Paclitaxel Protects against Isoproterenol-Induced Damage in Rat Myocardium: Its Heme-Oxygenase Mediated Role in Cardiovascular Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
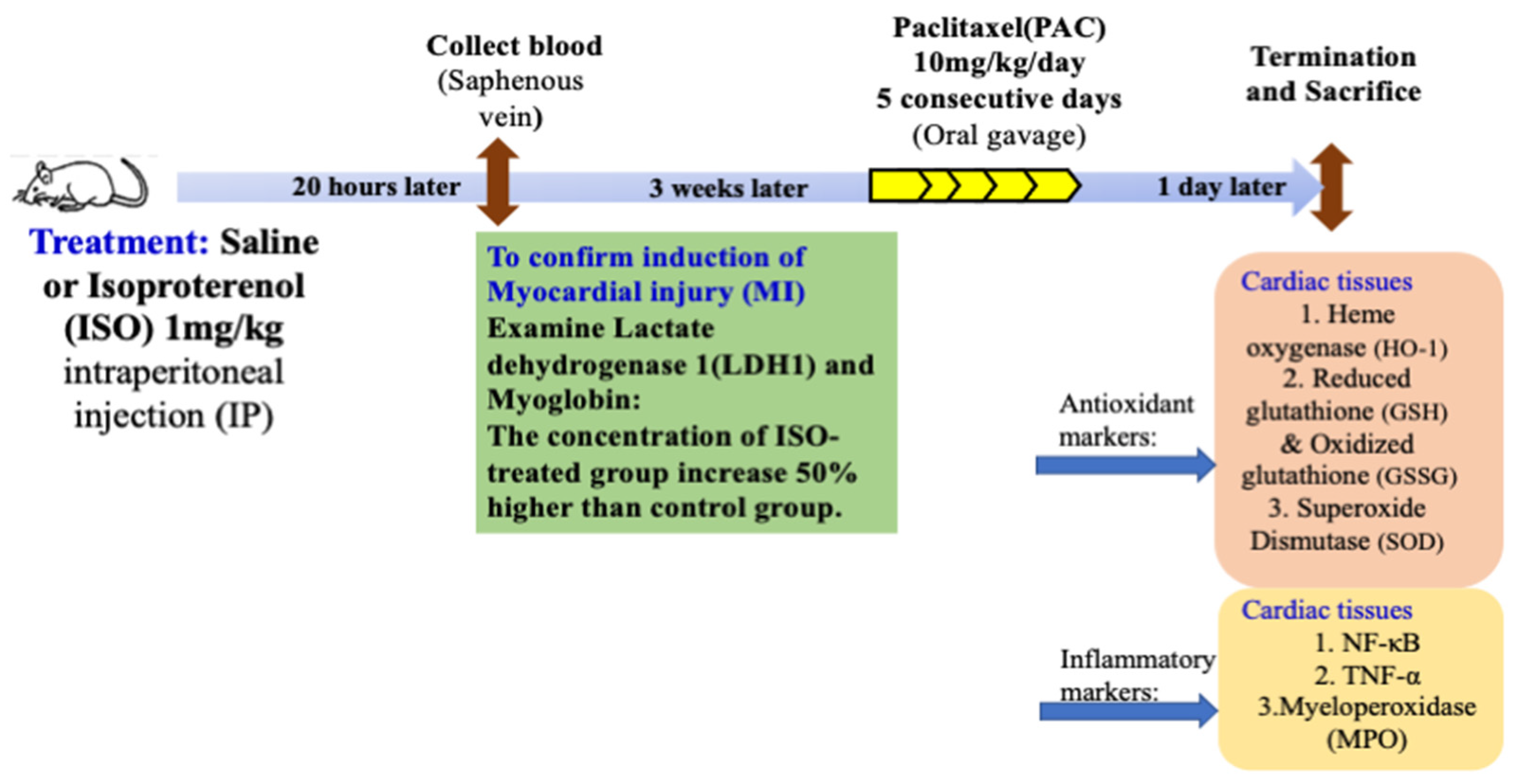
2.2. Experimental Protocol
2.3. Determination of Cardiac HO-1, NF-kB and TNF-α Concentrations
2.4. Measurement of Cardiac Total (GSH + GSSG) Level
2.5. Examination of Superoxide Dismutase (SOD) in Cardiac Tissue
2.6. Determination of Cardiac MPO Activity
2.7. Determination of Cardiac HO Activity
2.8. Statistical Analysis
3. Results
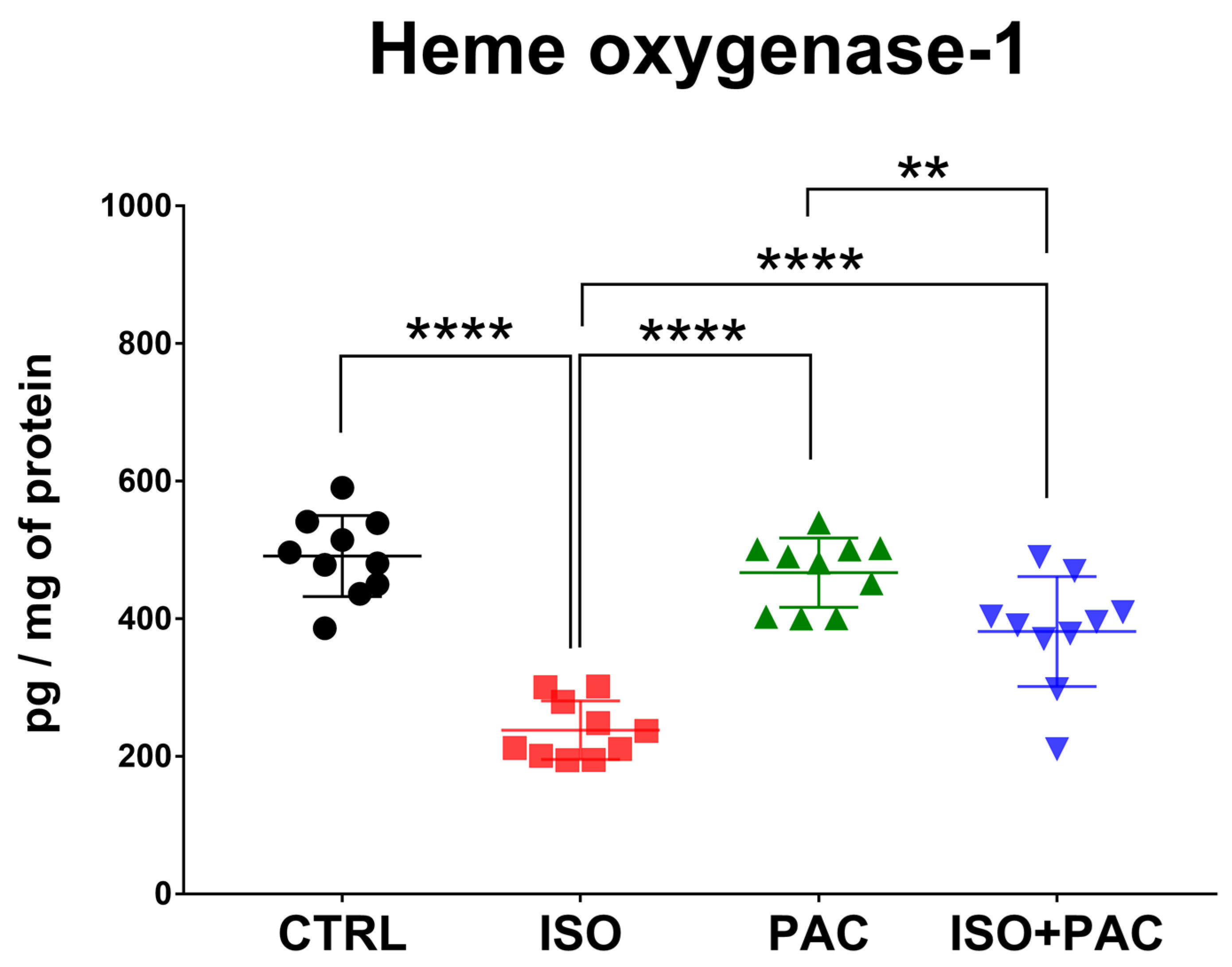
3.1. HO-1 Protein Expression
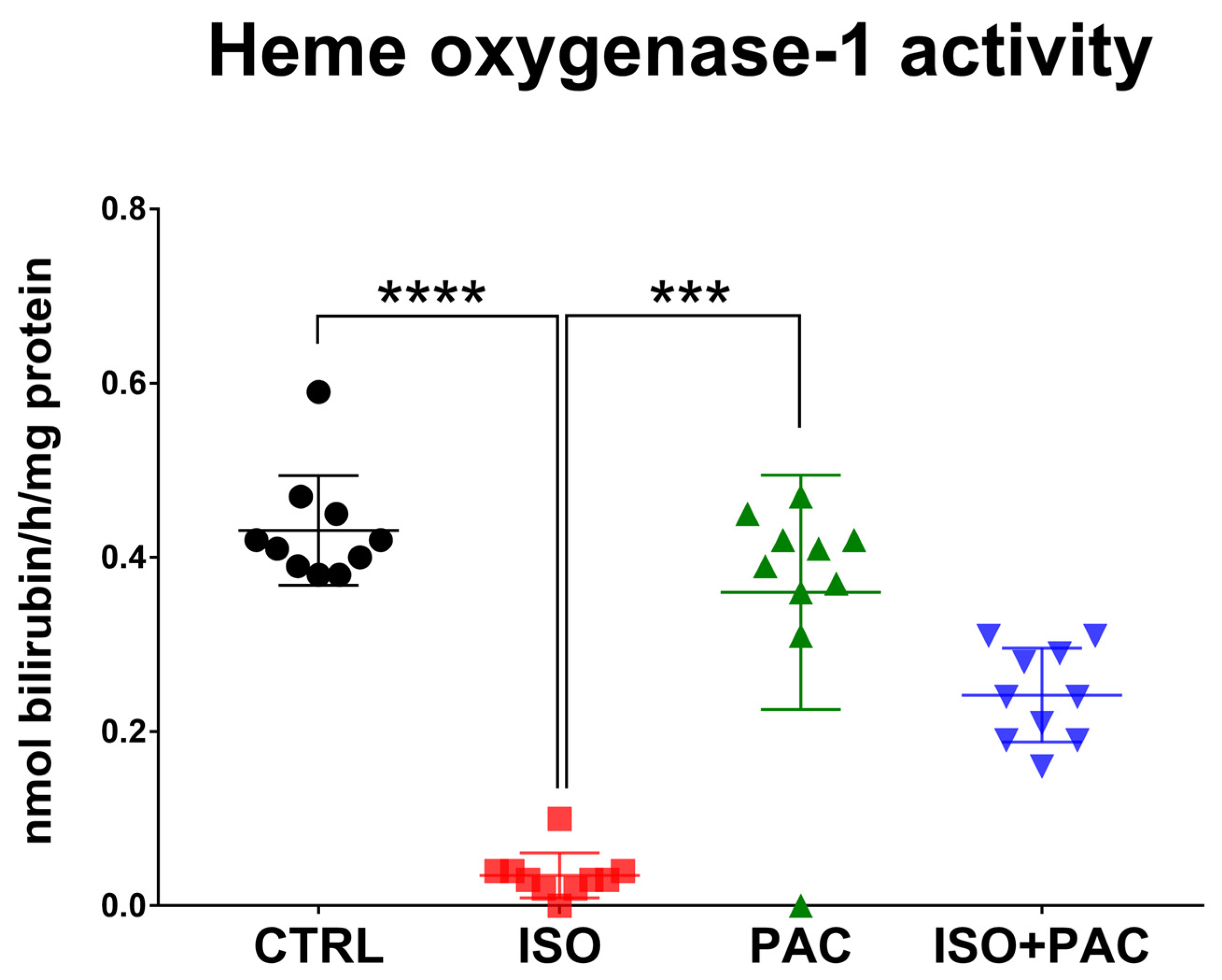
3.2. HO-1 Protein Activity
3.3. Superoxide Dismutase
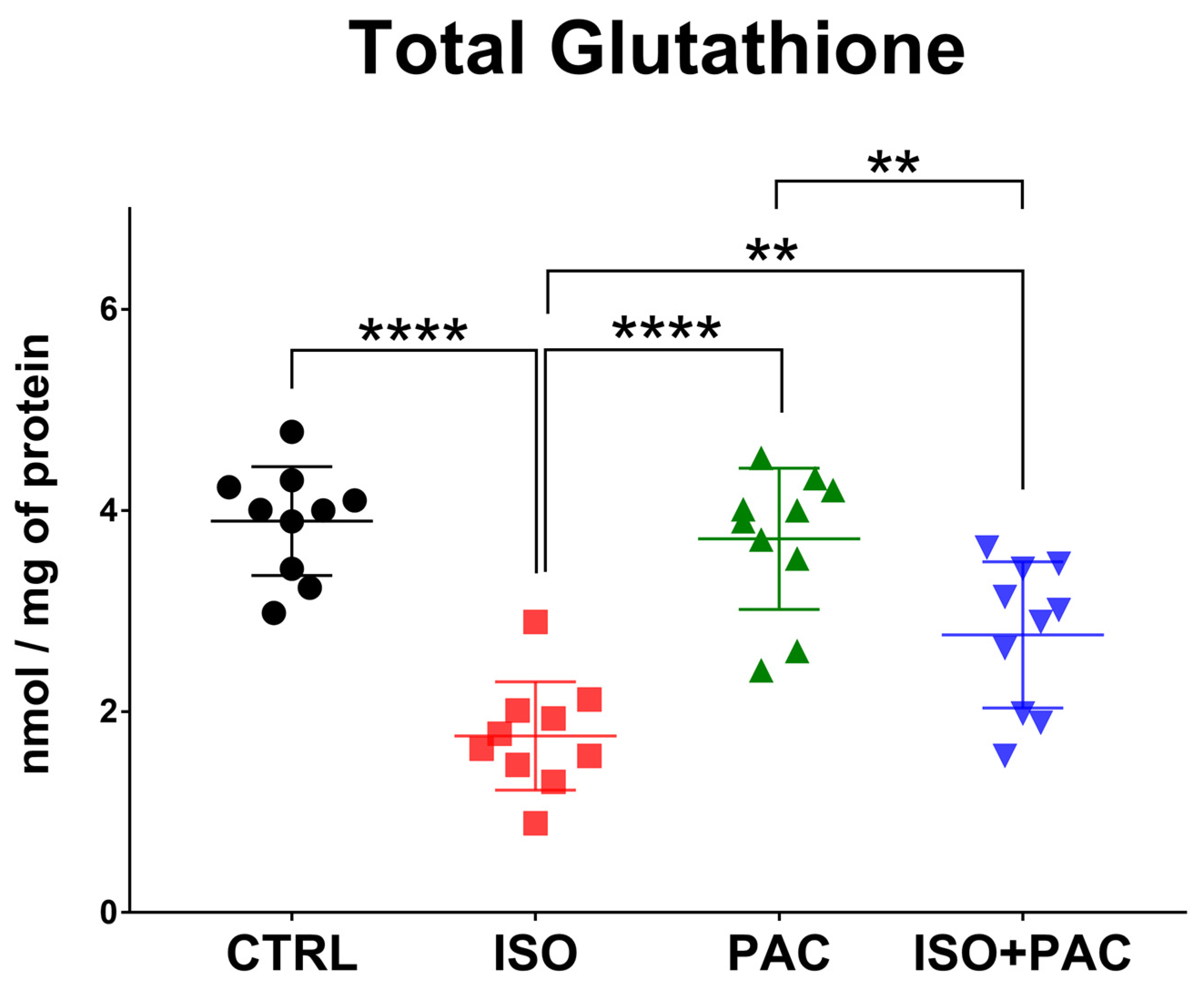
3.4. Total Glutathione
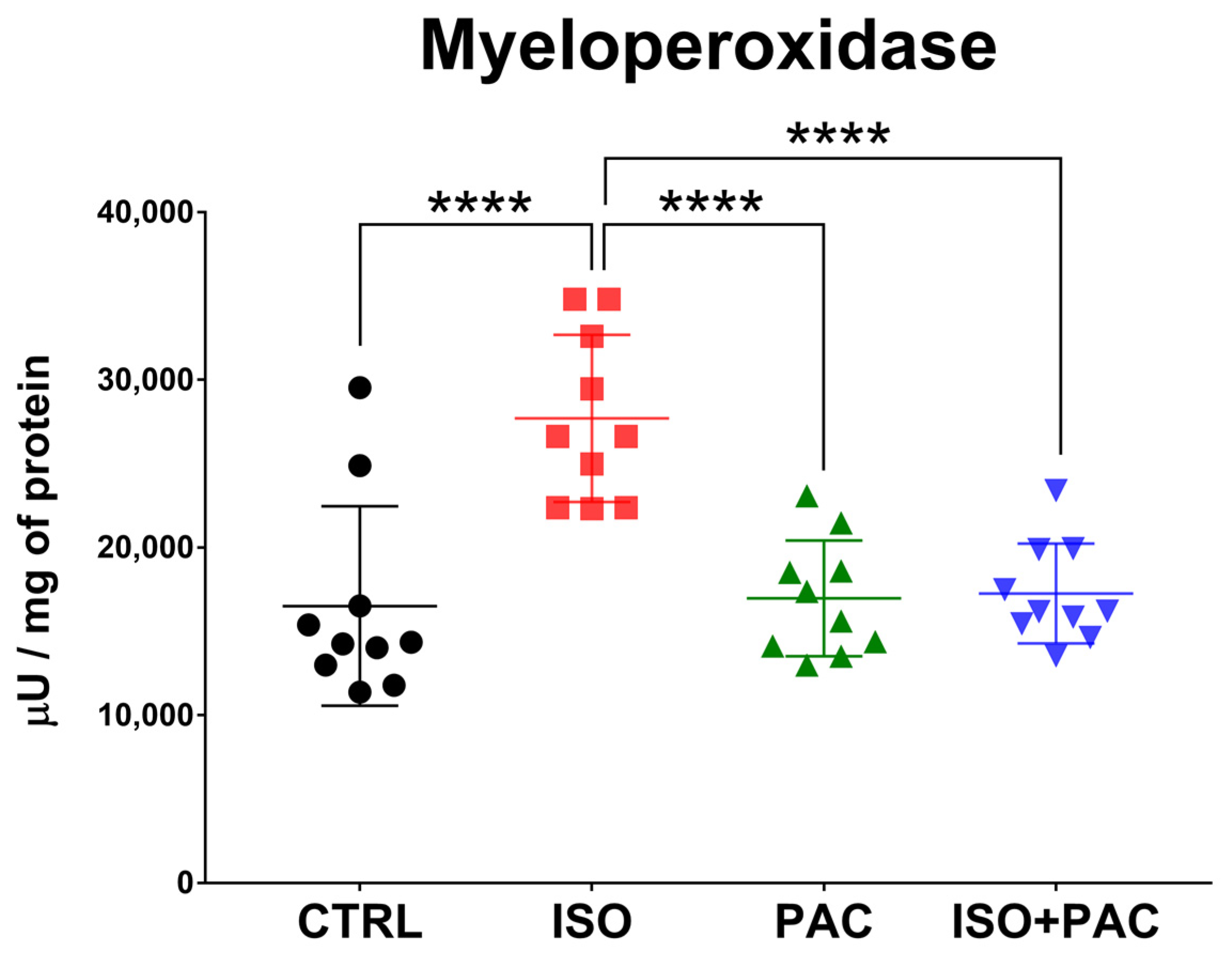
3.5. Myeloperoxidase
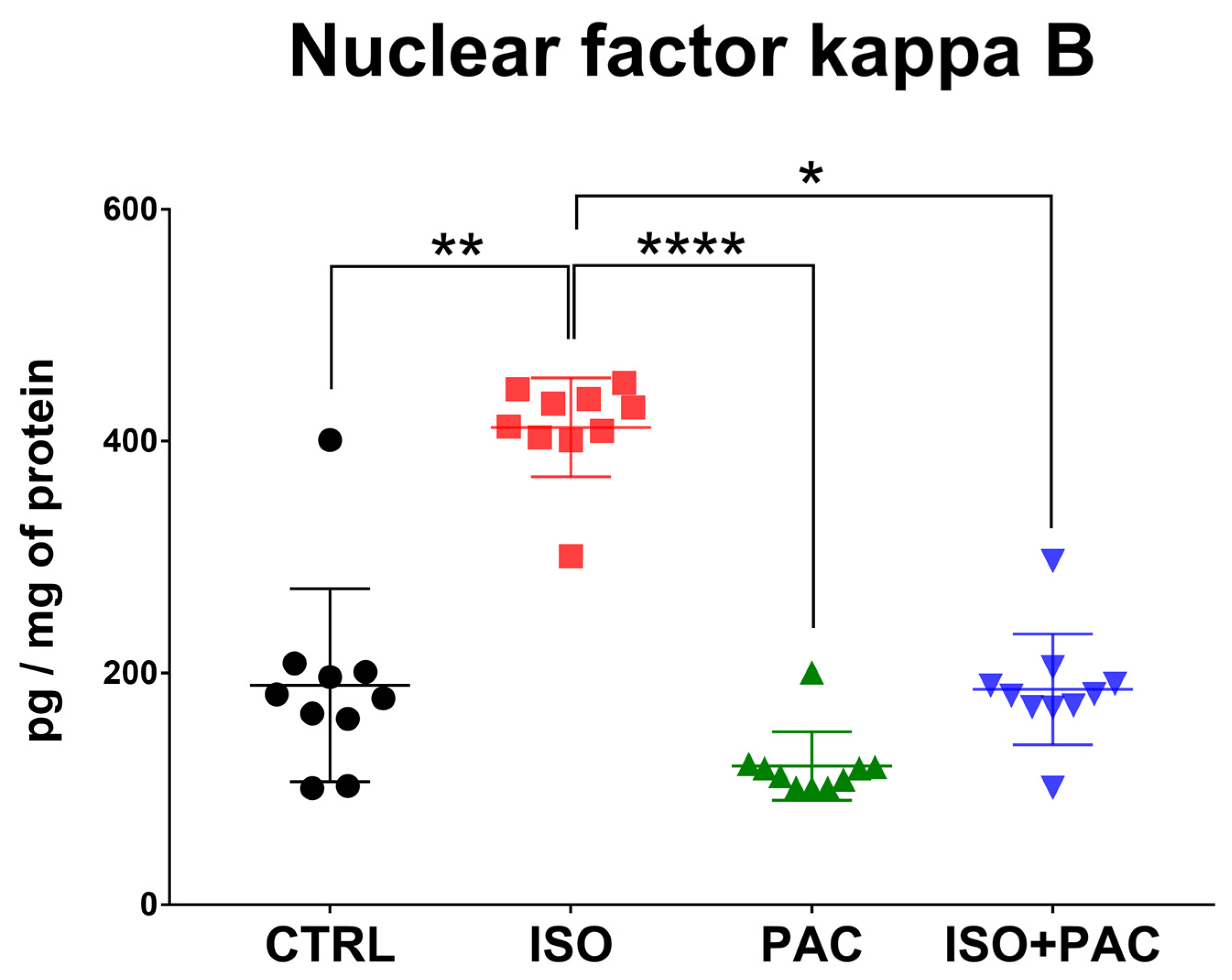
3.6. NF-κB Protein Expression
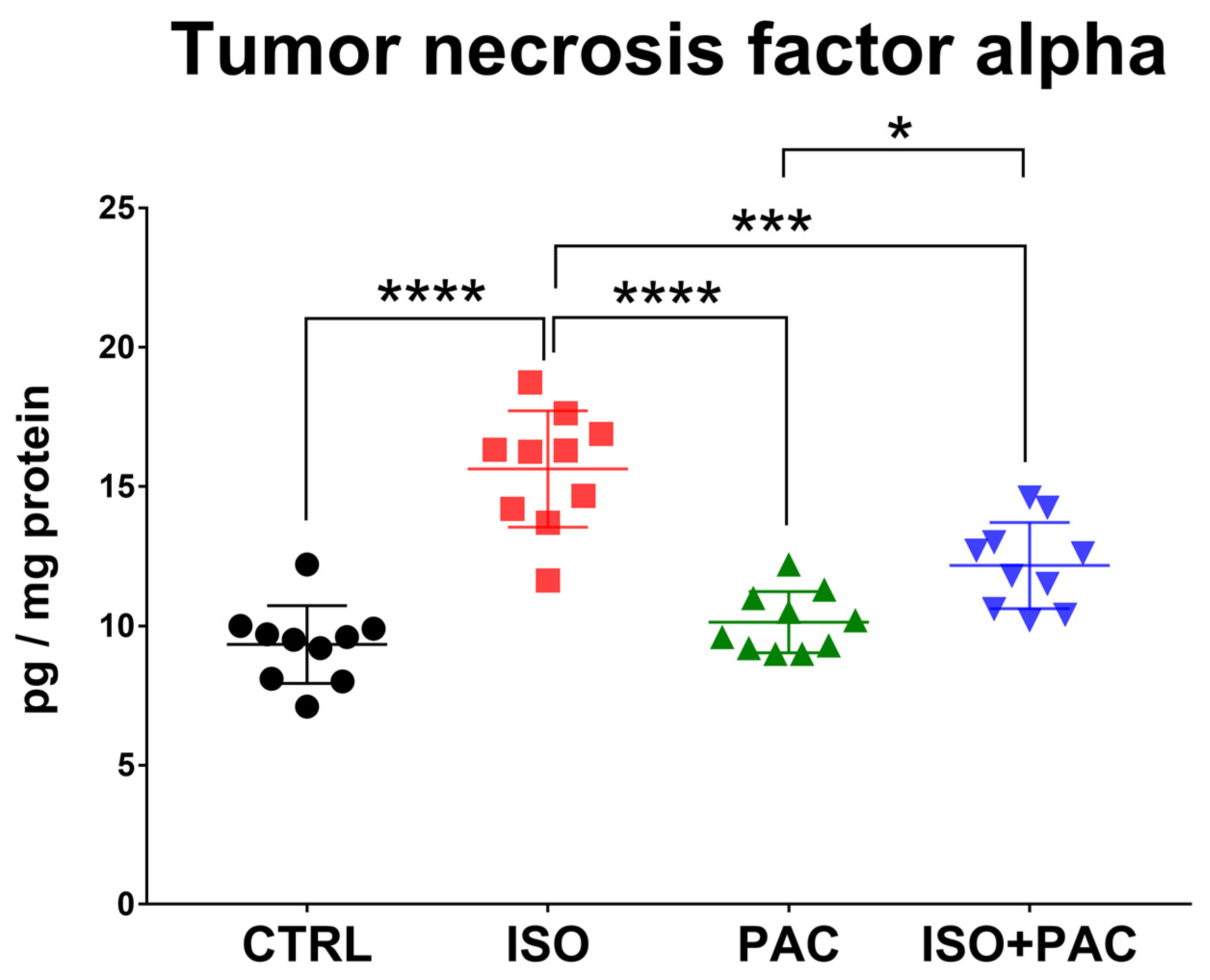
3.7. TNF-α Protein Expression
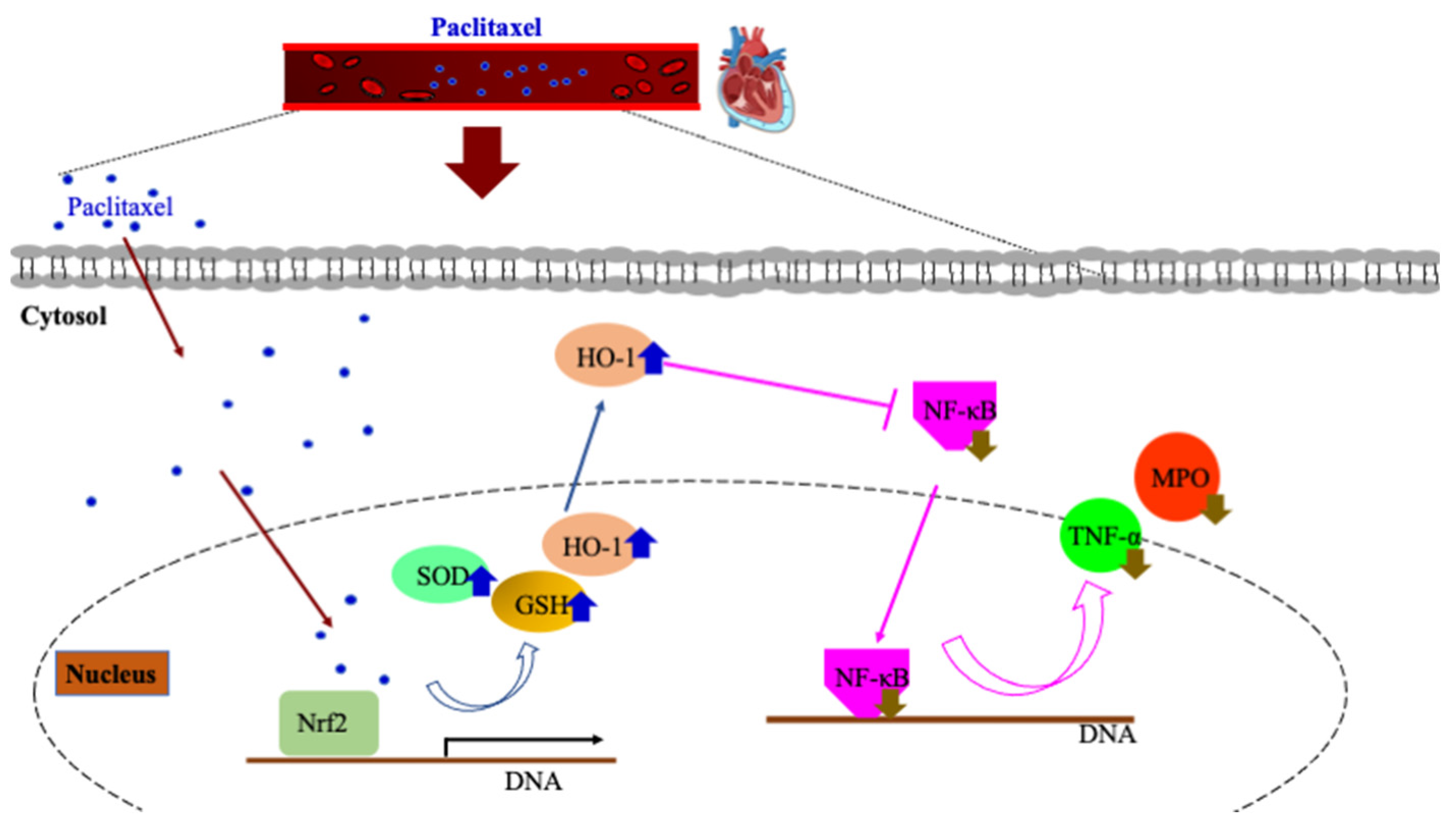
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumgartner, I.; Schindewolf, M. The paclitaxel story in cardiovascular medicine. Eur. Heart J. 2020, 41, 3740–3742. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, F.; Rivero, F.; Granada, J.F. Safety of Paclitaxel-Coated Balloons in the Coronary Arteries. J. Am. Coll. Cardiol. 2020, 75, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L.; Conte, M.S.; Murad, M.H. Critical review and evidence implications of paclitaxel drug-eluting balloons and stents in peripheral artery disease. J. Vasc. Surg. 2019, 70, 3–7. [Google Scholar] [CrossRef]
