The Effect of Astaxanthin on Mitochondrial Dynamics in Rat Heart Mitochondria under ISO-Induced Injury
Abstract
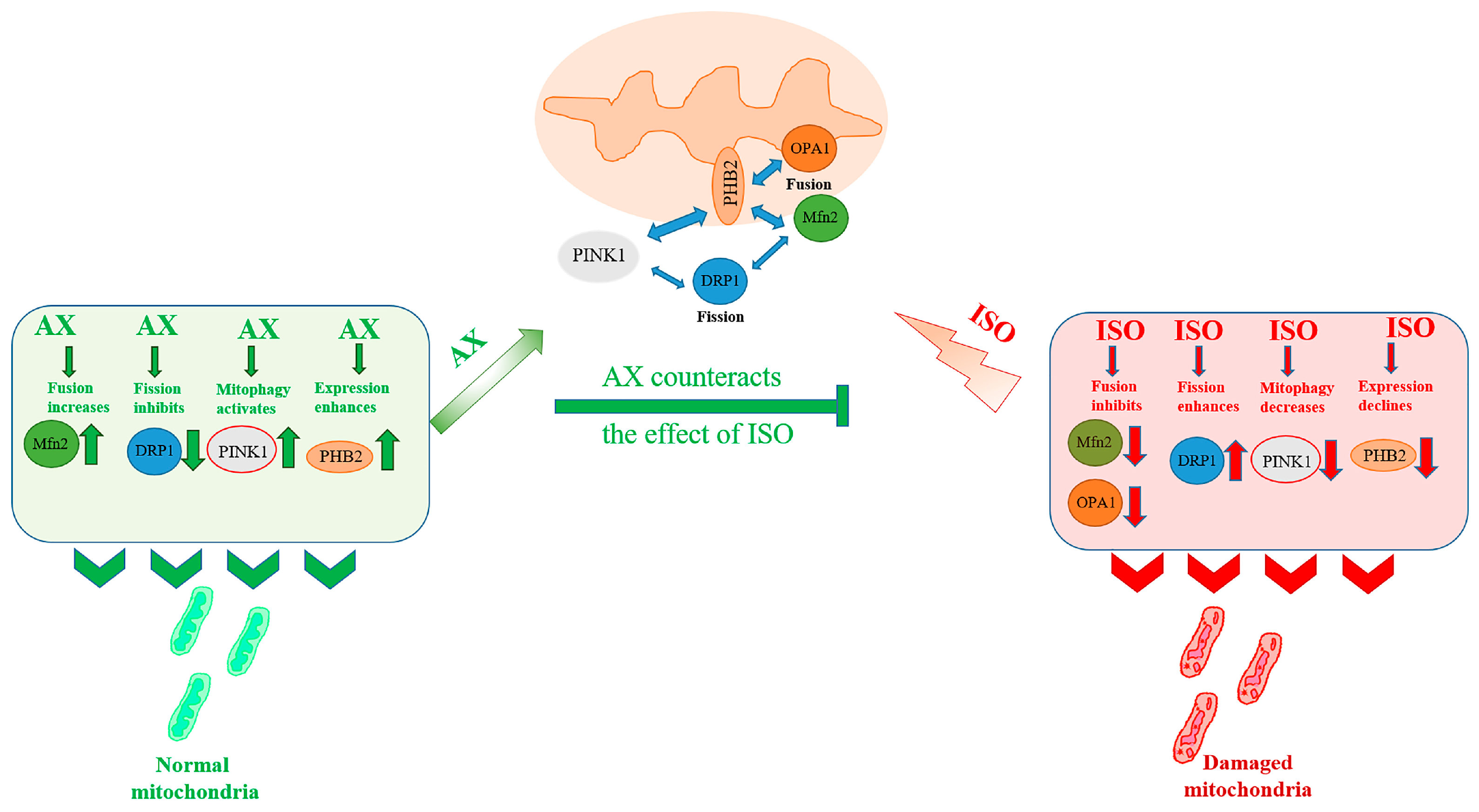
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Histological Analysis
2.3. Isolation of Rat Heart Mitochondria
2.4. Evaluation of the Mitochondrial Function
2.5. Electrophoresis and Immunoblotting
2.6. Statistical Analysis
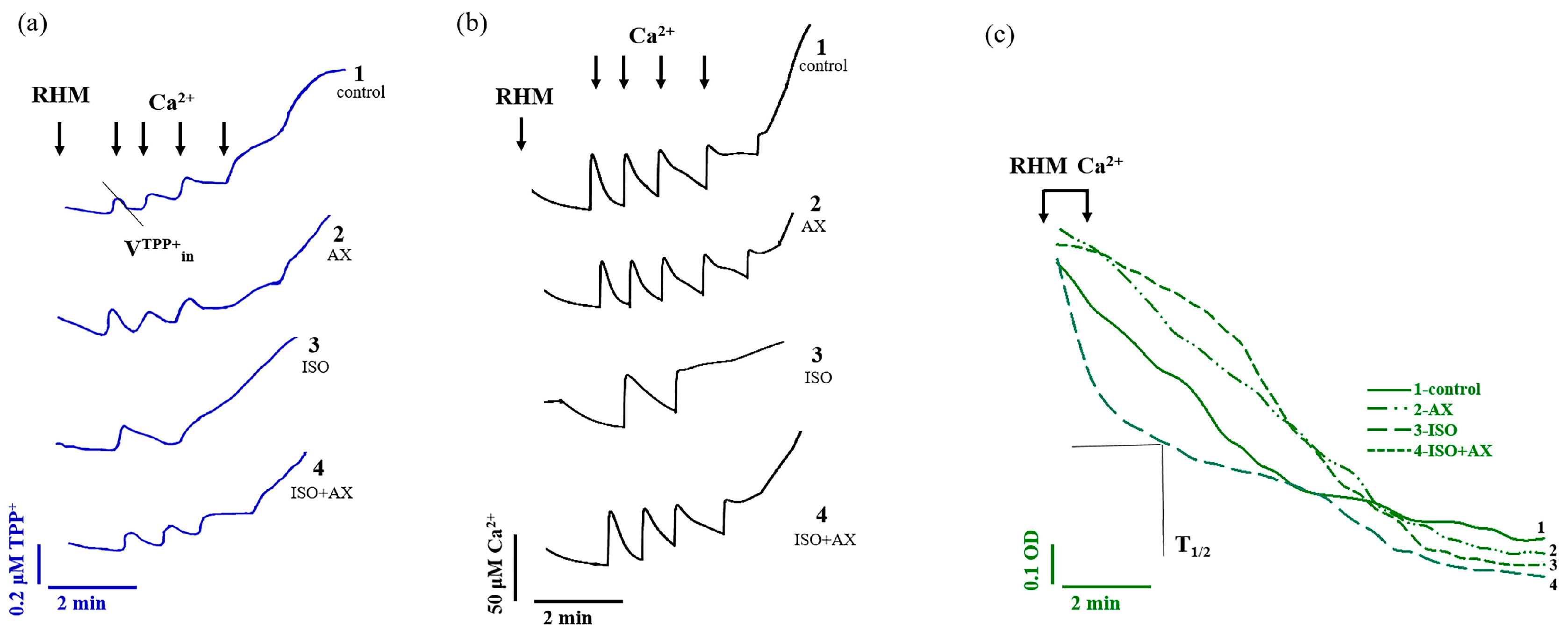
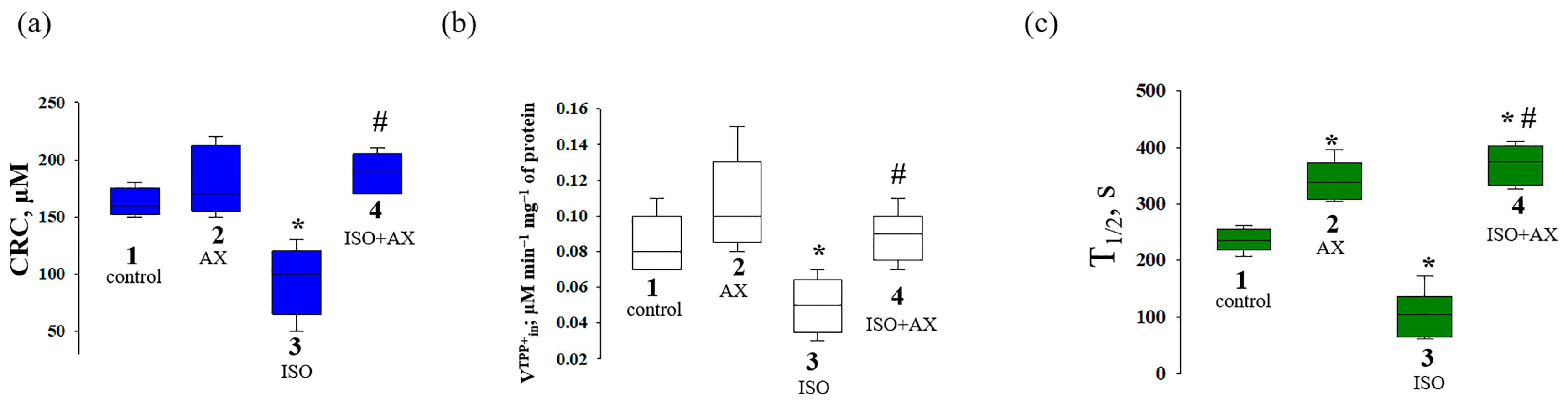
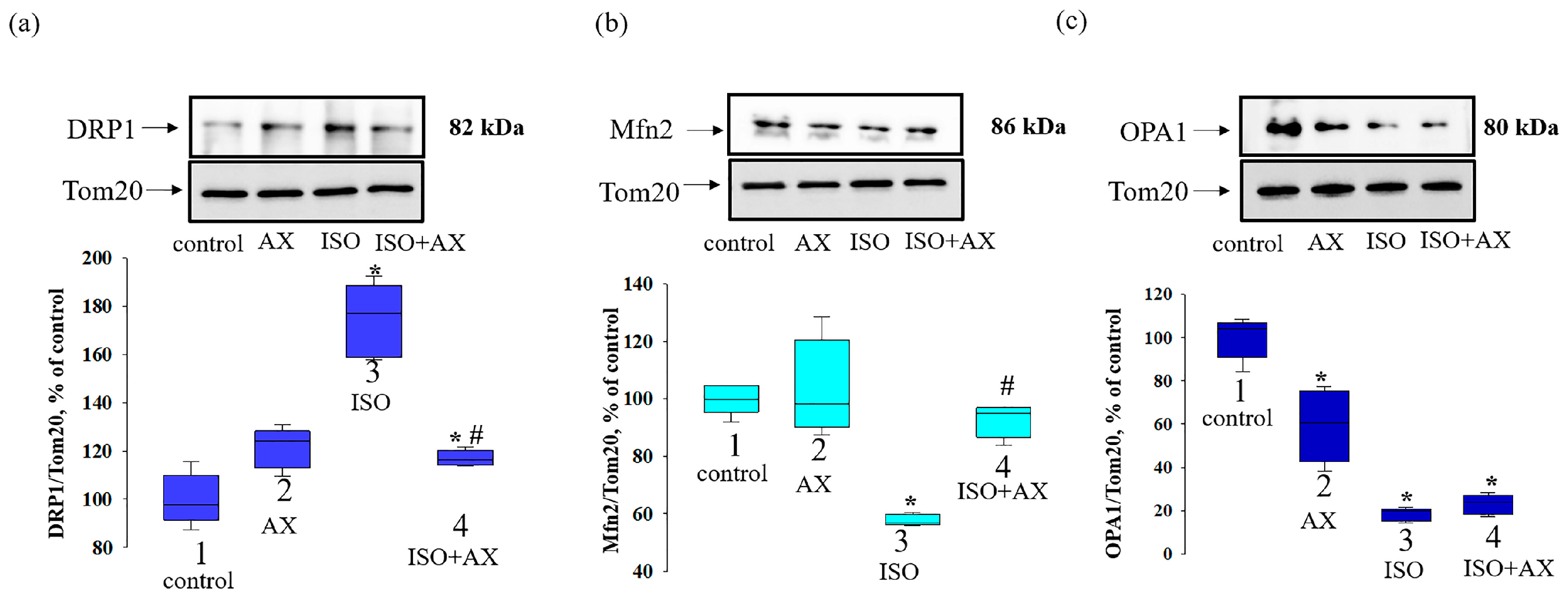
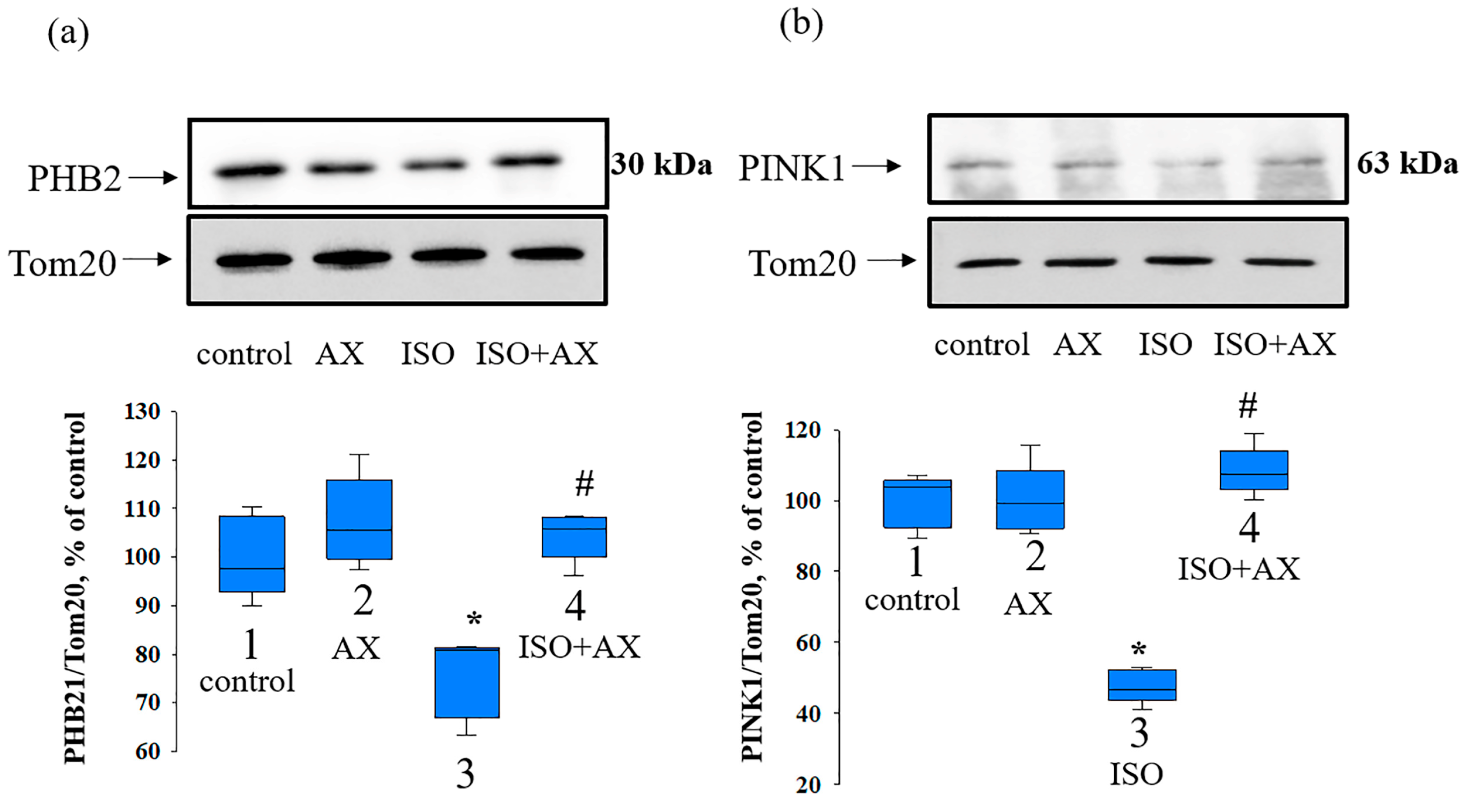
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hausenloy, D.J.; Botker, H.E.; Engstrom, T.; Erlinge, D.; Heusch, G.; Ibanez, B.; Kloner, R.A.; Ovize, M.; Yellon, D.M.; Garcia-Dorado, D. Targeting reperfusion injury in patients with st-segment elevation myocardial infarction: Trials and tribulations. Eur. Heart J. 2017, 38, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Javadov, S.; Kozlov, A.V.; Camara, A.K.S. Mitochondria in health and diseases. Cells 2020, 9, 1177. [Google Scholar] [CrossRef] [PubMed]
- van der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, a011072. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Lu, M.H.; Yuan, D.J.; Xu, D.E.; Yao, P.P.; Ji, W.L.; Chen, H.; Liu, W.L.; Yan, C.X.; Xia, Y.Y.; et al. Mitochondrial dysfunction in neural injury. Front. Neurosci. 2019, 13, 30. [Google Scholar] [CrossRef] [Green Version]
- Scheffer, D.D.L.; Garcia, A.A.; Lee, L.; Mochly-Rosen, D.; Ferreira, J.C.B. Mitochondrial fusion, fission, and mitophagy in cardiac diseases: Challenges and therapeutic opportunities. Antioxid. Redox Signal. 2022, 36, 844–863. