Monofloral Corn Poppy Bee-Collected Pollen—A Detailed Insight into Its Phytochemical Composition and Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
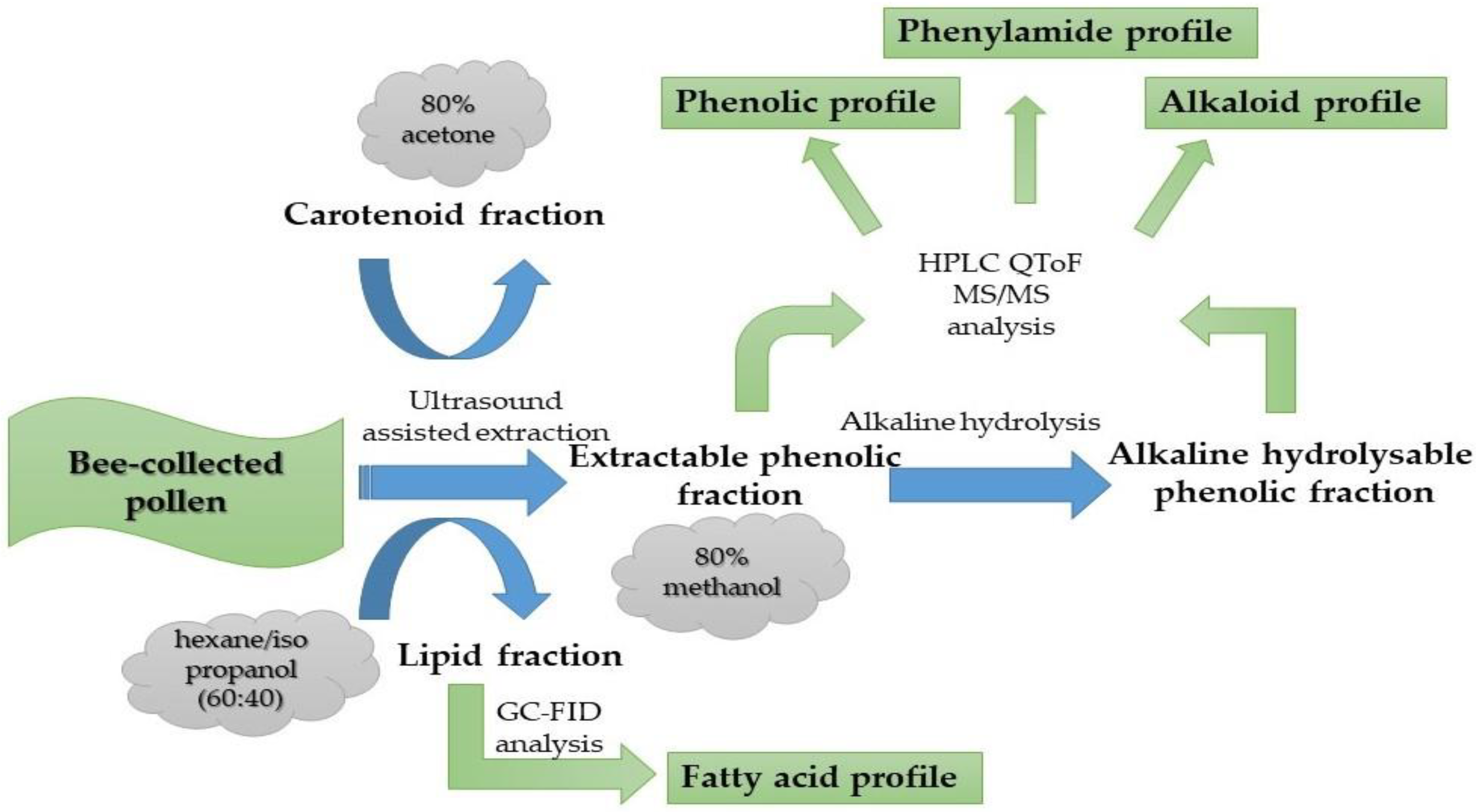
2.1. Collection and Extraction Procedures
2.2. General Phytochemical Characterization
2.3. A Detailed Profiling of Obtained Extracts
2.4. Antioxidant Properties of PBP
2.5. Statistical Analysis
3. Results
3.1. General Phytochemical Composition
3.2. UHPLC Phenolic Profile of PBP Extracts
3.3. UHPLC Phenylamide (Derivatives) Profile of PBP Extracts
3.4. UHPLC Alkaloid Profile of PBP Extracts
3.5. Fatty-Acid Profile of PBP Extract
3.6. Antioxidant Properties of PBP Phenolic Extract
4. Discussion
4.1. General Phytochemical Composition
4.2. UHPLC Phenolic Profile of PBP
4.3. UHPLC Phenylamide Profile of PBP
4.4. UHPLC Alkaloid Profile of PBP
4.5. GC-FID Fatty-Acid Profile of PBP
4.6. Antioxidant Properties of PBP Phenolic Extracts
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Temple, N.J. A Rational Definition for Functional Foods: A Perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Q.; Wang, K.; Marcucci, M.C.; Sawaya, A.C.H.F.; Hu, L.; Xue, X.-F.; Wu, L.-M.; Hu, F.-L. Nutrient-Rich Bee Pollen: A Treasure Trove of Active Natural Metabolites. J. Funct. Foods 2018, 49, 472–484. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Composition and Functionality of Bee Pollen: A Review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Ali Shariati, M.; Tešić, Ž.L.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient—The Present and Perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Ulusoy, E.; Kolayli, S. Phenolic Composition and Antioxidant Properties of Anzer Bee Pollen. J. Food Biochem. 2014, 38, 73–82. [Google Scholar] [CrossRef]
- Hemmami, H.; ben Seghir, B.; ben Ali, M.; Rebiai, A.; Zeghoud, S.; Brahmia, F. Phenolic Profile and Antioxidant Activity of Bee Pollen Extracts from Different Regions of Algeria. Ovidius Univ. Ann. Chem. 2020, 31, 93–98. [Google Scholar] [CrossRef]
- Waś, E.; Szczęsna, T.; Rybak-Chmielewska, H.; Teper, D.; Jaśkiewicz, K. Application of HPLC-DAD Technique for Determination of Phenolic Compounds in Bee Pollen Loads. J. Apic. Sci. 2017, 61, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Kao, Y.T.; Lu, M.J.; Chen, C. Preliminary Analyses of Phenolic Compounds and Antioxidant Activities in Tea Pollen Extracts. J. Food Drug Anal. 2011, 19, 470–477+540. [Google Scholar] [CrossRef]
- Alimoglu, G.; Guzelmeric, E.; Yuksel, P.I.; Celik, C.; Deniz, I.; Yesilada, E. Monofloral and Polyfloral Bee Pollens: Comparative Evaluation of Their Phenolics and Bioactivity Profiles. LWT 2021, 142, 110973. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Polyphenolic Profile and Antioxidant Properties of Bee-Collected Pollen from Sunflower (Helianthus annuus L.) Plant. LWT 2019, 112, 108244. [Google Scholar] [CrossRef]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Cells Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Ni, Y.-Q.; Liu, Y.-S. New Insights into the Roles and Mechanisms of Spermidine in Aging and Age-Related Diseases. Aging Dis. 2021, 12, 1948. [Google Scholar] [CrossRef]
- Pietrocola, F.; Castoldi, F.; Kepp, O.; Carmona-Gutierrez, D.; Madeo, F.; Kroemer, G. Spermidine Reduces Cancer-Related Mortality in Humans. Autophagy 2019, 15, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.B.; Liu, Q.; Ahn, J.H.; Jo, Y.H.; Turk, A.; Hong, I.P.; Han, S.M.; Hwang, B.Y.; Lee, M.K. Polyamine Derivatives from the Bee Pollen of Quercus mongolica with Tyrosinase Inhibitory Activity. Bioorg. Chem. 2018, 81, 127–133. [Google Scholar] [CrossRef]
- Kyselka, J.; Bleha, R.; Dragoun, M.; Bialasová, K.; Horáčková, Š.; Schätz, M.; Sluková, M.; Filip, V.; Synytsya, A. Antifungal Polyamides of Hydroxycinnamic Acids from Sunflower Bee Pollen. J. Agric. Food Chem. 2018, 66, 11018–11026. [Google Scholar] [CrossRef]
- Gardana, C.; Del Bo’, C.; Quicazán, M.C.; Corrrea, A.R.; Simonetti, P. Nutrients, Phytochemicals and Botanical Origin of Commercial Bee Pollen from Different Geographical Areas. J. Food Compos. Anal. 2018, 73, 29–38. [Google Scholar] [CrossRef]
- El Ghouizi, A.; El Menyiy, N.; Falcão, S.I.; Vilas-Boas, M.; Lyoussi, B. Chemical Composition, Antioxidant Activity, and Diuretic Effect of Moroccan Fresh Bee Pollen in Rats. Vet. World 2020, 13, 1251–1261. [Google Scholar] [CrossRef]
- Aylanc, V.; Tomás, A.; Russo-Almeida, P.; Falcão, S.I.; Vilas-Boas, M. Assessment of Bioactive Compounds under Simulated Gastrointestinal Digestion of Bee Pollen and Bee Bread: Bioaccessibility and Antioxidant Activity. Antioxidants 2021, 10, 651. [Google Scholar] [CrossRef]
