Mitochondrial ROS Accumulation Contributes to Maternal Hypertension and Impaired Remodeling of Spiral Artery but Not IUGR in a Rat PE Model Caused by Maternal Glucocorticoid Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Measurement of Blood Pressure
2.3. Uteroplacental Blood Perfusion Measurement
2.4. Measurement of Urinary Protein/Creatinine Concentration
2.5. Pathological Assessment of the Kidney
2.6. Pathological Immunostaining of Placental Tissue
2.7. Electron Microscopy
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Transcriptome Sequencing Analysis
2.10. Targeted Metabolomics
2.11. Cell Culture and In Vitro Trophoblast Migration and Invasion Assessment
2.12. Assay of Mitochondrial Oxygen Consumption
2.13. Mitochondrial Isolation and Function Determination
2.14. Total RNA Extraction and Quantitative Real-Time PCR (Q-PCR)
2.15. Mitochondrial DNA Copy Number Detection
2.16. Western Blotting Analysis
2.17. Statistical Analysis
3. Results
3.1. DEX Induces PE-like Features and Results in Changes in a Large Spectrum of the Transcriptome in Placentas of Pregnant Rats
3.2. Placental Mitochondrial Function and Morphology Are Impaired, and Mitochondrial ROS Production Is Significantly Increased in the DEX-Induced PE Model
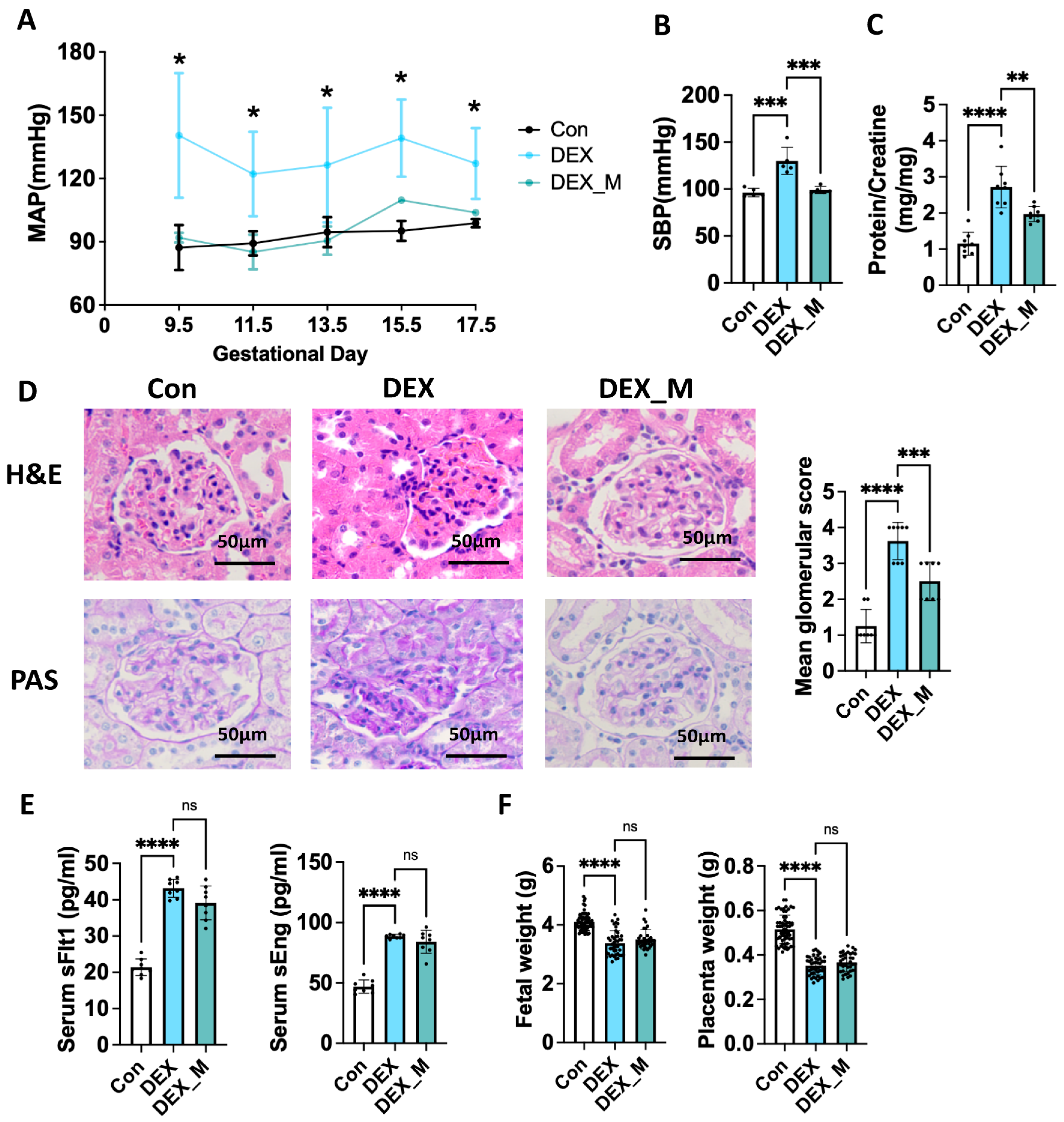
3.3. Scavenging mtROS Significantly Reverses Maternal Hypertension and Improves Renal Damage but Not IUGR and Elevated Circulatory sFlt-1 and sEng Levels in DEX-Induced PE Rats
3.4. Scavenging mtROS Significantly Partly Improves the Expression of the Factors in OXPHOS, Mitochondrial Function and Morphology in DEX Rats
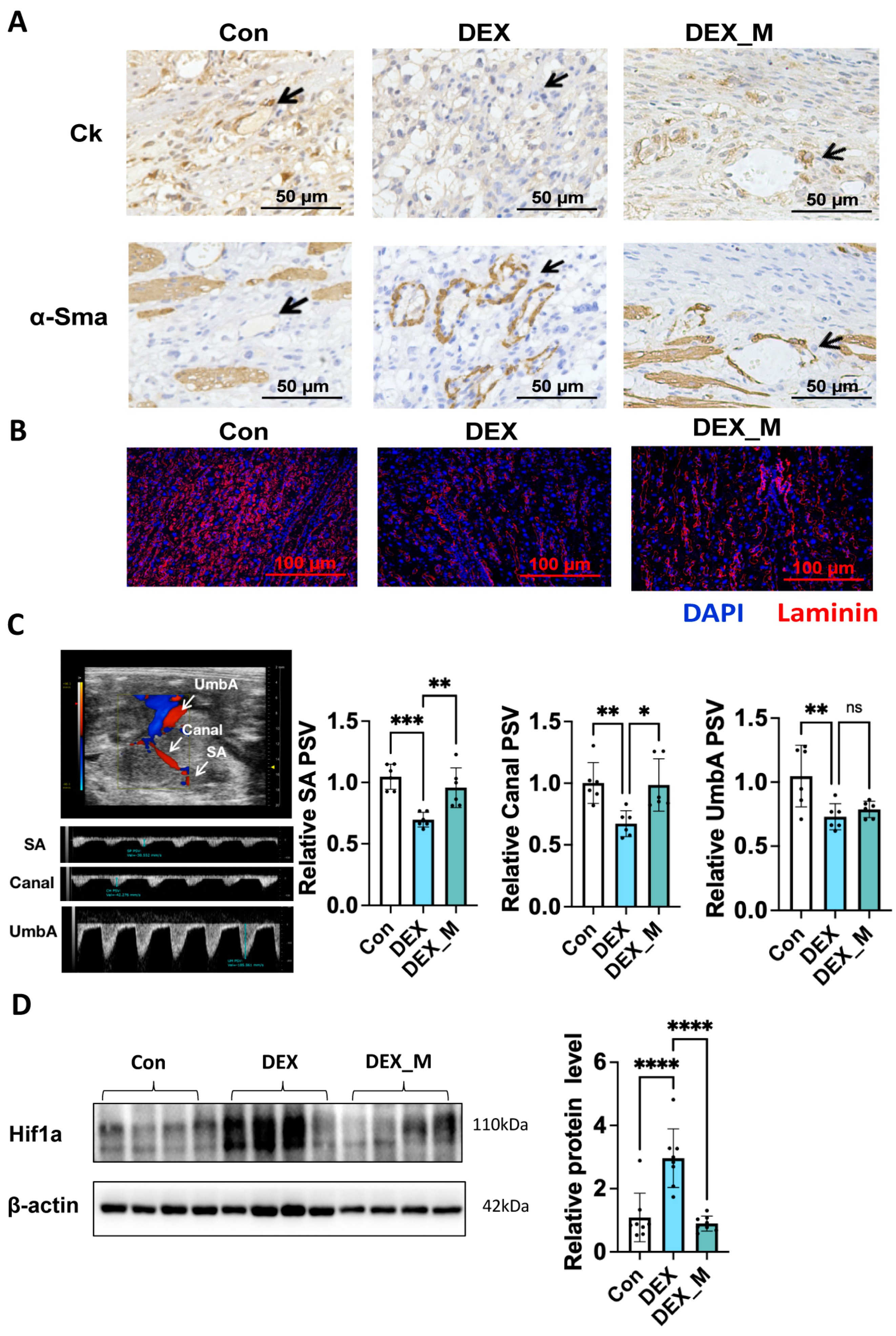
3.5. MtROS Accumulation Contributes to Impaired SA Remodeling, Uteroplacental Blood Flow and Placental Hypoxia but Not Fetal Blood Flow in DEX-Induced PE Rats
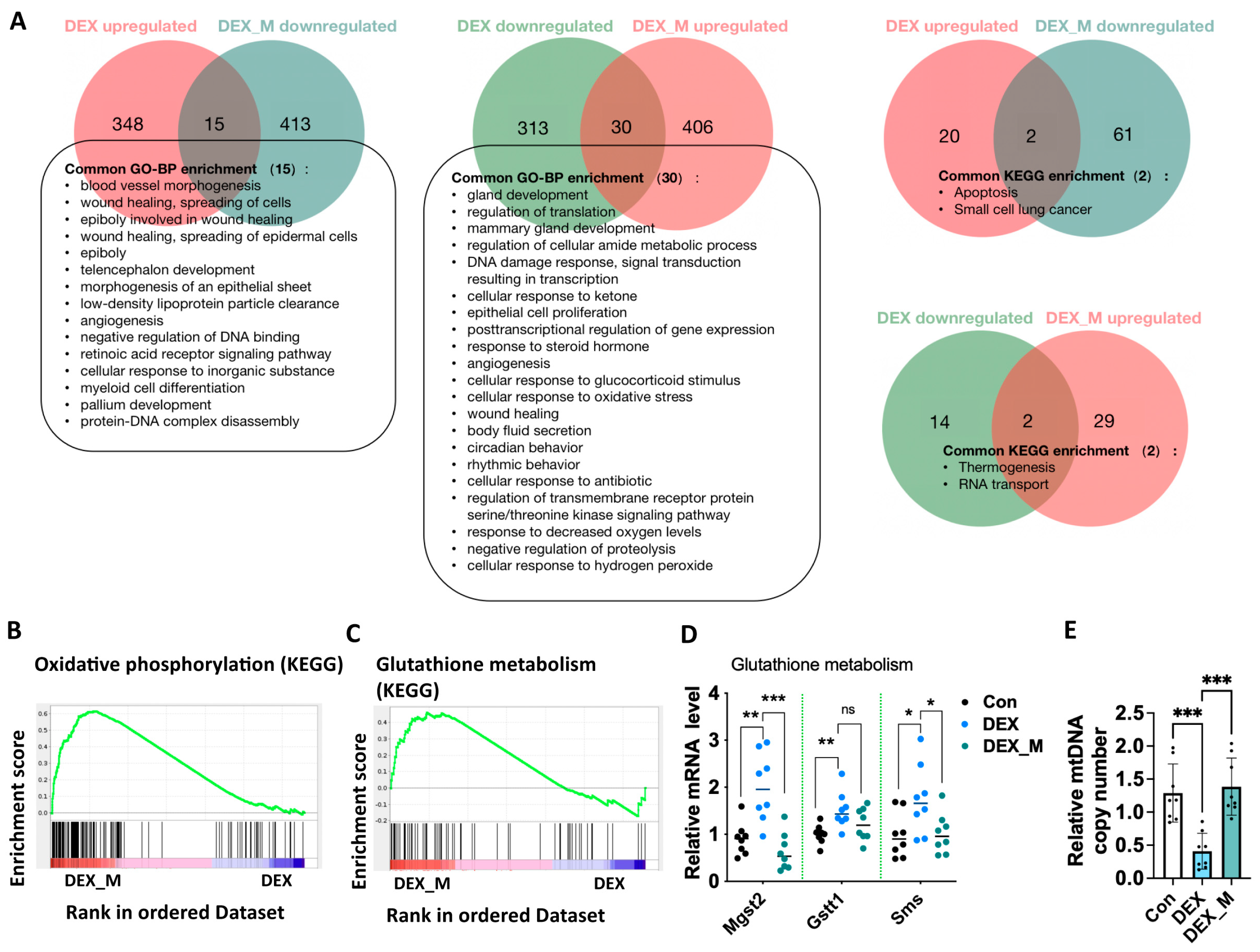
3.6. MitoTEMPO Treatment Reverses Some Pathways Including OXPHOS and Glutathione Pathways and Improves mtDNA Copy in DEX-Induced PE Rats
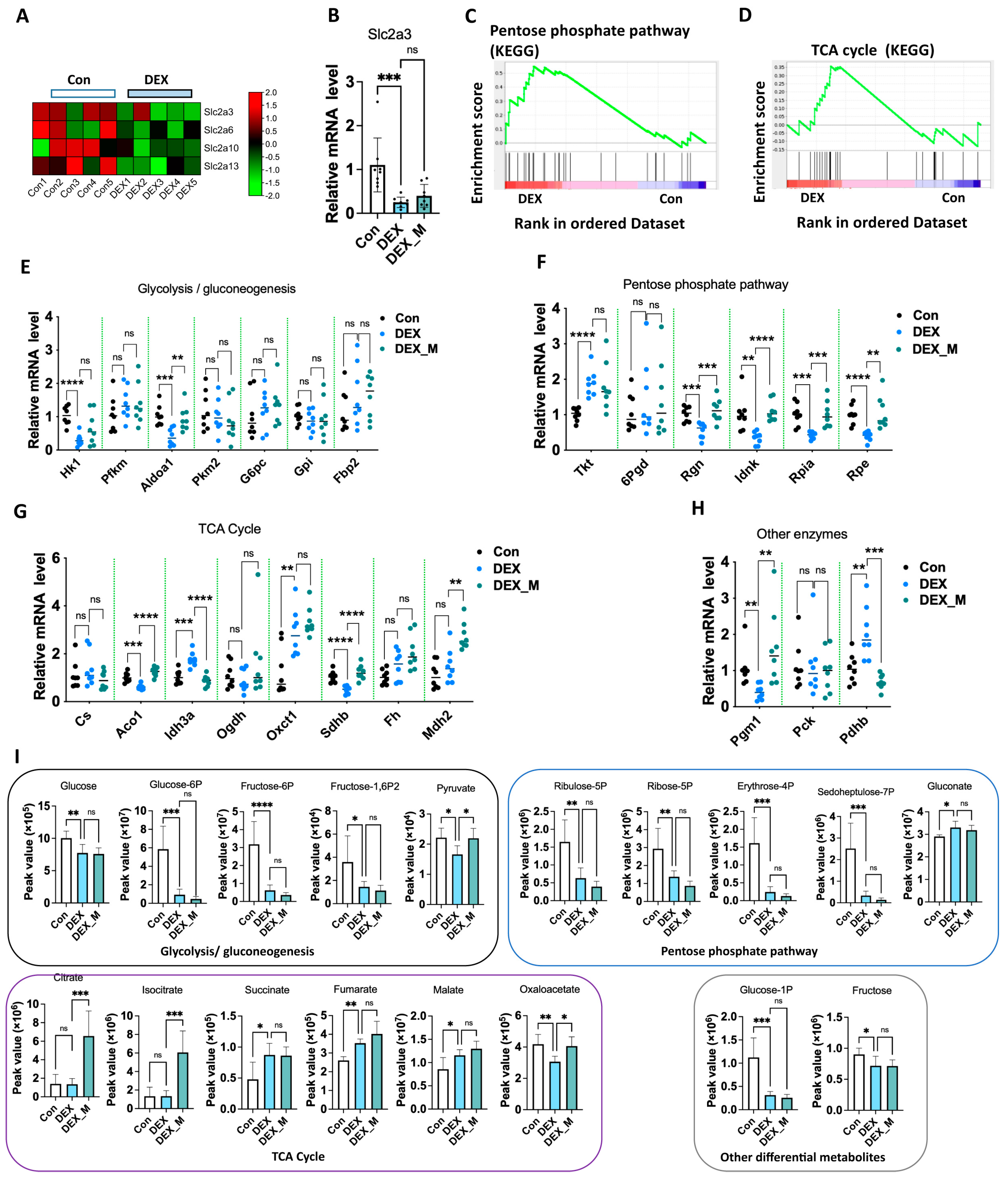
3.7. Impaired Glycolysis and Pentose Phosphate Pathway (PPP) Induced by DEX Might Not Be Associated with Excess ROS
3.8. Excess ROS May Not Contribute to DEX-Induced Alternation of Transcriptional Levels of Placental Cytokines, Prostaglandin Biosynthetic Process and Growth Factors and Increased Circulatory Levels of Proinflammatory Cytokines and PGE2 in DEX-Induced PE Rats
3.9. DEX Impairment of Human EVTs Function Is Associated with Excess ROS Due to Mitochondrial Dysfunction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.; Oparil, S. Preeclampsia—Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Gomez-Lopez, N.; Chaemsaithong, P.; Jaovisidha, A.; Gotsch, F.; Erez, O. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S844–S866. [Google Scholar] [CrossRef] [PubMed]
- Chappell, L.C.; Cluver, C.A.; Kingdom, J.; Tong, S. Pre-eclampsia. Lancet 2021, 398, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Solano, M.E.; Arck, P.C. Steroids, Pregnancy and Fetal Development. Front. Immunol. 2019, 10, 3017. [Google Scholar] [CrossRef]
- Solano, M.E.; Holmes, M.C.; Mittelstadt, P.R.; Chapman, K.E.; Tolosa, E. Antenatal endogenous and exogenous glucocorticoids and their impact on immune ontogeny and long-term immunity. Semin. Immunopathol. 2016, 38, 739–763. [Google Scholar] [CrossRef]
