Examining the Mechanisms behind Exercise’s Multifaceted Impacts on Body Composition, Cognition, and the Gut Microbiome in Cancer Survivors: Exploring the Links to Oxidative Stress and Inflammation
Abstract
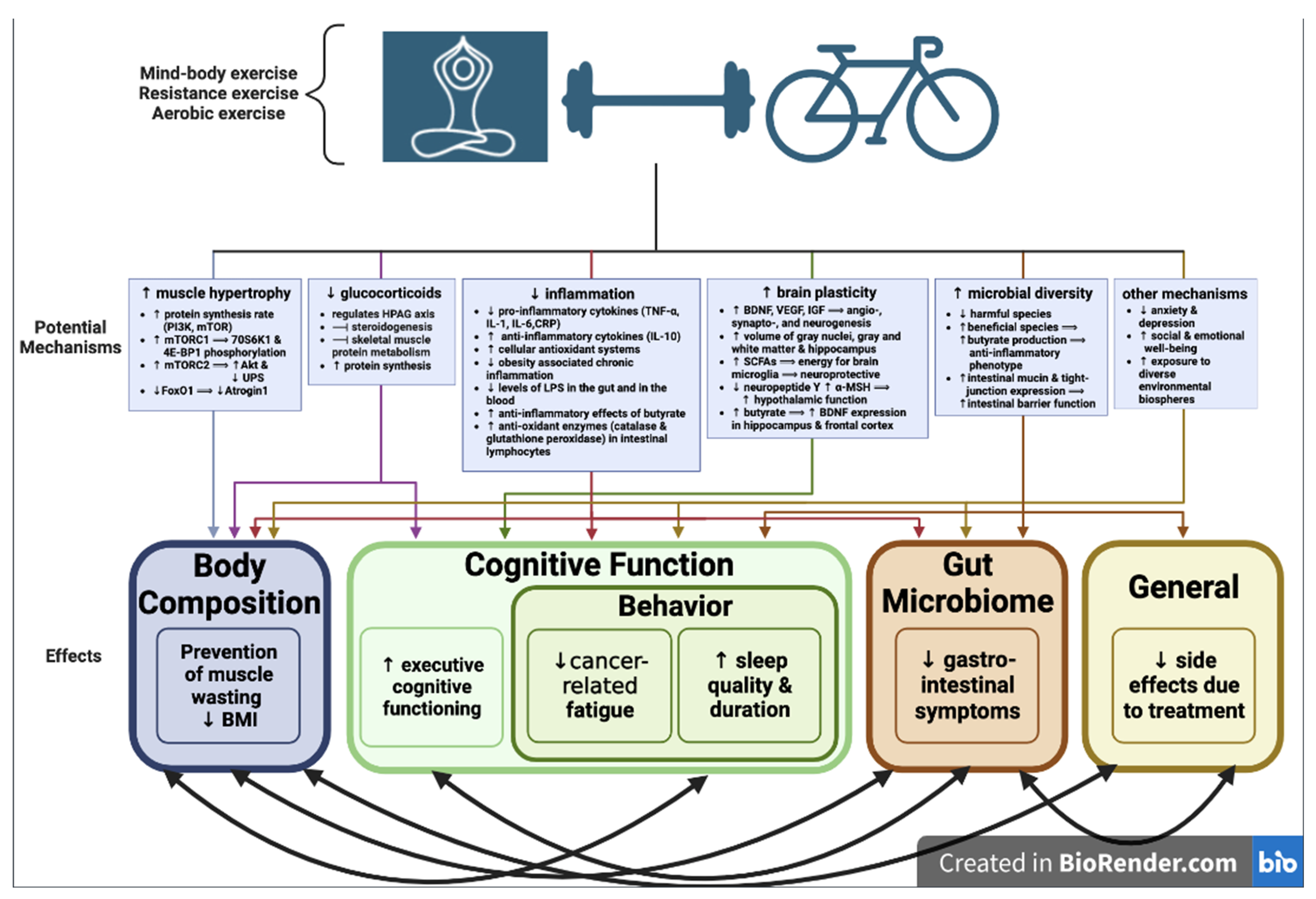
:1. Introduction
2. Results
2.1. Definition of Cancer Survival and Exercise
2.2. Exercise and Its Effect on Body Composition in Cancer Survivors
2.3. Exercise and Its Effect on Cognitive Outcomes in Cancer Survivors
2.3.1. Cancer-Related Fatigue
2.3.2. Cancer-Related Cognitive Impairment
2.4. Exercise and Its Effects on Gut Microbiome in Cancer Survivors
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Institute for Health Metrics and Evaluation (IHME). Findings from the Global Burden of Disease Study 2017. Available online: https://www.healthdata.org/sites/default/files/files/policy_report/2019/GBD_2017_Booklet.pdf (accessed on 5 April 2023).
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [Green Version]
- Cancer Research UK. Cancer Survival Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/survival (accessed on 10 May 2023).
- Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 4 May 2023).
