Oral Treatment with Plant-Derived Exosomes Restores Redox Balance in H2O2-Treated Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Studies
2.2. Collection and Processing of Murine Plasma from Blood Samples
2.3. Bone Marrow Cells Recovery from Mice
2.4. Ovarian Germ Cells Recovery from Mice
2.5. Splenocytes Recovery from Mice
2.6. Exocomplex®
2.7. Exocomplex® Isolation
2.8. Nanoparticle Tracking Analysis (NTA)
2.9. Trypan Blue Cell Counting
2.10. Cell Proliferation Assay
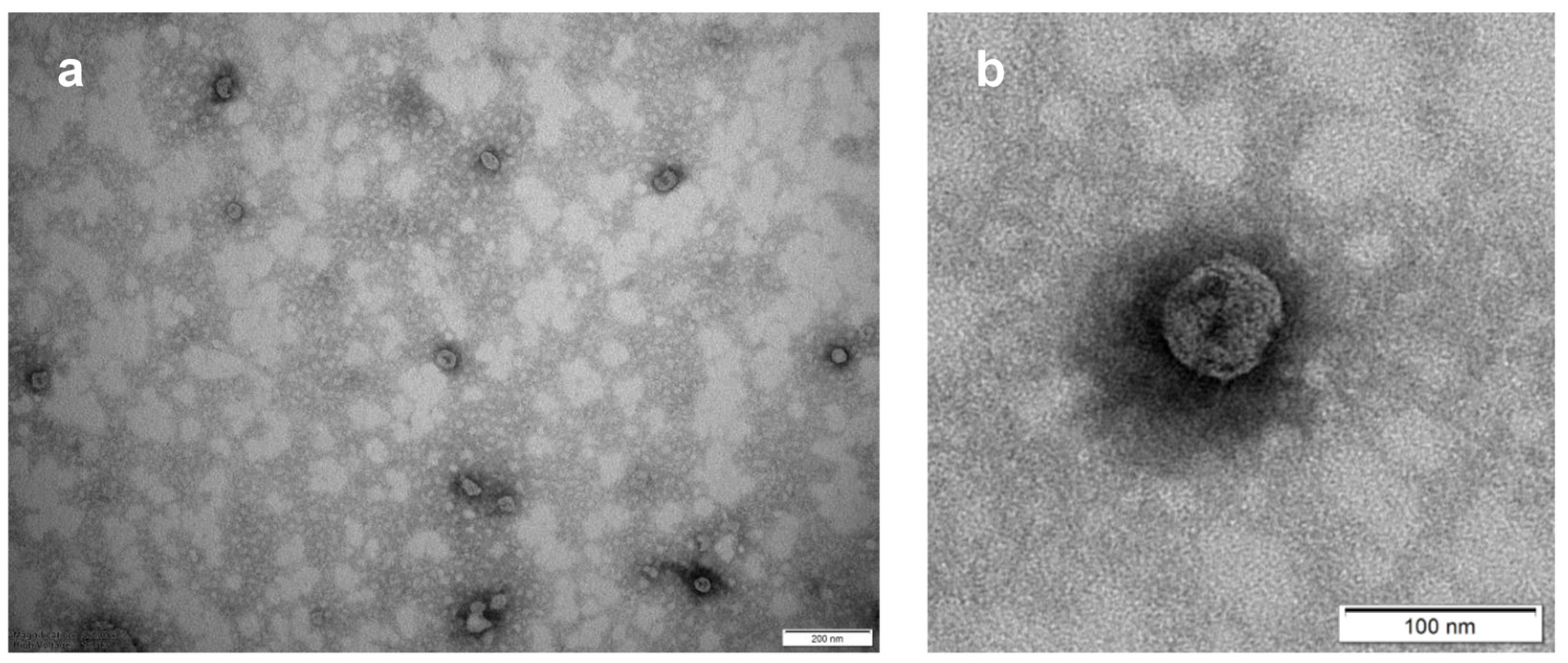
2.11. Transmission Electron Microscopy (TEM) of Exocomplex®
2.12. Total Antioxidant Power Assay (PAO Test Kit)
2.13. Ascorbic Acid Assay
2.14. Catalase Activity Assay
2.15. Reduced Glutathione (GSH) Detection and Quantification Assay
2.16. Superoxide Dismutase (SOD) Activity Assay
2.17. Total Reactive Oxygen Species (ROS) Assay
2.18. Detection of Telomeres by PNA Kit/FITC for Flow Cytometry
2.19. Lipid Peroxidation (MDA) Assay Kit
2.20. Freeze-Drying of Exocomplex® Samples
2.21. DNA Damage Assay Kit
2.22. Mitochondrial Membrane Potential Measurement
2.23. Mitochondrial Superoxide Detection
2.24. ATP Assay Kit
2.25. Melatonin Determination in Serum Samples
2.26. Serotonin Determination in Serum and Urine Samples
2.27. Total Ig Detection and Quantification in Serum Samples
2.28. Statistical Analysis
3. Results
3.1. Quantitative and Qualitative Analysis of the Exocomplex®
3.1.1. The Bioactives’ Content
3.1.2. Concentration and Size-Distribution
3.1.3. Morphological Characterization
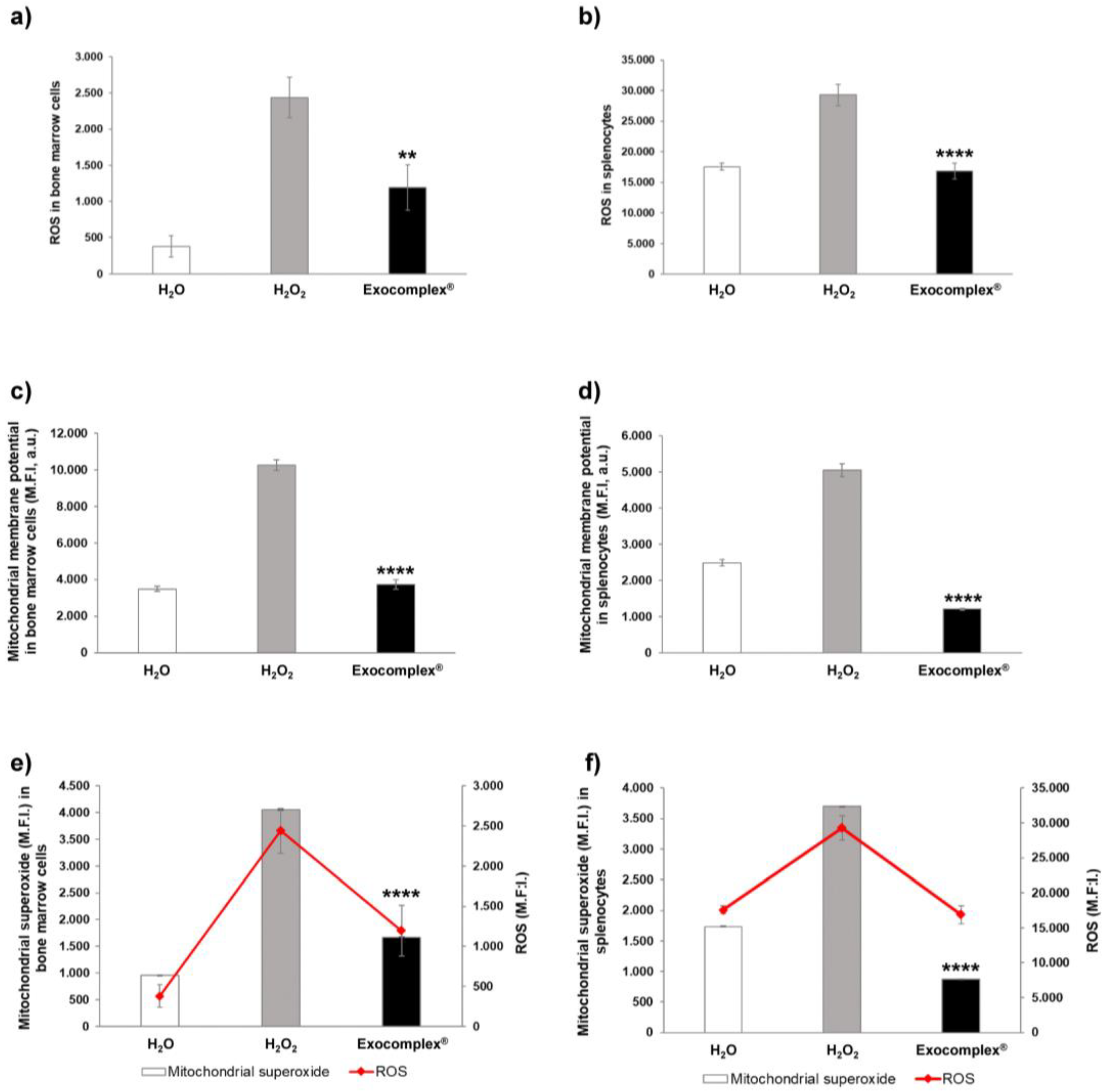
3.2. In Vivo Evaluation of the Exocomplex® in Mice Treated with H2O2
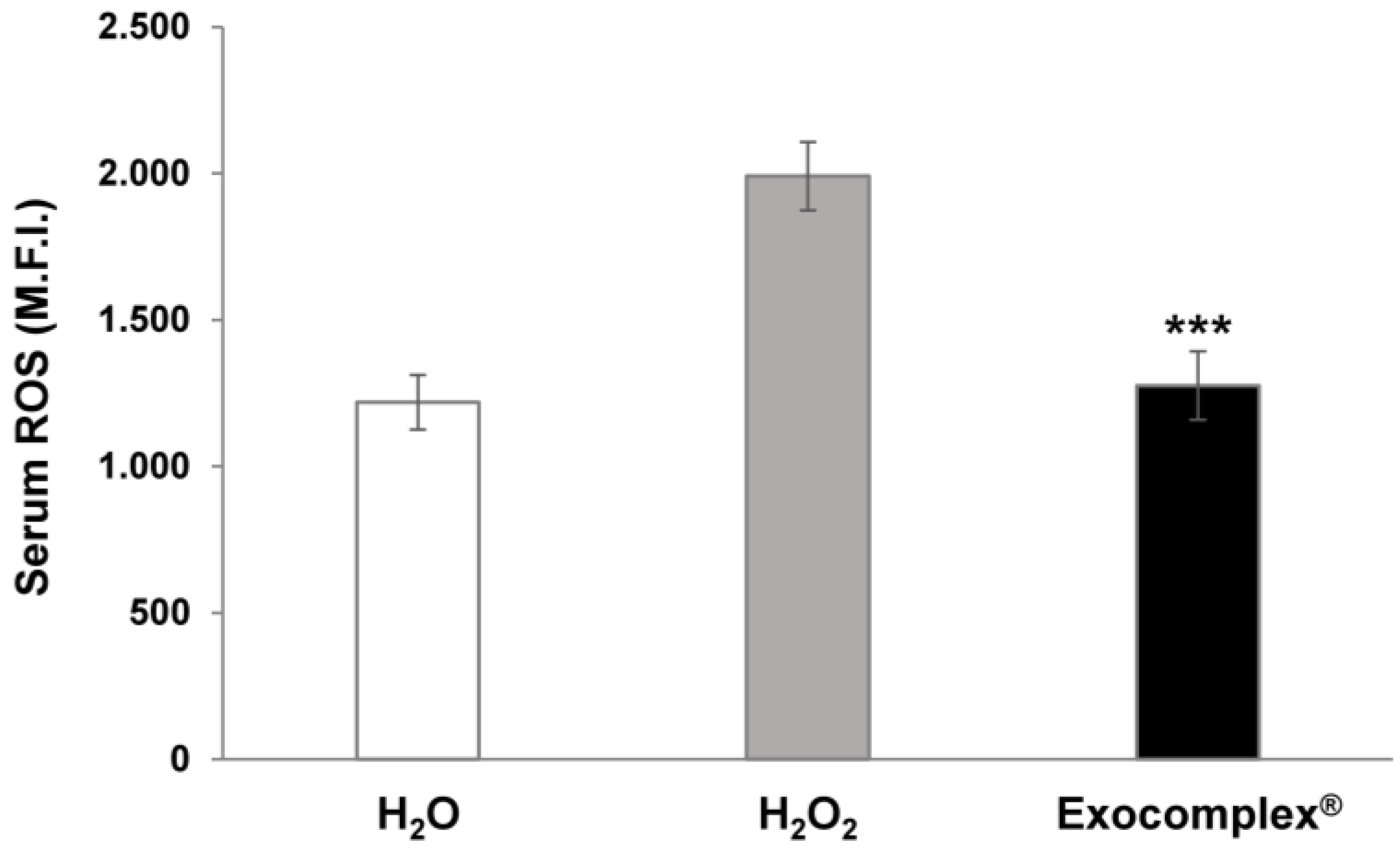
3.2.1. Antioxidant Effect of Exocomplex® on Serum ROS Levels In Vivo Treatment
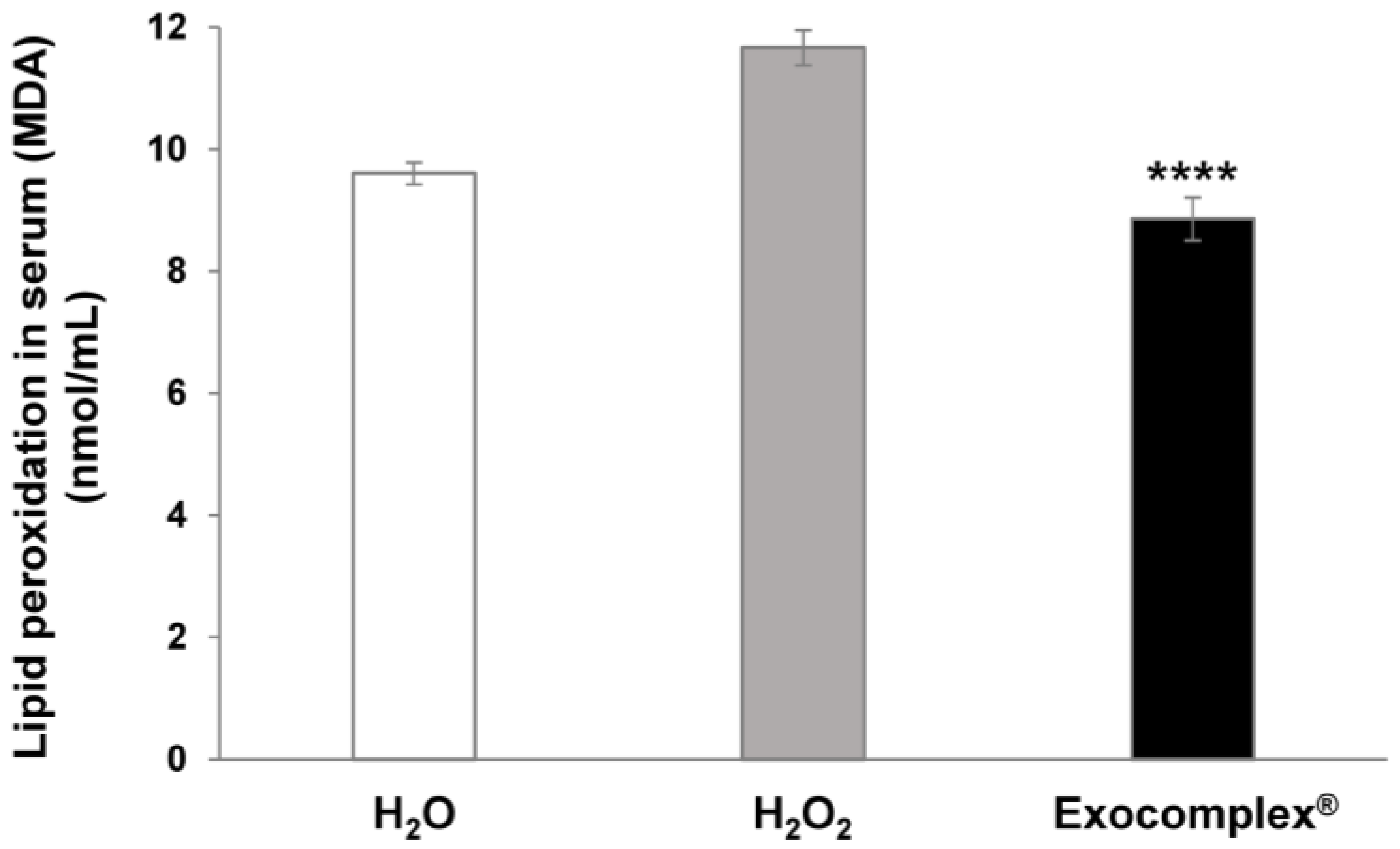
3.2.2. Antioxidant Effect of Exocomplex® on Serum Lipid Peroxidation In Vivo Treatment
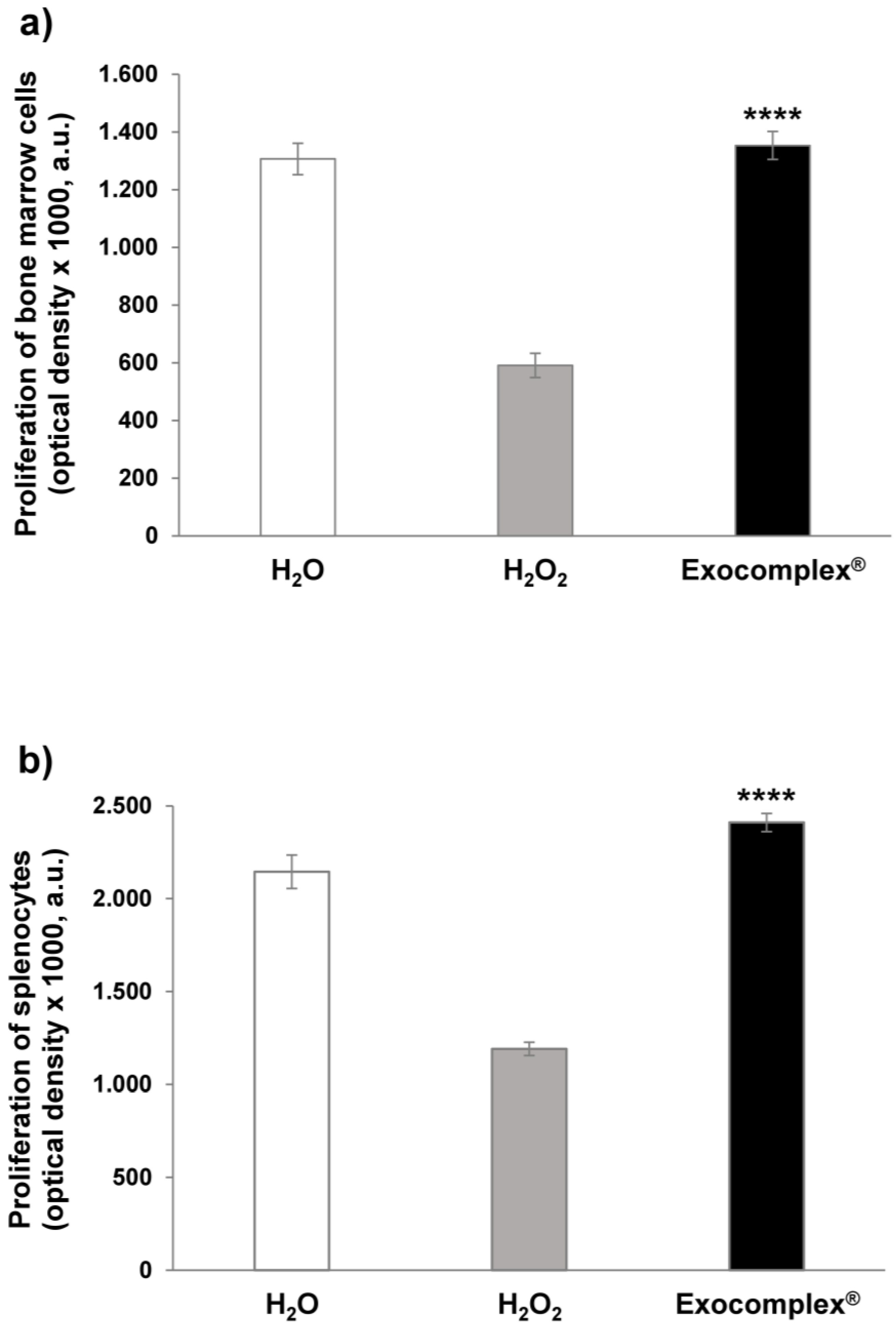
3.2.3. Effect of Exocomplex® Treatment on Cell Number and Cell Proliferation
3.2.4. Exocomplex® Treatment Reduces Oxidative Stress in Mitochondria of Bone Marrow Cells and Splenocytes
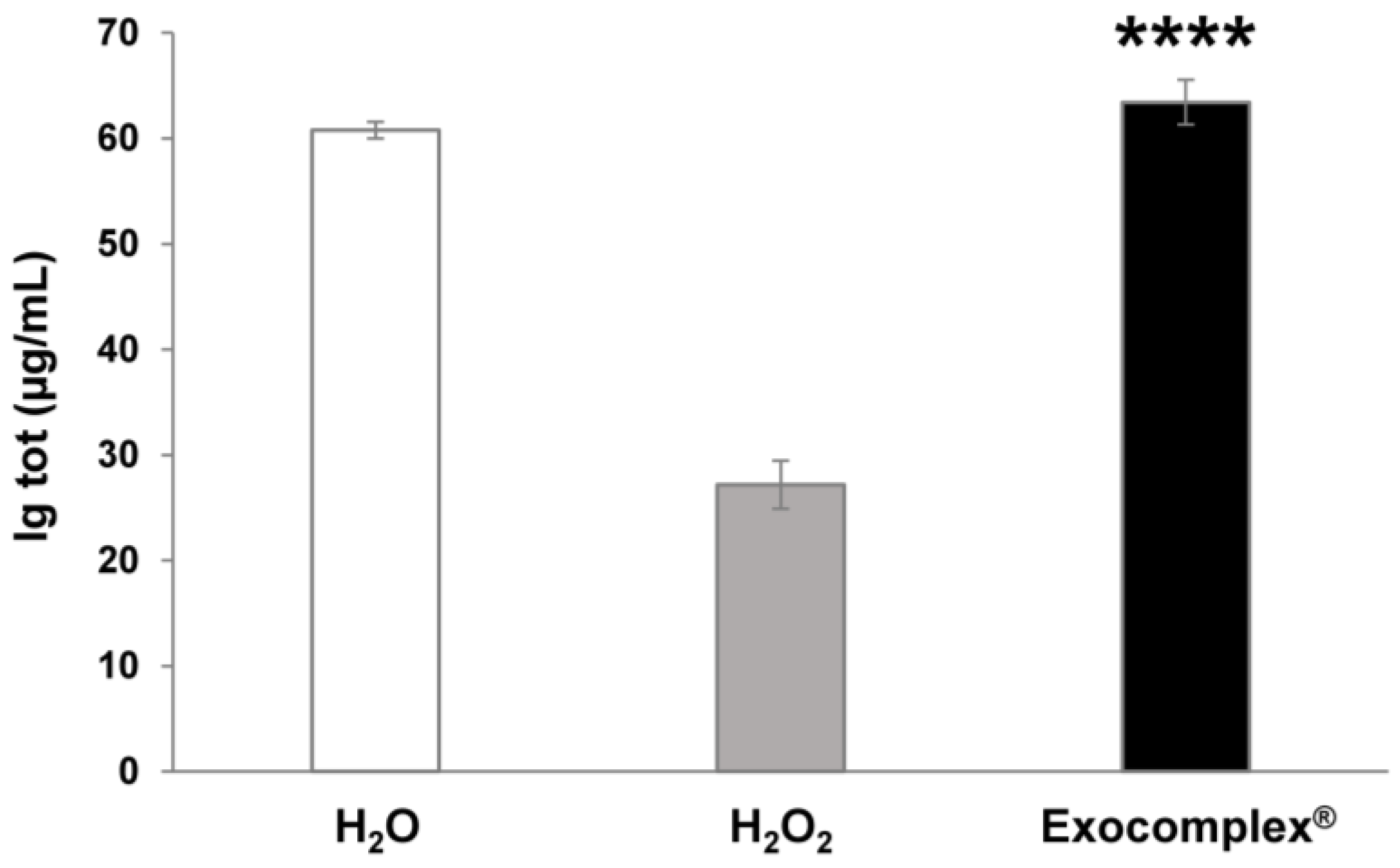
3.2.5. Exocomplex® Restores Physiological Concentration of Serum Immunoglobulins
3.2.6. Exocomplex® Administration Balances Cytotoxic Effect Induced by Oxidative Stress
