Neuroprotective and Antioxidant Properties of CholesteroNitrone ChN2 and QuinolylNitrone QN23 in an Experimental Model of Cerebral Ischemia: Involvement of Necrotic and Apoptotic Cell Death
Abstract
:1. Introduction
2. Materials and Methods
2.1. Neuroblastoma Cell Cultures
2.1.1. Treatment of Neuroblastoma Cell Cultures with Oligomycin–Rotenone (O-R)
2.1.2. Neuroblastoma Cell Cultures’ Exposure to Oxygen–Glucose Deprivation (OGD)
2.2. Evaluation of Cell Viability
2.3. Assessment of LDH Activity
2.4. Measurement of Caspase-3 Activity
2.5. Determination of Reactive Oxygen Species Formation
2.6. Statistical Analyses
3. Results
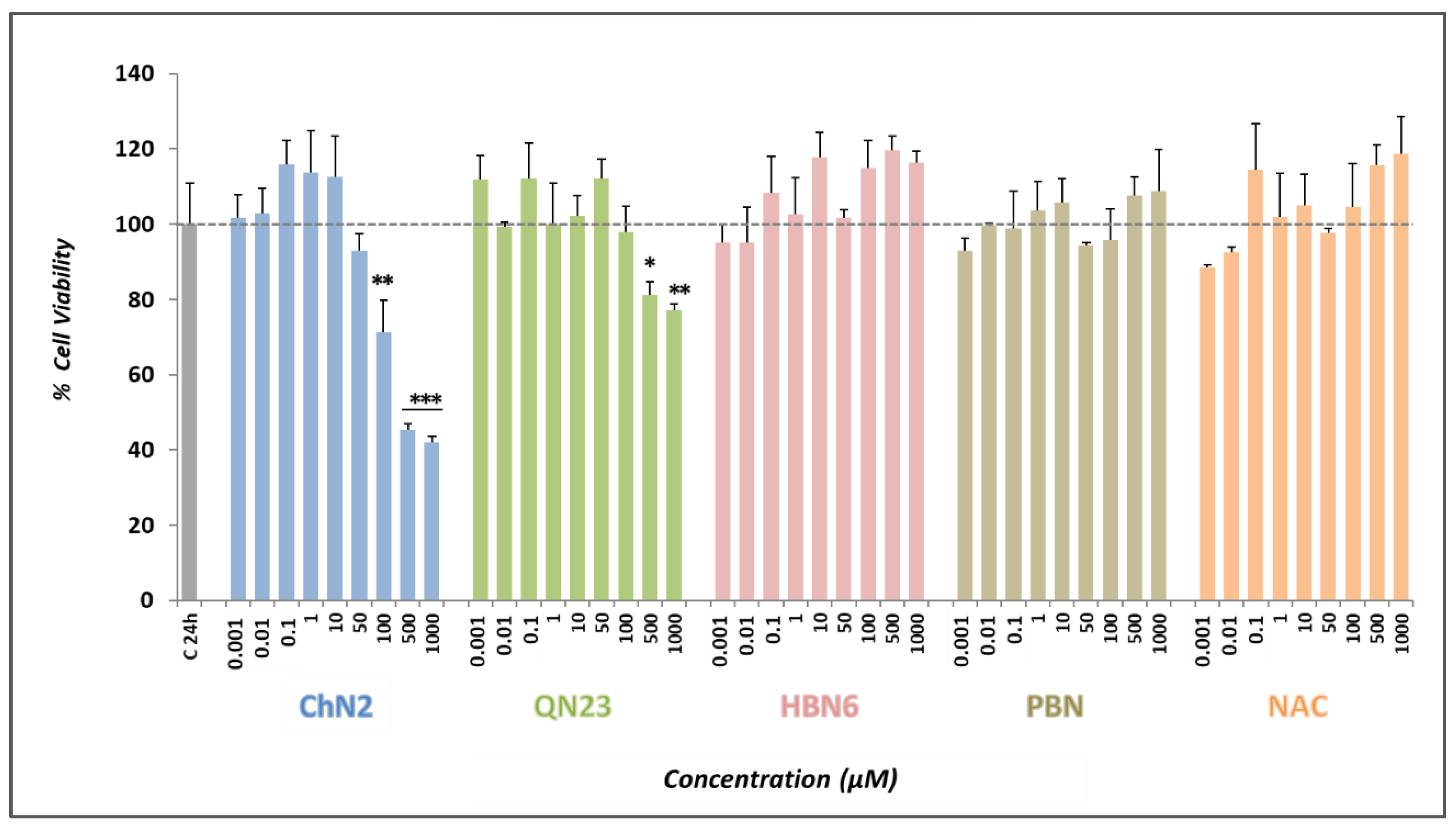
3.1. Basal Neurotoxicities of ChN2, QN23, HBN6, PBN, and NAC
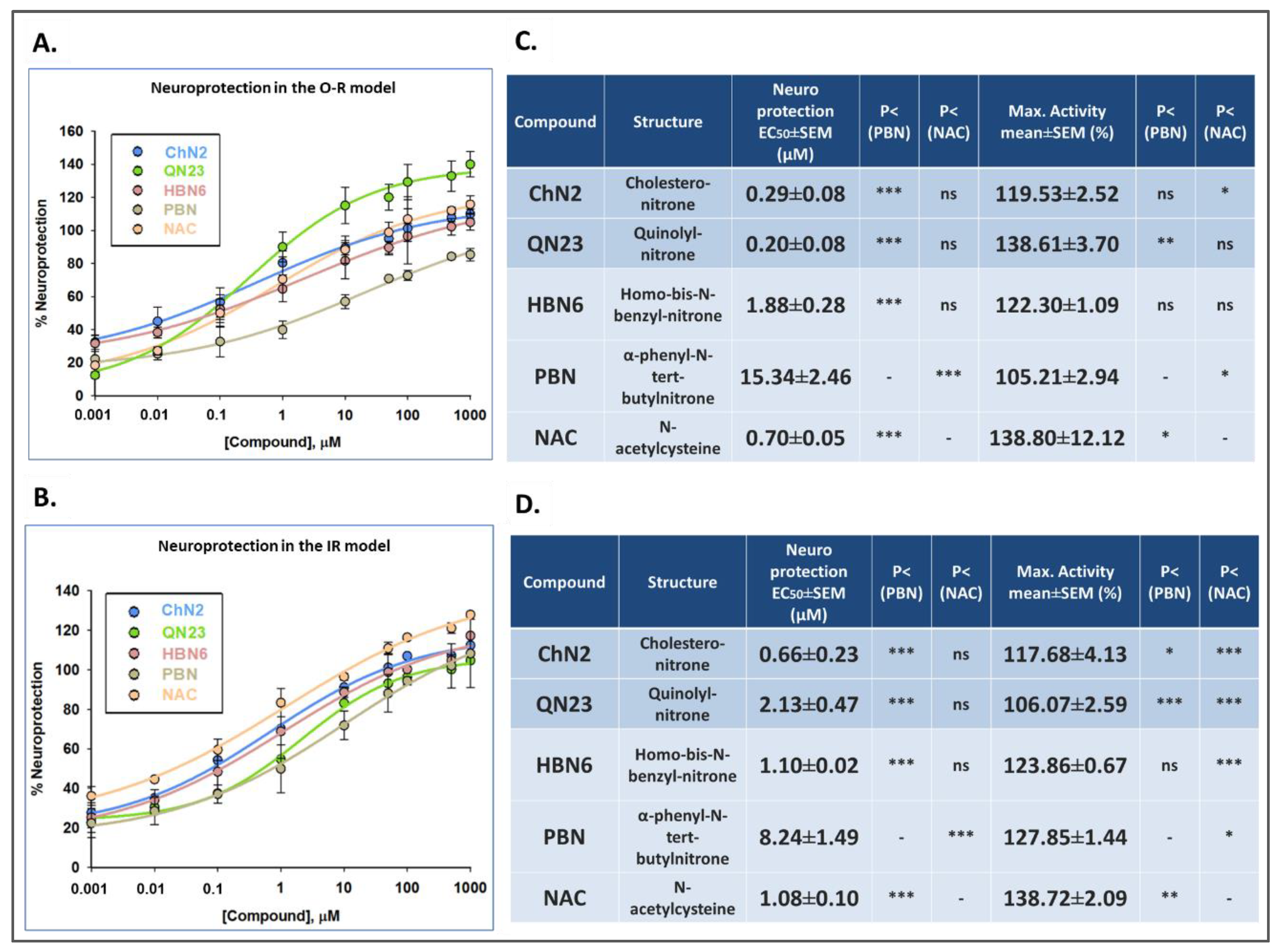
3.2. Neuroprotective Profiles of ChN2, QN23, HBN6, PBN, and NAC
3.2.1. Effect on Cell Viability
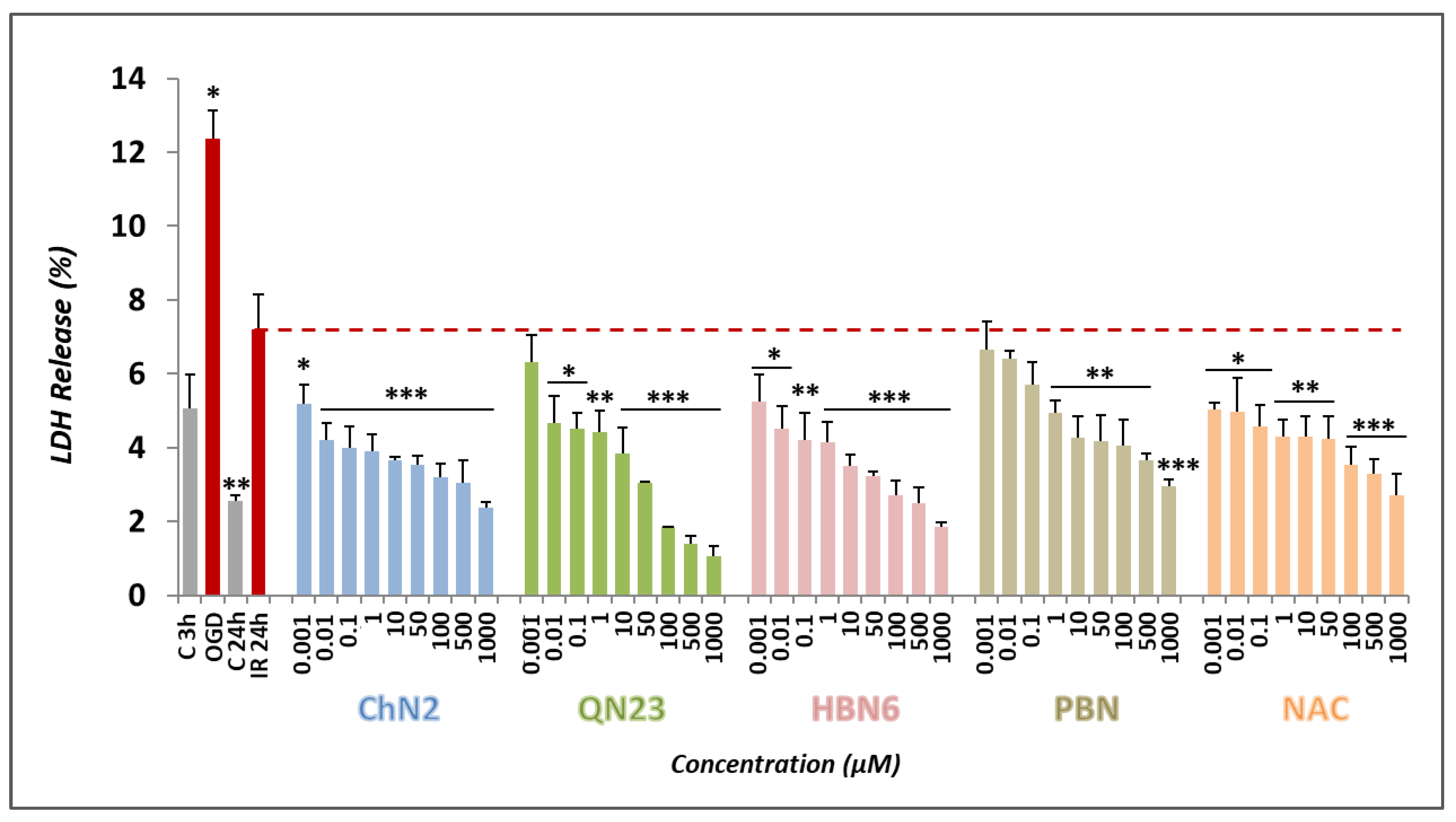
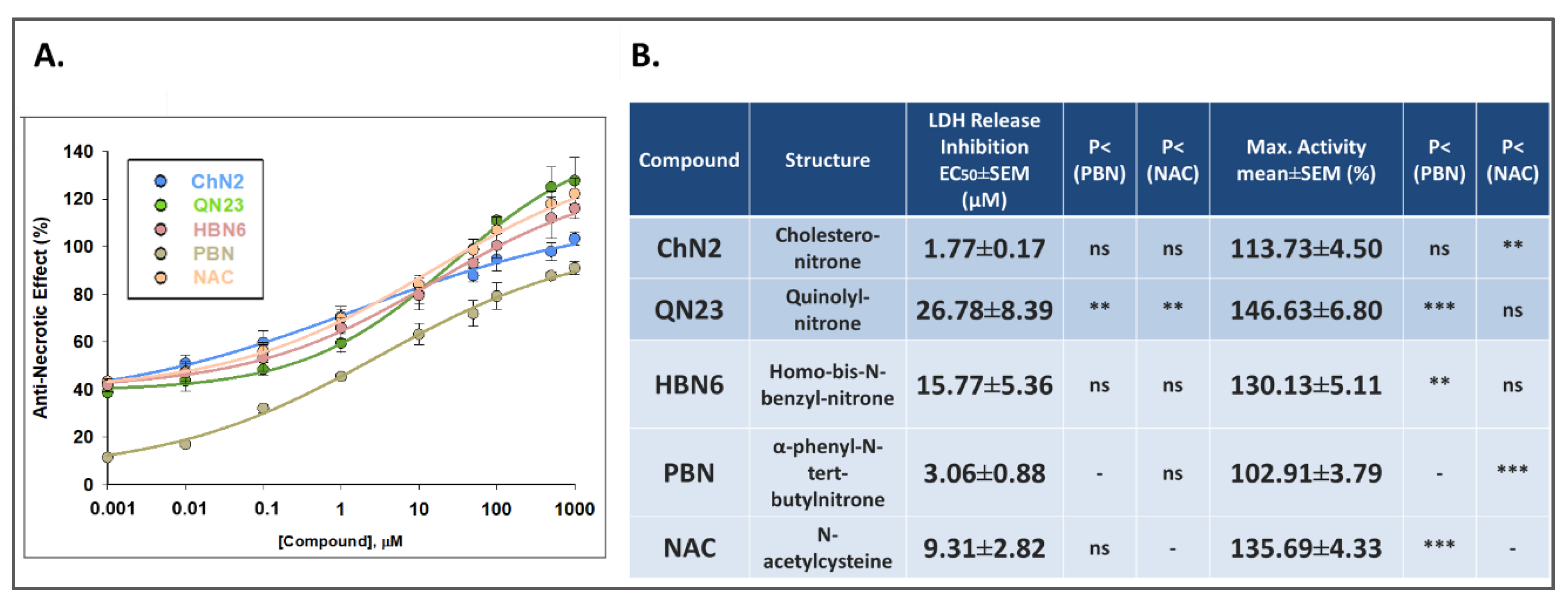
3.2.2. Effects on Necrotic Cell Death
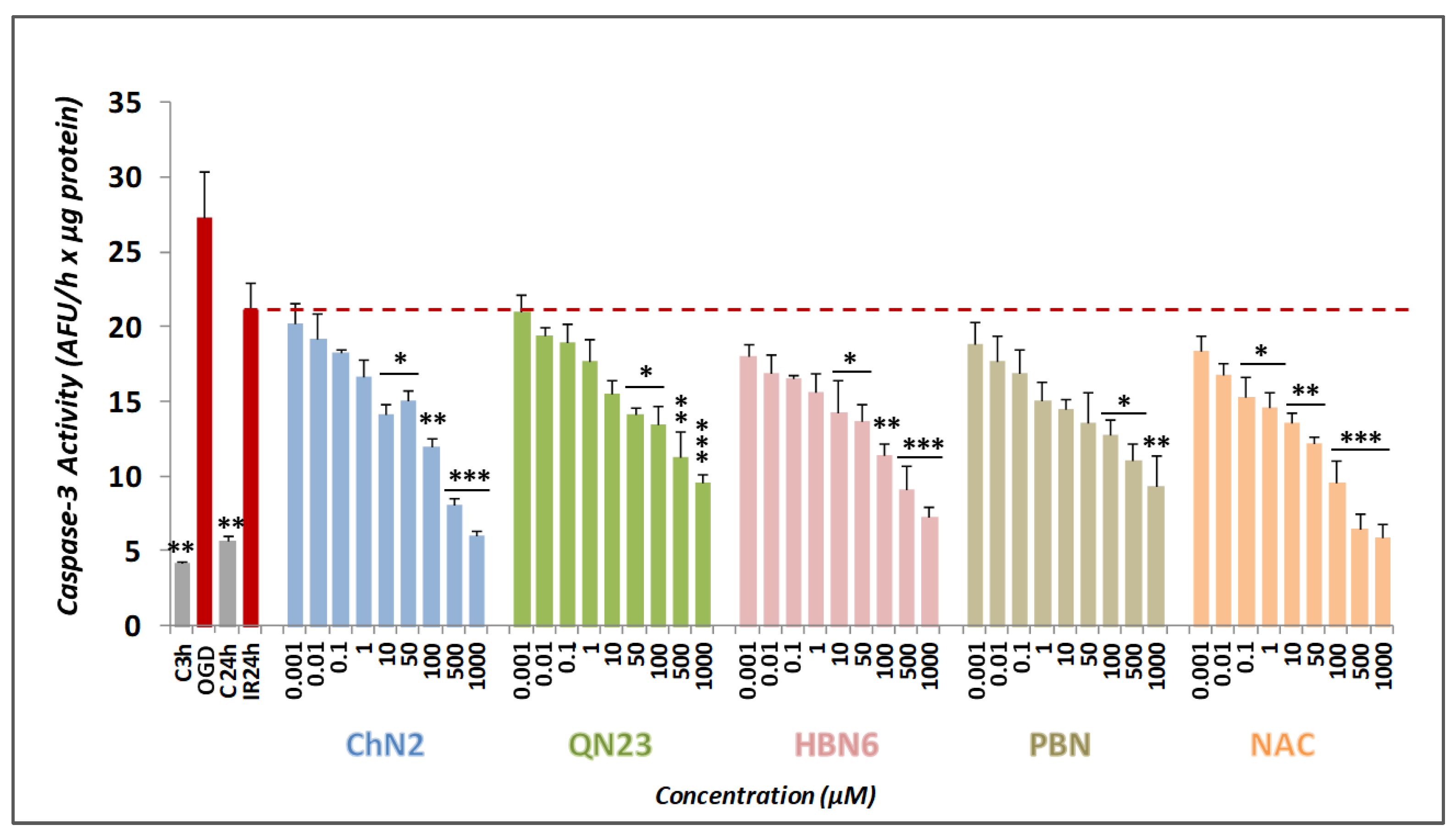
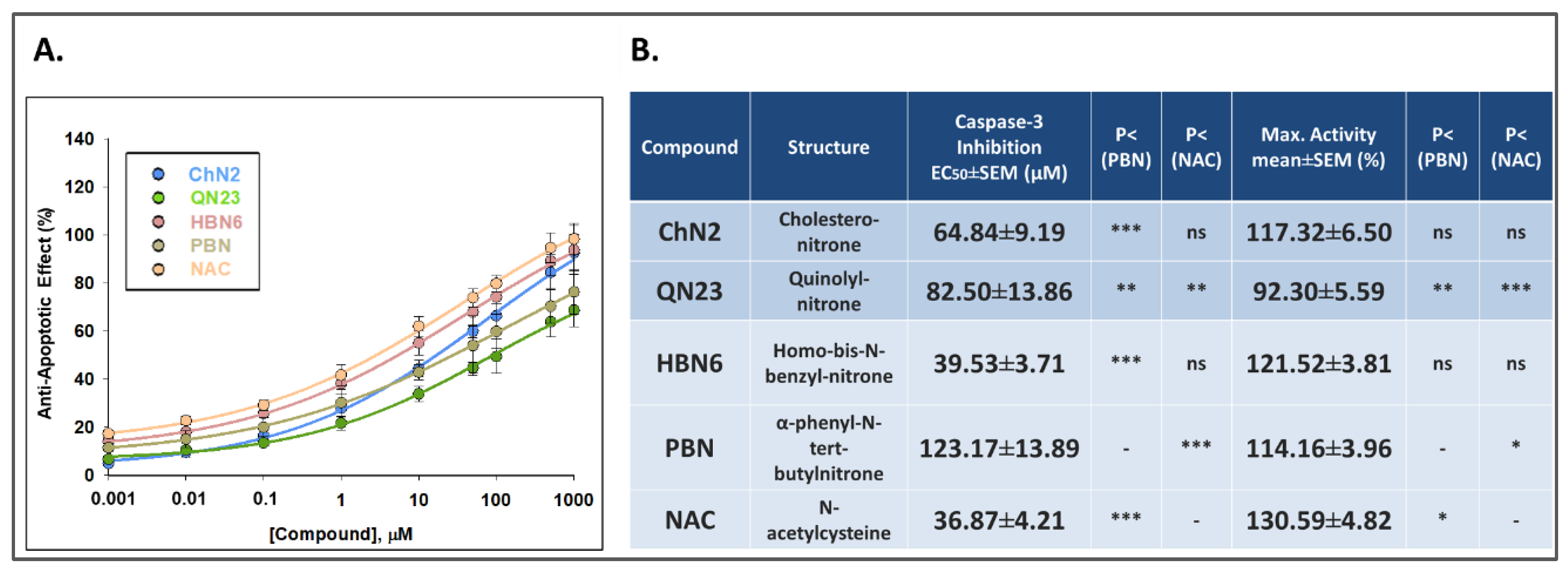
3.2.3. Effects on Apoptotic Cell Death
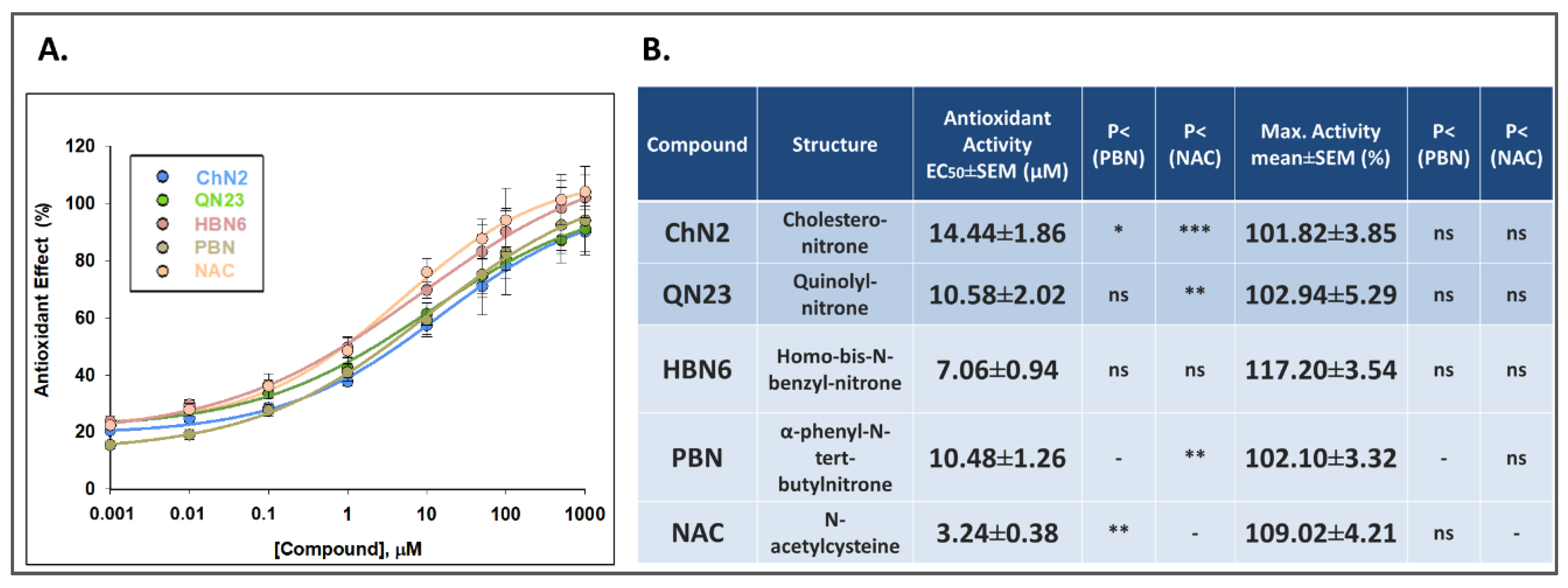
3.2.4. Antioxidant Effects
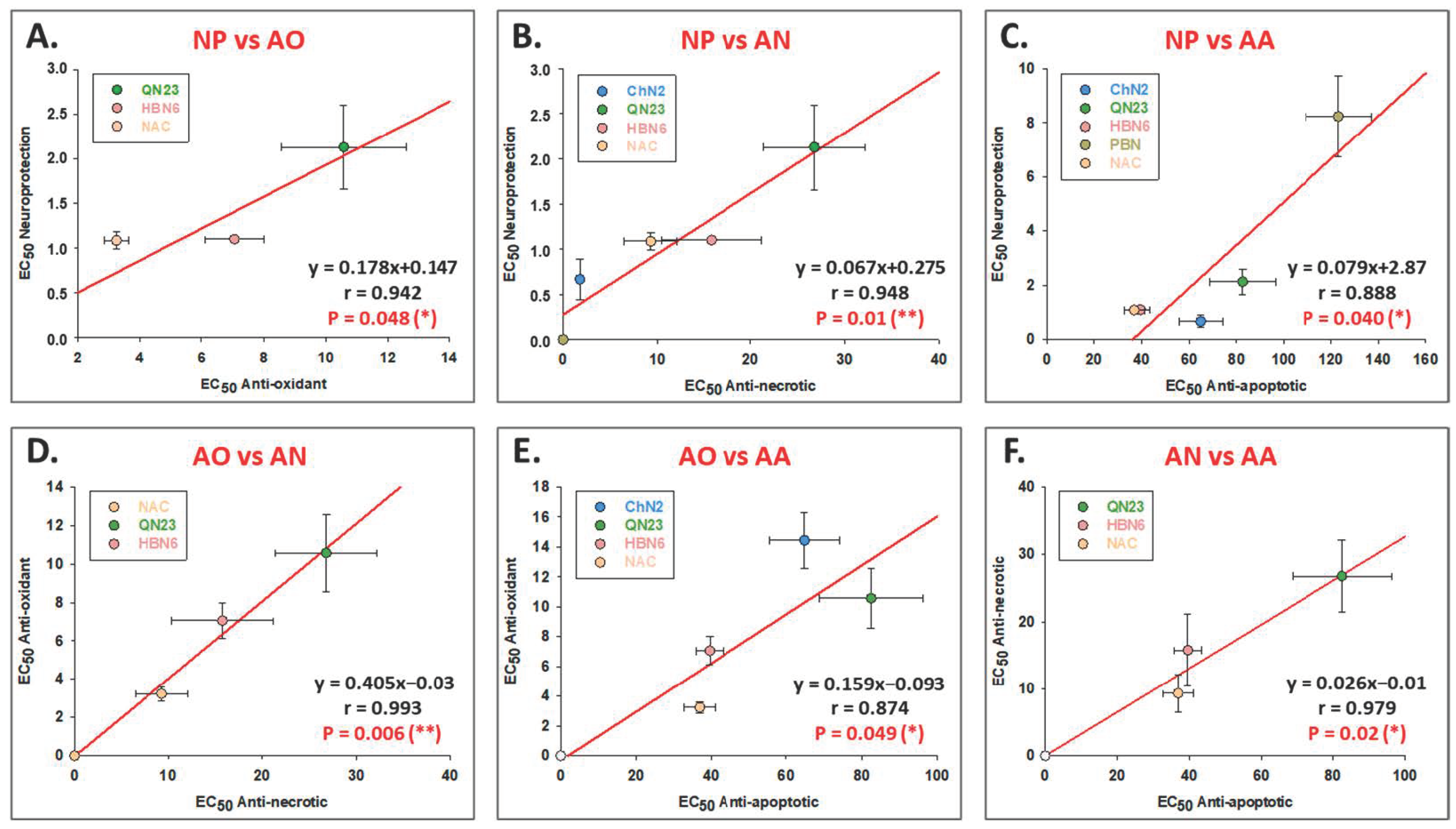
