Exploring the Potential of Bee-Derived Antioxidants for Maintaining Oral Hygiene and Dental Health: A Comprehensive Review
Abstract
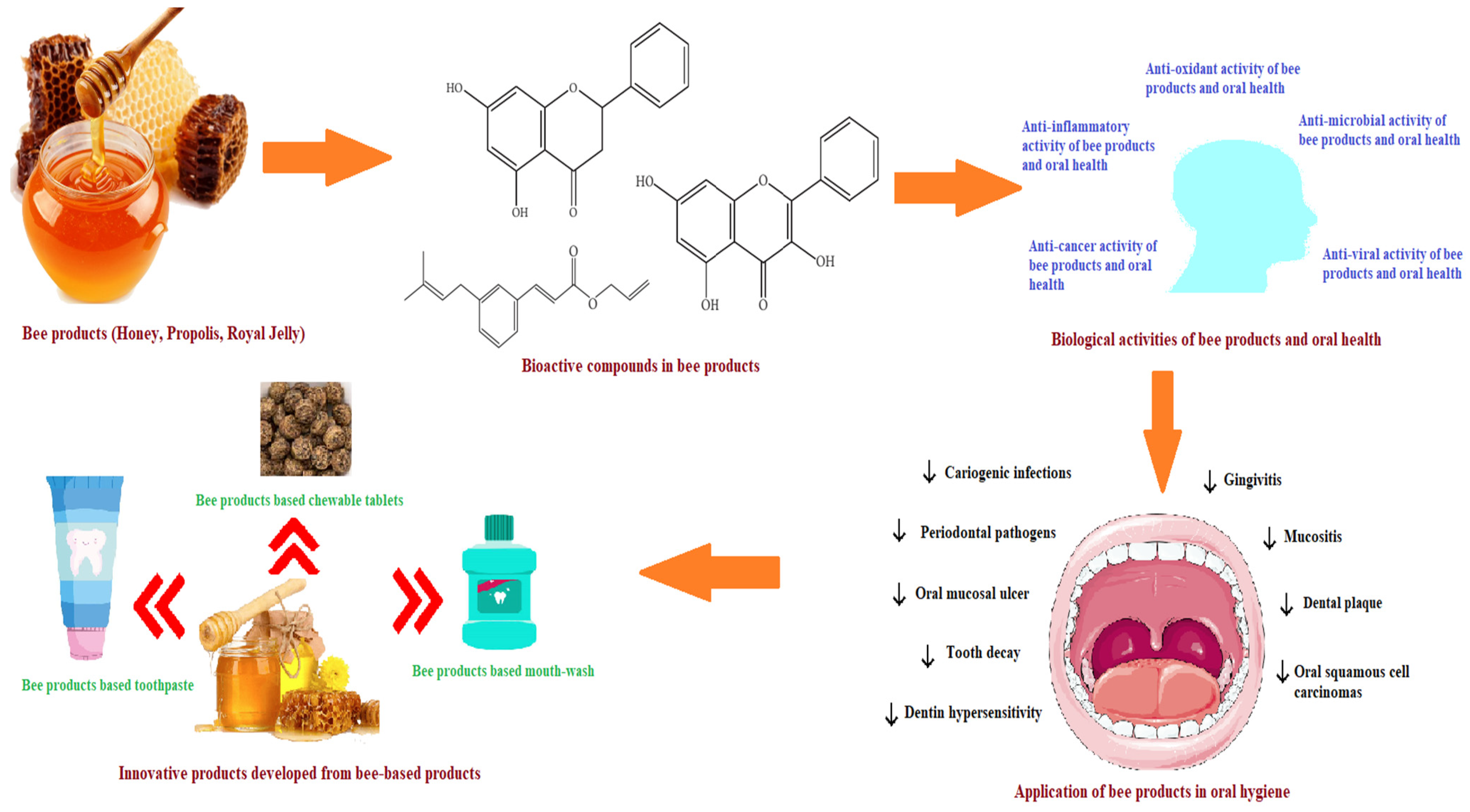
:1. Introduction
2. Bioactive Compounds of Bee Products in Relation to Oral Health
Bioactive Compounds
3. Biological Activities of Bee Products in Relation to Oral Health
3.1. Antioxidant Activity of Bee Products and Its Effects on Diseases of the Oral Cavity
3.2. Anti-Microbial Activity of Bee-Based Products and Its Effects on Diseases of the Oral Cavity
3.2.1. Anti-Bacterial Activity of Bee-Based Products and Its Effects on Diseases of the Oral Cavity
3.2.2. Anti-Fungal Activity of Bee-Based Products and Its Effects on Diseases of the Oral Cavity
3.2.3. Antiviral Activity of Bee-Based Products and Its Effects on Diseases of the Oral Cavity
3.2.4. Anti-Inflammatory Activity of Honey Bee-Derived Products and Its Effects on Diseases of the Oral Cavity
3.2.5. Anti-Cancer Activity of Bee Products in Relation to Oral Health
4. Applications of Bee-Based Products in Managing oral Diseases
4.1. Gingivitis
4.2. Dental Caries
4.3. Oral Cancer
4.4. Oral Malodor (Halitosis)
4.5. Oral Mucositis
4.6. Xerostomia
4.7. Dentin Hypersensitivity
5. Bee Products-Based Innovative Products for Oral Hygiene
5.1. Bee Product-Based Toothpaste
5.2. Bee Product-Based Mouthwash
5.3. Bee Product-Based Chewable Tablets or Chewing Gums
6. Safety Aspects of Honey and Bee Products
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Apimondia. Rodusele Stupului—Hrană, Sănătate, Frumuseţe; Apimondia: Bucureşti, Romania, 1989. [Google Scholar]
- Apimondia. Un Preţios Produs al Apiculturii. Propolisul; Apimondia: Bucureşti, Romania, 1990. [Google Scholar]
- Hellner, M.; Winter, D.; von Georgi, R.; Münstedt, K. Apitherapy: Usage and experience in German beekeepers. Evid.-Based Complement. Altern. Med. 2008, 5, 475–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurshid, Z.; Naseem, M.; Zafar, M.S.; Najeeb, S.; Zohaib, S. Propolis: A natural biomaterial for dental and oral healthcare. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 265–274. [Google Scholar] [CrossRef]
- Crane, E. History of Honey in Honey: A Comprehensive Survey; Crane, E., Ed.; Northern Bee Books: West Yorkshire, UK, 1975. [Google Scholar]
- Rossi, M.; Marrazzo, P. The potential of honeybee products for biomaterial applications. Biomimetics 2021, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Tappi, S.; Laghi, L.; Dettori, A.; Piana, L.; Ragni, L.; Rocculi, P. Investigation of water state during induced crystallization of honey. Food Chem. 2019, 294, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and its role in relieving multiple facets of atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pashte, V.V.; Pashte, S.V.; Said, P.P. Nutraceutical properties of natural honey to fight health issues: A comprehensive review. J. Pharmacogn. Phytochem. 2020, 9, 234–242. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [Green Version]
- Escuredo, O.; Seijo, M.C.; Salvador, J.; González-Martín, M.I. Near infrared spectroscopy for prediction of antioxidant compounds in the honey. Food Chem. 2013, 141, 3409–3414. [Google Scholar] [CrossRef]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef]
- Kasote, D.M. Propolis: A neglected product of value in the Indian beekeeping sector. Bee World 2017, 94, 80–83. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee products in dermatology and skin care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebadi, P.; Fazeli, M. Evaluation of the potential in vitro effects of propolis and honey on wound healing in human dermal fibroblast cells. S. Afr. J. Bot. 2021, 137, 414–422. [Google Scholar] [CrossRef]
- Buttstedt, A.; Mureşan, C.I.; Lilie, H.; Hause, G.; Ihling, C.H.; Schulze, S.-H.; Pietzsch, M.; Moritz, R.F. How honeybees defy gravity with royal jelly to raise queens. Curr. Biol. 2018, 28, 1095–1100.e1093. [Google Scholar] [CrossRef] [Green Version]
- Kunugi, H.; Ali, A.M. Royal jelly and its components promote healthy aging and longevity: From animal models to humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamakura, M. Royalactin induces queen differentiation in honeybees. Nature 2011, 473, 478–483. [Google Scholar] [CrossRef]
- Detienne, G.; De Haes, W.; Ernst, U.R.; Schoofs, L.; Temmerman, L. Royalactin extends lifespan of Caenorhabditis elegans through epidermal growth factor signaling. Exp. Gerontol. 2014, 60, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Kolayli, S.; Sahin, H.; Can, Z.; Yildiz, O.; Malkoc, M.; Asadov, A. A member of complementary medicinal food: Anatolian royal jellies, their chemical compositions, and antioxidant properties. J. Evid.-Based Complement. Altern. Med. 2016, 21, NP43–NP48. [Google Scholar] [CrossRef] [Green Version]
- WHO. Oral Health. EXECUTIVE BOARD 148th Session Provisional Agenda Item 6. Available online: https://apps.who.int/gb/ebwha/pdf_files/EB148/B148_8-en.pdf (accessed on 30 January 2023).
