, eneq005. [Google Scholar] [CrossRef]
- Wu, J.-W.; Hsieh, C.-L.; Wang, H.-Y.; Chen, H.-Y. Inhibitory Effects of Guava (Psidium guajava L.) Leaf Extracts and Its Active Compounds on the Glycation Process of Protein. Food Chem. 2009, 113, 78–84. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, L.; Liu, H.; Liu, D.; Wang, M.; Cai, J.; Liu, W.; Nie, W.; Zhang, Y.; Yu, X. Psidium guajava Flavonoids Prevent NLRP3 Inflammasome Activation and Alleviate the Pancreatic Fibrosis in a Chronic Pancreatitis Mouse Model. Am. J. Chin. Med. 2021, 49, 2001–2015. [Google Scholar] [CrossRef]
- Zioud, F.; Marzaioli, V.; El-Benna, J.; Bachoual, R. Punica granatum and Citrillus Colocynthis Aqueous Extracts Protect Mice from LPS-Induced Lung Inflammation and Inhibit Metalloproteinases-2 and -9. Indian J. Pharm. Educ. Res. 2019, 53, 503–510. [Google Scholar] [CrossRef]
- Kumagai, Y.; Nakatani, S.; Onodera, H.; Nagatomo, A.; Nishida, N.; Matsuura, Y.; Kobata, K.; Wada, M. Anti-Glycation Effects of Pomegranate (Punica granatum L.) Fruit Extract and Its Components in Vivo and in Vitro. J. Agric. Food Chem. 2015, 63, 7760–7764. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, S.; Lati, Y.; Aviram, M.; Fuhrman, B. Pomegranate Juice Polyphenols Induce a Phenotypic Switch in Macrophage Polarization Favoring a M2 Anti-Inflammatory State. BioFactors 2015, 41, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Pischetsrieder, M. Pomegranate (Punica granatum L.) Wine Polyphenols Affect Nrf2 Activation and Antioxidant Enzyme Expression in Human Neuroblastoma Cells (SH-SY5Y). J. Funct. Foods 2017, 38, 140–150. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, K. Protein Glycation Inhibitory and Antioxidative Activities of Some Plant Extracts in Vitro. J. Agric. Food Chem. 2003, 51, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Li, N.; Li, D.; Chen, L.; Deng, H.; Wang, S.; Tang, J.; Ouyang, W. Carnosic Acid Inhibits NLRP3 Inflammasome Activation by Targeting Both Priming and Assembly Steps. Int. Immunopharmacol. 2023, 116, 109819. [Google Scholar] [CrossRef]
- Martin, R.; Pierrard, C.; Lejeune, F.; Hilaire, P.; Breton, L.; Bernerd, F. Photoprotective Effect of a Water-Soluble Extract of Rosmarinus Officinalis L. against UV-Induced Matrix Metalloproteinase-1 in Human Dermal Fibroblasts and Reconstructed Skin. Eur. J. Dermatol. 2008, 18, 128–135. [Google Scholar]
- Yan, M.; Vemu, B.; Veenstra, J.; Petiwala, S.M.; Johnson, J.J. Carnosol, a Dietary Diterpene from Rosemary (Rosmarinus Officinalis) Activates Nrf2 Leading to Sestrin 2 Induction in Colon Cells. Integr. Mol. Med. 2018, 5, 1–7. [Google Scholar] [CrossRef]
- Bao, B.; Chen, Y.-G.; Zhang, L.; Na Xu, Y.L.; Wang, X.; Liu, J.; Qu, W. Momordica Charantia (Bitter Melon) Reduces Obesity-Associated Macrophage and Mast Cell Infiltration as Well as Inflammatory Cytokine Expression in Adipose Tissues. PLoS ONE 2013, 8, e84075. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [CrossRef]
- Guimarães, I.; Baptista-Silva, S.; Pintado, M.; Oliveira, A.L. Polyphenols: A Promising Avenue in Therapeutic Solutions for Wound Care. Appl. Sci. 2021, 11, 1230. [Google Scholar] [CrossRef]
- Kaparekar, P.S.; Anandasadagopan, S.K. The Potential Role of Bioactive Plant-Based Polyphenolic Compounds and Their Delivery Systems—As a Promising Opportunity for a New Therapeutic Solution for Acute and Chronic Wound Healing. Curr. Pharmacol. Rep. 2022, 8, 321–338. [Google Scholar] [CrossRef]