3.2.7. Exocomplex® Restores Physiological Levels of Serotonin and Melatonin in Murine Body Fluids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuo, C.-L.; Babuharisankar, A.P.; Lin, Y.-C.; Lien, H.-W.; Lo, Y.K.; Chou, H.-Y.; Tangeda, V.; Cheng, L.-C.; Cheng, A.N.; Lee, A.Y.-L. Mitochondrial Oxidative Stress in the Tumor Microenvironment and Cancer Immunoescape: Foe or Friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Almansa-Ordonez, A.; Bellido, R.; Vassena, R.; Barragan, M.; Zambelli, F. Oxidative Stress in Reproduction: A Mitochondrial Perspective. Biology 2020, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Xie, Z.; Zeng, H.; Wang, P.; Li, J.; Zheng, G.; Wang, S.; Cao, Q.; Li, M.; Liu, W.; et al. Oxidative Stress-Mediated Mitochondrial Dysfunction Facilitates Mesenchymal Stem Cell Senescence in Ankylosing Spondylitis. Cell Death Dis. 2020, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial Dysfunction and Oxidative Stress in Heart Disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The Impact of Oxidative DNA Damage and Stress on Telomere Homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Velarde, M.C.; Flynn, J.M.; Day, N.U.; Melov, S.; Campisi, J. Mitochondrial Oxidative Stress Caused by Sod2 Deficiency Promotes Cellular Senescence and Aging Phenotypes in the Skin. Aging 2012, 4, 3–12. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Masud, A.A.; Khan, M.I.; Islam, M.N.; Uddin, S.; Hossain, M.K. Role of Reactive Oxygen Species in Aging and Age-Related Diseases: A Review. ACS Appl. Bio Mater. 2022, 5, 4028–4054. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative Stress and Diabetes: Antioxidative Strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Andreotti, M.; Macchia, D.; Spada, M.; Fais, S. In Vivo Antiaging Effects of Alkaline Water Supplementation. J. Enzyme Inhib. Med. Chem. 2020, 35, 657–664. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Andreotti, M.; Spada, M.; Macchia, D.; Fais, S. Beneficial Effects of Fermented Papaya Preparation (FPP®) Supplementation on Redox Balance and Aging in a Mouse Model. Antioxidants 2020, 9, 144. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Anti-Aging and Anti-Tumor Effect of FPP® Supplementation. Eur. J. Transl. Myol. 2020, 30, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Kovačević, D.B.; Jambrak, A.R.