Effects of Hydrogen Gas Inhalation on Community-Dwelling Adults of Various Ages: A Single-Arm, Open-Label, Prospective Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Differential White Blood Cell Count Analysis
2.3. Total ROS Estimation
2.4. Nitrous Oxide Level Estimation
2.5. BDNF Level Measurement
2.6. BACE-1 Level Measurement
2.7. Detection of Inflammatory Cytokines, Aβ, and Tau Protein by Multiplex Assay
2.8. Data Management and Statistical Analysis
3. Results
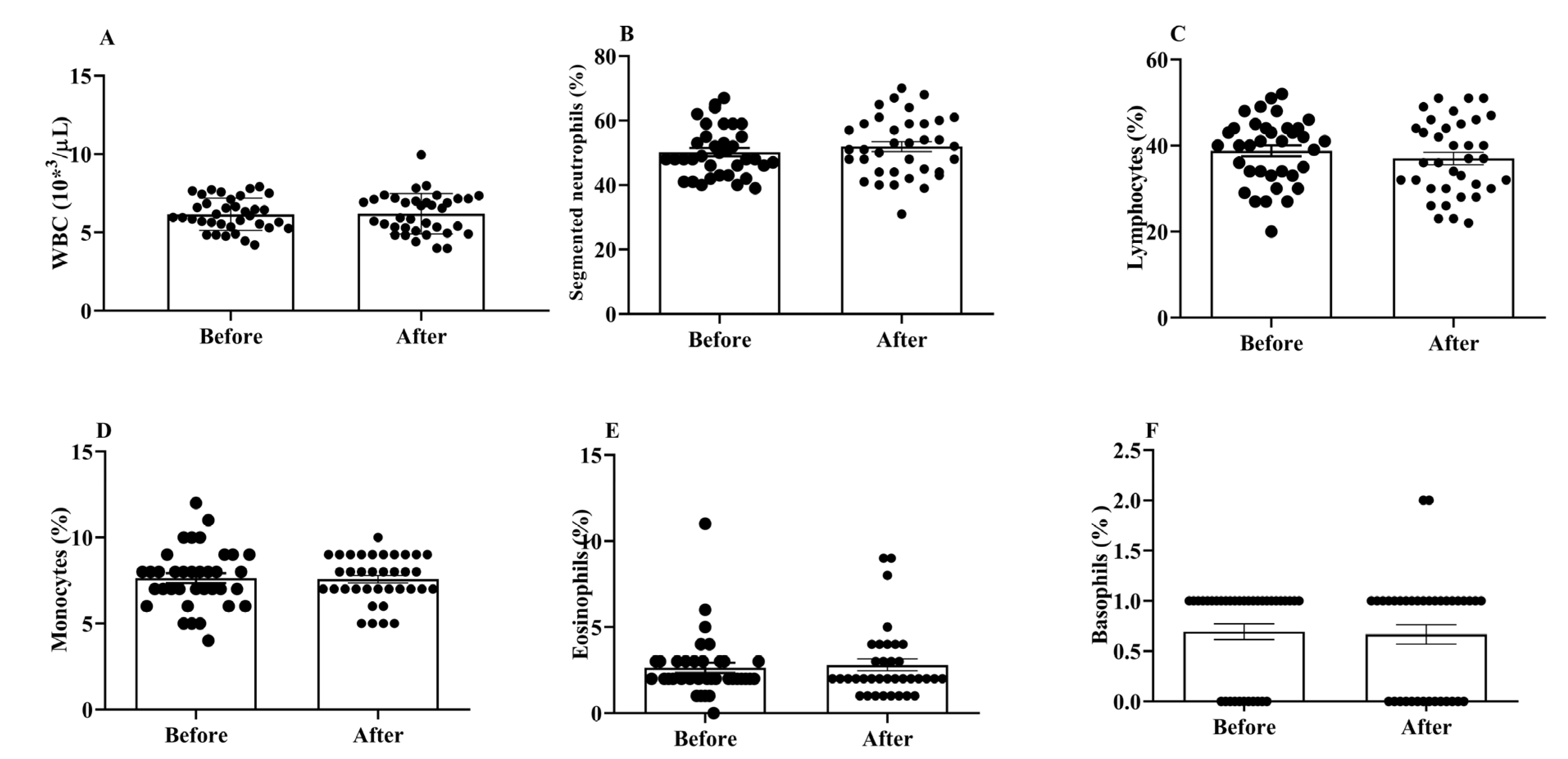
3.1. Effects of H2 Gas Inhalation on Total and Differential WBC Counts in Community-Dwelling Adults of Different Ages
3.2. Effects of H2 Gas Inhalation on ROS and NO in Community-Dwelling Adults of Different Ages
3.3. Effects of H2 Gas Inhalation on Serum BACE-1 Levels in Community-Dwelling Adults of Various Ages
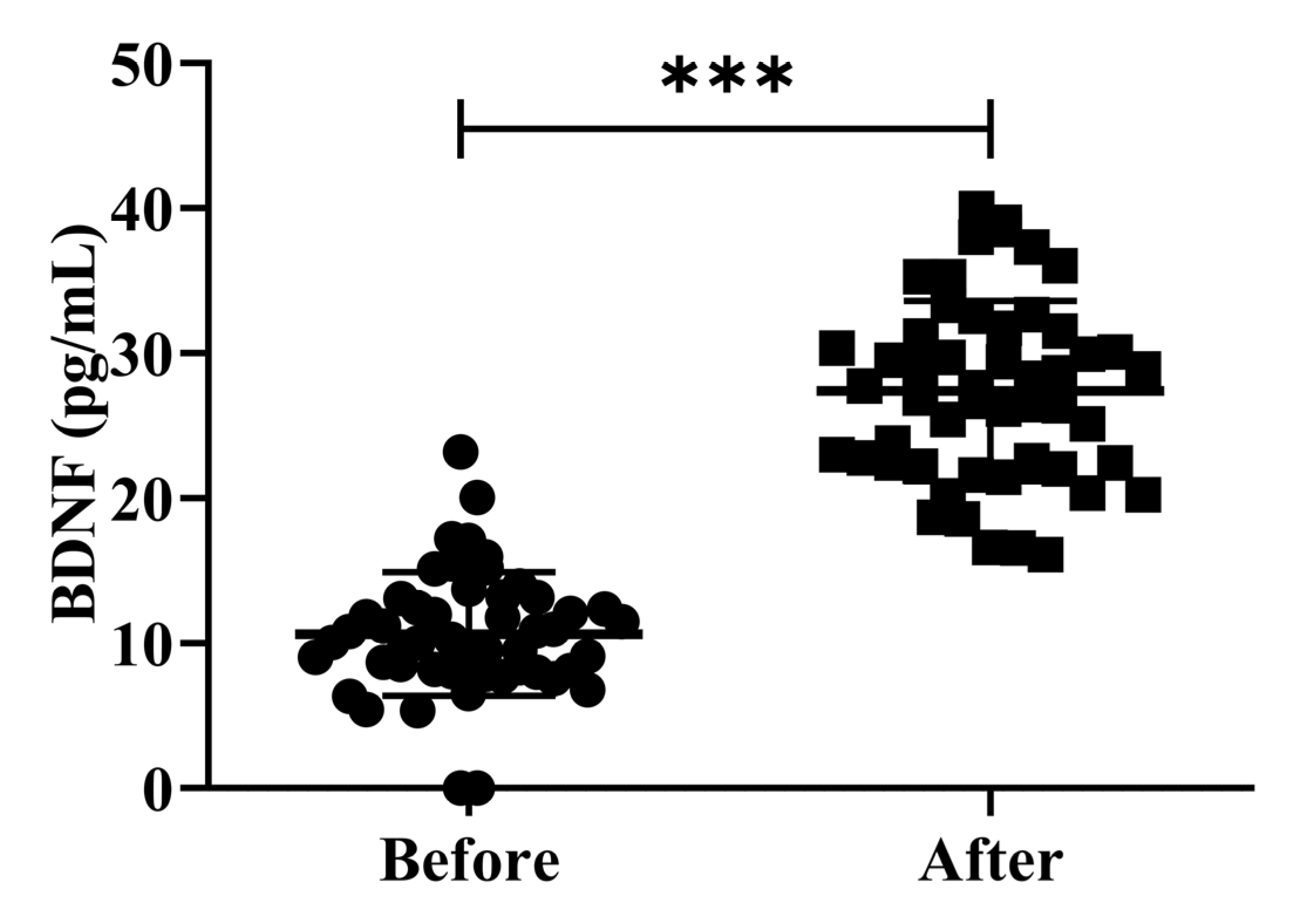
3.4. Effects of H2 Gas Inhalation on the Serum BDNF Levels in Community-Dwelling Adults of Various Ages
3.5. Effects of H2 Gas Inhalation on Serum Levels of Aβ-40, Aβ-42, t-Tau and p-Tau in Community-Dwelling Adults of Various Ages
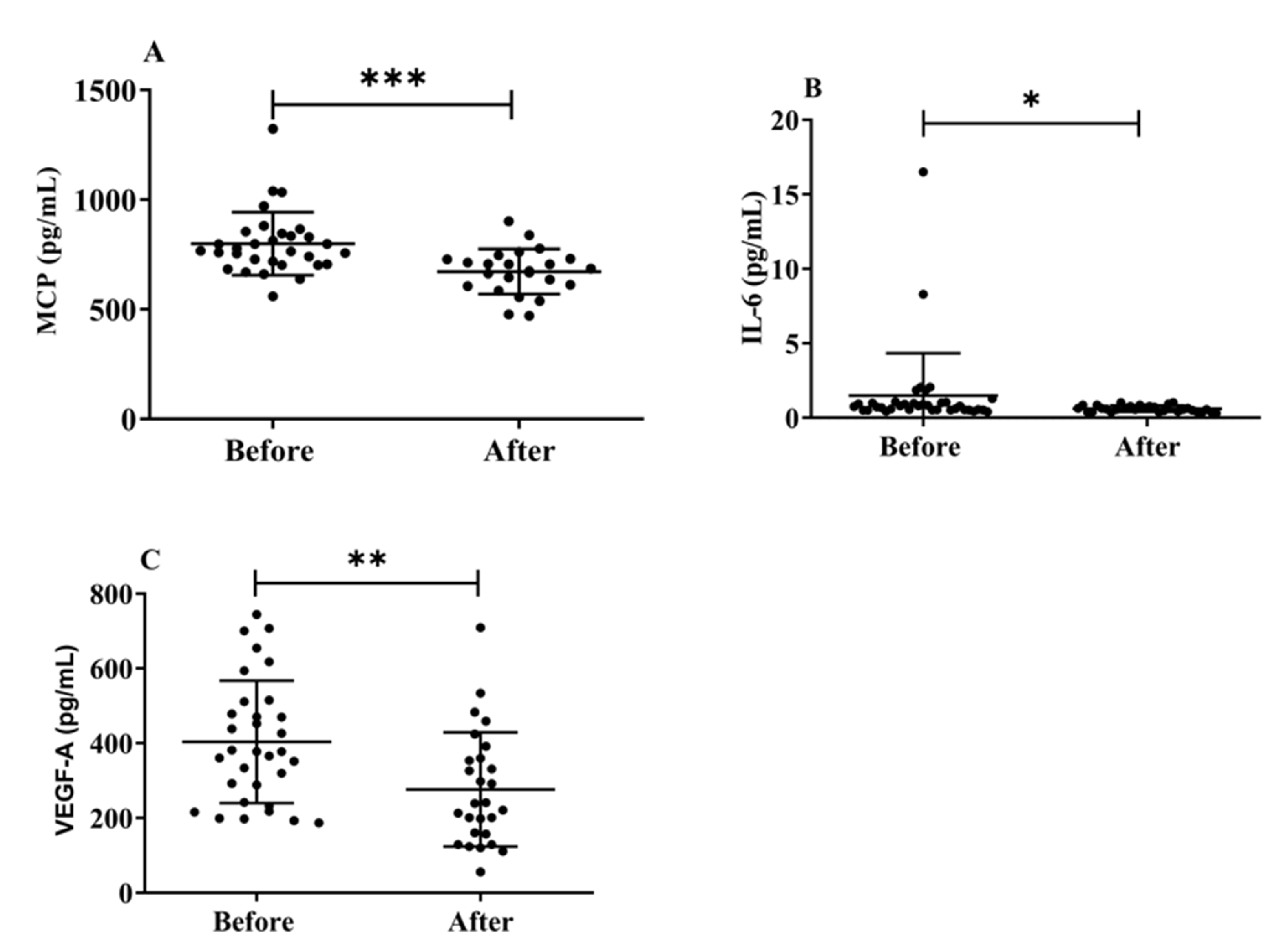
3.6. Effects of H2 Gas Inhalation on Serum Levels of MCP-1, IL-6, and VEGF-A in Community-Dwelling Adults of Various Ages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvey, R.J.; Skelton-Robinson, M.; Rossor, M.N. The prevalence and causes of dementia in people under the age of 65 years. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Shon, C.; Yoon, H. Health-economic burden of dementia in South Korea. BMC Geriatr. 2021, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Caselli, R.J.; Beach, T.G.; Yaari, R.; Reiman, E.M. Alzheimer’s disease a century later. J. Clin. Psychiatry 2006, 67, 1784–1800. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. A view on early diagnosis of dementias from neuropathology. In The Dementias: Early Diagnosis and Evaluation; CRC Press: Boca Raton, FL, USA, 2016; pp. 329–446. [Google Scholar]