- Cowell, W.; Deyssenroth, M.; Chen, J.; Wright, R.J. Maternal stress in relation to sex-specific expression of placental genes involved in nutrient transport, oxygen tension, immune response, and the glucocorticoid barrier. Placenta 2020, 96, 19–26. [Google Scholar] [CrossRef]
- Wieczorek, A.; Perani, C.V.; Nixon, M.; Constancia, M.; Sandovici, I.; Zazara, D.E.; Leone, G.; Zhang, M.-Z.; Arck, P.C.; Solano, M.E. Sex-specific regulation of stress-induced fetal glucocorticoid surge by the mouse placenta. Am. J. Physiol. Metab. 2019, 317, E109–E120. [Google Scholar] [CrossRef]
- Chen, Y.; He, Z.; Chen, G.; Liu, M.; Wang, H. Prenatal glucocorticoids exposure and fetal adrenal developmental programming. Toxicology 2019, 428, 152308. [Google Scholar] [CrossRef]
- Busada, J.T.; Cidlowski, J.A. Mechanisms of Glucocorticoid Action During Development. Curr. Top. Dev. Biol. Vol. 2017, 125, 147–170. [Google Scholar] [CrossRef]
- Zhang, D.; Zeng, J.; Miao, X.; Liu, H.; Ge, L.; Huang, W.; Jiao, J.; Ye, D. Glucocorticoid exposure induces preeclampsia via dampening 1,25-dihydroxyvitamin D3. Hypertens. Res. 2018, 41, 104–111. [Google Scholar] [CrossRef]
- Liu, H.; Huang, W.; Chen, L.; Xu, Q.; Ye, D.; Zhang, D. Glucocorticoid Exposure Induces Preeclampsia via DampeningLipoxin A4, an Endogenous Anti-Inflammatory and Proresolving Mediator. Front. Pharmacol. 2020, 11, 1131. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.; Zeng, J.; Miao, X.; Huang, W.; Chen, H.; Huang, Y.; Li, Y.; Ye, D. Glucocorticoid exposure in early placentation induces preeclampsia in rats via interfering trophoblast development. Gen. Comp. Endocrinol. 2016, 225, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, Y.; Hu, T.; Zhang, B.; Tang, Z.; Yao, R.; Huang, Y.; Fan, X.; Ni, X. Contribution of placental 11β-HSD2 to the pathogenesis of preeclampsia. FASEB J. 2020, 34, 15379–15399. [Google Scholar] [CrossRef]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef]
- Kisanga, E.P.; Tang, Z.; Guller, S.; Whirledge, S. Glucocorticoid signaling regulates cell invasion and migration in the human first-trimester trophoblast cell line Sw.71. Am. J. Reprod. Immunol. 2018, 80, e12974. [Google Scholar] [CrossRef]
- He, B.; Zhang, N.; Jia, Y.; Sun, Q.; Zhao, R. Glucocorticoid receptor-mediated insulin-like growth factor-I transcriptional regulation in BeWo trophoblast cells before and after syncytialisation. Steroids 2016, 115, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, O.; Powell, T.; Jansson, T. Glucocorticoid regulation of amino acid transport in primary human trophoblast cells. J. Mol. Endocrinol. 2019, 63, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Wang, Z.; Yee, H.; Ma, Y.; Swenson, N.; Yang, L.; Kadner, S.S.; Baergen, R.N.; Logan, S.; Garabedian, M.J.; et al. Expression and Regulation of Glucocorticoid Receptor in Human Placental Villous Fibroblasts. Endocrinology 2005, 146, 4619–4626. [Google Scholar] [CrossRef]
- Korgun, E.; Dohr, G.; Desoye, G.; Demir, R.; Kayisli, U.; Hahn, T. Expression of insulin, insulin-like growth factor I and glucocorticoid receptor in rat uterus and embryo during decidualization, implantation and organogenesis. Reproduction 2003, 125, 75–84. [Google Scholar] [CrossRef]
