Marine-Derived Bioactive Peptides Self-Assembled Multifunctional Materials: Antioxidant and Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
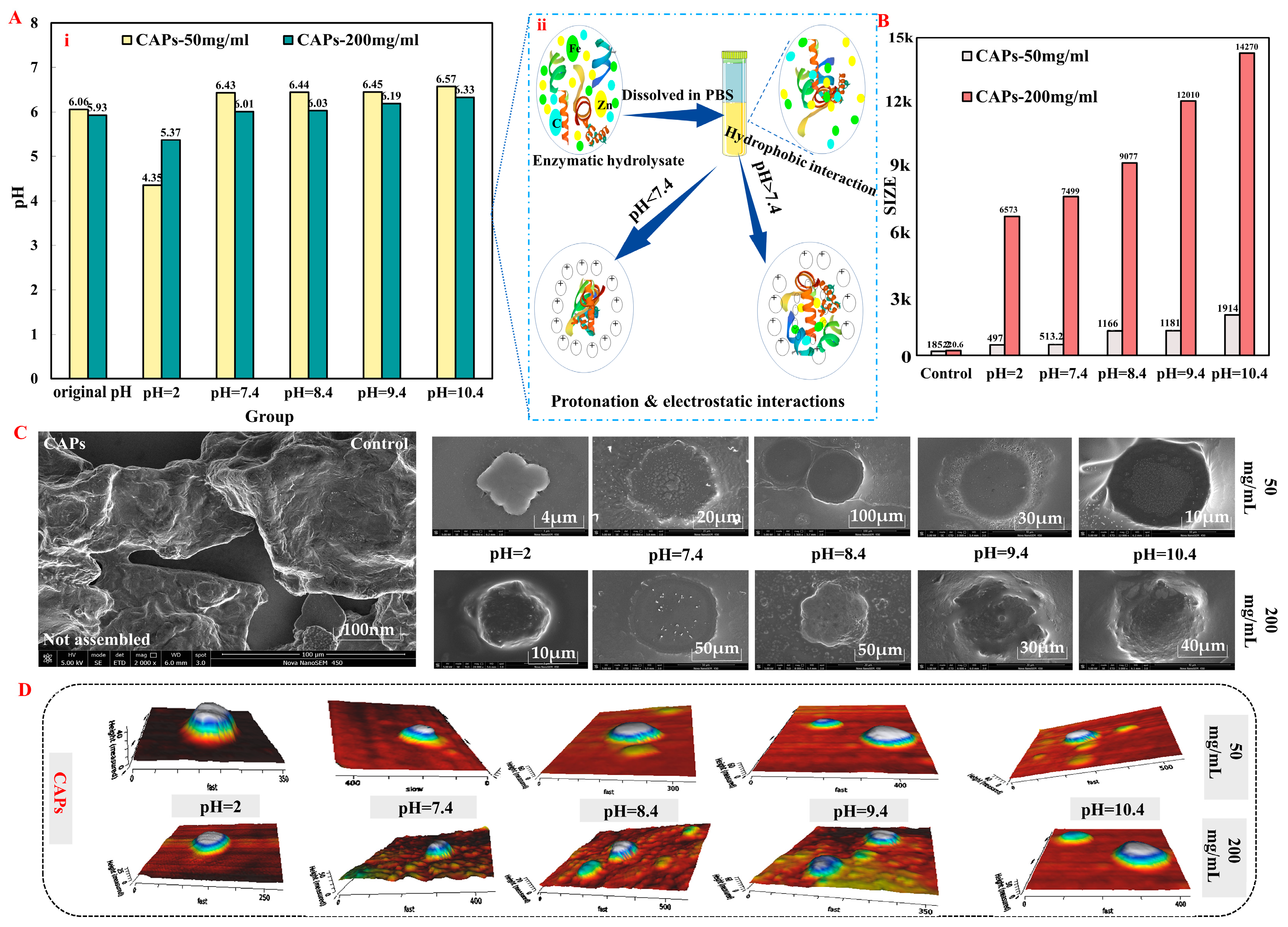
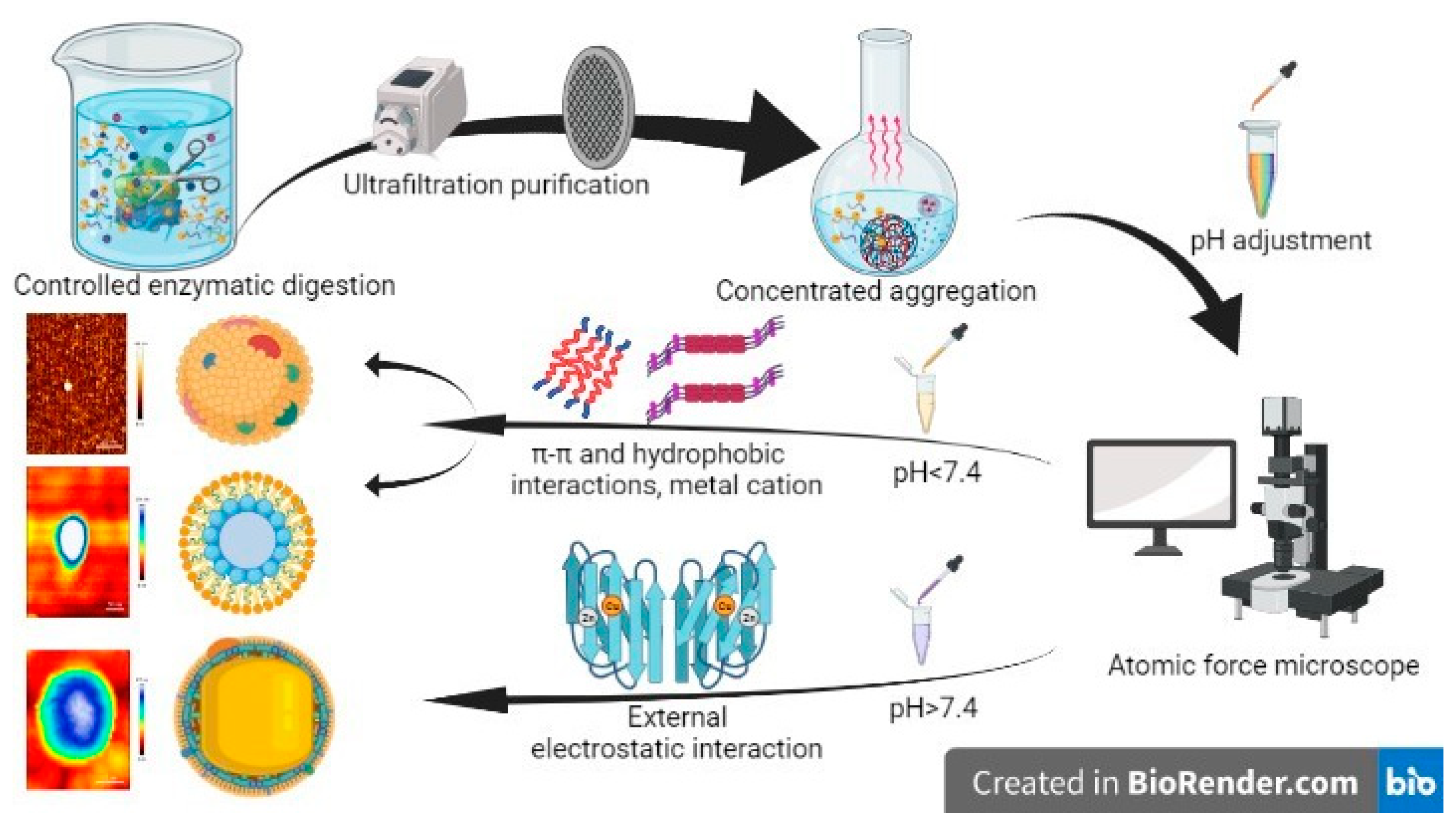
2.2. Preparation of Randomized Self-Assembling Materials (CAPs)
2.3. Physical and Chemical Analysis of CAPs
2.4. Verification System of In Vitro Healing Activity
2.4.1. Test of Antioxidant Activity
2.4.2. The Test of In Vitro Plasma Recalcification Time
2.4.3. Cell Proliferation and Toxicity Test
2.5. Animal Grouping and Experiment
2.6. Healing Rate
2.7. Histology
2.8. Immunohistochemical Method
2.9. Sirius Red Picric Acid Staining
2.10. Molecular Docking
2.11. Data Statistics and Analysis
3. Results
3.1. Analysis of CAPs Characterization Results
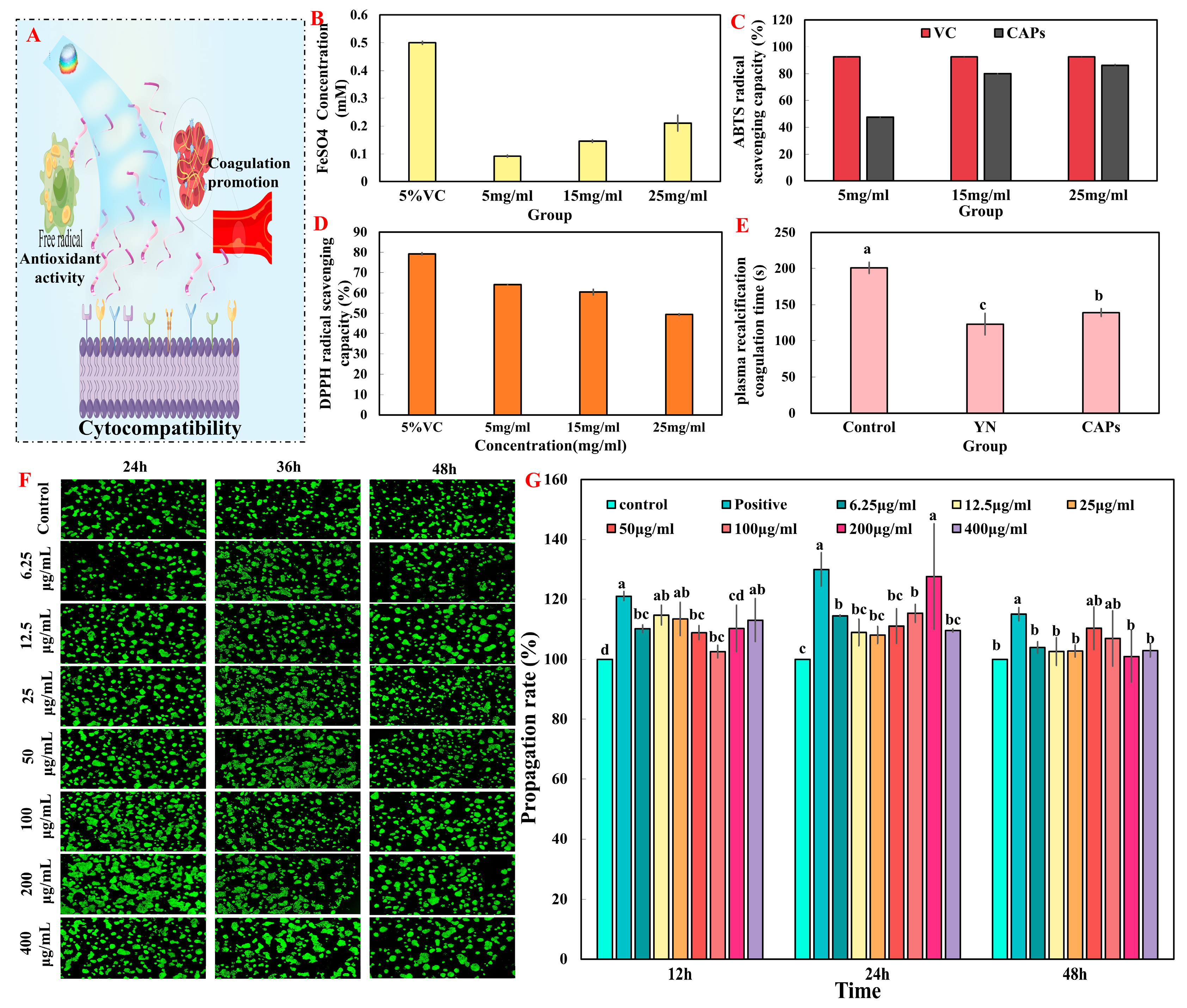
3.2. Verification System of In Vitro Healing Activity
3.2.1. Test of Antioxidant Activity
3.2.2. The Test of In Vitro Plasma Recalcification Time
3.2.3. Cell Proliferation and Toxicity Test In Vitro
3.3. Effects of CAPs on Animal Experiment
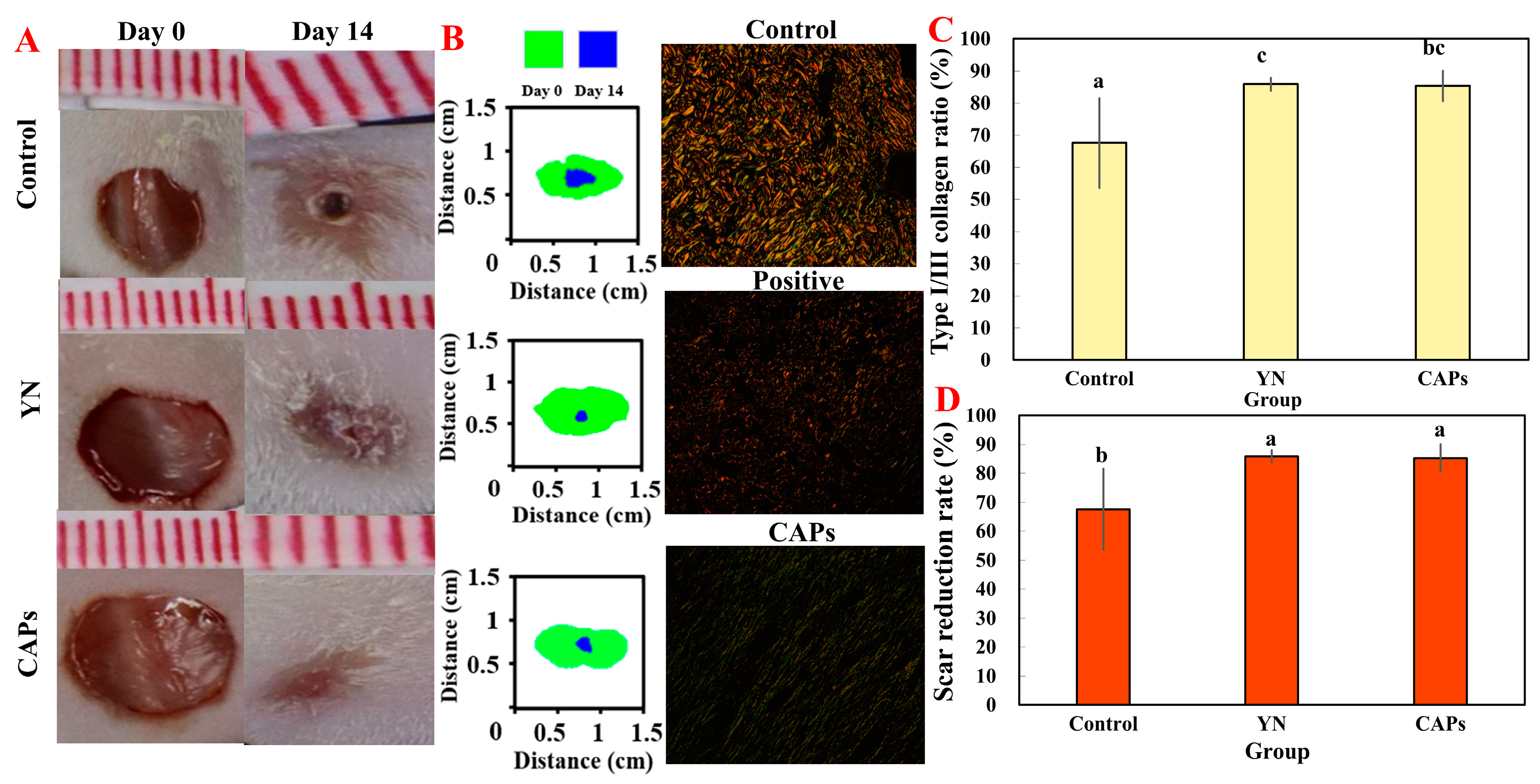
3.3.1. The Effect on Wound Healing
3.3.2. Effect of CAPs on Histopathological Analysis of Traumatic Surfaces in Mice
3.3.3. Effect of CAPs on FGF and CD31 in Mice Wounds
3.3.4. Effect of CAPs on Collagen and Wound Healing in Mice
3.3.5. Biosafety Assessment of CAPs In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.C.; Hebda, P.; Wells, A. Skin Wound Healing and Scarring: Fetal Wounds and Regenerative Restitution. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef]