- Fjellström, C.; Starkenberg, Å.; Wesslén, A.; Licentiate, M.S.; Bäckström, A.-C.T.; Faxén-Irving, G.; Fauth, E.; Hess, K.; Piercy, K.K.W.; Norton, M.C.; et al. Caregivers’ relationship closeness with the person with dementia predicts both positive and negative outcomes for caregivers’ physical health and psychological well-being. Aging Ment Health 2014, 33, 699–711. [Google Scholar] [CrossRef]
- Allan Butterfield, D. Amyloid β-peptide (1-42)-induced oxidative stress and neurotoxicity: Implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic. Res. 2002, 36, 1307–1313. [Google Scholar] [CrossRef]
- Barbagallo, M.; Marotta, F.; Dominguez, L.J. Oxidative Stress in Patients with Alzheimer’s Disease: Effect of Extracts of Fermented Papaya Powder. Mediat. Inflamm. 2015, 2015, 624801. [Google Scholar] [CrossRef]
- Yatin, S.M.; Yatin, M.; Aulick, T.; Ain, K.B.; Butterfield, D.A. Alzheimer’s amyloid β-peptide associated free radicals increase rat embryonic neuronal polyamine uptake and ornithine decarboxylase activity: Protective effect of vitamin E. Neurosci. Lett. 1999, 263, 17–20. [Google Scholar] [CrossRef]
- Drake, J.; Link, C.D.; Butterfield, D.A. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid β-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging 2003, 24, 415–420. [Google Scholar] [CrossRef]
- Cavedo, E.; Lista, S.; Khachaturian, Z.; Aisen, P.; Amouyel, P.; Herholz, K.; Jack, C., Jr.; Sperling, R.; Cummings, J.; Blennow, K.; et al. The road ahead to cure alzheimer’s disease: Development of biological markers and neuroimaging methods for prevention trials across all stages and target populations. J. Prev. Alzheimer’s Dis. 2014, 1, 181–202. [Google Scholar] [CrossRef]
- Gomez, W.; Morales, R.; Maracaja-Coutinho, V.; Parra, V.; Nassif, M. Down syndrome and Alzheimer’s disease: Common molecular traits beyond the amyloid precursor protein. Aging 2020, 12, 1011–1033. [Google Scholar] [CrossRef]
- Bogdanovic, N. Dementia, biomarker, genetics and experimental treatment. Eur. Geriatr. Med. 2015, 6, S161–S163. [Google Scholar] [CrossRef]
