The Preventive Mechanisms of Bioactive Food Compounds against Obesity-Induced Inflammation
Abstract
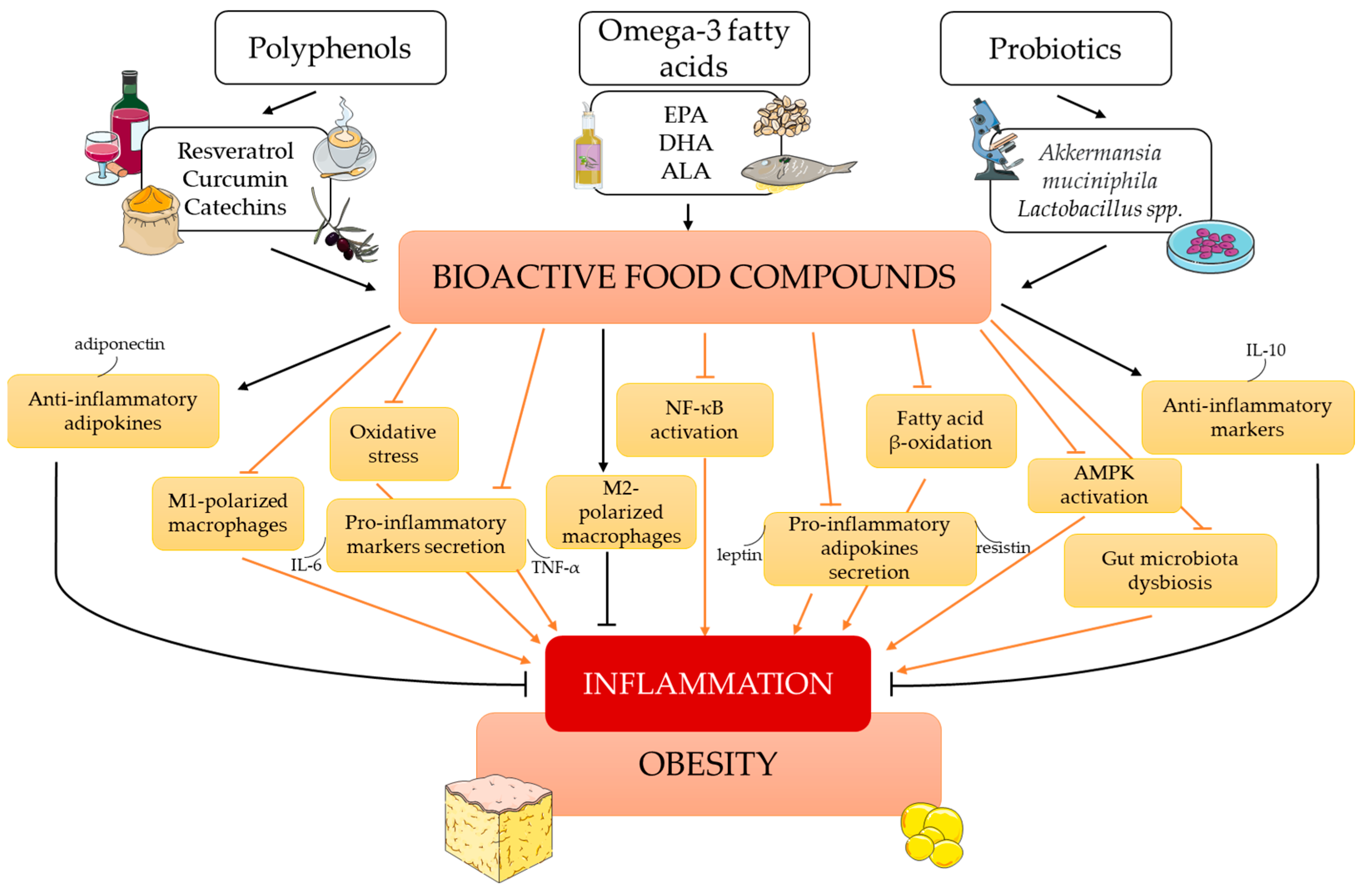
:1. Introduction
2. Oxidative Stress in Obesity
3. Antioxidant Potential of Bioactive Food Compounds in Obesity Management
4. Polyphenols
4.1. Resveratrol
| Bioactive Compound | Experimental Model | Results | References |
|---|---|---|---|
| Resveratrol | RAW264.7 cells and 3T3-L1 cells treated with RSVL (0.1, 1, 10 μM) for 1 h | RAW264.7 cells: ↓ LPS-stimulated IL-6 and TNF-α synthesis 3T3-L1 cells: ↓ proinflammatory factor (TNF-α, IL-6) production and changes in adipokine mRNA expression (upregulation for adiponectin and downregulation for resistin) ↓ NF-κB activation and ERK1/2 phosphorylation ↓ Phosphorylation of IRS-1 and AKT ↑ Insulin sensitivity in adipocytes | Kang et al. [96] |
| In vitro model of human AT treated with RSVL (10, 30, 100 μM) for 48 h | ↓ IL-6, IL-8, and MCP-1 levels in a concentration-dependent manner in adipocytes under inflammatory conditions ↓ NF-κB activity | Zagotta et al. [97] | |
| Male C57BL/6J mice (9 weeks old) on an HFDThree groups (n = 10), i.e., SD, HFD, and HFD + RSVL (0.4% RSVL), treated for 10 weeks | ↓ BW gain, ↓ visceral fat-pad weight, and ↓ TG, FFA, TC, glucose, TNF-α, and MCP1 concentrations ↓ Galanin-mediated signaling molecules (GalR1/2, PKCd, Cyc-D, E2F1, p-ERK) ↓ Key adipogenic gene expression (PPAR-g2, C/EBPα, SREBP-1c, FAS, LPL, aP2, leptin) in the epididymal AT ↓ Proinflammatory cytokines (TNF-α, IL-6, IFN-α, IFN-β) and their signaling molecules (NF-κB, TLR2/4, MyD88, Tirap, TRIF, TRAF6, IRF5, p-IRF3) | Kim et al. [100] | |
| Male C57BL/6 mice (6 weeks old) Mice were randomly divided into four groups (n = 10) and treated for 18 weeks as follows: SD, HFD (41.26% of calories from fat), HFD-RSVL/L (200 mg/kg/day), HFD-RSVL/H (400 mg/kg/day) | RSVL (400 mg/kg/day) ↓ Insulin resistance, ↓ TC, TG, LDL concentrations, ↑ HDL level, ↑ expression of pAkt, GLUT4, and IRS-1 in WAT, and ↓ serum proinflammatory cytokine levels (MCP-1, TNF-a, and IL-6), macrophage infiltration, and CCR2 expression in WAT | Ding et al. [109] | |
| High-fat, high-sugar diet-fed adult (7–13 years old) rhesus monkeys Monkeys were quasi-randomized into one of three groups and treated for 2 years: HF-HS diet + RSVL (n = 10), HF-HS diet + placebo (n = 10), and SD (n = 4) RSVL supplementation in doses of 80 mg and 480 mg/day for the first and second years, respectively | ↓ Adipocyte size and mRNA levels of IL-6, TNF-α, and IL-1β, ↑ SIRT1 expression, ↓ NF-κB activation, and ↑ insulin sensitivity in VAT of HF-HS animals | Jimenez-Gomez [103] | |
| Diabetic patients (n = 94) were randomly assigned to RSVL (n = 45) or placebo (n = 46) groups supplementing once daily with 200 mg of RSVL or cellulose capsules for 24 weeks, respectively A randomized, double-blinded, placebo-controlled parallel group trial | ↓ Plasma glucose, ↓ insulin, ↓ HOMA-IR, ↓ MDA, ↓ hs-CRP, ↓ TNF-α, and ↓ IL-6 RSVL supplementation regulated diabetes-associated miRNA levels (more than two-fold downregulation of miRNA-34a, miRNA-375, miRNA-21, and miRNA-192 and upregulation of miRNA-126 and miRNA-132 expression) | Mahjabeen et al. [110] | |
| Healthy, obese men (n = 11) supplementing with 150 mg of RSVL per day Randomized double-blind crossover study (30 days) | Activation of AMPK, ↑ SIRT1 and PGC-1α protein levels, ↑ citrate synthase activity in muscles, ↑ intramyocellular lipid levels, ↓ intrahepatic lipid content, ↓ circulating glucose and insulin levels, ↓ HOMA-IR value, ↓ TG, ↓ ALAT, and ↓ SBP and inflammation markers (↓ IL-6, IL-8, TNF-α) In the postprandial state: ↓ lipolysis, plasma fatty acid, and glycerol level in AT | Timmers et al. [106] | |
| Curcumin | Human monocytic THP-1 cells pretreated with CUR for 1 h and subsequently induced with PMA for 48 h Incubation of cells with CUR (in a dose of 0–100 μg) for 24-48 h | ↓ NLRP3 inflammasome expression, ↓ caspase-1 activation, ↓ IL-1β secretion, ↓ TLR4 expression, and ↓ NF-κB activation | Kong et al. [111] |
| TNF-α-stimulated 3T3-L1 adipocytes treated with 2–20 μM of curcumin (or RVSL) for 62 h | ↓ NF-κB activation, ↓ TNF-α, IL-1β, IL-6, and COX-2 gene expression, and ↓ IL-6 secretion | Gonzales et al. [112] | |
| HFD-induced obese (n = 5) and leptin-deficient ob/ob male C57BL/6J mice (n = 5) Standard diet (4% fat) ± curcumin 3% by weight HFD (35% fat) ± curcumin 3% by weight for 6 weeks | ↑ Foxo1 and adiponectin expression, ↓ infiltration of macrophages, ↑ circulating adiponectin levels, and ↓ MCP-1 in WAT ↓ TNF-α and MCP-1 expression and NF-κB activity in liver | Weisberg et al. [113] | |
| Male C57BL/6J mice (n = 12/group) LFD (10% kcal from fat), HFD (45% kcal from fat), and HFD + curcumin (4 g/kg diet) added 2 days/week for 28 weeks | ↓ Macrophage infiltration ↓ NF-κB expression and JNK signaling pathway activation in AT | Shao et al. [114] | |
| Obese individuals (males and females, n = 30) receiving 1g of CUR per day Randomized, double-blind, crossover | ↓ IL-1β, ↓ IL-4, and ↓ VEGF ↔ Other proinflammatory cytokine levels (e.g., IL-1, IL-6, TNF-α) | Ganjali et al. [115] | |
| Overweight/obese subjects with MetS (males and females, n = 117) taking 1 g/day of CUR (n = 59) or placebo (n = 58) for 8 weeks Randomized, double-blind, placebo-controlled trial | ↓ TNF-α, ↓ IL-6, ↓ TGF-β, and ↓ MCP-1 | Panahi et al. [116] | |
| Catechins | Palmitate-induced 3T3-L1 adipocytes incubated with epicatechin (EC) (0.1, 1 μM) for 24 h VAT from HFD-fed mice on a diet supplemented with 20 mg of EC per kg of body weight for 15 weeks | ↓ TNF-α, ↓ IL-6, ↓ MCP-1, ↑ adiponectin, ↓ F4/80, and ↓ NF-κB | Bettaieb et al. [117] |