- Eming, S.A.; Koch, M.; Krieger, A.; Brachvogel, B.; Kreft, S.; Bruckner-Tuderman, L.; Krieg, T.; Shannon, J.D.; Fox, J.W. Differential Proteomic Analysis Distinguishes Tissue Repair Biomarker Signatures in Wound Exudates Obtained from Normal Healing and Chronic Wounds. J. Proteom. Res. 2010, 9, 4758–4766. [Google Scholar] [CrossRef]
- Eming Sabine, A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265–266. [Google Scholar] [CrossRef]
- Li, H.S.; Li, B.Y.; Lv, D.L.; Li, W.H.; Lu, Y.F.; Luo, G.X. Biomaterials releasing drug responsively to promote wound healing via regulation of pathological microenvironment. Adv. Drug Deliv. Rev. 2023, 196, 114778. [Google Scholar] [CrossRef]
- Ji, S.; Zhou, S.; Zhang, X.; Chen, W.; Jiang, X. An oxygen-sensitive probe and a hydrogel for optical imaging and photodynamic antimicrobial chemotherapy of chronic wounds. Biomater. Sci 2022, 10, 2054–2061. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 147, 110468. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Z.; Tu, M.L.; Liu, H.X.; An, Y.; Du, M.; Zhu, B.W. A novel heptapeptide derived from Crassostrea gigas shows anticoagulant activity by targeting for thrombin active domain. Food Chem. 2021, 334, 127507. [Google Scholar] [CrossRef]
- Watanabe, M.; Fuda, H.; Jin, S.; Sakurai, T.; Ohkawa, F.; Hui, S.-P.; Takeda, S.; Watanabe, T.; Koike, T.; Chiba, H. Isolation and Characterization of a Phenolic Antioxidant from the Pacific Oyster (Crassostrea gigas). J. Agric. Food Chem. 2012, 60, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-R.; Yan, J.-N.; Sun, S.-G.; Tang, Y.; Shang, W.-H.; Li, A.-T.; Guo, X.-K.; Du, Y.-N.; Wu, H.-T.; Zhu, B.-W.; et al. Characteristic antioxidant activity and comprehensive flavor compound profile of scallop (Chlamys farreri) mantle hydrolysates-ribose Maillard reaction products. Food Chem. 2018, 261, 337–347. [Google Scholar] [CrossRef]