- Johnson, J.B.; Broszczak, D.A.; Mani, J.S.; Anesi, J.; Naiker, M. A Cut above the Rest: Oxidative Stress in Chronic Wounds and the Potential Role of Polyphenols as Therapeutics. J. Pharm. Pharmacol. 2021, 74, rgab038. [Google Scholar] [CrossRef]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The Biological Activities, Chemical Stability, Metabolism and Delivery Systems of Quercetin: A Review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Fu, J.; Huang, J.; Lin, M.; Xie, T.; You, T. Quercetin Promotes Diabetic Wound Healing via Switching Macrophages From M1 to M2 Polarization. J. Surg. Res. 2020, 246, 213–223. [Google Scholar] [CrossRef]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Chattoraj, S.S.; Chattoraj, A.; Burgess, J.R.; Curtis, J.L.; Martinez, F.J.; Zick, S.; Hershenson, M.B.; et al. Quercetin Prevents Progression of Disease in Elastase/LPS-Exposed Mice by Negatively Regulating MMP Expression. Respir. Res. 2010, 11, 131. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin Inhibits Advanced Glycation End Product Formation via Chelating Metal Ions, Trapping Methylglyoxal, and Trapping Reactive Oxygen Species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef]
- Li, R.; Chen, L.; Yao, G.-M.; Yan, H.-L.; Wang, L. Effects of Quercetin on Diabetic Retinopathy and Its Association with NLRP3 Inflammasome and Autophagy. Int. J. Ophthalmol. 2021, 14, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Zhu, Q.; Lu, Q.; Jiang, H.; Zhu, M.; Li, X.; Huang, G.; Xu, A. Quercetin Alleviates Rheumatoid Arthritis by Inhibiting Neutrophil Inflammatory Activities. J. Nutr. Biochem. 2020, 84, 108454. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Chung, M.-Y.; Lim, T.G.; Huh, W.B.; Lee, H.J.; Lee, K.W. Quercetin Suppresses Intracellular ROS Formation, MMP Activation, and Cell Motility in Human Fibrosarcoma Cells. J. Food Sci. 2013, 78, H1464–H1469. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Warabi, E.; Yanagawa, T.; Ma, D.; Itoh, K.; Ishii, Y.; Kawachi, Y.; Ishii, T. Essential Role of Nrf2 in Keratinocyte Protection from UVA by Quercetin. Biochem. Biophys. Res. Commun. 2009, 387, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-R.; Lee, J.Y.; Lee, H.-H.; Aryal, D.K.; Kim, Y.G.; Kim, S.K.; Woo, E.-R.; Kang, K.W. Antioxidative Effects of Quercetin-Glycosides Isolated from the Flower Buds of Tussilago Farfara L. Food Chem. Toxicol. 2006, 44, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Deng, G.; Liang, Q.; Chen, D.-F.; Guo, R.; Lai, R.-C. Antioxidant Activity of Quercetin and Its Glucosides from Propolis: A Theoretical Study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, İ.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound Healing Effect of Kaempferol in Diabetic and Nondiabetic Rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef]
- Ronsisvalle, S.; Panarello, F.; Longhitano, G.; Siciliano, E.A.; Montenegro, L.; Panico, A. Natural Flavones and Flavonols: Relationships among Antioxidant Activity, Glycation, and Metalloproteinase Inhibition. Cosmetics 2020, 7, 71. [Google Scholar] [CrossRef]
- Zhao, J.; Ling, L.; Zhu, W.; Ying, T.; Yu, T.; Sun, M.; Zhu, X.; Du, Y.; Zhang, L. M1/M2 Re-Polarization of Kaempferol Biomimetic NPs in Anti-Inflammatory Therapy of Atherosclerosis. J. Controlled Release 2023, 353, 1068–1083. [Google Scholar] [CrossRef]