- Wyatt, G.; Sikorskii, A.; Tesnjak, I.; Frambes, D.; Holmstrom, A.; Luo, Z.; Victorson, D.; Tamkus, D. A randomized clinical trial of caregiver-delivered reflexology for symptom management during breast cancer treatment. J. Pain Symptom Manag. 2017, 54, 670–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley des Varannes, S.; Le Vacon, F.; de La Cochetière, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Pham, F.; Moinard-Butot, F.; Coutzac, C.; Chaput, N. Cancer and immunotherapy: A role for microbiota composition. Eur. J. Cancer 2021, 155, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human gut microbiome and risk for colorectal cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef] [Green Version]
- Sabbatino, F.; Conti, V.; Liguori, L.; Polcaro, G.; Corbi, G.; Manzo, V.; Tortora, V.; Carlomagno, C.; Vecchione, C.; Filippelli, A.; et al. Molecules and Mechanisms to Overcome Oxidative Stress Inducing Cardiovascular Disease in Cancer Patients. Life 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Zraik, I.M.; Heß-Busch, Y. Management of chemotherapy side effects and their long-term sequelae. Urol. A 2021, 60, 862–871. [Google Scholar] [CrossRef]
- von Haehling, S.; Anker, S.D. Prevalence, incidence and clinical impact of cachexia: Facts and numbers-update 2014. J. Cachexia Sarcopenia Muscle 2014, 5, 261–263. [Google Scholar] [CrossRef]
- Pamoukdjian, F.; Bouillet, T.; Lévy, V.; Soussan, M.; Zelek, L.; Paillaud, E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin. Nutr. 2018, 37, 1101–1113. [Google Scholar] [CrossRef]
- Hardy, S.J.; Krull, K.R.; Wefel, J.S.; Janelsins, M. Cognitive Changes in Cancer Survivors. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 795–806. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Kohli, S.; Mohile, S.G.; Usuki, K.; Ahles, T.A.; Morrow, G.R. An update on cancer- and chemotherapy-related cognitive dysfunction: Current status. Semin Oncol. 2011, 38, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Országhová, Z.; Mego, M.; Chovanec, M. Long-Term Cognitive Dysfunction in Cancer Survivors. Front. Mol Biosci. 2021, 8, 770413. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Tan, C.C.; Zou, J.J.; Cao, X.P.; Tan, L. Sleep problems and risk of all-cause cognitive decline or dementia: An updated systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chu, S.; Gao, Y.; Ai, Q.; Liu, Y.; Li, X.; Chen, N. A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis. Cells 2019, 8, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGinnis, G.J.; Raber, J. CNS side effects of immune checkpoint inhibitors: Preclinical models, genetics and multimodality therapy. Immunotherapy 2017, 9, 929–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.H.; Chen, Z.; Chen, K.; Liao, F.T.; Chung, C.E.; Liu, X.; Lin, Y.C.; Keohavong, P.; Leikauf, G.D.; Di, Y.P. Lipopolysaccharide-Mediated Chronic Inflammation Promotes Tobacco Carcinogen-Induced Lung Cancer and Determines the Efficacy of Immunotherapy. Cancer Res. 2021, 81, 144–157. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. Correction to: ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 13. [Google Scholar] [CrossRef] [Green Version]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandrika, L.; Kumar, B.; Koul, S.; Maroni, P.; Koul, H.K. Oxidative stress in prostate cancer. Cancer Lett. 2009, 282, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B.; Vijayalekshmi, R.V.; Sung, B. Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin. Cancer Res. 2009, 15, 425–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Lambeth, J.D. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007, 43, 332–347. [Google Scholar] [CrossRef] [Green Version]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.; Chan, J.M. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J. Clin. Oncol. 2011, 29, 726–732. [Google Scholar] [CrossRef]
- Brown, J.C.; Damjanov, N.; Courneya, K.S.; Troxel, A.B.; Zemel, B.S.; Rickels, M.R.; Ky, B.; Rhim, A.D.; Rustgi, A.K.; Schmitz, K.H. A randomized dose-response trial of aerobic exercise and health-related quality of life in colon cancer survivors. Psychooncology 2018, 27, 1221–1228. [Google Scholar] [CrossRef]
- Montaño-Rojas, L.S.; Romero-Pérez, E.M.; Medina-Pérez, C.; Reguera-García, M.M.; de Paz, J.A. Resistance Training in Breast Cancer Survivors: A Systematic Review of Exercise Programs. Int. J. Environ. Res. Public Health 2020, 17, 6511. [Google Scholar] [CrossRef]
- Palma, S.; Hasenoehrl, T.; Jordakieva, G.; Ramazanova, D.; Crevenna, R. High-intensity interval training in the prehabilitation of cancer patients-a systematic review and meta-analysis. Support. Care Cancer 2021, 29, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.V.; Tripathy, D.; Bernstein, L.; Mortimer, J.E. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Butow, P.; Mullan, B.; Clarke, S.; Beale, P.; Pavlakis, N.; Kothe, E.; Lam, L.; Rosenthal, D. Impact of medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: A randomized controlled trial. Ann. Oncol. 2010, 21, 608–614. [Google Scholar] [CrossRef]