- Hao, L.; Wang, X.; Cao, Y.; Xu, J.; Xue, C. A comprehensive review of oyster peptides: Preparation, characterisation and bioactivities. Rev. Aquac. 2021, 14, 120–138. [Google Scholar] [CrossRef]
- Chen, C.; Liu, K.; Li, J.; Yan, X. Functional architectures based on self-assembly of bio-inspired dipeptides: Structure modulation and its photoelectronic applications. Adv. Colloid Interface Sci. 2015, 225, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Ulijn, R.V. Design of nanostructures based on aromatic peptide amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. [Google Scholar] [CrossRef]
- Vahedifar, A.; Wu, J. Self-assembling peptides: Structure, function, in silico prediction and applications. Trends Food Sci. Technol. 2021, 119, 476–494. [Google Scholar] [CrossRef]
- Votaw, N.L.; Collier, L.; Curvino, E.J.; Wu, Y.; Fries, C.N.; Ojeda, M.T.; Collier, J.H. Randomized peptide assemblies for enhancing immune responses to nanomaterials. Biomaterials 2021, 273, 120825. [Google Scholar] [CrossRef]
- Kong, D.; Ma, C.; Wang, W.; Liu, C.; Tian, Y.; Wang, T.; Zhao, Z.; Zhang, C.; Feng, H.; Chen, S. Two birds with one stone: Interfacial controls and pH response for long-term and high-efficiency Cu2O antibacterial materials. Chem. Eng. J. 2022, 427, 131734. [Google Scholar] [CrossRef]
- Yang, F.M.; Wang, Z.C.; Zhao, D.; Hu, L.; Cui, S.H.; Chen, L.Q.; Guo, T.T.; Pan, P.P.; Chen, J.D. Food-derived Crassostrea gigas peptides self-assembled supramolecules for scarless healing. Compos. Part B Eng. 2022, 246, 110265. [Google Scholar] [CrossRef]