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Wang, C.; Cleland, M.M.; Setoguchi, K.; Yokota, S.; Youle, R.J.; Mihara, K. Mff is an essential factor for mitochondrial recruitment of drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 2010, 191, 1141–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- Cipolat, S.; Rudka, T.; Hartmann, D.; Costa, V.; Serneels, L.; Craessaerts, K.; Metzger, K.; Frezza, C.; Annaert, W.; D’Adamio, L.; et al. Mitochondrial rhomboid parl regulates cytochrome c release during apoptosis via opa1-dependent cristae remodeling. Cell 2006, 126, 163–175. [Google Scholar] [CrossRef]
- Ni, H.M.; Williams, J.A.; Ding, W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015, 4, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Aung, L.H.H.; Jumbo, J.C.C.; Wang, Y.; Li, P. Therapeutic potential and recent advances on targeting mitochondrial dynamics in cardiac hypertrophy: A concise review. Mol. Ther. Nucleic Acids 2021, 25, 416–443. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, M.; Yan, R.; Shan, H.; Diao, J.; Wei, J. Inhibition of drp1 attenuates mitochondrial damage and myocardial injury in coxsackievirus b3 induced myocarditis. Biochem. Biophys. Res. Commun. 2017, 484, 550–556. [Google Scholar] [CrossRef]
- Escobar-Henriques, M.; Anton, F. Mechanistic perspective of mitochondrial fusion: Tubulation vs. Fragmentation. Biochim. Biophys. Acta 2013, 1833, 162–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, N.; Jofuku, A.; Eura, Y.; Mihara, K. Regulation of mitochondrial morphology by membrane potential, and drp1-dependent division and fzo1-dependent fusion reaction in mammalian cells. Biochem. Biophys. Res. Commun. 