3.3. Correlation Analyses between the Different Neuroprotective Properties of ChN2, QN23, HBN6, PBN and NAC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Fernández-Gajardo, R.; Gutiérrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative Stress and Pathophysiology of Ischemic Stroke: Novel Therapeutic Opportunities. CNS Neurol. Disord. Drug. Targets 2013, 12, 698–714. [Google Scholar] [CrossRef]
- Martínez-Alonso, E.; Escobar-Peso, A.; Ayuso, M.I.; Gonzalo-Gobernado, R.; Chioua, M.; Montoya, J.J.; Montaner, J.; Fernández, I.; Marco-Contelles, J.; Alcázar, A. Characterization of a CholesteroNitrone (ISQ-201), a Novel Drug Candidate for the Treatment of Ischemic Stroke. Antioxidants 2020, 9, 291. [Google Scholar] [CrossRef] [Green Version]
- Brouns, R.; De Deyn, P.P. The complexity of neurobiological processes in acute ischemic stroke. Clin. Neurol. Neurosurg. 2009, 111, 483–495. [Google Scholar] [CrossRef]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Love, S. Oxidative stress in brain ischemia. Brain Pathol. 1999, 9, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef]
- Latour, L.L.; Kang, D.W.; Ezzeddine, M.A.; Chalela, J.A.; Warach, S. Early blood-brain barrier disruption in human focal brain ischemia. Ann. Neurol. 2004, 56, 468–477. [Google Scholar] [CrossRef]
