The Benefits of Nanosized Magnesium Oxide in Fish Megalobrama amblycephala: Evidence in Growth Performance, Redox Defense, Glucose Metabolism, and Magnesium Homeostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish, Diet, and the Experimental Procedure
2.2. Sample Collection
2.3. Analysis of Diets and Body Composition
2.4. Analysis of Plasma Metabolites
2.5. Analysis of the Oxidative Stress-Related Parameters
2.6. RNA Isolation and Quantitative Real-Time-PCR
2.7. The Western Blotting Assay
2.8. Statistical Analysis
3. Results
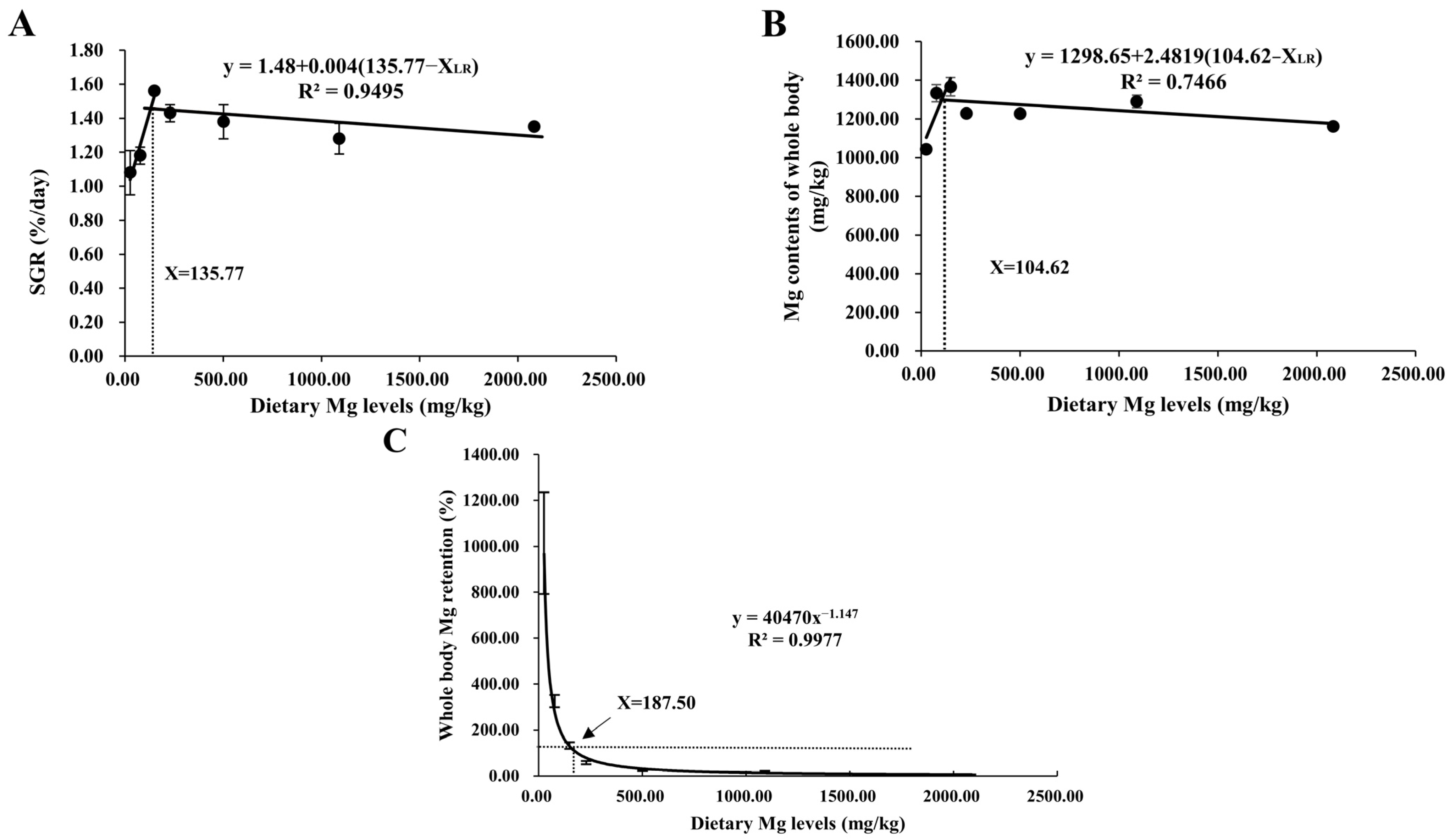
3.1. Growth Performance
3.2. Body Composition Analysis
3.3. Plasma Biochemical Parameters
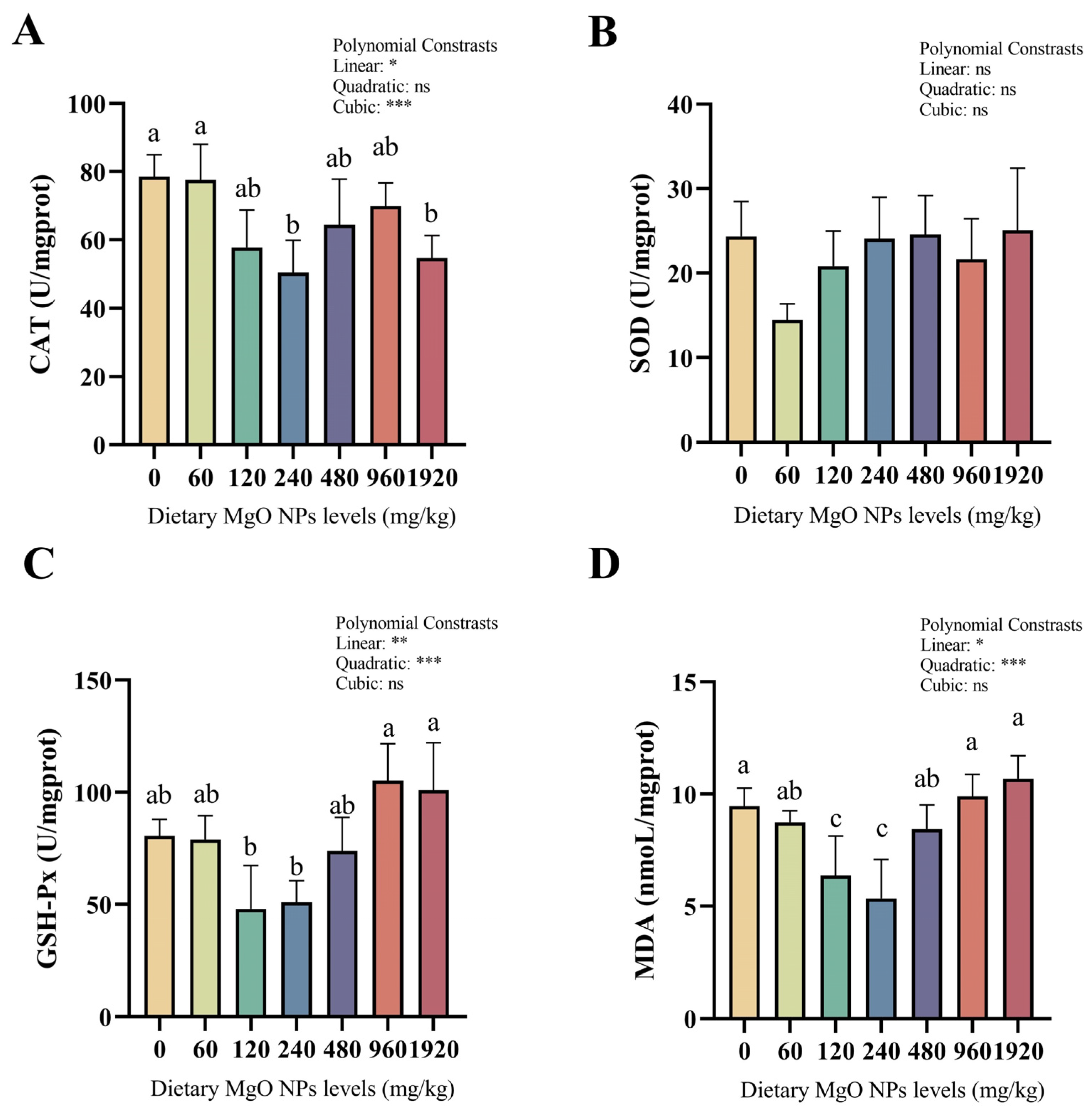
3.4. Antioxidant Enzyme Activities and the MDA Content
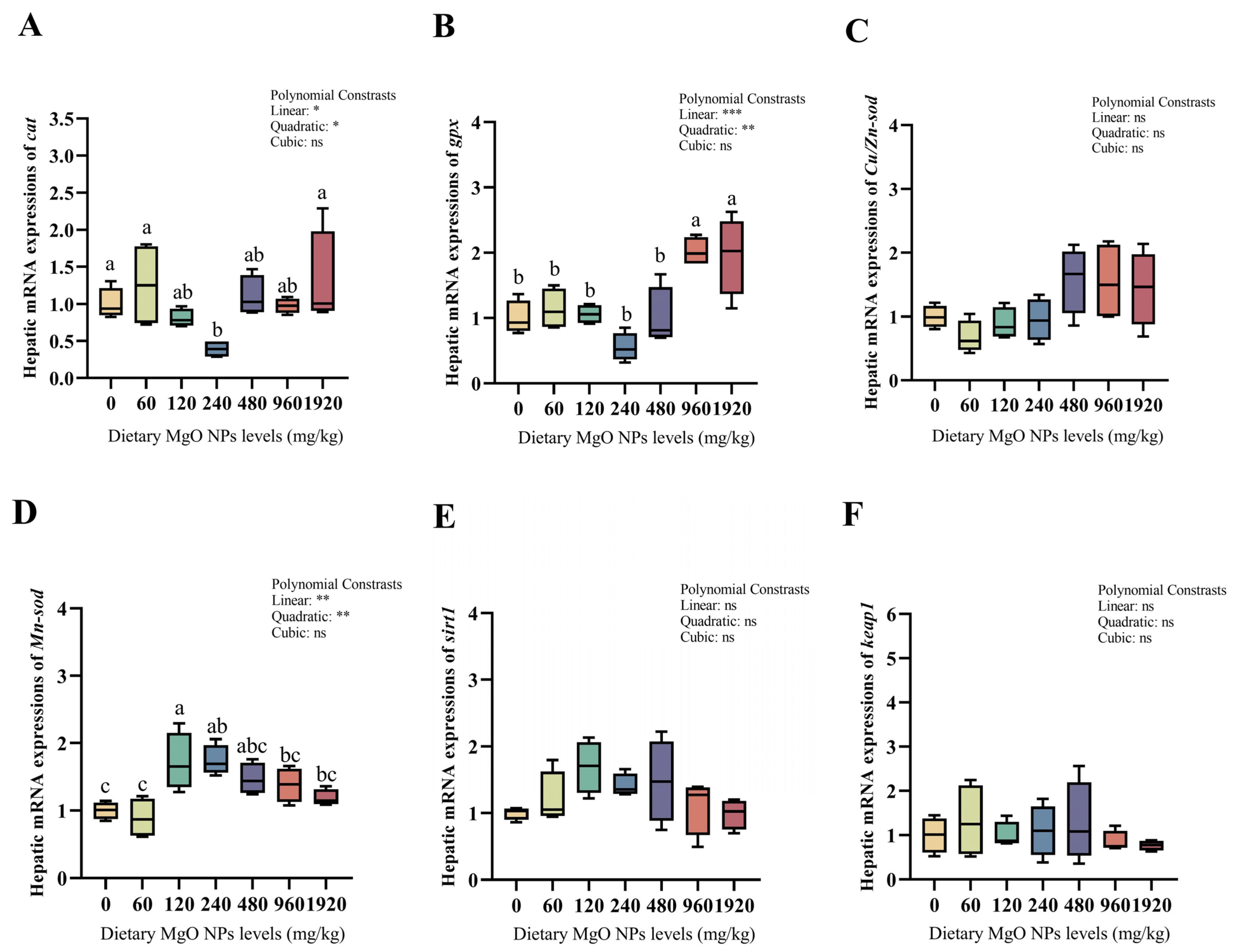
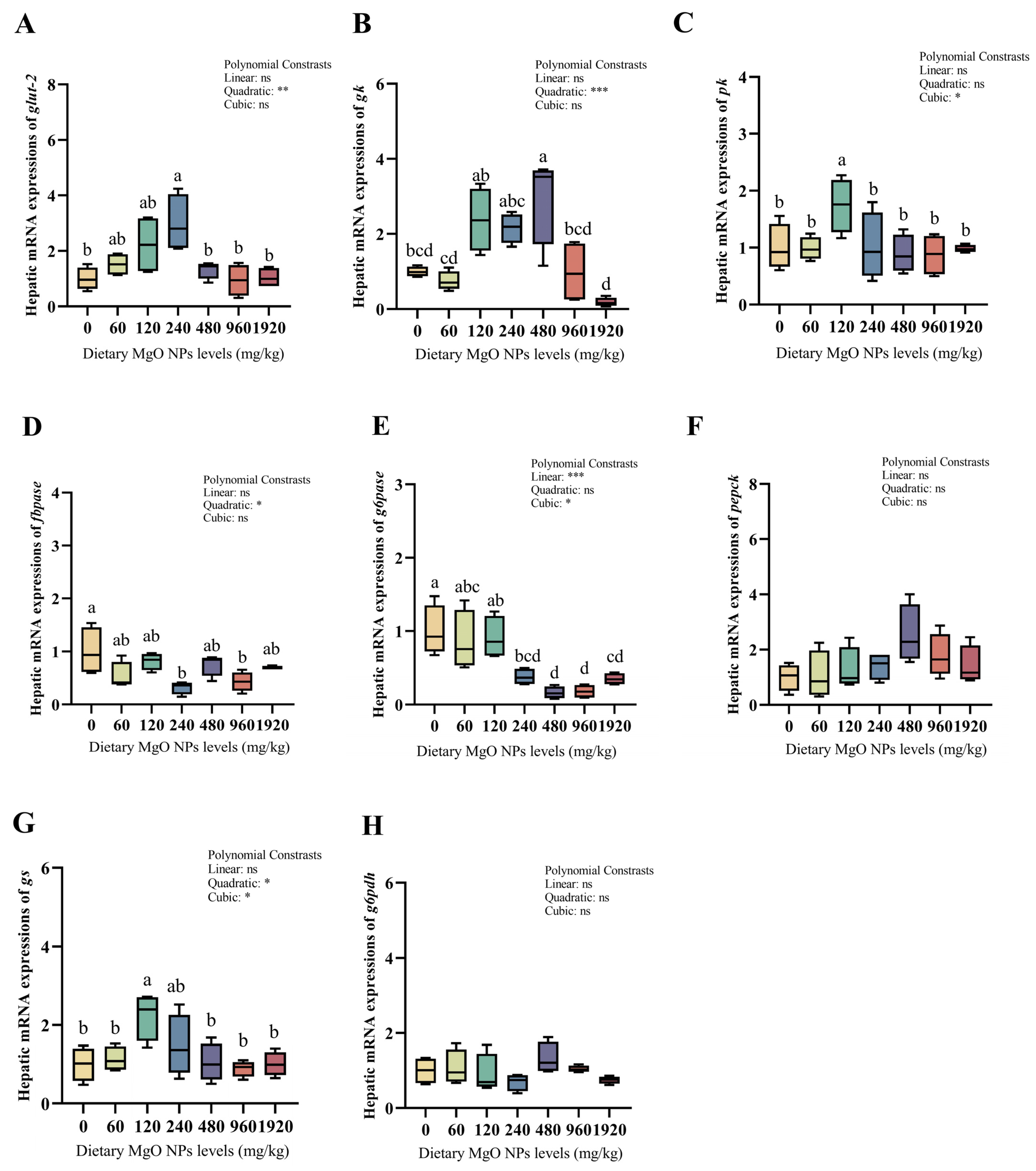
3.5. Redox Defense and Glucose Metabolism-Related Gene Expression
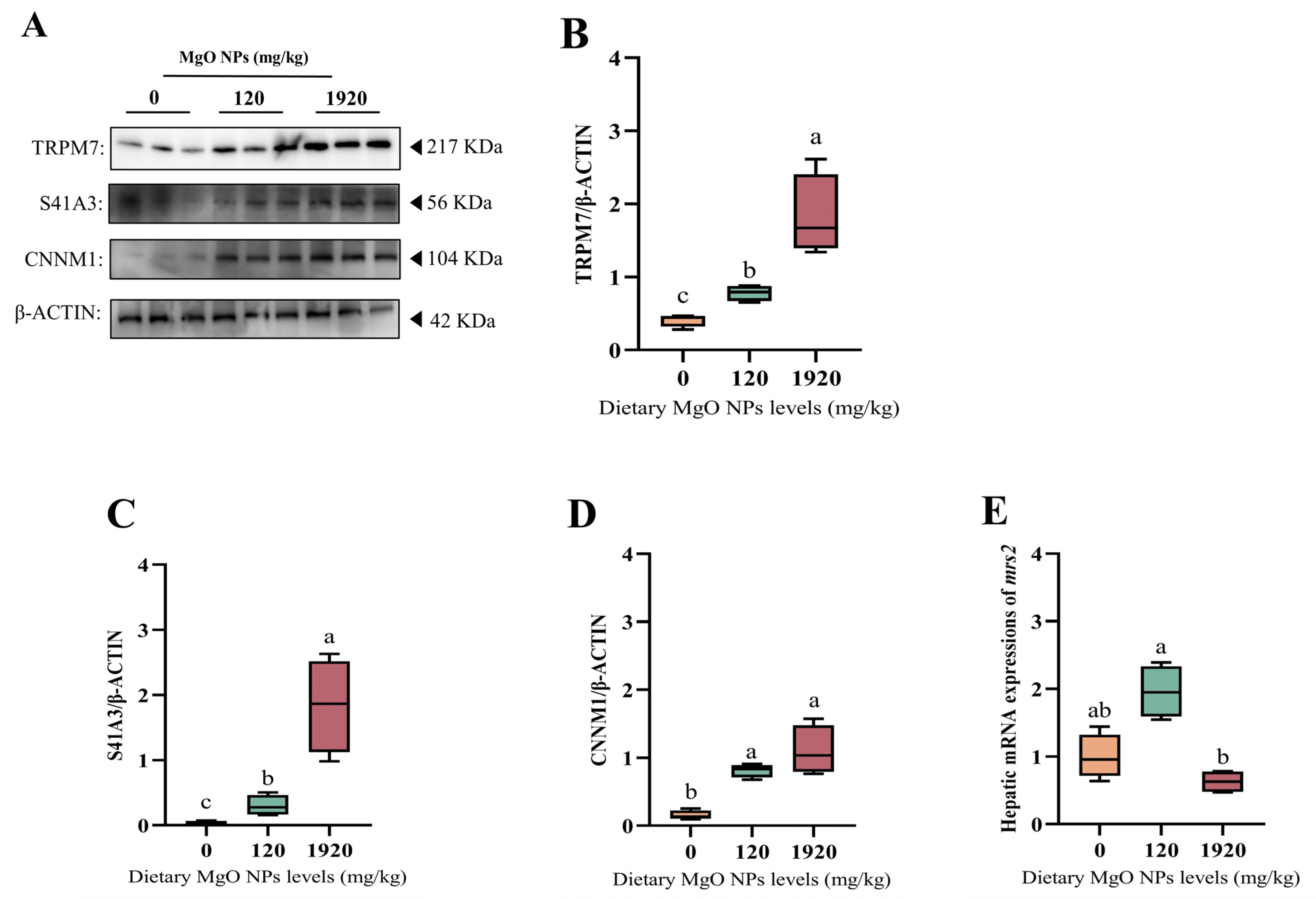
3.6. Magnesium Homeostasis-Related Protein and Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.B.; Luo, L.; Lin, S.M.; Chen, S.; Wang, Y.G.; Wen, H.; Hu, C.J. Dietary magnesium requirements of juvenile grass carp, Ctenopharyngodon idella. Aquacult. Nutr. 2011, 17, e691–e700. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.J.; Ai, Q.H.; Mai, K.S.; Zhang, W.B.; Zhang, L.; Zheng, S.X. Dietary selenium requirement for juvenile cobia, Rachycentron canadum L. Aquacult. Res. 2010, 41, e594–e601. [Google Scholar] [CrossRef]
- Kayne, L.H.; Lee, D.B.N. Intestinal magnesium absorption. Miner. Electrolyte Metab. 1993, 19, 210–217. [Google Scholar] [PubMed]
- Lin, Y.H.; Ku, C.Y.; Shiau, S.Y. Estimation of dietary magnesium requirements of juvenile tilapia, Oreochromis niloticus × Oreochromis aureus, reared in freshwater and seawater. Aquaculture 2013, 380, 47–51. [Google Scholar] [CrossRef]
- Davis, D.A.; Gatlin, D.M. Dietary mineral requirements of fish and marine crustaceans. Rev. Fish. Sci. 1996, 4, 75–99. [Google Scholar] [CrossRef]
- Lall, S.P. The minerals. In Fish Nutrition, 3rd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 259–308. [Google Scholar]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The involvement of Mg2+ in regulation of cellular and mitochondrial functions. Oxid. Med. Cell. Longev. 2017, 2017, 6797460. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Lei, B.; Zhuang, J.; Zhang, X.; Hu, C.; Cui, J.; Liu, Y. Magnesium-nitrogen co-doped carbon dots enhance plant growth through multifunctional regulation in photosynthesis. Chem. Eng. J. 2021, 442, 130114. [Google Scholar] [CrossRef]