2003, 301, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Gazaryan, I.G.; Brown, A.M. Intersection between mitochondrial permeability pores and mitochondrial fusion/fission. Neurochem. Res. 2007, 32, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Krestinin, R.; Baburina, Y.; Odinokova, I.; Kruglov, A.; Fadeeva, I.; Zvyagina, A.; Sotnikova, L.; Krestinina, O. Isoproterenol-induced permeability transition pore-related dysfunction of heart mitochondria is attenuated by astaxanthin. Biomedicines 2020, 8, 437. [Google Scholar] [CrossRef]
- Krestinina, O.; Baburina, Y.; Krestinin, R. Mitochondrion as a target of astaxanthin therapy in heart failure. Int. J. Mol. Sci. 2021, 22, 7964. [Google Scholar] [CrossRef] [PubMed]
- Baburina, Y.; Krestinin, R.; Odinokova, I.; Fadeeva, I.; Sotnikova, L.; Krestinina, O. The identification of prohibitin in the rat heart mitochondria in heart failure. Biomedicines 2021, 9, 1793. [Google Scholar] [CrossRef]
- Artal-Sanz, M.; Tavernarakis, N. Prohibitin and mitochondrial biology. Trends Endocrinol. Metab. 2009, 20, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Nijtmans, L.G.; de Jong, L.; Sanz, M.A.; Coates, P.J.; Berden, J.A.; Back, J.W.; Muijsers, A.O.; van der Spek, H.; Grivell, L.A. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J. 2000, 19, 2444–2451. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Chiang, W.C.; Sumpter, R., Jr.; Mishra, P.; Levine, B. Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell 2017, 168, 224–238.e210. [Google Scholar] [CrossRef] [Green Version]
- Akila, P.; Asaikumar, L.; Vennila, L. Chlorogenic acid ameliorates isoproterenol-induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes. Biomed. Pharmacother. 2017, 85, 582–591. [Google Scholar] [CrossRef]