- Chamorro, B.; Diez-Iriepa, D.; Merás-Sáiz, B.; Chioua, M.; García-Vieira, D.; Iriepa, I.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Martínez-Murillo, R.; González-Nieto, D.; et al. Synthesis, antioxidant properties and neuroprotection of α-phenyl-tert-butylnitrone derived HomoBisNitrones in in vitro and in vivo ischemia models. Sci. Rep. 2020, 10, 14150. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Morón-Oset, J.; Díaz-Castroverde, S.; García-Font, N.; Roncero, C.; López-Muñoz, F.; Marco Contelles, J.L.; Oset-Gasque, M.J. Neuroprotection by Phytoestrogens in the Model of Deprivation and Resupply of Oxygen and Glucose In Vitro: The Contribution of Autophagy and Related Signaling Mechanisms. Antioxidants 2020, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Q.; Guo, Y.; Li, X.; Zhang, G.-Q.; Li, P. Small molecules as modulators of regulated cell death against ischemia/reperfusion injury. Med. Res. Rev. 2022, 42, 2067–2101. [Google Scholar] [CrossRef] [PubMed]
- Tymianski, M. Novel Approaches to Neuroprotection Trials in Acute Ischemic Stroke. Stroke 2013, 44, 2942–2950. [Google Scholar] [CrossRef] [Green Version]
