A Review on the Involvement of Heat Shock Proteins (Extrinsic Chaperones) in Response to Stress Conditions in Aquatic Organisms
Abstract
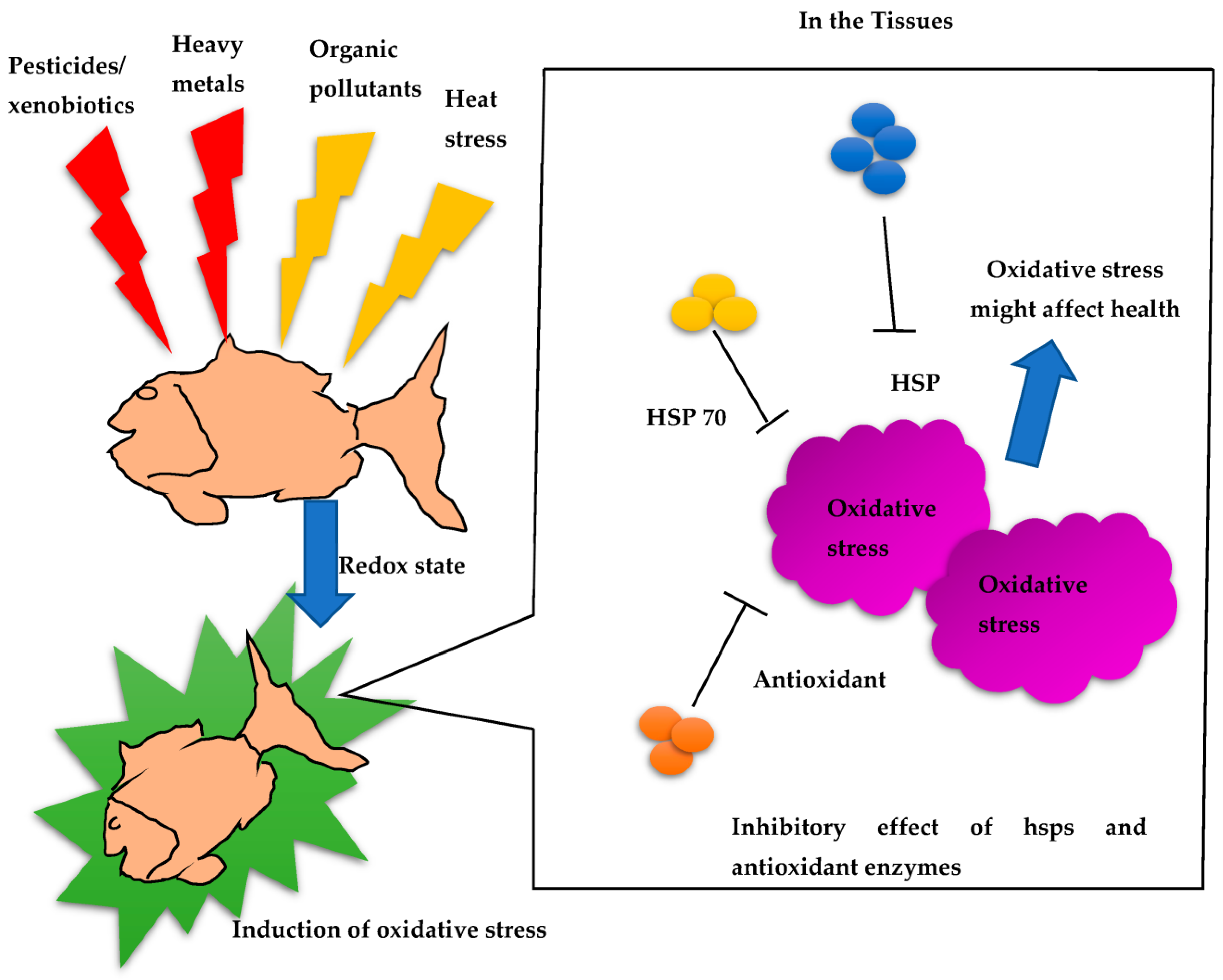
:1. Introduction
2. Different Types of Stress Factors Involved in the Expression of HSPs
2.1. Desiccation, Temperature, and Hypoxia/Anoxia Stress
2.2. Osmotic Stress
2.3. Ultraviolet Radiation Stress
2.4. Heavy Metal Stress
2.5. Effect of Endocrine Disruptor Chemicals in Heat Shock Proteins
2.6. Other Toxicants
3. The Role of Heat Shock Proteins in Aquaculture Disease Management
3.1. Immunology and Stress Response
3.2. Crustaceans: Exploring the Link between Environmental Stresses and Disease
Shellfish Diseases and the Role of Pathogens
3.3. Expression of Heat Shock Proteins in Fish
3.4. Expression of Heat Shock Proteins in Mollusk
3.5. Heat Shock Protein Expression in Insects
3.6. Heat Shock Proteins in Myxozoan Parasites (Cnidaria)
4. Defense Mechanisms of Heat Shock Proteins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, R.J.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. Stress; Acta: Montreal, QC, Canada, 1950. [Google Scholar]
- Easton, D.P.; Rutledge, P.S.; Spotila, J.R. Heat shock protein induction and induced thermal tolerance are independent in adult salamanders. J. Exp. Zool. 1987, 241, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Pickering, K.E.; Dickerson, R.R.; Huffman, G.J.; Boatman, J.F.; Schanot, A. Trace gas transport in the vicinity of frontal convective clouds. J. Geophys. Res. Atmos. 1988, 93, 759. [Google Scholar] [CrossRef]
- Sumpter, J.P. The endocrinology of stress. In Fish Stress and Health in Aquaculture; Cambridge University Press: Cambridge, UK, 1997; Volume 819, pp. 95–118. [Google Scholar]
- Wedemeyer, G.A. Effects of rearing conditions on the health and physiological quality of fish in intensive culture. In Fish Stress and Health in Aquaculture; Cambridge University Press: Cambridge, UK, 1997; pp. 35–71. [Google Scholar]
- Locke, M. The cellular stress response to exercise: Role of stress proteins. Exerc. Sport Sci. Rev. 1997, 25, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.K. New Jobs for Ancient Chaperones. Sci. Am. 2008, 299, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Martinod, S.R.; Bernard, B.; Serrar, M.; Gutierrez, G. Release of heat-shock protein Hsp72 after exercise and supplementation with an Opuntia ficus indica extract TEX-OE. In Proceedings of the American Association of Equine Practitioners, Orlando, FL, USA, 1–5 December 2007; Volume 53, pp. 72–76. [Google Scholar]
- Sandilands, J.; Drynan, K.; Roberts, R.J. Preliminary studies on the enhancement of storage time of chilled milt of Atlantic salmon, Salmo salar L., using an extender containing the Tex-OE®heat shock-stimulating factor. Aquac. Res. 2010, 41, 568–571. [Google Scholar] [CrossRef]
- Tomanek, L. Variation in the heat shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J. Exp. Biol. 2010, 213, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Somero, G.N. The Physiology of Global Change: Linking Patterns to Mechanisms. Annu. Rev. Mar. Sci. 2012, 4, 39–61. [Google Scholar] [CrossRef]
- Barber, R.D.; Harmer, D.W.; Coleman, R.A.; Clark, B.J.; Namachivayam, K.; Blanco, C.L.; MohanKumar, K.; Jagadeeswaran, R.; Vasquez, M.; McGill-Vargas, L.; et al. GAPDH as a housekeeping gene: Analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol. Genom. 2005, 21, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Huey, R.B. Physiological Consequences of Habitat Selection. Am. Nat. 1991, 137, S91–S115. [Google Scholar] [CrossRef] [Green Version]
- Morley, N.J.; Lewis, J.W. Temperature stress and parasitism of endothermic hosts under climate change. Trends Parasitol. 2014, 30, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Labaude, S.; Moret, Y.; Cézilly, F.; Reuland, C.; Rigaud, T. Variation in the immune state of Gammarus pulex (Crustacea, Amphipoda) according to temperature: Are extreme temperatures a stress? Dev. Comp. Immunol. 2017, 76, 25–33. [Google Scholar] [CrossRef]
- Cossins, A.R.; Bowler, K. Rate compensations and capacity adaptations. In Temperature Biology of Animals; Springer: Dordrecht, The Netherlands, 1987; pp. 155–203. [Google Scholar] [CrossRef]
- Montagna, M.C. Effect of temperature on the survival and growth of freshwater prawns Macrobrachium borellii and Palaemonetes argentinus (Crustacea, Palaemonidae). Iheringia Série Zool. 2011, 101, 233–238. [Google Scholar] [CrossRef]
- Hassan, S.; Habashy, W.; Ghoname, M.; Elnaggar, A. Blood hematology and biochemical of four laying hen strains exposed to acute heat stress. Int. J. Biometeorol. 2023, 67, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Bedulina, D.; Meyer, M.F.; Gurkov, A.; Kondratjeva, E.; Baduev, B.; Gusdorf, R.; Timofeyev, M.A. Intersexual differences of heat shock response between two amphipods (Eulimnogammarus verrucosus and Eulimnogammarus cyaneus) in Lake Baikal. PeerJ 2017, 5, e2864. [Google Scholar] [CrossRef] [Green Version]
- Pörtner, H. Climate variations and the physiological basis of temperature dependent biogeography: Systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 132, 739–761. [Google Scholar] [CrossRef]
- Morris, J.P.; Thatje, S.; Hauton, C. The use of stress-70 proteins in physiology: A re-appraisal. Mol. Ecol. 2013, 22, 1494–1502. [Google Scholar] [CrossRef]
- Madeira, D.; Mendonça, V.; Dias, M.; Roma, J.; Costa, P.M.; Larguinho, M.; Vinagre, C.; Diniz, M.S. Physiological, cellular and biochemical thermal stress response of intertidal shrimps with different vertical distributions: Palaemon elegans and Palaemon serratus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 183, 107–115. [Google Scholar] [CrossRef]
- Charmantier, G.U.Y. Ontogeny of osmoregulation in crustaceans: A review. Invertebr. Reprod. Dev. 1998, 33, 177–190. [Google Scholar] [CrossRef]
- Burton, R. Ionic regulation in fish: The influence of acclimation temperature on plasma composition and apparent set points. Comp. Biochem. Physiol. Part A Physiol. 1986, 85, 23–28. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Kong, X.; Zhang, D.; Pan, J.; Zhou, Y.; Wang, L.; Li, D.; Yang, X. ZmHSP16.9, a cytosolic class I small heat shock protein in maize (Zea mays), confers heat tolerance in transgenic tobacco. Plant Cell Rep. 2012, 31, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Zeng, C. The effects of salinity on the survival, growth and haemolymph osmolality of early juvenile blue swimmer crabs, Portunus pelagicus. Aquaculture 2006, 260, 151–162. [Google Scholar] [CrossRef]
- Fu, D.; Chen, J.; Zhang, Y.; Yu, Z. Cloning and expression of a heat shock protein (HSP) 90 gene in the haemocytes of Crassostrea hongkongensis under osmotic stress and bacterial challenge. Fish Shellfish Immunol. 2011, 31, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, Z.; Liu, Y.; Song, C.; Shi, G. Transcriptome Analysis and Discovery of Genes Involved in Immune Pathways from Hepatopancreas of Microbial Challenged Mitten Crab Eriocheir sinensis. PLoS ONE 2013, 8, e68233. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ye, H.; Huang, H.; Li, S.; Liu, X.; Zeng, X.; Gong, J. Expression of Hsp70 in the mud crab, Scylla paramamosain in response to bacterial, osmotic, and thermal stress. Cell Stress Chaperones 2013, 18, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.; Mu, C.; Zhang, C.; Wang, Y.; Song, W.; Li, R.; Wang, C. mRNA expression profiles of heat shock proteins of wild and salinity-tolerant swimming crabs, Portunus trituberculatus, subjected to low salinity stress. Genet. Mol. Res. 2014, 13, 6837–6847. [Google Scholar] [CrossRef]
- Kim, R.-O.; Rhee, J.-S.; Won, E.-J.; Lee, K.-W.; Kang, C.-M.; Lee, Y.-M.; Lee, J.-S. Ultraviolet B retards growth, induces oxidative stress, and modulates DNA repair-related gene and heat shock protein gene expression in the monogonont rotifer, Brachionus sp. Aquat. Toxicol. 2011, 101, 529–539. [Google Scholar] [CrossRef]
