Heme Oxygenase-1 Expression in Dendritic Cells Contributes to Protective Immunity against Herpes Simplex Virus Skin Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Virus Propagation
2.3. Viability and Maturation of HSV-Infected DCs
2.4. Caspase-3 Activity Assay and HSV Replication in Infected DCs
2.5. DC-T Cell Antigen Presentation Assays
2.6. HSV Skin Infection and T Cell Activation In Vivo
2.7. Statistical Analyses
3. Results
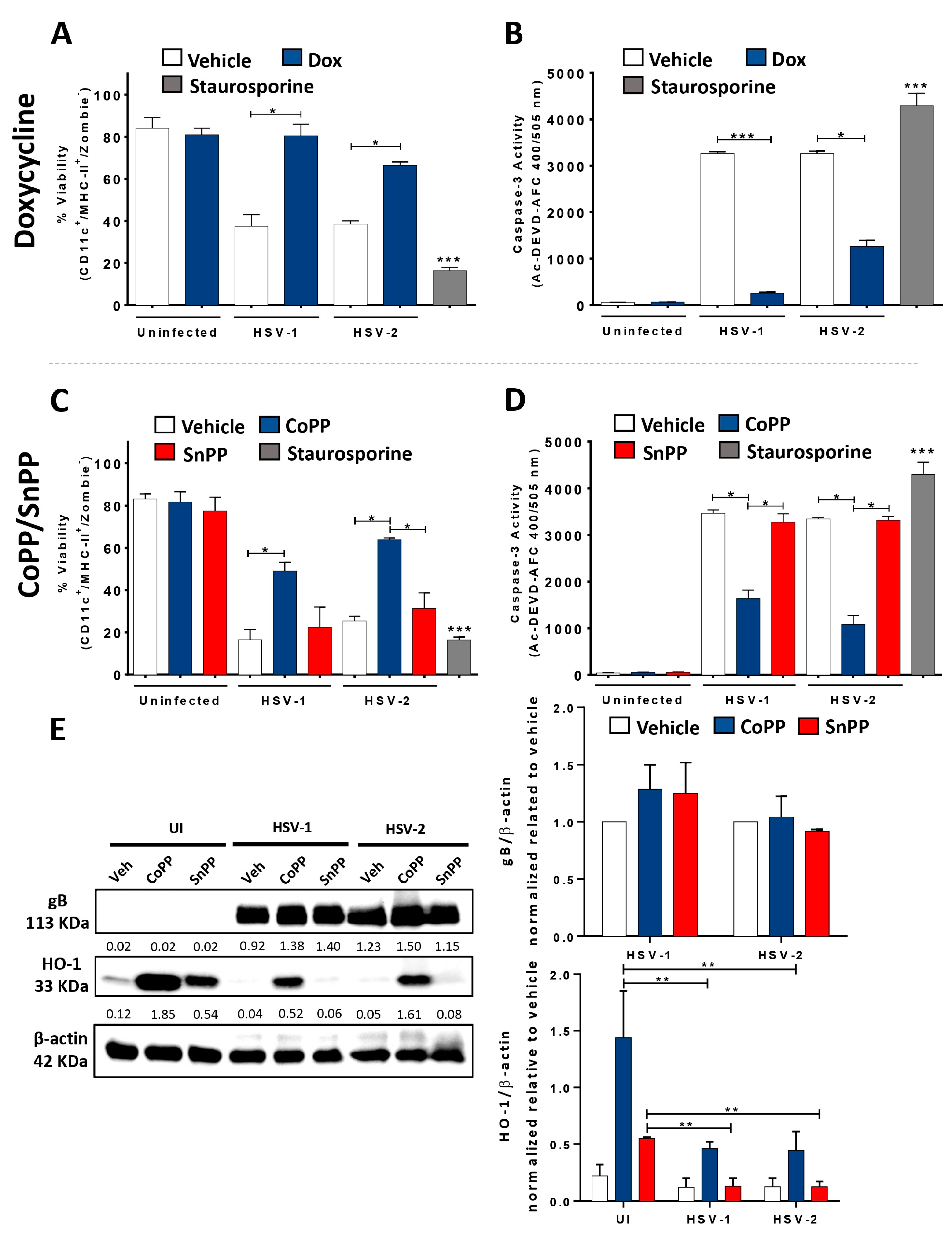
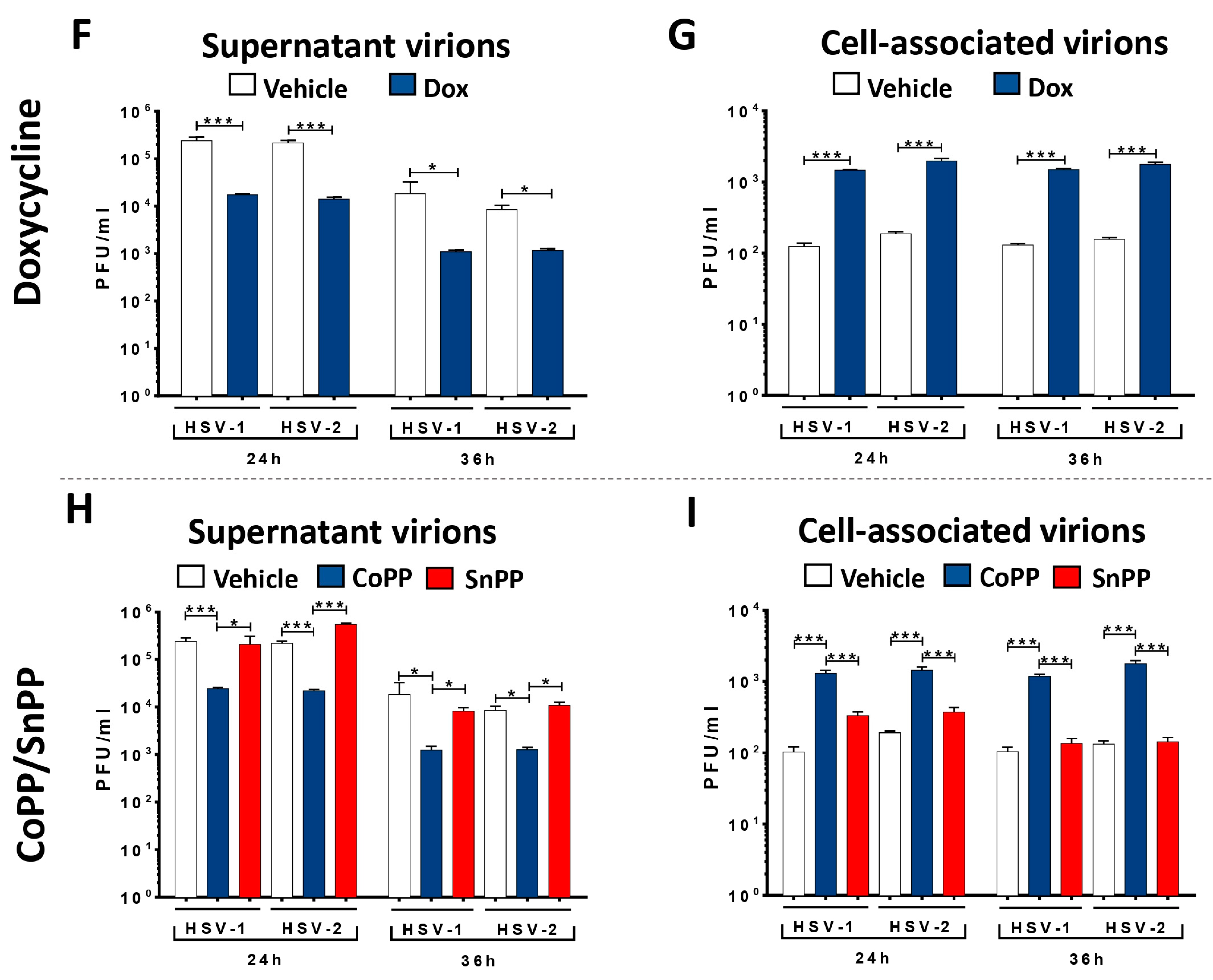
3.1. HO-1 Expression in HSV-Infected DCs Enhances Cell Viability and Hampers Viral Particle Egress without Affecting Viral Genome Replication or Transcription
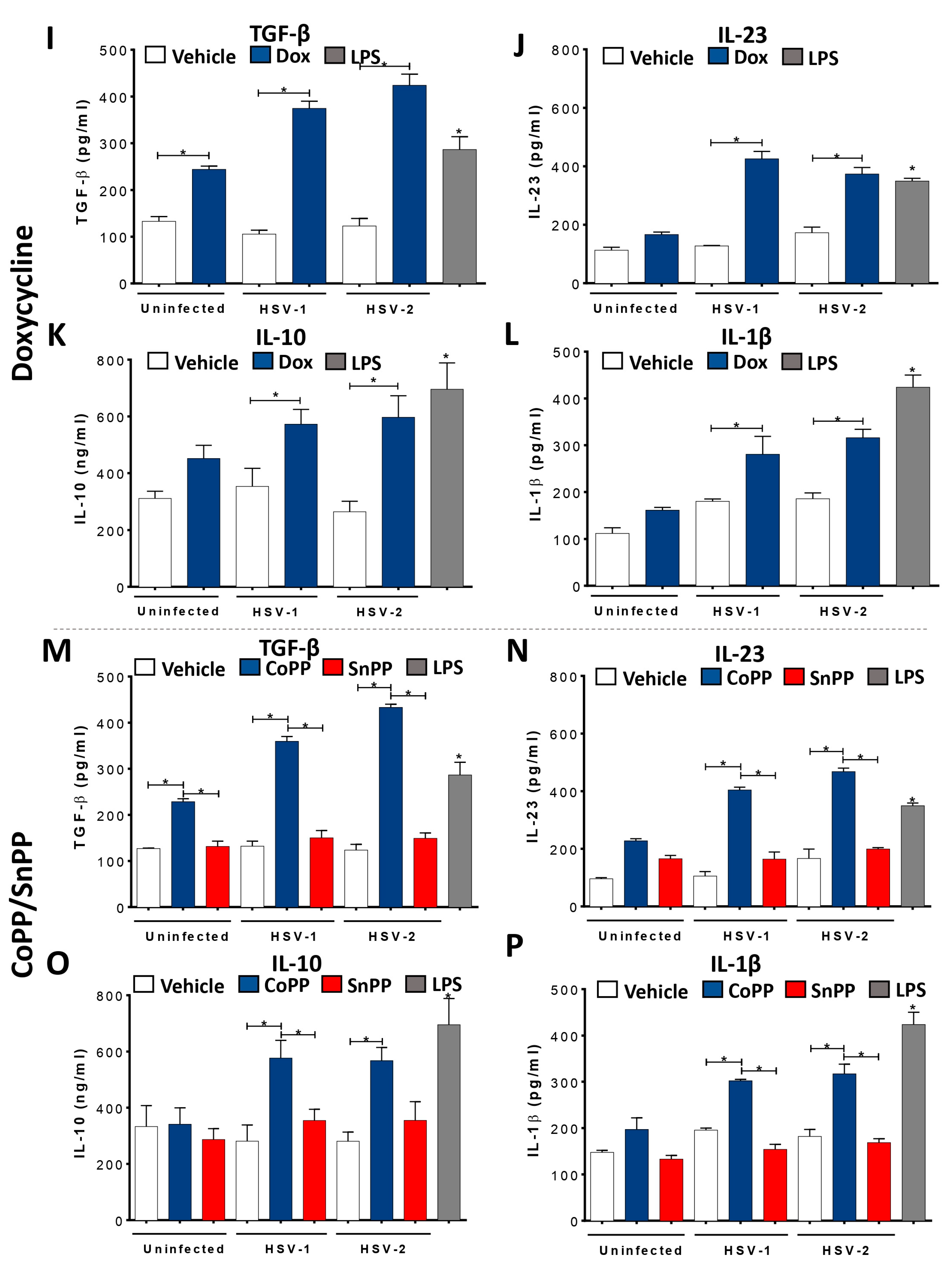
3.2. HO-1 Expression in HSV-Infected DCs Promotes an Anti-Inflammatory Phenotype
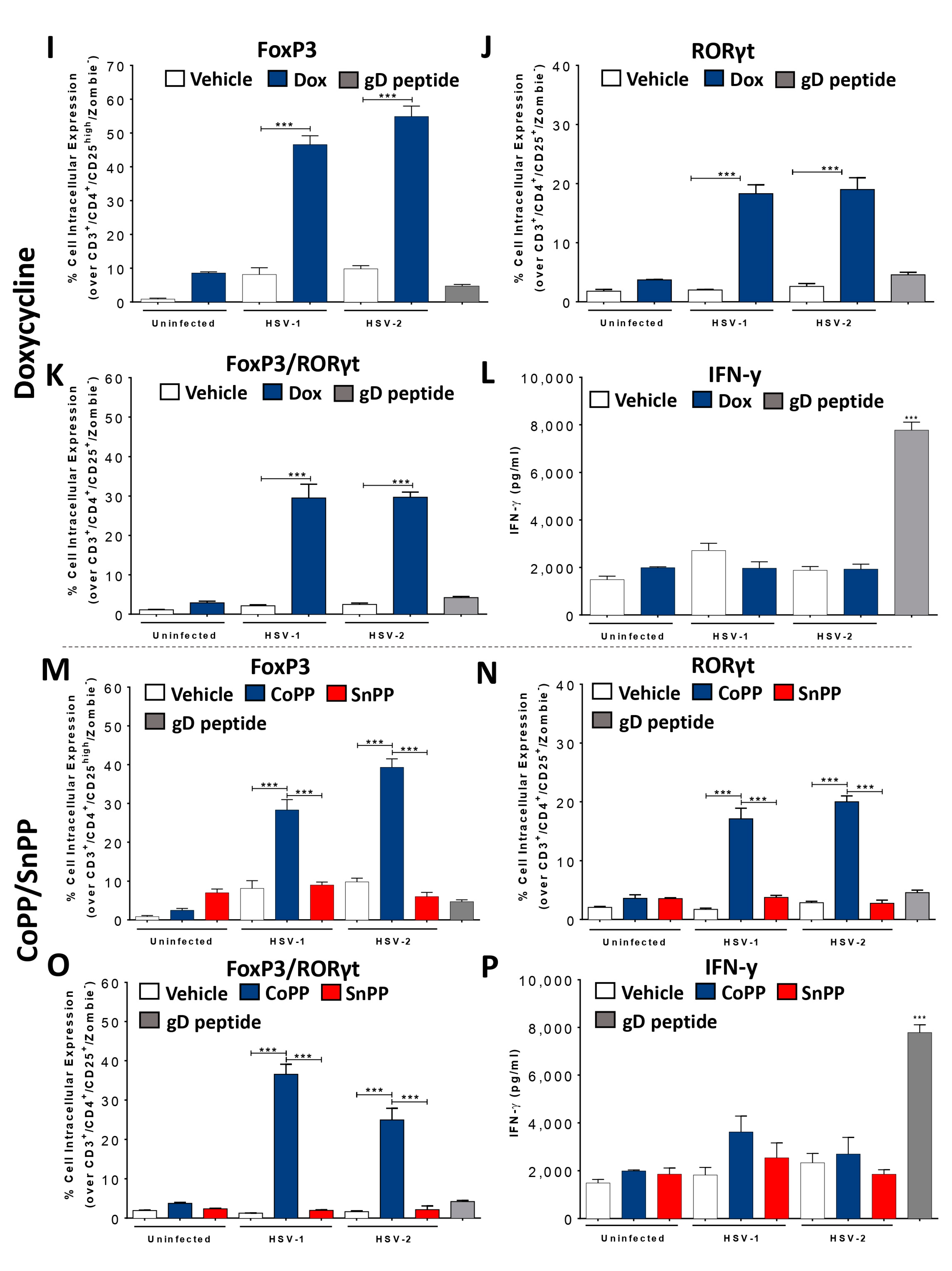
3.3. HO-1 Expression in HSV-Infected DCs Promotes CD4+ T Cell Activation In Vitro
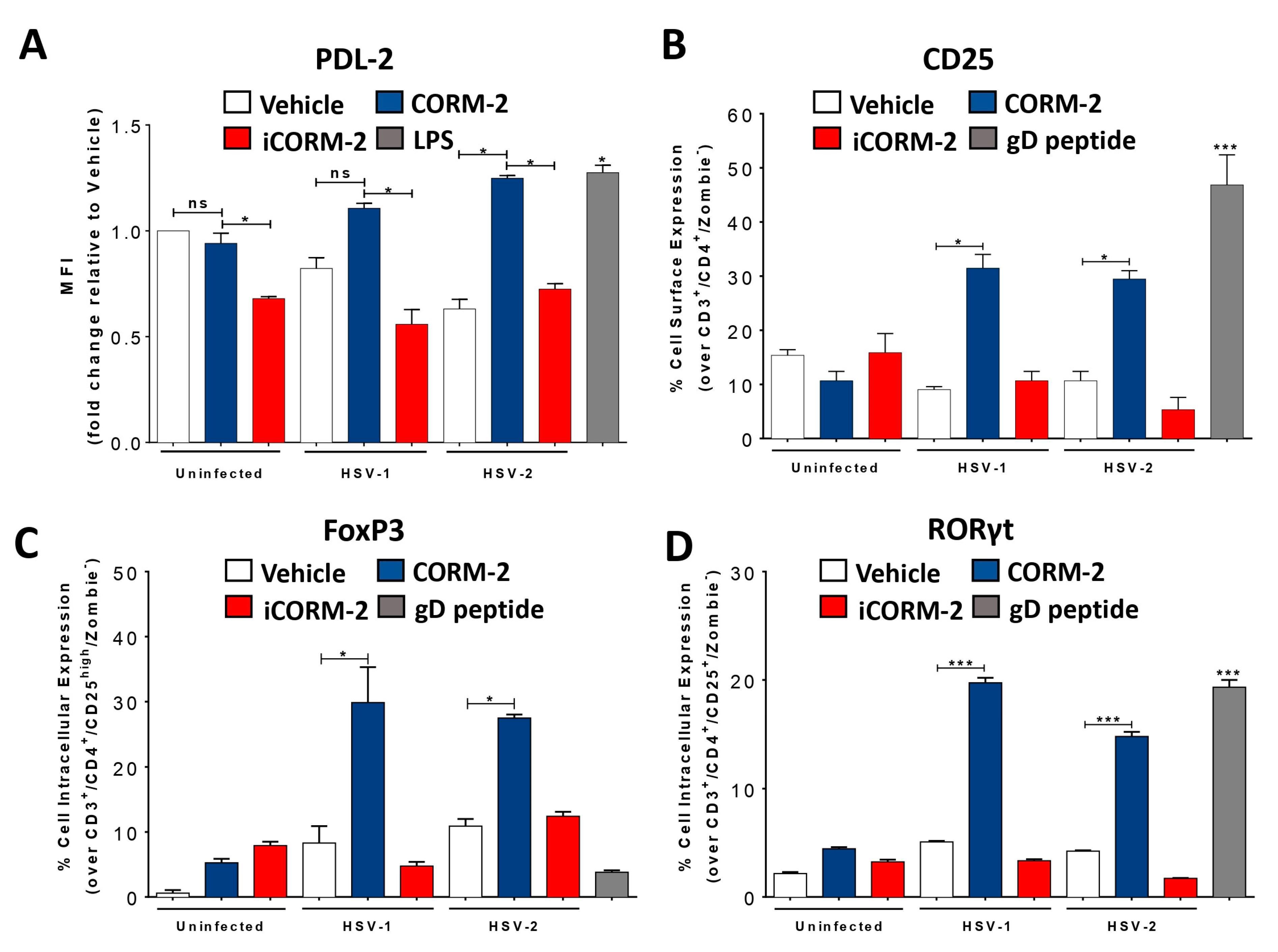
3.4. Carbon Monoxide Recapitulates the Effects of HO-1 Expression in HSV-Infected DCs
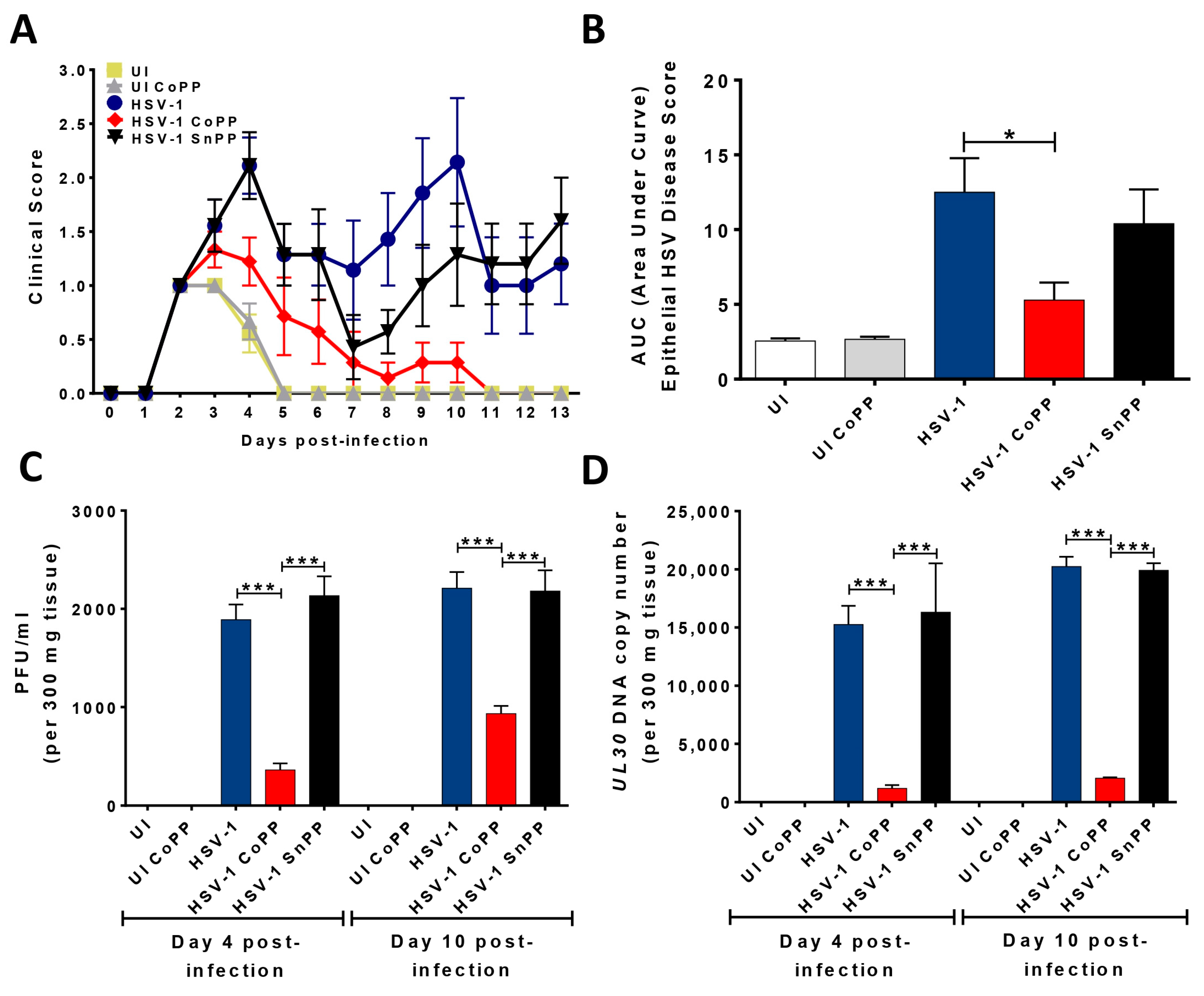
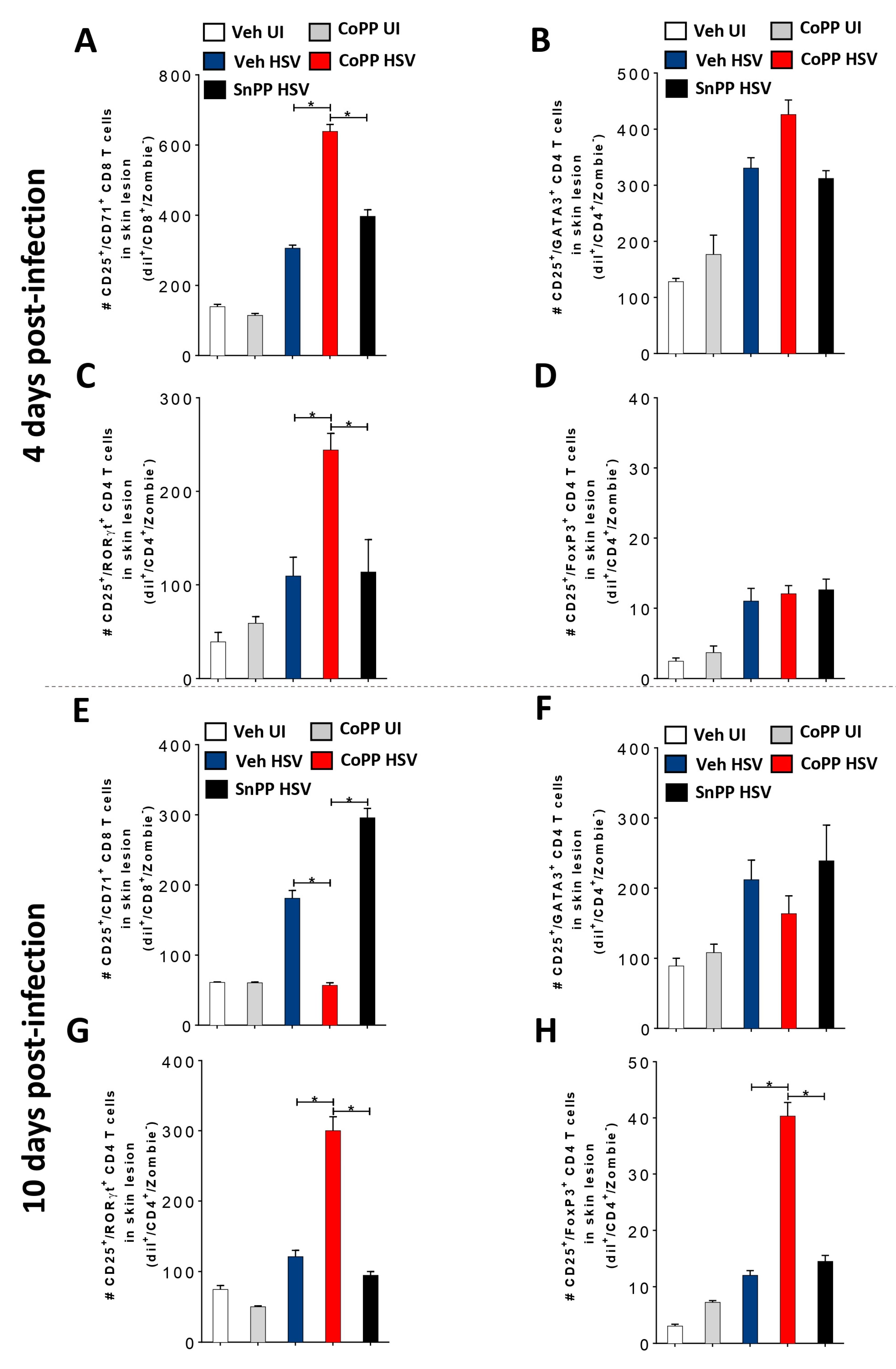
3.5. HO-1 Induction in HSV-Infected DCs Transferred into Mice Reduces Disease Severity and Modulates Virus-Specific T Cell Infiltration into HSV-1-Related Skin Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.F.; Reyes, A.; Farías, M.A.; Riedel, C.A.; Bueno, S.M.; Kalergis, A.M.; González, P.A. Crosstalk Between Epithelial Cells, Neurons and Immune Mediators in HSV-1 Skin Infection. Front. Immunol. 2021, 12, 662234. [Google Scholar] [CrossRef]
- Chung, E.; Sen, J. The ongoing pursuit of a prophylactic HSV vaccine. Rev. Med. Virol. 2012, 22, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.M.; Duarte, L.F.; Corrales, N.; Smith, P.C.; González, P.A. Cetylpyridinium chloride blocks herpes simplex virus replication in gingival fibroblasts. Antivir. Res. 2020, 179, 104818. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.L.; Shukla, D. Recent advances in vaccine development for herpes simplex virus types I and II. Hum. Vaccines Immunother. 2013, 9, 729–735. [Google Scholar] [CrossRef]
- Duarte, L.F.; Altamirano-Lagos, M.J.; Tabares-Guevara, J.H.; Opazo, M.C.; Díaz, M.; Navarrete, R.; Muza, C.; Vallejos, O.P.; Riedel, C.A.; Bueno, S.M.; et al. Asymptomatic Herpes Simplex Virus Type 1 Infection Causes an Earlier Onset and More Severe Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2021, 12, 635257. [Google Scholar] [CrossRef]
- Farías, M.; Duarte, L.; Tognarelli, E.; González, P. Herpes simplex virus interference with immunity: Focus on dendritic cells. Virulence 2021, 12, 2583–2607. [Google Scholar] [CrossRef]
- Tognarelli, E.I.; Palomino, T.F.; Corrales, N.; Bueno, S.M.; Kalergis, A.M.; González, P.A. Herpes Simplex Virus Evasion of Early Host Antiviral Responses. Front. Cell. Infect. Microbiol. 2019, 9, 127. [Google Scholar] [CrossRef]