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citrus Wastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green Alternative Methods for the Extraction of Antioxidant Bioactive Compounds from Winery Wastes and By-Products: A Review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N.; et al. Application of Non-Conventional Extraction Methods: Toward a Sustainable and Green Production of Valuable Compounds from Mushrooms. Food Eng. Rev. 2016, 8, 214–234. [Google Scholar] [CrossRef]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Režek Jambrak, A.; Granato, D.; Montesano, D.; Bursać Kovačević, D. Novel Food Processing and Extraction Technologies of High-Added Value Compounds from Plant Materials. Foods Basel Switz. 2018, 7, 106. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. The Potentiality of Plant-Derived Nanovesicles in Human Health—A Comparison with Human Exosomes and Artificial Nanoparticles. Int. J. Mol. Sci. 2022, 23, 4919. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Nanovesicles from Organic Agriculture-Derived Fruits and Vegetables: Characterization and Functional Antioxidant Content. Int. J. Mol. Sci. 2021, 22, 8170. [Google Scholar] [CrossRef] [PubMed]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Baldini, N.; Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Avnet, S. Exosome-like Nanovesicles Isolated from Citrus Limon L. Exert Antioxidative Effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of Therapeutic Agents by Nanoparticles Made of Grapefruit-Derived Lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.; Leone, A.; Ambrosone, A. Plant-Derived Nano and Microvesicles for Human Health and Therapeutic Potential in Nanomedicine. Pharmaceutics 2021, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Plant-Derived Edible Nanoparticles and MiRNAs: Emerging Frontier for Therapeutics and Targeted Drug-Delivery. ACS Sustain. Chem. Eng. 2019, 7, 8055–8069. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Xu, C.; Merlin, D. Plant Derived Edible Nanoparticles as a New Therapeutic Approach against Diseases. Tissue Barriers 2016, 4, e1134415. [Google Scholar] [CrossRef]
- Berger, E.; Colosetti, P.; Jalabert, A.; Meugnier, E.; Wiklander, O.P.B.; Jouhet, J.; Errazurig-Cerda, E.; Chanon, S.; Gupta, D.; Rautureau, G.J.P.; et al. Use of Nanovesicles from Orange Juice to Reverse Diet-Induced Gut Modifications in Diet-Induced Obese Mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 880–892. [Google Scholar] [CrossRef]