- Michael, A.E.; Papageorghiou, A.T. Potential significance of physiological and pharmacological glucocorticoids in early pregnancy. Hum. Reprod. Updat. 2008, 14, 497–517. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, P.; Myatt, L.; Sun, K. Roles of glucocorticoids in human parturition: A controversial fact? Placenta 2014, 35, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Waddell, B.J.; Benediktsson, R.; Brown, R.W.; Seckl, J.R. Tissue-Specific Messenger Ribonucleic Acid Expression of 11β-Hydroxysteroid Dehydrogenase Types 1 and 2 and the Glucocorticoid Receptor within Rat Placenta Suggests Exquisite Local Control of Glucocorticoid Action. Endocrinology 1998, 139, 1517–1523. [Google Scholar] [CrossRef]
- Natale, B.V.; Gustin, K.N.; Lee, K.; Holloway, A.C.; Laviolette, S.R.; Natale, D.R.C.; Hardy, D.B. Δ9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci. Rep. 2020, 10, 544. [Google Scholar] [CrossRef]
- Janbandhu, V.; Tallapragada, V.; Patrick, R.; Li, Y.; Abeygunawardena, D.; Humphreys, D.T.; Martin, E.M.; Ward, A.O.; Contreras, O.; Farbehi, N.; et al. Hif-1a suppresses ROS-induced proliferation of cardiac fibroblasts following myocardial infarction. Cell Stem Cell 2022, 29, 281–297.e12. [Google Scholar] [CrossRef]
- Liu, B.; Wang, D.; Cao, Y.; Wu, J.; Zhou, Y.; Wu, W.; Wu, J.; Zhou, J.; Qiu, J. MitoTEMPO protects against podocyte injury by inhibiting NLRP3 inflammasome via PINK1/Parkin pathway-mediated mitophagy. Eur. J. Pharmacol. 2022, 929, 175136. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Huang, Y.; Tang, Z.; Shan, Y.; Feng, D.; Wang, W.; Liu, J.; Huang, Y.; Gu, H.; Guo, D.; et al. Mitochondria Targeted Antioxidant Significantly Alleviates Preeclampsia Caused by 11β-HSD2 Dysfunction via OPA1 and MtDNA Maintenance. Antioxidants 2022, 11, 1505. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jiang, M.; Li, K.; Li, H.; Zhou, Y.; Xiao, X.; Xu, Y.; Krishfield, S.; Lipsky, P.E.; Tsokos, G.C.; et al. Glutathione peroxidase 4–regulated neutrophil ferroptosis induces systemic autoimmunity. Nat. Immunol. 2021, 22, 1107–1117. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Elhddad, A.; Lashen, H. Fetal growth in relation to maternal and fetal IGF-axes: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2013, 92, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Sandovici, I.; Georgopoulou, A.; Pérez-García, V.; Hufnagel, A.; López-Tello, J.; Lam, B.Y.; Schiefer, S.N.; Gaudreau, C.; Santos, F.; Hoelle, K.; et al. The imprinted Igf2-Igf2r axis is critical for matching placental microvasculature expansion to fetal growth. Dev. Cell 2022, 57, 63–79.e8. [Google Scholar] [CrossRef] [PubMed]
- Sferruzzi-Perri, A.N.; Sandovici, I.; Constancia, M.; Fowden, A.L. Placental phenotype and the insulin-like growth factors: Resource allocation to fetal growth. J. Physiol. 2017, 595, 5057–5093. [Google Scholar] [CrossRef] [PubMed]
- Shipley, J.M.; Mecham, R.P.; Maus, E.; Bonadio, J.; Rosenbloom, J.; McCarthy, R.T.; Baumann, M.L.; Frankfater, C.; Segade, F.; Shapiro, S.D. Developmental Expression of Latent Transforming Growth Factor β Binding Protein 2 and Its Requirement Early in Mouse Development. Mol. Cell. Biol. 2000, 20, 4879–4887. [Google Scholar] [CrossRef] [PubMed]
- Habib, W.A.; Brioude, F.; Edouard, T.; Bennett, J.T.; Lienhardt-Roussie, A.; Tixier, F.; Salem, J.; Yuen, T.; Azzi, S.; Le Bouc, Y.; et al. Genetic disruption of the oncogenic HMGA2–PLAG1–IGF2 pathway causes fetal growth restriction. Genet. Med. 2018, 20, 250–258. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, W.; Xu, X.; Huang, L.; Gao, Q.; Chen, H.; Sun, H.; Xia, Y.; Sha, J.; Wang, X.; et al. miR-141 Contributes to Fetal Growth Restriction by Regulating PLAG1 Expression. PLoS ONE 2013, 8, e58737. [Google Scholar] [CrossRef]