- Duan, L.; Xu, Y.; Li, M. Effects of Mind-Body Exercise in Cancer Survivors: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat. Med. 2020, 2020, 7607161. [Google Scholar] [CrossRef] [PubMed]
- Hojan, K.; Kwiatkowska-Borowczyk, E.; Leporowska, E.; Górecki, M.; Ozga-Majchrzak, O.; Milecki, T.; Milecki, P. Physical exercise for functional capacity, blood immune function, fatigue, and quality of life in high-risk prostate cancer patients during radiotherapy: A prospective, randomized clinical study. Eur. J. Phys. Rehabil. Med. 2016, 52, 489–501. [Google Scholar]
- Peters, H.P.; De Vries, W.R.; Vanberge-Henegouwen, G.P.; Akkermans, L.M. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut 2001, 48, 435–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traustadóttir, T.; Davies, S.S.; Su, Y.; Choi, L.; Brown-Borg, H.M.; Roberts, L.J.; Harman, S.M. Oxidative stress in older adults: Effects of physical fitness. Age 2012, 34, 969–982. [Google Scholar] [CrossRef] [Green Version]
- Nader, G.A. Molecular determinants of skeletal muscle mass: Getting the “AKT” together. Int. J. Biochem. Cell Biol. 2005, 37, 1985–1996. [Google Scholar] [CrossRef]
- He, F.; Li, J.; Liu, Z.; Chuang, C.C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [Green Version]
- Payne, J.K.; Held, J.; Thorpe, J.; Shaw, H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol. Nurs. Forum 2008, 35, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Carlson, L.E.; Zelinski, E.; Toivonen, K.; Flynn, M.; Qureshi, M.; Piedalue, K.A.; Grant, R. Mind-Body Therapies in Cancer: What Is the Latest Evidence? Curr. Oncol. Rep. 2017, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini-Laplagne, M.; Dupuy, O.; Sosner, P.; Bosquet, L. Effect of simultaneous exercise and cognitive training on executive functions, baroreflex sensitivity, and pre-frontal cortex oxygenation in healthy older adults: A pilot study. Geroscience 2023, 45, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Smoak, P.; Flores, V.; Harman, N.; Lisano, J.; Hayward, R.; Stewart, L.K. Structured Exercise in Cancer Survivors: Is it Enough for Neural, Mental Health and Well-being? Int. J. Exerc. Sci. 2021, 14, 162–176. [Google Scholar] [PubMed]
- Shukla, S.D.; Budden, K.F.; Neal, R.; Hansbro, P.M. Microbiome effects on immunity, health and disease in the lung. Clin. Transl. Immunol. 2017, 6, e133. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.Y.; Kim, W.; Nam, S.M.; Kim, D.W.; Chung, J.Y.; Choi, S.Y.; Yoon, Y.S.; Won, M.-H.; Hwang, I.K. Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem. Res. 2011, 36, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Griffin, E.W.; Bechara, R.G.; Birch, A.M.; Kelly, A.M. Exercise enhances hippocampal-dependent learning in the rat: Evidence for a BDNF-related mechanism. Hippocampus 2009, 19, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, C.S.; Carlson, R.W.; Are, M.; Baker, K.S.; Davis, E.; Edge, S.B.; Friedman, D.L.; Goldman, M.; Jones, L.; King, A.; et al. Survivorship: Introdcution and Definition. J. Natl. Compr. Canc. Netw. 2014, 12, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.; Pan, Y.; Zhong, T.; Zeng, Y.; Cheng, A.S.K. Effects of aerobic, resistance, and combined exercise on metabolic syndrome parameters and cardiovascular risk factors: A systematic review and network meta-analysis. Rev. Cardiovasc. Med. 2021, 22, 1523–1533. [Google Scholar] [CrossRef]
- Xavier, V.B.; Avanzi, O.; de Carvalho, B.D.M.C.; Alves, V.L.D.S. Combined aerobic and resistance training improves respiratory and exercise outcomes more than aerobic training in adolescents with idiopathic scoliosis: A randomised trial. J. Physiother. 2020, 66, 33–38. [Google Scholar] [CrossRef]
- Sigal, R.J.; Kenny, G.P.; Boulé, N.G.; Wells, G.A.; Prud’homme, D.; Fortier, M.; Reid, R.D.; Tulloch, H.; Coyle, D.; Phillips, P.; et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 357–369. [Google Scholar] [CrossRef]
- Gavala-González, J.; Torres-Pérez, A.; Fernández-García, J.C. Impact of Rowing Training on Quality of Life and Physical Activity Levels in Female Breast Cancer Survivors. Int. J. Environ. Res. Public Health 2021, 5, 7188. [Google Scholar] [CrossRef] [PubMed]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Cramer, H.; Lauche, R.; Klose, P.; Lange, S.; Langhorst, J.; Dobos, G.J. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst. Rev. 2017, 1, CD010802. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.Y.; Sabag, A.; Hao, W.L.; Zhang, L.N.; Jia, M.X.; Dai, N.; Zhang, H.; Ayati, Z.; Cheng, Y.J.; Zhang, C.H.; et al. Tai Chi for health and well-being: A bibliometric analysis of published clinical studies between 2010 and 2020. Complement Ther. Med. 2021, 60, 102748. [Google Scholar] [CrossRef] [PubMed]