- Khongkarat, P.; Ramadhan, R.; Phuwapraisirisan, P.; Chanchao, C. Safflospermidines from the Bee Pollen of Helianthus annuus L. Exhibit a Higher in Vitro Antityrosinase Activity than Kojic Acid. Heliyon 2020, 6, e03638. [Google Scholar] [CrossRef]
- Pernal, S.; Currie, R. The Influence of Pollen Quality on Foraging Behavior in Honeybees (Apis mellifera L.). Behav. Ecol. Sociobiol. 2001, 51, 53–68. [Google Scholar] [CrossRef]
- Muth, F.; Francis, J.S.; Leonard, A.S. Bees Use the Taste of Pollen to Determine Which Flowers to Visit. Biol. Lett. 2016, 12, 20160356. [Google Scholar] [CrossRef] [Green Version]
- Fatrcová-Šramková, K.; Nôžková, J.; Kačániová, M.; Máriássyová, M.; Rovná, K.; Stričík, M. Antioxidant and Antimicrobial Properties of Monofloral Bee Pollen. J. Environ. Sci. Health Part B 2013, 48, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qi, Y.; Ritho, J.; Zhang, Y.; Zheng, X.; Wu, L.; Li, Y.; Sun, L. Flavonoid Glycosides as Floral Origin Markers to Discriminate of Unifloral Bee Pollen by LC–MS/MS. Food Control 2015, 57, 54–61. [Google Scholar] [CrossRef]
- Sarabandi, K.; Akbarbaglu, Z.; Peighambardoust, S.H.; Ayaseh, A.; Jafari, S.M. Physicochemical, Antibacterial and Bio-Functional Properties of Persian Poppy-Pollen (Papaver bracteatum) Protein and Peptides. J. Food Measur. Character. 2023, in press. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Nedić, N.; Gašić, U.M.; Špirović Trifunović, B.; Vojt, D.; Tešić, Ž.L.; Pešić, M.B. Phytochemical Profile and Antioxidant Properties of Bee-Collected Artichoke (Cynara scolymus) Pollen. Antioxidants 2021, 10, 1091. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.; Mačukanović-Jocić, M.P.; Špirović Trifunović, B.D.; Vukašinović, I.; Pavlović, V.B.; Pešić, M.B. Fatty Acids of Maize Pollen—Quantification, Nutritional and Morphological Evaluation. J. Cereal. Sci. 2017, 77, 180–185. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Importance of Insoluble-Bound Phenolics to the Antioxidant Potential Is Dictated by Source Material. Antioxidants 2023, 12, 203. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Stanisavljević, N.S.; Gašić, U.M.; Lević, S.; Kojić, M.O.; Tešić, Ž.L.; Nedović, V.; Barać, M.B.; Pešić, M.B. Polyphenol Bioaccessibility and Antioxidant Properties of in Vitro Digested Spray-Dried Thermally-Treated Skimmed Goat Milk Enriched with Pollen. Food Chem. 2021, 351, 129310. [Google Scholar] [CrossRef]
- de Melo, B.K.C.; da Silva, J.A.; da Silva Gomes, R.D.; Custódio, P.P.; de Lira, G.A.; Ramalho, A.M.Z.; Gonçalves, M.C.; da Fonseca, S.B.; do Nascimento Rangel, A.H.; de Fátima Bezerra, M. Physicochemical Composition and Functional Properties of Bee Pollen Produced in Different Locations. Braz. J. Food Technol. 2023, 26. in press. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; de Freitas, A.d.S.; Barth, O.M.; de Almeida-Muradian, L.B. Multivariate Approach Based on Physicochemical Parameters and Biological Potential for the Botanical and Geographical Discrimination of Brazilian Bee Pollen. Food Biosci. 2018, 25, 91–110. [Google Scholar] [CrossRef] [Green Version]
- Asmae, E.G.; Nawal, E.M.; Bakour, M.; Lyoussi, B. Moroccan Monofloral Bee Pollen: Botanical Origin, Physicochemical Characterization, and Antioxidant Activities. J. Food Qual. 2021, 2021, 8877266. [Google Scholar] [CrossRef]
- Kaèániová, M.; Nô¦ková, J.; Fatrcová-Šramková, K.; Kropková, Z.; Kubincová, J. Antioxidant, Antimicrobial Activity and Heavy Metals Content in Pollen of Papaver somniferum L. Ecol. Chem. Eng. A 2010, 17, 97–105. [Google Scholar]
- Campos, M.G.; Webby, R.F.; Markham, K.R. The Unique Occurrence of the Flavone Aglycone Tricetin in Myrtaceae Pollen. Z. Naturforsh. C 2002, 57, 944–946. [Google Scholar] [CrossRef] [Green Version]
- Wollenweber, E.; Dörr, M. Occurrence and Distribution of the Flavone Tricetin and Its Methyl Derivatives as Free Aglycones. Nat. Prod. Commun. 2008, 3, 1293–1298. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Guo, K.; Liu, L.; Tian, W.; Xie, X.; Wen, S.; Wen, C. Integrated Transcriptomic and Metabolomic Data Reveal the Flavonoid Biosynthesis Metabolic Pathway in Perilla frutescens (L.) Leaves. Sci. Rep. 2020, 10, 16207. [Google Scholar] [CrossRef]
- Liu, Y.; Fernie, A.R.; Tohge, T. Diversification of Chemical Structures of Methoxylated Flavonoids and Genes Encoding Flavonoid-O-Methyltransferases. Plants 2022, 11, 564. [Google Scholar] [CrossRef]
- Negri, G.; Teixeira, E.W.; Florêncio Alves, M.L.T.M.; de Camargo Carmello Moreti, A.C.; Otsuk, I.P.; Borguini, R.G.; Salatino, A. Hydroxycinnamic Acid Amide Derivatives, Phenolic Compounds and Antioxidant Activities of Extracts of Pollen Samples from Southeast Brazil. J. Agric. Food Chem. 2011, 59, 5516–5522. [Google Scholar] [CrossRef]