- Yang, F.; Qin, X.; Zhang, T.; Zhang, C.; Lin, H. Effect of Oral Administration of Active Peptides of Pinctada Martensii on the Repair of Skin Wounds. Mar. Drugs 2019, 17, 697. [Google Scholar] [CrossRef]
- Wang, L.; Ma, M.; Yu, Z.; Du, S.K. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Wang, L.; Xu, C.; Mei, J.; Li, Y. Effects of germination and high hydrostatic pressure processing on mineral elements, amino acids and antioxidants in vitro bioaccessibility, as well as starch digestibility in brown rice (Oryza sativa L.). Food Chem. 2017, 214, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Aertgeerts, K.; Skene, R.; Yano, J.; Sang, B.-C.; Zou, H.; Snell, G.; Jennings, A.; Iwamoto, K.; Habuka, N.; Hirokawa, A.; et al. Structural Analysis of the Mechanism of Inhibition and Allosteric Activation of the Kinase Domain of HER2 Protein. J. Biol. Chem. 2011, 286, 18756–18765. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.K.; Tatulian, S.A. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q. Rev. Biophys. 1997, 30, 365–429. [Google Scholar] [CrossRef]
- Jackson, M.; Mantsch, H.H. The Use and Misuse of FTIR Spectroscopy in the Determination of Protein Structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Li, T.; Lu, X.-M.; Zhang, M.-R.; Hu, K.; Li, Z. Peptide-based nanomaterials: Self-assembly, properties and applications. Bioact. Mater. 2021, 11, 268–282. [Google Scholar] [CrossRef]
- Stechmiller, J.K. Understanding the Role of Nutrition and Wound Healing. Nutr. Clin. Pract. 2010, 25, 61–68. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, M.; Zhao, B.; Yang, F. Isolation of antioxidant peptides from yak casein hydrolysate. RSC Adv. 2020, 10, 19844–19851. [Google Scholar] [CrossRef]