- Shivakumar, K.; Kumar, B.P. Magnesium deficiency enhances oxidative stress and collagen synthesis in vivo in the aorta of rats. Int. J. Biochem. Cell Biol. 1997, 29, 1273–1278. [Google Scholar] [CrossRef]
- Tam, M.; Gomez, S.; Gonzalez-Gross, M.; Marcos, A. Possible roles of magnesium on the immune system. Eur. J. Clin. Nutr. 2003, 57, 1193–1197. [Google Scholar] [CrossRef]
- Takaya, J.; Higashino, H.; Kobayashi, Y. Intracellular magnesium and insulin resistance. Magnes. Res. 2004, 17, 126–136. [Google Scholar]
- Chen, H.Y.; Cheng, F.C.; Pan, H.C.; Hsu, J.C.; Wang, M.F. Magnesium enhances exercise performance via increasing glucose availability in the blood, muscle, and brain during exercise. PLoS ONE 2014, 9, e85486. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Keshavarz, M.; Sohanaki, H.; Dehpour, A.R.; Zahedi, A.S. Oral magnesium administration prevents vascular complications in STZ-diabetic rats. Life Sci. 2005, 76, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Kisters, K.; Gremmler, B.; Kozianka, J.; Hausberg, M. Magnesium deficiency and diabetes mellitus. Clin. Nephrol. 2006, 65, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch. Biochem. Biophys. 2007, 458, 40–47. [Google Scholar] [CrossRef]
- Reed, G.; Cefaratti, C.; Berti-Mattera, L.N.; Romani, A. Lack of insulin impairs Mg2+ homeostasis and transport in cardiac cells of streptozotocin-injected diabetic rats. J. Cell. Biochem. 2008, 104, 1034–1053. [Google Scholar] [CrossRef]
- Wei, C.C.; Wu, K.; Gao, Y.; Zhang, L.H.; Li, D.D.; Luo, Z. Magnesium reduces hepatic lipid accumulation in yellow catfish (Pelteobagrus fulvidraco) and modulates lipogenesis and lipolysis via PPARA, JAK-STAT, and AMPK pathways in hepatocytes. J. Nutr. 2017, 147, 1070–1078. [Google Scholar] [CrossRef]
- Bao, S.T.; Liu, X.C.; Huang, X.P.; Guan, J.F.; Xie, D.Z.; Li, S.A.; Xu, C. Magnesium supplementation in high carbohydrate diets: Implications on growth, muscle fiber development and flesh quality of Megalobrama amblycephala. Aquacult. Rep. 2022, 23, 101039. [Google Scholar] [CrossRef]
- Zhang, C.X.; Huang, F.; Li, J.; Wang, L.; Song, K.; Mai, K.S. Interactive effects of dietary magnesium and vitamin E on growth performance, body composition, blood parameters and antioxidant status in Japanese seabass (Lateolabrax japanicus). Aquacult. Nutr. 2016, 22, 708–722. [Google Scholar] [CrossRef]