- Gaafer, O.U.; Zimmers, T.A. Nutrition challenges of cancer cachexia. JPEN J. Parenter Enter. Nutr. 2021, 45, 16–25. [Google Scholar] [CrossRef]
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Burvenich, I.J.; Zhao, K.; Senko, C.; Glab, J.; Fogliaro, R.; Liu, Z.; Jose, I.; Puthalakath, H.; Hoogenraad, N.J.; et al. Identification of Potential Biomarkers for Cancer Cachexia and Anti-Fn14 Therapy. Cancers 2022, 14, 5533. [Google Scholar] [CrossRef]
- Constantin-Teodosiu, D.; Constantin, D. Molecular Mechanisms of Muscle Fatigue. Int. J. Mol. Sci. 2021, 22, 11587. [Google Scholar] [CrossRef]
- Vanhoutte, G.; van de Wiel, M.; Wouters, K.; Sels, M.; Bartolomeeussen, L.; De Keersmaecker, S.; Verschueren, C.; De Vroey, V.; De Wilde, A.; Smits, E.; et al. Cachexia in cancer: What is in the definition? BMJ Open Gastroenterol. 2016, 3, e000097. [Google Scholar] [CrossRef]
- Gingrich, A.; Volkert, D.; Kiesswetter, E.; Thomanek, M.; Bach, S.; Sieber, C.C.; Zopf, Y. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019, 19, 120. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Garcia, J.M. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options—A mini-review. Gerontology 2014, 60, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.L.; Lee, D.E.; Rosa-Caldwell, M.E.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Huseman, K.; Sataranatarajan, K.; Van Remmen, H.; Washington, T.A.; et al. Protein imbalance in the development of skeletal muscle wasting in tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2018, 9, 987–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, M.D.; Lecker, S.H.; Jagoe, R.T.; Navon, A.; Goldberg, A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 14440–14445. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- Lønbro, S.; Dalgas, U.; Primdahl, H.; Johansen, J.; Nielsen, J.L.; Aagaard, P.; Hermann, A.P.; Overgaard, J.; Overgaard, K. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy—Results from the randomized DAHANCA 25B trial. Radiother. Oncol. 2013, 108, 314–319. [Google Scholar] [CrossRef]
- Cormie, P.; Zopf, E.M. Exercise medicine for the management of androgen deprivation therapy-related side effects in prostate cancer. Urol. Oncol. 2020, 38, 62–70. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Goodman, C.A. The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev. Physiol. Biochem. Pharmacol. 2014, 166, 43–95. [Google Scholar] [CrossRef]
- Guertin, D.A.; Stevens, D.M.; Thoreen, C.C.; Burds, A.A.; Kalaany, N.Y.; Moffat, J.; Brown, M.; Fitzgerald, K.J.; Sabatini, D.M. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell 2006, 11, 859–871. [Google Scholar] [CrossRef] [Green Version]
- Léger, B.; Cartoni, R.; Praz, M.; Lamon, S.; Dériaz, O.; Crettenand, A.; Gobelet, C.; Rohmer, P.; Konzelmann, M.; Luthi, F.; et al. Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J. Physiol. 2006, 576, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Mascher, H.; Tannerstedt, J.; Brink-Elfegoun, T.; Ekblom, B.; Gustafsson, T.; Blomstrand, E. Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E43–E51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attaix, D.; Ventadour, S.; Codran, A.; Béchet, D.; Taillandier, D.; Combaret, L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem. 2005, 41, 173–186. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, B.D.; Caldow, M.K.; Brennan-Speranza, T.C.; Sbaraglia, M.; Jerums, G.; Garnham, A.; Wong, C.; Levinger, P.; Asrar Ul Haq, M.; Hare, D.L.; et al. Muscle atrophy in patients with Type 2 Diabetes Mellitus: Roles of inflammatory pathways, physical activity and exercise. Exerc. Immunol. Rev. 2016, 22, 94–109. [Google Scholar]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [Green Version]
- Cockram, P.E.; Kist, M.; Prakash, S.; Chen, S.H.; Wertz, I.E.; Vucic, D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021, 28, 591–605. [Google Scholar] [CrossRef]
- Paolucci, E.M.; Loukov, D.; Bowdish, D.M.E.; Heisz, J.J. Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 2018, 133, 79–84. [Google Scholar] [CrossRef]
- Ruiz-Iglesias, P.; Massot-Cladera, M.; Rodríguez-Lagunas, M.J.; Franch, À.; Camps-Bossacoma, M.; Pérez-Cano, F.J.; Castell, M. Protective Effect of a Cocoa-Enriched Diet on Oxidative Stress Induced by Intensive Acute Exercise in Rats. Antioxidants 2022, 11, 753. [Google Scholar] [CrossRef]
- de Boer, M.C.; Wörner, E.A.; Verlaan, D.; van Leeuwen, P.A.M. The Mechanisms and Effects of Physical Activity on Breast Cancer. Clin. Breast Cancer 2017, 17, 272–278. [Google Scholar] [CrossRef]