- Jung, G.L.; Bae, S.S.; Young, S.Y.; Ji, E.K.; Sung, W.Y.; Dong, W.J.; Jun, H.B.; Sung, W.P.; Young, H.K. Decreased Serum Brain-Derived Neurotrophic Factor Levels in Elderly Korean with Dementia. Psychiatry Investig. 2009, 6, 299–305. [Google Scholar] [CrossRef]
- Bauer, J.; Strauss, S.; Schreiter-Gasser, U.; Ganter, U.; Schlegel, P.; Witt, I.; Yolk, B.; Berger, M. Interleukin-6 and α-2-macroglobulin indicate an acute-phase state in Alzheimer’s disease cortices. FEBS Lett. 1991, 285, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, D.; Fenoglio, C.; Lovati, C.; Venturelli, E.; Guidi, I.; Corrà, B.; Scalabrini, D.; Clerici, F.; Mariani, C.; Bresolin, N.; et al. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol. Aging 2006, 27, 1763–1768. [Google Scholar] [CrossRef]
- Mahoney, E.R.; Dumitrescu, L.; Moore, A.M.; Cambronero, F.E.; De Jager, P.L.; Koran, M.E.I.; Petyuk, V.A.; Robinson, R.A.S.; Goyal, S.; Schneider, J.A.; et al. Brain expression of the vascular endothelial growth factor gene family in cognitive aging and alzheimer’s disease. Mol. Psychiatry 2020, 26, 888–896. [Google Scholar] [CrossRef]
- de Almodovar, C.R.; Lambrechts, D.; Mazzone, M.; Carmeliet, P. Role and Therapeutic Potential of VEGF in the Nervous System. Physiol. Rev. 2009, 89, 607–648. [Google Scholar] [CrossRef]
- Tayler, H.; Miners, J.S.; Güzel, Ö.; MacLachlan, R.; Love, S. Mediators of cerebral hypoperfusion and blood-brain barrier leakiness in Alzheimer’s disease, vascular dementia and mixed dementia. Brain Pathol. 2021, 31, e12935. [Google Scholar] [CrossRef]
- Ostojic, S.M. Molecular hydrogen: An inert gas turns clinically effective. Ann. Med. 2015, 47, 301–304. [Google Scholar] [CrossRef]
- Bhattacharyya, S.B.A.A.G.D.J.; Biswas, S.; Datta, A. Mode of Action of Endotoxin: Role of Free Radicals and Antioxidants. Curr. Med. Chem. 2005, 11, 359–368. [Google Scholar] [CrossRef]
- Zanini, D.; Todorovic, N.; Korovljev, D.; Stajer, V.; Ostojic, J.; Purac, J.; Kojic, D.; Vukasinovic, E.; Djordjievski, S.; Sopic, M.; et al. The effects of 6-month hydrogen-rich water intake on molecular and phenotypic biomarkers of aging in older adults aged 70 years and over: A randomized controlled pilot trial. Exp. Gerontol. 2021, 155, 111574. [Google Scholar] [CrossRef]