- Chen, X.; Liu, B.; Li, X.; An, T.T.; Zhou, Y.; Li, G.; Wu-Smart, J.; Alvarez, S.; Naldrett, M.J.; Eudy, J.; et al. Identification of Anti-inflammatory Vesicle-like Nanoparticles in Honey. J. Extracell. Vesicles 2021, 10, e12069. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lin, E.-Y.; Chiou, T.-W.; Harn, H.-J. Exosomes in Clinical Trial and Their Production in Compliance with Good Manufacturing Practice. Tzu Chi Med. J. 2020, 32, 113–120. [Google Scholar] [CrossRef]
- Sarwa, K.K.; Das, P.J.; Mazumder, B. A Nanovesicle Topical Formulation of Bhut Jolokia (Hottest Capsicum): A Potential Anti-Arthritic Medicine. Expert Opin. Drug Deliv. 2014, 11, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Sriwastva, M.K.; Deng, Z.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G.W.; Xu, F.; et al. Exosome-like Nanoparticles from Mulberry Bark Prevent DSS-induced Colitis via the AhR/COPS8 Pathway. EMBO Rep. 2022, 23, e53365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice From DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Chin, A.R.; Fong, M.Y.; Somlo, G.; Wu, J.; Swiderski, P.; Wu, X.; Wang, S.E. Cross-Kingdom Inhibition of Breast Cancer Growth by Plant MiR159. Cell Res. 2016, 26, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Rhee, W.J. Antioxidative Effects of Carrot-Derived Nanovesicles in Cardiomyoblast and Neuroblastoma Cells. Pharmaceutics 2021, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jung, J.-H.; Yoo, H.J.; Hyun, J.-K.; Park, J.-H.; Na, D.; Yeon, J.H. Anti-Metastatic Effects of Plant Sap-Derived Extracellular Vesicles in a 3D Microfluidic Cancer Metastasis Model. J. Funct. Biomater. 2020, 11, 49. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus Limon-Derived Nanovesicles Inhibit Cancer Cell Proliferation and Suppress CML Xenograft Growth by Inducing TRAIL-Mediated Cell Death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An Efficient Method to Isolate Lemon Derived Extracellular Vesicles for Gastric Cancer Therapy. J. Nanobiotechnology 2020, 18, 100. [Google Scholar] [CrossRef]
- Pinna, R.; Filigheddu, E.; Juliano, C.; Palmieri, A.; Manconi, M.; D’hallewin, G.; Petretto, G.; Maioli, M.; Caddeo, C.; Manca, M.L.; et al. Antimicrobial Effect of Thymus Capitatus and Citrus Limon Var. Pompia as Raw Extracts and Nanovesicles. Pharmaceutics 2019, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; de la Canal, L. Plant Extracellular Vesicles Are Incorporated by a Fungal Pathogen and Inhibit Its Growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef]