- Yu, X.; Chen, Y.; Li, Y.; Hong, J.; Hua, F. A bibliometric mapping study of the literature on oral health-related quality of life. J. Evid.-Based Dent. Pract. 2020, 23, 101780. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2020, 129, 108756. [Google Scholar] [CrossRef] [PubMed]
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial activity of bee-collected pollen and beebread: State of the art and future perspectives. Antibiotics 2020, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.; Mendes, M.A.; De Souza, B.M.; Konno, K.; César, L.l.M.M.; Malaspina, O.; Palma, M.S. Jelleines: A family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides 2004, 25, 919–928. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Chou, W.-M.; Widowati, D.A.; Lin, I.-P.; Peng, C.-C. 10-hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement. Altern. Med. 2018, 18, 202. [Google Scholar] [CrossRef]
- Filipič, B.; Gradišnik, L.; Rihar, K.; Šooš, E.; Pereyra, A.; Potokar, J. The influence of royal jelly and human interferon-alpha (HuIFN-αN3) on proliferation, glutathione level and lipid peroxidation in human colorectal adenocarcinoma cells in vitro. Arhiv za Higijenu Rada i Toksikologiju 2015, 66, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Shao, Q.; Geng, H.; Su, S. The effect of royal jelly on the growth of breast cancer in mice. Oncol. Lett. 2017, 14, 7615–7621. [Google Scholar] [CrossRef] [Green Version]
- Guendouz, M.; Haddi, A.; Grar, H.; Kheroua, O.; Saidi, D.; Kaddouri, H. Preventive effects of royal jelly against anaphylactic response in a murine model of cow’s milk allergy. Pharm. Biol. 2017, 55, 2145–2152. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, Y.; Kohno, K.; Inoue, S.-I.; Koya-Miyata, S.; Okamoto, I.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Oral administration of royal jelly inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int. Immunopharmacol. 2003, 3, 1313–1324. [Google Scholar] [CrossRef]
- Kai, H.; Motomura, Y.; Saito, S.; Hashimoto, K.; Tatefuji, T.; Takamune, N.; Misumi, S. Royal jelly enhances antigen-specific mucosal IgA response. Food Sci. Nutr. 2013, 1, 222–227. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Abdulrhman, M.; Elbarbary, N.S.; Amin, D.A.; Ebrahim, R.S. Honey and a mixture of honey, beeswax, and olive oil–propolis extract in treatment of chemotherapy-induced oral mucositis: A randomized controlled pilot study. Pediatr. Hematol. Oncol. 2012, 29, 285–292. [Google Scholar] [CrossRef]
- Kuo, Y.-Y.; Lin, H.-P.; Huo, C.; Su, L.-C.; Yang, J.; Hsiao, P.-H.; Chiang, H.-C.; Chung, C.-J.; Wang, H.-D.; Chang, J.-Y. Caffeic acid phenethyl ester suppresses proliferation and survival of TW2. 6 human oral cancer cells via inhibition of Akt signaling. Int. J. Mol. Sci. 2013, 14, 8801–8817. [Google Scholar] [CrossRef] [Green Version]
- Więckiewicz, W.; Miernik, M.; Więckiewicz, M.; Morawiec, T. Does propolis help to maintain oral health? Evid.-Based Complement. Altern. Med. 2013, 2013, 351062. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New insights into the biological and pharmaceutical properties of royal jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [Green Version]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Liu, J.-R.; Ye, Y.-L.; Lin, T.-Y.; Wang, Y.-W.; Peng, C.-C. Effect of floral sources on the antioxidant, antimicrobial, and anti-inflammatory activities of honeys in Taiwan. Food Chem. 2013, 139, 938–943. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Othman, N.H. Review of the medicinal effects of tualang honey and a comparison with manuka honey. Malays. J. Med. Sci. MJMS 2013, 20, 6. [Google Scholar] [PubMed]
- Olas, B. Honey and its phenolic compounds as an effective natural medicine for cardiovascular diseases in humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic profile and antioxidant properties of Polish honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Hung, H.-C.; Joshipura, K.J.; Jiang, R.; Hu, F.B.; Hunter, D.; Smith-Warner, S.A.; Colditz, G.A.; Rosner, B.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake and risk of major chronic disease. J. Natl. Cancer Inst. 2004, 96, 1577–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, S.; Banerjee, A.; Basu, K.; Sengupta, B.; Sengupta, P.K. Interaction of flavonoids with red blood cell membrane lipids and proteins: Antioxidant and antihemolytic effects. Int. J. Biol. Macromol. 2007, 41, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef] [Green Version]
- Curti, V.; Zaccaria, V.; Sokeng, A.J.T.; Dacrema, M.; Masiello, I.; Mascaro, A.; D’Antona, G.; Daglia, M. Bioavailability and In Vivo Antioxidant Activity of a Standardized Polyphenol Mixture Extracted from Brown Propolis. Int. J. Mol. Sci. 2019, 20, 1250. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Paşca, C.; Moise, A.R.; Bobiş, O. Plant Sources Responsible for the Chemical Composition and Main Bioactive Properties of Poplar-Type Propolis. Plants 2021, 10, 22. [Google Scholar] [CrossRef]
- Almaraz-Abarca, N.; da Graça Campos, M.; Avila-Reyes, J.A.; Naranjo-Jimenez, N.; Corral, J.H.; Gonzalez-Valdez, L.S. Antioxidant activity of polyphenolic extract of monofloral honeybee-collected pollen from mesquite (Prosopis juliflora, Leguminosae). J. Food Compos. Anal. 2007, 20, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, V.; Ahuja, A. Apitherapy-A sweet approach to dental diseases. Part II: Propolis. J. Adv. Oral Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- López-Gutiérrez, N.; del Mar Aguilera-Luiz, M.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Fast analysis of polyphenols in royal jelly products using automated TurboFlow™-liquid chromatography–Orbitrap high resolution mass spectrometry. J. Chromatogr. B 2014, 973, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Enayatullah, H.; Lv, Z.; Dai, H.; Wei, Q.; Shen, L.; Karwand, B.; Shi, F. Freeze-dried royal jelly proteins enhanced the testicular development and spermatogenesis in pubescent male mice. Animals 2019, 9, 977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Caravaca, A.; Gómez-Romero, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Advances in the analysis of phenolic compounds in products derived from bees. J. Pharm. Biomed. Anal. 2006, 41, 1220–1234. [Google Scholar] [CrossRef]
- Küçük, M.; Kolaylı, S.; Karaoğlu, Ş.; Ulusoy, E.; Baltacı, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Nagai, T.; Inoue, R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004, 84, 181–186. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef]
- Khalil, M.; Sulaiman, S.A. The potential role of honey and its polyphenols in preventing heart disease: A review. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, M.; Kaminska, D.; Matuszewska, E.; Hołderna-Kedzia, E.; Rogacki, J.; Matysiak, J. Promising antimicrobial properties of bioactive compounds from different honeybee products. Molecules 2021, 26, 4007. [Google Scholar] [CrossRef]
- Pérez-Pérez, E.; Vit, P.; Huq, F. Flavonoids and polyphenols in studies of honey antioxidant activity. Int. J. Med. Plant Altern. Med. 2013, 1, 63–72. [Google Scholar]
- Gheldof, N.; Wang, X.-H.; Engeseth, N.J. Buckwheat honey increases serum antioxidant capacity in humans. J. Agric. Food Chem. 2003, 51, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.; Kellett, J.; D’Cunha, N.M.; Toohey, K.; McKune, A.; Naumovski, N. The effect of honey as a treatment for oral ulcerative lesions: A systematic review. Explor. Res. Hypothesis Med. 2020, 5, 27–37. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant activity in bee products: A review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Propolis: A new frontier for wound healing? Burn. Trauma 2015, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Miguel, S.M.S.; Opperman, L.A.; Allen, E.P.; Svoboda, K.K. Use of antioxidants in oral healthcare. Compend. Contin. Educ. Dent. 2011, 32, E156–E159. [Google Scholar]
- Kuś, P.M.; Congiu, F.; Teper, D.; Sroka, Z.; Jerković, I.; Tuberoso, C.I.G. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT-Food Sci. Technol. 2014, 55, 124–130. [Google Scholar] [CrossRef]
- Dżugan, M.; Grabek-Lejko, D.; Swacha, S.; Tomczyk, M.; Bednarska, S.; Kapusta, I. Physicochemical quality parameters, antibacterial properties and cellular antioxidant activity of Polish buckwheat honey. Food Biosci. 2020, 34, 100538. [Google Scholar] [CrossRef]
- Kishore, R.K.; Halim, A.S.; Syazana, M.N.; Sirajudeen, K. Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr. Res. 2011, 31, 322–325. [Google Scholar] [CrossRef]
- Rosa, A.; Tuberoso, C.I.G.; Atzeri, A.; Melis, M.P.; Bifulco, E.; Dessì, M.A. Antioxidant profile of strawberry tree honey and its marker homogentisic acid in several models of oxidative stress. Food Chem. 2011, 129, 1045–1053. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; González-Paramás, A.M.; Damiani, E.; Astolfi, P.; Martinez-Sanchez, G.; Bompadre, S.; Quiles, J.L.; Santos-Buelga, C.; Battino, M. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem. Toxicol. 2012, 50, 1508–1516. [Google Scholar] [CrossRef]