- Feng, W.; Li, W. The study of iso induced heart failure rat model. Exp. Mol. Pathol. 2010, 88, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Odinokova, I.; Baburina, Y.; Kruglov, A.; Fadeeva, I.; Zvyagina, A.; Sotnikova, L.; Akatov, V.; Krestinina, O. Effect of melatonin on rat heart mitochondria in acute heart failure in aged rats. Int. J. Mol. Sci. 2018, 19, 1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, M.; Chott, A.; Fabiano, A.; Battifora, H. Effect of formalin tissue fixation and processing on immunohistochemistry. Am. J. Surg. Pathol. 2000, 24, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Lillie, R.D.; Fullmer, H.M. Histopathologic Technic and Practical Histochemistry; McGraw-Hill: San Francisco, CA, USA, 1976; p. 942. [Google Scholar]
- Azarashvili, T.; Grachev, D.; Krestinina, O.; Evtodienko, Y.; Yurkov, I.; Papadopoulos, V.; Reiser, G. The peripheral-type benzodiazepine receptor is involved in control of ca2+-induced permeability transition pore opening in rat brain mitochondria. Cell Calcium 2007, 42, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Webster, K.A. Mitochondrial membrane permeabilization and cell death during myocardial infarction: Roles of calcium and reactive oxygen species. Future Cardiol. 2012, 8, 863–884. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Z.; Jing, K.; Wu, H.; Wang, S.; Ye, L.; Li, Z.; Yang, C.; Pan, Q.; Liu, W.J.; Liu, H.F. Mechanisms and functions of mitophagy and potential roles in renal disease. Front. Physiol. 2020, 11, 935. [Google Scholar] [CrossRef]
- Ban, T.; Ishihara, T.; Kohno, H.; Saita, S.; Ichimura, A.; Maenaka, K.; Oka, T.; Mihara, K.; Ishihara, N. Molecular basis of selective mitochondrial fusion by heterotypic action between opa1 and cardiolipin. Nat. Cell Biol. 2017, 19, 856–863. [Google Scholar] [CrossRef]