- Schuh, C.M.A.P.; Aguayo, S.; Zavala, G.; Khoury, M. Exosome-like Vesicles in Apis Mellifera Bee Pollen, Honey and Royal Jelly Contribute to Their Antibacterial and pro-Regenerative Activity. J. Exp. Biol. 2019, 222, jeb208702. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Miller, D.P.; Kumar, A.; Teng, Y.; Sayed, M.; Mu, J.; Lei, C.; Sriwastva, M.K.; Zhang, L.; Yan, J.; et al. Plant-Derived Exosomal Nanoparticles Inhibit Pathogenicity of Porphyromonas Gingivalis. iScience 2019, 21, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, S.; Hossain, M.N.; Conese, M. Biological Properties and Therapeutic Effects of Plant-Derived Nanovesicles. Open Med. 2020, 15, 1096–1122. [Google Scholar] [CrossRef]
- Şahin, F.; Koçak, P.; Güneş, M.Y.; Özkan, İ.; Yıldırım, E.; Kala, E.Y. In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl. Biochem. Biotechnol. 2019, 188, 381–394. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.; Yu, H.; Shi, X.; Zhou, Y.; Alvarez, S.; Naldrett, M.J.; Kachman, S.D.; Ro, S.-H.; Sun, X.; et al. Therapeutic Potential of Garlic Chive-Derived Vesicle-like Nanoparticles in NLRP3 Inflammasome-Mediated Inflammatory Diseases. Theranostics 2021, 11, 9311–9330. [Google Scholar] [CrossRef]
- Yu, L.; Deng, Z.; Liu, L.; Zhang, W.; Wang, C. Plant-Derived Nanovesicles: A Novel Form of Nanomedicine. Front. Bioeng. Biotechnol. 2020, 8, 584391. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef]
- Orefice, N.S.; Di Raimo, R.; Mizzoni, D.; Logozzi, M.; Fais, S. Purposing Plant-Derived Exosomes-like Nanovesicles for Drug Delivery: Patents and Literature Review. Expert Opin. Ther. Pat. 2023, 33, 89–100. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. What We Know on the Potential Use of Exosomes for Nanodelivery. Semin. Cancer Biol. 2022, 86, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, M.; Merlin, D. Advances in Plant-Derived Edible Nanoparticle-Based Lipid Nano-Drug Delivery Systems as Therapeutic Nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Sharma, A.; Fish, B.L.; Moulder, J.E.; Medhora, M.; Baker, J.E.; Mader, M.; Cohen, E.P. Safety and Blood Sample Volume and Quality of a Refined Retro-Orbital Bleeding Technique in Rats Using a Lateral Approach. Lab Anim. 2014, 43, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.-H.; Wang, J.; Kim, Y.K.; Kwon, I.K. Isolation and Characterization of Ginseng-Derived Exosome-like Nanoparticles with Sucrose Cushioning Followed by Ultracentrifugation. SN Appl. Sci. 2022, 4, 63. [Google Scholar] [CrossRef]