- Negri, G.; Barreto, L.M.R.C.; Sper, F.L.; de Carvalho, C.; das Graças Ribeiro Campos, M. Phytochemical Analysis and Botanical Origin of Apis Mellifera Bee Pollen from the Municipality of Canavieiras, Bahia State, Brazil. Braz. J. Food Technol. 2018, 21, 17616. [Google Scholar] [CrossRef] [Green Version]
- Aylanc, V.; Larbi, S.; Calhelha, R.; Barros, L.; Rezouga, F.; Rodríguez-Flores, M.S.; Seijo, M.C.; El Ghouizi, A.; Lyoussi, B.; Falcão, S.I.; et al. Evaluation of Antioxidant and Anticancer Activity of Mono- and Polyfloral Moroccan Bee Pollen by Characterizing Phenolic and Volatile Compounds. Molecules 2023, 28, 835. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Escuredo, O.; Seijo, M.C.; Rojo, S.; Vilas-Boas, M.; Falcão, S.I. Phenolic Profile of Castanea Bee Pollen from the Northwest of the Iberian Peninsula. Separations 2023, 10, 270. [Google Scholar] [CrossRef]
- Gabriele, M.; Parri, E.; Felicioli, A.; Sagona, S.; Pozzo, L.; Biondi, C.; Domenici, V.; Pucci, L. Phytochemical Composition and Antioxidant Activity of Tuscan Bee Pollen of Different Botanic Origins. Ital. J. Food Sci. 2015, 27, 248–259. [Google Scholar]
- Chelucci, E.; Chiellini, C.; Cavallero, A.; Gabriele, M. Bio-Functional Activities of Tuscan Bee Pollen. Antioxidants 2023, 12, 115. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, M.; Zhu, X.; Liu, R.; Lu, Q. Metabolomics Reveals That Phenolamides Are the Main Chemical Components Contributing to the Anti-Tyrosinase Activity of Bee Pollen. Food Chem. 2022, 389, 133071. [Google Scholar] [CrossRef]
- Lopez, X.; Mujika, J.I.; Blackburn, G.M.; Karplus, M. Alkaline Hydrolysis of Amide Bonds: Effect of Bond Twist and Nitrogen Pyramidalization. J. Phys. Chem. A 2003, 107, 2304–2315. [Google Scholar] [CrossRef]
- Butnariu, M.; Quispe, C.; Herrera-Bravo, J.; Pentea, M.; Sarac, I.; Küşümler, A.S.; Özçelik, B.; Painuli, S.; Semwal, P.; Imran, M.; et al. Papaver Plants: Current Insights on Phytochemical and Nutritional Composition Along with Biotechnological Applications. Oxid. Med. Cell Longev. 2022, 2022, 2041769. [Google Scholar] [CrossRef]
- Végh, R.; Csóka, M.; Sörös, C.; Sipos, L. Food Safety Hazards of Bee Pollen—A Review. Trends Food Sci. Technol. 2021, 114, 490–509. [Google Scholar] [CrossRef]
- Faisal, S.; Badshah, S.L.; Kubra, B.; Emwas, A.-H.; Jaremko, M. Alkaloids as Potential Antivirals. A Comprehensive Review. Nat. Prod. Bioprospect. 2023, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Yoon, S.Y.; Park, S.J.; Park, Y.J. The Anticancer Effect of Natural Plant Alkaloid Isoquinolines. Int. J. Mol. Sci. 2021, 22, 1653. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, S.; Alam, S.; Sultana, A.; Raj, A.; Emon, N.U.; Richi, F.T.; Sharmin, T.; Moon, M.; Park, M.N.; Kim, B. Papaverine: A Miraculous Alkaloid from Opium and Its Multimedicinal Application. Molecules 2023, 28, 3149. [Google Scholar] [CrossRef] [PubMed]
- Manani, R.; Kazemzadeh, G.; Saberi, A.; Sadeghipour, F.; Rahmani, A. Effect of Local Papaverine on Arteriovenous Fistula Maturation in Patients with End-Stage Renal Disease. Braz. J. Nephrol. 2019, 41, 185–192. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Pešić, M.B.; Trbović, D.; Petronijević, R.; Dramićanin, A.M.; Milojković-Opsenica, D.M.; Tešić, Ž.L. The Fatty Acid Profile of Serbian Bee-Collected Pollen—A Chemotaxonomic and Nutritional Approach. J. Apic. Res. 2017, 56, 206. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Mărghitaş, L.A.; Dezmirean, D.S.; Dulf, F.V.; Bunea, A.; Socaci, S.A.; Bobiş, O. Predominant and Secondary Pollen Botanical Origins Influence the Carotenoid and Fatty Acid Profile in Fresh Honeybee-Collected Pollen. J. Agric. Food Chem. 2014, 62, 6306–6316. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Özkök, A.; Keskin, Ş.; Mayda, N.; Urcan, A.C.; Cornea-Cipcigan, M. Bee Collected Pollen as a Value-Added Product Rich in Bioactive Compounds and Unsaturated Fatty Acids: A Comparative Study from Turkey and Romania. LWT 2021, 149, 111925. [Google Scholar] [CrossRef]
- Satranský, M.; Fraňková, A.; Kuchtová, P.; Pazderů, K.; Capouchová, I. Oil Content and Fatty Acid Profile of Selected Poppy (Papaver somniferum L.) Landraces and Modern Cultivars. Plant Soil Environ. 2021, 67, 579–587. [Google Scholar] [CrossRef]
- Melo, D.; Álvarez-Ortí, M.; Nunes, M.A.; Espírito Santo, L.; Machado, S.; Pardo, J.E.; Oliveira, M.B.P.P. Nutritional and Chemical Characterization of Poppy Seeds, Cold-Pressed Oil, and Cake: Poppy Cake as a High-Fibre and High-Protein Ingredient for Novel Food Production. Foods 2022, 11, 3027. [Google Scholar] [CrossRef] [PubMed]