- Goenka, L.; Uppugunduri Satyanarayanab, C.R.; Kumar, S.; George, M. Neuroprotective agents in Acute Ischemic Stroke—A Reality Check. Biomed. Pharmacother. 2019, 109, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Savitz, S.I.; Baron, J.C.; Fisher, M. Stroke Treatment Academic Industry Roundtable X: Brain Cytoprotection Therapies in the Reperfusion Era. Stroke 2019, 50, 1026–1031. [Google Scholar] [CrossRef]
- Lyden, P.; Buchan, A.; Boltze, J.; Fisher, M. Top Priorities for Cerebroprotective Studies-A Paradigm Shift: Report From STAIR XI. Stroke 2021, 52, 3063–3071. [Google Scholar] [CrossRef]
- Samadi, A.; Soriano, E.; Revuelta, J.; Valderas, C.; Chioua, M.; Garrido, I.; Bartolomé, B.; Tomassolli, I.; Ismaili, L.; González-Lafuente, L.; et al. Synthesis, structure, theoretical and experimental in vitro antioxidant/pharmacological properties of a-aryl, N-alkylnitrones, as potential agents for the treatment of cerebral ischemia. Bioorg. Med. Chem. 2011, 19, 951–960. [Google Scholar] [CrossRef]
- Ayuso, M.I.; Chioua, M.; Martínez-Alonso, E.; Soriano, E.; Montaner, J.; Masjuán, J.; Hadjipavlou-Litina, D.J.; Marco-Contelles, J.; Alcázar, A. CholesteroNitrones for Stroke. J. Med. Chem. 2015, 58, 6704–6709. [Google Scholar] [CrossRef] [Green Version]