- Kim, B.-M.; Rhee, J.-S.; Lee, K.-W.; Kim, M.-J.; Shin, K.-H.; Lee, S.-J.; Lee, Y.-M.; Lee, J.-S. UV-B radiation-induced oxidative stress and p38 signaling pathway involvement in the benthic copepod Tigriopus japonicus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 167, 15–23. [Google Scholar] [CrossRef]
- Won, E.-J.; Han, J.; Lee, Y.; Kumar, K.S.; Shin, K.-H.; Lee, S.-J.; Park, H.G.; Lee, J.-S. In vivo effects of UV radiation on multiple endpoints and expression profiles of DNA repair and heat shock protein (Hsp) genes in the cycloid copepod Paracyclopina nana. Aquat. Toxicol. 2015, 165, 1–8. [Google Scholar] [CrossRef]
- Lacuna, D.G.; Uye, S.-I. Influence of mid-ultraviolet (UVB) radiation on the physiology of the marine planktonic copepod Acartia omorii and the potential role of photoreactivation. J. Plankton Res. 2001, 23, 143–156. [Google Scholar] [CrossRef]
- Yamuna, A.; Kabila, V.; Geraldine, P. Expression of heat shock protein 70 in freshwater prawn Macrobrachium malcolmsonii (H. Milne Edwards) following exposure to Hg and Cu. Indian J. Exp. Biol. 2000, 38, 921–925. [Google Scholar] [PubMed]
- Kim, B.-M.; Rhee, J.-S.; Jeong, C.-B.; Seo, J.S.; Park, G.S.; Lee, Y.-M.; Lee, J.-S. Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (hsp) modulation in the intertidal copepod Tigriopus japonicus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 166, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Pestana, J.L.; Novais, S.C.; Norouzitallab, P.; Vandegehuchte, M.B.; Bossier, P.; De Schamphelaere, K.A. Non-lethal heat shock increases tolerance to metal exposure in brine shrimp. Environ. Res. 2016, 151, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Zhang, M.-Z.; Zheng, C.-J.; Liu, J.; Hu, H.-J. Identification of two hsp90 genes from the marine crab, Portunus trituberculatus and their specific expression profiles under different environmental conditions. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 465–473. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kim, Y.-S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Procaccini, G.; Ianora, A. Gene expression patterns and stress response in marine copepods. Mar. Environ. Res. 2012, 76, 22–31. [Google Scholar] [CrossRef]
- Costa, E.M.F.; Spritzer, P.M.; Hohl, A.; Bachega, T.A.S.S. Effects of endocrine disruptors in the development of the female reproductive tract. Arq. Bras. Endocrinol. Metabol. 2014, 58, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Tu, W.; Niu, L.; Liu, W.; Xu, C. Embryonic exposure to butachlor in zebrafish (Danio rerio): Endocrine disruption, developmental toxicity and immunotoxicity. Ecotoxicol. Environ. Saf. 2013, 89, 189–195. [Google Scholar] [CrossRef]
- Lombardi, C.; Thompson, S.; Ritz, B.; Cockburn, M.; Heck, J.E. Residential proximity to pesticide application as a risk factor for childhood central nervous system tumors. Environ. Res. 2021, 197, 111078. [Google Scholar] [CrossRef]
- Defur, P.L. Use and Role of Invertebrate Models in Endocrine Disruptor Research and Testing. ILAR J. 2004, 45, 484–493. [Google Scholar] [CrossRef] [Green Version]
- Capkin, E.R.O.L.; Altinok, I.; Karahan, S. Water quality and fish size affect toxicity of endosulfan, an organochlorine pesticide, to rainbow trout. Chemosphere 2006, 64, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Park, T.-J.; Lee, Y.-M.; Park, H.G.; Yoon, Y.-D.; Lee, J.-S. Small Heat Shock Protein 20 Gene (Hsp20) of the Intertidal Copepod Tigriopus japonicus as a Possible Biomarker for Exposure to Endocrine Disruptors. Bull. Environ. Contam. Toxicol. 2006, 76, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Dorts, J.; Silvestre, F.; Tu, H.T.; Tyberghein, A.-E.; Phuong, N.T.; Kestemont, P. Oxidative stress, protein carbonylation and heat shock proteins in the black tiger shrimp, Penaeus monodon, following exposure to endosulfan and deltamethrin. Environ. Toxicol. Pharmacol. 2009, 28, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Cocci, P.; Capriotti, M.; Mosconi, G.; Palermo, F.A. Effects of endocrine disrupting chemicals on estrogen receptor alpha and heat shock protein 60 gene expression in primary cultures of loggerhead sea turtle (Caretta caretta) erythrocytes. Environ. Res. 2017, 158, 616–624. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.B.; Park, C.H.; Choi, J. Expression of heat shock protein and hemoglobin genes in Chironomus tentans (Diptera, chironomidae) larvae exposed to various environmental pollutants: A potential biomarker of freshwater biomonitoring. Chemosphere 2006, 65, 1074–1081. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A.; Poulet, S.A.; Carotenuto, Y.; Buttino, I.; Romano, G.; Casotti, R.; Pohnert, G.; Wichard, T.; Colucci-D’Amato, L.; et al. Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature 2004, 429, 403–407. [Google Scholar] [CrossRef]
- Hansen, B.H.; Altin, D.; Rørvik, S.F.; Øverjordet, I.B.; Olsen, A.J.; Nordtug, T. Comparative study on acute effects of water accommodated fractions of an artificially weathered crude oil on Calanus finmarchicus and Calanus glacialis (Crustacea: Copepoda). Sci. Total Environ. 2011, 409, 704–709. [Google Scholar] [CrossRef]
- Guo, H.; Xian, J.-A.; Li, B.; Ye, C.-X.; Wang, A.-L.; Miao, Y.-T.; Liao, S.-A. Gene expression of apoptosis-related genes, stress protein and antioxidant enzymes in hemocytes of white shrimp Litopenaeus vannamei under nitrite stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2013, 157, 366–371. [Google Scholar] [CrossRef]
- Ritossa, F. A new puffing pattern induced by temperature shock and DNP in drosophila. Cell. Mol. Life Sci. 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Sanders, B.M. Stress Proteins in Aquatic Organisms: An Environmental Perspective. Crit. Rev. Toxicol. 1993, 23, 49–75. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piano, A.; Franzellitti, S.; Tinti, F.; Fabbri, E. Sequencing and expression pattern of inducible heat shock gene products in the European flat oyster, Ostrea edulis. Gene 2005, 361, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, K.; Minami, M.; Minami, Y. Constantly Updated Knowledge of Hsp90. J. Biochem. 2005, 137, 443–447. [Google Scholar] [CrossRef]
- Habich, C.; Burkart, V. Heat shock protein 60: Regulatory role on innate immune cells. Cell. Mol. Life Sci. 2007, 64, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; MacRae, T.H. Heat Shock Proteins and Disease Control in Aquatic Organisms. J. Aquac. Res. Dev. 2011, s2, 6. [Google Scholar] [CrossRef] [Green Version]
- Chaurasia, M.K.; Nizam, F.; Ravichandran, G.; Arasu, M.V.; Al-Dhabi, N.A.; Arshad, A.; Elumalai, P.; Arockiaraj, J. Molecular importance of prawn large heat shock proteins 60, 70 and 90. Fish Shellfish Immunol. 2016, 48, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Becher, M.A.; Osborne, J.L.; Kennedy, P.J.; Aupinel, P.; Bretagnolle, V.; Brun, F.; Grimm, V.; Horn, J.; Requier, F. Predictive systems models can help elucidate bee declines driven by multiple combined stressors. Apidologie 2017, 48, 328–339. [Google Scholar] [CrossRef]
- Matilla-Vazquez, M.A.; Matilla, A.J. Ethylene: Role in plants under environmental stress. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: New York, NY, USA, 2013; Volume 2, pp. 189–222. [Google Scholar]
- Travis, J.M.J.; Travis, J.M.J.; Travis, J.M.J.; Travis, J.M.J. Climate change and habitat destruction: A deadly anthropogenic cocktail. Proc. R. Soc. B Boil. Sci. 2003, 270, 467–473. [Google Scholar] [CrossRef]
- Dehedin, A.; Piscart, C.; Marmonier, P. Seasonal variations of the effect of temperature on lethal and sublethal toxicities of ammonia for three common freshwater shredders. Chemosphere 2013, 90, 1016–1022. [Google Scholar] [CrossRef]
- Kozlowsky-Suzuki, B.; Koski, M.; Hallberg, E.; Wallén, R.; Carlsson, P. Glutathione transferase activity and oocyte development in copepods exposed to toxic phytoplankton. Harmful Algae 2009, 8, 395–406. [Google Scholar] [CrossRef]