- Lin, C.; Wu, F.; Zheng, T.; Wang, X.; Chen, Y.; Wu, X. Kaempferol Attenuates Retinal Ganglion Cell Death by Suppressing NLRP1/NLRP3 Inflammasomes and Caspase-8 via JNK and NF-ΚB Pathways in Acute Glaucoma. Eye 2019, 33, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xu, H.; Fan, P.-Z.; Xie, J.; He, J.; Yu, J.; Gu, X.; Zhang, C.-J. Kaempferol Blocks Neutrophil Extracellular Traps Formation and Reduces Tumour Metastasis by Inhibiting ROS-PAD4 Pathway. J. Cell. Mol. Med. 2020, 24, 7590–7599. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Sun, J.; Wei, J.; Zhang, X.; Chen, B.; Lin, Y. Kaempferol Protects Blood Vessels From Damage Induced by Oxidative Stress and Inflammation in Association With the Nrf2/HO-1 Signaling Pathway. Front. Pharmacol. 2020, 11, 1118. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a Flavonoid, as an Anticancer Agent: A Review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Cheng, H.-L.; Kuan, Y.-H.; Liang, T.-J.; Chao, Y.-Y.; Lin, H.-C. Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats. Biomedicines 2021, 9, 761. [Google Scholar] [CrossRef]
- Saragusti, A.C.; Ortega, M.G.; Cabrera, J.L.; Estrin, D.A.; Marti, M.A.; Chiabrando, G.A. Inhibitory Effect of Quercetin on Matrix Metalloproteinase 9 Activity Molecular Mechanism and Structure–Activity Relationship of the Flavonoid–Enzyme Interaction. Eur. J. Pharmacol. 2010, 644, 138–145. [Google Scholar] [CrossRef]
- Pandurangan, A.; Dharmalingam, P.; Sadagopan, S.; Ganapasam, S. Luteolin Inhibits Matrix Metalloproteinase 9 and 2 in Azoxymethane-Induced Colon Carcinogenesis. Hum. Exp. Toxicol. 2014, 33, 1176–1185. [Google Scholar] [CrossRef]
- Sarmah, S.; Das, S.; Roy, A.S. Protective Actions of Bioactive Flavonoids Chrysin and Luteolin on the Glyoxal Induced Formation of Advanced Glycation End Products and Aggregation of Human Serum Albumin: In Vitro and Molecular Docking Analysis. Int. J. Biol. Macromol. 2020, 165, 2275–2285. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.; Xu, S.; Shi, J.; Mao, X.; Yao, X.; Liu, C. Luteolin Alters Macrophage Polarization to Inhibit Inflammation. Inflammation 2020, 43, 95–108. [Google Scholar] [CrossRef]
- Lee, M.N.; Lee, Y.; Wu, D.; Pae, M. Luteolin Inhibits NLRP3 Inflammasome Activation via Blocking ASC Oligomerization. J. Nutr. Biochem. 2021, 92, 108614. [Google Scholar] [CrossRef]
- Jablonska, E.; Garley, M.; Surazynski, A.; Grubczak, K.; Iwaniuk, A.; Borys, J.; Moniuszko, M.; Ratajczak-Wrona, W. Neutrophil Extracellular Traps (NETs) Formation Induced by TGF-β in Oral Lichen Planus—Possible Implications for the Development of Oral Cancer. Immunobiology 2020, 225, 151901. [Google Scholar] [CrossRef]
- Zhang, B.-C.; Li, Z.; Xu, W.; Xiang, C.-H.; Ma, Y.-F. Luteolin Alleviates NLRP3 Inflammasome Activation and Directs Macrophage Polarization in Lipopolysaccharide-Stimulated RAW264.7 Cells. Am. J. Transl. Res. 2018, 10, 265–273. [Google Scholar] [PubMed]
- Cai, Q.; Rahn, R.O.; Zhang, R. Dietary Flavonoids, Quercetin, Luteolin and Genistein, Reduce Oxidative DNA Damage and Lipid Peroxidation and Quench Free Radicals. Cancer Lett. 1997, 119, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Lestari, M.L.A.D.; Indrayanto, G. Chapter Three—Curcumin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple Biological Activities of Curcumin: A Short Review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-Induced Angiogenesis Hastens Wound Healing in Diabetic Rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, M.; Saravanan, C.; Singh, S.K. Curcumin: A Potential Candidate for Matrix Metalloproteinase Inhibitors. Expert Opin. Ther. Targets 2012, 16, 959–972. [Google Scholar] [CrossRef]