- Yoritaka, A.; Takanashi, M.; Hirayama, M.; Nakahara, T.; Ohta, S.; Hattori, N. Pilot study of H2 therapy in Parkinson’s disease: A randomized double-blind placebo-controlled trial. Mov. Disord. 2013, 28, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-T.; Hu, C.-J.; Lin, H.-Y.; Wu, D. Effects of concomitant use of hydrogen water and photobiomodulation on Parkinson disease: A pilot study. Medicine 2021, 100, e24191. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.-S.; Bajgai, J.; You, I.-S.; Rahman, H.; Fadriquela, A.; Sharma, S.; Kwon, H.-U.; Lee, S.-Y.; Kim, C.-S.; Lee, K.-J. Therapeutic Effects of Hydrogen Gas Inhalation on Trimethyltin-Induced Neurotoxicity and Cognitive Impairment in the C57BL/6 Mice Model. Int. J. Mol. Sci. 2021, 22, 13313. [Google Scholar] [CrossRef] [PubMed]
- Buccellato, F.R.; D’anca, M.; Galimberti, D.; Fenoglio, C.; Scarpini, E. Role of Oxidative Damage in Alzheimer’s Disease and Neurodegeneration: From Pathogenic Mechanisms to Biomarker Discovery. Antioxidants 2021, 10, 1353. [Google Scholar] [CrossRef]
- Korovljev, D.; Trivic, T.; Òtajer, V.; Drid, P.; Sato, B.; Ostojic, S. Short-term H2 inhalation improves cognitive function in older women: A Pilot Study. Int. J. Gerontol. 2020, 14, 149–150. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Bobryshev, Y.V.; Ivanova, E.A.; Chistiakov, D.A.; Nikiforov, N.G.; Orekhov, A.N. Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. BioMed Res. Int. 2016, 2016, 9582430. [Google Scholar] [CrossRef]
- Rembach, A.; Watt, A.D.; Wilson, W.J.; Rainey-Smith, S.; Ellis, K.A.; Rowe, C.C.; Villemagne, V.L.; Macaulay, S.L.; Bush, A.I.; Martins, R.N.; et al. An increased neutrophil-lymphocyte ratio in Alzheimer’s disease is a function of age and is weakly correlated with neocortical amyloid accumulation. J. Neuroimmunol. 2014, 273, 65–71. [Google Scholar] [CrossRef]