- Junprung, W.; Supungul, P.; Tassanakajon, A. Structure, gene expression, and putative functions of crustacean heat shock proteins in innate immunity. Dev. Comp. Immunol. 2021, 115, 103875. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.-S.; Raisuddin, S.; Lee, K.-W.; Seo, J.S.; Ki, J.-S.; Kim, I.-C.; Park, H.G.; Lee, J.-S. Heat shock protein (Hsp) gene responses of the intertidal copepod Tigriopus japonicus to environmental toxicants. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Qiu, L.; Zhou, F.; Huang, J.; Guo, Y.; Yang, K. Molecular cloning and expression analysis of a heat shock protein (Hsp90) gene from black tiger shrimp (Penaeus monodon). Mol. Biol. Rep. 2009, 36, 127–134. [Google Scholar] [CrossRef]
- Qian, Z.; Liu, X.; Wang, L.; Wang, X.; Li, Y.; Xiang, J.; Wang, P. Gene expression profiles of four heat shock proteins in response to different acute stresses in shrimp, Litopenaeus vannamei. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 156, 211–220. [Google Scholar] [CrossRef]
- Guan, W.; Nong, W.; Wei, X.; Zhu, M.; Mao, L. Impacts of a novel live shrimp (Litopenaeus vannamei) water-free transportation strategy on flesh quality: Insights through stress response and oxidation in lipids and proteins. Aquaculture 2021, 533, 736168. [Google Scholar] [CrossRef]
- Mikulski, A.; Bernatowicz, P.; Grzesiuk, M.; Kloc, M.; Pijanowska, J. Differential Levels of Stress Proteins (HSPs) in Male and Female Daphnia magna in Response to Thermal Stress: A Consequence of Sex-Related Behavioral Differences? J. Chem. Ecol. 2011, 37, 670–676. [Google Scholar] [CrossRef] [Green Version]
- Haap, T.; Schwarz, S.; Köhler, H.-R. Metallothionein and Hsp70 trade-off against one another in Daphnia magna cross-tolerance to cadmium and heat stress. Aquat. Toxicol. 2016, 170, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, W.-J.; Zhu, X.-J.; Karouna-Renier, N.K.; Rao, R.K. Molecular cloning and expression of two HSP70 genes in the prawn, Macrobrachium rosenbergii. Cell Stress Chaperones 2004, 9, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Timofeyev, M.A.; Shatilina, Z.M.; Bedulina, D.S.; Protopopova, M.V.; Pavlichenko, V.V.; Grabelnych, O.I.; Kolesnichenko, A.V. Evaluation of biochemical responses in Palearctic and Lake Baikal endemic amphipod species exposed to CdCl2. Ecotoxicol. Environ. Saf. 2008, 70, 99–105. [Google Scholar] [CrossRef]
- Arts, M.-J.S.J.; Schill, R.O.; Knigge, T.; Eckwert, H.; Kammenga, J.E.; Köhler, H.-R. Stress Proteins (hsp70, hsp60) Induced in Isopods and Nematodes by Field Exposure to Metals in a Gradient near Avonmouth, UK. Ecotoxicology 2004, 13, 739–755. [Google Scholar] [CrossRef]
- Chang, E.S. Stressed-Out Lobsters: Crustacean Hyperglycemic Hormone and Stress Proteins. Integr. Comp. Biol. 2005, 45, 43–50. [Google Scholar] [CrossRef]
- Spees, J.L.; Chang, S.A.; Snyder, M.J.; Chang, E.S. Osmotic Induction of Stress-Responsive Gene Expression in the LobsterHomarus americanus. Biol. Bull. 2002, 203, 331–337. [Google Scholar] [CrossRef]
- Velázque-Amado, R.M.; Escamilla-Chimal, E.G.; Fanjul-Moles, M.L. Daily Light-Dark Cycles Influence Hypoxia-Inducible Factor 1 and Heat Shock Protein Levels in the Pacemakers of Crayfish. Photochem. Photobiol. 2012, 88, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Sheller, R.A.; Smyers, M.E.; Grossfeld, R.M.; Ballinger, M.L.; Bittner, G.D. Heat-shock proteins in axoplasm: High constitutive levels and transfer of inducible isoforms from glia. J. Comp. Neurol. 1998, 396, 1–11. [Google Scholar] [CrossRef]
- Clegg, J.S.; Jackson, S.A.; Van Hoa, N.; Sorgeloos, P. Thermal resistance, developmental rate and heat shock proteins in Artemia franciscana, from San Francisco Bay and southern Vietnam. J. Exp. Mar. Biol. Ecol. 2000, 252, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.-J.; Zheng, C.-Q.; Wang, Y.-W.; Meng, C.; Xie, X.-L.; Liu, H.-P. Differential protein expression using proteomics from a crustacean brine shrimp (Artemia sinica) under CO2-driven seawater acidification. Fish Shellfish Immunol. 2016, 58, 669–677. [Google Scholar] [CrossRef]
- Cottin, D.; Foucreau, N.; Hervant, F.; Piscart, C. Differential regulation of hsp70 genes in the freshwater key species Gammarus pulex (Crustacea, Amphipoda) exposed to thermal stress: Effects of latitude and ontogeny. J. Comp. Physiol. B 2015, 185, 303–313. [Google Scholar] [CrossRef]
- Bedulina, D.S.; Timofeyev, M.A.; Zimmer, M.; Zwirnmann, E.; Menzel, R.; Steinberg, C.E.W. Different natural organic matter isolates cause similar stress response patterns in the freshwater amphipod, Gammarus pulex. Environ. Sci. Pollut. Res. 2010, 17, 261–269. [Google Scholar] [CrossRef]
- Aruda, A.M.; Baumgartner, M.F.; Reitzel, A.M.; Tarrant, A.M. Heat shock protein expression during stress and diapause in the marine copepod Calanus finmarchicus. J. Insect Physiol. 2011, 57, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Frenkel, L.; Dimant, B.; Suárez, L.D.; Portiansky, E.L.; Delorenzi, A. Food odor, visual danger stimulus, and retrieval of an aversive memory trigger heat shock protein HSP70 expression in the olfactory lobe of the crab Chasmagnathus granulatus. Neuroscience 2012, 201, 239–251. [Google Scholar] [CrossRef]
- Cascella, K.; Jollivet, D.; Papot, C.; Léger, N.; Corre, E.; Ravaux, J.; Clark, M.S.; Toullec, J.-Y. Diversification, Evolution and Sub-Functionalization of 70kDa Heat-Shock Proteins in Two Sister Species of Antarctic Krill: Differences in Thermal Habitats, Responses and Implications under Climate Change. PLoS ONE 2015, 10, e0121642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colson-Proch, C.; Morales, A.; Hervant, F.; Konecny, L.; Moulin, C.; Douady, C.J. First cellular approach of the effects of global warming on groundwater organisms: A study of the HSP70 gene expression. Cell Stress Chaperones 2010, 15, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.T.; Chu, K.H. Characterization of heat shock protein 90 in the shrimpMetapenaeus ensis: Evidence for its role in the regulation of vitellogenin synthesis. Mol. Reprod. Dev. 2008, 75, 952–959. [Google Scholar] [CrossRef]
- Zheng, J.; Cao, J.; Mao, Y.; Su, Y.; Wang, J. Comparative transcriptome analysis provides comprehensive insights into the heat stress response of Marsupenaeus japonicus. Aquaculture 2019, 502, 338–346. [Google Scholar] [CrossRef]
- Li, J.; Han, J.; Chen, P.; Chang, Z.; He, Y.; Liu, P.; Wang, Q.; Li, J. Cloning of a heat shock protein 90 (HSP90) gene and expression analysis in the ridgetail white prawn Exopalaemon carinicauda. Fish Shellfish Immunol. 2012, 32, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yang, X.; Huang, Y.; Yan, G.; Cheng, Y. Oxidative stress and genotoxic effect of deltamethrin exposure on the Chinese mitten crab, Eriocheir sinensis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 212, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Thitamadee, S.; Prachumwat, A.; Srisala, J.; Jaroenlak, P.; Salachan, P.V.; Sritunyalucksana, K.; Flegel, T.W.; Itsathitphaisarn, O. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 2016, 452, 69–87. [Google Scholar] [CrossRef]
- Wang, W. Bacterial diseases of crabs: A review. J. Invertebr. Pathol. 2011, 106, 18–26. [Google Scholar] [CrossRef]
- Tran, L.; Nunan, L.; Redman, R.; Mohney, L.; Pantoja, C.; Fitzsimmons, K.; Lightner, D. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Org. 2013, 105, 45–55. [Google Scholar] [CrossRef]
- Smolowitz, R.M.; Bullis, R.A.; Abt, D.A. Pathologic Cuticular Changes of Winter Impoundment Shell Disease Preceding and During Intermolt in the American Lobster, Homarus americanus. Biol. Bull. 1992, 183, 99–112. [Google Scholar] [CrossRef]
- Vogan, C.; Costa-Ramos, C.; Rowley, A. A histological study of shell disease syndrome in the edible crab Cancer pagurus. Dis. Aquat. Org. 2001, 47, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huynh, T.G.; Hu, S.Y.; Chiu, C.S.; Truong, Q.P.; Liu, C.H. Bacterial population in intestines of white shrimp, Litopenaeus vannamei fed a synbiotic containing Lactobacillus plantarum and galactooligosaccharide. Aquac. Res. 2019, 50, 807–817. [Google Scholar] [CrossRef]