- Thompson, C.A.; Huibregtse, B.; Poff, B.; Wilson, G.J. Time Dependent Vascular and Myocardial Responses of a Second Generation, Small Vessel, Paclitaxel-Eluting Stent Platform. Catheter. Cardiovasc. Interv. 2009, 73, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Spargias, K.; Gyöngyösi, M.; Hemetsberger, R.; Posa, A.; Pavo, N.; Pavo, I.J.; Huber, K.; Petrasi, Z.; Petnehazy, O.; von Strandmann, R.P.; et al. Valvuloplasty with a Paclitaxel-Eluting Balloon Prevents Restenosis in an Experimental Animal Model of Aortic Stenosis. J. Heart Valve Dis. 2014, 23, 484–491. [Google Scholar]
- Pósa, A.; Nyolczas, N.; Hemetsberger, R.; Pavo, N.; Petneházy, Ö.; Petrasi, Z.; Sangiorgi, G.; Gyongyosi, M. Optimization of Drug-Eluting Balloon Use for Safety and Efficacy: Evaluation of the 2nd Generation Paclitaxel-Eluting DIOR-Balloon in Porcine Coronary Arteries. Catheter. Cardiovasc. Interv. 2010, 76, 395–403. [Google Scholar] [CrossRef]
- Beckman, J.A.; White, C.J. Paclitaxel-Coated Balloons and Eluting Stents: Is There a Mortality Risk in Patients with Peripheral Artery Disease? Circulation 2019, 140, 1342–1351. [Google Scholar] [CrossRef]
