Differential Effects of High Fat Diets on Resilience to H2O2-Induced Cell Death in Mouse Cerebral Arteries: Role for Processed Carbohydrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care and Use
2.2. Diet Compositions
2.3. Preparation of Isolated Posterior Cerebral Arteries
2.4. Vascular ROS Production
2.5. Cell Death
2.6. Mitochondrial Membrane Potential
2.7. Ca2+ Photometry
2.8. Experimental Interventions
2.9. Data Analysis and Statistics
3. Results
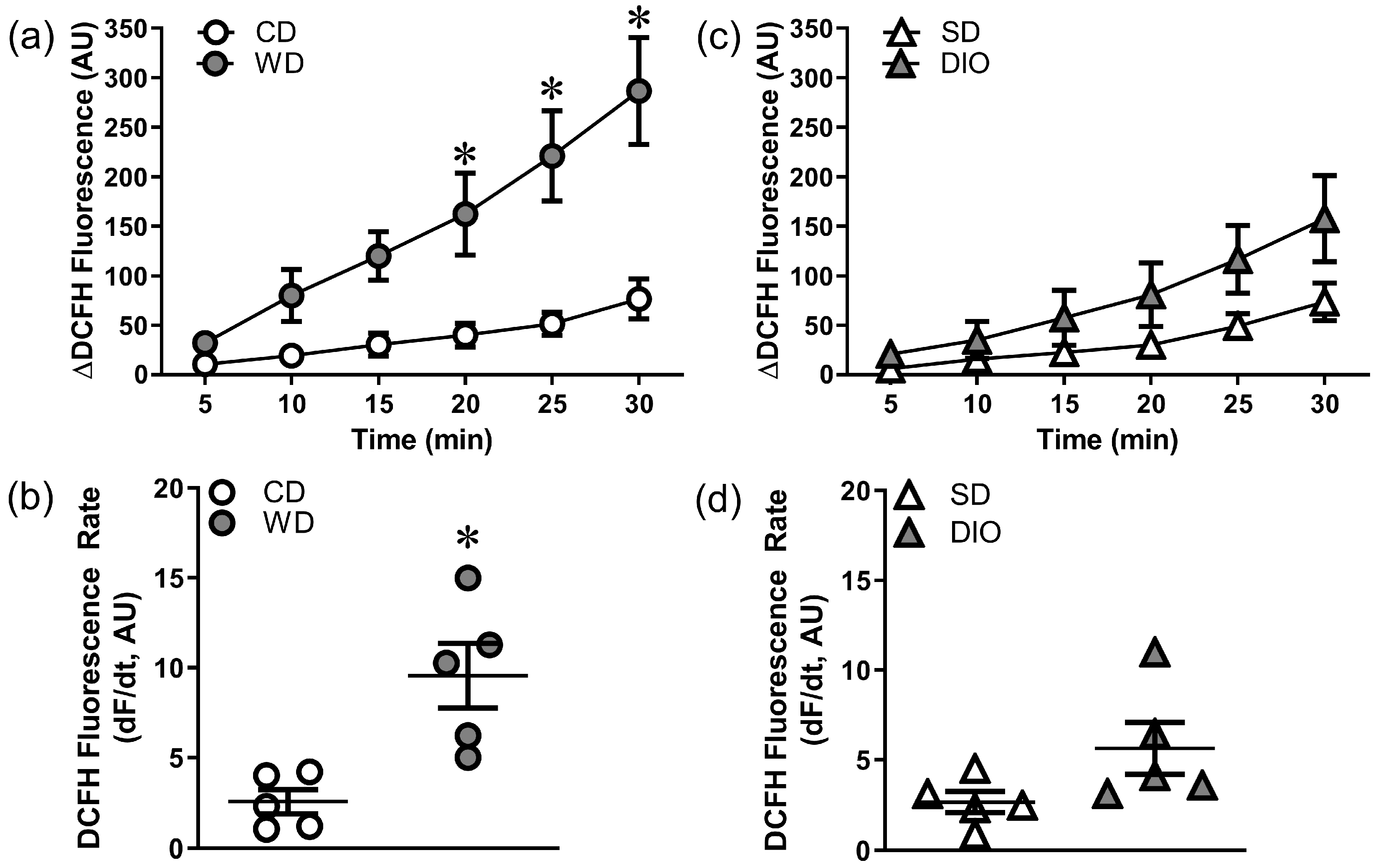
3.1. Effects of High Fat Diets on Vascular Oxidative Stress
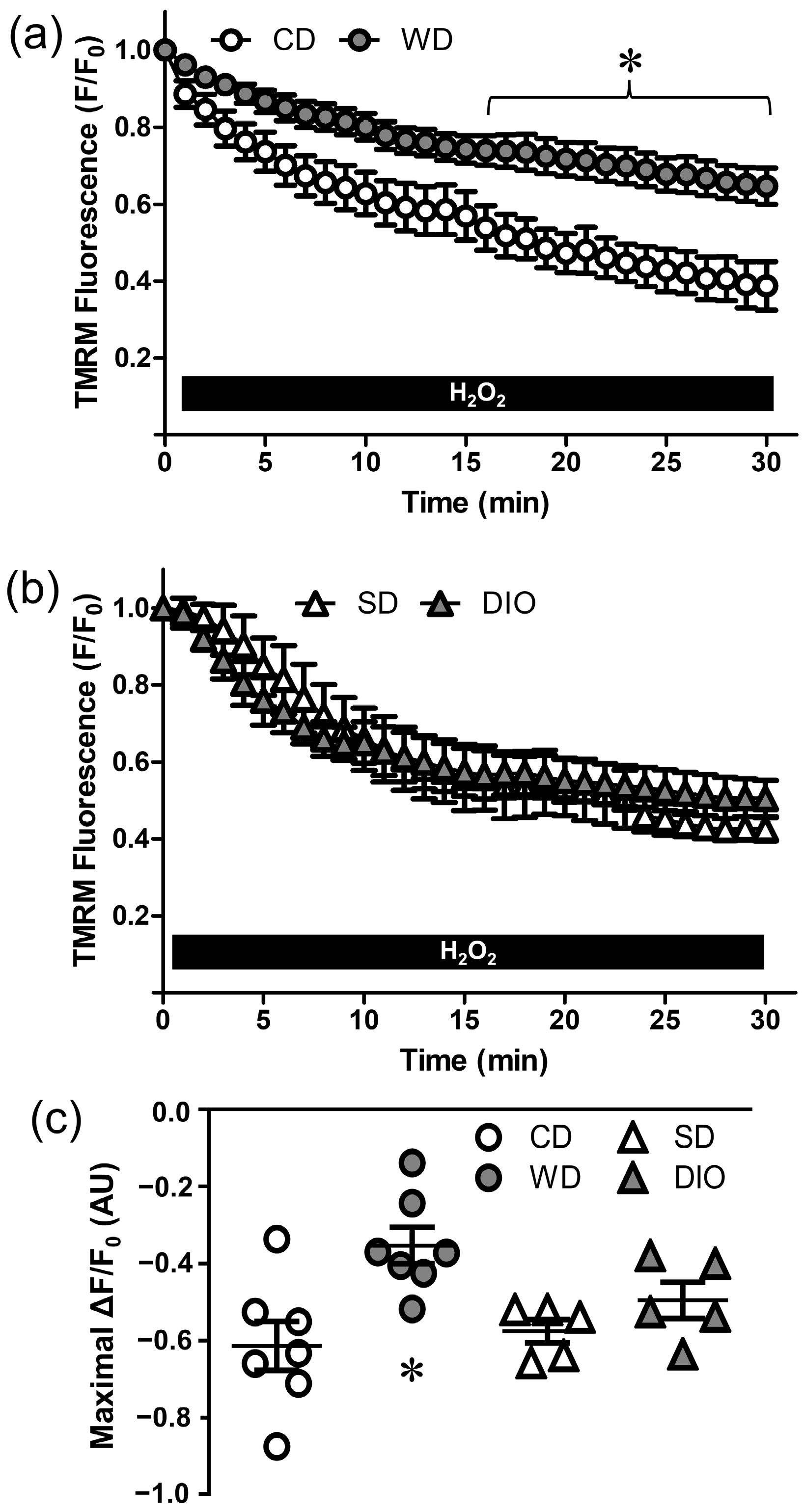
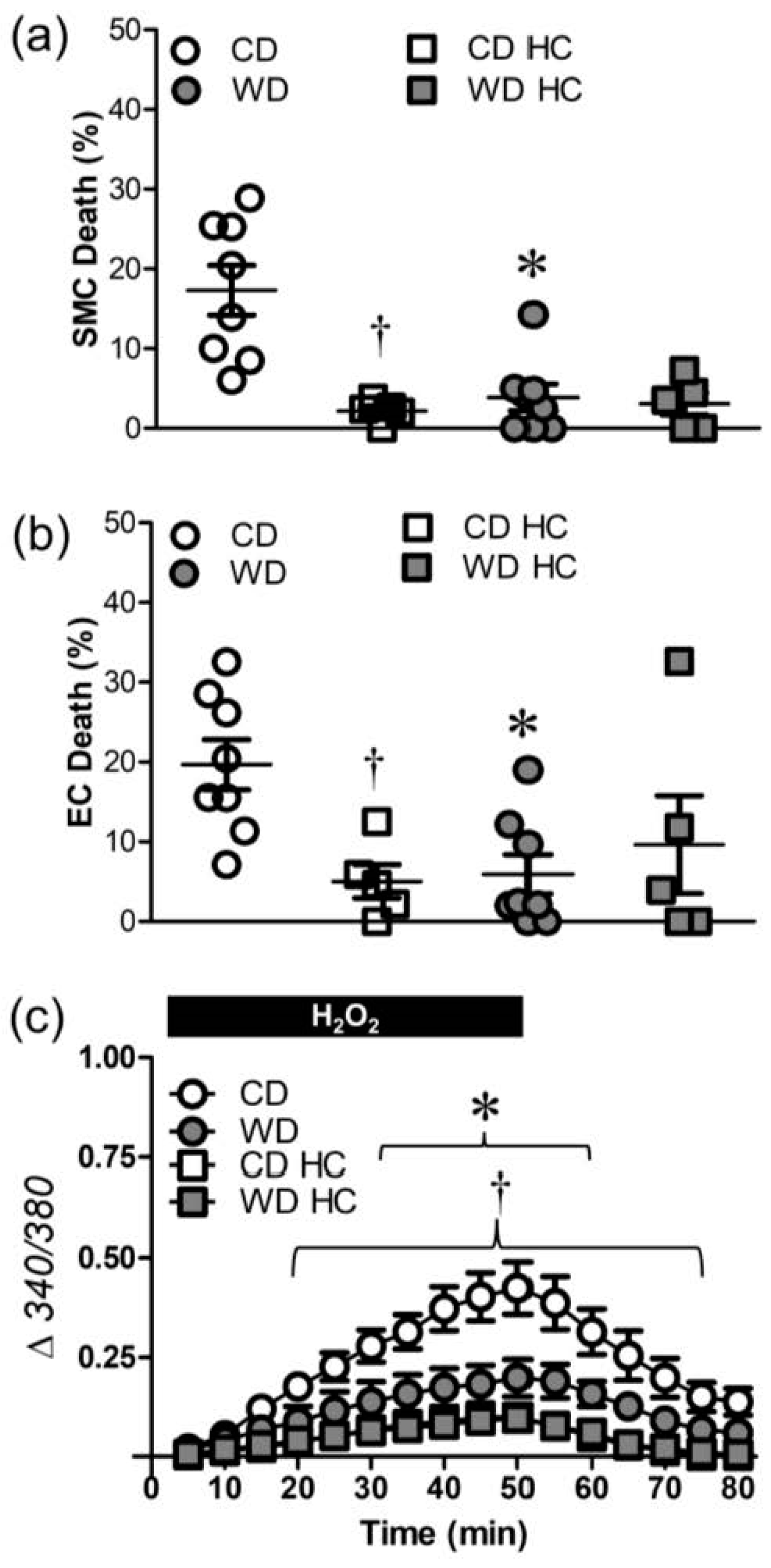
3.2. WD, but Not DIO, Increases Resilience to H2O2
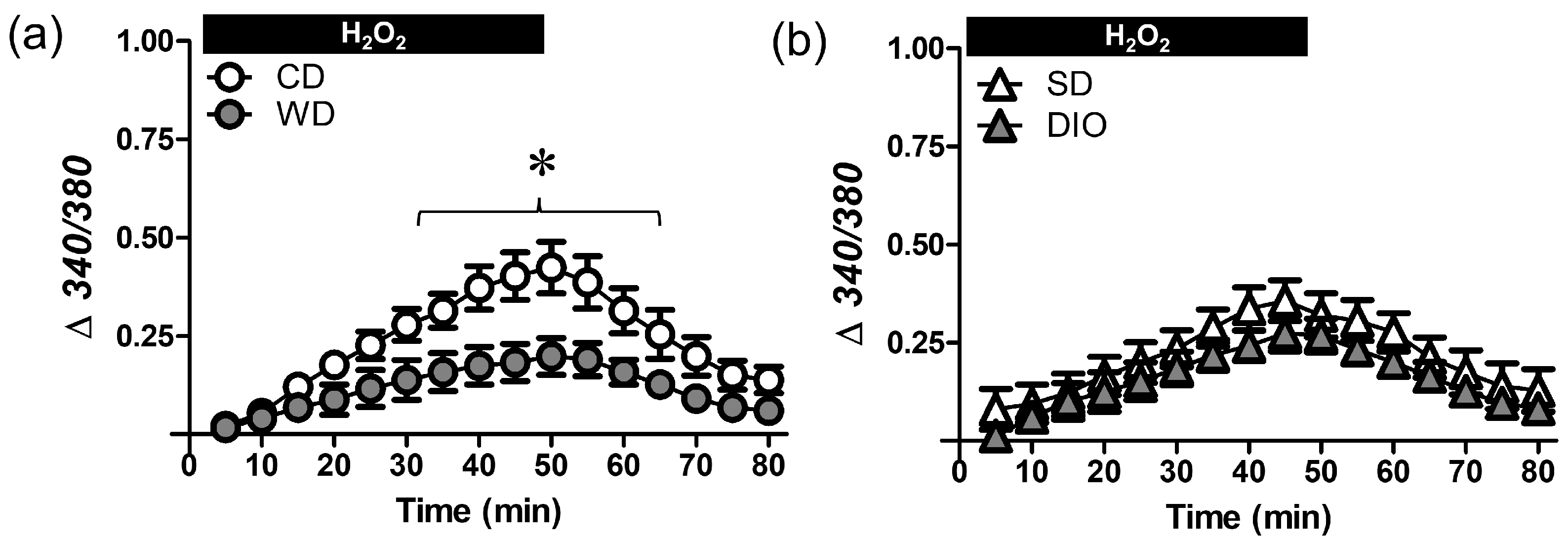
3.3. WD Attenuates the [Ca2+]i Response Induced by H2O2
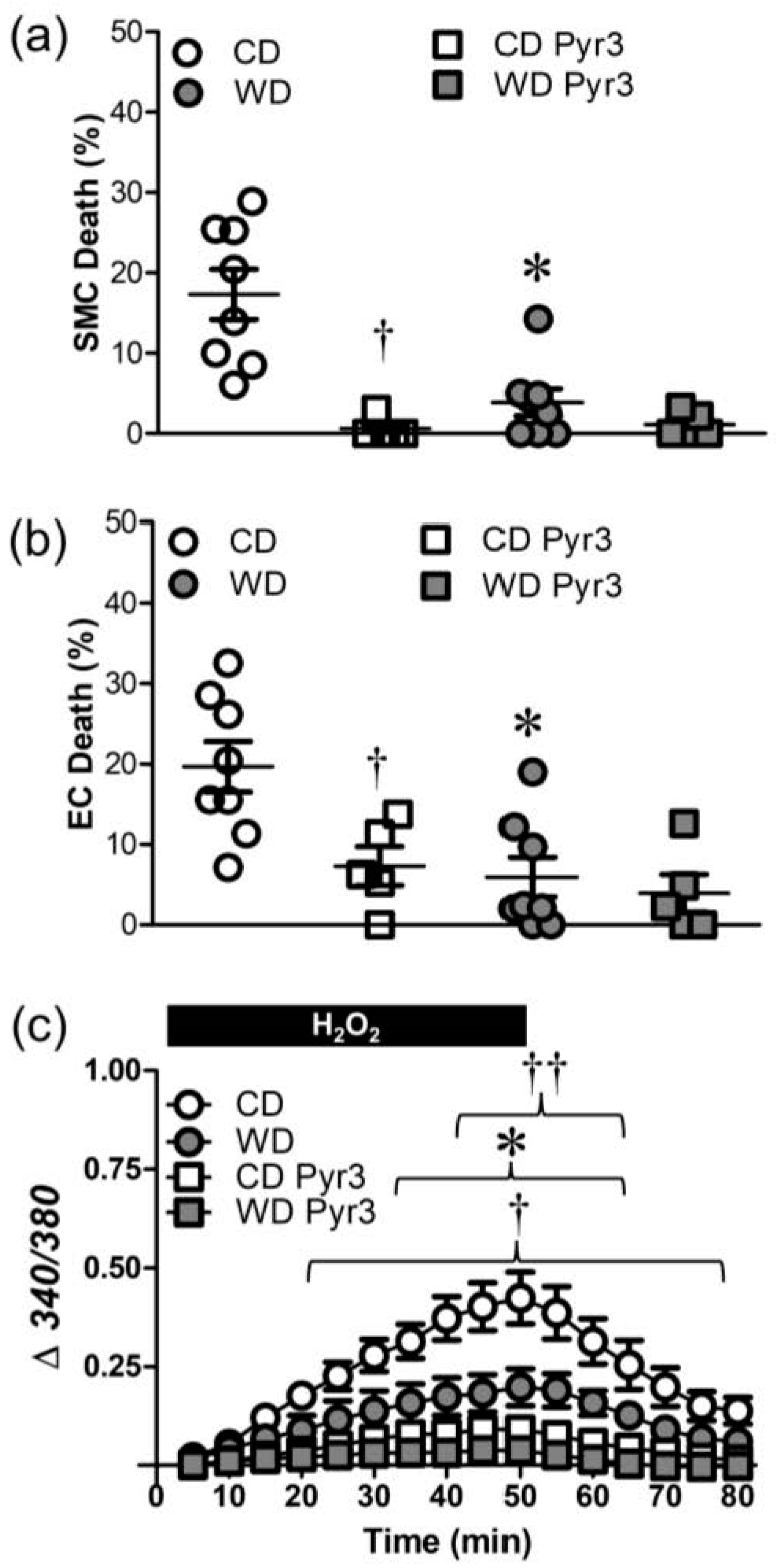
3.4. WD Diminishes Ca2+ Influx through TRP Channels Induced by H2O2
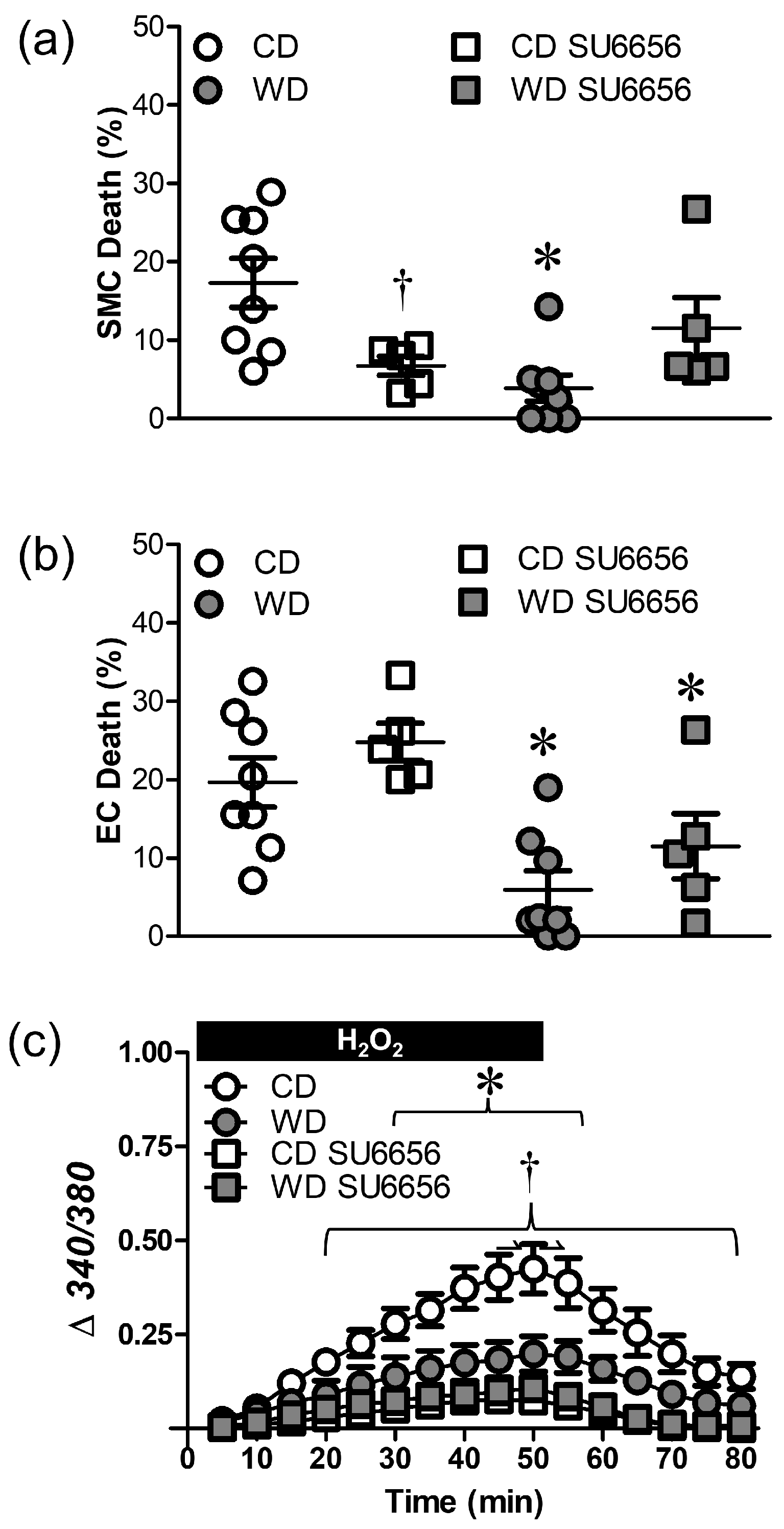
3.5. Src Kinases Contribute to Cell Death during H2O2 Exposure
4. Discussion
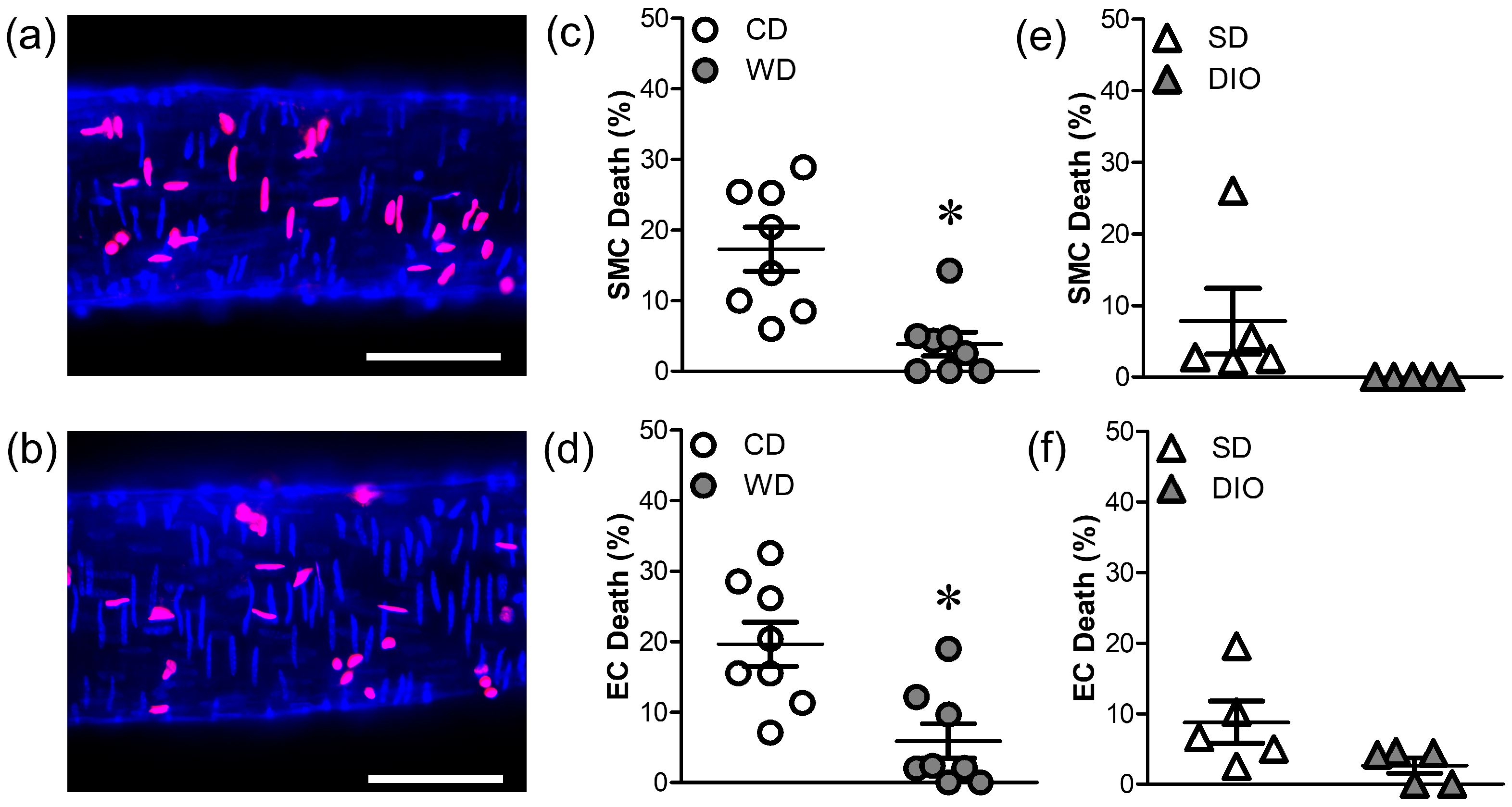
4.1. Effects of High Fat Diet on Oxidative Stress and Vascular Cell Death
4.2. Changes in TRPV4 and TRPC3 Channel-Mediated Ca2+ Entry Contribute to Differences in H2O2-Induced Cell Death
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rakhra, V.; Galappaththy, S.L.; Bulchandani, S.; Cabandugama, P.K. Obesity and western diet: How we got here. Mol. Med. 2020, 117, 536–538. [Google Scholar]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Mastsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Wonisch, W.; Falk, A.; Dundl, I.; Winklhofer-Roob, B.M.; Lindschinger, M. Oxidative stress increases continuously with BMI and age with unfavourable profiles in males. Aging Male 2012, 15, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Ghanim, H.; Ravishankar, S.; Sia, C.L.; Viswanathan, P.; Mohanty, P.; Dandona, P. Prolonged reactive oxygen species generation and nuclear factor-kappa B activation after a high-fat, high-carbohydrate meal in the obese. J. Clin. Endocrinol. Metab. 