- Ning, M.; Yuan, M.; Liu, M.; Gao, Q.; Wei, P.; Gu, W.; Wang, W.; Meng, Q. Characterization of cathepsin D from Eriocheir sinensis involved in Spiroplasma eriocheiris infection. Dev. Comp. Immunol. 2018, 86, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Angthong, P.; Roytrakul, S.; Jarayabhand, P.; Jiravanichpaisal, P. Involvement of a tachylectin-like gene and its protein in pathogenesis of acute hepatopancreatic necrosis disease (AHPND) in the shrimp, Penaeus monodon. Dev. Comp. Immunol. 2017, 76, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Todgham, A.; Ackerman, P.; Bibeau, M.; Nakano, K.; Schulte, P.; Iwama, G.K. Heat shock protein genes and their functional significance in fish. Gene 2002, 295, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Swain, D.P.; Sinclair, A.F.; Hanson, J.M. Evolutionary response to size-selective mortality in an exploited fish population. Proc. R. Soc. B Boil. Sci. 2007, 274, 1015–1022. [Google Scholar] [CrossRef]
- Iwama, G.K.; Vijayan, M.M.; Forsyth, R.B.; Ackerman, P.A. Heat Shock Proteins and Physiological Stress in Fish. Am. Zool. 1999, 39, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.K.; Sharma, J.G.; Chakrabarti, R. Simulation study of natural UV-B radiation on Catla catla and its impact on physiology, oxidative stress, Hsp 70 and DNA fragmentation. J. Photochem. Photobiol. B Biol. 2015, 149, 156–163. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Banerjee, S.; Bhattacharjee, S.; Mitra, T.; Purohit, G.K.; Sharma, A.P.; Karunakaran, D.; Mohanty, S. Muscle proteomics of the Indian major carp catla (Catla catla, Hamilton). J. Proteom. Bioinform. 2013, 6, 252–263. [Google Scholar]
- Purohit, G.K.; Mahanty, A.; Suar, M.; Sharma, A.P.; Mohanty, B.P.; Mohanty, S. Investigating hsp gene expression in liver of Channa striataunder heat stress for understanding the upper thermal acclimation. BioMed Res. Int. 2014, 2014, 381719. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Mohapatra, A.; Sahoo, P.K. Expression analysis of heat shock protein genes during Aeromonas hydrophila infection in rohu, Labeo rohita, with special reference to molecular characterization of Grp78. Cell Stress Chaperones 2015, 20, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, T.; Pal, A.K.; Chakraborty, S.K.; Manush, S.M.; Dalvi, R.S.; Apte, S.K.; Sahu, N.P.; Baruah, K. Biochemical and stress responses of rohu Labeo rohita and mrigal Cirrhinus mrigala in relation to acclimation temperatures. J. Fish Biol. 2009, 74, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Elicker, K.S.; Hutson, L.D. Genome-wide analysis and expression profiling of the small heat shock proteins in zebrafish. Gene 2007, 403, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, B.P.; Banerjee, S.; Sadhukhan, P.; Chowdhury, A.N.; Golder, D.; Bhattacharjee, S.; Bhowmick, S.; Manna, S.K.; Samanta, S. Pathophysiological Changes in Rohu (Labeo rohita, Hamilton) Fingerlings Following Arsenic Exposure. Natl. Acad. Sci. Lett. 2015, 38, 315–319. [Google Scholar] [CrossRef]
- Yengkokpam, S.; Pal, A.; Sahu, N.; Jain, K.; Dalvi, R.; Misra, S.; Debnath, D. Metabolic modulation in Labeo rohita fingerlings during starvation: Hsp70 expression and oxygen consumption. Aquaculture 2008, 285, 234–237. [Google Scholar] [CrossRef]
- Mahanty, A.; Mohanty, S.; Mohanty, B.P. Dietary supplementation of curcumin augments heat stress tolerance through upregulation of nrf-2-mediated antioxidative enzymes and hsps in Pethia sophore. Fish Physiol. Biochem. 2017, 43, 1131–1141. [Google Scholar] [CrossRef]
- Mitra, T.; Mahanty, A.; Ganguly, S.; Purohit, G.K.; Mohanty, S.; Parida, P.K.; Behera, P.R.; Raman, R.K.; Mohanty, B.P. Expression patterns of heat shock protein genes in Rita rita from natural riverine habitat as biomarker response against environmental pollution. Chemosphere 2018, 211, 535–546. [Google Scholar] [CrossRef]
- Garcia de la Serrana, D.; Johnston, I.A. Expression of heat shock protein (Hsp90) paralogues is regulated by amino acids in skeletal muscle of Atlantic salmon. PLoS ONE 2013, 8, e74295. [Google Scholar] [CrossRef] [Green Version]
- Oksala, N.K.; Ekmekçi, F.G.; Özsoy, E.; Kirankaya, S.; Kokkola, T.; Emecen, G.; Lappalainen, J.; Kaarniranta, K.; Atalay, M. Natural thermal adaptation increases heat shock protein levels and decreases oxidative stress. Redox Biol. 2014, 3, 25–28. [Google Scholar] [CrossRef]
- Jesus, T.F.; Inácio, A.; Coelho, M.M. Different levels of hsp70 and hsc70 mRNA expression in Iberian fish exposed to distinct river conditions. Genet. Mol. Biol. 2013, 36, 061–069. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.-L.; Yao, C.-L.; Wang, Z.-Y. Acute temperature and cadmium stress response characterization of small heat shock protein 27 in large yellow croaker, Larimichthys crocea. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Perezcasanova, J.C.; Rise, M.L.; Dixon, B.; Afonso, L.O.B.; Hall, J.R.; Johnson, S.C.; Gamperl, A.K. The immune and stress responses of Atlantic cod to long-term increases in water temperature. Fish Shellfish Immunol. 2008, 24, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Fangue, N.A.; Hofmeister, M.; Schulte, P.M. Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J. Exp. Biol. 2006, 209, 2859–2872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, H.; Watabe, S. Temperature-dependent enhancement of cell proliferation and mRNA expression for type I collagen and HSP70 in primary cultured goldfish cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 138, 221–228. [Google Scholar] [CrossRef]
- Kagawa, N. A Drastic Reduction in the Basal Level of Heat-shock Protein 90 in the Brain of Goldfish (Carassius auratus) after Administration of Geldanamycin. Zool. Sci. 2004, 21, 1085–1089. [Google Scholar] [CrossRef] [Green Version]
- Lund, S.G.; Tufts, B.L. The physiological effects of heat stress and the role of heat shock proteins in rainbow trout (Oncorhynchus f) red blood cells. Fish Physiol. Biochem. 2003, 29, 1–12. [Google Scholar] [CrossRef]
- Shi, H.; Liu, Z.; Zhang, J.; Kang, Y.; Wang, J.; Huang, J.; Wang, W. Effect of heat stress on heat-shock protein (Hsp60) mRNA expression in rainbow trout Oncorhynchus mykiss. Genet. Mol. Res. 2015, 14, 5280–5286. [Google Scholar] [CrossRef]
- Rendell, J.L.; Fowler, S.; Cockshutt, A.; Currie, S. Development-dependent differences in intracellular localization of stress proteins (hsps) in rainbow trout, Oncorhynchus mykiss, following heat shock. Comp. Biochem. Physiol. Part D Genom. Proteom. 2006, 1, 238–252. [Google Scholar] [CrossRef]
- Werner, I.; Linares-Casenave, J.; Van Eenennaam, J.P.; Doroshov, S.I. The Effect of Temperature Stress on Development and Heat-shock Protein Expression in Larval Green Sturgeon (Acipenser mirostris). Environ. Biol. Fishes 2007, 79, 191–200. [Google Scholar] [CrossRef]
- de la Vega, E.; Hall, M.R.; Degnan, B.M.; Wilson, K.J. Short-term hyperthermic treatment of Penaeus monodon increases expression of heat shock protein 70 (HSP70) and reduces replication of gill associated virus (GAV). Aquaculture 2006, 253, 82–90. [Google Scholar] [CrossRef]
- Weber, T.E.; Small, B.C.; Bosworth, B.G. Lipopolysaccharide regulates myostatin and MyoD independently of an increase in plasma cortisol in channel catfish (Ictalurus punctatus). Domest. Anim. Endocrinol. 2005, 28, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Song, L.; Weng, Z.; Liu, S.; Liu, Z. Hsp90, Hsp60 and sHsp families of heat shock protein genes in channel catfish and their expression after bacterial infections. Fish Shellfish Immunol. 2015, 44, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, S.; Geraldine, P. Heat shock protein induction in the freshwater prawn Macrobrachium malcolmsonii: Acclimation-influenced variations in the induction temperatures for Hsp70. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 140, 209–215. [Google Scholar] [CrossRef]
- Brown, H.M.; Briden, A.; Stokell, T.; Griffin, F.J.; Cherr, G.N. Thermotolerance and Hsp70 profiles in adult and embryonic California native oysters, Ostreola conchaphila (Carpenter, 1857). J. Shellfish Res. 2004, 23, 135–142. [Google Scholar]
- Osman, A.G.; Wuertz, S.; Mohammed-Geba, K. Lead-induced heat shock protein (HSP70) and metallothionein (MT) gene expression in the embryos of African catfish Clarias gariepinus (Burchell, 1822). Sci. Afr. 2019, 3, e00056. [Google Scholar] [CrossRef]