- Senila, L.; Neag, E.; Cadar, O.; Kovacs, M.H.; Becze, A.; Senila, M. Chemical, Nutritional and Antioxidant Characteristics of Different Food Seeds. Appl. Sci. 2020, 10, 1589. [Google Scholar] [CrossRef] [Green Version]
- Gavrilova, V.; Shelenga, T.; Porokhovinova, E.; Dubovskaya, A.; Kon’kova, N.; Grigoryev, S.; Podolnaya, L.; Konarev, A.; Yakusheva, T.; Kishlyan, N.; et al. The Diversity of Fatty Acid Composition in Traditional and Rare Oil Crops Cultivated in Russia. Biol. Commun. 2020, 65. [Google Scholar] [CrossRef] [Green Version]
- Grauso, L.; de Falco, B.; Motti, R.; Lanzotti, V. Corn Poppy, Papaver Rhoeas L.: A Critical Review of Its Botany, Phytochemistry and Pharmacology. Phytochem. Rev. 2021, 20, 227–248. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Pora, B.L.R.; Dong, K.; Hasjim, J. Health Benefits of Docosahexaenoic Acid and Its Bioavailability: A Review. Food Sci. Nutr. 2021, 9, 5229–5243. [Google Scholar] [CrossRef]
- Gercek, Y.C.; Celik, S.; Bayram, S. Screening of Plant Pollen Sources, Polyphenolic Compounds, Fatty Acids and Antioxidant/Antimicrobial Activity from Bee Pollen. Molecules 2021, 27, 117. [Google Scholar] [CrossRef] [PubMed]
- Dulger Altiner, D.; Sandikci Altunatmaz, S.; Sabuncu, M.; Aksu, F.; Sahan, Y. In-Vitro Bioaccessibility of Antioxidant Properties of Bee Pollen in Turkey. Food Sci. Technol.-Camp. 2021, 41, 133–141. [Google Scholar] [CrossRef]
- Bakour, M.; Laaroussi, H.; Ousaaid, D.; Oumokhtar, B.; Lyoussi, B. Antioxidant and Antibacterial Effects of Pollen Extracts on Human Multidrug-Resistant Pathogenic Bacteria. J. Food Qual. 2021, 2021, 5560182. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Assay Sample | TCC 1 [µg/g dw] | TPC [mg/g GAE dw] | TFC [mg/g QE dw] | HCA [mg/g CGAE dw] |
|---|---|---|---|---|
| I | / | 11.59 ± 0.24 a | 12.82 ± 1.36 | 5.96 ± 0.04 a |
| II | / | 2.46 ± 0.07 b | n.d. | 1.14 ± 0.03 b |
| III | 65.05 ± 0.71 | / | / | / |
| tR | Compound Name | Formula | Calculated Mass | m/z Exact Mass | mDa | MS2 Fragments (% Base PEAKS) | I (µg/kg dw) | II (µg/kg dw) |
|---|---|---|---|---|---|---|---|---|
| Organic acid | ||||||||
| 0.80 | Citric acid b | C6H7O7− | 191.0197 | 191.0211 | −1.38 | 103 (1), 111 (100), 112 (6) | 1464.18 | 8.64 |
| 0.67 | Gluconic acid b | C6H11O7− | 195.0510 | 195.0527 | −1.69 | 100 (25), 101 (82), 102 (6), 102 (3), 104 (7), 105 (6), 110 (7), 111 (8), 129 (100), 130 (4), 141 (6), 141 (6), 195 (12) | 1915.16 | / |
| Phenolic acid and derivatives | ||||||||
| 6.06 | Benzoic acid b | C7H5O2− | 121.0295 | 121.0303 | −0.82 | / | 28.70 | 251.96 |
| 4.25 | Hidroxybenzoic acid isomer I b | C7H5O3− | 137.0244 | 137.0253 | −0.85 | / | 38.12 | 98.04 |
| 8.15 | Hidroxybenzoic acid isomer II b | C7H5O3− | 137.0244 | 137.0258 | −1.34 | / | 2.69 | 1.37 |
| 2.49 | Dihidroxybenzoic acid isomer I a | C7H5O4− | 153.0193 | 153.0213 | −1.93 | 108 (100), 109 (84), 110 (7) | 143.41 | 10.35 |
| 6.84 | Dihidroxybenzoic acid isomer II a | C7H5O4− | 153.0193 | 153.0203 | −0.95 | 107 (100), 108 (10), 109 (8), 111 (2), 123 (5), 125 (9), 151 (54), 153 (5) | 153.77 | / |
| 7.31 | Diethoxybenzoate isomer I b | C11H13O4− | 209.0819 | 209.0833 | −1.35 | 101 (24), 103 (77), 106 (15), 106 (12), 117 (58), 118 (20), 118 (16), 119 (100), 120 (15), 121 (12), 122 (19), 129 (10), 143 (18), 150 (31) | 13.05 | / |
| 9.77 | Diethoxybenzoate isomer II b | C11H13O4− | 209.0819 | 209.0857 | −3.74 | 100 (56), 116 (67), 120 (69), 120 (57), 136 (57), 141 (60), 209 (100) | / | 5.81 |
| 7.15 | p−Coumaric acid a | C9H7O3− | 163.0401 | 163.0412 | −1.15 | 104 (2), 117 (8), 119 (100), 120 (11) | 567.10 | 335.32 |
| 6.13 | Aesculetin c | C9H5O4− | 177.0193 | 177.0212 | −1.85 | 105 (61), 106 (10), 107 (16), 107 (10), 108 (5), 117 (7), 121 (12), 122 (4), 133 (37), 134 (63), 135 (100), 136 (8), 148 (4), 149 (17), 177 (8) | / | 84.58 |
| 6.34 | Caffeic acid a | C9H7O4− | 179.0344 | 179.0397 | −5.30 | 106 (4), 107 (10), 108 (4), 109 (2), 117 (7), 133 (2), 134 (71), 135 (100), 136 (10) | 7.51 | / |
| 11.85 | Benzyl caffeate d | C16H13O4− | 269.0814 | 269.0900 | −8.58 | 106 (6), 132 (1), 133 (59), 134 (100), 135 (13), 161 (19), 161 (2), 162 (2), 183 (2), 197 (7) | 2.50 | / |
| 7.89 | Ferulic acid a | C10H9O4− | 193.0506 | 193.0526 | −1.96 | 106 (13), 108 (5), 108 (4), 117(8), 117(9), 118(3), 130(5), 131(3), 132 (4), 133 (57), 134 (100), 135 (8), 148 (3) | / | 125.32 |
| 8.49 | 5−Carboxyvanillic acid b | C9H7O6− | 211.0248 | 211.0271 | −2.32 | 107 (100), 108 (7), 108 (1), 109 (2), 123 (1), 151 (61), 152 (6) | / | 610.81 |
| Flavone | ||||||||
| 11.80 | Chrysin a | C15H9O4− | 253.0501 | 253.0541 | −4.01 | 101 (6), 107 (25), 119 (22), 143 (54), 144 (8), 145 (21), 151 (10), 165 (8), 167 (8), 180 (6), 181 (12), 185 (7), 209 (18), 253 (100), 254 (21) | 70.53 | / |