- An, P.; Zhou, X.; Du, Y.; Zhao, J.; Song, A.; Liu, H.; Ma, F.; Huang, G. Association of Neutrophil-Lymphocyte Ratio with Mild Cognitive Impairment in Elderly Chinese Adults: A Case-control Study. Curr. Alzheimer Res. 2020, 16, 1309–1315. [Google Scholar] [CrossRef]
- Gracy, R.W.; Talent, J.M.; Kong, Y.; Conrad, C.C. Reactive Oxygen Species: The unavoidable environmental insult? Mutat. Res./Fundam. Mol. Mech. Mutagen. 1999, 428, 17–22. [Google Scholar] [CrossRef]
- Jang, Y.C.; Lustgarten, M.S.; Liu, Y.; Muller, F.L.; Bhattacharya, A.; Liang, H.; Salmon, A.B.; Brooks, S.V.; Larkin, L.; Hayworth, C.R.; et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010, 24, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Puli, L.; Patil, C.R. Role of reactive oxygen species in the progression of Alzheimer’s disease. Drug Discov. Today 2021, 26, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Kokaia, Z.; Nawa, H.; Uchino, H.; Elmér, E.; Kokaia, M.; Carnahan, J.; Smith, M.-L.; Siesjö, B.; Lindvall, O. Regional brain-derived neurotrophic factor mRNA and protein levels following transient forebrain ischemia in the rat. Mol. Brain Res. 1996, 38, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Crutcher, K.A.; Scott, S.A.; Liang, S.; Everson, W.V.; Weingartner, J. Detection of NGF-like activity in human brain tissue: Increased levels in Alzheimer’s disease. J. Neurosci. 1993, 13, 2540–2550. [Google Scholar] [CrossRef]
- Bodendorf, U.; Fischer, F.; Bodian, D.; Multhaup, G.; Paganetti, P. A Splice Variant of β-Secretase Deficient in the Amyloidogenic Processing of the Amyloid Precursor Protein. J. Biol. Chem. 2001, 276, 12019–12023. [Google Scholar] [CrossRef]
- Vassar, R.; Kovacs, D.M.; Yan, R.; Wong, P.C. The β-secretase enzyme BACE in health and Alzheimer’s disease: Regulation, cell biology, function, and therapeutic potential. J. Neurosci. 2009, 29, 12787–12794. [Google Scholar] [CrossRef]