- Suazo, P.A.; Ibañez, F.J.; Retamal-Díaz, A.R.; Paz-Fiblas, M.V.; Bueno, S.M.; Kalergis, A.M.; González, P.A. Evasion of early antiviral responses by herpes simplex viruses. Mediat. Inflamm. 2015, 2015, 593757. [Google Scholar] [CrossRef]
- Retamal-Díaz, A.R.; Tognarelli, E.; Kalergis, A.M.; Bueno, S.M.; González, P.A. Immune Evasion by Herpes Simplex Viruses; IntechOpen: London, UK, 2016; ISBN 978-953-51-2611-9. [Google Scholar]
- Carreño, L.J.; González, P.A.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Modulation of the dendritic cell–T-cell synapse to promote pathogen immunity and prevent autoimmunity. Immunotherapy 2011, 3, 6–11. [Google Scholar] [CrossRef]
- Retamal-Díaz, A.R.; Kalergis, A.M.; Bueno, S.M.; González, P.A. A Herpes Simplex Virus Type 2 Deleted for Glycoprotein D Enables Dendritic Cells to Activate CD4+ and CD8+ T Cells. Front. Immunol. 2017, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- Retamal-Díaz, A.; Weiss, K.A.; Tognarelli, E.I.; Freire, M.; Bueno, S.M.; Herold, B.C.; Jacobs, W.R.J.; González, P.A. US6 Gene Deletion in Herpes Simplex Virus Type 2 Enhances Dendritic Cell Function and T Cell Activation. Front. Immunol. 2017, 8, 1523. [Google Scholar] [CrossRef]
- Hill, A.; Jugovic, P.; York, I.; Russ, G.; Bennink, J.; Yewdell, J.; Ploegh, H.; Johnson, D. Herpes simplex virus turns off the TAP to evade host immunity. Nature 1995, 375, 411–415. [Google Scholar] [CrossRef]
- Elboim, M.; Grodzovski, I.; Djian, E.; Wolf, D.G.; Mandelboim, O. HSV-2 specifically down regulates HLA-C expression to render HSV-2-infected DCs susceptible to NK cell killing. PLoS Pathog. 2013, 9, e1003226. [Google Scholar] [CrossRef]
- Matundan, H.; Ghiasi, H. Herpes Simplex Virus 1 ICP22 Suppresses CD80 Expression by Murine Dendritic Cells. J. Virol. 2019, 93, e01803-18. [Google Scholar] [CrossRef]
- Grosche, L.; Mühl-Zürbes, P.; Ciblis, B.; Krawczyk, A.; Kuhnt, C.; Kamm, L.; Steinkasserer, A.; Heilingloh, C.S. Herpes Simplex Virus Type-2 Paralyzes the Function of Monocyte-Derived Dendritic Cells. Viruses 2020, 12, 112. [Google Scholar] [CrossRef]
- Theodoridis, A.A.; Eich, C.; Figdor, C.G.; Steinkasserer, A. Infection of dendritic cells with herpes simplex virus type 1 induces rapid degradation of CYTIP, thereby modulating adhesion and migration. Blood 2011, 118, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Stefanidou, M.; Ramos, I.; Mas Casullo, V.; Trépanier, J.B.; Rosenbaum, S.; Fernandez-Sesma, A.; Herold, B.C. Herpes simplex virus 2 (HSV-2) prevents dendritic cell maturation, induces apoptosis, and triggers release of proinflammatory cytokines: Potential links to HSV-HIV synergy. J. Virol. 2013, 87, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Bosnjak, L.; Miranda-Saksena, M.; Koelle, D.M.; Boadle, R.A.; Jones, C.A.; Cunningham, A.L. Herpes Simplex Virus Infection of Human Dendritic Cells Induces Apoptosis and Allows Cross-Presentation via Uninfected Dendritic Cells. J. Immunol. 2005, 174, 2220–2227. [Google Scholar] [CrossRef]