| 8.49 | Tricetin e | C15H9O7− | 301.0354 | 301.0400 | −4.64 | 109 (12), 133 (100), 134 (11), 135 (29), 137 (37), 139 (28), 165 (22), 167 (21), 175 (7), 192 (7), 201 (8), 227 (7), 255 (15), 301 (45), 302 (10) | 3048.97 | / |
| 9.37 | Luteolin e | C15H9O6− | 285.0405 | 285.0446 | −4.11 | 107 (15), 121 (3), 133 (100), 134 (10), 149 (13), 151 (32), 152 (3), 175 (15), 199 (11), 201 (6), 217 (7), 241 (3), 243 (3), 285 (46), 286 (10) | 4398.15 | 209.24 |
| 7.00 | Apigenin 6,8−di−C−glucoside e | C27H29O15− | 593.1506 | 593.1572 | −6.58 | 133 (100), 133 (14), 134 (14), 135 (16), 179 (25), 299 (12), 300 (12), 353 (54), 383 (35), 473 (47), 503 (13) | 200.17 | / |
| Flavanone and derivatives | ||||||||
| 12.00 | Pinocembrin a | C15H11O4− | 255.0663 | 255.0693 | −3.00 | 107 (98), 108 (27), 135 (19), 136 (25), 145 (89), 151 (100), 169 (21), 171 (88), 172 (28), 183 (16), 185 (37), 211 (19), 213 (65), 255 (60) | 345.60 | / |
| 12.23 | Pinobanksin 3−O−acetate f | C17H13O6− | 313.0718 | 313.0751 | −3.29 | 107 (4), 143 (6), 145 (3), 151 (2), 165 (2), 181 (2), 185 (2), 197 (5), 209 (6), 211 (2), 211 (2), 253 (100), 254 (22), 255 (3), 271 (5) | 179.90 | / |
| Flavonols and derivatives | ||||||||
| 10.24 | Kaempferol a | C15H9O6− | 285.0405 | 285.0437 | −3.24 | 107 (8), 108 (5), 133 (10), 143 (7), 151 (8), 157 (6), 159 (8), 171 (7), 185 (12), 187 (10), 211 (8), 229 (10), 239 (8), 285 (100), 286 (23) | 414.85 | 364.717 |
| 11.73 | Kaempferol−methyl−ether g | C16H11O6− | 299.0561 | 299.0596 | −3.44 | 107 (5), 111 (8), 119 (27), 135 (15), 143 (6), 145 (4), 151 (10), 176 (33), 178 (100), 180 (11), 185 (5), 187 (37), 188 (6), 193 (11), 297 (9) | / | <LOQ |
| 8.43 | Kaempferol 7−O−hexoside g | C21H19O11− | 447.0927 | 447.1009 | −8.16 | 151 (1), 284 (5), 285 (100), 286 (20), 287 (3) | 412.69 | / |
| 8.16 | Kaempferol 3−O−hexoside g | C21H19O11− | 447.0927 | 447.1020 | −9.34 | 151 (3), 227 (19), 228 (3), 255 (36), 256 (13), 257 (3), 284 (100), 285 (42), 286 (7), 300 (4), 301 (3), 327 (2), 447 (15), 448 (5) | 232.39 | / |
| 6.53 | Kaempferol 3−O−(6″−pentosyl)hexoside g | C26H27O15− | 579.1350 | 579.1426 | −7.61 | 283 (3), 284 (100), 285 (23), 339 (10) | 710.39 | / |
| 7.82 | Kaempferol 3−O−(2″−pentosyl)hexoside g | C26H27O15− | 579.1350 | 579.1444 | −9.42 | 227 (4), 255 (8), 256 (3), 284 (100), 285 (36), 429 (3) | 885.46 | 700.168 |
| 7.99 | Kaempferol 3−O−(6″−rhamnosyl)hexoside g | C27H29O15− | 593.1506 | 593.1576 | −6.99 | 178 (2), 227 (3), 255 (6), 256 (2), 284 (100), 285 (30), 286 (5), 429 (3) | 103.68 | 74.06 |
| 7.51 | Kaempferol 3,7−di−O−hexoside g | C27H29O16− | 609.1456 | 609.1526 | −6.96 | 255 (5), 256 (1), 283 (31), 284 (8), 285 (12), 286 (2), 446 (27), 447 (18), 448.09702(4), 489 (2), 609 (100) | 714.93 | 311.17 |
| 8.36 | Kaempferol 3−O−(6″−pentosyl)acetyl−hexoside g | C28H29O16− | 621.1456 | 621.1550 | −9.39 | 151 (2), 227 (4), 255 (7), 256 (3), 284 (100), 285 (27), 286 (6), 286 (4), 435 (7) | / | 24.75 |
| 8.02 | Kaempferol 3−O−(2″−hexosyl)acetyl−hexoside g | C29H31O17− | 651.1561 | 651.1621 | −5.98 | 227 (4), 255 (9), 256 (2), 283 (11), 284 (100), 285 (42), 286 (7), 429 (2), 471 (4), 488 (6), 489 (3), 609 (2) | 1.99 | 21.40 |
| 7.04 | Kaempferol 3−O−(2″−hexosyl−6″−pentosyl)hexoside g | C32H37O20− | 741.1878 | 741.1950 | −7.16 | 116 (2), 116 (3), 151 (2), 255 (3), 283 (2), 284 (44), 285 (23), 286 (5), 561 (4), 625 (5), 741 (100) | 323.07 | / |
| 7.10 | Kaempferol 3−O−(2″,6″−di−hexosyl)hexoside g | C33H39O21− | 771.1984 | 771.2073 | −8.92 | 179 (1), 227 (1), 255 (3), 284 (23), 285 (23), 286 (3), 429 (2), 591 (2), 609 (7), 771 (100) | 36.42 | / |
| 8.28 | Quercetin 3−O−pentoside g | C20H17O11− | 433.0771 | 433.0825 | −5.37 | 133 (1), 165 (1), 300 (3), 301 (100), 302 (20), 303 (3) | 157.22 | / |
| 7.89 | Quercetin 3−O−hexoside g | C21H19O12− | 463.0877 | 463.0920 | −4.29 | 151 (4), 179 (3), 243 (1), 255 (6), 256 (1), 271 (11), 272 (3), 273 (1), 300 (100), 301 (46), 302 (9), 303 (1), 463 (3) | 23.07 | / |
| 7.47 | Quercetin 3−O−(6″−pentosyl)hexoside g | C26H27O16− | 595.1299 | 595.1384 | −8.53 | 178 (2), 255 (2), 271 (5), 299 (2), 300 (100), 301 (30) | 487.11 | / |
| 7.21 | Quercetin 3−O−(2″−hexosyl)hexoside g | C27H29O17− | 625.1405 | 625.1498 | −9.29 | 151 (1), 178 (4), 255 (2), 271 (5), 299 (3), 300 (100), 301 (38), 302 (7), 303 (1), 445 (2), 463 (11) | 577.59 | / |
| 9.22 | Isorhamnetin h | C16H11O7− | 315.0505 | 315.0553 | −4.83 | 134 (6), 136 (31), 165 (6), 199 (6), 200 (7), 201 (9), 202 (7), 216 (8), 227 (8), 228 (12), 243 (6), 272 (9), 299 (7), 300 (100), 301 (22) | 321.30 | / |