- Yan, C.; Gong, L.; Chen, L.; Xu, M.; Abou-Hamdan, H.; Tang, M.; Desaubry, L.; Song, Z. Phb2 (prohibitin 2) promotes pink1-prkn/parkin-dependent mitophagy by the parl-pgam5-pink1 axis. Autophagy 2020, 16, 419–434. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [Green Version]
- Busch, K.B.; Bereiter-Hahn, J.; Wittig, I.; Schagger, H.; Jendrach, M. Mitochondrial dynamics generate equal distribution but patchwork localization of respiratory complex i. Mol. Membr. Biol. 2006, 23, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Nawaz, A.; Hecht, K.; Tobe, K. Astaxanthin as a novel mitochondrial regulator: A new aspect of carotenoids, beyond antioxidants. Nutrients 2021, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Koshiba, T.; Detmer, S.A.; Kaiser, J.T.; Chen, H.; McCaffery, J.M.; Chan, D.C. Structural basis of mitochondrial tethering by mitofusin complexes. Science 2004, 305, 858–862. [Google Scholar] [CrossRef] [Green Version]
- Hom, J.; Sheu, S.S. Morphological dynamics of mitochondria—A special emphasis on cardiac muscle cells. J. Mol. Cell. Cardiol. 2009, 46, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Twig, G.; Elorza, A.; Molina, A.J.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.B.; Subrayan, S.; Lim, S.Y.; Yellon, D.M.; Davidson, S.M.; Hausenloy, D.J. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 2010, 121, 2012–2022. [Google Scholar] [CrossRef] [Green Version]
- Maneechote, C.; Palee, S.; Chattipakorn, S.C.; Chattipakorn, N. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J. Cell. Mol. Med. 2017, 21, 2643–2653. [Google Scholar] [CrossRef]