- Bischoff, D.S.; Zhu, J.H.; Makhijani, N.S.; Kumar, A.; Yamaguchi, D.T. Angiogenic CXC chemokine expression during differentiation of human mesenchymal stem cells towards the osteoblastic lineage. J. Cell. Biochem. 2008, 103, 812–824. [Google Scholar] [CrossRef]
- Yang, F.; Li, L.; Chen, Y.; Wang, B.; Wang, J. Peptides from the tryptic hydrolysate of cartilaginous proteins of Raja porosa and their antioxidant activities. J. Fish. China 2019, 43, 1245–1254. [Google Scholar] [CrossRef]
- Hwang, J.-W.; Lee, S.-J.; Kim, Y.-S.; Kim, E.-K.; Ahn, C.-B.; Jeon, Y.-J.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Purification and characterization of a novel peptide with inhibitory effects on colitis induced mice by dextran sulfate sodium from enzymatic hydrolysates of Crassostrea gigas. Fish Shellfish Immunol. 2012, 33, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, P.; Cai, T.; Xu, S.; Hong, J.; Wang, S. Isolation, identification and characterization of antioxidative carrot seed peptides. Food Ferment. Ind. 2019, 45, 95–100. [Google Scholar] [CrossRef]
- Wang, Y.; Conlon, J.M. Purification and Structural Characterization of Vasoactive Intestinal Polypeptide from the Trout and Bowfin. Gen. Comp. Endocrinol. 1995, 98, 94–101. [Google Scholar] [CrossRef]
- Kobbi, S.; Balti, R.; Bougatef, A.; Le Flem, G.; Firdaous, L.; Bigan, M.; Chataigné, G.; Chaabouni, S.; Dhulster, P.; Nedjar, N. Antibacterial activity of novel peptides isolated from protein hydrolysates of RuBisCO purified from green juice alfalfa. J. Funct. Foods 2015, 18, 703–713. [Google Scholar] [CrossRef]