- Deilamy, P.H.; Mousavi, S.M.; Zakeri, M.; Keyvanshokooh, S.; Kochanian, P. Synergistic effects of selenium and magnesium nanoparticles on growth, digestive enzymes, some serum biochemical parameters and immunity of Asian sea bass (Lates calcarifer). Biol. Trace Elem. Res. 2021, 199, 3102–3111. [Google Scholar] [CrossRef]
- Shaukat, A.; Anwar, H.; Mahmood, A.; Hussain, G.; Rasul, A.; Ijaz, M.U.; Faisal, M.N.; Ibrahim, M.; Ali, A. Synthesis cum characterization of MgO and MnO nanoparticles and their assessment as antidiabetic and antioxidative agents in diabetic rat model. Phys. B Condens. Matter 2021, 602, 412570. [Google Scholar] [CrossRef]
- Shaukat, A.; Hussain, G.; Irfan, S.; Ijaz, M.U.; Anwar, H. Therapeutic potential of MgO and MnO nanoparticles within the context of thyroid profile and pancreatic histology in a diabetic rat model. Dose Response 2022, 20, 15593258221128743. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K.; Law, M.C. Cytotoxicity analysis of morphologically different sol-gel-synthesized MgO nanoparticles and their in vitro insulin resistance reversal ability in adipose cells. Appl. Biochem. Biotechnol. 2020, 190, 1385–1410. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gautam, A.; Kumar, V.; Guleria, P. In vitro exposed magnesium oxide nanoparticles enhanced the growth of legume Macrotyloma uniflorum. Environ. Sci. Pollut. Res. Int. 2022, 29, 13635–13645. [Google Scholar] [CrossRef]
- Alkhudhayri, A.; Thagfan, F.A.; Al-Quraishy, S.; Abdel-Gaber, R.; Dkhil, M.A. Assessment of the oxidative status and goblet cell response during eimeriosis and after treatment of mice with magnesium oxide nanoparticles. Saudi. J. Biol. Sci. 2022, 29, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Chinese Fisheries Year Book; China Agriculture Press: Beijing, China, 2021. [Google Scholar]
- Dong, Q.; Nie, C.H.; Wu, Y.M.; Zhang, D.Y.; Wang, X.D.; Tu, T.; Jin, J.; Tian, Z.Y.; Liu, J.Q.; Xiao, Z.Y.; et al. Generation of blunt snout bream without intermuscular bones by runx2b gene mutation. Aquaculture 2023, 567, 739263. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of superoxide dismutase anion radical in autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 42, 469–474. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar]