| 8.29 | Isorhamnetin 3−O−hexoside h | C22H21O12− | 477.1033 | 477.1120 | −8.67 | 215 (1), 243 (2), 255 (4), 271 (17), 272 (6), 299 (100), 300 (48), 301 (9), 302 (1), 314 (49), 315 (21), 316 (4), 462 (4) | <LOQ | / |
| 7.82 | Isorhamnetin 3−O−(2″−pentosyl)hexoside h | C27H29O16− | 609.1456 | 609.1538 | −8.24 | 209 (2), 271 (6), 272 (2), 299 (44), 300 (20), 301 (3), 313 (2), 314 (100), 315 (44), 316 (8), 429 (5) | 53.85 | 1.78 |
| 7.69 | Isorhamnetin 3−O−(2″−hexosyl)rhamnoside h | C28H31O16− | 623.1612 | 623.1695 | −8.28 | 209 (2), 271 (4), 272 (2), 299 (40), 300 (19), 301 (9), 314 (100), 315 (29), 316 (5), 459 (3) | / | <LOQ |
| 8.29 | Isorhamnetin 3−O−(2″−rhamnosyl)hexoside h | C28H31O16− | 623.1612 | 623.1699 | −8.70 | 137 (2), 271 (4), 299 (47), 300 (17), 301 (5), 313 (2), 314 (100), 315 (35), 316 (7), 443 (4) | / | <LOQ |
| 7.42 | Isorhamnetin 3−O−(2″−hexosyl)hexoside h | C28H31O17− | 639.1561 | 639.1619 | −5.84 | 209 (2), 271 (5), 272 (1), 299 (41), 300 (19), 301 (3), 313 (2), 314 (100), 315 (43), 316 (8), 459 (5), 624 (2) | <LOQ | / |
| 7.34 | Isorhamnetin 3−O−(2″−hexosyl−6″−pentosyl)hexoside h | C33H39O21− | 771.1984 | 771.2048 | −6.43 | 209 (1), 271 (2), 299 (12), 300 (7), 313 (1), 314 (24), 315 (17), 316 (3), 459 (2), 756 (1), 771 (100) | 47.42 | / |
| Other phenolics | ||||||||
| 7.01 | (Epi)catechin 3−O−gallate g | C22H17O10− | 441.0827 | 441.0853 | −2.55 | 123 (28), 125 (32), 151 (16), 163 (15), 178 (35), 179 (41), 189 (62), 219 (32), 231 (32), 255 (37), 261 (17), 299 (28), 341 (16), 343 (100), 344 (22) | / | 50.81 |
| TOTAL | 18,083.0 | 3290.3 | ||||||
| tR | Base Fragment | Formula | Calculated Mass | ppm | mDa | Compound Name | m/z Exact Mass | MS2 Fragments | I | II | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8.53 | 287.0553 | C21H21O11+ | 449.1084 | 6.04 | 2.71 | Cyanidin 3-O-glucoside | 449.1111 | 287 (100), 288, 289 | / | + | |
| 6.57 | 287.0549 | C26H29O15+ | 581.1506 | 4.05 | 2.35 | Cyanidin 3-O-(6″-pentosyl)hexoside isomer I | 581.153 | 287 (100), 288, 289 | + | / | |
| 7.81 | 287.0549 | C26H29O15+ | 581.1506 | 4.05 | 2.35 | Cyanidin 3-O-(6″-pentosyl)hexoside isomer II | 581.1531 | 287 (100), 288, 289 | / | + | |
| 7.30 | 303.0503 | C27H31O17+ | 627.1561 | 0.92 | 0.58 | Delphinidin 3-O-(6″-O-hexosyl)hexoside | 627.1563 | 303 (100), 304, 305, 145, 127 | + | / |
| tR | Base Fragment | Formula | Calculated Mass | ppm | mDa | Compound Name | m/z Exact Mass | MS2 Fragments | I | II |
|---|---|---|---|---|---|---|---|---|---|---|
| 5.74 | 147.0444 | C13H19N2O2+ | 235.1447 | −4.48 | −1.05 | Coumaroyl putrescine | 235.1436 | 147 (100), 119, 148, 120, 149 | + | |
| 4.52 | 100.0000 | C12H29N4O+ | 245.2341 | −3.82 | −0.94 | Acetyl spermine | 245.2332 | 100 (100), 112, 113, 101, 129, 171 | + | |
| 3.24 | 163.0381 | C13H19N2O3+ | 251.1396 | 2.12 | 0.53 | Caffeoyl putrescine isomer I | 251.1401 | 163 (100), 135, 145, 117, 164, 120, 107, 146, 136, 118, 165 | + | |
| 4.29 | 163.0385 | C13H19N2O3+ | 251.1396 | 2.12 | 0.53 | Caffeoyl putrescine isomer II | 251.1401 | 163 (100), 135, 145, 117, 164, 146, 107, 136, 118, 165, 121 | + | |
| 1.40 | 147.0438 | C16H26N3O2+ | 292.2025 | 0.68 | 0.2 | Coumaroyl spermidine | 292.2027 | 147 (100), 119, 204, 148, 112, 205, 120, 129, 149 | + | |
| 2.16 | 177.0555 | C17H28N3O3+ | 322.2131 | −2.38 | −0.77 | Feruloyl spermidine | 322.2123 | 177 (100), 145, 234, 178, 117, 146, 149, 112, 235, 129, 146 | + | |
| 2.35 | 100.0764 | C21H35N4O3+ | 391.2709 | −1.06 | −0.42 | Coumaroyl acetyl spermine isomer I | 391.2705 | 100 (100), 204, 147, 171, 129, 205, 275, 112, 148, 172, 119, 276, 155, 130, 206 | + | |
| 3.16 | 100.0764 | C21H35N4O3+ | 391.2709 | −1.06 | −0.42 | Coumaroyl acetyl spermine isomer II | 391.2705 | 100 (100), 204, 147, 171, 205, 275, 129, 101, 112, 148, 172, 276, 119, 206 | + | |
| 4.51 | 100.0756 | C21H35N4O3+ | 391.2709 | −1.06 | −0.42 | Coumaroyl acetyl spermine isomer III | 391.2705 | 100 (100), 204, 147, 171, 205, 275, 129, 101, 112, 148, 172, 276, 119, 206 | + | |
| 5.50 | 204.1032 | C21H35N4O3+ | 391.2709 | −1.06 | −0.42 | Coumaroyl acetyl spermine isomer IV | 391.2705 | 204 (100), 147, 100, 205, 171, 129, 275, 245, 112, 148, 119, 276, 391, 172, 155 | + | |
| 8.00 | 147.0442 | C21H35N4O3+ | 391.2709 | −1.06 | −0.42 | Coumaroyl acetyl spermine isomer V | 391.2705 | 147 (100), 204, 100, 171, 129, 112, 245, 275, 205, 148, 374, 119, 228, 391, 276, 246, 172, 154 | + | |
| 2.80 | 100.0760 | C21H35N4O4+ | 407.2658 | 1.4 | 0.57 | Caffeoyl acetyl spermine | 407.2664 | 100 (100), 220, 163, 171, 291, 221, 129, 112, 101, 172, 164, 292, 145, 222, 130, 166, 212, 135, 144, 155, 113 | + | |
| 9.21 | 177.0538 | C23H27N2O5+ | 411.192 | 3.66 | 1.5 | Coumaroyl feruloyl putrescine | 411.1935 | 177 (100), 147, 145, 178, 148, 218, 235, 117, 414, 121, 119, 370, 146, 109, 149, 119, 146, 107, 414, 265 | + | |