| Adipocytes co-cultured with LPS-induced macrophages incubated with 50 μM of EC for 1 h WAT from HFD-fed C57BL/6J mice supplemented with 20 mg of EC per kg of body weight for 12 weeks | ↓ TNF-α, ↓ IL-6, ↓ CCL19, ↓ Rantes, ↓ Ip-10, ↓ Saa3, ↓ Lbp, and ↓ Socs3 ↓ TNF-α, ↓ IL-6, ↓ MCP-1, and ↓ Saa3 | Sano et al. [118] | |
| TNF-α- and GC-(4->8)-GCG-induced 3T3-L1 adipocytes (10, 20 μg/mL) for 24 h Serum and WAT from HFD-fed male C57BL/6 mice supplemented with GC-(4->8)-GCG in a dose of 40 or 80 mg/kg/day for 8 weeks | ↓ IL-6, ↓ COX-2, ↓ MCP-1, ↓ TNF-α, ↓ F4/80, ↓ CD11b, ↓ NF-κB, ↓ JAK, ↓ STAT3, and ↓ MAPKs | Peng et al. [119] | |
| Female SD rats (3 months old) on an HFD (n = 12/group) Groups included LFD and HFD or HFD + 0.5% w/v GTCs for 8 months (4 months with GTCs) | ↓ BW and %FM and ↑%FFM ↓ IGF-I, ↓ leptin, and ↓ proinflammatory cytokines (IL-1α, IL-2, IL-4, IL-10, GM-CSF, IFN-γ, TNF-α) ↑ GPX protein expression in liver | Shen et al. [120] | |
| Obese, hypertensive subjects (n = 56) taking GTCs (379 mg GTCs/d) or placebo for 3 months Double-blinded, placebo-controlled trial | ↓ Inflammation and oxidative stress (↓ TNF-α, ↓ CRP, ↑ TAS) ↔ BMI, waist circumference, creatinine, and glucose ↓ SBP and DBP ↓ TCH, LDL-C, and TG and ↑ HDL-C ↓ Serum insulin and ↓ HOMA-IR | Bogdański et al. [121] | |
| Obese patients with metabolic syndrome (n = 35) consuming green tea (928 mg total catechins in 4 cups/d) or GTCs (870 mg total catechins in 2 capsules/d) or placebo (4 cups water/d) for 8 weeks Randomized controlled trial | ↔ Inflammatory markers (adiponectin, hs-CRP, IL-6, IL-1β, sVCAM-1, sICAM-1, leptin, and leptin/adiponectin ratio) ↔ Waist circumference, SBP, DBP, TG, HDL, and glucose ↓ Plasma serum amyloid alpha | Basu et al. [122] |
4.2. Curcumin
4.3. Catechins
5. Omega-3 Fatty Acids
| Bioactive Compound | Experimental Model | Results | References |
|---|---|---|---|
| Omega-3 fatty acids | Co-culture model of murine 3T3-L1 adipocytes and RAW 264.7 macrophages incubated with 125 μM albumin-complexed DHA, EPA, palmitic acid (PA), or albumin alone (control) for 12 h | DHA: ↓ IL-6, MCP-1 ↓ mRNA of Mcp1, iNOS, TNF-α, and NF-κB ↑ mRNA of Tgfb1 and IL-10 | Boer et al. [161] |
| Human adipose tissue (n = 8) and primary adipocyte cultures treated with endotoxin-free BSA conjugated with SFAs (lauric acid (LA) and palmitic acid (PA)) and PUFAs (EPA, DHA, and oleic acid (OA)) in a dose of 5 or 10 μM with or without LPS for 6 and 12 h | PUFA: ↓ TNF-α, MCP-1, IL-6 | Murumalla et al. [178] | |
| SVF and adipose tissue from male HFHS-fed C57BL/6J mice on a diet supplemented with EPA (5% wt/wt) for 24 weeks 3T3-L1 adipocytes exposed to 250 μM palmitate for 30, 60, or 24 h with or without a 6 h pretreatment with 50 μM EPA-Na | EPA: ↓ IL-6, ↓ TNF-a, ↓ MCP-1, ↓ CLSs, ↓ CD11c, ↑ CD206 ↓ NF-κB, ↓ JNK | Yamada et al. [162] | |
| Four groups of C57BL/6 mice were fed LFD or HFD for 8 weeks. Two more groups of HFD were supplemented with n-3 PUFAs incorporated in the form of either phospholipids (n-3PL) or triacylglycerols (n-3TG) | PUFAs: ↓ MCP-1, IL-6, leptin, and 4-HNE, ↓ expression of MCP-1 and IL-6 in AT, and ↓ adipocyte size n-3PL: ↑ tocopherols in AT | Awada et al. [163] | |
| Severely obese nondiabetic patients (n = 55) scheduled to undergo elective bariatric surgery, taking 3.36 g n-3/d (EPA, DHA, n = 27) or an equivalent amount of butterfat as control (n = 28) for 8 weeks Randomized controlled trial | DHA and EPA: ↓ IL-6, ↓ TG, and ↓ expression of MCP-1, CCl-3, IL-6, and CD40 ↑ Expression of ADIPOQ (adiponectin) in SAT | Itariu et al. [165] | |
| Overweight/obese pregnant women (n = 49) randomly assigned to receive DHA plus EPA (2 g/day) or the equivalent of a placebo twice a day from week 10–16 to term A randomized, double-masked controlled trial | DHA and EPA: ↓ hs-CRP ↓ TLR4, ↓ IL6, IL8, and TNF-α expression in maternal AT and placenta | Haghiac et al. [167] |
6. Probiotics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayoral, L.P.-C.; Andrade, G.M.; Mayoral, E.P.-C.; Huerta, T.H.; Canseco, S.P.; Rodal Canales, F.J.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Pérez Santiago, A.D.; Alpuche, J.J.; et al. Obesity Subtypes, Related Biomarkers & Heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef] [PubMed]
- ICD-11 for Mortality and Morbidity Statistics. Available online: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/149403041 (accessed on 16 February 2023).
- World Obesity Day 2022–Accelerating Action to Stop Obesity. Available online: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity (accessed on 16 February 2023).
- Lopez-Jimenez, F.; Almahmeed, W.; Bays, H.; Cuevas, A.; Di Angelantonio, E.; le Roux, C.W.; Sattar, N.; Sun, M.C.; Wittert, G.; Pinto, F.J.; et al. Obesity and Cardiovascular Disease: Mechanistic Insights and Management Strategies. A Joint Position Paper by the World Heart Federation and World Obesity Federation. Eur. J. Prev. Cardiol. 2022, 29, 2218–2237. [Google Scholar] [CrossRef] [PubMed]
- Panuganti, K.K.; Nguyen, M.; Kshirsagar, R.K. Obesity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bogdański, P.; Filipiak, K.J.; Kowalska, I.; Lew-Starowicz, M.; Madej, P.; Mamcarz, A.; Mastalerz-Migas, A.; Ostrowska, L.; Wyleżoł, M.; Zgliczyński, W. Interdyscyplinarne stanowisko w sprawie rozpoznawania i leczenia otyłości. Forum Zaburzeń Metab. 2020, 11, 47–54. [Google Scholar]
- Stupnicki, R.; Tomaszewski, P. Body Mass Index and Body Fat Content in Adults. Hygeia Public Health. 2016, 51, 335–338. Available online: http://www.h-ph.pl/pdf/hyg-2016/hyg-2016-4-335.pdf (accessed on 1 May 2023).