- Arena, S.K.; Doherty, D.J.; Bellford, A.; Hayman, G. Effects of Aerobic Exercise on Oxidative Stress in Patients Diagnosed with Cancer: A Narrative Review. Cureus 2019, 11, e5382. [Google Scholar] [CrossRef] [Green Version]
- Menconi, M.; Fareed, M.; O’Neal, P.; Poylin, V.; Wei, W.; Hasselgren, P.O. Role of glucocorticoids in the molecular regulation of muscle wasting. Crit. Care Med. 2007, 35, S602–S608. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Castells, J.; Allibert, V.; Emerit, A.; Zolotoff, C.; Cardot-Ruffino, V.; Gallot, Y.S.; Vernus, B.; Chauvet, V.; Bartholin, L.; et al. Hypothalamic-pituitary-adrenal axis activation and glucocorticoid-responsive gene expression in skeletal muscle and liver of Apc mice. J. Cachexia Sarcopenia Muscle 2022, 13, 1686–1703. [Google Scholar] [CrossRef]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C.; Viru, A. Twenty-four-hour cortisol response to multiple daily exercise sessions of moderate and high intensity. Clin. Physiol. 1999, 19, 178–182. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Glucocorticoids, stress, and their adverse neurological effects: Relevance to aging. Exp. Gerontol. 1999, 34, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, C.G.; Milbury, K.; Chandwani, K.D.; Chaoul, A.; Perkins, G.; Nagarathna, R.; Haddad, R.; Nagendra, H.R.; Raghuram, N.V.; Spelman, A.; et al. Examining Mediators and Moderators of Yoga for Women With Breast Cancer Undergoing Radiotherapy. Integr. Cancer Ther. 2016, 15, 250–262. [Google Scholar] [CrossRef] [Green Version]
- Ho, R.T.; Fong, T.C.; Cheung, I.K.; Yip, P.S.; Luk, M.Y. Effects of a Short-Term Dance Movement Therapy Program on Symptoms and Stress in Patients With Breast Cancer Undergoing Radiotherapy: A Randomized, Controlled, Single-Blind Trial. J. Pain Symptom Manag. 2016, 51, 824–831. [Google Scholar] [CrossRef] [Green Version]
- Carek, P.J.; Laibstain, S.E.; Carek, S.M. Exercise for the treatment of depression and anxiety. Int. J. Psychiatry Med. 2011, 41, 15–28. [Google Scholar] [CrossRef]
- Aversa, Z.; Costelli, P.; Muscaritoli, M. Cancer-induced muscle wasting: Latest findings in prevention and treatment. Ther. Adv. Med. Oncol. 2017, 9, 369–382. [Google Scholar] [CrossRef] [Green Version]
- Argilés, J.M.; Busquets, S.; López-Soriano, F.J.; Costelli, P.; Penna, F. Are there any benefits of exercise training in cancer cachexia? J. Cachexia Sarcopenia Muscle 2012, 3, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thong, M.S.Y.; van Noorden, C.J.F.; Steindorf, K.; Arndt, V. Cancer-Related Fatigue: Causes and Current Treatment Options. Curr. Treat. Options Oncol. 2020, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Von Ah, D.; Habermann, B.; Carpenter, J.S.; Schneider, B.L. Impact of perceived cognitive impairment in breast cancer survivors. Eur. J. Oncol. Nurs. 2013, 17, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef]
- Lee, C.H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef] [Green Version]
- Rakel, R.E. Depression. Prim. Care 1999, 26, 211–224. [Google Scholar] [CrossRef]
- Roila, F.; Fumi, G.; Ruggeri, B.; Antonuzzo, A.; Ripamonti, C.; Fatigoni, S.; Cavanna, L.; Gori, S.; Fabi, A.; Marzano, N.; et al. Prevalence, characteristics, and treatment of fatigue in oncological cancer patients in Italy: A cross-sectional study of the Italian Network for Supportive Care in Cancer (NICSO). Support. Care Cancer 2019, 27, 1041–1047. [Google Scholar] [CrossRef]
- Wang, X.S.; Zhao, F.; Fisch, M.J.; O’Mara, A.M.; Cella, D.; Mendoza, T.R.; Cleeland, C.S. Prevalence and characteristics of moderate to severe fatigue: A multicenter study in cancer patients and survivors. Cancer 2014, 120, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Pierce, B.L.; Ballard-Barbash, R.; Bernstein, L.; Baumgartner, R.N.; Neuhouser, M.L.; Wener, M.H.; Baumgartner, K.B.; Gilliland, F.D.; Sorensen, B.E.; McTiernan, A.; et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 2009, 27, 3437–3444. [Google Scholar] [CrossRef] [Green Version]
- Hiensch, A.E.; Mijwel, S.; Bargiela, D.; Wengström, Y.; May, A.M.; Rundqvist, H. Inflammation Mediates Exercise Effects on Fatigue in Patients with Breast Cancer. Med. Sci. Sports Exerc. 2021, 53, 496–504. [Google Scholar] [CrossRef]
- Laird, K.T.; Paholpak, P.; Roman, M.; Rahi, B.; Lavretsky, H. Mind-Body Therapies for Late-Life Mental and Cognitive Health. Curr. Psychiatry Rep. 2018, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.E. Distress Management Through Mind-Body Therapies in Oncology. J. Natl. Cancer Inst. Monogr. 2017, 2017, lgx009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, K.C.; Nijeboer, S.; Dixon, M.L.; Floman, J.L.; Ellamil, M.; Rumak, S.P.; Sedlmeier, P.; Christoff, K. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci. Biobehav. Rev. 2014, 43, 48–73. [Google Scholar] [CrossRef]