- Bradford, M. A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gao, Z.X.; Luo, W.; Liu, H.; Zeng, C.; Liu, X.; Yi, S.; Wang, W. Transcriptome analysis and SSR/SNP markers information of the blunt snout bream (Megalobrama amblycephala). PLoS ONE 2012, 7, e42637. [Google Scholar] [CrossRef] [PubMed]
- Poppi, D.A.; Moore, S.S.; Glencross, B.D. Redefining the requirement for total sulfur amino acids in the diet of barramundi (Lates calcarifer) including assessment of the cystine replacement value. Aquaculture 2017, 471, 213–222. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Fan, Z.; Wu, D.; Li, J.N.; Wang, L.S. Dietary magnesium requirement on dietary minerals and physiological function of juvenile hybrid sturgeon (Acipenser schrenckii♀ × Acipenser baerii♂). Aquacult. Int. 2021, 29, 1697–1709. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirement of Fish and Shrimp; National Academic Press: Washington, DC, USA, 2011. [Google Scholar]
- Liang, J.J.; Tian, L.X.; Liu, Y.J.; Yang, H.J.; Liang, G.Y. Dietary magnesium requirement and effects on growth and tissue magnesium content of juvenile grass carp (Ctenopharyngodon idella). Aquacult. Nutr. 2012, 18, 56–64. [Google Scholar] [CrossRef]
- Shim, K.F.; Ng, S.H. Magnesium requirement of the guppy (Poecilia reticulata Peters). Aquaculture 1988, 73, 131–141. [Google Scholar] [CrossRef]
- Srinivasan, V.; Bhavan, P.S.; Rajkumar, G.; Satgurunathan, T.; Muralisankar, T. Dietary supplementation of magnesium oxide (MgO) nanoparticles for better survival and growth of the freshwater prawn Macrobrachium rosenbergii Post-larvae. Biol. Trace Elem. Res. 2017, 177, 196–208. [Google Scholar] [CrossRef]
- Dabrowska, H.; Meyer-Burgdorff, K.H.; Gunther, K.D. Magnesium status in freshwater fish, common carp (Cyprinus carpio, L.) and the dietary protein-magnesium interaction. Fish Physiol. Biochem. 1991, 9, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Kandeepan, C. Dietary magnesium requirement of Cyprinus carpio VAR. communis. Indian J. Natur. Sci. 2013, 3, 1236–1243. [Google Scholar]
- Antony Jesu Prabhu, P.; Schrama, J.W.; Kaushik, S.J. Mineral requirements of fish: A systematic review. Rev. Aquac. 2016, 8, 172–219. [Google Scholar] [CrossRef]