- Lee, E.Y.; Yoon, K.-H. Epidemic Obesity in Children and Adolescents: Risk Factors and Prevention. Front. Med. 2018, 12, 658–666. [Google Scholar] [CrossRef]
- Littleton, S.H.; Berkowitz, R.I.; Grant, S.F.A. Genetic Determinants of Childhood Obesity. Mol. Diagn. Ther. 2020, 24, 653–663. [Google Scholar] [CrossRef]
- Melchior, V.; Fuchs, S.; Scantamburlo, G. Obesity and eating disorders. Rev. Med. Liege 2021, 76, 134–139. [Google Scholar]
- Cockerham, W.C. Theoretical Approaches to Research on the Social Determinants of Obesity. Am. J. Prev. Med. 2022, 63 (Suppl. S1), S8–S17. [Google Scholar] [CrossRef]
- Tomiyama, A.J. Stress and Obesity. Annu. Rev. Psychol. 2019, 70, 703–718. [Google Scholar] [CrossRef] [Green Version]
- Perry, C.; Guillory, T.S.; Dilks, S.S. Obesity and Psychiatric Disorders. Nurs. Clin. N. Am. 2021, 56, 553–563. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.T.; Marsche, G. Obesity-Related Changes in High-Density Lipoprotein Metabolism and Function. Int. J. Mol. Sci. 2020, 21, 8985. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, Y.; Huang, Y.-M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.-L. Obesity in Patients with COVID-19: A Systematic Review and Meta-Analysis. Metabolism 2020, 113, 154378. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Song, S.; Li, X.; Geng, C.; Wang, C. Excessive Body Fat at a Young Age Increases the Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis. Nutr. Cancer 2021, 73, 1601–1612. [Google Scholar] [CrossRef]
- Alimoradi, Z.; Golboni, F.; Griffiths, M.D.; Broström, A.; Lin, C.-Y.; Pakpour, A.H. Weight-Related Stigma and Psychological Distress: A Systematic Review and Meta-Analysis. Clin. Nutr. 2020, 39, 2001–2013. [Google Scholar] [CrossRef]
- Peeters, A.; Barendregt, J.J.; Willekens, F.; Mackenbach, J.P.; Al Mamun, A.; Bonneux, L.; NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group. Obesity in Adulthood and Its Consequences for Life Expectancy: A Life-Table Analysis. Ann. Intern. Med. 2003, 138, 24–32. [Google Scholar] [CrossRef]
- Murawska-Ciałowicz, E. Adipose Tissue-Morphological and Biochemical Characteristic of Different Depots. Postep. Hig. Med. Dosw. 2017, 71, 466–484. [Google Scholar] [CrossRef]
- Kolb, H. Obese Visceral Fat Tissue Inflammation: From Protective to Detrimental? BMC Med. 2022, 20, 494. [Google Scholar] [CrossRef]
- Thomas, E.L.; Frost, G.; Taylor-Robinson, S.D.; Bell, J.D. Excess Body Fat in Obese and Normal-Weight Subjects. Nutr. Res. Rev. 2012, 25, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Balistreri, C.R.; Caruso, C.; Candore, G. The Role of Adipose Tissue and Adipokines in Obesity-Related Inflammatory Diseases. Mediat. Inflamm. 2010, 2010, 802078. [Google Scholar] [CrossRef] [Green Version]
- Vykoukal, D.; Davies, M.G. Vascular Biology of Metabolic Syndrome. J. Vasc. Surg. 2011, 54, 819–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skowrońska, B.; Fichna, M.; Fichna, B. The role of adipose tissue in the endocrine system. Endokrynol. Otył. Zab. Przem. Mat. 2005, 1, 21–29. Available online: http://www.kzf.ump.edu.pl/Lekarski%20I%20rok/Rola%20tkanki.pdf (accessed on 1 May 2023).
- Gugliucci, A. Biomarkers of Dysfunctional Visceral Fat. Adv. Clin. Chem. 2022, 109, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar] [CrossRef]
- Yasmeen, R.; Fukagawa, N.K.; Wang, T.T. Establishing Health Benefits of Bioactive Food Components: A Basic Research Scientist’s Perspective. Curr. Opin. Biotechnol. 2017, 44, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayarathne, S.; Koboziev, I.; Park, O.-H.; Oldewage-Theron, W.; Shen, C.-L.; Moustaid-Moussa, N. Anti-Inflammatory and Anti-Obesity Properties of Food Bioactive Components: Effects on Adipose Tissue. Prev. Nutr. Food Sci. 2017, 22, 251. [Google Scholar] [CrossRef] [Green Version]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Hauck, A.K.; Huang, Y.; Hertzel, A.V.; Bernlohr, D.A. Adipose Oxidative Stress and Protein Carbonylation. J. Biol. Chem. 2019, 294, 1083–1088. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Berg, A.H.; Iyengar, P.; Lam, T.K.T.; Giacca, A.; Combs, T.P.; Rajala, M.W.; Du, X.; Rollman, B.; Li, W.; et al. The Hyperglycemia-Induced Inflammatory Response in Adipocytes: The Role of Reactive Oxygen Species. J. Biol. Chem. 2005, 280, 4617–4626. [Google Scholar] [CrossRef] [Green Version]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose Tissue in Obesity-Related Inflammation and Insulin Resistance: Cells, Cytokines, and Chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Góralska, M.; Majewska-Szczepanik, M.; Szczepanik, M. Immunological mechanisms involved in obesity and their role in metabolic syndrome. Postep. Hig. Med. Dosw. 2015, 69, 1384–1404. [Google Scholar]
- Salisbury, D.; Bronas, U. Reactive Oxygen and Nitrogen Species: Impact on Endothelial Dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.S.; Kolesnik, T.B.; Kuang, Z.; D’Cruz, A.A.; Blewitt, M.E.; Masters, S.L.; Low, A.; Willson, T.; Norton, R.S.; Nicholson, S.E. TLR Regulation of SPSB1 Controls Inducible Nitric Oxide Synthase Induction. J. Immunol. 2011, 187, 3798–3805. [Google Scholar] [CrossRef] [Green Version]
- Lucas, K.; Maes, M. Role of the Toll Like Receptor (TLR) Radical Cycle in Chronic Inflammation: Possible Treatments Targeting the TLR4 Pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Tripathi, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in Metabolism, Inflammation, and Disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef] [Green Version]
- Alessi, M.-C.; Poggi, M.; Juhan-Vague, I. Plasminogen Activator Inhibitor-1, Adipose Tissue and Insulin Resistance. Curr. Opin. Lipidol. 2007, 18, 240–245. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2019, 10, 1638. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Gámez-Nava, J.I.; Díaz-de la Cruz, E.N.; Cardona-Muñoz, E.G.; Becerra-Alvarado, I.N.; Aceves-Aceves, J.A.; Sánchez-Rodríguez, E.N.; Miranda-Díaz, A.G. The Effect of Visceral Abdominal Fat Volume on Oxidative Stress and Proinflammatory Cytokines in Subjects with Normal Weight, Overweight and Obesity. Diabetes Metab. Syndr. Obes. 2020, 13, 1077–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila-Escalante, M.L.; Coop-Gamas, F.; Cervantes-Rodríguez, M.; Méndez-Iturbide, D.; Aranda-González, I.I. The Effect of Diet on Oxidative Stress and Metabolic Diseases-Clinically Controlled Trials. J. Food Biochem. 2020, 44, e13191. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Ho, C.-T.; Wang, Y. Preventive Mechanism of Bioactive Dietary Foods on Obesity-Related Inflammation and Diseases. Food Funct. 2018, 9, 6081–6095. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Zolghadri, S.; Stanek, A. Beneficial Effects of Anti-Inflammatory Diet in Modulating Gut Microbiota and Controlling Obesity. Nutrients 2022, 14, 3985. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Kenđel Jovanović, G.; Mrakovcic-Sutic, I.; Pavičić Žeželj, S.; Šuša, B.; Rahelić, D.; Klobučar Majanović, S. The Efficacy of an Energy-Restricted Anti-Inflammatory Diet for the Management of Obesity in Younger Adults. Nutrients 2020, 12, 3583. [Google Scholar] [CrossRef]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Moreno, E.; Arias-Rico, J.; Jiménez-Sánchez, R.; Estrada, D.; Jiménez-Osorio, A.; Zafra-Rojas, Q.; Ariza Ortega, J.A.; Flores-Chávez, O.; Morales-Castillejos, L.; Sandoval-Gallegos, E. Role of Bioactive Compounds in Obesity: Metabolic Mechanism Focused on Inflammation. Foods 2022, 11, 1232. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Antioxidants in Personalized Nutrition and Exercise. Adv. Nutr. 2018, 9, 813–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Gao, L.; Huang, W.; Ma, Y.; Muhammad, I.; Hanif, A.; Ding, Z.; Guo, X. Antioxidant Properties of Lactic Acid Bacteria Isolated from Traditional Fermented Yak Milk and Their Probiotic Effects on the Oxidative Senescence of Caenorhabditis Elegans. Food Funct. 2022, 13, 3690–3703. [Google Scholar] [CrossRef]
- Slattery, C.; Cotter, P.D.; O’Toole, P.W. Analysis of Health Benefits Conferred by Lactobacillus Species from Kefir. Nutrients 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Xie, D.; Hu, J.; Yang, Z.; Wu, T.; Xu, W.; Meng, Q.; Cao, K.; Luo, X. Vitamin Supplementation Protects against Nanomaterial-Induced Oxidative Stress and Inflammation Damages: A Meta-Analysis of In Vitro and In Vivo Studies. Nutrients 2022, 14, 2214. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.A.; Chun, O.K. Vitamin C and Heart Health: A Review Based on Findings from Epidemiologic Studies. Int. J. Mol. Sci. 2016, 17, 1328. [Google Scholar] [CrossRef] [Green Version]
- Filgueiras, M.S.; Rocha, N.P.; Novaes, J.F.; Bressan, J. Vitamin D Status, Oxidative Stress, and Inflammation in Children and Adolescents: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 660–669. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bellentani, S. Nutraceutical Approach to Non-Alcoholic Fatty Liver Disease (NAFLD): The Available Clinical Evidence. Nutrients 2018, 10, 1153. [Google Scholar] [CrossRef] [Green Version]
- Mézes, M.; Erdélyi, M. Antioxidant effect of the fibre content of foods. Orv. Hetil. 2018, 159, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D.A. The Antioxidant Glutathione. Vitam. Horm. 2023, 121, 109–141. [Google Scholar] [CrossRef] [PubMed]