- Fan, Y.; Tang, Y.Y.; Ma, Y.; Posner, M.I. Mucosal immunity modulated by integrative meditation in a dose-dependent fashion. J. Altern. Complement Med. 2010, 16, 151–155. [Google Scholar] [CrossRef]
- Glaser, R. Stress-associated immune dysregulation and its importance for human health: A personal history of psychoneuroimmunology. Brain Behav. Immun. 2005, 19, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Kesler, S.R.; Ahles, T.A.; Morrow, G.R. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry 2014, 26, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, J.; Monje, M.; Wefel, J.; Meyers, C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist 2008, 13, 1285–1295. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Tao, M.L.; Hu, W.; Belin, T.R.; Sepah, S.; Cole, S.; Aziz, N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res. 2009, 15, 5534–5540. [Google Scholar] [CrossRef] [Green Version]
- Campbell, K.L.; Zadravec, K.; Bland, K.A.; Chesley, E.; Wolf, F.; Janelsins, M.C. The Effect of Exercise on Cancer-Related Cognitive Impairment and Applications for Physical Therapy: Systematic Review of Randomized Controlled Trials. Phys. Ther. 2020, 100, 523–542. [Google Scholar] [CrossRef]
- Tomasello, B.; Malfa, G.A.; Strazzanti, A.; Gangi, S.; Di Giacomo, C.; Basile, F.; Renis, M. Effects of physical activity on systemic oxidative/DNA status in breast cancer survivors. Oncol. Lett. 2017, 13, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Atherton, P.J.; Smith, K. Muscle protein synthesis in response to nutrition and exercise. J. Physiol. 2012, 590, 1049–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raber, J.; Akana, S.F.; Bhatnagar, S.; Dallman, M.F.; Wong, D.; Mucke, L. Hypothalamic-pituitary-adrenal dysfunction in Apoe(-/-) mice: Possible role in behavioral and metabolic alterations. J. Neurosci. 2000, 20, 2064–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, N.M.; Landfield, P.W. Stress hormones and brain aging: Adding injury to insult? Nat. Neurosci. 1998, 1, 3–4. [Google Scholar] [CrossRef] [PubMed]
- De Nys, L.; Anderson, K.; Ofosu, E.F.; Ryde, G.C.; Connelly, J.; Whittaker, A.C. The effects of physical activity on cortisol and sleep: A systematic review and meta-analysis. Psychoneuroendocrinology 2022, 143, 105843. [Google Scholar] [CrossRef] [PubMed]
- Stillman, C.M.; Esteban-Cornejo, I.; Brown, B.; Bender, C.M.; Erickson, K.I. Effects of Exercise on Brain and Cognition Across Age Groups and Health States. Trends Neurosci. 2020, 43, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.R.; Loman, B.R.; Bailey, M.T.; Pyter, L.M. Gut microbiota-immune-brain interactions in chemotherapy-associated behavioral comorbidities. Cancer 2018, 124, 3990–3999. [Google Scholar] [CrossRef] [Green Version]
- Grant, C.V.; Loman, B.R.; Bailey, M.T.; Pyter, L.M. Manipulations of the gut microbiome alter chemotherapy-induced inflammation and behavioral side effects in female mice. Brain Behav. Immun. 2021, 95, 401–412. [Google Scholar] [CrossRef]
- Deleemans, J.M.; Chleilat, F.; Reimer, R.A.; Henning, J.W.; Baydoun, M.; Piedalue, K.A.; McLennan, A.; Carlson, L.E. The chemo-gut study: Investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; study protocol. BMC Cancer 2019, 19, 1243. [Google Scholar] [CrossRef] [Green Version]
- Tlaskalová-Hogenová, H.; Stěpánková, R.; Kozáková, H.; Hudcovic, T.; Vannucci, L.; Tučková, L.; Rossmann, P.; Hrnčíř, T.; Kverka, M.; Zákostelská, Z.; et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell. Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Fermentation in the human large intestine: Its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 2011, 45, S120–S127. [Google Scholar] [CrossRef] [Green Version]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonel, A.J.; Alvarez-Leite, J.I. Butyrate: Implications for intestinal function. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Aczel, D.; Gyorgy, B.; Bakonyi, P.; BukhAri, R.; Pinho, R.; Boldogh, I.; Yaodong, G.; Radak, Z. The Systemic Effects of Exercise on the Systemic Effects of Alzheimer’s Disease. Antioxidants 2022, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, M.; Hamada, T. Acute exercise increases brain region-specific expression of MCT1, MCT2, MCT4, GLUT1, and COX IV proteins. J. Appl. Physiol. 2014, 116, 1238–1250. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Allen, A.P.; Dinan, T.G.; Clarke, G.; Cryan, J.F. A psychology of the human brain-gut-microbiome axis. Soc. Pers. Psychol. Compass 2017, 11, e12309. [Google Scholar] [CrossRef] [Green Version]
- Royes, L.F.F. Cross-talk between gut and brain elicited by physical exercise. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165877. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [Green Version]
- Raber, J.; Sharpton, T.J. Gastrointestinal Dysfunction in Neurological and Neurodegenerative Disorders. 2023, in press.