- Kilasoniya, A.; Garaeva, L.; Shtam, T.; Spitsyna, A.; Putevich, E.; Moreno-Chamba, B.; Salazar-Bermeo, J.; Komarova, E.; Malek, A.; Valero, M.; et al. Potential of Plant Exosome Vesicles from Grapefruit (Citrus × Paradisi) and Tomato (Solanum lycopersicum) Juices as Functional Ingredients and Targeted Drug Delivery Vehicles. Antioxidants 2023, 12, 943. [Google Scholar] [CrossRef]
- Kocholata, M.; Prusova, M.; Auer Malinska, H.; Maly, J.; Janouskova, O. Comparison of Two Isolation Methods of Tobacco-Derived Extracellular Vesicles, Their Characterization and Uptake by Plant and Rat Cells. Sci. Rep. 2022, 12, 19896. [Google Scholar] [CrossRef]
- Danino, O.; Svetitsky, S.; Kenigsberg, S.; Levin, A.; Journo, S.; Gold, A.; Drexler, M.; Snir, N.; Elkayam, O.; Fischer, B.; et al. Inhibition of Nucleotide Pyrophosphatase/Phosphodiesterase 1: Implications for Developing a Calcium Pyrophosphate Deposition Disease Modifying Drug. Rheumatology 2018, 57, 1472–1480. [Google Scholar] [CrossRef]
- Nemidkanam, V.; Chaichanawongsaroj, N. Characterizing Kaempferia Parviflora Extracellular Vesicles, a Nanomedicine Candidate. PLoS ONE 2022, 17, e0262884. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, S.; Koo, Y.; Yang, A.; Dai, Y.; Khant, H.; Osman, S.R.; Chowdhury, M.; Wei, H.; Li, Y.; et al. Characterization of and Isolation Methods for Plant Leaf Nanovesicles and Small Extracellular Vesicles. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102271. [Google Scholar] [CrossRef]
- Bruno, S.P.; Paolini, A.; D’Oria, V.; Sarra, A.; Sennato, S.; Bordi, F.; Masotti, A. Extracellular Vesicles Derived From Citrus Sinensis Modulate Inflammatory Genes and Tight Junctions in a Human Model of Intestinal Epithelium. Front. Nutr. 2021, 8, 778998. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Fais, S. Nanovesicules Deriving from Biological Plants as Natural Carriers of Phyto-Complexes for Nutraceutical, Cosmetic and Regenerative Use. 2022. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022157726 (accessed on 15 May 2023).