- Alizadeh, M.; Kheirouri, S. Curcumin against Advanced Glycation End Products (AGEs) and AGEs-Induced Detrimental Agents. Crit. Rev. Food Sci. Nutr. 2019, 59, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Z.; Zhang, Z.; Ma, L. Inhibitory Effects of Curcumin Derivatives on Nonenzymatic Glucosylation in Vitro. Front. Chem. China 2006, 1, 227–231. [Google Scholar] [CrossRef]
- Liu, W.; Ma, H.; DaSilva, N.A.; Rose, K.N.; Johnson, S.L.; Zhang, L.; Wan, C.; Dain, J.A.; Seeram, N.P. Development of a Neuroprotective Potential Algorithm for Medicinal Plants. Neurochem. Int. 2016, 100, 164–177. [Google Scholar] [CrossRef]
- Hu, T.-Y.; Liu, C.-L.; Chyau, C.-C.; Hu, M.-L. Trapping of Methylglyoxal by Curcumin in Cell-Free Systems and in Human Umbilical Vein Endothelial Cells. J. Agric. Food Chem. 2012, 60, 8190–8196. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hu, Y.; Zhao, Y.; Cheng, M.; Qin, H.; Cheng, T.; Wang, Q.; Peng, X.; Zhang, X. Curcumin Attenuates Titanium Particle-Induced Inflammation by Regulating Macrophage Polarization In Vitro and In Vivo. Front. Immunol. 2017, 8, 55. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Abdollahi, E.; Nikfar, B.; Chaichian, S.; Ekhlasi-Hundrieser, M. Curcumin as a Potential Modulator of M1 and M2 Macrophages: New Insights in Atherosclerosis Therapy. Heart Fail. Rev. 2019, 24, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhou, J.; Li, H.; Gao, Y.; Xu, C.; Zhao, S.; Chen, Y.; Cai, W.; Wu, J. Curcumin Suppresses NLRP3 Inflammasome Activation and Protects against LPS-Induced Septic Shock. Mol. Nutr. Food Res. 2015, 59, 2132–2142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shi, S.; Jiang, P.; Huang, X.; Zhao, J.; Jin, Y.; Shen, Y.; Zhou, X.; Liu, H.; Cai, J. Curcumin Alleviates Hepatic Ischemia-Reperfusion Injury by Inhibiting Neutrophil Extracellular Traps Formation. J. Investig. Surg. 2023, 36, 2164813. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Li, S.; Ma, Y.; Hu, D.; Xiao, F. Curcumin Hinders PBDE-47-Induced Neutrophil Extracellular Traps Release via Nrf2-Associated ROS Inhibition. Ecotoxicol. Environ. Saf. 2021, 225, 112779. [Google Scholar] [CrossRef] [PubMed]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin Improves Wound Healing by Modulating Collagen and Decreasing Reactive Oxygen Species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nonaka, G.-I.; Nishioka, I. Tannins and Related Compounds. XL.: Revision of the Structures of Punicalin and Punicalagin, and Isolation and Characterization of 2-O-Galloylpunicalin from the Bark of Punica granatum L. Chem. Pharm. Bull. 1986, 34, 650–655. [Google Scholar] [CrossRef]
- Rozadi, N.; Oktavia, S.; Fauziah, F. Pharmacological Activities of Punicalagin: A Review. J. Drug Deliv. Ther. 2022, 12, 148–155. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, R.; Singh, V.; Mazumder, A.; Mazumder, R.; Kumar, A. Wound Healing Activity of Punicalin and Punicalagin Isolated from Punica granatum L. Rasayan J. Chem. 2022, 15, 183–189. [Google Scholar] [CrossRef]
- Tang, J.; Li, B.; Hong, S.; Liu, C.; Min, J.; Hu, M.; Li, Y.; Liu, Y.; Hong, L. Punicalagin Suppresses the Proliferation and Invasion of Cervical Cancer Cells through Inhibition of the β-Catenin Pathway. Mol. Med. Rep. 2017, 16, 1439–1444. [Google Scholar] [CrossRef]
- Liu, W.; Ma, H.; Frost, L.; Yuan, T.; Dain, J.A.; Seeram, N.P. Pomegranate Phenolics Inhibit Formation of Advanced Glycation Endproducts by Scavenging Reactive Carbonyl Species. Food Funct. 2014, 5, 2996–3004. [Google Scholar] [CrossRef]
- Ge, G.; Bai, J.; Wang, Q.; Liang, X.; Tao, H.; Chen, H.; Wei, M.; Niu, J.; Yang, H.; Xu, Y.; et al. Punicalagin Ameliorates Collagen-Induced Arthritis by Downregulating M1 Macrophage and Pyroptosis via NF-ΚB Signaling Pathway. Sci. China Life Sci. 2022, 65, 588–603. [Google Scholar] [CrossRef]