- Wirth, E.; Latzko, E. Partial Purification and Properties of Spinach Leaf Glutathione Reductase. Z. Pflanzenphysiol. 1978, 89, 69–75. [Google Scholar] [CrossRef]
- Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G.; Merillon, J.M. (Eds.) Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic Syndrome, Mediterranean Diet, and Polyphenols: Evidence and Perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. PTR 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Williamson, G. The Role of Polyphenols in Modern Nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Cutrim, C.S.; Cortez, M.A.S. A Review on Polyphenols: Classification, Beneficial Effects and Their Application in Dairy Products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Pedret, A.; Valls, R.M.; Fernández-Castillejo, S.; Catalán, Ú.; Romeu, M.; Giralt, M.; Lamuela-Raventós, R.M.; Medina-Remón, A.; Arija, V.; Aranda, N.; et al. Polyphenol-Rich Foods Exhibit DNA Antioxidative Properties and Protect the Glutathione System in Healthy Subjects. Mol. Nutr. Food Res. 2012, 56, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- Zeriouh, W.; Nani, A.; Belarbi, M.; Dumont, A.; de Rosny, C.; Aboura, I.; Ghanemi, F.Z.; Murtaza, B.; Patoli, D.; Thomas, C.; et al. Phenolic Extract from Oleaster (Olea Europaea Var. Sylvestris) Leaves Reduces Colon Cancer Growth and Induces Caspase-Dependent Apoptosis in Colon Cancer Cells via the Mitochondrial Apoptotic Pathway. PLoS ONE 2017, 12, e0170823. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, Y.; Ohishi, T.; Nakamura, Y.; Fukutomi, R.; Miyoshi, N. Anti-Cancer Effects of Dietary Polyphenols via ROS-Mediated Pathway with Their Modulation of MicroRNAs. Molecules 2022, 27, 3816. [Google Scholar] [CrossRef] [PubMed]
- González-Castejón, M.; Rodriguez-Casado, A. Dietary Phytochemicals and Their Potential Effects on Obesity: A Review. Pharmacol. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Leiherer, A.; Mündlein, A.; Drexel, H. Phytochemicals and Their Impact on Adipose Tissue Inflammation and Diabetes. Vasc. Pharmacol. 2013, 58, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus quadripetalus) Inhibit Adipogenesis and Lipogenesis in 3T3-L1 Cells. Food Chem. 2014, 148, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Paiva, N. Stress-Induced Phenylpropanoid Metabolism. Plant. Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel Insights of Dietary Polyphenols and Obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of Three Wine-Related Polyphenols in Three Different Matrices by Healthy Subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Moreno-Labanda, J.F.; Mallavia, R.; Pérez-Fons, L.; Lizama, V.; Saura, D.; Micol, V. Determination of Piceid and Resveratrol in Spanish Wines Deriving from Monastrell (Vitis vinifera L.) Grape Variety. J. Agric. Food Chem. 2004, 52, 5396–5403. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of Adipose Tissue Inflammation by Bioactive Food Compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Floyd, Z.E.; Wang, Z.Q.; Kilroy, G.; Cefalu, W.T. Modulation of Peroxisome Proliferator-Activated Receptor Gamma Stability and Transcriptional Activity in Adipocytes by Resveratrol. Metabolism 2008, 57 (Suppl. S1), S32–S38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayalam, S.; Yang, J.-Y.; Ambati, S.; Della-Fera, M.A.; Baile, C.A. Resveratrol Induces Apoptosis and Inhibits Adipogenesis in 3T3-L1 Adipocytes. Phytother. Res. 2008, 22, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Nagayama, D.; Ishihara, N.; Tanaka, S.; Watanabe, R.; Watanabe, Y.; Sato, Y.; Yamaguchi, T.; Ban, N.; Kawana, H.; et al. Resveratrol Attenuates Triglyceride Accumulation Associated with Upregulation of Sirt1 and Lipoprotein Lipase in 3T3-L1 Adipocytes. Mol. Genet. Metab. Rep. 2017, 12, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Huang, B.; Choi, S.-K.; Seo, J.-S. Anti-Obesity Effect of Resveratrol-Amplified Grape Skin Extracts on 3T3-L1 Adipocytes Differentiation. Nutr. Res. Pract. 2012, 6, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Szkudelska, K.; Szkudelski, T. Resveratrol, Obesity and Diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef]
- Gomez-Zorita, S.; Tréguer, K.; Mercader, J.; Carpéné, C. Resveratrol Directly Affects In Vitro Lipolysis and Glucose Transport in Human Fat Cells. J. Physiol. Biochem. 2013, 69, 585–593. [Google Scholar] [CrossRef]
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Resveratrol, a Naturally Occurring Diphenolic Compound, Affects Lipogenesis, Lipolysis and the Antilipolytic Action of Insulin in Isolated Rat Adipocytes. J. Steroid Biochem. Mol. Biol. 2009, 113, 17–24. [Google Scholar] [CrossRef]
- Park, H.J.; Yang, J.-Y.; Ambati, S.; Della-Fera, M.A.; Hausman, D.B.; Rayalam, S.; Baile, C.A. Combined Effects of Genistein, Quercetin, and Resveratrol in Human and 3T3-L1 Adipocytes. J. Med. Food 2008, 11, 773–783. [Google Scholar] [CrossRef]
- Mercader, J.; Palou, A.; Bonet, M.L. Resveratrol Enhances Fatty Acid Oxidation Capacity and Reduces Resistin and Retinol-Binding Protein 4 Expression in White Adipocytes. J. Nutr. Biochem. 2011, 22, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Heng, W.; Yuan, A.; Baolin, L.; Fang, H. Resveratrol Modulates Adipokine Expression and Improves Insulin Sensitivity in Adipocytes: Relative to Inhibition of Inflammatory Responses. Biochimie 2010, 92, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Zagotta, I.; Dimova, E.Y.; Debatin, K.-M.; Wabitsch, M.; Kietzmann, T.; Fischer-Posovszky, P. Obesity and Inflammation: Reduced Cytokine Expression Due to Resveratrol in a Human In Vitro Model of Inflamed Adipose Tissue. Front. Pharmacol. 2015, 6, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullberg, K.B.; Olholm, J.; Paulsen, S.K.; Foldager, C.B.; Lind, M.; Richelsen, B.; Pedersen, S.B. Resveratrol Has Inhibitory Effects on the Hypoxia-Induced Inflammation and Angiogenesis in Human Adipose Tissue In Vitro. Eur. J. Pharm. Sci. 2013, 49, 251–257. [Google Scholar] [CrossRef]
- Bujanda, L.; Hijona, E.; Larzabal, M.; Beraza, M.; Aldazabal, P.; García-Urkia, N.; Sarasqueta, C.; Cosme, A.; Irastorza, B.; González, A.; et al. Resveratrol Inhibits Nonalcoholic Fatty Liver Disease in Rats. BMC Gastroenterol. 2008, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Jin, Y.; Choi, Y.; Park, T. Resveratrol Exerts Anti-Obesity Effects via Mechanisms Involving down-Regulation of Adipogenic and Inflammatory Processes in Mice. Biochem. Pharmacol. 2011, 81, 1343–1351. [Google Scholar] [CrossRef]
- Franco, J.G.; Lisboa, P.C.; Lima, N.S.; Amaral, T.A.S.; Peixoto-Silva, N.; Resende, A.C.; Oliveira, E.; Passos, M.C.F.; Moura, E.G. Resveratrol Attenuates Oxidative Stress and Prevents Steatosis and Hypertension in Obese Rats Programmed by Early Weaning. J. Nutr. Biochem. 2013, 24, 960–966. [Google Scholar] [CrossRef]
- Carreras, A.; Zhang, S.X.; Peris, E.; Qiao, Z.; Wang, Y.; Almendros, I.; Gozal, D. Effect of Resveratrol on Visceral White Adipose Tissue Inflammation and Insulin Sensitivity in a Mouse Model of Sleep Apnea. Int. J. Obes. 2015, 39, 418–423. [Google Scholar] [CrossRef]
- Jimenez-Gomez, Y.; Mattison, J.A.; Pearson, K.J.; Martin-Montalvo, A.; Palacios, H.H.; Sossong, A.M.; Ward, T.M.; Younts, C.M.; Lewis, K.; Allard, J.S.; et al. Resveratrol Improves Adipose Insulin Signaling and Reduces the Inflammatory Response in Adipose Tissue of Rhesus Monkeys on High-Fat, High-Sugar Diet. Cell Metab. 2013, 18, 533–545. [Google Scholar] [CrossRef] [Green Version]
- Tabrizi, R.; Tamtaji, O.R.; Lankarani, K.B.; Mirhosseini, N.; Akbari, M.; Dadgostar, E.; Peymani, P.; Asemi, Z. The Effects of Resveratrol Supplementation on Biomarkers of Inflammation and Oxidative Stress among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Food Funct. 2018, 9, 6116–6128. [Google Scholar] [CrossRef]
- Koushki, M.; Dashatan, N.A.; Meshkani, R. Effect of Resveratrol Supplementation on Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Ther. 2018, 40, 1180–1192.e5. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konings, E.; Timmers, S.; Boekschoten, M.V.; Goossens, G.H.; Jocken, J.W.; Afman, L.A.; Müller, M.; Schrauwen, P.; Mariman, E.C.; Blaak, E.E. The Effects of 30 Days Resveratrol Supplementation on Adipose Tissue Morphology and Gene Expression Patterns in Obese Men. Int. J. Obes. 2014, 38, 470–473. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.; Shen, J.; Wan, L.; Zhu, Y.; Yi, T.; Xiao, Z. The Beneficial Effects of Quercetin, Curcumin, and Resveratrol in Obesity. Oxid. Med. Cell. Longev. 2017, 2017, 1459497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, S.; Jiang, J.; Wang, Z.; Zhang, G.; Yin, J.; Wang, X.; Wang, S.; Yu, Z. Resveratrol Reduces the Inflammatory Response in Adipose Tissue and Improves Adipose Insulin Signaling in High-Fat Diet-Fed Mice. PeerJ 2018, 6, e5173. [Google Scholar] [CrossRef]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of Resveratrol Supplementation in Regulation of Glucose Hemostasis, Inflammation and Oxidative Stress in Patients with Diabetes Mellitus Type 2: A Randomized, Placebo-Controlled Trial. Complement. Ther. Med. 2022, 66, 102819. [Google Scholar] [CrossRef]