- Pejznochova, M.; Tesarova, M.; Hansikova, H.; Magner, M.; Honzik, T.; Vinsova, K.; Hajkova, Z.; Havlickova, V.; Zeman, J. Mitochondrial DNA content and expression of genes involved in mtDNA transcription, regulation and maintenance during human fetal development. Mitochondrion 2010, 10, 321–329. [Google Scholar] [CrossRef]
- Atlass, J.; Menke, M.; Parks, W.T.; Catov, J.M. Pre-conception blood pressure and evidence of placental malperfusion. BMC Pregnancy Childbirth 2020, 20, 25. [Google Scholar] [CrossRef]
- Mennuni, S.; Rubattu, S.; Pierelli, G.; Tocci, G.; Fofi, C.; Volpe, M. Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 2014, 28, 74–79. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Laresgoiti-Servitje, E.; Gomez-Lopez, N. The Pathophysiology of Preeclampsia Involves Altered Levels of Angiogenic Factors Promoted by Hypoxia and Autoantibody-Mediated Mechanisms. Biol. Reprod. 2012, 87, 36. [Google Scholar] [CrossRef]
- Denney, J.M.; Bird, C.; Gendron-Fitzpatrick, A.; Sampene, E.; Bird, I.M.; Shah, D.M. Renin-angiotensin system transgenic mouse model recapitulates pathophysiology similar to human preeclampsia with renal injury that may be mediated through VEGF. Am. J. Physiol. Renal Physiol. 2017, 312, F445–F455. [Google Scholar] [CrossRef] [PubMed]
- Santana-Garrido, Á.; Reyes-Goya, C.; Espinosa-Martín, P.; Sobrevia, L.; Beltrán, L.M.; Vázquez, C.M.; Mate, A. Oxidative and Inflammatory Imbalance in Placenta and Kidney of sFlt1-Induced Early-Onset Preeclampsia Rat Model. Antioxidants 2022, 11, 1608. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmed, A. Elevated Placental Soluble Vascular Endothelial Growth Factor Receptor-1 Inhibits Angiogenesis in Preeclampsia. Circ. Res. 2004, 95, 884–891. [Google Scholar] [CrossRef] [PubMed]
- West, A.P. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology 2017, 391, 54–63. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Nikiforov, N.G.; Eid, A.H.; Nedosugova, L.V.; Starodubova, A.V.; Popkova, T.V.; Bezsonov, E.E.; Orekhov, A.N. Mitochondrial Dysfunction and Chronic Inflammation in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2021, 22, 3923. [Google Scholar] [CrossRef]
- Davizon-Castillo, P.; McMahon, B.; Aguila, S.; Bark, D.; Ashworth, K.; Allawzi, A.; Campbell, R.A.; Montenont, E.; Nemkov, T.; D’alessandro, A.; et al. TNF-α-driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood 2019, 134, 727–740. [Google Scholar] [CrossRef]
- Zhai, Q.; Chen, X.; Fei, D.; Guo, X.; He, X.; Zhao, W.; Shi, S.; Gooding, J.J.; Jin, F.; Jin, Y.; et al. Nanorepairers Rescue Inflammation-Induced Mitochondrial Dysfunction in Mesenchymal Stem Cells. Adv. Sci. 2022, 9, e2103839. [Google Scholar] [CrossRef]
- Kadiyala, V.; Sasse, S.K.; Altonsy, M.O.; Berman, R.; Chu, H.W.; Phang, T.L.; Gerber, A.N. Cistrome-based Cooperation between Airway Epithelial Glucocorticoid Receptor and NF-κB Orchestrates Anti-inflammatory Effects. J. Biol. Chem. 2016, 291, 12673–12687. [Google Scholar] [CrossRef]
- Butler, M.; Vervoort, B.M.; Schenau, D.S.V.I.; Jongeneel, L.; van der Zwet, J.C.; Marke, R.; Meijerink, J.P.; Scheijen, B.; van der Meer, L.T.; van Leeuwen, F.N. Reversal of IKZF1-induced glucocorticoid resistance by dual targeting of AKT and ERK signaling pathways. Front. Oncol. 2022, 12, 905665. [Google Scholar] [CrossRef]