- Hu, X.Z.; Liao, M.R.; Gong, H.N.; Zhang, L.; Cox, H.; Waigh, T.A.; Lu, J.R. Recent advances in short peptide self-assembly: From rational design to novel applications. Cur.r Opin. Colloid. 2020, 45, 1–13. [Google Scholar] [CrossRef]
- Singh, R.; Kumar Mishra, N.; Kumar, V.; Vinayak, V.; Ballabh Joshi, K. Transition Metal Ion–Mediated Tyrosine-Based Short-Peptide Amphiphile Nanostructures Inhibit Bacterial Growth. ChemBioChem 2018, 19, 1630–1637. [Google Scholar] [CrossRef]
- Singh, R.; Mishra, N.K.; Gupta, P.; Joshi, K.B. Self-assembly of a Sequence-shuffled Short Peptide Amphiphile Triggered by Metal Ions into Terraced Nanodome-like Structures. Chem.–Asian J. 2020, 15, 531–539. [Google Scholar] [CrossRef]
- Bayer, T.; Pfaff, L.; Branson, Y.; Becker, A.; Wu, S.; Bornscheuer, U.T.; Wei, R. Biosensor and chemo-enzymatic one-pot cascade applications to detect and transform PET-derived terephthalic acid in living cells. iScience 2022, 25, 104326. [Google Scholar] [CrossRef]
- Hills, R.D.; Brooks, C.L. Hydrophobic Cooperativity as a Mechanism for Amyloid Nucleation. J. Mol. Biol. 2007, 368, 894–901. [Google Scholar] [CrossRef]
- Oliva, N.; Almquist, B.D. Spatiotemporal delivery of bioactive molecules for wound healing using stimuli-responsive biomaterials. Adv. Drug Deliv. Rev. 2020, 161, 22–41. [Google Scholar] [CrossRef]
- Anlas, C.; Bakirel, T.; Ustun-Alkan, F.; Celik, B.; Baran, M.Y.; Ustuner, O.; Kuruuzum-Uz, A. In vitro evaluation of the therapeutic potential of Anatolian kermes oak (Quercus coccifera L.) as an alternative wound healing agent. Ind. Crop Prod. 2019, 137, 24–32. [Google Scholar] [CrossRef]