- Tognarelli, E.I.; Retamal-Díaz, A.; Farías, M.A.; Duarte, L.F.; Palomino, T.F.; Ibañez, F.J.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M.; González, P.A. Pharmacological Inhibition of IRE-1 Alpha Activity in Herpes Simplex Virus Type 1 and Type 2-Infected Dendritic Cells Enhances T Cell Activation. Front. Immunol. 2021, 12, 764861. [Google Scholar] [CrossRef]
- Ibáñez, F.J.; Farías, M.A.; Retamal-Díaz, A.; Espinoza, J.A.; Kalergis, A.M.; González, P.A. Pharmacological Induction of Heme Oxygenase-1 Impairs Nuclear Accumulation of Herpes Simplex Virus Capsids upon Infection. Front. Microbiol. 2017, 8, 2108. [Google Scholar] [CrossRef] [PubMed]
- Keyse, S.M.; Tyrrell, R.M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc. Natl. Acad. Sci. USA 1989, 86, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Alam, J.; Choi, A.M.K. Heme Oxygenase-1/Carbon Monoxide: From Basic Science to Therapeutic Applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef]
- Louise, L.; Dunn, R.G.M.; Jun, N.; Hafizah, A.H.; Christopher, R.P. Roland, Stocker New Insights into Intracellular Locations and Functions of Heme Oxygenase-1. Antioxid. Redox Signal. 2014, 20, 1723–1742. [Google Scholar] [CrossRef]
- Espinoza, J.A.; León, M.A.; Céspedes, P.F.; Gómez, R.S.; Canedo-Marroquín, G.; Riquelme, S.A.; Salazar-Echegarai, F.J.; Blancou, P.; Simon, T.; Anegon, I.; et al. Heme Oxygenase-1 Modulates Human Respiratory Syncytial Virus Replication and Lung Pathogenesis during Infection. J. Immunol. 2017, 199, 212–223. [Google Scholar] [CrossRef]
- Shan, Y.; Lambrecht, R.W.; Donohue, S.E.; Bonkovsky, H.L. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 2651–2653. [Google Scholar] [CrossRef]
- Anderson, K.E.; Simionatto, C.S.; Drummond, G.S.; Kappas, A. Tissue distribution and disposition of tin-protoporphyrin, a potent competitive inhibitor of heme oxygenase. J. Pharmacol. Exp. Ther. 1984, 228, 327–333. [Google Scholar]
- Mueller, S.N.; Heath, W.R.; Mclain, J.D.; Carbone, F.R.; Jones, C.M. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol. Cell Biol. 2002, 80, 156–163. [Google Scholar] [CrossRef]
- Collins, N.; Jiang, X.; Zaid, A.; Macleod, B.L.; Li, J.; Park, C.O.; Haque, A.; Bedoui, S.; Heath, W.R.; Mueller, S.N.; et al. Skin CD4+ memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat. Commun. 2016, 7, 11514. [Google Scholar] [CrossRef]
- Clement, C.; Tiwari, V.; Scanlan, P.M.; Valyi-Nagy, T.; Yue, B.Y.J.T.; Shukla, D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J. Cell Biol. 2006, 174, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Cheshenko, N.; Trepanier, J.B.; González, P.A.; Eugenin, E.A.; Jacobs, W.R.; Herold, B.C. Herpes simplex virus type 2 glycoprotein h interacts with integrin αvβ3 to facilitate viral entry and calcium signaling in human genital tract epithelial cells. J. Virol. 2014, 88, 10026–10038. [Google Scholar] [CrossRef]