- Oppong, E.; Flink, N.; Cato, A.C. Molecular mechanisms of glucocorticoid action in mast cells. Mol. Cell. Endocrinol. 2013, 380, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Zeyen, L.; Seternes, O.M.; Mikkola, I. Crosstalk between p38 MAPK and GR Signaling. Int. J. Mol. Sci. 2022, 23, 3322. [Google Scholar] [CrossRef] [PubMed]
- Mark, P.; Lewis, J.; Jones, M.; Keelan, J.; Waddell, B. The inflammatory state of the rat placenta increases in late gestation and is further enhanced by glucocorticoids in the labyrinth zone. Placenta 2013, 34, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Mirazi, N.; Alfaidy, N.; Martin, R.; Challis, J.R.G. Effects of Dexamethasone and Sulfasalazine on Prostaglandin E2 Output by Human Placental Cells In Vitro. J. Soc. Gynecol. Investig. 2004, 11, 22–26. [Google Scholar] [CrossRef]
- Whittle, W.; Patel, F.; Alfaidy, N.; Holloway, A.; Fraser, M.; Gyomorey, S.; Lye, S.; Gibb, W.; Challis, J. Glucocorticoid Regulation of Human and Ovine Parturition: The Relationship Between Fetal Hypothalamic-Pituitary-Adrenal Axis Activation and Intrauterine Prostaglandin Production. Biol. Reprod. 2001, 64, 1019–1032. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Peng, H.; Liu, H.; Cheng, Q.; Cheng, X.; Zeng, P.; Wu, P.; Chen, H.; Huang, Y.; et al. Glucocorticoids Sensitize Rat Placental Inflammatory Responses via Inhibiting Lipoxin A4 Biosynthesis. Biol. Reprod. 2014, 90, 74. [Google Scholar] [CrossRef]
- Czamara, D.; Dieckmann, L.; Röh, S.; Kraemer, S.; Rancourt, R.C.; Sammallahti, S.; Kajantie, E.; Laivuori, H.; Eriksson, J.G.; Räikkönen, K.; et al. Betamethasone administration during pregnancy is associated with placental epigenetic changes with implications for inflammation. Clin. Epigenet. 2021, 13, 165. [Google Scholar] [CrossRef]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef]
- Harizi, H.; Gualde, N. Pivotal role of PGE2 and IL-10 in the cross-regulation of dendritic cell-derived inflammatory mediators. Cell. Mol. Immunol. 2006, 3, 271–277. [Google Scholar]
- Fülöp, V.; Vermes, G.; Demeter, J. The relationship between inflammatory and immunological processes during pregnancy. Practical aspects. Orv. Hetil. 2019, 160, 1247–1259. [Google Scholar] [CrossRef]
- Vesce, F.; Battisti, C.; Crudo, M. The Inflammatory Cytokine Imbalance for Miscarriage, Pregnancy Loss and COVID-19 Pneumonia. Front. Immunol. 2022, 13, 861245. [Google Scholar] [CrossRef] [PubMed]
- Equils, O.; Kellogg, C.; McGregor, J.; Gravett, M.; Neal-Perry, G.; Gabay, C. The role of the IL-1 system in pregnancy and the use of IL-1 system markers to identify women at risk for pregnancy complications†. Biol. Reprod. 2020, 103, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.Y.; Guild, S.-J.; Barrett, C.; Chen, Q.; McCowan, L.; Jordan, V.; Chamley, L. Tumor Necrosis Factor-Alpha, Interleukin-6, and Interleukin-10 Levels are Altered in Preeclampsia: A Systematic Review and Meta-Analysis. Am. J. Reprod. Immunol. 2013, 70, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.P.; Miles, T.M.; Benyo, D.F. Circulating Levels of Immunoreactive Cytokines in Women with Preeclampsia. Am. J. Reprod. Immunol. 1998, 40, 102–111. [Google Scholar] [CrossRef]
- Benyo, D.F.; Smarason, A.; Redman, C.W.G.; Sims, C.; Conrad, K.P. Expression of Inflammatory Cytokines in Placentas from Women with Preeclampsia. J. Clin. Endocrinol. Metab. 2001, 86, 2505–2512. [Google Scholar] [CrossRef]
- Aggarwal, R.; Jain, A.K.; Mittal, P.; Kohli, M.; Jawanjal, P.; Rath, G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 2019, 33, e22834. [Google Scholar] [CrossRef]