2007, 92, 4476–4479. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.L.; Norhaizan, M.E. Effect of high-fat diets on oxidative stress, cellular inflammatory response, and cognitive function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [Green Version]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Asargar, M.A.; Masood, A.; Sofi, M.A.; Banie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Inzucchi, S.E.; Sawan, C.; Macko, R.F.; Furie, K.L. Obesity: A stubbornly obvious target for stroke prevention. Stroke 2012, 44, 278–286. [Google Scholar] [CrossRef]
- Kurth, T.; Gaziano, J.M.; Berger, K.; Kase, C.S.; Rexrode, K.M.; Cook, N.R.; Buring, J.E.; Manson, J.E. Body mass index and the risk of stroke in men. Arch. Intern. Med. 2002, 162, 2557–2562. [Google Scholar] [CrossRef]
- Rodrigo, R.; Fernandez-Gajardo, R.; Guitierrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeuric opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Sua, J.; Liu, W.; Altura, B.T.; Altura, B.M. Hydrogen peroxide induces apoptosis in cerebral vascular smooth muscle cells: Possible relation to neurodegenerative diseases and strokes. Brain Res. Bull. 2003, 62, 101–106. [Google Scholar] [CrossRef]
- Konno, T.; Melo, E.P.; Chambers, J.E.; Avezov, E. Intracellular sources of ROS/H2O2 in health and neurodegeneration: Spotlight on endoplasmic reticulum. Cells 2021, 10, 233. [Google Scholar] [CrossRef]
- Norton, C.E.; Sinkler, S.Y.; Jacobsen, N.L.; Segal, S.S. Advanced age protects resistance arteries of mouse skeletal muscle from oxidative stress through attenuating apoptosis induced by hydrogen peroxide. J. Physiol. 2019, 597, 3801–3816. [Google Scholar] [CrossRef]