- Rodríguez-Sinovas, A.; Abad, E.; Sánchez, J.A.; Sanz, C.F.; Inserte, J.; Ruiz-Meana, M.; Alburquerque-Béjar, J.J.; García-Dorado, D. Microtubule stabilization with paclitaxel does not protect against infarction in isolated rat hearts. Exp. Physiol. 2015, 100, 23–34. [Google Scholar] [CrossRef]
- Steiner, S.; Schmidt, A.; Zeller, T.; Tepe, G.; Thieme, M.; Maiwald, L.; Schröder, H.; Euringer, W.; Ulrich, M.; Brechtel, K.; et al. COMPARE: Prospective, Randomized, Non-Inferiority Trial of High- vs. Low-Dose Paclitaxel Drug-Coated Balloons for Femoropopliteal Interventions. Eur. Heart J. 2020, 41, 2541–2552. [Google Scholar] [CrossRef]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Karnabatidis, D. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2018, 7, e011245. [Google Scholar] [CrossRef]
- Zeller, T.; Micari, A.; Scheinert, D.; Baumgartner, I.; Bosiers, M.; Vermassen, F.E.; Banyai, M.; Shishehbor, M.H.; Wang, H.; Brodmann, M.; et al. The IN.PACT DEEP Clinical Drug-Coated Balloon Trial: 5-Year Outcomes. JACC Cardiovasc. Interv. 2020, 13, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Freisinger, E.; Koeppe, J.; Gerss, J.; Goerlich, D.; Malyar, N.M.; Marschall, U.; Faldum, A.; Reinecke, H. Mortality after use of paclitaxel-based devices in peripheral arteries: A real-world safety analysis. Eur. Heart J. 2020, 41, 3732–3739. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.-M.; Wang, Q.; You, H.-Y.; Li, J.; Yang, Z.