- Bu, C.; Wang, Z.; Ren, Y.; Chen, D.; Jiang, S.-W. Syncytin-1 nonfusogenic activities modulate inflammation and contribute to preeclampsia pathogenesis. Cell. Mol. Life Sci. 2022, 79, 290. [Google Scholar] [CrossRef]
- Michalczyk, M.; Celewicz, A.; Celewicz, M.; Woźniakowska-Gondek, P.; Rzepka, R. The Role of Inflammation in the Pathogenesis of Preeclampsia. Mediat. Inflamm. 2020, 2020, 3864941. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhao, Y. Inflammation in Preeclampsia: Genetic Biomarkers, Mechanisms, and Therapeutic Strategies. Front. Immunol. 2022, 13, 883404. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Nicolaides, K.H.; Poon, L.C. Prevention of preeclampsia with aspirin. Am. J. Obstet. Gynecol. 2022, 226, S1108–S1119. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Zhong, Y.; Li, Q.; Wu, M.; Yang, L.; Liu, X.; Zou, L. Metformin Corrects Glucose Metabolism Reprogramming and NLRP3 Inflammasome-Induced Pyroptosis via Inhibiting the TLR4/NF-κB/PFKFB3 Signaling in Trophoblasts: Implication for a Potential Therapy of Preeclampsia. Oxid. Med. Cell. Longev. 2021, 2021, 1806344. [Google Scholar] [CrossRef]
- Chakraborty, S.; Islam, S.; Saha, S.; Ain, R. Dexamethasone-induced Intra-Uterine Growth Restriction impacts NOSTRIN and its downstream effector genes in the rat mesometrial uterus. Sci. Rep. 2018, 8, 8342. [Google Scholar] [CrossRef] [PubMed]
- Sugden, M.C.; Langdown, M.L. Possible involvement of PKC isoforms in signalling placental apoptosis in intrauterine growth retardation. Mol. Cell. Endocrinol. 2001, 185, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ain, R.; Canham, L.N.; Soares, M.J. Dexamethasone-induced intrauterine growth restriction impacts the placental prolactin family, insulin-like growth factor-II and the Akt signaling pathway. J. Endocrinol. 2005, 185, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Alqaryyan, M.; Kilarkaje, N.; Mouihate, A.; Al-Bader, M.D. Dexamethasone-Induced Intrauterine Growth Restriction Is Associated with Altered Expressions of Metastasis Tumor Antigens and Cell Cycle Control Proteins in Rat Placentas. Reprod. Sci. 2017, 24, 1164–1175. [Google Scholar] [CrossRef]
- Er, H.; Acar, N.; Kipmen-Korgun, D.; Celik-Ozenci, C.; Ustunel, I.; Asar, M.; Korgun, E.T. Determination of PCNA, cyclin D3, p27, p57 and apoptosis rate in normal and dexamethasone-induced intrauterine growth restricted rat placentas. Acta Histochem. 2015, 117, 137–147. [Google Scholar] [CrossRef]
- Dardevet, D.; Sornet, C.; Savary, I.; Debras, E.; Patureau-Mirand, P.; Grizard, J. Glucocorticoid effects on insulin- and IGF-I-regulated muscle protein metabolism during aging. J. Endocrinol. 1998, 156, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.S. Insulin and glucocorticoid-dependent suppression of the IGF-I system in diabetic wounds. Surgery 2000, 127, 687–695. [Google Scholar] [CrossRef]
- Kühnel, E.; Kleff, V.; Stojanovska, V.; Kaiser, S.; Waldschütz, R.; Herse, F.; Plösch, T.; Winterhager, E.; Gellhaus, A. Placental-Specific Overexpression of sFlt-1 Alters Trophoblast Differentiation and Nutrient Transporter Expression in an IUGR Mouse Model. J. Cell. Biochem. 2017, 118, 1316–1329. [Google Scholar] [CrossRef]
- Liu, L.; Wang, R.; Xu, R.; Chu, Y.; Gu, W. Procyanidin B2 ameliorates endothelial dysfunction and impaired angiogenesis via the Nrf2/PPARγ/sFlt-1 axis in preeclampsia. Pharmacol. Res. 2022, 177, 106127. [Google Scholar] [CrossRef]