- Gouras, G.K.; Tsai, J.; Naslund, J.; Vincent, B.; Edgar, M.; Checler, F.; Greenfield, J.P.; Haroutunian, V.; Buxbaum, J.D.; Xu, H.; et al. Intraneuronal Aβ42 Accumulation in Human Brain. Am. J. Pathol. 2000, 156, 15–20. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, S.J.; Hong, S.; Kim, Y. Diagnosis of Alzheimer’s disease utilizing amyloid and tau as fluid biomarkers. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Fandos, N.; Pérez-Grijalba, V.; Pesini, P.; Olmos, S.; Bossa, M.; Villemagne, V.L.; Doecke, J.; Fowler, C.; Masters, C.L.; Sarasa, M.; et al. Plasma amyloid β 42/40 ratios as biomarkers for amyloid β cerebral deposition in cognitively normal individuals. Alzheimer’s Dementia: Diagn. Assess. Dis. Monit. 2017, 8, 179–187. [Google Scholar] [CrossRef]
- van Oijen, M.; Hofman, A.; Soares, H.D.; Koudstaal, P.J.; Breteler, M.M. Plasma Aβ1–40 and Aβ1–42 and the risk of dementia: A prospective case-cohort study. Lancet Neurol. 2006, 5, 655–660. [Google Scholar] [CrossRef]
- Mayeux, R.; Honig, L.S.; Tang, M.-X.; Manly, J.; Stern, Y.; Schupf, N.; Mehta, P.D. Plasma A 40 and A 42 and Alzheimer’s disease: Relation to age, mortality, and risk. Neurology 2003, 61, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Sämgård, K.; Zetterberg, H.; Blennow, K.; Hansson, O.; Minthon, L.; Londos, E. Cerebrospinal fluid total tau as a marker of Alzheimer’s disease intensity. Int. J. Geriatr. Psychiatry 2010, 25, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.A.; Appleby, B.S.; Safar, J.; Leverenz, J.B. Rapidly Progressive Alzheimer’s Disease in Two Distinct Autopsy Cohorts. J. Alzheimer’s Dis. 2018, 64, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, S.; Chertkow, H.; Kergoat, M.-J.; Gauthier, S.; Belleville, S. Patterns of Cognitive Decline Prior to Dementia in Persons with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2015, 47, 901–913. [Google Scholar] [CrossRef]
- Pomara, N.; Willoughby, L.M.; Sidtis, J.J.; Mehta, P.D. Selective Reductions in Plasma Aβ 1-42 in Healthy Elderly Subjects During Longitudinal Follow-Up: A Preliminary Report. Am. J. Geriatr. Psychiatry 2005, 13, 914–917. [Google Scholar] [CrossRef]