- Shaw, R.L.; Norton, C.E.; Segal, S.S. Apoptosis in resistance arteries induced by hydrogen peroxide: Greater resilience of endothelium versus smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1625–H1633. [Google Scholar] [CrossRef]
- Norton, C.E.; Shaw, R.L.; Mittler, R.; Segal, S.S. Endothelial cells promote smooth muscle cell resilience to H2O2-induced cell death in mouse cerebral arteries. Acta Physiol. 2022, 235, e13819. [Google Scholar] [CrossRef]
- Norton, C.E.; Jacobsen, N.L.; Sinkler, S.Y.; Manrique-Acevedo, C.; Segal, S.S. Female sex and Western-style diet protect mouse resistance arteries during acute oxidative stress. Am. J. Physiol. Cell Physiol. 2020, 318, C627–C639. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, D.; Shi, L.; Tu, Q.; Yu, Y.; Chen, J. AdipoRon accelerates bone repair of calvarial defect in diet-induced obesity mice. Heliyon 2023, 9, e13975. [Google Scholar] [CrossRef] [PubMed]
- Manrique, C.; DeMarco, V.G.; Aroor, A.R.; Mugerfield, I.; Garro, M.; Habibi, J.; Hayden, M.R.; Sowers, J.R. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a western diet. Endocrinology 2013, 154, 3632–3642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manrique, C.; Habibi, J.; Aroor, A.R.; Jia, G.; Hayden, M.R.; Garro, M.; Martinez-Lemus, L.A.; Ramirez-Perez, F.I.; Klein, T.; Meininger, G.A.; et al. Dipeptidyl peptidase-4 inhibition with linagliptin prevents western diet-induced vascular abnormalities in female mice. Cardiovasc. Diabetol. 2016, 15, 94. [Google Scholar] [CrossRef] [Green Version]
- Socha, M.J.; Boerman, E.M.; Behringer, E.J.; Shaw, R.L.; Domeier, T.L.; Segal, S.S. Advanced age protects microvascular endothelium from aberrant Ca2+ influx and cell death induced by hydrogen peroxide. J. Physiol. 2015, 593, 2155–2169. [Google Scholar] [CrossRef] [Green Version]