- Chioua, M.; Sucunza, D.; Soriano, E.; Hadjipavlou-Litina, D.; Alcázar, A.; Ayuso, A.; Oset-Gasque, M.J.; González, M.P.; Monjas, L.; Rodríguez-Franco, M.I.; et al. α-Aryl-N-alkyl nitrones, as potential agents for stroke treatment: Synthesis, theoretical calculations, antioxidant, anti-inflammatory, neuroprotective and brain-blood barrier permeability properties. J. Med. Chem 2012, 55, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Chioua, M.; Martínez-Alonso, E.; Gonzalo-Gobernado, R.; Ayuso, M.I.; Escobar-Peso, A.; Infantes, L.; Hadjipavlou-Litina, D.; Montoya, J.J.; Montaner, J.; Alcázar, A.; et al. New Quinolylnitrones for Stroke Therapy: Antioxidant and Neuroprotective (Z)-N-tert-Butyl-1-(2-chloro-6-methoxyquinolin-3-yl)methanimine Oxide as a New Lead-Compound for Ischemic Stroke Treatment. J. Med. Chem. 2019, 62, 2184–2201. [Google Scholar] [CrossRef]
- Chamorro, B.; Izquierdo-Bermejo, S.; Serrano, J.; Hadjipavlou-Litina, D.; Chioua, M.; López-Muñoz, F.; Marco-Contelles, J.; Martínez-Murillo, R.; Oset-Gasque, M.J. Neuroprotective and antioxidant properties of new quinolylnitrones in in vitro and in vivo cerebral ischemia models. Sci. Rep. 2023, 13, 2865. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alonso, E.; Escobar-Peso, A.; Aliena-Valero, A.; Torregrosa, G.; Chioua, M.; Fernández-Serra, R.; González-Nieto, D.; Ouahid, Y.; Salom, J.B.; Masjuan, J.; et al. Preclinical Characterization of Antioxidant Quinolyl Nitrone QN23 as a New Candidate for the Treatment of Ischemic Stroke. Antioxidants 2022, 11, 1186. [Google Scholar] [CrossRef]
- Diez-Iriepa, D.; Chamorro, B.; Talaván, M.; Chioua, M.; Iriepa, I.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Marco-Contelles, J.; Oset-Gasque, M.J. Homo-Tris-Nitrones Derived from α-Phenyl-N-tert-butylnitrone: Synthesis, Neuroprotection and Antioxidant Properties. Int. J. Mol. Sci. 2020, 21, 7949. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Mediavilla, L.; Cuarental, L.; Głowacka, I.E.; Marco-Contelles, J.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Oset-Gasque, M.J. Synthesis and Neuroprotective Properties of N-Substituted C-Dialkoxyphosphorylated Nitrones. ACS Omega 2019, 4, 8581–8587. [Google Scholar] [CrossRef] [Green Version]
- Chien-Hung, L.; Nicol, C.J.B.; Cheng, Y.-C.; Yen, C.; Wang, Y.-S.; Chiang, M.-C. Neuroprotective effects of resveratrol against oxygen glucose deprivation induced mitochondrial dysfunction by activation of AMPK in SH-SY5Y cells with 3D gelatin scaffold. Brain Res. 2020, 1726, 146492. [Google Scholar] [CrossRef]