- Kong, F.; Ye, B.; Cao, J.; Cai, X.; Lin, L.; Huang, S.; Huang, W.; Huang, Z. Curcumin Represses NLRP3 Inflammasome Activation via TLR4/MyD88/NF-ΚB and P2X7R Signaling in PMA-Induced Macrophages. Front. Pharmacol. 2016, 7, 369. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, A.M.; Orlando, R.A. Curcumin and Resveratrol Inhibit Nuclear Factor-KappaB-Mediated Cytokine Expression in Adipocytes. Nutr. Metab. 2008, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary Curcumin Significantly Improves Obesity-Associated Inflammation and Diabetes in Mouse Models of Diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef] [Green Version]
- Shao, W.; Yu, Z.; Chiang, Y.; Yang, Y.; Chai, T.; Foltz, W.; Lu, H.; Fantus, I.G.; Jin, T. Curcumin Prevents High Fat Diet Induced Insulin Resistance and Obesity via Attenuating Lipogenesis in Liver and Inflammatory Pathway in Adipocytes. PLoS ONE 2012, 7, e28784. [Google Scholar] [CrossRef]
- Ganjali, S.; Sahebkar, A.; Mahdipour, E.; Jamialahmadi, K.; Torabi, S.; Akhlaghi, S.; Ferns, G.; Parizadeh, S.M.R.; Ghayour-Mobarhan, M. Investigation of the Effects of Curcumin on Serum Cytokines in Obese Individuals: A Randomized Controlled Trial. Sci. World J. 2014, 2014, 898361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcumin on Serum Cytokine Concentrations in Subjects with Metabolic Syndrome: A Post-Hoc Analysis of a Randomized Controlled Trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Cremonini, E.; Kang, H.; Kang, J.; Haj, F.G.; Oteiza, P.I. Anti-Inflammatory Actions of (-)-Epicatechin in the Adipose Tissue of Obese Mice. Int. J. Biochem. Cell. Biol. 2016, 81 Pt B, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Sano, T.; Nagayasu, S.; Suzuki, S.; Iwashita, M.; Yamashita, A.; Shinjo, T.; Sanui, T.; Kushiyama, A.; Kanematsu, T.; Asano, T.; et al. Epicatechin Downregulates Adipose Tissue CCL19 Expression and Thereby Ameliorates Diet-Induced Obesity and Insulin Resistance. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Jia, Y.; Hu, T.; Du, J.; Wang, Y.; Cheng, B.; Li, K. GC-(4→8)-GCG, A Proanthocyanidin Dimer from Camellia Ptilophylla, Modulates Obesity and Adipose Tissue Inflammation in High-Fat Diet Induced Obese Mice. Mol. Nutr. Food Res. 2019, 63, e1900082. [Google Scholar] [CrossRef]
- Shen, C.-L.; Cao, J.J.; Dagda, R.Y.; Chanjaplammootil, S.; Lu, C.; Chyu, M.-C.; Gao, W.; Wang, J.-S.; Yeh, J.K. Green Tea Polyphenols Benefits Body Composition and Improves Bone Quality in Long-Term High-Fat Diet-Induced Obese Rats. Nutr. Res. 2012, 32, 448–457. [Google Scholar] [CrossRef]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green Tea Extract Reduces Blood Pressure, Inflammatory Biomarkers, and Oxidative Stress and Improves Parameters Associated with Insulin Resistance in Obese, Hypertensive Patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Basu, A.; Du, M.; Sanchez, K.; Leyva, M.J.; Betts, N.M.; Blevins, S.; Wu, M.; Aston, C.E.; Lyons, T.J. Green Tea Minimally Affects Biomarkers of Inflammation in Obese Subjects with Metabolic Syndrome. Nutrition 2011, 27, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B. Targeting Inflammation-Induced Obesity and Metabolic Diseases by Curcumin and Other Nutraceuticals. Annu. Rev. Nutr. 2010, 30, 173–199. [Google Scholar] [CrossRef] [Green Version]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From Kitchen to Clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, R.; Tamura, Y.; Yamanaka, H.; Kikuzaki, H.; Nakatani, N. Two Curcuminoid Pigments from Curcuma Domestica. Phytochemistry 1993, 33, 501–502. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T., 4th; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose Escalation of a Curcuminoid Formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermorken, A.J.M.; Zhu, J.; Van de Ven, W.J.M.; Cui, Y.; Fryns, J.P. Curcumin for the Prevention of Progression in Monoclonal Gammopathy of Undetermined Significance: A Word of Caution. Exp. Ther. Med. 2010, 1, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [Green Version]
- Meydani, M.; Hasan, S.T. Dietary Polyphenols and Obesity. Nutrients 2010, 2, 737–751. [Google Scholar] [CrossRef] [Green Version]
- Kocaadam, B.; Şanlier, N. Curcumin, an Active Component of Turmeric (Curcuma longa), and Its Effects on Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin Inhibits Adipogenesis in 3T3-L1 Adipocytes and Angiogenesis and Obesity in C57/BL Mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef] [Green Version]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.-J. Nano-Curcumin Improves Glucose Indices, Lipids, Inflammation, and Nesfatin in Overweight and Obese Patients with Non-Alcoholic Fatty Liver Disease (NAFLD): A Double-Blind Randomized Placebo-Controlled Clinical Trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of Curcumin Supplementation on Markers of Inflammation and Oxidative Stress among Healthy Overweight and Obese Girl Adolescents: A Randomized Placebo-Controlled Clinical Trial. Phytother. Res. 2019, 33, 2015–2022. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J.J.A.; Abbott, K.A.; Garg, M.L. Anti-Inflammatory Effects of Oral Supplementation with Curcumin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2021, 79, 1043–1066. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Razi, B.; Aslani, S.; Abbasifard, M.; Imani, D.; Sathyapalan, T.; Sahebkar, A. Effect of Curcumin on Proinflammatory Cytokines: A Meta-Analysis of Randomized Controlled Trials. Cytokine 2021, 143, 155541. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.G. Curcumin and Obesity. BioFactors 2013, 39, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, Q.Y.; Tsang, D.; Huang, Y. Degradation of Green Tea Catechins in Tea Drinks. J. Agric. Food Chem. 2001, 49, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Miyoshi, N.; Isemura, M. Health-Promoting Effects of Green Tea. Proc. Jpn. Acad. Ser. B 2012, 88, 88–101. [Google Scholar] [CrossRef] [Green Version]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial Effects of Green Tea: A Literature Review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Della-Fera, M.A.; Baile, C.A. Green Tea Polyphenol Epigallocatechin Gallate Inhibits Adipogenesis and Induces Apoptosis in 3T3-L1 Adipocytes. Obes. Res. 2005, 13, 982–990. [Google Scholar] [CrossRef]
- Stangl, V.; Dreger, H.; Stangl, K.; Lorenz, M. Molecular Targets of Tea Polyphenols in the Cardiovascular System. Cardiovasc. Res. 2007, 73, 348–358. [Google Scholar] [CrossRef]
- Axling, U.; Olsson, C.; Xu, J.; Fernandez, C.; Larsson, S.; Ström, K.; Ahrné, S.; Holm, C.; Molin, G.; Berger, K. Green Tea Powder and Lactobacillus Plantarum Affect Gut Microbiota, Lipid Metabolism and Inflammation in High-Fat Fed C57BL/6J Mice. Nutr. Metab. 2012, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-K.; Cheung, C.; Reuhl, K.R.; Liu, A.B.; Lee, M.-J.; Lu, Y.-P.; Yang, C.S. Effects of Green Tea Polyphenol(-)-Epigallocatechin-3-Gallate on a Newly Developed High-Fat/Western-Style Diet-Induced Obesity and Metabolic Syndrome in Mice. J. Agric. Food Chem. 2011, 59, 11862–11871. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Lee, J.-Y.; Chung, M.-Y.; Park, Y.-K.; Bower, A.M.; Koo, S.I.; Giardina, C.; Bruno, R.S. Green Tea Extract Suppresses NFκB Activation and Inflammatory Responses in Diet-Induced Obese Rats with Nonalcoholic Steatohepatitis. J. Nutr. 2012, 142, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Alipour, M.; Motevalli, M.S.; Chebbi, A.; Laher, I.; Zouhal, H. Does Green Tea Extract Enhance the Anti-Inflammatory Effects of Exercise on Fat Loss? Br. J. Clin. Pharmacol. 2020, 86, 753–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Grubbs, B.; Motevalli, M.S.; Baker, J.S.; Laher, I.; Zouhal, H. Effects of Green Tea Extract Supplementation and Endurance Training on Irisin, pro-Inflammatory Cytokines, and Adiponectin Concentrations in Overweight Middle-Aged Men. Eur. J. Appl. Physiol. 2020, 120, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Fukino, Y.; Shimbo, M.; Aoki, N.; Okubo, T.; Iso, H. Randomized Controlled Trial for an Effect of Green Tea Consumption on Insulin Resistance and Inflammation Markers. J. Nutr. Sci. Vitaminol. 2005, 51, 335–342. [Google Scholar] [CrossRef]
- Rasaei, N.; Asbaghi, O.; Samadi, M.; Setayesh, L.; Bagheri, R.; Gholami, F.; Soveid, N.; Casazza, K.; Wong, A.; Suzuki, K.; et al. Effect of Green Tea Supplementation on Antioxidant Status in Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Antioxidants 2021, 10, 1731. [Google Scholar] [CrossRef]
- Serban, C.; Sahebkar, A.; Antal, D.; Ursoniu, S.; Banach, M. Effects of Supplementation with Green Tea Catechins on Plasma C-Reactive Protein Concentrations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrition 2015, 31, 1061–1071. [Google Scholar] [CrossRef]
- Asbaghi, O.; Fouladvand, F.; Gonzalez, M.J.; Aghamohammadi, V.; Choghakhori, R.; Abbasnezhad, A. The Effect of Green Tea on C-Reactive Protein and Biomarkers of Oxidative Stress in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2019, 46, 210–216. [Google Scholar] [CrossRef]
- Hwang, J.-T.; Park, I.-J.; Shin, J.-I.; Lee, Y.K.; Lee, S.K.; Baik, H.W.; Ha, J.; Park, O.J. Genistein, EGCG, and Capsaicin Inhibit Adipocyte Differentiation Process via Activating AMP-Activated Protein Kinase. Biochem. Biophys. Res. Commun. 2005, 338, 694–699. [Google Scholar] [CrossRef]