- Kothary, R.K.; Jones, D.; Candido, E.P. 70-Kilodalton heat shock polypeptides from rainbow trout: Characterization of cDNA sequences. Mol. Cell. Biol. 1984, 4, 1785–1791. [Google Scholar] [PubMed] [Green Version]
- Murtha, J.M.; Keller, E.T. Characterization of the heat shock response in mature zebrafish (Danio rerio). Exp. Gerontol. 2003, 38, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; Yamamoto, Y.; Jeffery, W.R.; Krone, P.H. Zebrafish Hsp70 is required for embryonic lens formation. Cell Stress Chaperones 2005, 10, 66–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taherian, A.; Krone, P.H.; Ovsenek, N. A comparison of Hsp90 α and Hsp90 β interactions with cochaperones and substrates. Biochem. Cell Biol. 2008, 86, 37–45. [Google Scholar] [CrossRef]
- Krone, P.; Sass, J. Hsp 90α and Hsp 90β Genes Are Present in the Zebrafish and Are Differentially Regulated in Developing Embryos. Biochem. Biophys. Res. Commun. 1994, 204, 746–752. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, C.; Ye, X.; Zou, S.; Lu, M.; Liu, Z.; Tian, Y. Characterization of four heat-shock protein genes from Nile tilapia (Oreochromis niloticus) and demonstration of the inducible transcriptional activity of Hsp70 promoter. Fish Physiol. Biochem. 2014, 40, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Dini, L.; Lanubile, R.; Tarantino, P.; Mandich, A.; Cataldi, E. Expression of stress proteins 70 in tilapia (Oreochromis mossambicus) during confinement and crowding stress. Ital. J. Zool. 2006, 73, 117–124. [Google Scholar] [CrossRef]
- Al-Salman, A.N.; Ghaida’a Jassim, A.; Al-Niaeem, K.S. Comet and Micronucleus Assays for Detecting Benzo (a) Pyrene Genotoxicity in Blood Cells of Nile Tilapia (Oreochromis niloticus) from the Shatt Al-Arab River in Southern Iraq. J. Pharm. Negat. Results 2022, 13, 1606–1614. [Google Scholar]
- Molina, A.; Biemar, F.; Müller, F.; Iyengar, A.; Prunet, P.; Maclean, N.; Martial, J.A.; Muller, M. Cloning and expression analysis of an inducibleHSP70gene from tilapia fish. FEBS Lett. 2000, 474, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Zuanazzi, J.S.G.; De Lara, J.A.F.; Goes, E.S.D.R.; Almeida, F.L.A.; De Oliveira, C.A.L.; Ribeiro, R.P. Anoxia stress and effect on flesh quality and gene expression of tilapia. Food Sci. Technol. 2019, 39, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Tine, M.; Bonhomme, F.; McKenzie, D.J.; Durand, J.-D. Differential expression of the heat shock protein Hsp70 in natural populations of the tilapia, Sarotherodon melanotheron, acclimatised to a range of environmental salinities. BMC Ecol. 2010, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.; Zhang, G.; Yin, S.; Wang, L. The role of three heat shock protein genes in the immune response to Aeromonas hydrophila challenge in marbled eel, Anguilla marmorata. R. Soc. Open Sci. 2016, 3, 160375. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.Y.; Kim, J.Y. Responses of HSP gene expressions to elevated water temperature in the Nile tilapia Oreochromis niloticus. Dev. Reprod. 2010, 14, 179–184. [Google Scholar]
- Wei, T.; Gao, Y.; Wang, R.; Xu, T. A heat shock protein 90 β isoform involved in immune response to bacteria challenge and heat shock from Miichthys miiuy. Fish Shellfish Immunol. 2013, 35, 429–437. [Google Scholar] [CrossRef]
- Gao, X.Q.; Fei, F.; Huang, B.; Meng, X.S.; Zhang, T.; Zhao, K.F.; Chen, H.-B.; Xing, R.; Liu, B.L. Alterations in hematological and biochemical parameters, oxidative stress, and immune response in Takifugu rubripes under acute ammonia exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 243, 108978. [Google Scholar] [CrossRef]
- Karouna-Renier, N.K.; Zehr, J.P. Short-term exposures to chronically toxic copper concentrations induce HSP70 proteins in midge larvae (Chironomus tentans). Sci. Total Environ. 2003, 312, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Luan, W.; Zhang, C.; Zhang, J.; Wang, B.; Xie, Y.; Li, S.; Xiang, J. Cloning of cytoplasmic heat shock protein 90 (FcHSP90) from Fenneropenaeus chinensis and its expression response to heat shock and hypoxia. Cell Stress Chaperones 2009, 14, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmisano, A.N.; Winton, J.R.; Dickhoff, W.W. Tissue-Specific Induction of Hsp90 mRNA and Plasma Cortisol Response in Chinook Salmon following Heat Shock, Seawater Challenge, and Handling Challenge. Mar. Biotechnol. 2000, 2, 329–338. [Google Scholar] [CrossRef]
- Hofmann, G.; Buckley, B.; Airaksinen, S.; Keen, J.; Somero, G. Heat-shock protein expression is absent in the antarctic fish Trematomus bernacchii (family Nototheniidae). J. Exp. Biol. 2000, 203, 2331–2339. [Google Scholar] [CrossRef]
- Dyer, S.D.; Dickson, K.L.; Zimmerman, E.G.; Sanders, B.M. Tissue-specific patterns of synthesis of heat-shock proteins and thermal tolerance of the fathead minnow (Pimephales promelas). Can. J. Zool. 1991, 69, 2021–2027. [Google Scholar] [CrossRef]
- Seebaugh, D.R.; Wallace, W.G. Assimilation and subcellular partitioning of elements by grass shrimp collected along an impact gradient. Aquat. Toxicol. 2009, 93, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zarate, J.; Bradley, T.M. Heat shock proteins are not sensitive indicators of hatchery stress in salmon. Aquaculture 2003, 223, 175–187. [Google Scholar] [CrossRef]
- Forsyth, R.B.; Candido, E.P.M.; Babich, S.L.; Iwama, G.K. Stress Protein Expression in Coho Salmon with Bacterial Kidney Disease. J. Aquat. Anim. Health 1997, 9, 18–25. [Google Scholar] [CrossRef]
- Ackerman, P.A.; Iwama, G.K. Physiological and Cellular Stress Responses of Juvenile Rainbow Trout to Vibriosis. J. Aquat. Anim. Health 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Deane, E.E.; Woo, N.Y.S. Evidence for disruption of Na+-K+-ATPase and hsp70 during vibriosis of sea bream, Sparus (= Rhabdosargus) sarba Forsskål. J. Fish Dis. 2005, 28, 239–251. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Van Damme, E.J.; Sorgeloos, P.; Bossier, P. Non-lethal heat shock protects gnotobiotic Artemia franciscana larvae against virulent Vibrios. Fish Shellfish Immunol. 2007, 22, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-Y.; Kang, S.-T.; Chen, W.-Y.; Hsu, T.-C.; Lo, C.-F.; Liu, K.-F.; Chen, L.-L. Identification of the small heat shock protein, HSP21, of shrimp Penaeus monodon and the gene expression of HSP21 is inactivated after white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol. 2008, 25, 250–257. [Google Scholar] [CrossRef]
- Wilhelm, V.; Miquel, A.; Burzio, L.O.; Rosemblatt, M.; Engel, E.; Valenzuela, S.; Parada, G.; Valenzuela, P.D. A vaccine against the salmonid pathogen Piscirickettsia salmonis based on recombinant proteins. Vaccine 2006, 24, 5083–5091. [Google Scholar] [CrossRef]
- Plant, K.P.; LaPatra, S.E.; Cain, K.D. Vaccination of rainbow trout, Oncorhynchus mykiss (Walbaum), with recombinant and DNA vaccines produced to Flavobacterium psychrophilum heat shock proteins 60 and 70. J. Fish Dis. 2009, 32, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Ryckaert, J.; Pasmans, F.; Tobback, E.; Duchateau, L.; Decostere, A.; Haesebrouck, F.; Sorgeloos, P.; Bossier, P. Heat shock proteins protect platyfish (Xiphophorus maculatus) from Yersinia ruckeri induced mortality. Fish Shellfish Immunol. 2010, 28, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.I.; Phan, T.; Sokolova, I.M. Long-Term Acclimation to Different Thermal Regimes Affects Molecular Responses to Heat Stress in a Freshwater Clam Corbicula Fluminea. Sci. Rep. 2016, 6, 39476. [Google Scholar] [CrossRef] [Green Version]
- Sleight, V.A.; Peck, L.S.; Dyrynda, E.A.; Smith, V.J.; Clark, M.S. Cellular stress responses to chronic heat shock and shell damage in temperate Mya truncata. Cell Stress Chaperones 2018, 23, 1003–1017. [Google Scholar] [CrossRef] [Green Version]