- González, P.A.; Prado, C.E.; Leiva, E.D.; Carreño, L.J.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc. Natl. Acad. Sci. USA 2008, 105, 14999–15004. [Google Scholar] [CrossRef] [PubMed]
- Kraft, R.M.; Nguyen, M.L.; Yang, X.-H.; Thor, A.D.; Blaho, J.A. Caspase 3 activation during herpes simplex virus 1 infection. Virus Res. 2006, 120, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Namvar, L.; Olofsson, S.; Bergström, T.; Lindh, M. Detection and typing of Herpes Simplex virus (HSV) in mucocutaneous samples by TaqMan PCR targeting a gB segment homologous for HSV types 1 and 2. J. Clin. Microbiol. 2005, 43, 2058–2064. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Castillo, E.; Duarte, L.F.; Corrales, N.; Álvarez, D.M.; Farías, M.A.; Henríquez, A.; Smith, P.C.; Agurto-Muñoz, C.; González, P.A. Anti-herpetic Activity of Macrocystis pyrifera and Durvillaea antarctica Algae Extracts Against HSV-1 and HSV-2. Front. Microbiol. 2020, 11, 2006. [Google Scholar] [CrossRef]
- Petro, C.; González, P.A.; Cheshenko, N.; Jandl, T.; Khajoueinejad, N.; Bénard, A.; Sengupta, M.; Herold, B.C.; Jacobs, W.R. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. eLife 2015, 4, e06054. [Google Scholar] [CrossRef]
- Cai, J.; Wang, D.; Zhang, G.; Guo, X. The Role Of PD-1/PD-L1 Axis In Treg Development And Function: Implications For Cancer Immunotherapy. OncoTargets Ther. 2019, 12, 8437–8445. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, X.; Lan, P.; Li, J.; Dou, Y.; Chen, W.; Ishii, N.; Chen, S.; Xia, B.; Chen, K.; et al. OX40 Costimulation Inhibits Foxp3 Expression and Treg Induction via BATF3-Dependent and Independent Mechanisms. Cell Rep. 2018, 24, 607–618. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Rana, B.M.J.; Walker, J.A.; Kerscher, B.; Knolle, M.D.; Jolin, H.E.; Serrao, E.M.; Haim-Vilmovsky, L.; Teichmann, S.A.; Rodewald, H.-R.; et al. Tissue-Restricted Adaptive Type 2 Immunity Is Orchestrated by Expression of the Costimulatory Molecule OX40L on Group 2 Innate Lymphoid Cells. Immunity 2018, 48, 1195–1207.e6. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Arbieva, Z.H.; Maienschein-Cline, M.; Ganesh, B.B.; Ramasamy, S.; Prabhakar, B.S. Induction of Antigen-Independent Proliferation of Regulatory T-Cells by TNF Superfamily Ligands OX40L and GITRL. Methods Mol. Biol. Clifton N. J. 2021, 2248, 63–71. [Google Scholar] [CrossRef]
- Marinelarena, A.; Bhattacharya, P.; Kumar, P.; Maker, A.V.; Prabhakar, B.S. Identification of a Novel OX40L+ Dendritic Cell Subset That Selectively Expands Regulatory T cells. Sci. Rep. 2018, 8, 14940. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, M.; Yang, S.; Shi, J.; Ji, T.; Ding, W.; Jiang, L.; Fan, Z.; Chen, J.; Lu, Y. Butyric Acid Protects Against Renal Ischemia–Reperfusion Injury by Adjusting the Treg/Th17 Balance via HO-1/p-STAT3 Signaling. Front. Cell Dev. Biol. 2021, 9, 733308. [Google Scholar] [CrossRef]