- Lo, J.; Liu, C.-C.; Li, Y.-S.; Lee, P.-Y.; Liu, P.-L.; Wu, P.-C.; Lin, T.-C.; Chen, C.-S.; Chiu, C.-C.; Lai, Y.-H.; et al. Punicalagin Attenuates LPS-Induced Inflammation and ROS Production in Microglia by Inhibiting the MAPK/NF-ΚB Signaling Pathway and NLRP3 Inflammasome Activation. J. Inflamm. Res. 2022, 15, 5347–5359. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tao, X.; Men, X.; Xu, Z.; Wang, T. In Vitro and in Vivo Antioxidant Activities of Three Major Polyphenolic Compounds in Pomegranate Peel: Ellagic Acid, Punicalin, and Punicalagin. J. Integr. Agric. 2017, 16, 1808–1818. [Google Scholar] [CrossRef]
- Aloqbi, A.; Omar, U.; Yousr, M.; Grace, M.; Lila, M.A.; Howell, N. Antioxidant Activity of Pomegranate Juice and Punicalagin. Nat. Sci. 2016, 08, 235. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Hou, X.; Li, D.; He, S.; Wan, C.; Yin, P.; Liu, M.; Liu, F.; Xu, J. Punicalagin Induces Nrf2/HO-1 Expression via Upregulation of PI3K/AKT Pathway and Inhibits LPS-Induced Oxidative Stress in RAW264.7 Macrophages. Mediators Inflamm. 2015, 2015, e380218. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-Scavenging Hydrogel to Promote Healing of Bacteria Infected Diabetic Wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- Al-Irayfawee, N.; Al-Biatey, D.; Hussein, Z. Effectiveness of Punica granatum and Propolis: A New Dressing Method in Management of Diabetic Foot Ulceration. Indian J. Forensic Med. Toxicol. 2019, 13, 314. [Google Scholar] [CrossRef]
- Mokhtari, M.; Razzaghi, R.; Momen-Heravi, M. The Effects of Curcumin Intake on Wound Healing and Metabolic Status in Patients with Diabetic Foot Ulcer: A Randomized, Double-Blind, Placebo-Controlled Trial. Phytother. Res. 2021, 35, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Kesavan, R.; Kavitha, K.V.; Kumpatla, S. A Pilot Study on the Effects of a Polyherbal Formulation Cream on Diabetic Foot Ulcers. Indian J. Med. Res. 2011, 134, 168–173. [Google Scholar] [PubMed]
- Carvalho, M.T.B.; Araújo-Filho, H.G.; Barreto, A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S.; Barreto, R.S.S. Wound Healing Properties of Flavonoids: A Systematic Review Highlighting the Mechanisms of Action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef]
- Lodhi, S.; Singhai, A.K. Wound Healing Effect of Flavonoid Rich Fraction and Luteolin Isolated from Martynia Annua Linn. on Streptozotocin Induced Diabetic Rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Huang, C.-N.; Liao, C.-K.; Chang, H.-M.; Kuan, Y.-H.; Tseng, T.-J.; Yen, K.-J.; Yang, K.-L.; Lin, H.-C. Effects of Rutin on Wound Healing in Hyperglycemic Rats. Antioxidants 2020, 9, 1122. [Google Scholar] [CrossRef]
- Özay, Y.; Güzel, S.; Erdoğdu, İ.H.; Yıldırım, Z.; Pehlivanoğlu, B.; Aydın Türk, B.; Darcan, S. Evaluation of the Wound Healing Properties of Luteolin Ointments on Excision and Incision Wound Models in Diabetic and Non-Diabetic Rats. Rec. Nat. Prod. 2018, 12, 350–366. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or Signalling Molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and Antiradical Activities of Flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Singh, M.P.; Gupta, A.; Sisodia, S.S. Wound Healing Activity of Terminalia Bellerica Roxb. and Gallic Acid in Experimentally Induced Diabetic Animals. J. Complement. Integr. Med. 2020, 17, 20190133. [Google Scholar] [CrossRef]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Nitiruangjaras, A.; Reanmongkol, W. Wound Healing Activities of Standardized Pomegranate Rind Extract and Its Major Antioxidant Ellagic Acid in Rat Dermal Wounds. J. Nat. Med. 2014, 68, 377–386. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chang, L.-S. Gallic Acid Downregulates Matrix Metalloproteinase-2 (MMP-2) and MMP-9 in Human Leukemia Cells with Expressed Bcr/Abl. Mol. Nutr. Food Res. 2012, 56, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-T.; Wang, C.-Y.; Yang, R.-C.; Wu, H.-T.; Yang, S.-H.; Cheng, Y.-C.; Pang, J.-H.S. Ellagic Acid, the Active Compound of Phyllanthus Urinaria, Exerts In Vivo Anti-Angiogenic Effect and Inhibits MMP-2 Activity. Evid. Based Complement. Altern. Med. 2011, 2011, enep207. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Guan, N.; Gao, X.; Zhang, W.; Xue, G.; Wang, L. Gallic Acid Inhibits M1 Macrophage Polarization via Adenosine 5′-Monophosphate-Activated Protein Kinase/Signal Transducers and Activators of Transcription 1 Pathway. Indian J. Pharm. Sci. 2022, 84, 284–293. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and Hydrolysable Tannins as Antioxidants Influencing the Health. Mini Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, Q.; Tian, T.; Chang, Y.; Li, Y.; Duan, L.-R.; Li, H.; Wang, S.-W. Gastroprotective Effect of Gallic Acid against Ethanol-Induced Gastric Ulcer in Rats: Involvement of the Nrf2/HO-1 Signaling and Anti-Apoptosis Role. Biomed. Pharmacother. 2020, 126, 110075. [Google Scholar] [CrossRef]
- Altamimi, J.Z.; AlFaris, N.A.; Alshammari, G.M.; Alagal, R.I.; Aljabryn, D.H.; Aldera, H.; Alrfaei, B.M.; Alkhateeb, M.A.; Yahya, M.A. Ellagic Acid Protects against Diabetic Nephropathy in Rats by Regulating the Transcription and Activity of Nrf2. J. Funct. Foods 2021, 79, 104397. [Google Scholar] [CrossRef]
- Tsai, F.-S.; Lin, L.-W.; Wu, C.-R. Lupeol and Its Role in Chronic Diseases. Drug Discov. Mother Nat. 2016, 929, 145–175. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Chen, J.; Chen, T.; Shi, Z.; Lei, M.; Zhang, Y.; Bai, P.; Li, Y.; Fei, X. The Pentacyclic Triterpene Lupeol Switches M1 Macrophages to M2 and Ameliorates Experimental Inflammatory Bowel Disease. Int. Immunopharmacol. 2016, 30, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Junior, M.S.; Pereira, E.P.; de Amorim, V.C.M.; Reis, L.T.C.; do Nascimento, R.P.; da Silva, V.D.A.; Costa, S.L. Lupeol Inhibits LPS-Induced Neuroinflammation in Cerebellar Cultures and Induces Neuroprotection Associated to the Modulation of Astrocyte Response and Expression of Neurotrophic and Inflammatory Factors. Int. Immunopharmacol. 2019, 70, 302–312. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, C.; Hao, J.; Zhang, M.; Wang, Z.; Yin, T.; Lin, K.; Liu, W.; Jiang, Q.; Li, Z.; et al. Beneficial Consequences of Lupeol on Middle Cerebral Artery-Induced Cerebral Ischemia in the Rat Involves Nrf2 and P38 MAPK Modulation. Metab. Brain Dis. 2020, 35, 841–848. [Google Scholar] [CrossRef]
- Beserra, F.P.; Vieira, A.J.; Gushiken, L.F.S.; de Souza, E.O.; Hussni, M.F.; Hussni, C.A.; Nóbrega, R.H.; Martinez, E.R.M.; Jackson, C.J.; de Azevedo Maia, G.L.; et al. Lupeol, a Dietary Triterpene, Enhances Wound Healing in Streptozotocin-Induced Hyperglycemic Rats with Modulatory Effects on Inflammation, Oxidative Stress, and Angiogenesis. Oxid. Med. Cell. Longev. 2019, 2019, e3182627. [Google Scholar] [CrossRef] [PubMed]

| Scientific Name | Mechanism of Action | Extract | Model | Ref. | Extract Composition | Ref. | |
|---|---|---|---|---|---|---|---|