- Quiles, J.M.; Gustafsson, A.B. The role of mitochondrial fission in cardiovascular health and disease. Nat. Rev. Cardiol. 2022, 19, 723–736. [Google Scholar] [CrossRef]
- Huang, C.; Wen, C.; Yang, M.; Li, A.; Fan, C.; Gan, D.; Li, Q.; Zhao, J.; Zhu, L.; Lu, D. Astaxanthin improved the cognitive deficits in app/ps1 transgenic mice via selective activation of mtor. J. Neuroimmune Pharmacol. 2021, 16, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, T.; Model, K.; Langer, T. Formation of membrane-bound ring complexes by prohibitins in mitochondria. Mol. Biol. Cell 2005, 16, 248–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Jian, C.; Peng, Q.; Hou, T.; Wu, K.; Shang, B.; Zhao, M.; Wang, Y.; Zheng, W.; Ma, Q.; et al. Prohibitin 2 deficiency impairs cardiac fatty acid oxidation and causes heart failure. Cell Death Dis. 2020, 11, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, D.; Tangutur, A.D.; Khatua, T.N.; Saxena, P.; Banerjee, S.K.; Bhadra, M.P. A proteomic view of isoproterenol induced cardiac hypertrophy: Prohibitin identified as a potential biomarker in rats. J. Transl. Med. 2013, 11, 130. [Google Scholar] [CrossRef] [Green Version]
- Artal-Sanz, M.; Tsang, W.Y.; Willems, E.M.; Grivell, L.A.; Lemire, B.D.; van der Spek, H.; Nijtmans, L.G. The mitochondrial prohibitin complex is essential for embryonic viability and germline function in caenorhabditis elegans. J. Biol. Chem. 2003, 278, 32091–32099. [Google Scholar] [CrossRef] [Green Version]
- Merkwirth, C.; Dargazanli, S.; Tatsuta, T.; Geimer, S.; Lower, B.; Wunderlich, F.T.; von Kleist-Retzow, J.C.; Waisman, A.; Westermann, B.; Langer, T. Prohibitins control cell proliferation and apoptosis by regulating opa1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008, 22, 476–488. [Google Scholar] [CrossRef] [Green Version]