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomosci Lek. 2004, 57, 453–455. [Google Scholar]
- Finkel, T. Signal Transduction by Reactive Oxygen Species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.S.; Shi, J.; Murad, E.; Whalen, A.M.; Sun, C.Q.; Polavarapu, R.; Parthasarathy, S.; Petros, J.A.; Lambeth, J.D. Hydrogen Peroxide Mediates the Cell Growth and Transformation Caused by the Mitogenic Oxidase Nox1. Proc. Natl. Acad. Sci. USA 2001, 98, 5550–5555. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H. Exogenous H2O2 Induces Growth Inhibition and Cell Death of Human Pulmonary Artery Smooth Muscle Cells via Glutathione Depletion. Mol. Med. Rep. 2016, 14, 936–942. [Google Scholar] [CrossRef]
- Ang, H.Y.; Subramani, T.; Yeap, S.K.; Omar, A.R.; Ho, W.Y.; Abdullah, M.P.; Alitheen, N.B. Immunomodulatory Effects of Potentilla Indica and Dendrophthoe Pentandra on Mice Splenocytes and Thymocytes. Exp. Ther. Med. 2014, 7, 1733–1737. [Google Scholar] [CrossRef]
- Azadmehr, A.; Hajiaghaee, R.; Hassan, A.T.; Vesiehsari, M.J.; Oladnabidozin, M. Splenocyte Proliferation, NK Cell Activation and Cytokines Production by Extract of Scrophularia Variegata; an in Vitro Study on Mice Spleen Cells. Res. J. Pharmacogn. 2016, 3, 9–15. [Google Scholar]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial Membrane Potential Probes and the Proton Gradient: A Practical Usage Guide. BioTechniques 2011, 50, 98–115. [Google Scholar] [CrossRef]
- Izyumov, D.S.; Avetisyan, A.V.; Pletjushkina, O.Y.; Sakharov, D.V.; Wirtz, K.W.; Chernyak, B.V.; Skulachev, V.P. “Wages of Fear”: Transient Threefold Decrease in Intracellular ATP Level Imposes Apoptosis. Biochim. Biophys. Acta BBA Bioenerg. 2004, 1658, 141–147. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- McMahon, B.H.; Fabian, M.; Tomson, F.; Causgrove, T.P.; Bailey, J.A.; Rein, F.N.; Dyer, R.B.; Palmer, G.; Gennis, R.B.; Woodruff, W.H. FTIR Studies of Internal Proton Transfer Reactions Linked to Inter-Heme Electron Transfer in Bovine Cytochrome c Oxidase. Biochim. Biophys. Acta BBA Bioenerg. 2004, 1655, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Traber, M.G. Proceedings of the International Symposium on Natural Antioxidants, 1st ed.; AOCS Publishing: Urbana, IL, USA, 1996; ISBN 978-1-4398-3205-9. [Google Scholar]
- Starkov, A.A.; Fiskum, G. Regulation of Brain Mitochondrial H2O2 Production by Membrane Potential and NAD(P)H Redox State: ROS Production by Brain Mitochondria. J. Neurochem. 2003, 86, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P. Role of Uncoupled and Non-Coupled Oxidations in Maintenance of Safely Low Levels of Oxygen and Its One-Electron Reductants. Q. Rev. Biophys. 1996, 29, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Affourtit, C.; Esteves, T.C.; Green, K.; Lambert, A.J.; Miwa, S.; Pakay, J.L.; Parker, N. Mitochondrial Superoxide: Production, Biological Effects, and Activation of Uncoupling Proteins. Free Radic. Biol. Med. 2004, 37, 755–767. [Google Scholar] [CrossRef]
- Harman, D. The Biologic Clock: The Mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in Plants: Biosynthesis and Physiological Role in Environmental Stress Tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Ercal, N.; Neal, R.; Treeratphan, P.; Lutz, P.M.; Hammond, T.C.; Dennery, P.A.; Spitz, D.R. A Role for Oxidative Stress in Suppressing Serum Immunoglobulin Levels in Lead-Exposed Fisher 344 Rats. Arch. Environ. Contam. Toxicol. 2000, 39, 251–256. [Google Scholar] [CrossRef]
- Singer, R.E.; Moss, K.; Beck, J.D.; Offenbacher, S. Association of Systemic Oxidative Stress with Suppressed Serum IgG to Commensal Oral Biofilm and Modulation by Periodontal Infection. Antioxid. Redox Signal. 2009, 11, 2973–2983. [Google Scholar] [CrossRef]
- Kemkes-Grottenthaler, A. Postponing or Rejecting Parenthood? Results of A Survey among Female Academic Professionals. J. Biosoc. Sci. 2003, 35, 213–226. [Google Scholar] [CrossRef]
- Van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their Relevance during Oocyte Ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef] [PubMed]
- Kordowitzki, P. Oxidative Stress Induces Telomere Dysfunction and Shortening in Human Oocytes of Advanced Age Donors. Cells 2021, 10, 1866. [Google Scholar] [CrossRef]
- Schulpis, K.; Papassotiriou, I.; Tsakiris, S. 8-Hydroxy-2-Desoxyguanosine Serum Concentrations as a Marker of DNA Damage in Patients with Classical Galactosaemia. Acta Paediatr. 2006, 95, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Watanabe, M.; Qureshi, A.R.; Mukai, H.; Machowska, A.; Heimbürger, O.; Barany, P.; Lindholm, B.; Stenvinkel, P. Serum 8-Hydroxydeoxyguanosine, a Marker of Oxidative DNA Damage, Is Associated with Mortality Independent of Inflammation in Chronic Kidney Disease. Eur. J. Intern. Med. 2019, 68, 60–65. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′ -Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Chuma, M.; Hige, S.; Nakanishi, M.; Ogawa, K.; Natsuizaka, M.; Yamamoto, Y.; Asaka, M. 8-Hydroxy-2′-Deoxy-Guanosine Is a Risk Factor for Development of Hepatocellular Carcinoma in Patients with Chronic Hepatitis C Virus Infection. J. Gastroenterol. Hepatol. 2008, 23, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elela, D.H.; El-Edel, R.H.; Shalaby, A.S.; Fouaad, M.A.; Sonbol, A.A. Telomere Length and 8-Hydroxy-2-Deoxyguanosine as Markers for Early Prediction of Alzheimer Disease. Indian J. Psychiatry 2020, 62, 678–683. [Google Scholar] [CrossRef]
- Kerr, J.B.; Hutt, K.J.; Michalak, E.M.; Cook, M.; Vandenberg, C.J.; Liew, S.H.; Bouillet, P.; Mills, A.; Scott, C.L.; Findlay, J.K.; et al. DNA Damage-Induced Primordial Follicle Oocyte Apoptosis and Loss of Fertility Require TAp63-Mediated Induction of Puma and Noxa. Mol. Cell 2012, 48, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.J.; Badia, P. Sleep-Promoting and Hypothermic Effects of Daytime Melatonin Administration in Humans. Sleep 1997, 20, 124–131. [Google Scholar] [CrossRef]