- Snow, J.B.; Norton, C.E.; Sands, M.A.; Weise Cross, L.; Yan, S.; Herbert, L.M.; Sheak, J.R.; Gonzales Bosc, L.V.; Walker, B.R.; Kanagy, N.L.; et al. Intermittent hypoxia augments pulmonary vasoconstrictor reactivity through PCKβ/mitochondrial oxidant signaling. Am. J. Respir. Cell Mol. Biol. 2020, 62, 732–746. [Google Scholar] [CrossRef]
- Narayanan, D.; Xi, Q.; Pfeffer, L.M.; Jagger, J.H. Mitochondria control functional CaV1.2 expression in smooth muscle cells of cerebral arteries. Circ. Res. 2010, 107, 631–641. [Google Scholar] [CrossRef]
- Loor, G.; Kondapalli, J.; Iwase, H.; Chandel, N.S.; Waypa, G.B.; Guzy, R.D.; Vanden Hoek, T.L.; Schumacker, P.T. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim. Biophys. Acta 2011, 1813, 1382–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamuru, S.; Li, X.; Attene-Ramos, M.S.; Huang, R.; Lu, J.; Shou, L.; Shen, M.; Tice, R.R.; Austin, C.P.; Xia, M. Application of a homogenous membrane potential assay to assess mitochondrial function. Physiol. Genom. 2012, 44, 495–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonkusare, S.K.; Bonev, A.D.; Ledoux, J.; Liedtke, W.; Kotlikoff, M.I.; Heppner, T.; Hill-Eubanks, D.C.; Nelson, M.T. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 2012, 336, 597–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochukov, M.Y.; Balasubramanian, A.; Abramowitz, J.; Birnbaumer, L.; Marelli, S.P. Activation of endothelial transient receptor potential C3 chanel is required for small conductance calcium-activated potassium channel activation and sustained endothelial hyperpolarization and vasodilation of cerebral artery. J. Am. Heart Assoc. 2014, 3, e000913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blake, R.A.; Broome, M.A.; Liu, X.; Wu, J.; Gishizky, M.; Sun, L.; Courtneidge, S.A. SU6656, a selective Src family kinase inhibitor, used to probe growth factor signaling. Mol. Cell Biol. 2000, 20, 9018–9027. [Google Scholar] [CrossRef] [Green Version]