- Kotsakiozi, P.; Parmakelis, A.; Aggeli, I.-K.; Gaitanaki, C.; Giokas, S.; Valakos, E.D. Water balance and expression of heat-shock protein 70 in Codringtonia species: A study within a phylogenetic framework. J. Molluscan Stud. 2015, 81, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Ivanina, A.V.; Cherkasov, A.S.; Sokolova, I.M. Effects of cadmium on cellular protein and glutathione synthesis and expression of stress proteins in eastern oysters, Crassostrea virginica Gmelin. J. Exp. Biol. 2008, 211, 577–586. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Pan, H.; Pan, B.; Bu, W. Identification and functional characterization of three TLR signaling pathway genes in Cyclina sinensis. Fish Shellfish Immunol. 2016, 50, 150–159. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jeong, S.-Y.; Kim, P.-J.; Dahms, H.-U.; Han, K.-N. Bio-effect-monitoring of long-term thermal wastes on the oyster, Crassostrea gigas, using heat shock proteins. Mar. Pollut. Bull. 2017, 119, 359–364. [Google Scholar] [CrossRef]
- Jung, M.-Y.; Lee, Y.-M. Expression profiles of heat shock protein gene families in the monogonont rotifer Brachionus koreanus—Exposed to copper and cadmium. Toxicol. Environ. Health Sci. 2012, 4, 235–242. [Google Scholar] [CrossRef]
- Farcy, E.; Serpentini, A.; Fiévet, B.; Lebel, J.-M. Identification of cDNAs encoding HSP70 and HSP90 in the abalone Haliotis tuberculata: Transcriptional induction in response to thermal stress in hemocyte primary culture. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 146, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Tissiéres, A.; Mitchell, H.K.; Tracy, U.M. Protein synthesis in salivary glands of Drosophila melanogaster: Relation to chromosome puffs. J. Mol. Biol. 1974, 84, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, S. Regulation of protein synthesis during heat shock. Nature 1981, 293, 311–314. [Google Scholar] [CrossRef]
- Lakhotia, S.C. Forty years of the 93D puff of Drosophila melanogaster. J. Biosci. 2011, 36, 399–423. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J.-S. Heat shock protein genes in the green alga Tetraselmis suecica and their role against redox and non-redox active metals. Eur. J. Protistol. 2019, 69, 37–51. [Google Scholar] [CrossRef]
- Martín-Folgar, R.; Martínez-Guitarte, J.-L. Cadmium alters the expression of small heat shock protein genes in the aquatic midge Chironomus riparius. Chemosphere 2017, 169, 485–492. [Google Scholar] [CrossRef]
- Martínez-Paz, P.; Morales, M.; Martín, R.; Martínez-Guitarte, J.L.; Morcillo, G. Characterization of the small heat shock protein Hsp27 gene in Chironomus riparius (Diptera) and its expression profile in response to temperature changes and xenobiotic exposures. Cell Stress Chaperones 2014, 19, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Wang, X.; Wang, X.; Lei, C.; Zhu, F. Starvation-, thermal- and heavy metal- associated expression of four small heat shock protein genes in Musca domestica. Gene 2018, 642, 268–276. [Google Scholar] [CrossRef]
- Kjærsgaard, A.; Blanckenhorn, W.U.; Pertoldi, C.; Loeschcke, V.; Kaufmann, C.; Hald, B.; Pagès, N.; Bahrndorff, S. Plasticity in behavioural responses and resistance to temperature stress in Musca domestica. Anim. Behav. 2015, 99, 123–130. [Google Scholar] [CrossRef]
- Tang, T.; Wu, C.; Li, J.; Ren, G.; Huang, D.; Liu, F. Stress-induced HSP70 from Musca domestica plays a functionally significant role in the immune system. J. Insect Physiol. 2012, 58, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rohilla, M.S.; Tiwari, P.K. Developmental and hyperthermia-induced expression of the heat shock proteins HSP60 and HSP70 in tissues of the housefly Musca domestica: An in vitro study. Genet. Mol. Biol. 2007, 30, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Colinet, H.; Hance, T. Male Reproductive Potential of Aphidius colemani (Hymenoptera: Aphidiinae) Exposed to Constant or Fluctuating Thermal Regimens. Environ. Entomol. 2009, 38, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Economou, K.; Kotsiliti, E.; Mintzas, A.C. Stage and cell-specific expression and intracellular localization of the small heat shock protein Hsp27 during oogenesis and spermatogenesis in the Mediterranean fruit fly, Ceratitis capitata. J. Insect Physiol. 2017, 96, 64–72. [Google Scholar] [CrossRef]
- Yocum, G.D.; Joplin, K.H.; Denlinger, D.L. Upregulation of a 23 kDa small heat shock protein transcript during pupal diapause in the flesh fly, Sarcophaga crassipalpis. Insect Biochem. Mol. Biol. 1998, 28, 677–682. [Google Scholar] [CrossRef]
- Chen, E.-H.; Hou, Q.-L. Identification and expression analysis of cuticular protein genes in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Pestic. Biochem. Physiol. 2021, 178, 104943. [Google Scholar] [CrossRef]
- Wrońska, A.K.; Boguś, M.I. Heat shock proteins (HSP 90, 70, 60, and 27) in Galleria mellonella (Lepidoptera) hemolymph are affected by infection with Conidiobolus coronatus (Entomophthorales). PLoS ONE 2020, 15, e0228556. [Google Scholar] [CrossRef] [Green Version]
- Shivam, S.; Ertl, R.; Sexl, V.; El-Matbouli, M.; Kumar, G. Differentially expressed transcripts of Tetracapsuloides bryosalmonae (Cnidaria) between carrier and dead-end hosts involved in key biological processes: Novel insights from a coupled approach of FACS and RNA sequencing. Vet. Res. 2023, 54, 1–19. [Google Scholar] [CrossRef]
- Engman, D.M.; Dragon, E.A.; Donelson, J.E. Human humoral immunity to hsp70 during Trypanosoma cruzi infection. J. Immunol. 1990, 144, 3987–3991. [Google Scholar] [CrossRef]
- Hedstrom, R.; Culpepper, J.; Schinski, V.; Agabian, N.; Newport, G. Schistosome heat-shock proteins are immunologically distinct host-like antigens. Mol. Biochem. Parasitol. 1988, 29, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kosakyan, A.; Alama-Bermejo, G.; Bartošová-Sojková, P.; Born-Torrijos, A.; Šíma, R.; Nenarokova, A.; Eszterbauer, E.; Bartholomew, J.; Holzer, A.S. Selection of suitable reference genes for gene expression studies in myxosporean (Myxozoa, Cnidaria) parasites. Sci. Rep. 2019, 9, 15073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukimi, Y.; Okabe, S. Recent Advances in Gastrointestinal Pathophysiology: Role of Heat Shock Proteins in Mucosal Defense and Ulcer Healing. Biol. Pharm. Bull. 2001, 24, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitrovska, M.; Dervisevik, M.; Cipanovska, N.; Gerazova, K.; Dinevska-Kjovkarovska, S.; Miova, B. Physiological and pharmacological inductors of HSP70 enhance the antioxidative defense mechanisms of the liver and pancreas in diabetic rats. Can. J. Physiol. Pharmacol. 2018, 96, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Ventura, M.; Canchaya, C.; Zhang, Z.; Fitzgerald, G.F.; van Sinderen, D. Molecular characterization of hsp20, encoding a small heat shock protein of Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 2007, 73, 4695–4703. [Google Scholar] [CrossRef] [Green Version]
- Khaskheli, G.B.; Zuo, F.; Yu, R.; Chen, S. Overexpression of Small Heat Shock Protein Enhances Heat- and Salt-Stress Tolerance of Bifidobacterium longum NCC2705. Curr. Microbiol. 2015, 71, 8–15. [Google Scholar] [CrossRef]
- Ojima, N. Rainbow trout hspb1 (hsp27): Identification of two mRNA splice variants that show predominant expression in muscle tissues. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 277–285. [Google Scholar] [CrossRef]
- Ojima, N.; Oohara, I. Isolation and characterization of a genomic fosmid clone containing hspb1 (hsp27) from the Pacific bluefin tuna Thunnus orientalis. Mar. Genom. 2008, 1, 87–93. [Google Scholar] [CrossRef]
- Zhang, K.; Ezemaduka, A.N.; Wang, Z.; Hu, H.; Shi, X.; Liu, C.; Lu, X.; Fu, X.; Chang, Z.; Yin, C.-C. A Novel Mechanism for Small Heat Shock Proteins to Function as Molecular Chaperones. Sci. Rep. 2015, 5, srep08811. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Qiao, Y.; He, J.; Wang, Q.; Chen, Z.; Ni, F.; Liu, Y.; Liu, X.; Zhang, Q.; Wang, X. Characterisation, evolution and expression analysis of heat shock protein 20 genes from Japanese flounder (Paralichthys olivaceus) in response to Edwardsiella tarda infection. Aquaculture 2020, 529, 735722. [Google Scholar] [CrossRef]
- Li, J.; Mak, Y.L.; Chang, Y.-H.; Xiao, C.; Chen, Y.-M.; Shen, J.; Wang, Q.; Ruan, Y.; Lam, P.K.S. Uptake and Depuration Kinetics of Pacific Ciguatoxins in Orange-Spotted Grouper (Epinephelus coioides). Environ. Sci. Technol. 2020, 54, 4475–4483. [Google Scholar] [CrossRef] [PubMed]