- Zhang, Q.; Cui, T.; Chang, Y.; Zhang, W.; Li, S.; He, Y.; Li, B.; Liu, L.; Wang, G.; Gao, T.; et al. HO-1 regulates the function of Treg: Association with the immune intolerance in vitiligo. J. Cell. Mol. Med. 2018, 22, 4335–4343. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Chang, J.-H.; Chuang, H.-C.; Fan, C.-K.; Hou, T.-Y.; Lin, C.-L.; Lee, Y.-L. Schisandrin B promotes Foxp3+ regulatory T cell expansion by activating heme oxygenase-1 in dendritic cells and exhibits immunomodulatory effects in Th2-mediated allergic asthma. Eur. J. Pharmacol. 2022, 918, 174775. [Google Scholar] [CrossRef] [PubMed]
- Klinger, M.; Kim, J.K.; Chmura, S.A.; Barczak, A.; Erle, D.J.; Killeen, N. Thymic OX40 expression discriminates cells undergoing strong responses to selection ligands. J. Immunol. Baltim. Md. 1950 2009, 182, 4581–4589. [Google Scholar] [CrossRef]
- Gagliani, N.; Amezcua Vesely, M.C.; Iseppon, A.; Brockmann, L.; Xu, H.; Palm, N.W.; de Zoete, M.R.; Licona-Limón, P.; Paiva, R.S.; Ching, T.; et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 2015, 523, 221–225. [Google Scholar] [CrossRef]
- Chauveau, C.; Rémy, S.; Royer, P.J.; Hill, M.; Tanguy-Royer, S.; Hubert, F.-X.; Tesson, L.; Brion, R.; Beriou, G.; Gregoire, M.; et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood 2005, 106, 1694–1702. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Pogu, J.; Anegon, I.; Bueno, S.M.; Kalergis, A.M. Carbon monoxide impairs mitochondria-dependent endosomal maturation and antigen presentation in dendritic cells. Eur. J. Immunol. 2015, 45, 3269–3288. [Google Scholar] [CrossRef]
- Wong, T.-H.; Chen, H.-A.; Gau, R.-J.; Yen, J.-H.; Suen, J.-L. Heme Oxygenase-1-Expressing Dendritic Cells Promote Foxp3+ Regulatory T Cell Differentiation and Induce Less Severe Airway Inflammation in Murine Models. PLoS ONE 2016, 11, e0168919. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Luo, Z.; Li, W.; Li, X.; Dallmann, R.; Kurihara, H.; Li, Y.-F.; He, R.-R. Disturbed Yin–Yang balance: Stress increases the susceptibility to primary and recurrent infections of herpes simplex virus type 1. Acta Pharm. Sin. B 2020, 10, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Cotter, C.R.; Kim, W.; Nguyen, M.L.; Yount, J.S.; López, C.B.; Blaho, J.A.; Moran, T.M. The Virion Host Shutoff Protein of Herpes Simplex Virus 1 Blocks the Replication-Independent Activation of NF-κB in Dendritic Cells in the Absence of Type I Interferon Signaling. J. Virol. 2011, 85, 12662–12672. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cheng, J.; Xu, X.; Li, X.; Zhang, J.; Ma, D.; Jiang, G.; Liao, Y.; Fan, S.; Niu, Z.; et al. HSV-1 Infection of Epithelial Dendritic Cells Is a Critical Strategy for Interfering with Antiviral Immunity. Viruses 2022, 14, 1046. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Andres-Ejarque, R.; Ale, H.B.; Grys, K.; Tosi, I.; Solanky, S.; Ainali, C.; Catak, Z.; Sreeneebus, H.; Saklatvala, J.; Dand, N.; et al. Enhanced NF-κB signaling in type-2 dendritic cells at baseline predicts non-response to adalimumab in psoriasis. Nat. Commun. 2021, 12, 4741. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Yrjänheikki, J.; Keinänen, R.; Pellikka, M.; Hökfelt, T.; Koistinaho, J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. USA 1998, 95, 15769–15774. [Google Scholar] [CrossRef]
- Santa-Cecília, F.V.; Socias, B.; Ouidja, M.O.; Sepulveda-Diaz, J.E.; Acuña, L.; Silva, R.L.; Michel, P.P.; Del-Bel, E.; Cunha, T.M.; Raisman-Vozari, R. Doxycycline Suppresses Microglial Activation by Inhibiting the p38 MAPK and NF-kB Signaling Pathways. Neurotox. Res. 2016, 29, 447–459. [Google Scholar] [CrossRef]
- Tardif, V.; Riquelme, S.A.; Remy, S.; Carreño, L.J.; Cortés, C.M.; Simon, T.; Hill, M.; Louvet, C.; Riedel, C.A.; Blancou, P.; et al. Carbon monoxide decreases endosome-lysosome fusion and inhibits soluble antigen presentation by dendritic cells to T cells. Eur. J. Immunol. 2013, 43, 2832–2844. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Carreño, L.J.; Espinoza, J.A.; Mackern-Oberti, J.P.; Alvarez-Lobos, M.M.; Riedel, C.A.; Bueno, S.M.; Kalergis, A.M. Modulation of antigen processing by haem-oxygenase 1. Implications on inflammation and tolerance. Immunology 2016, 149, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Mangano, K.; Quattrocchi, C.; Motterlini, R.; Di Marco, R.; Magro, G.; Penacho, N.; Romao, C.C.; Nicoletti, F. Prevention of clinical and histological signs of proteolipid protein (PLP)-induced experimental allergic encephalomyelitis (EAE) in mice by the water-soluble carbon monoxide-releasing molecule (CORM)-A1. Clin. Exp. Immunol. 2011, 163, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, I.; Saksida, T.; Mangano, K.; Vujicic, M.; Stojanovic, I.; Nicoletti, F.; Stosic-Grujicic, S. Pharmacological application of carbon monoxide ameliorates islet-directed autoimmunity in mice via anti-inflammatory and anti-apoptotic effects. Diabetologia 2014, 57, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.; Cavalli, E.; Mammana, S.; Basile, M.S.; Caltabiano, R.; Pesce, A.; Puleo, S.; Atanasov, A.G.; Magro, G.; Nicoletti, F.; et al. Involvement of the Nrf2/HO-1/CO axis and therapeutic intervention with the CO-releasing molecule CORM-A1, in a murine model of autoimmune hepatitis. J. Cell. Physiol. 2018, 233, 4156–4165. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.-Z.; Yang, B.-S.; Li, H.; Zhang, Y.-F.; Pei, F.-H.; Zhu, A.-C.; Wang, X.-R.; Liu, B.-R. The therapeutic effect of CORM-3 on acute liver failure induced by lipopolysaccharide/D-galactosamine in mice. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 73–80. [Google Scholar] [CrossRef]