- Buettner, R.; Scholmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, H.; Singh, N. Hydrogen peroxide induces apoptosis in HeLa cells through mitochondrial pathway. Mitochondrion 2007, 7, 367–373. [Google Scholar] [CrossRef]
- Ermak, G.; Davies, K.J. Calcium and oxidative stress: From cell signaling to cell death. Mol. Immunol. 2001, 38, 713–721. [Google Scholar] [CrossRef]
- Lin, H.Y.; Shen, S.C.; Lin, C.W.; Yang, L.Y.; Chen, Y.C. Baicalein inhibition of hydrogen peroxide-induced apoptosis via ROS-dependent heme oxygenase 1 gene expression. Mol. Cell Res. 2007, 1773, 1073–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegierski, T.; Lewandrowski, U.; Muller, B.; Sickmann, A.; Walz, G. Tyrosine phosphorylation modulates the activity of TRPV4 in response to definded stimuli. J. Biol. Chem. 2009, 284, 2923–2933. [Google Scholar] [CrossRef] [Green Version]
- Rossig, L.; Dimmeler, S.; Zeiher, A.M. Apoptosis in the vascular wall and atherosclerosis. Basic Res. Cardiol. 2001, 96, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Sugamura, K.; Keaney, J.F. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Muneer, P.M.; Chandra, N.; Haorah, J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2015, 51, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.; Franca, L.M.; Zhang, Y.; Paes, A.M.A.; Gerdes, A.M.; Carrillo-Sepulveda, M.A. Western diet triggers Toll-like receptor 4 signaling-induced endothelial dysfunction in female Wistar rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1735–H1747. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and oxidative stress: A general overview of mechanisms and implications in human disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Strom, J.; Xu, B.; Tian, X.; Chen, M.Q. Nrf2 protects mitochondrial decay by oxidative stress. FASEB J. 2016, 30, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, N.; Maurya, C.K.; Arha, D.; Avisetti, D.R.; Parathapan, A.; Raj, P.S.; Raghu, K.G.; Kalivendi, S.V.; Tammrakar, A.K. Fructose induces mitochondrial dysfunction and triggers apoptosis in skeletal muscle cells by provoking oxidative stress. Apoptosis 2015, 20, 930–947. [Google Scholar] [CrossRef]