- Kim, H.; Hiraishi, A.; Tsuchiya, K.; Sakamoto, K. (-)Epigallocatechin Gallate Suppresses the Differentiation of 3T3-L1 Preadipocytes through Transcription Factors FoxO1 and SREBP1c. Cytotechnology 2010, 62, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Murase, T.; Misawa, K.; Haramizu, S.; Hase, T. Catechin-Induced Activation of the LKB1/AMP-Activated Protein Kinase Pathway. Biochem. Pharmacol. 2009, 78, 78–84. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, P.J.; Shin, H.J.; Kim, Y.-K.; Shin, D.W.; Shin, E.S.; Lee, H.H.; Lee, B.G.; Baik, J.-H.; Lee, T.R. (-)-Catechin Suppresses Expression of Kruppel-like Factor 7 and Increases Expression and Secretion of Adiponectin Protein in 3T3-L1 Cells. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1166–E1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flachs, P.; Rossmeisl, M.; Bryhn, M.; Kopecky, J. Cellular and Molecular Effects of N-3 Polyunsaturated Fatty Acids on Adipose Tissue Biology and Metabolism. Clin. Sci. 2009, 116, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 Fatty Acids in Obesity and Metabolic Syndrome: A Mechanistic Update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; West, S.G.; Gillies, P.J.; Kris-Etherton, P.M. Dietary α-Linolenic Acid Reduces Inflammatory and Lipid Cardiovascular Risk Factors in Hypercholesterolemic Men and Women. J. Nutr. 2004, 134, 2991–2997. [Google Scholar] [CrossRef] [Green Version]
- Egert, S.; Baxheinrich, A.; Lee-Barkey, Y.H.; Tschoepe, D.; Wahrburg, U.; Stratmann, B. Effects of an Energy-Restricted Diet Rich in Plant-Derived α-Linolenic Acid on Systemic Inflammation and Endothelial Function in Overweight-to-Obese Patients with Metabolic Syndrome Traits. Br. J. Nutr. 2014, 112, 1315–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauls, S.D.; Rodway, L.A.; Winter, T.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Anti-Inflammatory Effects of α-Linolenic Acid in M1-like Macrophages Are Associated with Enhanced Production of Oxylipins from α-Linolenic and Linoleic Acid. J. Nutr. Biochem. 2018, 57, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Boer, A.A.D.; Monk, J.M.; Robinson, L.E. Docosahexaenoic Acid Decreases Pro-Inflammatory Mediators in an In Vitro Murine Adipocyte Macrophage Co-Culture Model. PLoS ONE 2014, 9, e85037. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Umemoto, T.; Kakei, M.; Momomura, S.-I.; Kawakami, M.; Ishikawa, S.-E.; Hara, K. Eicosapentaenoic Acid Shows Anti-Inflammatory Effect via GPR120 in 3T3-L1 Adipocytes and Attenuates Adipose Tissue Inflammation in Diet-Induced Obese Mice. Nutr. Metab. 2017, 14, 33. [Google Scholar] [CrossRef] [Green Version]
- Awada, M.; Meynier, A.; Soulage, C.O.; Hadji, L.; Géloën, A.; Viau, M.; Ribourg, L.; Benoit, B.; Debard, C.; Guichardant, M.; et al. N-3 PUFA Added to High-Fat Diets Affect Differently Adiposity and Inflammation When Carried by Phospholipids or Triacylglycerols in Mice. Nutr. Metab. 2013, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Tapia, G.; Valenzuela, R.; Espinosa, A.; Romanque, P.; Dossi, C.; Gonzalez-Mañán, D.; Videla, L.A.; D’Espessailles, A. N-3 Long-Chain PUFA Supplementation Prevents High Fat Diet Induced Mouse Liver Steatosis and Inflammation in Relation to PPAR-α Upregulation and NF-ΚB DNA Binding Abrogation. Mol. Nutr. Food Res. 2014, 58, 1333–1341. [Google Scholar] [CrossRef]
- Itariu, B.K.; Zeyda, M.; Hochbrugger, E.E.; Neuhofer, A.; Prager, G.; Schindler, K.; Bohdjalian, A.; Mascher, D.; Vangala, S.; Schranz, M.; et al. Long-Chain n-3 PUFAs Reduce Adipose Tissue and Systemic Inflammation in Severely Obese Nondiabetic Patients: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2012, 96, 1137–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellulu, M.S.; Khaza’ai, H.; Patimah, I.; Rahmat, A.; Abed, Y. Effect of Long Chain Omega-3 Polyunsaturated Fatty Acids on Inflammation and Metabolic Markers in Hypertensive and/or Diabetic Obese Adults: A Randomized Controlled Trial. Food Nutr. Res. 2016, 60, 29268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghiac, M.; Yang, X.; Presley, L.; Smith, S.; Dettelback, S.; Minium, J.; Belury, M.A.; Catalano, P.M.; Hauguel-de Mouzon, S. Dietary Omega-3 Fatty Acid Supplementation Reduces Inflammation in Obese Pregnant Women: A Randomized Double-Blind Controlled Clinical Trial. PLoS ONE 2015, 10, e0137309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browning, L.M.; Krebs, J.D.; Moore, C.S.; Mishra, G.D.; O’Connell, M.A.; Jebb, S.A. The Impact of Long Chain N-3 Polyunsaturated Fatty Acid Supplementation on Inflammation, Insulin Sensitivity and CVD Risk in a Group of Overweight Women with an Inflammatory Phenotype. Diabetes Obes. Metab. 2007, 9, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Kratz, M.; Kuzma, J.N.; Hagman, D.K.; van Yserloo, B.; Matthys, C.C.; Callahan, H.S.; Weigle, D.S. N3 PUFAs Do Not Affect Adipose Tissue Inflammation in Overweight to Moderately Obese Men and Women. J. Nutr. 2013, 143, 1340–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bragt, M.C.E.; Mensink, R.P. Comparison of the Effects of N-3 Long Chain Polyunsaturated Fatty Acids and Fenofibrate on Markers of Inflammation and Vascular Function, and on the Serum Lipoprotein Profile in Overweight and Obese Subjects. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 966–973. [Google Scholar] [CrossRef] [Green Version]
- Schweitzer, G.R.B.; Rios, I.N.M.S.; Gonçalves, V.S.S.; Magalhães, K.G.; Pizato, N. Effect of N-3 Long-Chain Polyunsaturated Fatty Acid Intake on the Eicosanoid Profile in Individuals with Obesity and Overweight: A Systematic Review and Meta-Analysis of Clinical Trials. J. Nutr. Sci. 2021, 10, e53. [Google Scholar] [CrossRef]
- Kavyani, Z.; Musazadeh, V.; Fathi, S.; Hossein Faghfouri, A.; Dehghan, P.; Sarmadi, B. Efficacy of the Omega-3 Fatty Acids Supplementation on Inflammatory Biomarkers: An Umbrella Meta-Analysis. Int. Immunopharmacol. 2022, 111, 109104. [Google Scholar] [CrossRef]
- Su, H.; Liu, R.; Chang, M.; Huang, J.; Jin, Q.; Wang, X. Effect of Dietary Alpha-Linolenic Acid on Blood Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Nutr. 2018, 57, 877–891. [Google Scholar] [CrossRef]
- Weatherill, A.R.; Lee, J.Y.; Zhao, L.; Lemay, D.G.; Youn, H.S.; Hwang, D.H. Saturated and Polyunsaturated Fatty Acids Reciprocally Modulate Dendritic Cell Functions Mediated through TLR4. J. Immunol. 2005, 174, 5390–5397. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-Inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Eicosapentaenoic and Docosahexaenoic Acid Derived Specialised Pro-Resolving Mediators: Concentrations in Humans and the Effects of Age, Sex, Disease and Increased Omega-3 Fatty Acid Intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, F. N-3 Polyunsaturated Fatty Acids and Inflammation in Obesity: Local Effect and Systemic Benefit. Biomed. Res. Int. 2015, 2015, 581469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murumalla, R.K.; Gunasekaran, M.K.; Padhan, J.K.; Bencharif, K.; Gence, L.; Festy, F.; Césari, M.; Roche, R.; Hoareau, L. Fatty Acids Do Not Pay the Toll: Effect of SFA and PUFA on Human Adipose Tissue and Mature Adipocytes Inflammation. Lipids Health Dis. 2012, 11, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alili, R.; Belda, E.; Fabre, O.; Pelloux, V.; Giordano, N.; Legrand, R.; Bel Lassen, P.; Swartz, T.D.; Zucker, J.-D.; Clément, K. Characterization of the Gut Microbiota in Individuals with Overweight or Obesity during a Real-World Weight Loss Dietary Program: A Focus on the Bacteroides 2 Enterotype. Biomedicines 2021, 10, 16. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional Interactions between the Gut Microbiota and Host Metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Vandeputte, D.; Kathagen, G.; D’hoe, K.; Vieira-Silva, S.; Valles-Colomer, M.; Sabino, J.; Wang, J.; Tito, R.Y.; De Commer, L.; Darzi, Y.; et al. Quantitative Microbiome Profiling Links Gut Community Variation to Microbial Load. Nature 2017, 551, 507–511. [Google Scholar] [CrossRef]
- Goffredo, M.; Mass, K.; Parks, E.J.; Wagner, D.A.; McClure, E.A.; Graf, J.; Savoye, M.; Pierpont, B.; Cline, G.; Santoro, N. Role of Gut Microbiota and Short Chain Fatty Acids in Modulating Energy Harvest and Fat Partitioning in Youth. J. Clin. Endocrinol. Metab. 2016, 101, 4367–4376. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- Gentile, C.L.; Weir, T.L. The Gut Microbiota at the Intersection of Diet and Human Health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [Green Version]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The Neuroactive Potential of the Human Gut Microbiota in Quality of Life and Depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO (2006) Probiotics in Food Health and Nutritional Properties and Guidelines for Evaluation. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food In-cluding Powder Milk with Live Lactic Acid Bacteria, Cordoba, Argentina, 1–4 October 2001 [and] Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London, Ontario, Canada, 30 April–1 May 2002. FAO Food and Nutrition Paper 85, Food and Agriculture Organization of the United Nations, World Health Organization, Rome. References-Scientific Research Publishing. Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/referencespapers.aspx?referenceid=2833926 (accessed on 20 April 2023).