- van Lint, A.; Ayers, M.; Brooks, A.G.; Coles, R.M.; Heath, W.R.; Carbone, F.R. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. J. Immunol. 2004, 172, 392–397. [Google Scholar] [CrossRef]
- Mathias, R.A.; Weinberg, A.; Boguniewicz, M.; Zaccaro, D.J.; Armstrong, B.; Schneider, L.C.; Hata, T.R.; Hanifin, J.M.; Beck, L.A.; Barnes, K.C.; et al. Atopic dermatitis complicated by eczema herpeticum is associated with HLA B7 and reduced interferon-γ-producing CD8+ T cells. Br. J. Dermatol. 2013, 169, 700–703. [Google Scholar] [CrossRef]
- Espinoza, J.A.; González, P.A.; Kalergis, A.M. Modulation of Antiviral Immunity by Heme Oxygenase-1. Am. J. Pathol. 2017, 187, 487–493. [Google Scholar] [CrossRef]
- Xu, H.; Agalioti, T.; Zhao, J.; Steglich, B.; Wahib, R.; Vesely, M.C.A.; Bielecki, P.; Bailis, W.; Jackson, R.; Perez, D.; et al. The induction and function of the anti-inflammatory fate of TH17 cells. Nat. Commun. 2020, 11, 3334. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Benavides, G.A.; Metge, B.J.; Swain, C.A.; Kammerud, S.C.; Alsheikh, H.A.; Elhamamsy, A.; Chen, D.; Darley-Usmar, V.; Rathmell, J.C.; et al. Hedgehog Signaling Regulates Treg to Th17 Conversion Through Metabolic Rewiring in Breast Cancer. Cancer Immunol. Res. 2023, 11, 687–702. [Google Scholar] [CrossRef]
- Diller, M.L.; Kudchadkar, R.R.; Delman, K.A.; Lawson, D.H.; Ford, M.L. Balancing Inflammation: The Link between Th17 and Regulatory T Cells. Mediat. Inflamm. 2016, 2016, e6309219. [Google Scholar] [CrossRef] [PubMed]
- Shevyrev, D.; Tereshchenko, V.; Kozlov, V.; Sizikov, A.; Chumasova, O.; Koksharova, V. T-regulatory cells from patients with rheumatoid arthritis retain suppressor functions in vitro. Exp. Ther. Med. 2021, 21, 209. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ojalvo, D.; Tyler, S.R.; Aranda, C.J.; Wang, J.; Sicherer, S.; Sampson, H.A.; Wood, R.A.; Burks, A.W.; Jones, S.M.; Leung, D.Y.M.; et al. Allergen recognition by specific effector Th2 cells enables IL-2-dependent activation of regulatory T-cell responses in humans. Allergy 2022, 251–252, 103–106. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Yu, U.; Zhang, G.; White, R.; Sparwasser, T.; Alexander, S.I.; Jones, C.A. Treg depletion attenuates the severity of skin disease from ganglionic spread after HSV-2 flank infection. Virology 2013, 447, 9–20. [Google Scholar] [CrossRef]
- Bomprezzi, R. Dimethyl fumarate in the treatment of relapsing–remitting multiple sclerosis: An overview. Ther. Adv. Neurol. Disord. 2015, 8, 20–30. [Google Scholar] [CrossRef]
- Mrowietz, U.; Altmeyer, P.; Bieber, T.; Röcken, M.; Schopf, R.E.; Sterry, W. Treatment of psoriasis with fumaric acid esters (Fumaderm®). JDDG J. Dtsch. Dermatol. Ges. 2007, 5, 716–717. [Google Scholar] [CrossRef]