-Y. Stabilizing Microtubules Decreases Myocardial Ischaemia-Reperfusion Injury. J. Int. Med. Res. 2011, 39, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, Y.; Wang, Q.; Wang, R.; Guo, S.; Zhao, X.; Zhang, Y.; Tong, D.; Yang, Z. Taxol Prevents Myocardial Ischemia-reperfusion Injury by Inducing JNK-Mediated HO-1 Expression. Pharm. Biol. 2016, 54, 555–560. [Google Scholar] [CrossRef]
- Kumazawa, A.; Katoh, H.; Nonaka, D.; Watanabe, T.; Saotome, M.; Urushida, T.; Satoh, H.; Hayashi, H. Microtubule Disorganization Affects the Mitochondrial Permeability Transition Pore in Cardiac Myocytes. Circ. J. 2014, 78, 1206–1215. [Google Scholar] [CrossRef]
- Ren, S.; Huang, T.; Ou, D.; Feng, L.; Huang, S.; Zhou, C.; Ge, L. Inhibition of TNF-α and JNK Signaling Pathway Can Reduce Paclitaxel-Induced Apoptosis of Mouse Cardiomyocytes. Appl. Bionics Biomech. 2022, 2022, 8460121. [Google Scholar] [CrossRef]
- Kang, Y.; Cai, Y.; Pan, W. Rapamycin and Paclitaxel Affect Human Aortic Smooth Muscle Cells-Derived Foam Cells Viability and Proliferation. Braz. J. Cardiovasc. Surg. 2022, 37, 200–206. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Abduh, M.S.; Abukhalil, M.H.; Aladaileh, S.H.; Hanieh, H.; Mahmoud, A.M. Umbelliferone Prevents Isoproterenol-Induced Myocardial Injury by Upregulating Nrf2/HO-1 Signaling, and Attenuating Oxidative Stress, Inflammation, and Cell Death in Rats. Biomed. Pharmacother. 2022, 149, 112900. [Google Scholar] [CrossRef]