- Yuslianti, E.R.; Bachtiar, B.M.; Sastradipura, D.F.S.; Sutjiatmo, A.B. Antioxidant Activity of Rambutan Honey: The free radical-scavenging activity in vitro and lipid peroxidation inhibition of oral mucosa wound tissue in vivo. Res. J. Med. Plant 2015, 9, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Toczewska, J.; Maciejczyk, M.; Konopka, T.; Zalewska, A. Total oxidant and antioxidant capacity of gingival crevicular fluid and saliva in patients with periodontitis: Review and clinical study. Antioxidants 2020, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yao, H.; Yao, Y.; Fai, L.Y.; Zhang, Z. Protection of dietary polyphenols against oral cancer. Nutrients 2013, 5, 2173–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Saluja, M.; Agarwal, G.; Alam, M. Green tea: A boon for periodontal and general health. J. Indian Soc. Periodontol. 2012, 16, 161. [Google Scholar] [PubMed]
- Khan, H.; Sureda, A.; Belwal, T.; Çetinkaya, S.; Süntar, İ.; Tejada, S.; Devkota, H.P.; Ullah, H.; Aschner, M. Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 2019, 18, 647–657. [Google Scholar] [CrossRef]
- Abbasi, A.J.; Mohammadi, F.; Bayat, M.; Gema, S.M.; Ghadirian, H.; Seifi, H.; Bayat, H.; Bahrami, N. Applications of Propolis in Dentistry: A Review. Ethiop. J. Health Sci. 2018, 28, 505–512. [Google Scholar] [CrossRef]
- Babaee, N.; Hosseinkazemi, H.; Pouramir, M.; Baboli, O.K.; Salehi, M.; Khadir, F.; Bijani, A.; Mehryari, M. Salivary oxidant/antioxidant status and hematological parameters in patients with recurrent aphthous stomatitis. Casp. J. Intern. Med. 2016, 7, 13. [Google Scholar]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Boisard, S.; Le Ray, A.-M.; Gatto, J.; Aumond, M.-C.; Blanchard, P.; Derbré, S.; Flurin, C.; Richomme, P. Chemical composition, antioxidant and anti-AGEs activities of a French poplar type propolis. J. Agric. Food Chem. 2014, 62, 1344–1351. [Google Scholar] [CrossRef] [Green Version]
- Fabris, S.; Bertelle, M.; Astafyeva, O.; Gregoris, E.; Zangrando, R.; Gambaro, A.; Lima, G.P.P.; Stevanato, R. Antioxidant Properties and Chemical Composition Relationship of Europeans and Brazilians Propolis; Scientific Research Publishing: Wuhan, China, 2013. [Google Scholar]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Naik, P.; Vishma, B.; Salian, S.R.; Devkar, R.A.; Khan, S.; Mutalik, S.; Kalthur, G.; Adiga, S.K. Mitigating effect of Indian propolis against mitomycin C induced bone marrow toxicity. Cytotechnology 2016, 68, 1789–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonamigo, T.; Campos, J.F.; Alfredo, T.M.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; de Picoli Souza, K.; Dos Santos, E.L. Antioxidant, cytotoxic, and toxic activities of propolis from two native bees in Brazil: Scaptotrigona depilis and Melipona quadrifasciata anthidioides. Oxidative Med. Cell. Longev. 2017, 2017, 1038153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özkök, D.; Silici, S. Antioxidant activities of honeybee products and their mixtures. Food Sci. Biotechnol. 2017, 26, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kocot, J.; Kielczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxidative Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Aghel, S.; Pouramir, M.; Moghadamnia, A.A.; Moslemi, D.; Molania, T.; Ghassemi, L.; Motallebnejad, M. Effect of Iranian propolis on salivary total antioxidant capacity in gamma-irradiated rats. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 235. [Google Scholar]
- El-Sharkawy, H.M.; Anees, M.M.; Van Dyke, T.E. Propolis Improves Periodontal Status and Glycemic Control in Patients With Type 2 Diabetes Mellitus and Chronic Periodontitis: A Randomized Clinical Trial. J. Periodontol. 2016, 87, 1418–1426. [Google Scholar] [CrossRef]
- Giammarinaro, E.; Marconcini, S.; Genovesi, A.; Poli, G.; Lorenzi, C.; Covani, U. Propolis as an adjuvant to non-surgical periodontal treatment: A clinical study with salivary anti-oxidant capacity assessment. Minerva Stomatol. 2018, 67, 183–188. [Google Scholar] [CrossRef]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Silici, S.; Ekmekcioglu, O.; Eraslan, G.; Demirtas, A. Antioxidative effect of royal jelly in cisplatin-induced testes damage. Urology 2009, 74, 545–551. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, B.Y.; Deng, Y.; Park, H.G.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Antioxidant capacity of major royal jelly proteins of honeybee (Apis mellifera) royal jelly. J. Asia-Pac. Entomol. 2020, 23, 445–448. [Google Scholar] [CrossRef]
- Liu, J.-R.; Yang, Y.-C.; Shi, L.-S.; Peng, C.-C. Antioxidant properties of royal jelly associated with larval age and time of harvest. J. Agric. Food Chem. 2008, 56, 11447–11452. [Google Scholar] [CrossRef] [PubMed]
- Balkanska, R.; Marghitas, L.-A.; Pavel, C.I. Antioxidant activity and total polyphenol content of royal jelly from Bulgaria. Int. J. Curr. Microbiol. Appl. Sci 2017, 6, 578–585. [Google Scholar] [CrossRef]
- Uçar, M.; Barlak, Y. The Antioxidant Activity of Water, DMSO and Methanol Extracts of Royal Jelly from Bursa Province in Turkey. Pharm. Drug Regul. Aff. J. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Aparna, S.; Srirangarajan, S.; Malgi, V.; Setlur, K.P.; Shashidhar, R.; Setty, S.; Thakur, S. A Comparative Evaluation of the Antibacterial Efficacy of Honey In Vitro and Antiplaque Efficacy in a 4-Day Plaque Regrowth Model In Vivo: Preliminary Results. J. Periodontol. 2012, 83, 1116–1121. [Google Scholar] [CrossRef]
- Moussa, A.; Noureddine, D.; Saad, A.; Abdelmelek, M.; Abdelkader, B. Antifungal activity of four honeys of different types from Algeria against pathogenic yeast: Candida albicans and Rhodotorula sp. Asian Pac. J. Trop. Biomed. 2012, 2, 554–557. [Google Scholar] [CrossRef] [Green Version]
- Awad, O.G.A.-N.; Hamad, A.-M.H. Honey can help in herpes simplex gingivostomatitis in children: Prospective randomized double blind placebo controlled clinical trial. Am. J. Otolaryngol. 2018, 39, 759–763. [Google Scholar] [CrossRef]
- Candiracci, M.; Piatti, E.; Dominguez-Barragán, M.; García-Antrás, D.; Morgado, B.; Ruano, D.; Gutiérrez, J.F.; Parrado, J.; Castaño, A.l. Anti-inflammatory activity of a honey flavonoid extract on lipopolysaccharide-activated N13 microglial cells. J. Agric. Food Chem. 2012, 60, 12304–12311. [Google Scholar] [CrossRef]
- Ghashm, A.A.; Othman, N.H.; Khattak, M.N.; Ismail, N.M.; Saini, R. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complement. Altern. Med. 2010, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Skaba, D.; Morawiec, T.; Tanasiewicz, M.; Mertas, A.; Bobela, E.; Szliszka, E.; Skucha-Nowak, M.; Dawiec, M.; Yamamoto, R.; Ishiai, S. Influence of the toothpaste with brazilian ethanol extract propolis on the oral cavity health. Evid.-Based Complement. Altern. Med. 2013, 2013, 215391. [Google Scholar] [CrossRef] [Green Version]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother. Res. 2010, 24, S20–S28. [Google Scholar] [CrossRef] [PubMed]
- Bretz, W.A.; Paulino, N.; Nör, J.E.; Moreira, A. The effectiveness of propolis on gingivitis: A randomized controlled trial. J. Altern. Complement. Med. 2014, 20, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.-Y.; Jim, W.-T.; Su, L.-C.; Chung, C.-J.; Lin, C.-Y.; Huo, C.; Tseng, J.-C.; Huang, S.-H.; Lai, C.-J.; Chen, B.-C. Caffeic acid phenethyl ester is a potential therapeutic agent for oral cancer. Int. J. Mol. Sci. 2015, 16, 10748–10766. [Google Scholar] [CrossRef] [Green Version]
- Moghim, H.; Taghipour, S.; Kheiri, S.; Khabbazi, H.; Baradaran, A. Antifungal effects of iranian propolis extract and royal jelly against candida albicans in-vitro. Int. J. Prev. Med. 2021, 12, 163. [Google Scholar] [PubMed]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Izuta, H.; Chikaraishi, Y.; Shimazawa, M.; Mishima, S.; Hara, H. 10-Hydroxy-2-decenoic acid, a major fatty acid from royal jelly, inhibits VEGF-induced angiogenesis in human umbilical vein endothelial cells. Evid.-Based Complement. Altern. Med. 2009, 6, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Garcia, G.; Ramos, F.; Maldonado, J.; Fernandez, A.; Yáñez, J.; Hernandez, L.; Gaytán, P. Prevalence of two Entamoeba gingivalis ST1 and ST2-kamaktli subtypes in the human oral cavity under various conditions. Parasitol. Res. 2018, 117, 2941–2948. [Google Scholar] [CrossRef]
- Teles, R.; Teles, F.; Frias-Lopez, J.; Paster, B.; Haffajee, A. Lessons learned and unlearned in periodontal microbiology. Periodontology 2000, 62, 95–162. [Google Scholar] [CrossRef]
- Prabu, G.; Gnanamani, A.; Sadulla, S. Guaijaverin–a plant flavonoid as potential antiplaque agent against Streptococcus mutans. J. Appl. Microbiol. 2006, 101, 487–495. [Google Scholar] [CrossRef]
- McGrath, L.J.; Becker-Dreps, S.; Pate, V.; Brookhart, M.A. Trends in antibiotic treatment of acute otitis media and treatment failure in children, 2000–2011. PLoS ONE 2013, 8, e81210. [Google Scholar] [CrossRef] [Green Version]
- Hwang, A.Y.; Gums, J.G. The emergence and evolution of antimicrobial resistance: Impact on a global scale. Bioorg. Med. Chem. 2016, 24, 6440–6445. [Google Scholar] [CrossRef]