- Nanduri, A.; Saleem, S.; Khalaf, M. Severe hypermagnesemia induced by magnesium oxide ingestion: A case series. Chest 2020, 158, A1016. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, S.M.; Huang, C.H. Dietary magnesium requirement of soft-shelled turtles, Pelodiscus sinensis, fed diets containing exogenous phytate. Aquaculture 2014, 432, 80–84. [Google Scholar] [CrossRef]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, S.R. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997, 16, 5572–5581. [Google Scholar] [CrossRef] [PubMed]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yañez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; Ooijen, G.V. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, N.; Bobby, Z.; Sridhar, M.G. Increased glycation of hemoglobin in chronic renal failure: Corrected. potential role of oxidative stress. Arch. Med. Res. 2008, 39, 277–284. [Google Scholar] [CrossRef]
- Corica, F.; Allegra, A.; Di Benedetto, A.; Giacobbe, M.S.; Romano, G.; Cucinotta, D. Effects of oral magnesium supplementation on plasma lipid concentrations in patients with non-insulin-dependent diabetes mellitus. Magnesium Res. 1994, 7, 43–47. [Google Scholar]
- Gueux, E.; Mazur, A.; Cardot, P.; Rayssiguier, Y. Magnesium deficiency affects plasma lipoprotein composition in rats. J. Nutr. 1991, 121, 1222–1227. [Google Scholar] [CrossRef]
- Garcia, L.A.; Dejong, S.C.; Martin, S.M.; Smith, R.S.; Buettner, G.R.; Kerber, R.E. Magnesium reduces free radicals in an in vivo coronary occlusion-reperfusion model. J. Am. Coll. Cardiol. 1998, 32, 536–539. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J.; Galioto, A.; Ferlisi, A.; Cani, C.; Malfa, L.; Pineo, A.; Paolisso, G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol. Asp. Med. 2003, 24, 39–52. [Google Scholar] [CrossRef]
- Brown, K.A.; Didion, S.P.; Andresen, J.J.; Faraci, F.M. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: Evidence for MnSOD haploinsufficiency. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1941–1946. [Google Scholar] [CrossRef]
- Keen, C.L.; Ensunsa, J.L.; Clegg, M.S. Manganese metabolism in animals and humans including the toxicity of manganese. Met. Ions. Biol. Syst. 2000, 37, 89–121. [Google Scholar]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Gommers, L.M.; Hoenderop, J.G.; Bindels, R.J.; de Baaij, J.H. Hypomagnesemia in type 2 diabetes: A vicious circle? Diabetes 2016, 65, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, V.L.; MacDonald, P.E.; Klip, A. The cell biology of systemic insulin function. J. Cell Biol. 2018, 217, 2273–2289. [Google Scholar] [CrossRef]
- Kostov, K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: Focusing on the processes of insulin secretion and signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef] [PubMed]