- Borgeraas, H.; Johnson, L.K.; Skattebu, J.; Hertel, J.K.; Hjelmesaeth, J. Effects of Probiotics on Body Weight, Body Mass Index, Fat Mass and Fat Percentage in Subjects with Overweight or Obesity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obes. Rev. 2018, 19, 219–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, P.M.; Telo, G.H.; Ramalho, R.; Sbaraini, M.; Leivas, G.; Martins, A.F.; Schaan, B.D. The Effect of Probiotics, Prebiotics or Synbiotics on Metabolic Outcomes in Individuals with Diabetes: A Systematic Review and Meta-Analysis. Diabetologia 2020, 64, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Szulińska, M.; Łoniewski, I.; Skrypnik, K.; Sobieska, M.; Korybalska, K.; Suliburska, J.; Bogdański, P. Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women-A 12-Week Placebo-Controlled and Randomized Clinical Study. Nutrients 2018, 10, E1672. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Li, X.; Tan, F.; Zhou, X.; Mu, J.; Zhao, X. Lactobacillus Fermentum CQPC07 Attenuates Obesity, Inflammation and Dyslipidemia by Modulating the Antioxidant Capacity and Lipid Metabolism in High-Fat Diet Induced Obese Mice. J. Inflamm. 2021, 18, 5. [Google Scholar] [CrossRef]
- Rani, K.; Ali, S.A.; Kaul, G.; Behare, P.V. Protective Effect of Probiotic and Prebiotic Fermented Milk Containing Lactobacillus Fermentum against Obesity-induced Hepatic Steatosis and Inflammation. J. Food Biochem. 2022, 46, e14509. [Google Scholar] [CrossRef]
- Molina-Tijeras, J.A.; Diez-Echave, P.; Vezza, T.; Hidalgo-García, L.; Ruiz-Malagón, A.J.; Rodríguez-Sojo, M.J.; Romero, M.; Robles-Vera, I.; García, F.; Plaza-Diaz, J.; et al. Lactobacillus Fermentum CECT5716 Ameliorates High Fat Diet-Induced Obesity in Mice through Modulation of Gut Microbiota Dysbiosis. Pharmacol. Res. 2021, 167, 105471. [Google Scholar] [CrossRef]
- Kang, Y.; Kang, X.; Yang, H.; Liu, H.; Yang, X.; Liu, Q.; Tian, H.; Xue, Y.; Ren, P.; Kuang, X.; et al. Lactobacillus Acidophilus Ameliorates Obesity in Mice through Modulation of Gut Microbiota Dysbiosis and Intestinal Permeability. Pharmacol. Res. 2022, 175, 106020. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Sun, R.; Wang, M.; Wang, K.; Li, Y.; Shang, H.; Hou, J.; Jiang, Z. Lactobacillus Plantarum 23-1 Improves Intestinal Inflammation and Barrier Function through the TLR4/NF-ΚB Signaling Pathway in Obese Mice. Food Funct. 2022, 13, 5971–5986. [Google Scholar] [CrossRef]
- Park, M.; Park, E.-J.; Kim, S.-H.; Lee, H.-J. Lactobacillus Plantarum ATG-K2 and ATG-K6 Ameliorates High-Fat with High-Fructose Induced Intestinal Inflammation. Int. J. Mol. Sci. 2021, 22, 4444. [Google Scholar] [CrossRef]
- Long, X.; Zeng, X.; Tan, F.; Yi, R.; Pan, Y.; Zhou, X.; Mu, J.; Zhao, X. Lactobacillus plantarum KFY04 Prevents Obesity in Mice through the PPAR Pathway and Alleviates Oxidative Damage and Inflammation. Food Funct. 2020, 11, 5460–5472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Takano, T.; Zhou, Y.; Wang, R.; Toshimitsu, T.; Sashihara, T.; Tanokura, M.; Miyakawa, T.; Nakajima-Adachi, H.; Hachimura, S. Orally Administered Lactiplantibacillus plantarum OLL2712 Decreased Intestinal Permeability, Especially in the Ileum: Ingested Lactic Acid Bacteria Alleviated Obesity-Induced Inflammation by Collaborating with Gut Microbiota. Front. Immunol. 2023, 14, 1123052. [Google Scholar] [CrossRef] [PubMed]
- Joung, H.; Chu, J.; Kim, B.-K.; Choi, I.-S.; Kim, W.; Park, T.-S. Probiotics Ameliorate Chronic Low-Grade Inflammation and Fat Accumulation with Gut Microbiota Composition Change in Diet-Induced Obese Mice Models. Appl. Microbiol. Biotechnol. 2021, 105, 1203–1213. [Google Scholar] [CrossRef]
- Yoshitake, R.; Hirose, Y.; Murosaki, S.; Matsuzaki, G. Heat-Killed Lactobacillus plantarum L-137 Attenuates Obesity and Associated Metabolic Abnormalities in C57BL/6 J Mice on a High-Fat Diet. Biosci. Microbiota Food Health 2021, 40, 84–91. [Google Scholar] [CrossRef]
- Yang, B.; Zheng, F.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus Reuteri FYNLJ109L1 Attenuating Metabolic Syndrome in Mice via Gut Microbiota Modulation and Alleviating Inflammation. Foods 2021, 10, 2081. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Jiménez, C.; Martínez-Ramírez, M.J.; Del Castillo-Codes, I.; Arraiza-Irigoyen, C.; Tercero-Lozano, M.; Camacho, J.; Chueca, N.; García, F.; Olza, J.; Plaza-Díaz, J.; et al. Lactobacillus Reuteri V3401 Reduces Inflammatory Biomarkers and Modifies the Gastrointestinal Microbiome in Adults with Metabolic Syndrome: The PROSIR Study. Nutrients 2019, 11, 1761. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-T.; Chiou, S.-Y.; Hsu, A.-H.; Lin, Y.-C.; Lin, J.-S. Lactobacillus rhamnosus Strain LRH05 Intervention Ameliorated Body Weight Gain and Adipose Inflammation via Modulating the Gut Microbiota in High-Fat Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2022, 66, e2100348. [Google Scholar] [CrossRef]
- Wang, T.; Yan, H.; Lu, Y.; Li, X.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lü, X. Anti-Obesity Effect of Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 on High-Fat and High-Fructose Diet Mice Base on Inflammatory Response Alleviation and Gut Microbiota Regulation. Eur. J. Nutr. 2020, 59, 2709–2728. [Google Scholar] [CrossRef]
- Kawano, M.; Miyoshi, M.; Ogawa, A.; Sakai, F.; Kadooka, Y. Lactobacillus gasseri SBT2055 Inhibits Adipose Tissue Inflammation and Intestinal Permeability in Mice Fed a High-Fat Diet. J. Nutr. Sci. 2016, 5, e23. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-S.; Lim, S.K.; Lee, J.; Park, H.K.; Kwon, M.-S.; Yun, M.; Kim, N.; Oh, Y.J.; Choi, H.-J. Latilactobacillus sakei WIKIM31 Decelerates Weight Gain in High-Fat Diet-Induced Obese Mice by Modulating Lipid Metabolism and Suppressing Inflammation. J. Microbiol. Biotechnol. 2021, 31, 1568–1575. [Google Scholar] [CrossRef]
- Park, J.E.; Oh, S.-H.; Cha, Y.-S. Lactobacillus Brevis OPK-3 from Kimchi Prevents Obesity and Modulates the Expression of Adipogenic and Pro-Inflammatory Genes in Adipose Tissue of Diet-Induced Obese Mice. Nutrients 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, Y.; Lu, X.; Wang, X.; Wang, X.; Chen, L.; Guo, H.; Zhang, M.; Li, Y. Bifidobacterium Animalis Subsp. Lactis A6 Alleviates Obesity Associated with Promoting Mitochondrial Biogenesis and Function of Adipose Tissue in Mice. Molecules 2020, 25, 1490. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Zheng, A.; Ni, L.; Wu, L.; Hu, L.; Zhao, Y.; Fu, Z.; Ni, Y. Bifidobacterium animalis subsp. lactis Lkm512 Attenuates Obesity-Associated Inflammation and Insulin Resistance Through the Modification of Gut Microbiota in High-Fat Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2022, 66, 2100639. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kong, Q.; Cui, S.; Li, X.; Gu, Z.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Bifidobacterium Adolescentis Isolated from Different Hosts Modifies the Intestinal Microbiota and Displays Differential Metabolic and Immunomodulatory Properties in Mice Fed a High-Fat Diet. Nutrients 2021, 13, 1017. [Google Scholar] [CrossRef]
- Bernini, L.J.; Simão, A.N.C.; Alfieri, D.F.; Lozovoy, M.A.B.; Mari, N.L.; de Souza, C.H.B.; Dichi, I.; Costa, G.N. Beneficial Effects of Bifidobacterium Lactis on Lipid Profile and Cytokines in Patients with Metabolic Syndrome: A Randomized Trial. Effects of Probiotics on Metabolic Syndrome. Nutrition 2016, 32, 716–719. [Google Scholar] [CrossRef]
- Weng, G.; Huang, J.; Ma, X.; Song, M.; Yin, Y.; Deng, D.; Deng, J. Brevibacillus laterosporus BL1, a Promising Probiotic, Prevents Obesity and Modulates Gut Microbiota in Mice Fed a High-Fat Diet. Front. Nutr. 2022, 9, 1050025. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, D.; Liu, J.; Sun, X.; Gao, F.; Duan, H.; Dong, L.; Wang, X.; Wu, C. Pediococcus pentosaceus PR-1 Modulates High-Fat-Died-Induced Alterations in Gut Microbiota, Inflammation, and Lipid Metabolism in Zebrafish. Front. Nutr. 2023, 10, 1087703. [Google Scholar] [CrossRef]