- Brown, H.L.; Roberts, A.E.L.; Cooper, R.; Jenkins, R. A review of selected bee products as potential anti-bacterial, anti-fungal, and anti-viral agents. Med. Res. Arch. 2016, 4, 1–20. [Google Scholar]
- Topcuoglu, N.; Ozan, F.; Ozyurt, M.; Kulekci, G. In Vitro antibacterial effects of glassionomer cement containing ethanolic extract of propolis on Streptococcus mutans. Eur. J. Dent. 2012, 6, 428–433. [Google Scholar] [CrossRef]
- Dzidic, M.; Collado, M.C.; Abrahamsson, T.; Artacho, A.; Stensson, M.; Jenmalm, M.C.; Mira, A. Oral microbiome development during childhood: An ecological succession influenced by postnatal factors and associated with tooth decay. ISME J. 2018, 12, 2292–2306. [Google Scholar] [CrossRef] [Green Version]
- Tanner, A.; Kressirer, C.A.; Faller, L.L. Understanding Caries From the Oral Microbiome Perspective. J. Calif. Dent. Assoc. 2016, 44, 437–446. [Google Scholar] [CrossRef]
- Dustmann, J.H. Antibacterial effect of honey. Apiacta 1979, 14, 7–11. [Google Scholar]
- Kiamco, M.M.; Atci, E.; Mohamed, A.; Call, D.R.; Beyenal, H. Hyperosmotic agents and antibiotics affect dissolved oxygen and PH concentration gradients in Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 2017, 83, e02783-16. [Google Scholar] [CrossRef] [Green Version]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M. Antibacterial activity of different blossom honeys: New findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef] [Green Version]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive components and antioxidant and antibacterial activities of different varieties of honey: A screening prior to clinical application. J. Agric. Food Chem. 2018, 67, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Molan, P.C. The antibacterial activity of honey: 1. The nature of the antibacterial activity. Bee World 1992, 73, 5–28. [Google Scholar] [CrossRef]
- Brudzynski, K.; Abubaker, K.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef] [Green Version]
- Rabie, E.; Serem, J.C.; Oberholzer, H.M.; Gaspar, A.R.M.; Bester, M.J. How methylglyoxal kills bacteria: An ultrastructural study. Ultrastruct. Pathol. 2016, 40, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Terio, V.; Bozzo, G.; Ceci, E.; Savarino, A.E.; Barrasso, R.; Di Pinto, A.; Mottola, A.; Marchetti, P.; Tantillo, G.; Bonerba, E. Methylglyoxal (MGO) in Italian Honey. Appl. Sci. 2021, 11, 831. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Koochak, H.; Seyyednejad, S.M.; Motamedi, H. Preliminary study on the antibacterial activity of some medicinal plants of Khuzestan (Iran). Asian Pac. J. Trop. Med. 2010, 3, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Pieper, B. Honey-based dressings and wound care: An option for care in the United States. J. Wound Ostomy Cont. Nurs. 2009, 36, 60–66. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Investigating the antimicrobial activity of natural honey and its effects on the pathogenic bacterial infections of surgical wounds and conjunctiva. J. Med. Food 2004, 7, 210–222. [Google Scholar] [CrossRef]
- Shiga, H.; Jo, A.; Terao, K.; Nakano, M.; Oshima, T.; Maeda, N. Decrease of halitosis by intake of Manuka honey. Gen. Sess. IADR Barc. 2010, 14, 234–238. [Google Scholar]
- Patel, R.; Thaker, V.; Patel, V.; Shukla, P.; Bhatnagar, P.; Patel, A. In-Vitro study of changing antibiotic sensitivity and resistance by honey on gingival inflammation during orthodontic treatment a preliminary report. Orthod. Cyber J. 2010, 20, 3–8. [Google Scholar]
- Eick, S.; Schäfer, G.; Kwieciński, J.; Atrott, J.; Henle, T.; Pfister, W. Honey—A potential agent against Porphyromonas gingivalis: An in vitro study. BMC Oral Health 2014, 14, 24. [Google Scholar] [CrossRef] [Green Version]
- Boyanova, L.; Ilieva, J.; Gergova, G.; Vladimirov, B.; Nikolov, R.; Mitov, I. Honey and green/black tea consumption may reduce the risk of Helicobacter pylori infection. Diagn. Microbiol. Infect. Dis. 2015, 82, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Bouchelaghem, S. Propolis characterization and antimicrobial activities against Staphylococcus aureus and Candida albicans: A review. Saudi J. Biol. Sci. 2022, 29, 1936–1946. [Google Scholar] [CrossRef]
- Popova, M.P.; Bankova, V.S.; Bogdanov, S.; Tsvetkova, I.; Naydenski, C.; Marcazzan, G.L.; Sabatini, A.-G. Chemical characteristics of poplar type propolis of different geographic origin. Apidologie 2007, 38, 306–311. [Google Scholar] [CrossRef] [Green Version]
- Mirzoeva, O.; Grishanin, R.; Calder, P. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Koo, H.; Cury, J.A.; Rosalen, P.L.; Ambrosano, G.M.; Ikegaki, M.; Park, Y.K. Effect of a mouthrinse containing selected propolis on 3-day dental plaque accumulation and polysaccharide formation. Caries Res. 2002, 36, 445–448. [Google Scholar] [CrossRef]
- Duailibe, S.A.d.C.; Gonçalves, A.G.; Ahid, F.J.M. Effect of a propolis extract on Streptococcus mutans counts in vivo. J. Appl. Oral Sci. 2007, 15, 420–423. [Google Scholar] [CrossRef] [Green Version]
- do Amaral, R.C.; Gomes, R.T.; Rocha, W.M.S.; Lemos, S.; Abreu, R.; Santos, V.R. Periodontitis treatment with Brazilian green propolis gel. Pharmacologyonline 2006, 3, 336–341. [Google Scholar]
- Chandna, P.; Adlakha, V.K.; Das, S.; Singh, S. Complementary and Alternative Medicine (CAM): A review of propolis in dentistry. Technology 2014, 4, 675–685. [Google Scholar]
- Castro, S.L. Propolis: Biological and pharmacological activities. Therapeutic uses of this bee-product. Annu. Rev. Biomed. Sci. 2001, 3, 49–83. [Google Scholar] [CrossRef]
- Coutinho, D.; Karibasappa, S.N.; Mehta, D.S. Royal jelly antimicrobial activity against periodontopathic bacteria. J. Interdiscip. Dent. 2018, 8, 18. [Google Scholar]
- Sugiyama, T.; Takahashi, K.; Mori, H. Royal jelly acid, 10-hydroxy-trans-2-decenoic acid, as a modulator of the innate immune responses. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2012, 12, 368–376. [Google Scholar] [CrossRef]
- Terada, Y.; Narukawa, M.; Watanabe, T. Specific hydroxy fatty acids in royal jelly activate TRPA1. J. Agric. Food Chem. 2011, 59, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Khosla, A.; Gupta, S.J.; Jain, A.; Shetty, D.C.; Sharma, N. Evaluation and comparison of the antimicrobial activity of royal jelly—A holistic healer against periodontopathic bacteria: An in vitro study. J. Indian Soc. Periodontol. 2020, 24, 221–226. [Google Scholar] [CrossRef]
- Baker, J.L.; Bor, B.; Agnello, M.; Shi, W.; He, X. Ecology of the oral microbiome: Beyond bacteria. Trends Microbiol. 2017, 25, 362–374. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, D.; Zaman, S.; Naqvi, S.; Sheikh, M.; Ali, G. Studies on the antimicrobial activity of honey. Pak. J. Pharm. Sci. 1995, 8, 51–62. [Google Scholar]
- White, J.W., Jr.; Subers, M.H.; Schepartz, A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta (BBA)-Spec. Sect. Enzymol. Subj. 1963, 73, 57–70. [Google Scholar] [CrossRef]
- Bang, L.M.; Buntting, C.; Molan, P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J. Altern. Complement. Med. 2003, 9, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Irish, J.; Carter, D.A.; Shokohi, T.; Blair, S.E. Honey has an antifungal effect against Candida species. Med. Mycol. 2006, 44, 289–291. [Google Scholar] [CrossRef] [Green Version]
- English, H.; Pack, A.; Molan, P.C. The effects of manuka honey on plaque and gingivitis: A pilot study. J. Int. Acad. Periodontol. 2004, 6, 63–67. [Google Scholar]
- Takaisi-Kikuni, N.B.; Schilcher, H. Electron microscopic and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Medica 1994, 60, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Feres, M.; Figueiredo, L.C.; Barreto, I.; Coelho, M.; Araujo, M.; Cortelli, S.C. In vitro antimicrobial activity of plant extracts and propolis in saliva samples of healthy and periodontally-involved subjects. J. Int. Acad. Periodontol. 2005, 7, 90–96. [Google Scholar]
- Santos, V.; Pimenta, F.; Aguiar, M.; Do Carmo, M.; Naves, M.; Mesquita, R. Oral candidiasis treatment with Brazilian ethanol propolis extract. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2005, 19, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Bachanová, K.; Klaudiny, J.; Kopernický, J.; Šimúth, J. Identification of honeybee peptide active against Paenibacillus larvae larvae through bacterial growth-inhibition assay on polyacrylamide gel. Apidologie 2002, 33, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Bílikova, K.; Huang, S.-C.; Lin, I.-P.; Šimuth, J.; Peng, C.-C. Structure and antimicrobial activity relationship of royalisin, an antimicrobial peptide from royal jelly of Apis mellifera. Peptides 2015, 68, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Hetru, C. Antimicrobial peptides from insects. In Molecular Mechanisms of Immune Responses in Insects; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemistry and bioactivity of royal jelly from Greece. J. Agric. Food Chem. 2005, 53, 8987–8992. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Zhao, F. Phage–bacteria interaction network in human oral microbiome. Environ. Microbiol. 2016, 18, 2143–2158. [Google Scholar] [CrossRef]