- Stefani, A.; Brusa, L.; Olivola, E.; Pierantozzi, M.; Martorana, A. CSF and clinical hallmarks of subcortical dementias: Focus on DLB and PDD. J. Neural Transm. 2012, 119, 861–875. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Sanchez-Sanchez, J.L.; Giudici, K.V.; Guyonnet, S.; Delrieu, J.; Li, Y.; Bateman, R.J.; Parini, A.; Vellas, B.; Barreto, P.D.S.; Carrié, I.; et al. Plasma MCP-1 and changes on cognitive function in community-dwelling older adults. Alzheimer’s Res. Ther. 2022, 14, 5. [Google Scholar] [CrossRef]
- Beazley-Long, N.; Hua, J.; Jehle, T.; Hulse, R.P.; Dersch, R.; Lehrling, C.; Bevan, H.; Qiu, Y.; Lagrèze, W.A.; Wynick, D.; et al. VEGF-A165b Is an Endogenous Neuroprotective Splice Isoform of Vascular Endothelial Growth Factor A in Vivo and in Vitro. Am. J. Pathol. 2013, 183, 918–929. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chatterjee, M.; Twaalfhoven, H.; Milan, M.D.C.; Teunissen, C.E.; Scheltens, P.; Fontijn, R.D.; van Der Flier, W.M.; de Vries, H.E. Vascular Endothelial Growth Factor remains unchanged in cerebrospinal fluid of patients with Alzheimer’s disease and vascular dementia. Alzheimer’s Res. Ther. 2018, 10, 58. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Zeng, F.-F.; Chen, Z.-W.; Wang, C.-Y.; Zhao, B.; Li, K.-S. Vascular Endothelial Growth Factor Gene Promoter Polymorphisms and Alzheimer’s Disease Risk: A Meta-Analysis. CNS Neurosci. Ther. 2013, 19, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Bouvet, P.; Chounlamountri, N.; Watrin, C.; Besançon, R.; Pinatel, D.; Meyronet, D.; Honnorat, J.; Buisson, A.; Salin, P.-A.; et al. VEGF counteracts amyloid-β-induced synaptic dysfunction. Cell Rep. 2021, 35, 109121. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]




| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patients aged 40 to 70 years can walk voluntarily. | Those who have a history of AD disease. |
| Patients who are willing or able to follow the doctor’s instructions, including joint movements. | Those who have participated in other clinical trials within 6 months of participating in clinical trials. |