| Parameters | Group № | |||
|---|---|---|---|---|
| 1 (Control) | 2 (AX) | 3 (ISO) | 4 (ISO + AX) | |
| Vst2, ng-atom O min−1 mg−1 of protein | 7.25 ± 0.46 | 8.33 ± 0.47 | 4.52 ± 0.28 * | 7.11 ± 0.32 # |
| Vst3, ng-atom O min−1 mg−1 of protein | 43.70 ± 2.28 | 48.71 ± 4.11 | 34.36 ± 4.01 * | 40.98 ± 2.75 # |
| Vst4, ng-atom O min−1 mg−1 of protein | 7.48 ± 0.31 | 9.56 ± 0.37 | 12.58 ± 0.83 * | 8.51 ± 0.86 # |
| Respiratory control index | 5.84 ± 0.88 | 5.10 ± 0.51 | 2.73 ± 0.21 * | 4.81 ± 0.51 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krestinin, R.; Baburina, Y.; Odinokova, I.; Kruglov, A.; Sotnikova, L.; Krestinina, O. The Effect of Astaxanthin on Mitochondrial Dynamics in Rat Heart Mitochondria under ISO-Induced Injury. Antioxidants 2023, 12, 1247. https://doi.org/10.3390/antiox12061247
Krestinin R, Baburina Y, Odinokova I, Kruglov A, Sotnikova L, Krestinina O. The Effect of Astaxanthin on Mitochondrial Dynamics in Rat Heart Mitochondria under ISO-Induced Injury. Antioxidants. 2023; 12(6):1247. https://doi.org/10.3390/antiox12061247
Chicago/Turabian StyleKrestinin, Roman, Yulia Baburina, Irina Odinokova, Alexey Kruglov, Linda Sotnikova, and Olga Krestinina. 2023. "The Effect of Astaxanthin on Mitochondrial Dynamics in Rat Heart Mitochondria under ISO-Induced Injury" Antioxidants 12, no. 6: 1247. https://doi.org/10.3390/antiox12061247
APA StyleKrestinin, R., Baburina, Y., Odinokova, I., Kruglov, A., Sotnikova, L., & Krestinina, O. (2023). The Effect of Astaxanthin on Mitochondrial Dynamics in Rat Heart Mitochondria under ISO-Induced Injury. Antioxidants, 12(6), 1247. https://doi.org/10.3390/antiox12061247