- Sällberg, M. Oral viral infections of children. Periodontol. 2000 2009, 49, 87–95. [Google Scholar] [CrossRef]
- Presti, R.M.; Handley, S.; Droit, L.; Ghannoum, M.; Jacobson, M.; Shiboski, C.H.; Webster-Cyriaque, J.; Brown, T.; Michael, T.Y.; Overton, E.T. Alterations in the oral microbiome in HIV-infected participants after ART administration are influenced by immune status. AIDS 2018, 32, 1279. [Google Scholar] [CrossRef]
- Santosh, A.B.R.; Muddana, K. Viral infections of oral cavity. J. Fam. Med. Prim. Care 2020, 9, 36. [Google Scholar] [CrossRef]
- Asai, D.; Nakashima, H. Pathogenic viruses commonly present in the oral cavity and relevant antiviral compounds derived from natural products. Medicines 2018, 5, 120. [Google Scholar] [CrossRef] [Green Version]
- Nagi, R.; Patil, D.J.; Rakesh, N.; Jain, S.; Sahu, S. Natural agents in the management of oral mucositis in cancer patients-systematic review. J. Oral Biol. Craniofac. Res. 2018, 8, 245–254. [Google Scholar] [CrossRef]
- Münstedt, K. Bee products and the treatment of blister-like lesions around the mouth, skin and genitalia caused by herpes viruses—A systematic review. Complement. Ther. Med. 2019, 43, 81–84. [Google Scholar] [CrossRef]
- Pagani, L. Effects of propolis flavonoids on virus infectivity and replication. Microbiologica 1990, 13, 207–213. [Google Scholar]
- Serkedjieva, J.; Manolova, N.; Bankova, V. Anti-influenza virus effect of some propolis constituents and their analogues (esters of substituted cinnamic acids). J. Nat. Prod. 1992, 55, 294–297. [Google Scholar] [CrossRef]
- Woźniak, M.; Sip, A.; Mrówczyńska, L.; Broniarczyk, J.; Waśkiewicz, A.; Ratajczak, I. Biological Activity and Chemical Composition of Propolis from Various Regions of Poland. Molecules 2022, 28, 141. [Google Scholar] [CrossRef]
- Hashemipour, M.A.; Tavakolineghad, Z.; Arabzadeh, S.; Iranmanesh, Z.; Nassab, S. Antiviral Activities of Honey, Royal Jelly, and Acyclovir Against HSV-1. Wounds Compend. Clin. Res. Pract. 2014, 26, 47–54. [Google Scholar]
- Ingawale, D.K.; Mandlik, S.K.; Patel, S.S. An emphasis on molecular mechanisms of anti-inflammatory effects and glucocorticoid resistance. J. Complement. Integr. Med. 2015, 12, 1–13. [Google Scholar] [CrossRef]
- Yang, D.; Elner, S.G.; Bian, Z.-M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Halim, D.S.; Mahanani, E.S.; Saini, R.; Omar, M.; bt Ibrahi, N.R.; Alam, M.K. A comparison study on the effectiveness of local honey and salicylate gel for treatment of minor recurrent aphtous stomatitis. Int. Med. J. 2013, 20, 770–772. [Google Scholar]
- Song, J.J.; Twumasi-Ankrah, P.; Salcido, R. Systematic review and meta-analysis on the use of honey to protect from the effects of radiation-induced oral mucositis. Adv. Ski. Wound Care 2012, 25, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, Q.; Ding, Y.; Bao, C.; Li, W. Mangiferin ameliorates Porphyromonas gingivalis-induced experimental periodontitis by inhibiting phosphorylation of nuclear factor-κB and Janus kinase 1–signal transducer and activator of transcription signaling pathways. J. Periodontal Res. 2017, 52, 1–7. [Google Scholar] [CrossRef]
- Fuji, A.; Kobayashi, S.; Kuboyama, N.; Furukawa, Y.; Kaneko, Y.; Ishihama, S. Augmantation of wound healing by royal jelly in streptozocindiabetic rats. J. Pharmacol. 1990, 53, 331–337. [Google Scholar]
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef]
- Shillitoe, E.J. The microbiome of oral cancer. In Critical Reviews™ in Oncogenesis; Begell House: Danbury, CT, USA, 2018; Volume 23. [Google Scholar]
- Tamura, T.; Fujii, A.; Kuboyama, N. Effects of royal jelly on experimental transplantable tumours. In Proceedings of the XXXth International Apicultural Congress, Nagoya, Japan, 10–16 October 1985; Constantinescu, C., Ed.; Apimondia Publ House: Bucharest, Romania, 1985; pp. 474–477. [Google Scholar]
- Afrin, S.; Haneefa, S.M.; Fernandez-Cabezudo, M.J.; Giampieri, F.; Al-Ramadi, B.K.; Battino, M. Therapeutic and preventive properties of honey and its bioactive compounds in cancer: An evidence-based review. Nutr. Res. Rev. 2020, 33, 50–76. [Google Scholar] [CrossRef]
- Saxena, S.; Gautam, S.; Maru, G.; Kawle, D.; Sharma, A. Suppression of error prone pathway is responsible for antimutagenic activity of honey. Food Chem. Toxicol. 2012, 50, 625–633. [Google Scholar] [CrossRef]
- Meister, A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol. Ther. 1991, 51, 155–194. [Google Scholar] [CrossRef]
- Orsolic, N.; Tadic, Z.; Benkovic, V.; Horvat, A.; Lojkic, M.; Basic, I. Radioprotective effect of a water-soluble derivative of propolis in mice. Mellifera 2004, 4, 45–52. [Google Scholar]
- Chung, L.C.; Chiang, K.C.; Feng, T.H.; Chang, K.S.; Chuang, S.T.; Chen, Y.J.; Tsui, K.H.; Lee, J.C.; Juang, H.H. Caffeic acid phenethyl ester upregulates N-myc downstream regulated gene 1 via ERK pathway to inhibit human oral cancer cell growth in vitro and in vivo. Mol. Nutr. Food Res. 2017, 61, 1600842. [Google Scholar] [CrossRef] [PubMed]
- Taheri, J.B.; Azimi, S.; Rafieian, N.; Zanjani, H.A. Herbs in dentistry. Int. Dent. J. 2011, 61, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Fujii, A.; Kuboyama, N. Antitumor effects of royal jelly (RJ). Nihon Yakurigaku Zasshi. Folia Pharmacol. Jpn. 1987, 89, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oršolić, N.; Terzić, S.; Šver, L.; Bašić, I. Honey-bee products in prevention and/or therapy of murine transplantable tumours. J. Sci. Food Agric. 2005, 85, 363–370. [Google Scholar] [CrossRef]
- Aljaghwani, A.; Allemailem, K.S.; Aljaghwani, L.F.; Alrumaihi, F.; Joseph, R.J.; Khan, A.A.; Rahmani, A.H.; Almatroudi, A. Antimicrobial Effect of Different Types of Honey on Selected ATCC Bacterial Strains. Pharmacogn. J. 2021, 13, 217–225. [Google Scholar] [CrossRef]
- Atwa, A.-D.A.; AbuShahba, R.Y.; Mostafa, M.; Hashem, M.I. Effect of honey in preventing gingivitis and dental caries in patients undergoing orthodontic treatment. Saudi Dent. J. 2014, 26, 108–114. [Google Scholar] [CrossRef]
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in oral health and care: A mini review. J. Oral Biosci. 2019, 61, 32–36. [Google Scholar] [CrossRef]
- Beegum, N.; Nandan, N.; Vishwanathan, S. Honey the paradisiacal panacea-A Review. J. Ayurveda Integr. Med. Sci. 2019, 4, 273–280. [Google Scholar]
- Sanghavi, T.; Shah, N.; Parekh, V.; Singbal, K. Evaluation and comparison of efficacy of three different storage media, coconut water, propolis, and oral rehydration solution, in maintaining the viability of periodontal ligament cells. J. Conserv. Dent. JCD 2013, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Kamburoğlu, K.; Özen, T. Analgesic effect of Anatolian propolis in mice. Agri 2011, 23, 47–50. [Google Scholar]
- Dhanesuan, N.; Srisuparbh, D.; Tiranathanagul, S.; Rungsiyanont, S. The In Vitro Effect of Royal Jelly, Apis mellifera, on Proliferation of Human Gingival, Periodontal Ligament Fibroblasts and Human Bone Cells. Thai Pharm. Health Sci. J. 2011, 6, 182–187. [Google Scholar]
- Qamar, Z.; Alghonaim, M.; Almohana, S.; Almohana, A.; Zeeshan, T. Potential biochemical effects of honey in oral health care: A review. Int. Food Res. J. 2021, 28, 23–30. [Google Scholar] [CrossRef]
- Singhal, R.; Siddibhavi, M.; Sankeshwari, R.; Patil, P.; Jalihal, S.; Ankola, A. Effectiveness of three mouthwashes—Manuka honey, Raw honey, and Chlorhexidine on plaque and gingival scores of 12-15-year-old school children: A randomized controlled field trial. J. Indian Soc. Periodontol. 2018, 22, 34–39. [Google Scholar] [CrossRef]
- Beena, J.P.; Sahoo, P.; Konde, S.; Raj, N.S.; Kumar, N.C.; Agarwal, M. Manuka Honey: A Potent Cariostatic Agent—An in vitro Study. Int. J. Clin. Pediatr. Dent. 2018, 11, 105. [Google Scholar]
- Xu, X.; Zhou, X.D.; Wu, C.D. Tea catechin epigallocatechin gallate inhibits Streptococcus mutans biofilm formation by suppressing gtf genes. Arch. Oral Biol. 2012, 57, 678–683. [Google Scholar] [CrossRef]
- Koo, H.; Schobel, B.; Scott-Anne, K.; Watson, G.; Bowen, W.; Cury, J.; Rosalen, P.; Park, Y. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J. Dent. Res. 2005, 84, 1016–1020. [Google Scholar] [CrossRef]
- .Duarte, S.; Rosalen, P.L.; Hayacibara, M.F.; Cury, J.A.; Bowen, W.H.; Marquis, R.; Rehder, V.L.; Sartoratto, A.; Ikegaki, M.; Koo, H. The influence of a novel propolis on mutans streptococci biofilms and caries development in rats. Arch. Oral Biol. 2006, 51, 15–22. [Google Scholar] [CrossRef]
- Hayacibara, M.F.; Koo, H.; Rosalen, P.L.; Duarte, S.; Franco, E.M.; Bowen, W.H.; Ikegaki, M.; Cury, J.A. In Vitro and In Vivo effects of isolated fractions of Brazilian propolis on caries development. J. Ethnopharmacol. 2005, 101, 110–115. [Google Scholar] [CrossRef]
- Nam, S.; Choi, Y.; Jang, S.; Shim, Y.; Han, G. Antimicrobial activity of propolis on different oral bacteria. Indian J. Sci. Technol. 2016, 9, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kuang, X.; Chen, V.; Xu, X. Novel approaches to the control of oral microbial biofilms. BioMed Res. Int. 2018, 2018, 6498932. [Google Scholar] [CrossRef] [Green Version]