- Brownfoot, F.; Tong, S.; Hannan, N.; Hastie, R.; Cannon, P.; Tuohey, L.; Kaitu’U-Lino, T. YC-1 reduces placental sFlt-1 and soluble endoglin production and decreases endothelial dysfunction: A possible therapeutic for preeclampsia. Mol. Cell. Endocrinol. 2015, 413, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.; Aiken, C.E.; Charnock-Jones, D.S.; Smith, G.C. Placental energy metabolism in health and disease—Significance of development and implications for preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S928–S944. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, J.; Baker, P.N.; Tong, C. Revisiting preeclampsia: A metabolic disorder of the placenta. FEBS J. 2022, 289, 336–354. [Google Scholar] [CrossRef] [PubMed]
- Stanirowski, P.J.; Lipa, M.; Bomba-Opoń, D.; Wielgoś, M. Expression of placental glucose transporter proteins in pregnancies complicated by fetal growth disorders. Adv. Protein Chem. Struct. Biol. 2021, 123, 95–131. [Google Scholar] [CrossRef]
- Baumann, M.U.; Deborde, S.; Illsley, N.P. Placental Glucose Transfer and Fetal Growth. Endocrine 2002, 19, 13–22. [Google Scholar] [CrossRef]
- Lüscher, B.P.; Marini, C.; Joerger-Messerli, M.S.; Huang, X.; Hediger, M.A.; Albrecht, C.; Baumann, M.U.; Surbek, D.V. Placental glucose transporter (GLUT)-1 is down-regulated in preeclampsia. Placenta 2017, 55, 94–99. [Google Scholar] [CrossRef]
- Bloxam, D.L.; Bullen, B.E.; Walters, B.N.; Lao, T.T. Placental glycolysis and energy metabolism in preeclampsia. Am. J. Obstet. Gynecol. 1987, 157, 97–101. [Google Scholar] [CrossRef]
- Inuyama, M.; Ushigome, F.; Emoto, A.; Koyabu, N.; Satoh, S.; Tsukimori, K.; Nakano, H.; Ohtani, H.; Sawada, Y. Characteristics ofl-lactic acid transport in basal membrane vesicles of human placental syncytiotrophoblast. Am. J. Physiol. Cell Physiol. 2002, 283, C822–C830. [Google Scholar] [CrossRef]
- Lv, H.; Tong, J.; Yang, J.; Lv, S.; Li, W.-P.; Zhang, C.; Chen, Z.-J. Dysregulated Pseudogene HK2P1 May Contribute to Preeclampsia as a Competing Endogenous RNA for Hexokinase 2 by Impairing Decidualization. Hypertension 2018, 71, 648–658. [Google Scholar] [CrossRef]
- Abdulhadi, N. Glucose 6 phosphate dehydrogenase (G6PD) deficiency is a possible risk factor for the development of preeclampsia. Med. Hypotheses 2004, 62, 780–782. [Google Scholar] [CrossRef]
- Afzal-Ahmed, I.; Mann, G.E.; Shennan, A.H.; Poston, L.; Naftalin, R.J. Preeclampsia inactivates glucose-6-phosphate dehydrogenase and impairs the redox status of erythrocytes and fetal endothelial cells. Free. Radic. Biol. Med. 2007, 42, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Liu, L.; Ni, C.-X.; Zhang, Y.; Su, W.-J.; Lian, Y.-J.; Peng, W.; Zhang, J.-P.; Jiang, C.-L. Dexamethasone rapidly inhibits glucose uptake via non-genomic mechanisms in contracting myotubes. Arch. Biochem. Biophys. 2016, 603, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Alawadhi, M.; Mouihate, A.; Kilarkaje, N.; Al-Bader, M. Progesterone partially recovers placental glucose transporters in dexamethasone-induced intrauterine growth restriction. Reprod. Biomed. Online 2022, 44, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.A.; Jawad, M. Stimulation of human chorionic gonadotropin secretion by glucocorticoids. Am. J. Obstet. Gynecol. 1982, 142, 344–349. [Google Scholar] [CrossRef]
- Goodwin, J.E. Glucocorticoids and the Cardiovascular System. Adv. Exp. Med. Biol. Vol. 2015, 872, 299–314. [Google Scholar] [CrossRef]