- Shen, X.R.; Chen, X.L.; Xie, H.X.; He, Y.; Chen, W.; Luo, Q.; Yuan, W.H.; Tang, X.; Hou, D.Y.; Jiang, D.W.; et al. Beneficial effects of a novel shark-Dskin collagen dressing for the promotion of seawater immersion wound healing. Mil. Med. Res. 2017, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, D.; Caballero, J. Is It Reliable to Use Common Molecular Docking Methods for Comparing the Binding Affinities of Enantiomer Pairs for Their Protein Target? Int. J. Mol. Sci. 2016, 17, 525. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, Y.; Liu, S.; Hu, S.; Zhao, B.; Li, P.; Du, S. Effects of Panax Notoginseng Saponins on Esterases Responsible for Aspirin Hydrolysis In Vitro. Int. J. Mol. Sci. 2018, 19, 3144. [Google Scholar] [CrossRef] [PubMed]



| Sequence | Peptide Sequence of CAPs | Molecular Mass (Da) | Score |
|---|---|---|---|
| 1 | Leu-Gln-Glu-Lys-Glu-Glu-Glu-Phe-Asp-Asn-Thr-Arg-Arg-Asn-His-Gln | 2071.967 | 52.05 |
| 2 | Leu-Asp-Val-Asn-His-Asp-Gly-Lys-Ile-Ser-Ile-Glu-Asp-Val-Glu-Glu-Ser-Arg-Asn-Lys | 2296.129 | 51.43 |
| 3 | Leu-Asp-Glu-Leu-Glu-Asp-Asn-Leu-Glu-Arg-Glu-Lys-Lys | 1629.821 | 48.84 |
| 4 | Ile-Gln-Asp-Lys-Glu-Gly-Ile-Pro-Pro-Asp-Gln-Gln-Arg | 1522.774 | 47.67 |
| 5 | Asp-Glu-Leu-Glu-Asp-Asn-Leu-Glu-Arg-Glu-Lys-Lys | 1516.737 | 45.66 |
| 6 | Leu-Gln-Glu-Lys-Glu-Glu-Glu-Phe-Asp-Asn-Thr-Arg-Arg | 1692.807 | 45.54 |
| 7 | Leu-Glu-Lys-Ser-Tyr-Glu-Leu-Pro-Asp-Gly-Gln-Val-Ile-Thr | 1590.814 | 45.34 |
| 8 | Ile-Glu-Glu-Asp-Ala-Gly-Leu-Gly-Asn-Gly-Gly-Leu-Gly-Arg | 1356.663 | 45.24 |
| 9 | Leu-Arg-Glu-Lys-Asp-Glu-Glu-Ile-Asp-Ser-Ile-Arg-Lys-Ser-Ser | 1803.933 | 44.6 |