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef]
- Sabit, H.; Cevik, E.; Tombuloglu, H. Colorectal cancer: The epigenetic role of microbiome. World J. Clin. Cases 2019, 7, 3683–3697. [Google Scholar] [CrossRef] [PubMed]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid. Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Kim, H.J.; Leeds, P.; Chuang, D.M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009, 110, 1226–1240. [Google Scholar] [CrossRef] [Green Version]
- Sampsell, K.; Wang, W.; Ohland, C.; Mager, L.F.; Pett, N.; Lowry, D.E.; Sales, K.M.; McNeely, M.L.; McCoy, K.D.; Culos-Reed, S.N.; et al. Exercise and Prebiotic Fiber Provide Gut Microbiota-Driven Benefit in a Survivor to Germ-Free Mouse Translational Model of Breast Cancer. Cancers 2022, 14, 2722. [Google Scholar] [CrossRef] [PubMed]
- Himbert, C.; Stephens, W.Z.; Gigic, B.; Hardikar, S.; Holowatyj, A.N.; Lin, T.; Ose, J.; Swanson, E.; Ashworth, A.; Warby, C.A.; et al. Differences in the gut microbiome by physical activity and BMI among colorectal cancer patients. Am. J. Cancer Res. 2022, 12, 4789–4801. [Google Scholar]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [Green Version]
- Gisolfi, C.V. Is the GI System Built For Exercise? News Physiol. Sci. 2000, 15, 114–119. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal complaints during exercise: Prevalence, etiology, and nutritional recommendations. Sports Med. 2014, 44 (Suppl. S1), S79–S85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lira, F.S.; Rosa, J.C.; Pimentel, G.D.; Souza, H.A.; Caperuto, E.C.; Carnevali, L.C.; Seelaender, M.; Damaso, A.R.; Oyama, L.M.; de Mello, M.T.; et al. Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis. 2010, 9, 82. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Liu, A.; Zong, W.; Dai, L.; Liu, Y.; Luo, R.; Ge, S.; Dong, G. Moderate exercise ameliorates osteoarthritis by reducing lipopolysaccharides from gut microbiota in mice. Saudi J. Biol. Sci. 2021, 28, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Goetz, L.; Pervaiz, N.; Packer, N.; Guan, J. Freewheel training decreases pro- and increases anti-inflammatory cytokine expression in mouse intestinal lymphocytes. Brain Behav. Immun. 2010, 24, 1105–1115. [Google Scholar] [CrossRef]
- Ropelle, E.R.; da Silva, A.S.R.; Cintra, D.E.; de Moura, L.P.; Teixeira, A.M.; Pauli, J.R. Physical Exercise: A Versatile Anti-Inflammatory Tool Involved in the Control of Hypothalamic Satiety Signaling. Exerc. Immunol. Rev. 2021, 27, 7–23. [Google Scholar]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef]
- Carnier, J.; de Mello, M.T.; Ackel-DElia, C.; Corgosinho, F.C.; Campos, R.M.; Sanches, P.e.L.; Masquio, D.C.; Bueno, C.R.; Ganen, A.e.P.; Martins, A.C.; et al. Aerobic training (AT) is more effective than aerobic plus resistance training (AT+RT) to improve anorexigenic/orexigenic factors in obese adolescents. Appetite 2013, 69, 168–173. [Google Scholar] [CrossRef]
- Della Guardia, L.; Codella, R. Exercise Restores Hypothalamic Health in Obesity by Reshaping the Inflammatory Network. Antioxidants 2023, 12, 297. [Google Scholar] [CrossRef]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Hill, E.E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A.C. Exercise and circulating cortisol levels: The intensity threshold effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Segal, R.J.; Mackey, J.R.; Gelmon, K.; Reid, R.D.; Friedenreich, C.M.; Ladha, A.B.; Proulx, C.; Vallance, J.K.; Lane, K.; et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J. Clin. Oncol. 2007, 25, 4396–4404. [Google Scholar] [CrossRef] [PubMed]
- Mutrie, N.; Campbell, A.M.; Whyte, F.; McConnachie, A.; Emslie, C.; Lee, L.; Kearney, N.; Walker, A.; Ritchie, D. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: Pragmatic randomised controlled trial. BMJ 2007, 334, 517. [Google Scholar] [CrossRef] [Green Version]
- Ciria, L.F.; Román-Caballero, R.; Vadillo, M.A.; Holgado, D.; Luque-Casado, A.; Perakakis, P.; Sanabria, D. An umbrella review of randomized control trials on the effects of physical exercise on cognition. Nat. Hum. Behav. 2023, 7, 928–941. [Google Scholar] [CrossRef]
- Stern, Y.; MacKay-Brandt, A.; Lee, S.; McKinley, P.; McIntyre, K.; Razlighi, Q.; Agarunov, E.; Bartels, M.; Sloan, R.P. Effect of aerobic exercise on cognition in younger adults: A randomized clinical trial. Neurology 2019, 92, e905–e916. [Google Scholar] [CrossRef]
- De la Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; García-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, A.G.; et al. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci. 2020, 9, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Kraft, E. Cognitive function, physical activity, and aging: Possible biological links and implications for multimodal interventions. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2012, 19, 248–263. [Google Scholar] [CrossRef]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018, 361, eaan8821. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.M.; Berg Miller, M.E.; Pence, B.D.; Whitlock, K.; Nehra, V.; Gaskins, H.R.; White, B.A.; Fryer, J.D.; Woods, J.A. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J. Appl. Physiol. 2015, 118, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