| Aloe vera |
| Gel extract | STZ rats | [96] | Apigenin | Quercetin | [97] |
| Myricetin | Rutin | ||||||
| Isovitexin | Epicatechin | ||||||
| Kaempferol | p-coumarin | ||||||
| Luteolin | Cafeic acid | ||||||
| Ferulic acid | |||||||
| Annona Squamosa |
| Ethanolic leaf extract | STZ rats | [98] | Rutin | Quercetin | [99,100] |
| Kaempferol | Isorhamnetin | ||||||
| Carica papaya |
| Juice of unripe fruit | STZ rats | [94] | Caffeic acid | Ferulic acid | [101,102,103] |
| p-coumaric acid | Quercetin | ||||||
| Rutin | Kaempferol | ||||||
| Myricetin | |||||||
| Curcuma longa |
| Aqueous extract | STZ rats | [104] | Curcumin | [105] | |
| Demethoxycurcumin | |||||||
| Bisdemethoxycurcumin | |||||||
| Momordica charantia |
| Aqueous extract of dried fruits | STZ rats | [106] | Caffeic acid | Catechin | [107,108] |
| Epicatechin | Charantin | ||||||
| Aqueous fruit extract | STZ rats | [109] | Chlorogenic acid | Gallic acid | ||
| Ferulic acid | |||||||
| Moringa oleifera |
| Methanolic extract of dried leaves | STZ rats | [110] | Quercetin glycoside Kaempferol glycoside Vicenin-2 | [111] | |
| Aqueous fraction of methanolic leaf extract | STZ and NAD rats | [112] | ||||
| Aqueous extract of leaves | STZ rats | Citric acid | Quinic acid | [113] | ||
| [114] | Vicenin-2 | Vitexin | |||||
| Quercetin glycoside | Luteolin glycoside | ||||||
| Psidium guajava L. |
| Acetone extract of leaves | Alloxan rats | [115] | Guavanoic acid | guavacoumaric acid | [116] |
| Myricetin | Quercetin | ||||||
| Luteolin | Kaempferol | ||||||
| Punica granatum |
| Ethanolic extract of dried skin fruit | Alloxan rats | [117] | Punicalagin | Gallic acid | [118] |
| Ellagic acid | Rutin | ||||||
| Quercetin | |||||||
| Rosmarinus officinalis |
| Aqueous extract of the aerial part | Alloxan mice | [119] | Kaempferol glycoside | Quercetin glycoside | [120] |
| Gallic acid | Quercetin | ||||||
| Gentisic acid | Epicatechin | ||||||
| Rutin | Ellagic acid | ||||||
| Rosmarinic acid | |||||||
| Plants | Extracts | Model | Effect | References |
|---|---|---|---|---|
| Aloe vera | Gel | LPS activated PBMC | Decrease MMP9 activity and expression | [121] |
| Annona squamosa | Leaves extract | Rats exposed to UVB | Decrease MMP1 expression | [126] |
| Carica papaya | Ethanolic dried leaves extract | NHDFs cells exposed to UVB | Decrease MMP1 and MMP3 expression | [127] |
| Curcuma longa | Curcuminoids | Keratinocytes HaCaT and Fibroblasts | Decrease MMP1 expression | [129] |
| Momordica charantia | Ethanolic leaves extract | PLS10 cells | Decrease MMP2 and MMP9 secretion | [135] |
| Moringa oleifa | Isothiocyanate of seeds | Keratinocytes HaCaT | Decrease MMP1, MMP3, and MMP9 expression | [140] |
| Psidium guajava | Aqueous leaves extract | DU-145 cells | Decrease MMP2 and MMP9 expression | [144] |
| Punica granatum | Aqueous peels extract | LPS-induced lung inflammation mouse model | Inhibition MMP2 and MMP9 activities | [147] |
| Rosmarinus officinalis | Aqueous extract | Fibroblast and 3d reconstructed skin model | Decrease MMP1 expression and activation | [153] |
| Plants | Extracts | Model | Effect | References |
|---|---|---|---|---|
| Aloe vera | Methanolic extract | BSA + Glucose | Inhibit AGE and N∊-Carboxymethyl-lysine (CML) formation | [122] |
| Carica papaya | Fruit juice | Aldolase reductase and Sorbitol dehydrogenase activity assay | Inhibit enzymes of the polyol pathway, which is involved in the formation of AGEs | [128] |
| Curcuma longa | Curcumin | Diabetic rats | Decrease AGE-Collagen | [130] |