- Liesa, M.; Shirihai, O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef] [Green Version]
- Thakore, P.; Earley, S. Transient receptor potential channels and endothelial cell calcium signaling. Compr. Physiol. 2019, 9, 1249–1277. [Google Scholar]
- Ma, X.; Cheng, K.T.; Wong, C.O.; O’Neil, R.G.; Birnbaumer, L.; Ambudkar, I.S.; Yao, X. Heteromeric TRPV4-C1 channels contribute to store-operated Ca2+ entry in vascular endothelial cells. Cell Calcium 2011, 50, 502–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, J.S.; Lee, K.J.; Huang, M.; Cho, C.H.; Lee, E.H. Heteromeric TRPC3 with TRPC1 formed via its ankyrin repeats regulates the resting cytosolic Ca2+ levels in skeletal muscle. Biochem. Biophys. Res. Commun. 2014, 44, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Uchida, Y.; Tachikawa, M.; Ohtsuki, S.; Couraud, P.O.; Suzuki, T.; Terasaki, T. Oxidative stress-induced activation of Abl and Src kinases rapidly induces P-glycoprotein internalization via phosphorylation of caveolin-1 on tyrosine-14, decreasing cortisol efflux at the blood–brain barrier. J. Cereb. Blood Flow Metab. 2020, 40, 420–436. [Google Scholar] [CrossRef]
- Chan, H.; Chou, H.; Duran, M.; Gruenewald, J.; Waterfield, M.D.; Ridley, A.; Timms, J.F. Major role of epidermal growth factor receptor and Src kinases in promoting oxidative stress-dependent loss of adhesion and apoptosis in epithelial cells. J. Biol. Chem. 2009, 285, 4307–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasquez, G.; Wedel, B.J.; Kawasaki, B.T.; St John Bird, G.; Putney, J.W. Obligatory role of Src kinase in the signaling mechanism for TRPC3 cation channels. J. Biol. Chem. 2004, 279, 40521–40528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, B.T.; Liao, Y.; Birnbaumer, L. Role of Src in C3 transient receptor potential channel function and evidence for a heterogeneous makeup of receptor- and store-operated Ca2+ entry channels. Proc. Natl. Acad. Sci. USA 2006, 103, 335–340. [Google Scholar] [CrossRef]