- Rungrassamee, W.; Leelatanawit, R.; Jiravanichpaisal, P.; Klinbunga, S.; Karoonuthaisiri, N. Expression and distribution of three heat shock protein genes under heat shock stress and under exposure to Vibrio harveyi in Penaeus monodon. Dev. Comp. Immunol. 2010, 34, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Arockiaraj, J.; Easwvaran, S.; Vanaraja, P.; Singh, A.; Othman, R.Y.; Bhassu, S. Prophenoloxidase activating enzyme-III from giant freshwater prawn Macrobrachium rosenbergii: Characterization, expression and specific enzyme activity. Mol. Biol. Rep. 2011, 39, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Whang, I.; Lee, J. Molecular and functional characterization of HdHSP20: A biomarker of environmental stresses in disk abalone Haliotis discus discus. Fish Shellfish Immunol. 2012, 33, 48–59. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Liu, J.; Yu, Y.; Huang, Y.; Huang, X.; Wei, J.; Qin, Q. Singapore Grouper Iridovirus (SGIV) Inhibited Autophagy for Efficient Viral Replication. Front. Microbiol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Wang, H.-S.; Wang, X.-H.; Zhou, C.-S.; Huang, L.-H.; Zhang, S.-F.; Guo, W.; Kang, L. cDNA cloning of heat shock proteins and their expression in the two phases of the migratory locust. Insect Mol. Biol. 2007, 16, 207–219. [Google Scholar] [CrossRef]
- Mahanty, A.; Purohit, G.K.; Yadav, R.P.; Mohanty, S.; Mohanty, B.P. hsp90 and hsp47 appear to play an important role in minnow Puntius sophore for surviving in the hot spring run-off aquatic ecosystem. Fish Physiol. Biochem. 2017, 43, 89–102. [Google Scholar] [CrossRef]
- Seo, J.S.; Lee, Y.-M.; Park, H.G.; Lee, J.-S. The intertidal copepod Tigriopus japonicus small heat shock protein 20 gene (Hsp20) enhances thermotolerance of transformed Escherichia coli. Biochem. Biophys. Res. Commun. 2006, 340, 901–908. [Google Scholar] [CrossRef]
- Rhee, J.-S.; Kim, R.-O.; Choi, H.-G.; Lee, J.; Lee, Y.-M.; Lee, J.-S. Molecular and biochemical modulation of heat shock protein 20 (Hsp20) gene by temperature stress and hydrogen peroxide (H2O2) in the monogonont rotifer, Brachionus sp. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 19–27. [Google Scholar] [CrossRef]


| Species | Stress Factor | Type of HSP | Protein Response | References |
|---|---|---|---|---|
| Tigriopus japonicus | Environmental toxicants (heat, heavy metals, and endocrine disrupting chemicals (EDCs) | Hsp70 | Upregulation | [37,47,68] |
| Heavy metal stress | Hsp105/Hsp90/Hsp70 | Upregulation | ||
| Endocrine disruptors | Hsp20 | Upregulation | ||
| Penaeus monodon | Heat treatment | Hsp90 | Upregulation | [48,69] |
| pH challenge, osmotic stress, and heavy metal exposure | Hsp60 and Hsp10 | Upregulation | ||
| Salinity stress | Hsp21 | Upregulation | ||
| Oxidative stress: endosulfan and deltamethrin | Hsp90 | - | ||
| Litopenaeus vannamei | Thermal | Hsp70 | Upregulation | [53,70,71] |
| Nitrite-N stress | Hsp70 | Upregulation | ||
| Cold shock at 13 °C | Hsp70 | Upregulation | ||
| WSSV infection | LvHSP70 | Tenfold upregulation | ||
| Daphnia magna | Environmental stresses (cyanobacteria, predation from fish, toxic compounds, temperature) | Hsp60s | Upregulation | [72,73] |
| Cadmium and heat stress | Hsp70 | Upregulation | ||
| Environmental | Hsp70 | Upregulation | ||
| Portunus trituberculatus | Salinity stress | Hsp90, Hsp60 | Upregulation | [31] |
| Salinity stress | Hsp70 | Upregulation | ||
| Macrobrachium malcolmsonii | Hg and Cu | Hsp70 | Upregulation | [36] |
| Macrobrachium rosenbergii | Hsp70/Hsc70 | Upregulation | [74] | |
| Amphipods | Cadmium chloride and temperature stresses | Induced by both temperature and toxic stresses | Upregulation | [75] |
| Palaemon elegans | Thermal stress | No significant result | [23] | |
| Palaemon serratus | ||||
| Paracyclopina nana | UV radiation | Hsp60 | Upregulation | [34] |
| Porcellio scaber | Metals | Lower hsp70 levels | Downregulation | [76] |
| Homarus americanus | Acute thermal stress, osmotic stress, molting stress | Significant induction of heat shock, hypo-, and hyper-osmotic responses | Upregulation | [77] |
| Nephrops norvegicus | ||||
| Homarus americanus | Equivalent temperature shift | Hsp70 | Upregulation | [77,78] |
| Thermal shifts | Hsp90/Hsp70/Hsc70 | Upregulation | ||
| Procambarus clarkii | Extreme light | Hsp70 | Upregulation | [79] |
| Help medial giant axons to maintain essential structures and functions | Hsp70 | Upregulation | [80] | |
| Artemia franciscana | Long-term anoxia | Substantial amounts of p26 translocated into nuclei of anoxic brine shrimp embryos | Upregulation | [81] |
| Cd and Zn acute exposure and non-lethal heat shock | Hsp production | Upregulation | [38] | |
| Artemia sinica | CO2-driven seawater acidification | Upregulated in all treatments | Upregulation | [82] |
| Gammarus pulex | Thermal stress | Hsc70 | Upregulation | [83] |
| Dissolved humic substances (HSs) | Significantly increased expression of Hsp70 | Upregulation | [84] | |
| Gammarus lacustris Eulimnogammarus cyaneus E. verrucosus | Involved in stress defense system | Hsp70/sHsp | Upregulation | [75] |
| Calanus finmarchicus | Diapause | Hsp70 | Upregulation | [85] |
| Neohelicegranulatus | Food | Hsp70 | Upregulation | [86] |
| Portunus trituberculatus | Salinity | Hsp70 | Upregulation | [31] |
| Pachygrapsus marmoratus | Temperature, salinity, and pH | Hsp70 | Upregulation | [23] |
| Antarctic krills (Euphausia superba and E. crystallorophias) | Thermal shock | Hsp70 | Upregulation | [87] |
| E. verrucosus and E. cyaneus | Acute thermal stress | Hsp70 | Upregulation | [20] |
| Scylla serrata | Temperature, pathogen, salinity, nitrite stress | Hsp70 | Upregulation | [30] |
| Niphargus virei and N. rhenorhodanensis | Thermal stress | Hsp70 | Upregulation | [88] |
| Eriocheir sinensis | Both low and high salinity | Hsp70 | Upregulation | [26] |
| Oniscus asellus | Organic chemicals, metals | Hsp70 | Upregulation | [76] |
| Metapenaeus ensis | Exogenous estradiol-17β | Hsp90 | Upregulation | [89] |
| Marsupenaeus japonicus | Mjhsp60, Mjhsp70, Mjhsp90 | Upregulation | [90] | |
| Exopalaemon carinicauda | pH and ammonia-N stresses | Hsp90 | Upregulation | [91] |
| Eriocheir sinensis | Glyphosate | Hsp20, Hsp60, Hsp70, HSP90 | Upregulation | [92] |
| Deltamethrin | Hsp60, Hsp70, Hsp90 | Upregulation |
| Species | Tissue | Stressor | HSPs | References |
|---|---|---|---|---|
| Catla catla | Larvae | UV-B radiation | Hsp70 | [104,105] |
| Muscle | Hsp27, Hsp47, Hsp60, Hsp70, Hsp90, Hsp110 | |||
| Channa striata | Gill, muscle | Heat stress | Hsp27, Hsp47, Hsp60, Hsp70, Hsp78, Hsp90, Hsp110 | [106] |
| Cirrhinus mrigala | Liver, gill, brain, kidney | Heat stress | Hsp70 | [107,108] |
| Danio rerio | Embryo | Hspb1, Hspb2, Hspb3, Hspb4, Hspb5a, Hspb5b, Hspb6, Hspb7, Hspb8, Hspb9, Hspb11, Hspb12, Hspb15 | [109] | |
| Labeo rohita | Liver | Arsenic | hsp47, hsp60, hsp70, hsc71, hsp78, hsp90 | [107,108,110,111] |
| Liver | Starvation/fasting | Hsp70 | ||
| Liver, anterior kidney, spleen | Aeromonas hydrophila infection | Hsp30, Hsp70, Hsp90 | ||
| Pethia sophore | Liver, gill, muscle | Heat stress | Hsp27, Hsp47, Hsp60, Hsp70, Hsp78, Hsp90, Hsp110 | [112] |
| Rita rita | Liver, gill | Pollution | Hsp27, Hsp47, Hsp60, Hsp70, Hsp90, Hsp110 | [113] |
| Salmo salar | Skeletal muscle | Starvation/fasting | Hsp90α1a, Hsp90α1b, Hsp90α2a, Hsp90α2b, Hsp90ß1a | [114] |
| Garra rufa | Muscle | Naturally living in a hot spring temp. (34.4 °C) | Hsp70, Hsp60, Hsp90, Hsc70, Grp75 | [115] |
| Squalius torgalensis and Squalius carolitertii | Pectoral, pelvic, upper caudal fins, muscle | 20, 25, 30, and 35 °C for 1 °C per day | Hsp70, Hsc70 | [116] |
| Larimichthys crocea | Muscle, brain, liver, spleen, kidney, gill, and blood | Low temp. (19 °C) and high temp. (27 and 31 °C) | Hsp27 | [117] |
| Gadus morhua | Plasma | Increased temp., 2 °C (2 °C/h) and control 10 °C | Hsp70 | [118] |
| Fundulus heteroclitus | Whole organism | Thermal stress from 2 to 34 °C | Hsp70 and Hsp90 | [119] |