- Holloway, P.M.; Gavins, F.N. Modeling ischemic stroke in vitro: Status quo and future perspectives. Stroke 2016, 47, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.R.; Hu, L.S.; Li, G.Y. SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar] [PubMed]
- Kruger, T.M.; Bell, K.J.; Lansakara, T.I.; Tivanski, A.V.; Doorn, J.A.; Stevens, L.L. Reduced Extracellular Matrix Stiffness Prompts SH-SY5Y Cell Softening and Actin Turnover To Selectively Increase Aβ(1–42) Endocytosis. ACS Chem. Neurosci. 2019, 10, 1284–1293. [Google Scholar] [CrossRef]
- Chan, F.K.-G.; Moriwaki, K.; De Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Pradillo, J.M.; García-Culebras, A.; Cuartero, M.I.; Peña-Martínez, C.; Moro, M.A.; Lizasoain, I.; Moraga, A. Del laboratorio a la clínica en el ictus isquémico agudo. Modelos experimentales in vitro e in vivo. Rev. Neurol. 2022, 75, 283–293. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, B.; Izquierdo-Bermejo, S.; Martín-de-Saavedra, M.D.; López-Muñoz, F.; Chioua, M.; Marco-Contelles, J.; Oset-Gasque, M.J. Neuroprotective and Antioxidant Properties of CholesteroNitrone ChN2 and QuinolylNitrone QN23 in an Experimental Model of Cerebral Ischemia: Involvement of Necrotic and Apoptotic Cell Death. Antioxidants 2023, 12, 1364. https://doi.org/10.3390/antiox12071364