- Takahashi, N.; Mizuno, Y.; Kozai, D.; Yamomoto, S.; Kiyonaka, S.; Shibata, T.; Uchida, K.; Mori, Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels 2008, 2, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Inoue, R.; Mori, T.; Takahashi, N.; Yamomoto, S.; Hara, Y.; Tominaga, M.; Shimizu, S.; Sato, Y.; Mori, Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2006, 2, 596–607. [Google Scholar] [CrossRef]
- Anderon, M.E. Oxidant stress promotes disease by activating CaMKII. J. Mol. Cell. Cardiol. 2015, 89, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Mishra, R.; Simonson, M.S. Ca2+/calmodulin-dependent protein kinase II stimulates c-fos transcription and DNA synthesis by a Src-based mechanism in glomerular mesangial cells. J. Am. Soc. Nephrol. 2003, 14, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Ottolini, M.; Hong, K.; Cope, E.L.; Daneva, Z.; DeLalio, L.J.; Sokolowski, J.D.; Marziano, C.; Nguyen, N.Y.; Altschmied, J.; Haendeler, J.; et al. Local peroxynitrite impairs endothelial TRPV4 channels and elevates blood pressure in obesity. Circulation 2021, 141, 1318–1333. [Google Scholar] [CrossRef] [PubMed]
- Startek, J.B.; Boonen, B.; Talavera, K.; Meseguer, V. TRP channels as sensors of chemically-induced changes in cell membrane mechanical properties. Int. J. Mol. Sci. 2019, 20, 371. [Google Scholar] [CrossRef] [Green Version]
- Holzer, R.G.; Park, E.J.; Li, N.; Tran, H.; Chen, M.; Choi, C.; Solina, G.; Karin, M. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell 2011, 147, 173–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauerhofer, C.; Afonyushkin, T.; Oskolkova, O.V.; Hellauer, K.; Gesslbauer, B.; Schmerda, J.; Schmerda, J.; Ke, Y.; Zimmer, A.; Birukova, A.A.; et al. Low concentrations of oxidized phospholipids increase stress tolerance of endothelial cells. Antioxidants 2022, 11, 1741. [Google Scholar] [CrossRef]