- Kim, D.-W.; Kwon, J.-S.; Kim, Y.-G.; Kim, M.S.; Lee, G.-S.; Youn, T.-J.; Cho, M.-C. Novel Oral Formulation of Paclitaxel Inhibits Neointimal Hyperplasia in a Rat Carotid Artery Injury Model. Circulation 2004, 109, 1558–1563. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M.K. Heme Oxygenase-1/Carbon Monoxide: From Basic Science to Therapeutic Applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Saji, K.; Fukumoto, Y.; Suzuki, J.; Fukui, S.; Nawata, J.; Shimokawa, H. Colchicine, a Microtubule Depolymerizing Agent, Inhibits Myocardial Apoptosis in Rats. Tohoku J. Exp. Med. 2007, 213, 139–148. [Google Scholar] [CrossRef]
- Eltobshy, S.A.; Hussein, A.M.; Elmileegy, A.A.; Askar, M.H.; Khater, Y.; Metias, E.F.; Helal, G.M. Effects of Heme Oxygenase-1 Upregulation on Isoproterenol-Induced Myocardial Infarction. Korean J. Physiol. Pharmacol. 2019, 23, 203–217. [Google Scholar] [CrossRef]
- Harvey, C.J.; Thimmulappa, R.K.; Singh, A.; Blake, D.J.; Ling, G.; Wakabayashi, N.; Fujii, J.; Myers, A.; Biswal, S. Nrf2-Regulated Glutathione Recycling Independent of Biosynthesis Is Critical for Cell Survival during Oxidative Stress. Free Radic. Biol. Med. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the Heart of Oxidative Stress and Cardiac Protection. Physiol. Genom. 2018, 50, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Abukhalil, M.H.; Hussein, O.E.; Aladaileh, S.H.; Althunibat, O.Y.; Al-Amarat, W.; Saghir, S.A.; Alfwuaires, M.A.; Algefare, A.I.; Alanazi, K.M.; Al-Swailmi, F.K.; et al. Visnagin Prevents Isoproterenol-Induced Myocardial Injury by Attenuating Oxidative Stress and Inflammation and Upregulating Nrf2 Signaling in Rats. J. Biochem. Mol. Toxicol. 2021, 35, e22906. [Google Scholar] [CrossRef] [PubMed]