- Sricholpech, M.; Srisupabh, D. Royal jelly promotes the viability and proliferation of periodontal ligament fibroblasts in an in vitro tooth avulsion simulation. Mahidol Dent. J. 2015, 35, 47–56. [Google Scholar]
- Scully, C.; Bagan, J. Oral squamous cell carcinoma: Overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009, 15, 388–399. [Google Scholar] [CrossRef]
- Borse, V.; Konwar, A.N.; Buragohain, P. Oral cancer diagnosis and perspectives in India. Sens. Int. 2020, 1, 100046. [Google Scholar] [CrossRef]
- Al-Koshab, M.; Alabsi, A.M.; Bakri, M.M.; Naicker, M.S.; Seyedan, A. Chemopreventive activity of Tualang honey against oral squamous cell carcinoma—In Vivo. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 484–492. [Google Scholar] [CrossRef]
- Mahmood, R.; Asif, J.A.; Shahidan, W.N.S. Stingless-bee (Trigona itama) honey adversely impacts the growth of oral squamous cell carcinoma cell lines (HSC-2). Eur. J. Integr. Med. 2020, 37, 101162. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mandal, M. Antiproliferative effects of honey and of its polyphenols: A review. J. Biomed. Biotechnol. 2009, 2009, 830616. [Google Scholar] [CrossRef] [Green Version]
- Dornelas, C.A.; Fechine-Jamacaru, F.V.; Albuquerque, I.L.; Magalhães, H.I.F.; Souza, A.J.S.D.; Alves, L.A.; Almeida, P.R.C.D.; Lemos, T.L.G.D.; Castro, J.D.V.D.; Moraes, M.E.A. Chemoprevention with green propolis green propolis extracted in L-lysine versus carcinogenesis promotion with L-lysine in N-Butyl-N-[4-hydroxybutyl] nitrosamine (BBN) induced rat bladder cancer. Acta Cir. Bras. 2012, 27, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.-Y.; Yang, H.-W.; Chu, Y.-H.; Chang, Y.-C.; Hsieh, M.-J.; Chou, M.-Y.; Yeh, K.-T.; Lin, Y.-M.; Yang, S.-F.; Lin, C.-W. Caffeic Acid phenethyl ester inhibits oral cancer cell metastasis by regulating matrix metalloproteinase-2 and the mitogen-activated protein kinase pathway. Evid.-Based Complement. Altern. Med. 2012, 2012, 732578. [Google Scholar] [CrossRef] [Green Version]
- Yanagita, M.; Kojima, Y.; Mori, K.; Yamada, S.; Murakami, S. Osteoinductive and anti-inflammatory effect of royal jelly on periodontal ligament cells. Biomed. Res. 2011, 32, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Drain, J.; Fleming, M.O. Palliative management of malodorous squamous cell carcinoma of the oral cavity with Manuka honey. J. Wound Ostomy Cont. Nurs. 2015, 42, 190–192. [Google Scholar] [CrossRef]
- Lusby, P.; Coombes, A.; Wilkinson, J. Honey: A potent agent for wound healing? J. WOCN 2002, 29, 295–300. [Google Scholar] [CrossRef]
- Kim, K.-E.; Kang, J.-O.; Park, Y.-D. Effect of mouthrinse containing propolis on oral malodor. Int. J. Clin. Prev. Dent. 2014, 10, 179–184. [Google Scholar] [CrossRef]
- Biswal, B.M.; Zakaria, A.; Ahmad, N.M. Topical application of honey in the management of radiation mucositis. A preliminary study. Support. Care Cancer 2003, 11, 242–248. [Google Scholar] [CrossRef]
- Raeessi, M.A.; Raeessi, N.; Panahi, Y.; Gharaie, H.; Davoudi, S.M.; Saadat, A.; Karimi Zarchi, A.A.; Raeessi, F.; Ahmadi, S.M.; Jalalian, H. “Coffee plus honey” versus “topical steroid” in the treatment of chemotherapy-induced oral mucositis: A randomised controlled trial. BMC Complement. Altern. Med. 2014, 14, 293. [Google Scholar] [CrossRef] [Green Version]
- Davari, A.; Ataei, E.; Assarzadeh, H. Dentin hypersensitivity: Etiology, diagnosis and treatment; a literature review. J. Dent. 2013, 14, 136. [Google Scholar]
- Erdem, O.; Gungormus, Z. The effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holist. Nurs. Pract. 2014, 28, 242–246. [Google Scholar] [CrossRef]
- Yamauchi, K.; Kogashiwa, Y.; Moro, Y.; Kohno, N. The effect of topical application of royal jelly on chemoradiotherapy-induced mucositis in head and neck cancer: A preliminary study. Int. J. Otolaryngol. 2014, 2014, 974967. [Google Scholar] [CrossRef] [Green Version]
- Wojtaszek, C.; Kochis, L.; Cunningham, R. Nutrition Impact Systems in the Oncology Patient. Oncol. Issues 2002, 17, 15–17. [Google Scholar]
- Charalambous, A.; Lambrinou, E.; Katodritis, N.; Vomvas, D.; Raftopoulos, V.; Georgiou, M.; Paikousis, L.; Charalambous, M. The effectiveness of thyme honey for the management of treatment-induced xerostomia in head and neck cancer patients: A feasibility randomized control trial. Eur. J. Oncol. Nurs. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Sateriale, D.; Facchiano, S.; Colicchio, R.; Pagliuca, C.; Varricchio, E.; Paolucci, M.; Volpe, M.G.; Salvatore, P.; Pagliarulo, C. In Vitro synergy of polyphenolic extracts from honey, myrtle and pomegranate against oral pathogens, S. mutans and R. dentocariosa. Front. Microbiol. 2020, 11, 1465. [Google Scholar] [CrossRef]
- Akber, A.; Ahmed, S.; Umer Hasan, S.M. A comparative study on the effect of propolis and dentine bonding agent in treating dentine hypersensitivity. J. Pak. Med. Assoc. 2022, 72, 2417–2421. [Google Scholar] [CrossRef]
- Tavares, J.A.O.; da Silva, F.A.; Santos, T.M.L.; Caneppele, T.M.F.; Augusto, M.G. The effectiveness of propolis extract in reducing dentin hypersensitivity: A systematic review. Arch. Oral Biol. 2021, 131, 105248. [Google Scholar] [CrossRef]
- Mehta, P.; Vimala, N.; Mandke, L. An insight into dentin desensitizing agents-In vivo study. Indian J. Dent. Res. 2013, 24, 571. [Google Scholar] [CrossRef]
- Purra, A.R.; Mushtaq, M.; Acharya, S.R.; Saraswati, V. A comparative evaluation of propolis and 5.0% potassium nitrate as a dentine desensitizer: A clinical study. J. Indian Soc. Periodontol. 2014, 18, 466. [Google Scholar] [CrossRef]
- Askari, M.; Yazdani, R. Comparison of two desensitizing agents for decreasing dentin hypersensitivity following periodontal surgeries: A randomized clinical trial. Quintessence Int. 2019, 50, 320–329. [Google Scholar]
- Maity, S.; Priyadharshini, V.; Basavaraju, S. A comparative evaluation of propolis and light-cured ormocer-based desensitizer in reducing dentin hypersensitivity. J. Indian Soc. Periodontol. 2020, 24, 441. [Google Scholar] [CrossRef]
- Nayak, P.A.; Nayak, U.A.; Mythili, R. Effect of Manuka honey, chlorhexidine gluconate and xylitol on the clinical levels of dental plaque. Contemp. Clin. Dent. 2010, 1, 214. [Google Scholar] [CrossRef]
- Alibasyah, Z.M.; Saputri, D.; Alviana, V. The Comparison Between Dental Plaque Score Before and After Gargling with Tongra Original Honey 5% Solution (Study of Student in Dentistry of Syiah Kuala University). Biomed. Pharmacol. J. 2018, 11, 381–385. [Google Scholar] [CrossRef]
- Nik Man, N.M.; Hassan, R.; Ang, C.Y.; Abdullah, A.D.; Mohd Radzi, M.A.; Sulaiman, S.A. Antileukemic Effect of Tualang Honey on Acute and Chronic Leukemia Cell Lines. Biomed. Res. Int. 2015, 2015, 307094. [Google Scholar] [CrossRef] [Green Version]
- Yılmaz, N.; Nisbet, Ö.; Nisbet, C.; Ceylan, G.; Hoşgör, F.; Dede, Ö.D. Biochemical evaluation of the therapeutic effectiveness of honey in oral mucosal ulcers. Bosn. J. Basic Med. Sci. 2009, 9, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, R.; Senpuku, H.; Ohnishi, M.; Takai, H.; Ogata, Y. Effect of topical administration of propolis in chronic periodontitis. Odontology 2020, 108, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, A.J.; Pakfetrat, A.; Tonkaboni, A.; Aledavood, S.A.; Najafi, M.F.; Delavarian, Z.; Shakeri, M.T.; Mohtashami, A. Preventing and therapeutic effect of propolis in radiotherapy induced mucositis of head and neck cancers: A triple-blind, randomized, placebo-controlled trial. Iran. J. Cancer Prev. 2015, 8, e4019. [Google Scholar]
- Pina, G.d.; Lia, E.N.; Berretta, A.A.; Nascimento, A.P.; Torres, E.C.; Buszinski, A.F.; de Campos, T.A.; Coelho, E.B.; Martins, V.d.P. Efficacy of propolis on the denture stomatitis treatment in older adults: A multicentric randomized trial. Evid.-Based Complement. Altern. Med. 2017, 2017, 8971746. [Google Scholar] [CrossRef] [PubMed]
- Netto, C.A.; Marcucci, M.C.; Paulino, N.; Anido-Anido, A.; Amore, R.; de Mendonça, S.; Neto, L.B.; Bretz, W.A. Effects of typified propolis on mutans streptococci and lactobacilli: A randomized clinical trial. Braz. Dent. Sci. 2013, 16, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, E.M.R.; da Silva, J.L.D.C.; Silva, F.F.; De Luca, M.P.; Lorentz, T.C.M.; Santos, V.R. Clinical evidence of the efficacy of a mouthwash containing propolis for the control of plaque and gingivitis: A phase II study. Evid.-Based Complement. Altern. Med. 2011, 2011, 750249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Faveri, M.; Pupio, G.C.; Koo, H.; Bueno-Silva, B.; de Oliveira, K.M.; Figueiredo, L.C.; Rosalen, P.L.; Hayacibara, R.M.; Fujimaki, M. The effect of Brazilian propolis type-3 against oral microbiota and volatile sulfur compounds in subjects with morning breath malodor. Clin. Oral Investig. 2021, 26, 1531–1541. [Google Scholar] [CrossRef]
- Barnett, M.L. The rationale for the daily use of an antimicrobial mouthrinse. J. Am. Dent. Assoc. 2006, 137, S16–S21. [Google Scholar] [CrossRef]