| 10 | Ile-Ser-Ile-Glu-Asp-Val-Glu-Glu-Ser-Arg-Asn-Lys | 1417.705 | 44.16 |
| 11 | Leu-Asp-Glu-Leu-Glu-Asp-Asn-Leu-Glu-Arg-Glu-Lys | 1501.726 | 43.9 |
| 12 | Ala-Ala-Asp-Glu-Ser-Glu-Arg-Asn-Arg-Lys-Val | 1273.638 | 43.89 |
| 13 | Asp-Val-Asn-His-Asp-Gly-Lys-Ile-Ser-Ile-Glu | 1225.594 | 43.8 |
| 14 | Leu-Arg-Glu-Lys-Asp-Glu-Glu-Ile-Asp-Ser-Ile-Arg-Lys-Ser | 1716.901 | 43.56 |
| 15 | Glu-Lys-Ser-Tyr-Glu-Leu-Pro-Asp-Gly-Gln | 1164.53 | 43.46 |
| 16 | His-Gly-Asp-Ser-Asp-Leu-Gln-Leu-Glu-Arg | 1168.5472 | 43.42 |
| 17 | Leu-Glu-Lys-Ser-Tyr-Glu-Leu-Pro-Asp-Gly-Gln-Val | 1376.6824 | 43.15 |
| 18 | Glu-Lys-Ser-Tyr-Glu-Leu-Pro-Asp-Gly-Gln-Val-Ile | 1376.6824 | 43.05 |
| 19 | Leu-Asp-Val-Asn-His-Asp-Gly-Lys-Ile-Ser-Ile-Glu | 1338.6779 | 42.73 |
| 20 | Ile-Thr-Gly-Glu-Ser-Gly-Ala-Gly-Lys-Thr-Glu-Asn | 1162.5466 | 42.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Cui, S.; Chen, L.; Zheng, S.; Zhao, D.; Yin, X.; Yang, F.; Chen, J. Marine-Derived Bioactive Peptides Self-Assembled Multifunctional Materials: Antioxidant and Wound Healing. Antioxidants 2023, 12, 1190. https://doi.org/10.3390/antiox12061190
Yu D, Cui S, Chen L, Zheng S, Zhao D, Yin X, Yang F, Chen J. Marine-Derived Bioactive Peptides Self-Assembled Multifunctional Materials: Antioxidant and Wound Healing. Antioxidants. 2023; 12(6):1190. https://doi.org/10.3390/antiox12061190
Chicago/Turabian StyleYu, Dingyi, Shenghao Cui, Liqi Chen, Shuang Zheng, Di Zhao, Xinyu Yin, Faming Yang, and Jingdi Chen. 2023. "Marine-Derived Bioactive Peptides Self-Assembled Multifunctional Materials: Antioxidant and Wound Healing" Antioxidants 12, no. 6: 1190. https://doi.org/10.3390/antiox12061190