- Sandamali, J.A.N.; Hewawasam, R.P.; Jayatilaka, K.A.P.W.; Mudduwa, L.K.B. Cinnamomum zeylanicum Blume (Ceylon cinnamon) Bark Extract Attenuates Doxorubicin Induced Cardiotoxicity in Wistar Rats. Saudi Pharm. J. 2021, 29, 820–832. [Google Scholar] [CrossRef]
- Heslop, C.L.; Frohlich, J.J.; Hill, J.S. Myeloperoxidase and C-Reactive Protein Have Combined Utility for Long-Term Prediction of Cardiovascular Mortality after Coronary Angiography. J. Am. Coll. Cardiol. 2010, 55, 1102–1109. [Google Scholar] [CrossRef]
- Sharma, L.K.; Lu, J.; Bai, Y. Mitochondrial Respiratory Complex I: Structure, Function and Implication in Human Diseases. Curr. Med. Chem. 2009, 16, 1266–1277. [Google Scholar] [CrossRef]
- Issan, Y.; Kornowski, R.; Aravot, D.; Shainberg, A.; Laniado-Schwartzman, M.; Sodhi, K.; Abraham, N.G.; Hochhauser, E. Heme Oxygenase-1 Induction Improves Cardiac Function following Myocardial Ischemia by Reducing Oxidative Stress. PLoS ONE 2014, 9, e92246. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF-kappa B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Gianni, L.; Kearns, C.M.; Giani, A.; Capri, G.; Viganó, L.; Lacatelli, A.; Bonadonna, G.; Egorin, M.J. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J. Clin. Oncol. 