- Colombo, A.; Tanner, A. The role of bacterial biofilms in dental caries and periodontal and peri-implant diseases: A historical perspective. J. Dent. Res. 2019, 98, 373–385. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, CD008676. [Google Scholar] [CrossRef]
- Lim, K.-S.; Kam, P. Chlorhexidine-pharmacology and clinical applications. Anaesth. Intensive Care 2008, 36, 502–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osso, D.; Kanani, N. Antiseptic mouth rinses: An update on comparative effectiveness, risks and recommendations. Am. Dent. Hyg. Assoc. 2013, 87, 10–18. [Google Scholar]
- Mude, G.; Pise, S.; Thombare, G. Formulation and evaluation of polyherbal toothpaste and comparative study with marketed formulations. Int. J. Creat. Res. Thoughts 2020, 8, 3796–3806. [Google Scholar]
- Morawiec, T.; Dziedzic, A.; Niedzielska, I.; Mertas, A.; Tanasiewicz, M.; Skaba, D.; Kasperski, J.; Machorowska-Pieniążek, A.; Kucharzewski, M.; Szaniawska, K. The biological activity of propolis-containing toothpaste on oral health environment in patients who underwent implant-supported prosthodontic rehabilitation. Evid.-Based Complement. Altern. Med. 2013, 2013, 704947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiatrak, K.; Morawiec, T.; Rój, R.; Kownacki, P.; Nitecka-Buchta, A.; Niedzielski, D.; Wychowański, P.; Machorowska-Pieniążek, A.; Cholewka, A.; Baldi, D. Evaluation of Effectiveness of a Toothpaste Containing Tea Tree Oil and Ethanolic Extract of Propolis on the Improvement of Oral Health in Patients Using Removable Partial Dentures. Molecules 2021, 26, 4071. [Google Scholar] [CrossRef] [PubMed]
- Victorino, F.R.; Bramante, C.M.; Watanabe, E.; Ito, I.Y.; Franco, S.L.; Hidalgo, M.M. Antibacterial activity of propolis-based toothpastes for endodontic treatment. Braz. J. Pharm. Sci. 2009, 45, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Luo, S.; Susha, Y.L. Propolis Toothpaste and Preparation Method Thereof. CN102283795A, 22 August 2011. [Google Scholar]
- Zhang, Y.; Zhang, Y. Propolis Toothpaste and Preparation Method Thereof. CN110755355A, 6 August 2019. [Google Scholar]
- Alviano, W.; Mendonça-Filho, R.; Alviano, D.; Bizzo, H.; Souto-Padrón, T.; Rodrigues, M.; Bolognese, A.; Alviano, C.; Souza, M. Antimicrobial activity of Croton cajucara Benth linalool-rich essential oil on artificial biofilms and planktonic microorganisms. Oral Microbiol. Immunol. 2005, 20, 101–105. [Google Scholar] [CrossRef]
- Yoo, C.-S.; Chae, S.-K.; Lee, H.-G.; Kim, C.-H.; Kim, D.-K.; Kim, W.-G.; Kim, K.-E.; Ahn, H.-K.; Kim, J.-E.; Oh, Y.-J. The inhibition effects of cetylpyridinium chloride in dentifrices on plaque formation: Clinical test. Int. J. Clin. Prev. Dent. 2014, 10, 9–14. [Google Scholar] [CrossRef]
- Halboub, E.; Al-Maweri, S.A.; Al-Wesabi, M.; Al-Kamel, A.; Shamala, A.; Al-Sharani, A.; Koppolu, P. Efficacy of propolis-based mouthwashes on dental plaque and gingival inflammation: A systematic review. BMC Oral Health 2020, 20, 198. [Google Scholar] [CrossRef]
- Murray, M.; Worthington, H.; Blinkhorn, A. A study to investigate the effect of a propolis-containing mouthrinse on the inhibition of de novo plaque formation. J. Clin. Periodontol. 1997, 24, 796–798. [Google Scholar] [CrossRef]
- Dehghani, M.; Abtahi, M.; Hasanzadeh, N.; Farahzad, Z.; Noori, M.; Noori, M. Effect of Propolis mouthwash on plaque and gingival indices over fixed orthodontic patients. J. Clin. Exp. Dent. 2019, 11, e244–e249. [Google Scholar] [CrossRef] [PubMed]
- Santiago, K.B.; Piana, G.M.; Conti, B.J.; Cardoso, E.d.O.; Murbach Teles Andrade, B.F.; Zanutto, M.R.; Mores Rall, V.L.; Fernandes, A., Jr.; Sforcin, J.M. Microbiological control and antibacterial action of a propolis-containing mouthwash and control of dental plaque in humans. Nat. Prod. Res. 2018, 32, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.; Manjunath, S.; Shivanagendra, S.; Kumar, S.D.; Shekar, S.S. Health from the hive: 5% Propolis mouth wash as an adjunct in the treatment of chronic generalized gingivitis-a randomized controlled clinical trial. Dentistry 2019, 9, 1–6. [Google Scholar] [CrossRef]
- Ke, W.; Duan, Y. Propolis Mouthwash and Preparation Method Thereof. CN104739738A, 27 December 2013. [Google Scholar]
- Tulsani, S.G.; Chikkanarasaiah, N.; Siddaiah, S.B.; Krishnamurthy, N.H. The effect of Propolis and Xylitol chewing gums on salivary Streptococcus mutans count: A clinical trial. Indian J. Dent. Res. 2014, 25, 737. [Google Scholar] [CrossRef]
- Liu, G. Compound Bee Product Chewable Tablet. CN107198189B, 26 June 2017. [Google Scholar]
- Fan, S. Chewable Propolis Tablet and Production Technology Thereof. CN102326723A, 31 May 2011. [Google Scholar]
- Ibrahim, P. Method for Producing Chewing Gum Using Propolis in Solid Resin Form. WO2020101601, 15 November 2018. [Google Scholar]
- Liu, S.; Liu, Y. Brazil Green Propolis Toothpaste. CN107412138B, 6 September 2017. [Google Scholar]
- Wang, J. Manuka Honey Toothpaste for Pregnant Women and Preparation Method of Toothpaste. CN105287328A, 23 September 2015. [Google Scholar]
- Sung, K.H. A composition for Cleaning Oral Cavity Containing Water-Soluble Propolis Extract. KR100719306B1, 4 November 2004. [Google Scholar]
- Feng, M. Hive Honey Chewing Gum and Preparation Method. CN107751533A, 10 October 2017. [Google Scholar]
- Koca, I.; Koca, A.F. Poisoning by mad honey: A brief review. Food Chem. Toxicol. 2007, 45, 1315–1318. [Google Scholar] [CrossRef] [PubMed]
- Silici, S.; Atayoglu, A.T. Mad honey intoxication: A systematic review on the 1199 cases. Food Chem. Toxicol. 2015, 86, 282–290. [Google Scholar] [CrossRef]
- Edgar, J.A.; Roeder, E.; Molyneux, R.J. Honey from plants containing pyrrolizidine alkaloids: A potential threat to health. J. Agric. Food Chem. 2002, 50, 2719–2730. [Google Scholar] [CrossRef]
- Fields, B.A.; Reeve, J.; Bartholomaeus, A.; Mueller, U. Human pharmacokinetic study of tutin in honey; a plant-derived neurotoxin. Food Chem. Toxicol. 2014, 72, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Larsen, L.; Joyce, N.I.; Sansom, C.E.; Cooney, J.M.; Jensen, D.J.; Perry, N.B. Sweet poisons: Honeys contaminated with glycosides of the neurotoxin tutin. J. Nat. Prod. 2015, 78, 1363–1369. [Google Scholar] [CrossRef]
- Islam, M.N.; Khalil, M.I.; Islam, M.A.; Gan, S.H. Toxic compounds in honey. J. Appl. Toxicol. 2014, 34, 733–742. [Google Scholar] [CrossRef]
- Fearnley, J. Bee Propolis: Natural Healing from the Hive; Souvenir: London, UK, 2001. [Google Scholar]
- Walgrave, S.E.; Warshaw, E.M.; Glesne, L.A. Allergic contact dermatitis from propolis. Dermatitis 2005, 16, 209–215. [Google Scholar] [PubMed]
- Rosmilah, M.; Shahnaz, M.; Patel, G.; Lock, J.; Rahman, D.; Masita, A.; Noormalin, A. Characterization of major allergens of royal jelly Apis mellifera. Trop. Biomed. 2008, 25, 243–251. [Google Scholar] [PubMed]

| Source of Bee Product | Group | Bioactive Compounds | Biological Activity | References |
|---|---|---|---|---|
| Honey | Flavonoids | flavonoles (a,b,c,g quercetin, b,g galangin, b,f,g fisetin, b myricetin) flavanones (a,b pinocembrin, c pinobanksin, e naringenin, c,e hesperetin) flavones (c,f apigenin, b,g acacetin, b,c,f chrysin, b,c,e Luteolin, c genistein, b wagonin) and b caffeic acid phenethyl ester | a anti-microbial b anti-cancer c anti-inflammatory d anti-fungal e antioxidant f anti-bacterial g anti-allergic h anti-genotoxic i neuroprotective j anti-anxiolytic k chemoprotective l anti-proliferative | [61,62,63,64,65] |
| Phenolic acid | h,i p-coumaric acid, j gallic acid, e,k,l ellagic acid, c,e,i ferulic acid, b,e syringic acid | [64] | ||
| Propolis | Flavonoids | flavonoles (a,b,c,g quercetin, b,g galangin, b,f,g fisetin) flavanones(a,b pinocembrin) flavones (c,f Apigenin, b,g acacetin, b,c,f chrysin) and b caffeic acid phenethyl ester (CAPE) | [61,62,63] | |
| Phenolic acid | a 2,2-dimethyl-8-phenylchromene, a,b,c 4-hydroxy-3,5-diprenyl cinnamic acid (artepillin C), a 3-prenyl cinnamic acid allyl ester, b kaempferide, d benzofuran, | [61] | ||
| Terpenoid | d isocupressic acid, b symphyoreticulic acid, a,b,e procrim a and b, a,b,e lupeol, d farnesol | [61,66] | ||
| Royal jelly | Flavonoids | flavonoles (e.g., a,b,c,g quercetin, b kaempherol, b,g galangin and b,f,g fisetin) flavanones (e.g., a,b pinocembrin, c naringin, c,e hesperidin and isosakuranetin) flavones (e.g., c,f apigenin, b,g acacetin, b,c,f chrysin and b,c,e luteolin) | [67] | |
| Phenolic acid | 2,2-dimethyl-8-prenylchromene, 3-prenyl cinnamic acid allyl ester, artepillin C | [63] | ||
| Terpenoid | isocupressic acid, labdane diterpenoid | [63] |
| Type of Bee Product | Type of Extract | Bioactive Compounds | Type of Study | Biological Activity | Key Findings | References |
|---|---|---|---|---|---|---|
| Honey | Aqueous extract | Mixture of phenols and flavonoids | In vitro and in vivo | Antioxidant | In male Wistar rats, application of 1 mg/mL honey showed 45.3% DPPH inhibition. Significant (p = 0.028) reduction in lipid per oxidation in oral mucosa wound tissue was observed. | [75] |