- Garfinkle, L.; Garfinkle, D. Magnesium regulation of the glycolytic pathway and the enzymes involved. Magnesium 1985, 4, 60–72. [Google Scholar]
- Niemeyer, H.; Cardenas, M.L.; Rabajille, E.; Ureta, T.; Clark-Turri, L.; Penaranda, J. Sigmoidal Kinetics of glucokinase. Enzyme 1975, 20, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Benkovic, S.J.; deMaine, M. Mechanism of action of fructose 1, 6-bisphosphatase. Adv. Enzymol. Relat. Areas Mol. Biol. 1982, 53, 45–82. [Google Scholar]
- Armoni, M.; Harel, C.; Karnieli, E. Transcriptional regulation of the GLUT4 gene: From PPAR-γ and FOXO1 to FFA and inflammation. Trends Endocrinol. Metab. 2007, 18, 100–107. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7-Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta 2007, 1772, 813–821. [Google Scholar] [CrossRef]
- Chiang, Y.F.; Chen, H.Y.; Lee, I.T.; Chien, L.S.; Huang, J.H.; Kolisek, M.; Cheng, F.C.; Tsai, S.W. Magnesium-responsive genes are downregulated in diabetic patients after a three-month exercise program on a bicycle ergometer. J. Chin. Med. Assoc. 2019, 82, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Tabata, S.; Shindo, Y.; Hotta, K.; Suzuki, K.; Soga, T.; Oka, K. Mitochondrial Mg2+ homeostasis decides cellular energy metabolism and vulnerability to stress. Sci. Rep. 2016, 6, 30027. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Contents (%) | Proximate Analysis | Contents |
|---|---|---|---|
| Fish meal | 3 | Moisture (%) | 9.54 |
| Casein | 30 | Crude protein (%) | 31.16 |
| Gelatin | 7.5 | Ether extract (%) | 6.86 |
| Corn starch | 38.3 | Crude fiber (%) | 14.76 |
| Fish oil | 3.1 | Ash (%) | 3.49 |
| Soybean oil | 3.1 | Energy (MJ/kg) | 20.73 |
| Cellulose | 10 | ||
| Premix without magnesium | 1.2 | ||
| Calcium biphosphate | 1.8 | ||
| Carboxymethyl cellulose | 2 |
| Target Genes | Forward (5′-3′) | Reverse (5′-3′) | Accession Numbers or Reference |
|---|---|---|---|
| glut-2 | ACGCACCCGATGTGAAAGT | TTGGACAGCAGCATTGATT | KC513421.1 |
| gk | AAAATGCTGCCCACTTAT | AATGCCCTTATCCAAATC | KJ141202.1 |
| pk | GCCGAGAAAGTCTTCATCGCACAG | CGTCCAGAACCGCATTAGCCAC | [34] |
| fbpase | TACCCAGATGTCACAGAAT | CACTCATACAACAGCCTCA | KJ743995.1 |
| g6pase | TGAGACCCGGTTTTATGGAG | CATGCAGACCACCAGCTCTA | [34] |
| pepck | TGGCCCGTGTGGAGAGTAAAA | ATGTGTTCTGCCAGCCAG | [34] |
| gs | CCTCCAGTAACAACTCACAACA | CAGATAGATTGGTGGTTACGC | [34] |
| g6pdh | AGGTAAAGGTGCTGAAGT | AAATGTAGCCTGAGTGGA | KJ743994.1 |