1995, 13, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Radeleff, B.; Lopez-Benitez, R.; Stampfl, U.; Stampfl, S.; Sommer, C.; Thierjung, H.; Berger, I.; Kauffmann, G.; Richter, G.M. Paclitaxel-induced arterial wall toxicity and inflammation: Tissue uptake in various dose densities in a Minipig Model. J. Vasc. Interv. Radiol. 2010, 21, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Bartekova, M.; Radosinska, J.; Jelemensky, M.; Dhalla, N.S. Role of cytokines and inflammation in heart function during health and disease. Heart Fail. Rev. 2018, 23, 733–758. [Google Scholar] [CrossRef]
- Szekely, Y.; Arbel, Y. A Review of Interleukin-1 in Heart Disease: Where Do We Stand Today? Cardiol. Ther. 2018, 7, 25–44. [Google Scholar] [CrossRef] [PubMed]

| Mean ± SD | Control | ISO | PAC | ISO + PAC |
|---|---|---|---|---|
| MPO (µU/mg of protein) | 16,511 ± 5944 | 27,697 ± 4979 | 16,967 ± 3453 | 17,260 ± 2979 |
| HO-1activity (nmol bilirubin/h/mg protein) | 0.431 ± 0.0629 | 0.035 ± 0.0259 | 0.36 ± 0.1346 | 0.242 ± 0.05391 |
| HO-1 (pg/mg of protein) | 491.1 ± 58.87 | 237.8 ± 42.52 | 467 ± 50.29 | 381.4 ± 79.82 |
| Total Glutathione (nmol/mg of protein) | 3.894 ± 0.5405 | 1.758 ± 0.5383 | 3.718 ± 0.7018 | 2.762 ± 0.7259 |
| SOD (ng/mL) | 141.9 ± 3.1 | 64.6 ± 4.624 | 132 ± 9.95 | 109.1 ± 14.96 |
| TNFα (pg/mg protein) | 9.33 ± 1.395 | 15.63 ± 2.089 | 10.13 ± 1.103 | 12.16 ± 1.542 |