- Gillman, M.W.; Cupples, L.A.; Millen, B.E.; Ellison, R.C.; Wolf, P.A. Inverse association of dietary fat with development of ischemic stroke in men. JAMA 1997, 278, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Oesch, L.; Tatlisumak, T.; Arnold, M.; Sarikaya, H. Obesity paradox in stroke—Myth or reality? A systematic review. PLoS ONE 2017, 12, e0171334. [Google Scholar] [CrossRef] [Green Version]
| Group | BW (g) | n |
|---|---|---|
| CD | 29.9 ± 0.3 | 15 |
| WD | 45.2 ± 0.9 * | 15 |
| SD | 33.6 ± 1.1 | 5 |
| DIO | 45.1 ± 1.6 # | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norton, C.E.; Shaw, R.L.; Segal, S.S. Differential Effects of High Fat Diets on Resilience to H2O2-Induced Cell Death in Mouse Cerebral Arteries: Role for Processed Carbohydrates. Antioxidants 2023, 12, 1433. https://doi.org/10.3390/antiox12071433
Norton CE, Shaw RL, Segal SS. Differential Effects of High Fat Diets on Resilience to H2O2-Induced Cell Death in Mouse Cerebral Arteries: Role for Processed Carbohydrates. Antioxidants. 2023; 12(7):1433. https://doi.org/10.3390/antiox12071433
Chicago/Turabian StyleNorton, Charles E., Rebecca L. Shaw, and Steven S. Segal. 2023. "Differential Effects of High Fat Diets on Resilience to H2O2-Induced Cell Death in Mouse Cerebral Arteries: Role for Processed Carbohydrates" Antioxidants 12, no. 7: 1433. https://doi.org/10.3390/antiox12071433
APA StyleNorton, C. E., Shaw, R. L., & Segal, S. S. (2023). Differential Effects of High Fat Diets on Resilience to H2O2-Induced Cell Death in Mouse Cerebral Arteries: Role for Processed Carbohydrates. Antioxidants, 12(7), 1433. https://doi.org/10.3390/antiox12071433