- Iuchi, T.; Akaike, M.; Mitsui, T.; Ohshima, Y.; Shintani, Y.; Azuma, H.; Matsumoto, T. Glucocorticoid Excess Induces Superoxide Production in Vascular Endothelial Cells and Elicits Vascular Endothelial Dysfunction. Circ. Res. 2003, 92, 81–87. [Google Scholar] [CrossRef]
- Shukla, V.; Soares, M.J. Modeling Trophoblast Cell-Guided Uterine Spiral Artery Transformation in the Rat. Int. J. Mol. Sci. 2022, 23, 2947. [Google Scholar] [CrossRef]
- Lee, S.-R.; Kim, H.-K.; Song, I.-S.; Youm, J.; Dizon, L.A.; Jeong, S.-H.; Ko, T.-H.; Heo, H.-J.; Ko, K.S.; Rhee, B.D.; et al. Glucocorticoids and their receptors: Insights into specific roles in mitochondria. Prog. Biophys. Mol. Biol. 2013, 112, 44–54. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Tarca, A.L.; Kekesi, K.A.; Xu, Y.; Xu, Z.; Juhasz, K.; Bhatti, G.; Leavitt, R.J.; Gelencser, Z.; et al. Integrated Systems Biology Approach Identifies Novel Maternal and Placental Pathways of Preeclampsia. Front. Immunol. 2018, 9, 1661. [Google Scholar] [CrossRef]
- Jena, M.K.; Sharma, N.R.; Petitt, M.; Maulik, D.; Nayak, N.R. Pathogenesis of Preeclampsia and Therapeutic Approaches Targeting the Placenta. Biomolecules 2020, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Marín, R.; Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Sobrevia, L. Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165961. [Google Scholar] [CrossRef] [PubMed]
- Vaka, V.R.; McMaster, K.M.; Cunningham, M.W., Jr.; Ibrahim, T.; Hazlewood, R.; Usry, N.; Cornelius, D.C.; Amaral, L.M.; LaMarca, B. Role of Mitochondrial Dysfunction and Reactive Oxygen Species in Mediating Hypertension in the Reduced Uterine Perfusion Pressure Rat Model of Preeclampsia. Hypertension 2018, 72, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, P.; Zhu, F.; Liao, J.; Wu, Y.; Hu, M.; Fu, H.; Qiao, J.; Lin, L.; Huang, B.; et al. The Potent Antioxidant MitoQ Protects Against Preeclampsia During Late Gestation but Increases the Risk of Preeclampsia When Administered in Early Pregnancy. Antioxid. Redox Signal. 2021, 34, 118–136. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, J.; Huang, Y.; Wang, G.; Tang, Z.; Shan, Y.; Shen, S.; Ni, X. Mitochondrial ROS Accumulation Contributes to Maternal Hypertension and Impaired Remodeling of Spiral Artery but Not IUGR in a Rat PE Model Caused by Maternal Glucocorticoid Exposure. Antioxidants 2023, 12, 987. https://doi.org/10.3390/antiox12050987
Long J, Huang Y, Wang G, Tang Z, Shan Y, Shen S, Ni X. Mitochondrial ROS Accumulation Contributes to Maternal Hypertension and Impaired Remodeling of Spiral Artery but Not IUGR in a Rat PE Model Caused by Maternal Glucocorticoid Exposure. Antioxidants. 2023; 12(5):987. https://doi.org/10.3390/antiox12050987
Chicago/Turabian StyleLong, Jing, Yan Huang, Gang Wang, Zhengshan Tang, Yali Shan, Shiping Shen, and Xin Ni. 2023. "Mitochondrial ROS Accumulation Contributes to Maternal Hypertension and Impaired Remodeling of Spiral Artery but Not IUGR in a Rat PE Model Caused by Maternal Glucocorticoid Exposure" Antioxidants 12, no. 5: 987. https://doi.org/10.3390/antiox12050987
APA StyleLong, J., Huang, Y., Wang, G., Tang, Z., Shan, Y., Shen, S., & Ni, X. (2023). Mitochondrial ROS Accumulation Contributes to Maternal Hypertension and Impaired Remodeling of Spiral Artery but Not IUGR in a Rat PE Model Caused by Maternal Glucocorticoid Exposure. Antioxidants, 12(5), 987. https://doi.org/10.3390/antiox12050987