| NFκB (pg/mg of protein) | 189.4 ± 83.26 | 411.8 ± 42.69 | 119.6 ± 29.52 | 185.7 ± 47.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matusovits, D.; Murlasits, Z.; Kupai, K.; Baráth, Z.; Kang, H.L.; Osváth, P.; Szűcs, M.; Priksz, D.; Juhász, B.; Radák, Z.; et al. Paclitaxel Protects against Isoproterenol-Induced Damage in Rat Myocardium: Its Heme-Oxygenase Mediated Role in Cardiovascular Research. Antioxidants 2023, 12, 1129. https://doi.org/10.3390/antiox12051129
Matusovits D, Murlasits Z, Kupai K, Baráth Z, Kang HL, Osváth P, Szűcs M, Priksz D, Juhász B, Radák Z, et al. Paclitaxel Protects against Isoproterenol-Induced Damage in Rat Myocardium: Its Heme-Oxygenase Mediated Role in Cardiovascular Research. Antioxidants. 2023; 12(5):1129. https://doi.org/10.3390/antiox12051129
Chicago/Turabian StyleMatusovits, Danica, Zsolt Murlasits, Krisztina Kupai, Zoltán Baráth, Hsu Lin Kang, Péter Osváth, Miklós Szűcs, Dániel Priksz, Béla Juhász, Zsolt Radák, and et al. 2023. "Paclitaxel Protects against Isoproterenol-Induced Damage in Rat Myocardium: Its Heme-Oxygenase Mediated Role in Cardiovascular Research" Antioxidants 12, no. 5: 1129. https://doi.org/10.3390/antiox12051129
APA StyleMatusovits, D., Murlasits, Z., Kupai, K., Baráth, Z., Kang, H. L., Osváth, P., Szűcs, M., Priksz, D., Juhász, B., Radák, Z., Várkonyi, T., Pavo, I., & Pósa, A. (2023). Paclitaxel Protects against Isoproterenol-Induced Damage in Rat Myocardium: Its Heme-Oxygenase Mediated Role in Cardiovascular Research. Antioxidants, 12(5), 1129. https://doi.org/10.3390/antiox12051129