| Carassius auratus | Cells derived from caudal fin | 4 h heat shock form 20 to 40 °C | Hsp30, Hsp70 mRNA | [120,121] |
| Brain | 2 h heat shock from 22 to 32 °C | Hsp72, hsp90 | ||
| Oncorhynchus mykiss | Red blood cell | 8 h heat shock from 10 to 30 °C | Hsp70 mRNA | [122,123,124] |
| Gill, liver, spleen, heart, and head kidney | 18 °C were exposed to an elevated temp. (25 °C) | Hsp60 mRNA | ||
| Liver and heart tissues | 8 h heat shock from 13 to 25 °C with 18–24 h recovery | Hsp70, Hsp90 | ||
| Acipense medtrostrs | Whole larvae | 3 day heat shock from 17 to 26 °C at 1.5 °C/h | Hsp72, Hsp78, Hsp89 | [125] |
| Labeo rohita | Kidney, gill, liver, and brain | 30 day heat shock at 31, 33, and 36 °C | Hsp70 | [107] |
| Penaeus monodon | Tail muscle | 24 h heat shock from 29 to 35 °C | Hsp70 | [126] |
| Ictalurus punctuatus | Muscle | Exposure to low temp. from 25 to 10.5 °C for 14 and 28 days | Hsp70 mRNA | [127,128] |
| Tissue | Bacterial infections | Hsp90, hsp60, and shsp families | ||
| Macrobrachium malcolmsonni | Gill and heart | 3 h heat shock from 25 to 32–34 °C and 30 to 36–38 °C with 1 h recovery | Hsp70 | [129] |
| Macrobrachium rosenbergii | Hepatopancreas and thoracic glands | 2 h heat shock form 25 to 30 and 35 °C | Hsp70 mRNA | [74] |
| Ostrea conchaphila | Gill | 1 h heat shock from 12–15 to 33–38 °C | Hsp70 | [130] |
| Ostrea edulis | Gill | 1 h heat shock from 18 to 34 °C with 24 h recovery at 18 °C | Hsp70 | [57] |
| Channa striata | Gill | Heat shock treatment at 36 °C for 4/15/30 days | Hsp60, Hsp70, Hsp78 | [106] |
| Clarias gariepinus | Embryos | Heavy metals | HSP70 | [131] |
| Rainbow trout | Cultured trout cell line | Heat shock and sodium arsenite | Rapid synthesis of trout Hsp70 mRNA | [132] |
| Danio rerio | Brain | 37 °C heat stress | Hsp47 | [133] |
| Embryos | Environmental stress | Hsp70 | [134,135,136] | |
| Early-stage embryos | Heat shock | Hsp90α and Hsp90β genes | ||
| Embryonic development | Hsp47, Hsp70, and Hsp90 | |||
| Embryonic development | Hsp90 alpha and Hsp90 beta genes | |||
| Oreochromis niloticus | Liver, head kidney, spleen, and gill | Streptococcus agalactiae | Hs70 family, Hsc70-1, Hsc70-2, and Hsc70-3 | [137,138,139] |
| Liver, brain, and gill | Cortisol | Hsp70 | ||
| Muscle, gill, and liver | Different degrees of heat (10, 15, 35, 39 °C) | Hsp70 | ||
| Oreochromis niloticus fingerlings | All organs | Hyperthermal-induced stress | HSP70 | [140] |
| Garra rufa | Liver | Elevated water temperature | Hsp70, Hsp60, Hsp90, Hsc70, and Grp75 | [115] |
| Oreochromis niloticus | Anoxia stress | Hsp70 | [141] | |
| Sarotherodon melanotheron | Gills | Environmental salinity | Hsp70 | [142] |
| Anguilla marmorata | Liver, intestine, muscle, and heart | Aeromonas hydrophila challenge | Amhsp90, Amhsp70 | [143] |
| Oncorhynchus mykiss | Gill, liver, spleen, heart, and head kidney | Elevated temperature | Hsp60 | [123] |
| Oreochromis niloticus | Gonad, liver, and muscle | Elevated water temperature | Hsp90 | [144] |
| Miichthys miiuy | Liver, spleen, and kidney tissue | Bacterial infection | Heat shock protein 90b isoform | [145] |
| Boleophthalmus pectinirostris | Gill, liver tissues | Heat stress conditions | Hsp90AB | [146] |
| Dreissena polymorpha and midge larvae Chironomus tentans | Hsp70 | [147] | ||
| Fenneropenaeus chinensis | Microbial pathogens | Hsp70 | [148] | |
| Heat shock and hypoxia | Hsp70 | |||
| Portunus trituberculatus | Different environmental conditions | Hsp90 genes | [39] | |
| Chinook salmon | Heat shock | Hsp90 genes | [149] | |
| Cyprinus carpio | Gill | Ammonia stress | Hsp70 | [60] |
| Trematomus bernacchii | Cold shock | [150] | ||
| Pimephales promelas | Gill, muscle, and brain | 28, 31, and 33 °C | [151] | |
| Palaemonetes pugio | Muscle | Heat, cadmium, atrazine, and bunker fuel | [152] | |
| Salmo salar L. | Anesthesia, formalin exposure, hypoxia, handling, crowding, and cold shock | Hsp70 | [153] | |
| Oncorhynchus kisutch | Kidney and liver | Renibacterium salmoninarum | Hsp70 | [154] |
| Rainbow trout | Anterior kidney | Vibrio anguillarum | Hsp70 | [155] |
| Sparus sarba Forsskål | Kidney and liver tissue | Vibrio anguillarum | Hsp90 and Hsp60 | [156] |
| Brine shrimp/Vibrio model | Heat shock at 37 °C Vibrio campbelli or Vibrio proteolyticus | Hsp70 upregulation | [157] | |
| Hypothermic shock or acute osmotic | Hsp70 No change | |||
| Penaeus monodon | WSSV | Hsp21 | [158] | |
| Vide supra | Hsp gene downregulation | [156] | ||
| Salmonids | Piscirickettsia salmonis | Hsp60 and HSP70 | [159] | |
| Oncorhychus mykiss (Walbaum) | Fish pathogen Flavobacterium psychrophilum | Hsp60 and Hsp70 | [160] | |
| Brine shrimp | Vibrio infection | Hsp70 | [157] | |
| Xiphophorus maculates | Escherichia coli | Hsps | [157] | |
| Heat-shock-stimulated bacteria | Hsps | [161] |
| Species | Tissue | Stressor | HSPs | Expression | References |
|---|---|---|---|---|---|
| Corbicula fluminea | - | High thermal | HSP70, HSP90, and HSP60 | Upregulation | [162] |
| Mya truncata | - | Chronic heat shock | Upregulation | [163] | |
| Codringtonia | Foot, digestive gland, and genitalia | Short-term heat | HSP70 | Upregulation | [164] |
| Crassostrea virginica and Mercenaria mercenaria | - | - | HSP60, HSP90, and HSP70 | Upregulation | [165] |
| Cyclina sinensis | Hemocytes, hepatopancreas | Cd Vibrio anguillarum | HSP70 | Upregulation | [166] |
| Crassostrea gigas | Long-term thermal waste | HSP70 and HSP90 | Upregulation | [167] | |
| Mid-intertidal limpet Cellana toreuma | Thermal conditions | HSP70 and HSP90 | Upregulation | [158] | |
| B. koreanus | Environmental stressors were reported in copper and UV-exposed | HSP | Upregulation | [168] | |
| Haliotis tuberculata | Thermal stress | HSP70 | Upregulation | [169] |
| Species | Stress Factor | Type of HSP | Protein Response | References |
|---|---|---|---|---|
| Tetraselmis suecica | Redox- and non-redox-active metals | Small TsHSP20 and large TsHSP70 and 100 | Fluctuations | [173] |
| Chironomus riparius | Cadmium | Seven sHSP genes (HSP17, HSP21, HSP22, HSP23, HSP24, HSP27, HSP34) | Downregulation | [174,175] |
| Temperature variations | HSP27 | Upregulation | ||
| Cadmium | HSP27 | Upregulation | ||
| Musca domestica | Thermal and heavy metal | MdomHSP10, MdomHSP27, MdomHSP27.1, MdomHSP27 | Downregulation | [176,177,178,179,180] |
| Starvation, unsuitable temperatures, bacterial and hazard metal challenge | upregulation | |||
| Insecticide dimethoate and alkylbenzene sulfonate heat shock, Cd stress, and bacterial challenge | HSP70 and HSP60 | Upregulation | ||
| Development and maturation of eggs | HSP60 | upregulation | ||
| Stress conditions | Small HSPs | Upregulation | ||
| Drosophila melanogaster | Expressed highly in gonads and nervous system | HSP23, HSP26, and HSP27 | Upregulation | [181] |
| Sarcophaga crassipalpis | Cold-induced diapause | HSP23 | Upregulation | [182] |
| Plutella xylostella | Heavy metals | sHSPs | Upregulation | [183] |
| Galleria mellonella | Conidiobolus coronatus-induced infection | HSP90, HSP70, HSP60, HSP27 | Upregulation | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.-S. A Review on the Involvement of Heat Shock Proteins (Extrinsic Chaperones) in Response to Stress Conditions in Aquatic Organisms. Antioxidants 2023, 12, 1444. https://doi.org/10.3390/antiox12071444
Jeyachandran S, Chellapandian H, Park K, Kwak I-S. A Review on the Involvement of Heat Shock Proteins (Extrinsic Chaperones) in Response to Stress Conditions in Aquatic Organisms. Antioxidants. 2023; 12(7):1444. https://doi.org/10.3390/antiox12071444
Chicago/Turabian StyleJeyachandran, Sivakamavalli, Hethesh Chellapandian, Kiyun Park, and Ihn-Sil Kwak. 2023. "A Review on the Involvement of Heat Shock Proteins (Extrinsic Chaperones) in Response to Stress Conditions in Aquatic Organisms" Antioxidants 12, no. 7: 1444. https://doi.org/10.3390/antiox12071444
APA StyleJeyachandran, S., Chellapandian, H., Park, K., & Kwak, I.-S. (2023). A Review on the Involvement of Heat Shock Proteins (Extrinsic Chaperones) in Response to Stress Conditions in Aquatic Organisms. Antioxidants, 12(7), 1444. https://doi.org/10.3390/antiox12071444