Chamorro B, Izquierdo-Bermejo S, Martín-de-Saavedra MD, López-Muñoz F, Chioua M, Marco-Contelles J, Oset-Gasque MJ. Neuroprotective and Antioxidant Properties of CholesteroNitrone ChN2 and QuinolylNitrone QN23 in an Experimental Model of Cerebral Ischemia: Involvement of Necrotic and Apoptotic Cell Death. Antioxidants. 2023; 12(7):1364. https://doi.org/10.3390/antiox12071364
Chicago/Turabian StyleChamorro, Beatriz, Sara Izquierdo-Bermejo, María Dolores Martín-de-Saavedra, Francisco López-Muñoz, Mourad Chioua, José Marco-Contelles, and María Jesús Oset-Gasque. 2023. "Neuroprotective and Antioxidant Properties of CholesteroNitrone ChN2 and QuinolylNitrone QN23 in an Experimental Model of Cerebral Ischemia: Involvement of Necrotic and Apoptotic Cell Death" Antioxidants 12, no. 7: 1364. https://doi.org/10.3390/antiox12071364
APA StyleChamorro, B., Izquierdo-Bermejo, S., Martín-de-Saavedra, M. D., López-Muñoz, F., Chioua, M., Marco-Contelles, J., & Oset-Gasque, M. J. (2023). Neuroprotective and Antioxidant Properties of CholesteroNitrone ChN2 and QuinolylNitrone QN23 in an Experimental Model of Cerebral Ischemia: Involvement of Necrotic and Apoptotic Cell Death. Antioxidants, 12(7), 1364. https://doi.org/10.3390/antiox12071364