- McArthur, A.J.; Gillette, M.U.; Prosser, R.A. Melatonin Directly Resets the Rat Suprachiasmatic Circadian Clock in Vitro. Brain Res. 1991, 565, 158–161. [Google Scholar] [CrossRef]
- Attenburrow, M.E.J.; Cowen, P.J.; Sharpley, A.L. Low Dose Melatonin Improves Sleep in Healthy Middle-Aged Subjects. Psychopharmacology 1996, 126, 179–181. [Google Scholar] [CrossRef]
- Brzezinski, A.; Vangel, M.G.; Wurtman, R.J.; Norrie, G.; Zhdanova, I.; Ben-Shushan, A.; Ford, I. Effects of Exogenous Melatonin on Sleep: A Meta-Analysis. Sleep Med. Rev. 2005, 9, 41–50. [Google Scholar] [CrossRef]
- Lockley, S.; Skene, D.; James, K.; Thapan, K.; Wright, J.; Arendt, J. Melatonin Administration Can Entrain the Free-Running Circadian System of Blind Subjects. J. Endocrinol. 2000, 164, R1–R6. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Geiger, D.A.; Schwagerl, A.L.; Leclair, O.U.; Killiany, R.; Taylor, J.A.; Rosene, D.L.; Moss, M.B.; Madras, B.K. Melatonin Promotes Sleep in Three Species of Diurnal Nonhuman Primates. Physiol. Behav. 2002, 75, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.V.; Mosser, E.A.; Oikonomou, G.; Prober, D.A. Melatonin Is Required for the Circadian Regulation of Sleep. Neuron 2015, 85, 1193–1199. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One Molecule, Many Derivatives: A Never-Ending Interaction of Melatonin with Reactive Oxygen and Nitrogen Species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Melhuish Beaupre, L.M.; Brown, G.M.; Gonçalves, V.F.; Kennedy, J.L. Melatonin’s Neuroprotective Role in Mitochondria and Its Potential as a Biomarker in Aging, Cognition and Psychiatric Disorders. Transl. Psychiatry 2021, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Vašíček, O.; Lojek, A.; Číž, M. Serotonin and Its Metabolites Reduce Oxidative Stress in Murine RAW264.7 Macrophages and Prevent Inflammation. J. Physiol. Biochem. 2020, 76, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Measurement of Antioxidant Ability of Melatonin and Serotonin by the DMPD and CUPRAC Methods as Trolox Equivalent. J. Enzyme Inhib. Med. Chem. 2008, 23, 871–876. [Google Scholar] [CrossRef]
- Sarikaya, S.; Gulcin, I. Radical Scavenging and Antioxidant Capacity of Serotonin. Curr. Bioact. Compd. 2013, 9, 143–152. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Bocca, B.; Di Raimo, R.; Petrucci, F.; Caimi, S.; Alimonti, A.; Falchi, M.; Cappello, F.; Campanella, C.; et al. Human Primary Macrophages Scavenge AuNPs and Eliminate It through Exosomes. A Natural Shuttling for Nanomaterials. Eur. J. Pharm. Biopharm. 2019, 137, 23–36. [Google Scholar] [CrossRef]
- Federici, C.; Petrucci, F.; Caimi, S.; Cesolini, A.; Logozzi, M.; Borghi, M.; D’Ilio, S.; Lugini, L.; Violante, N.; Azzarito, T.; et al. Exosome Release and Low PH Belong to a Framework of Resistance of Human Melanoma Cells to Cisplatin. PLoS ONE 2014, 9, e88193. [Google Scholar] [CrossRef] [PubMed]
- Iessi, E.; Logozzi, M.; Lugini, L.; Azzarito, T.; Federici, C.; Spugnini, E.P.; Mizzoni, D.; Di Raimo, R.; Angelini, D.F.; Battistini, L.; et al. Acridine Orange/Exosomes Increase the Delivery and the Effectiveness of Acridine Orange in Human Melanoma Cells: A New Prototype for Theranostics of Tumors. J. Enzyme Inhib. Med. Chem. 2017, 32, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.P.; Benbrook, C.M.; Iii, E.G.; Benbrook, K.L. Pesticide Residues in Conventional, Integrated Pest Management (IPM)-Grown and Organic Foods: Insights from Three US Data Sets. Food Addit. Contam. 2002, 19, 427–446. [Google Scholar] [CrossRef]
- Cristache, S.-E.; Vuță, M.; Marin, E.; Cioacă, S.-I.; Vuţă, M. Organic versus Conventional Farming—A Paradigm for the Sustainable Development of the European Countries. Sustainability 2018, 10, 4279. [Google Scholar] [CrossRef]
- Wu, G.; Lupton, J.R.; Turner, N.D.; Fang, Y.-Z.; Yang, S. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Berwal, M.; Ram, C. Superoxide Dismutase: A Stable Biochemical Marker for Abiotic Stress Tolerance in Higher Plants. In Abiotic and Biotic Stress in Plants; Bosco de Oliveira, A., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-811-2. [Google Scholar]
- Scandalios, J.G. Oxidative Stress: Molecular Perception and Transduction of Signals Triggering Antioxidant Gene Defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free Radicals, Antioxidants, and Nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Tan, S.; Yang, Y.; Yang, W.; Han, Y.; Huang, L.; Yang, R.; Hu, Z.; Tao, Y.; Liu, L.; Li, Y.; et al. Exosomal Cargos-Mediated Metabolic Reprogramming in Tumor Microenvironment. J. Exp. Clin. Cancer Res. CR 2023, 42, 59. [Google Scholar] [CrossRef]




| Mean | Std Err | |
|---|---|---|
| SOD (U/mL) | 557 | 13 |
| GSH (µM) | 552 | 12 |
| CATALASE (mU/mL) | 1713 | 30 |
| ASCORBIC ACID (µg) | 32 | 0.01 |
| MELATONIN (ng) | 1.81 | 0.02 |
| TOTAL ANTIOXIDANT
CAPACITY (mM) | 620.5 | 27.8 |
| PHENOLIC COMPOUNDS (mM) | 769.3 | 49.1 |
| ATP (µM) | 81.6 | 8.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Raimo, R.; Mizzoni, D.; Spada, M.; Dolo, V.; Fais, S.; Logozzi, M. Oral Treatment with Plant-Derived Exosomes Restores Redox Balance in H2O2-Treated Mice. Antioxidants 2023, 12, 1169. https://doi.org/10.3390/antiox12061169
Di Raimo R, Mizzoni D, Spada M, Dolo V, Fais S, Logozzi M. Oral Treatment with Plant-Derived Exosomes Restores Redox Balance in H2O2-Treated Mice. Antioxidants. 2023; 12(6):1169. https://doi.org/10.3390/antiox12061169
Chicago/Turabian StyleDi Raimo, Rossella, Davide Mizzoni, Massimo Spada, Vincenza Dolo, Stefano Fais, and Mariantonia Logozzi. 2023. "Oral Treatment with Plant-Derived Exosomes Restores Redox Balance in H2O2-Treated Mice" Antioxidants 12, no. 6: 1169. https://doi.org/10.3390/antiox12061169
APA StyleDi Raimo, R., Mizzoni, D., Spada, M., Dolo, V., Fais, S., & Logozzi, M. (2023). Oral Treatment with Plant-Derived Exosomes Restores Redox Balance in H2O2-Treated Mice. Antioxidants, 12(6), 1169. https://doi.org/10.3390/antiox12061169