| Persons who can comply with concomitantly permitted drugs, prohibited drugs, and relief drugs. | Patients who have another autoimmune disease. |
| Persons who can maintain the same exercise and activity during the clinical trial period. | Patients with tumors other than neurodegenerative diseases. |
| Fully understand the purpose and procedure of this clinical trial. | Pregnant and lactating women. |
| Variable | Total | Female | Male | |
|---|---|---|---|---|
| Gender | 51 | 39 (76.47%) | 11 (21.57%) | |
| Age (years) | 53.98 ± 8.86 | 56.15 ± 8.89 | 46.09 ± 7.91 | |
| Education | 51 | Graduation | Graduation | |
| Blood Pressure (mmHg) | Systolic | 123.43 ± 8.44 | 122.82 ± 7.80 | 125.63 ± 6.93 |
| Diastolic | 78.07 ± 7.60 | 77.67 ± 7.79 | 79.54 ± 4.20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.H.; Bajgai, J.; Sharma, S.; Jeong, E.-S.; Goh, S.H.; Jang, Y.-G.; Kim, C.-S.; Lee, K.-J. Effects of Hydrogen Gas Inhalation on Community-Dwelling Adults of Various Ages: A Single-Arm, Open-Label, Prospective Clinical Trial. Antioxidants 2023, 12, 1241. https://doi.org/10.3390/antiox12061241
Rahman MH, Bajgai J, Sharma S, Jeong E-S, Goh SH, Jang Y-G, Kim C-S, Lee K-J. Effects of Hydrogen Gas Inhalation on Community-Dwelling Adults of Various Ages: A Single-Arm, Open-Label, Prospective Clinical Trial. Antioxidants. 2023; 12(6):1241. https://doi.org/10.3390/antiox12061241
Chicago/Turabian StyleRahman, Md. Habibur, Johny Bajgai, Subham Sharma, Eun-Sook Jeong, Seong Hoon Goh, Yeon-Gyu Jang, Cheol-Su Kim, and Kyu-Jae Lee. 2023. "Effects of Hydrogen Gas Inhalation on Community-Dwelling Adults of Various Ages: A Single-Arm, Open-Label, Prospective Clinical Trial" Antioxidants 12, no. 6: 1241. https://doi.org/10.3390/antiox12061241
APA StyleRahman, M. H., Bajgai, J., Sharma, S., Jeong, E.-S., Goh, S. H., Jang, Y.-G., Kim, C.-S., & Lee, K.-J. (2023). Effects of Hydrogen Gas Inhalation on Community-Dwelling Adults of Various Ages: A Single-Arm, Open-Label, Prospective Clinical Trial. Antioxidants, 12(6), 1241. https://doi.org/10.3390/antiox12061241