| Study | Sample Size | Health Status | Exercise Intervention | Relevant Tests and Measures | Relevant Findings Due to Exercise Intervention | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exercise Type | Frequency | Session Time | Total Duration | Measures | Assessments | Beneficial Effects | Unaffected | |||
| Brown (2018) [32] | 39 | Colon cancer (stage I-III) | Moderate:Aerobic, home treadmill | Depends | 150 mins OR 300 mins/week | 6 months | / | SF-36, PSQI, FSI, FACT-C, | Physical component SF-36, FACT-C, PSQI, and FSI | Mental component SF-36 |
| Courneya (2007) [156] | 242 | Breast cancer (stage I-IIIa) | Moderate: Resistance OR Aerobic | 3x/week | 15–45 mins | 9–24 weeks | / | FACT-A, self-esteem | Self-esteem | FACT-A |
| Dieli-Conwrigth (2018) [35] | 100 | Breast cancer (stage 0-III) BMI > 25.0 kg/m2 | Moderate to vigorous: Aerobic and resistance | 3x/week | 150 mins aerobic 2/3 days resistance | 16 weeks | Insulin, leptin, adiponectin: Blood sample (2x) | / | Reduction in all biomarkers; improved body composition | / |
| Gavala-González (2021) [54] | 28 | Breast cancer (stage I-IV) | Moderate:Combined (Rowing) | 3x/week | 60–90 min | 12 weeks | / | IPAQ-SF, SF-36 | QOL, overall perceived health, physical activity | / |
| Himbert (2022) [141] | 179 | Colon cancer (stage I-IV) | Moderate to vigorous:Various types | Depends | Depends | Depends | Gut microbiota analysis: Stool sample (1x) | / | Increased diversity of gut microbiome | / |
| Ho (2016) [91] | 121 | Breast cancer | Dance movement therapy | 2x/week | 90 mins | 3 weeks | Cortisol: Saliva sample (5x–diurnal slope) | PSQI, BFI | Steeper cortisol slope | / |
| Hojan (2016) [38] | 54 | High-risk prostate cancer | Moderate:Aerobic & resistance | 5x/week | 50 mins | 12–20 weeks | IL-1ß, IL-6, TNF-α: Blood sample (2x) | FACT-F, QOL (EORTC) | Decreased inflammatory markers; improved fatigue and QOL after RT | Blood markers before RT |
| Kenfield (2011) [31] | 2705 | Non-metastatic prostate cancer | General physical activity | Depends | Depends | Depends | Risk of advanced prostate cancer | Overall survival | Overall survival; reduced risk of advanced prostate cancer with vigorous activity | / |
| Lønbro (2013) [69] | 41 | Head and neck cancer | Progressive resistance training | 2–3x/week | Depends | 12 weeks | Body lean mass, muscle strength | QOL (EORTC) | Lean body mass, muscle strength; QOL cognition | / |
| Mutrie (2007) [157] | 177 | Breast cancer (stage 0-III) | Moderate: Walking/cycling/aerobics | 3x/week | 45 min | 12 weeks | / | FACT-G, BDI, BMI | Improved BMI and BDI | FACT-G |
| Oh (2010) [36] | 162 | Various cancers (mainly breast and colonic cancer) | Medical qigong | 2x/week | 90 min | 10 weeks | CRP: Blood sample (2x) | FACT-G, FACT-F, mood state | Reduced CRP; improved QOL and FACT-F, reduced mood disturbance | Anger and hostility, Confusion (mood) |
| Payne (2008) [43] | 20 | Breast cancer and hormonal treatment | Moderate: Aerobic, walking activity | 4x/week | 20 min | 14 weeks | Cortisol, serotonin, bilirubin: Blood sample (2x) | PSQI, PRFS | Decreased cortisol, serotonin and bilirubin; improved PSQI | / |
| Ratcliff (2016) [90] | 163 | Breast cancer (stage 0-III) | Mind–body, yoga | 3x/week | 60 min | 6 weeks | Cortisol: Saliva sample (5x– diurnal slope) | PSQI, SF-36 | Yoga caused steeper cortisol slope; yoga improved PSQI | PSQI, mental component SF-36 |
| Sampsell (2022) [140] | 10 | Breast cancer | Mild to moderate: Aerobic and strength | 2x/week | 60 min | 12 weeks | Gut microbiota analysis: Stool sample (3x) | FACT-G | Trend toward increased a-diversity between 0 and 12 weeks | FACT-G, human gut microbiome |
| Smoak (2021) [46] | 183 | Various cancers | Resistance and aerobic | 3x/week | 60 min | 12 weeks | BDNF, NGF: Blood sample (2x) | QOL, fatigue, depression | BDNF increase (in treatment); QOL, fatigue (no treatment) | Depression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, B.; Winters-Stone, K.M.; Raber, J. Examining the Mechanisms behind Exercise’s Multifaceted Impacts on Body Composition, Cognition, and the Gut Microbiome in Cancer Survivors: Exploring the Links to Oxidative Stress and Inflammation. Antioxidants 2023, 12, 1423. https://doi.org/10.3390/antiox12071423
Matei B, Winters-Stone KM, Raber J. Examining the Mechanisms behind Exercise’s Multifaceted Impacts on Body Composition, Cognition, and the Gut Microbiome in Cancer Survivors: Exploring the Links to Oxidative Stress and Inflammation. Antioxidants. 2023; 12(7):1423. https://doi.org/10.3390/antiox12071423
Chicago/Turabian StyleMatei, Benjamin, Kerri M. Winters-Stone, and Jacob Raber. 2023. "Examining the Mechanisms behind Exercise’s Multifaceted Impacts on Body Composition, Cognition, and the Gut Microbiome in Cancer Survivors: Exploring the Links to Oxidative Stress and Inflammation" Antioxidants 12, no. 7: 1423. https://doi.org/10.3390/antiox12071423
APA StyleMatei, B., Winters-Stone, K. M., & Raber, J. (2023). Examining the Mechanisms behind Exercise’s Multifaceted Impacts on Body Composition, Cognition, and the Gut Microbiome in Cancer Survivors: Exploring the Links to Oxidative Stress and Inflammation. Antioxidants, 12(7), 1423. https://doi.org/10.3390/antiox12071423