| Momordica charantia | Methanolic extract | Lysozyme + Methylglyoxal | Inhibit AGE and CML formation | [136] |
| Moringa oleifa | Methanolic and aqueous extracts | BSA+ Fructose | Inhibit CML formation and crosslinking breaker capacity | [141] |
| Psidium guajava L. | Aqueous extract | BSA + Glucose | Inhibit AGE formation | [145] |
| Punica granatum | Fruit juice | BSA + Glucose/Fructose | Inhibit glyceraldehyde-derived AGE formation | [148] |
| Rosmarinus officinalis | Aqueous extract | BSA+ Fructose | Inhibit AGE formation | [151] |
| Plants | Extracts | Model | Effect | References |
|---|---|---|---|---|
| Aloe vera | Gel | Rats | Modulate macrophages polarization | [123] |
| Curcuma longa | Gel | Mice and RAW264.7 macrophages | Enhance M2 | [132] |
| Momordica charantia | Fruit powder | Mice | Enhance M2 and decrease M1 | [155] |
| Punica granatum | Juice | J774A.1 macrophages | Enhance M2: increase IL-10 and decrease IL-6 | [149] |
| Plants | Extracts | Model | Effect | References |
|---|---|---|---|---|
| Aloe vera | Gel | THP1 macrophages | Decrease IL-1β and NLRP3 compounds Decrease NF-κB and MAPK activation | [124] |
| Curcuma longa | Curcumin | Depressive rats model | Inhibit NLRP3 Decrease mature IL-1β production | [133] |
| Momordica charantia | Acetonic fraction of fruit extract | RAW264.7 Mice | Decrease NLRP3 expression | [137,138] |
| Moringa oleifa | Ethanolic extract | Methotrexate-Induced Oxidative Stress and Apoptosis on Mouse Spleen | Decrease NLRP3 expression Increase antioxydant enzymes | [142] |
| Psidium guajava | Flavonoids leaf extract | Chronic Pancreatitis Mouse Model | Decrease NLRP3 expression Decrease IL-1β and IL-18 expression | [146] |
| Rosmarinus officinalis | Carnosic acid | Human macrophages | Inhibit NLRP3 activation Decrease mitochondrial ROS production | [152] |
| Plants | Extracts | Model | Effect | References |
|---|---|---|---|---|
| Aloe vera | Polysaccharides | Acute colitis in mice | Enhance Nrf2 expression | [125] |
| Curcuma longa | Curcumin | Lymphoma ascites cells | Enhance antioxydant enzyme expression Decrease proinflammatory cytokines | [134,156] |
| Momordica charantia | Polysaccharides | Hypothalamus rats | Increase antioxydant enzyme expression Improve Nrf2 translocation | [139] |
| Moringa oleifa | Aqueous leaves extract | Hepatic steatosis mices | Increase Nrf2 expression | [143] |
| Punica granatum | Fruit wine | SH-SY5Ycells | Increase Nrf2 translocation Inhibit NF-κB | [150] |
| Rosmarinus officinalis | Carnosol | HCT116 cells | Increase Nrf2 activation | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accipe, L.; Abadie, A.; Neviere, R.; Bercion, S. Antioxidant Activities of Natural Compounds from Caribbean Plants to Enhance Diabetic Wound Healing. Antioxidants 2023, 12, 1079. https://doi.org/10.3390/antiox12051079
Accipe L, Abadie A, Neviere R, Bercion S. Antioxidant Activities of Natural Compounds from Caribbean Plants to Enhance Diabetic Wound Healing. Antioxidants. 2023; 12(5):1079. https://doi.org/10.3390/antiox12051079
Chicago/Turabian StyleAccipe, Laura, Alisson Abadie, Remi Neviere, and Sylvie Bercion. 2023. "Antioxidant Activities of Natural Compounds from Caribbean Plants to Enhance Diabetic Wound Healing" Antioxidants 12, no. 5: 1079. https://doi.org/10.3390/antiox12051079
APA StyleAccipe, L., Abadie, A., Neviere, R., & Bercion, S. (2023). Antioxidant Activities of Natural Compounds from Caribbean Plants to Enhance Diabetic Wound Healing. Antioxidants, 12(5), 1079. https://doi.org/10.3390/antiox12051079