| 6.60 | 100.0768 | C23H37N4O4+ | 433.2815 | 3.74 | 1.62 | Coumaroyl diacetyl spermine isomer I | 433.2831 | 100 (100), 147, 171, 204, 287, 433, 288, 172, 148, 101, 434, 129, 119, 317, 205, 270, 188, 269, 275 | + | |
| 6.87 | 100.0771 | C23H37N4O4+ | 433.2815 | 3.74 | 1.62 | Coumaroyl diacetyl spermine isomer II | 433.2831 | 100 (100), 147, 171, 204, 287, 433, 148, 288, 172, 101, 434, 205, 119, 416, 317, 120, 188, 112, 270, 275, 203 | + | |
| 7.15 | 100.0762 | C23H37N4O4+ | 433.2815 | 18.51 | 8.02 | Coumaroyl diacetyl spermine isomer III | 433.2895 | 100 (100), 147, 171, 204, 287, 416, 433, 417, 317, 270, 205, 172, 148, 288, 112, 434, 101, 373, 119, 269, 154 | + | |
| 7.82 | 147.0442 | C25H32N3O4+ | 438.2393 | 4.38 | 1.92 | Dicoumaroyl spermidine | 438.2412 | 147 (100), 204, 292, 205, 275, 218, 148, 293, 438, 119, 221, 129, 421, 112, 276, 146, 439, 203 | + | + |
| 9.23 | 177.0541 | C24H29N2O6+ | 441.2026 | 3.71 | 1.64 | Diferuloyl putrescine | 441.2042 | 177 (100), 145, 178, 116, 265, 117, 146, 248, 163, 444, 149, 441, 149, 443, 266, 241, 179, 136, 133 | + | |
| 7.89 | 204.1019 | C30H41N4O5+ | 537.3077 | 11.55 | 6.2 | Dicoumaroyl acetyl spermine | 537.3139 | 204 (100), 391, 537, 147, 275, 538, 392, 205, 171, 520, 276, 539, 129, 245, 100, 317, 148, 373, 374, 112, 519, 119, 521, 393, 203 | + | + |
| 8.05 | 567.3178 | C31H43N4O6+ | 567.3183 | 8.35 | 4.74 | Coumaroyl feruloyl acetyl spermine | 567.323 | 567 (100), 204, 568, 177, 391, 421, 234, 147, 275, 205, 392, 422, 569, 171, 145, 305, 550, 245, 235, 129, 178 | + | |
| 9.12 | 433.2816 | C32H43N4O6+ | 579.3183 | 5.77 | 3.34 | Dicoumaroyl diacetyl spermine | 579.3216 | 433 (100), 416, 434, 147, 204, 519, 287, 417, 171, 100, 415, 313, 520, 435, 537, 317, 275, 148, 205 | + | + |
| 9.93 | 438.2377 | C34H38N3O6+ | 584.2761 | 1.26 | 0.74 | Tricoumaroyl spermidine | 584.2768 | 438 (100), 204, 147, 439, 420, 292, 275, 421, 585, 440, 205, 218, 293, 586, 130, 148, 422, 276, 119, 318 | + | |
| 9.07 | 601.3018 | C35H43N4O5+ | 599.3233 | 2.76 | 1.65 | Dicoumaroyl benzoyl spermine | 599.325 | 601 (100), 599, 204, 602, 453, 275, 233, 600, 147, 162, 454, 276, 205, 234, 435, 203, 379, 603, 436, 129, 148, 105 | + | |
| 8.65 | 641.3340 | C37H45N4O6+ | 641.3339 | 4.82 | 3.09 | Tricoumaroyl spermine | 641.3370 | 641 (100), 275, 204, 642, 495, 147, 496, 276, 643, 477, 203, 205, 478, 129, 421, 497, 644, 148, 112, 119, 349 | + | + |
| 9.67 | 537.3072 | C39H47N4O7+ | 683.3445 | 4.57 | 3.13 | Tricoumaroyl acetyl spermine | 683.3476 | 537 (100), 538, 204, 519, 391, 520, 147, 275, 392, 374, 521, 205, 276, 417, 683 | + | + |
| 9.87 | 369.2245 | C40H49N4O8+ | 713.355 | 1.63 | 1.16 | Dicoumaroyl acetyl feruloyl spermine | 713.3562 | 369 (100), 537, 370, 567, 538, 568, 367, 519, 177, 204, 275, 391, 520, 549, 539, 550, 569, 368, 147 | + | |
| 10.42 | 641.3330 | C46H51N4O8+ | 787.3707 | 3.57 | 2.81 | Tetracoumaroyl spermine | 787.3735 | 641 (100), 642, 623, 275, 643, 204, 624, 495, 478, 147, 322, 276, 477, 625, 479 | + | |
| Nonidentified phenylamide | ||||||||||
| 5.94 | 315.1083 | / | / | / | / | Coumaroyl phenylamide derivatives | 330.1366 | 315 (100), 297, 330, 298, 147, 314, 296, 152, 316, 312, 190, 129, 331, 204, 271, 299, 123, 280, 188, 282, 269, 171, 137 | + | |
| 9.71 | 147.0443 | / | / | / | / | Coumaroyl phenylamide derivatives | 342.1786 | 147 (100), 119, 204, 148, 100, 112, 171, 129, 120, 205, 245, 175 | + | |
| tR | Base Fragment | Formula | Calculated Mass | ppm | mDa | Compound Name | m/z Exact Mass | MS2 Fragments | I | II |
|---|---|---|---|---|---|---|---|---|---|---|
| Benzylisoquinoline alkaloids | ||||||||||
| 5.88 | 107.0498 | C16H18NO3+ | 272.1287 | −2.09 | −0.57 | Norcoclaurine | 272.1281 | 107 (100), 143, 161, 108, 115, 123, 145, 209, 194, 237, 144, 191, 127, 240, 162, 133, 121, 131, 117, 181, 164, 116, 149, 226, 219, 255, 147 | + | |
| 6.78 | 107.0498 | C17H20NO3+ | 286.1443 | 3.43 | 0.98 | Coclaurine | 286.1453 | 107 (100), 100, 143, 108, 209, 175, 237, 115, 137, 194, 191, 145, 160, 254, 171, 131, 144, 181, 219, 238, 210, 239, 154, 121, 176, 178 | + | |
| 6.76 | 107.0500 | C18H22NO3+ | 300.16 | 3.44 | 1.03 | N−methylcoclaurine | 300.1610 | 107 (100), 237, 175, 143, 108, 209, 197, 137, 121, 145, 115, 269, 238, 131, 160, 254, 191, 194, 179, 163, 144, 176, 178, 239, 225 | + | + |
| 6.41 | 123.0440 | C18H22NO4+ | 316.1549 | 8.59 | 2.72 | 3′−Hydroxy−N−methylcoclaurine | 316.1576 | 123 (100), 192, 143, 175, 137, 177, 193, 253, 207, 115, 124, 161, 225, 179, 176, 178, 144, 285, 213, 160, 235, 149, 241 | + | |