| cat | CAGTGCTCCTGATACCCAGC | TTCTGACACAGACGCTCTCG | XM_048158628.1 |
| Cu/Zn-sod | AGTTGCCATGTGCACTTTTCT | AGGTGCTAGTCGAGTGTTAGG | KF479046.1 |
| Mn-sod | AGCTGCACCACAGCAAGCAC | TCCTCCACCATTCGGTGACA | KF195932.1 |
| gpx | GAACGCCCACCCTCTGTTTG | CGATGTCATTCCGGTTCACG | KF378713.1 |
| sirt1 | TCGGTTCATTCAGCAGCACA | ATGATGATCTGCCACAGCGT | MT518159.1 |
| keap1 | AATATCCGCCGGCTGTGTAG | TGAGTCCGAGGTGTTTCGTG | XM_048200093.1 |
| ef1-α | CTTCTCAGGCTGACTGTGC | CCGCTAGCATTACCCTCC | X77689.1 |
| Dietary MgO NPs Levels (mg/kg) | IW (g) | SR (%) | FW (g) | SGR (%/d) | FI (g per Fish) | FCR | PER |
|---|---|---|---|---|---|---|---|
| 0 | 12.14 ± 0.22 | 57.14 ± 4.12 b | 30.45 ± 3.67 c | 1.08 ± 0.13 b | 46.40 ± 8.29 c | 2.55 ± 0.04 b | 1.97 ± 0.13 ab |
| 60 | 12.24 ± 0.29 | 71.43 ± 8.25 ab | 32.92 ± 1.55 bc | 1.18 ± 0.05 b | 51.15 ± 2.41 c | 2.48 ± 0.06 b | 1.91 ± 0.01 ab |
| 120 | 12.57 ± 0.22 | 90.48 ± 2.38 a | 46.45 ± 2.01 a | 1.56 ± 0.02 a | 61.79 ± 2.84 bc | 1.83 ± 0.10 c | 2.24 ± 0.12 a |
| 240 | 12.48 ± 0.05 | 88.10 ± 2.38 a | 41.56 ± 2.82 ab | 1.43 ± 0.05 ab | 81.48 ± 3.42 ab | 2.83 ± 0.24 b | 1.51 ± 0.11 bc |
| 480 | 12.38 ± 0.27 | 83.33 ± 6.30 a | 39.77 ± 2.59 abc | 1.38 ± 0.10 ab | 68.61 ± 5.87 bc | 2.51 ± 0.06 b | 1.72 ± 0.04 b |
| 960 | 12.57 ± 0.08 | 59.52 ± 2.38 b | 36.97 ± 4.26 abc | 1.28 ± 0.09 ab | 67.95 ± 9.88 bc | 2.76 ± 0.14 b | 1.61 ± 0.14 bc |
| 1920 | 12.57 ± 0.14 | 57.14 ± 4.12 b | 38.95 ± 0.58 abc | 1.35 ± 0.01 ab | 97.70 ± 4.38 a | 3.70 ± 0.18 a | 1.20 ± 0.06 c |
| Polynomial contrasts | |||||||
| Linear | ns | ns | * | * | *** | *** | *** |
| Quadratic | ns | *** | ** | ** | ns | *** | * |
| Cubic | ns | ns | ns | ns | ns | ns | ns |
| Dietary MgO NPs Levels (mg/kg) | Moisture (%) | Crude Protein (%) | Crude Lipid (%) | Ash (%) | Mg Contents (mg/kg) | Mg Retention (%) |
|---|---|---|---|---|---|---|
| 0 | 70.32 ± 1.25 | 14.48 ± 0.72 b | 10.81 ± 0.66 | 5.44 ± 0.29 | 1043.61 ± 13.48 c | 1013.21 ± 221.45 a |
| 60 | 71.53 ± 1.09 | 16.20 ± 0.06 ab | 11.53 ± 0.29 | 4.56 ± 0.27 | 1333.10 ± 43.79 a | 325.64 ± 26.88 b |
| 120 | 72.07 ± 1.81 | 17.70 ± 0.58 a | 7.81 ± 0.41 | 5.26 ± 0.40 | 1366.26 ± 47.43 a | 133.11 ± 14.19 b |
| 240 | 71.09 ± 1.79 | 15.40 ± 0.58 ab | 8.51 ± 1.55 | 5.28 ± 0.12 | 1229.08 ± 16.62 ab | 59.55 ± 7.12 b |
| 480 | 70.67 ± 0.59 | 16.01 ± 0.41 ab | 9.76 ± 0.73 | 5.35 ± 0.47 | 1228.01 ± 12.05 ab | 23.51 ± 0.76 b |
| 960 | 70.95 ± 0.96 | 16.52 ± 0.21 ab | 9.69 ± 0.94 | 4.39 ± 0.24 | 1290.08 ± 32.85 ab | 21.47 ± 1.65 b |