- Kasarełło, K.; Jesion, A.; Tyszkowska, K.; Matusik, K.; Czarzasta, K.; Wrzesień, R.; Cudnoch-Jedrzejewska, A. Effect of dimethyl fumarate on heme oxygenase-1 expression in experimental allergic encephalomyelitis in rats. Folia Neuropathol. 2017, 55, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Chora, Â.A.; Fontoura, P.; Cunha, A.; Pais, T.F.; Cardoso, S.; Ho, P.P.; Lee, L.Y.; Sobel, R.A.; Steinman, L.; Soares, M.P. Heme oxygenase–1 and carbon monoxide suppress autoimmune neuroinflammation. J. Clin. Investig. 2007, 117, 438–447. [Google Scholar] [CrossRef]
- Cross, S.A.; Cook, D.R.; Chi, A.W.S.; Vance, P.J.; Kolson, L.L.; Wong, B.J.; Jordan-Sciutto, K.L.; Kolson, D.L. Dimethyl Fumarate, an Immune Modulator and Inducer of the Antioxidant Response, Suppresses HIV Replication and Macrophage-Mediated Neurotoxicity: A Novel Candidate for HIV Neuroprotection. J. Immunol. 2011, 187, 5015–5025. [Google Scholar] [CrossRef]
- Heiligenhaus, A.; Li, H.; Schmitz, A.; Wasmuth, S.; Bauer, D. Improvement of herpetic stromal keratitis with fumaric acid derivate is associated with systemic induction of T helper 2 cytokines. Clin. Exp. Immunol. 2005, 142, 180–187. [Google Scholar] [CrossRef]
- Sicurella, M.; Pula, W.; Musiał, K.; Cieślik-Boczula, K.; Sguizzato, M.; Bondi, A.; Drechsler, M.; Montesi, L.; Esposito, E.; Marconi, P. Ethosomal Gel for Topical Administration of Dimethyl Fumarate in the Treatment of HSV-1 Infections. Int. J. Mol. Sci. 2023, 24, 4133. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tognarelli, E.I.; Duarte, L.F.; Farías, M.A.; Cancino, F.A.; Corrales, N.; Ibáñez, F.J.; Riedel, C.A.; Bueno, S.M.; Kalergis, A.M.; González, P.A. Heme Oxygenase-1 Expression in Dendritic Cells Contributes to Protective Immunity against Herpes Simplex Virus Skin Infection. Antioxidants 2023, 12, 1170. https://doi.org/10.3390/antiox12061170
Tognarelli EI, Duarte LF, Farías MA, Cancino FA, Corrales N, Ibáñez FJ, Riedel CA, Bueno SM, Kalergis AM, González PA. Heme Oxygenase-1 Expression in Dendritic Cells Contributes to Protective Immunity against Herpes Simplex Virus Skin Infection. Antioxidants. 2023; 12(6):1170. https://doi.org/10.3390/antiox12061170
Chicago/Turabian StyleTognarelli, Eduardo I., Luisa F. Duarte, Mónica A. Farías, Felipe A. Cancino, Nicolás Corrales, Francisco J. Ibáñez, Claudia A. Riedel, Susan M. Bueno, Alexis M. Kalergis, and Pablo A. González. 2023. "Heme Oxygenase-1 Expression in Dendritic Cells Contributes to Protective Immunity against Herpes Simplex Virus Skin Infection" Antioxidants 12, no. 6: 1170. https://doi.org/10.3390/antiox12061170
APA StyleTognarelli, E. I., Duarte, L. F., Farías, M. A., Cancino, F. A., Corrales, N., Ibáñez, F. J., Riedel, C. A., Bueno, S. M., Kalergis, A. M., & González, P. A. (2023). Heme Oxygenase-1 Expression in Dendritic Cells Contributes to Protective Immunity against Herpes Simplex Virus Skin Infection. Antioxidants, 12(6), 1170. https://doi.org/10.3390/antiox12061170