| 7.13 | 192.1013 | C19H24NO4+ | 330.1705 | 2.02 | 0.67 | Reticuline | 330.1712 | 192 (100), 137, 143, 178, 123, 175, 151, 206, 193, 330, 189, 177, 138, 299, 179, 180, 152, 167, 115, 176, 285, 267, 227, 239, 207, 255, 149, 145 | + | + |
| 8.30 | 192.1013 | C20H22NO4+ | 340.1549 | 3.58 | 1.22 | Papaverine | 340.1561 | 192 (100), 165, 193, 340, 150, 149, 166, 177, 190, 176, 341, 292, 135, 324, 105, 119, 151, 148, 293, 133, 325 | + | + |
| 8.12 | 192.1020 | C20H24NO4+ | 342.1705 | 9.25 | 3.17 | 3,4−Dihydropapaverine | 342.1737 | 192 (100), 165, 193, 342, 150, 177, 190, 151, 166, 176, 343, 310, 327, 148, 137, 326, 194, 105, 294, 312, +178, 133, 131 | + | + |
| 7.41 | 137.0609 | C20H26NO4+ | 344.1862 | 0.92 | 0.32 | Tetrahydropapaverine | 344.1865 | 137 (100), 206, 189, 151, 192, 174, 175, 143, 282, 158, 190, 207, 138, 298, 313, 165, 193, 191, 281, 152, 159, 176, 344, 241, 253, 177 | + | + |
| 8.15 | 189.0784 | C20H20NO5+ | 354.1341 | 7.21 | 2.55 | Papaveraldine | 354.1367 | 189 (100), 188, 354, 149, 206, 275, 190, 165, 355, 247, 295, 336, 265, 135, 207, 235, 175, 293, 276, 267, 177, 195, 323, 295, 150, 178, 237, 305, 321, 107, 306, 337, 324, 311 | + | + |
| 8.07 | 206.1170 | C21H28NO4+ | 358.2018 | 2.7 | 0.97 | Laudanosine | 358.2028 | 206 (100), 151, 189, 207, 165, 174, 190, 158, 296, 152, 191, 327, 281, 159, 297, 312, 192, 150, 175, 136, 177, 284, 107, 145, 193, 135 | + | + |
| Berberine alkaloids | ||||||||||
| 8.54 | 176.0710 | C19H18NO4+ | 324.1236 | 5.61 | 1.82 | Stylopine | 324.1254 | 176 (100), 149, 324, 177, 119, 325, 178, 150, 174, 135, 249, 189, 277, 120, 326, 151, 188, 219, 175, 307 | + | + |
| 7.60 | 178.0883 | C19H20NO4+ | 326.1392 | −1.63 | −0.53 | Nandinine | 326.1387 | 178 (100), 151, 326, 179, 163, 176, 119, 149, 327, 152, 311, 135, 219, 191, 177, 277, 180, 294, 136 | + | + |
| 7.45 | 178.0867 | C19H22NO4+ | 328.1549 | 4.32 | 1.42 | Scoulerine | 328.1563 | 178 (100), 151, 179, 328, 163, 119, 180, 176, 329, 152, 313, 296, 137, 164, 191, 312, 177, 190, 136, 298, 135, 279 | + | + |
| 7.73 | 192.1018 | C20H22NO4+ | 340.1549 | 4.46 | 1.52 | Canadine | 340.1564 | 192 (100), 193, 340, 177, 341, 190, 194, 178, 149, 191 | + | |
| 8.40 | 320.0908 | C20H20NO6+ | 370.1291 | 3.88 | 1.44 | Papaverrubin E | 370.1305 | 320 (100), 321, 338, 177, 352, 176, 322, 292, 353, 174, 339, 149, 190, 303, 293, 291, 262, 290, 263, 135, 310, 178, 308 | + | + |
| Other isoquinoline alkaloids | ||||||||||
| 6.50 | 123.0447 | C18H20NO4+ | 314.1392 | 2.76 | 0.87 | Laurolitsine | 314.1401 | 123 (100), 298, 192, 299, 143, 175, 178, 314, 137, 151, 300, 253, 285, 179, 177, 107, 207, 193, 115, 176, 161, 315, 152, 225, 124, 213, 270, 284, 316, 235, 254, 283, 241, 227, 255, 237, 256, 209, 301, 286, 282, 296, 223, 252 | + | |
| 8.74 | 352.1181 | C21H22NO6+ | 384.1447 | 3.61 | 1.39 | Hydrastine | 384.1461 | 352 (100), 190, 320, 353, 188, 334, 303, 291, 263, 321, 189, 191, 293, 235, 176, 322, 304, 149, 335, 294, 292, 233, 324 | + | + |
| Fatty Acid (FA) | % of Total Fatty Acids |
|---|---|
| Capric acid (C10:0) | 0.91 ± 0.14 h |
| Palmitic acid (C16:0) | 10.92 ± 0.43 d |
| Stearic acid (C18:0) | 13.72 ± 0.78 c |
| Oleic acid (C18:1) | 10.73 ± 0.74 d |
| Linoleic acid (C18:2) | 7.25 ± 0.48 e |
| α-Linolenic acid (C18:3) | 22.98 ± 0.82 b |
| Eicosadienoic acid (C20:2) | 5.20 ± 0.22 f |
| Erucic acid (C22:1) | 3.01 ± 0.12 g |
| Docosahexaenoic acid (C22:3) | 25.26 ± 0.62 a |
| Total SFAs * | 25.55 |
| Total UFAs | 74.45 |
| Assay Sample | TAC 1 [mg/g AAE dw] | FRP [mg/g AAE dw] | CUPRAC [mg/g AAE dw] | DPPH∙ [µmol/g TE dw] |
|---|---|---|---|---|
| I | 28.92 ± 1.06 a | 5.58 ± 0.04 a | 69.00 ± 0.96 a | 16.71 ± 0.87 a |
| II | 0.92 ± 0.01 b | 0.35 ± 0.03 b | 22.78 ± 0.66 b | 2.94 ± 0.12 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostić, A.Ž.; Milinčić, D.D.; Špirović Trifunović, B.; Nedić, N.; Gašić, U.M.; Tešić, Ž.L.; Stanojević, S.P.; Pešić, M.B. Monofloral Corn Poppy Bee-Collected Pollen—A Detailed Insight into Its Phytochemical Composition and Antioxidant Properties. Antioxidants 2023, 12, 1424. https://doi.org/10.3390/antiox12071424
Kostić AŽ, Milinčić DD, Špirović Trifunović B, Nedić N, Gašić UM, Tešić ŽL, Stanojević SP, Pešić MB. Monofloral Corn Poppy Bee-Collected Pollen—A Detailed Insight into Its Phytochemical Composition and Antioxidant Properties. Antioxidants. 2023; 12(7):1424. https://doi.org/10.3390/antiox12071424
Chicago/Turabian StyleKostić, Aleksandar Ž., Danijel D. Milinčić, Bojana Špirović Trifunović, Nebojša Nedić, Uroš M. Gašić, Živoslav Lj. Tešić, Sladjana P. Stanojević, and Mirjana B. Pešić. 2023. "Monofloral Corn Poppy Bee-Collected Pollen—A Detailed Insight into Its Phytochemical Composition and Antioxidant Properties" Antioxidants 12, no. 7: 1424. https://doi.org/10.3390/antiox12071424