| 1920 | 71.36 ± 2.20 | 15.66 ± 0.26 ab | 10.02 ± 1.86 | 4.37 ± 0.51 | 1162.20 ± 14.63 bc | 5.79 ± 0.98 b |
| Polynomial contrasts | ||||||
| Linear | ns | ns | ns | ns | ns | *** |
| Quadratic | ns | * | ns | ns | *** | *** |
| Cubic | ns | ns | ns | ns | *** | * |
| Dietary MgO NPs Levels (mg/kg) | GLU (mmol/L) | GSP (umol/L) | TCHO (mmol/L) | TRIG (mmol/L) | Mg (mmol/L) |
|---|---|---|---|---|---|
| 0 | 5.35 ± 0.60 a | 381.75 ± 21.07 a | 5.44 ± 0.29 | 3.23 ± 0.16 a | 1.21 ± 0.01 c |
| 60 | 5.05 ± 0.50 ab | 342.75 ± 11.40 a | 4.56 ± 0.27 | 2.26 ± 0.19 b | 1.25 ± 0.02 c |
| 120 | 3.67 ± 0.34 abc | 189.00 ± 3.85 b | 5.26 ± 0.40 | 1.96 ± 0.21 b | 1.38 ± 0.03 bc |
| 240 | 3.32 ± 0.28 bc | 197.00 ± 5.31 b | 5.28 ± 0.12 | 1.60 ± 0.23 b | 1.41 ± 0.03 bc |
| 480 | 5.13 ± 0.47 ab | 338.50 ± 49.12 a | 5.35 ± 0.47 | 2.06 ± 0.21 b | 1.48 ± 0.05 b |
| 960 | 5.28 ± 0.22 ab | 365.50 ± 28.61 a | 4.39 ± 0.24 | 2.29 ± 0.23 b | 1.75 ± 0.09 a |
| 1920 | 2.78 ± 0.72 c | 191.75 ± 8.64 b | 4.37 ± 0.51 | 1.58 ± 0.16 b | 1.41 ± 0.05 bc |
| Polynomial contrasts | |||||
| Linear | ns | ns | ns | * | *** |
| Quadratic | *** | *** | ns | ns | * |
| Cubic | ns | ns | ns | ns | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, Z.; Deng, Y.; He, C.; Liu, W.; Li, X. The Benefits of Nanosized Magnesium Oxide in Fish Megalobrama amblycephala: Evidence in Growth Performance, Redox Defense, Glucose Metabolism, and Magnesium Homeostasis. Antioxidants 2023, 12, 1350. https://doi.org/10.3390/antiox12071350
Zhang L, Liu Z, Deng Y, He C, Liu W, Li X. The Benefits of Nanosized Magnesium Oxide in Fish Megalobrama amblycephala: Evidence in Growth Performance, Redox Defense, Glucose Metabolism, and Magnesium Homeostasis. Antioxidants. 2023; 12(7):1350. https://doi.org/10.3390/antiox12071350
Chicago/Turabian StyleZhang, Ling, Zishang Liu, Ying Deng, Chaofan He, Wenbin Liu, and Xiangfei Li. 2023. "The Benefits of Nanosized Magnesium Oxide in Fish Megalobrama amblycephala: Evidence in Growth Performance, Redox Defense, Glucose Metabolism, and Magnesium Homeostasis" Antioxidants 12, no. 7: 1350. https://doi.org/10.3390/antiox12071350
APA StyleZhang, L., Liu, Z., Deng, Y., He, C., Liu, W., & Li, X. (2023). The Benefits of Nanosized Magnesium Oxide in Fish Megalobrama amblycephala: Evidence in Growth Performance, Redox Defense, Glucose Metabolism, and Magnesium Homeostasis. Antioxidants, 12(7), 1350. https://doi.org/10.3390/antiox12071350