- Everard, A.; Matamoros, S.; Geurts, L.; Delzenne, N.M.; Cani, P.D. Saccharomyces Boulardii Administration Changes Gut Microbiota and Reduces Hepatic Steatosis, Low-Grade Inflammation, and Fat Mass in Obese and Type 2 Diabetic Db/Db Mice. mBio 2014, 5, e01011–e01014. [Google Scholar] [CrossRef] [Green Version]
- Jian, H.; Liu, Y.; Wang, X.; Dong, X.; Zou, X. Akkermansia muciniphila as a Next-Generation Probiotic in Modulating Human Metabolic Homeostasis and Disease Progression: A Role Mediated by Gut–Liver–Brain Axes? Int. J. Mol. Sci. 2023, 24, 3900. [Google Scholar] [CrossRef]
- Wu, W.; Lv, L.; Shi, D.; Ye, J.; Fang, D.; Guo, F.; Li, Y.; He, X.; Li, L. Protective Effect of Akkermansia muciniphila against Immune-Mediated Liver Injury in a Mouse Model. Front. Microbiol. 2017, 8, 1804. [Google Scholar] [CrossRef]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Keshavarz Azizi Raftar, S.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Ahmadi Badi, S.; Khatami, S.; et al. Akkermansia muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019, 10, 2155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, W.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Liu, R.; Hong, J. Akkermansia muciniphila Improves Metabolic Profiles by Reducing Inflammation in Chow Diet-Fed Mice. J. Mol. Endocrinol. 2017, 58, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ba, T.; Cheng, Y.; Zhang, P.; Chang, X. Probiotics Alleviate Adipose Inflammation in High-Fat Diet–Induced Obesity by Restoring Adipose Invariant Natural Killer T Cells. Nutrition 2021, 89, 111285. [Google Scholar] [CrossRef] [PubMed]
- Szulińska, M.; Łoniewski, I.; Van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.-C.; Fang, T.-J.; Ho, H.-H.; Chen, J.-F.; Kuo, Y.-W.; Huang, Y.-Y.; Tsai, S.-Y.; Wu, S.-F.; Lin, H.-C.; Yeh, Y.-T. A Multi-Strain Probiotic Blend Reshaped Obesity-Related Gut Dysbiosis and Improved Lipid Metabolism in Obese Children. Front. Nutr. 2022, 9, 922993. [Google Scholar] [CrossRef]
- Minami, J.; Kondo, S.; Yanagisawa, N.; Odamaki, T.; Xiao, J.; Abe, F.; Nakajima, S.; Hamamoto, Y.; Saitoh, S.; Shimoda, T. Oral Administration of Bifidobacterium Breve B-3 Modifies Metabolic Functions in Adults with Obese Tendencies in a Randomised Controlled Trial. J. Nutr. Sci. 2015, 4, e17. [Google Scholar] [CrossRef] [Green Version]


| Probiotics | Experimental Model | Results | References |
|---|---|---|---|
| L. fermentum CQPC07 (LF-CQPC07) in 2 doses: low dose (LD) of 1.0 × 108 CFU/kg or high dose of (HD) −1.0 × 109 CFU/kg) | 6-wk-old C57BL/6 J mice (n = 60) on HFD supplemented with CQPC07 in LD or HD or placebo by 7 weeks | ↓ IL-1β, TNF-α, IL-6, IFN-γ, ↑ IL-10, and IL-4 ↑ mRNA expression of CAT, GSH1, SOD1, SOD 2, and GSH-Px | Wu et al. [190] |
| L. plantarum L-137 (heat-killed; 0.002%) | Wk-old C57BL/6 J mice (n = 60) on HFD supplemented with L. plantarum L-137 or placebo | ↓ Expression of inflammation-related genes (F4/80, CD11c, and IL-1β) in the epididymal adipose tissue ↓ LBP | Yoshitake et al. [199] |
| L. reuteri V3401 (5 × 109 CFU/d) | Adults with MetS following healthy lifestyle recommendation (n = 53) supplemented with L. reuteri V3401 or placebo for 12 weeks Randomized, crossover, placebo-controlled, single-center trial | ↓ IL-6 and sVCAM No significant effect on LPS, LBP, IL-8, CRP, TNF-α, MCP-1, or sICAM | Tenorio-Jiménez et al. [201] |
| BB. breve B-3 (5 × 1010 CFU/d) | Adult overweight volunteers (n = 52) receiving supplements with B. breve B-3 or placebo for 12 weeks Randomized, double-blind, placebo-controlled trial | ↓ hs-CRP | Minami et al. [222] |
| VSL#3 VSL#3 was a high-concentration (1.5 × 109 CFU/mouse/d) mixture of viable, lyophilized Lactobacillus, Bifidobacterium, and Streptococcus thermophilus | Wild-type (WT) (6-wk-old) male C57BL/6 mice (n = 16) and CD1d knockout (iNKT-cell-deficient) mice (n = 16) on an HFD or a normal-fat diet by 8 weeks CD1dKO (n = 16) and WT mice (n = 16) were then administered VSL#3 probiotics (active or heat—control group) by oral gavage for 4 weeks | VSL#3: ↓ adipose iNKT cell depletion and ↓ IL-6 and TNF- α Higher preventive effect against severe obesity development in wild-type mice than CD1dKO mice | Wang et al. [219] |
| Multi-species probiotic Ecologic® Barrier containing BB. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, Lactococcus lactis W19, and Lactococcus lactis W58 in 2 doses: LD (2.5 × 109 CFU/d) or HD (1 × 1010 CFU/d) | Postmenopausal women with obesity (n = 81) receiving supplements with multi-species probiotic Ecologic® Barrier in a low dose (LD) or a high dose (HD) or placebo for 12 weeks Randomized, double-blind, placebo-controlled clinical study | ↓ IL-6 in both LD and HD groups ↓ TNF-α in HD group | Szulińska et al. [189] |
| Three-strain probiotic including L. salivarius AP-32 (109 CFU/d), L. rhamnosus bv-77 (109 CFU/d), and BB. animalis CP-9 (8 × 109 CFU/d) | Overweight/obese children aged 6–18 years (n = 82) receiving supplements with a three-strain probiotic or placebo for 3 months Randomized, double-blind, placebo-controlled clinical study | ↓ Leptin and TNF-α and ↑ adiponectin | Chen et al. [221] |
| A. muciniphila (109 CFU/200 μL) or extracellular vesicles (EVs) of A. muciniphila (10 μg protein/200 μL EVs) | 8-wk-old male mice (n = 30) on HFD (group 1) or normal-fat diet (group 2) for 3 months After weight gain, both groups were divided into 3 subgroups receiving supplements with live A. muciniphila or EVs or placebo for 5 weeks | A. muciniphila: ↓ mRNA expression of TLR-4 and IL-6 genes in EAT, but no effect on TNF-α expression, ↑ expression of PPAR-α and PPAR-γ in adipose tissue, and ↓ TGF-β expression in EAT EVs: ↓ TNF-α, IL-6, and TLR-4 expression in HFD mice (greater than A. muciniphila), induced overexpression of PPAR-α in EAT in obese groups | Ashrafian et al. [216] |
| A. muciniphila (TCC BAA-835) 2 × 108 CFUs/200 μL | 6-wk-old male specific-pathogen-free (SPF)-grade C57BL/6 mice (n = 20) on normal chow diet supplemented with A. muciniphila or placebo for 5 weeks | ↓ Plasma levels of lipopolysaccharide (LPS)-binding protein (LBP) and leptin and inactivation of LPS/LBP downstream signaling (e.g., decreased phospho-JNK and increased IKBA expression) in liver and muscle ↑ Anti-inflammatory factors such as α-tocopherol and β-sitosterol | Zhao et al. [217] |
| A. muciniphila MucT 3 × 109 CFU/day | 4-5-wk-old C57BL/6 mice (n = 22) with acute liver injury administrated A. muciniphila MucT or placebo by oral gavage for 14 days | ↓ Proinflammatory cytokines MIP-1a, MIP-1b, and KC ↓ IFN-γ, IL-2, IL-1β, and IL-12p40 No effect on TNF-α ↓ Chemokines: MCP-1 | Wu et al. [215] |
| A.muciniphila (alive or pasteurized) (1010 bacteria per day) | Adults with overweight/obesity and metabolic disorders (n = 32) receiving supplements with live or pasteurized A.muciniphila or placebo for 3 months Randomized double-blind placebo-controlled proof-of-concept trial | Only pasteurized A.muciniphila led to significant results: ↓ LPS, ↓ DPP-IV activity, and ↓ sCD40L levels and chemokine GRO No significant effect but a trend toward ↓ CRP and MCP-1 | Depommier et al. [218] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelczyńska, M.; Moszak, M.; Wesołek, A.; Bogdański, P. The Preventive Mechanisms of Bioactive Food Compounds against Obesity-Induced Inflammation. Antioxidants 2023, 12, 1232. https://doi.org/10.3390/antiox12061232
Pelczyńska M, Moszak M, Wesołek A, Bogdański P. The Preventive Mechanisms of Bioactive Food Compounds against Obesity-Induced Inflammation. Antioxidants. 2023; 12(6):1232. https://doi.org/10.3390/antiox12061232
Chicago/Turabian StylePelczyńska, Marta, Małgorzata Moszak, Agnieszka Wesołek, and Paweł Bogdański. 2023. "The Preventive Mechanisms of Bioactive Food Compounds against Obesity-Induced Inflammation" Antioxidants 12, no. 6: 1232. https://doi.org/10.3390/antiox12061232
APA StylePelczyńska, M., Moszak, M., Wesołek, A., & Bogdański, P. (2023). The Preventive Mechanisms of Bioactive Food Compounds against Obesity-Induced Inflammation. Antioxidants, 12(6), 1232. https://doi.org/10.3390/antiox12061232