| Aqueous extract | Flavonoids | In vitro and in vivo | Anti-bacterial | Honey mouth rinse showed antibacterial characteristics and was found effective against oral infections (in vitro). Plaque development was also inhibited (in vivo). | [100] | |
| Aqueous extract | Phenolic acids, Flavonoids | In vitro | Anti-fungal | The Algerian honeys with different concentrations (undiluted, 10%, 30%, 50% and 70% w/v) were tested against Candida albicans and Rhodotorula sp. Both species had MICs of 70.09–93.48% and 4.90–99.70% v/v, respectively. | [101] | |
| Aqueous extract | Flavonoids | Clinical trial | Antiviral | In children with primary herpetic gingivostomatitis, combining honey with oral acyclovir can yield better results than acyclovir alone. | [102] | |
| Aqueous extract | Flavonoids | In vitro | Anti-inflammatory | Honey flavonoid extract (HFE) considerably reduce release of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin -1 (IL-1), according to the findings. The formation of reactive oxygen intermediates and the expression of inducible nitric oxide synthase (iNOS) were also dramatically reduced. | [103] | |
| Aqueous extract | Phenolic acids | In vitro | Anti-cancer | Oral squamous cell carcinoma (OSCC) and osteosarcoma (HOS) cell lines showed maximum suppression of cell growth of 80% at a concentration of 15%. | [104] | |
| Propolis | Aqueous extract | Flavonoids | Clinical trial | Antioxidant and anti-inflammatory | Propolis reduced and delayed radiation-induced mucositis in rats by being able to prevent reduction in salivary antioxidant levels. | [91] |
| 3% ethanolic extract (EEP) | Flavonoids:—kaempferol and quercetin and cinnamic acid derivatives | Clinical trial | Anti-bacterial | At 50 mg/L, EEP had a time-dependent microbiological effect with anti-bacterial efficacy against Gram-positive bacteria. Hygienic treatments containing 3% EEP effectively assist plaque clearance and enhance the condition of the marginal periodontium. | [105] | |
| 70% ethanol | Esters of phenolic acids (caffeates and ferulates) and flavonoids | In vitro | Anti-bacterial, Anti-fungal and Antiviral | All the studied samples exhibited significant activity against fungal and Gram-positive bacterial test strains, with the majority also showing antiviral activity. | [106] | |
| Aqueous and ethanol extracts | Galangin and chrysin | In vitro | Antiviral | In viral suspension experiments, both propolis extracts were found to have significant antiviral efficacy against HSV-1, with plaque formation reduced by >98%. | [107] | |
| Aqueous extracts | Artepillin C | Clinical trial | Anti-inflammatory | Rinse products containing Brazilian green propolis high in artepillin C reduced gingivitis to the same extent as a NaF/cetylpyridinium chloride rinse or a chlorhexidine solution in randomized, double-blind, placebo-controlled studies. | [108] | |
| Ethanol extracts | Caffeic acid phenethyl ester | In vitro | Anti-cancer | In TW2.6 human oral squamous cell carcinoma (OSCC) cells, propolis extracted caffeic acid phenethyl ester CAPE treatment reduced cell proliferation and colony formation in a dose-dependent manner. CAPE treatment reduced the number of G1 phase cells, increased the number of G2/M phase cells, and caused death in TW2.6 cells. | [109] | |
| Royal Jelly | Aqueous extracts | I-IV jellein peptides | In vitro | Anti-bacterial | Four peptides were isolated from honey bee and Royal Jelly presented exclusively antimicrobial activities against Gram-positive and Gram-negative bacteria. | [29] |
| Aqueous extracts | Phenolic compounds | In vitro | Anti-fungal | The MIC, MIC50 and MFC of Royal Jelly on Candida albicans were 80, 103 and 160 mg/mL, respectively, while the MIC, MIC50 and MFC of Iranian Propolis alcoholic extract were 0.030, 0.0618 and 0.0833 mg/mL, respectively. | [110] | |
| Aqueous extracts | Major royal jelly protein 3 (MRJP3) | Clinical trial | Anti-inflammatory | When RJ suspension was given to a culture of mouse peritoneal macrophages activated with lipopolysaccharide and IFN-gamma, the production of proinflammatory cytokines such as TNF-α, IL-6 and IL-1 was efficiently reduced in a dose-dependent manner without causing macrophage cytotoxicity. | [111] | |
| Aqueous extracts | 10-Hydroxy-2-decenoic acid | In vitro | Anti-cancer | The 10-HDA at 20 M or higher significantly suppressed proliferation and migration of cancerous cells. | [112] |
| Type of Bee Product | Disease Targeted | Whole Bee Product/Extracts and Dose | Key Findings | Reference |
|---|---|---|---|---|
| Honey | Radiotherapy-induced xerostomia | Individuals suffering from neck and head cancer were randomly allocated to the control group (oral rinses with saline) and the intervention group (oral rinses with 20 mL of thyme honey diluted in 100 mL of purified water). Patients were required to perform oral rinses just before, immediately after and 6 h after the radiotherapy session. Radiation-induced xerostomia was assessed starting from the 4th week of radiotherapy, one and six months after the completion of radiotherapy | Thyme honey was found useful in lowering or stabilizing the degree of xerostomia over time, with progressive improvement. The better management of xerostomia showed significant effects on overall quality of life. | [228] |
| Chemotherapy-induced mucositis | Patients randomly assigned to one of three treatment groups: Group 1—Empirical dosage of 0.5 g honey/kg (max.15 g) was applied topically three times daily to the afflicted oral mucosa for 10 days, or until healing. Group 2—An empirical dose of 0.25 g/kg (max. 5 g) of a 4:2:1 mixture of honey, olive oil-propolis extract, and beeswax (HOPE) administered topically three times daily to the diseased oral mucosa for 10 days, or until healing. Group 3—Served as the control group where benzocaine 7.5% gel was topically applied three times daily to the afflicted oral mucosa. | Honey (Group 1) resulted in faster healing than HOPE (Group 2) or control (Group 3) in both grades of mucositis (p > 0.05). | [37] | |
| Dental Plaque | At the commencement of the trial, each patient underwent a professional prophylaxis to completely remove plaque and calculus from the teeth. The patients were randomly assigned to below groups: Group 1—Manuka honey was applied gently to gingival sulcus of the teeth, and the procedure was repeated twice after 5 min. The honey was applied twice a day after meals. Group 2—Rinsing with 0.2% chlorhexidine (10 mL) twice a day for 60 s followed by its expectoration. Group 3—Chewing the xylitol chewing gum for 5 min, thrice a day after meals. Following the experimental time, the plaque scores were determined. | The mean plaque scores for Groups 1, 2 and 3 were correspondingly 1.37, 1.35 and 1.57. The study indicated that manuka honey and chlorhexidine mouthwash significantly reduced plaque development compared to xylitol chewing gum. | [236] | |
| Dental Plaque | Dental plaque score was recorded in individuals before and after gargling with Tongra original honey 5% solution for six days. | Gargling with Tongra original honey 5% solution effectively decreased the dental plaque score. | [237] | |
| Oral squamous cell carcinoma (OSCC) and human osteosarcoma (HOS) | Different Tualang honey (1–20%) concentrations were administered to OSCC and HOS cell lines at different intervals of 3, 6, 12, 24, 48 and 72 h. | Tualang honey had an anti-proliferative effect on both cell lines. Maximum inhibition (≥80%) of cell growth recorded at a dose of 15%. | [238] | |
| Oral mucosal ulcers | For the oral mucosal ulcer model, excisional wounds were conducted on 30 Wistar albino rats (240–30 g) and they were separated into 3 groups: Group 1—Apitherapeutic agent or honey treatment (0.1 ml, 2 × 1). Group 2—Glyceroloxytriester (TGO) (0.1 ml, 2 × 1) was used to treat locally. Control Group 3—On the 7th day, biopsy samples were collected from the right buccal mucosa, and on the 14th day, samples were taken from the left buccal mucosa. | Only on the 7th day a significant difference documented between groups 1 and 3, whereas on day 14, no significant difference was noted among the groups. Honey was found to be efficient in the therapy of oral mucosal ulcers and showed a greater therapeutic benefit than glyceroloxytriester (TGO). | [239] | |
| Propolis | Gingivitis | One of the twins got 2% pure propolis for rinsing during the gingivitis induction period while the other received a color-matched 0.05% sodium fluoride + 0.05% cetylpyridinium chloride for rinsing (positive control). For 21 days, patients were advised to rinse 20 mL of respective rinses twice a day for 30 s each time. | During a 3-week no-hygiene period, a 2% typified propolis rinse performed similar to positive control rinse. | [240] |
| Radiotherapy-induced mucositis | Patients were divided into two groups at random: The Case group (n = 10) received 15 mL of water-based propolis mouthwash three times daily, while the Control group (n = 10) received 15 mL of placebo mouthwash. | Propolis water extract effectively prevented and cured radiotherapy-induced mucositis. | [241] | |
| Denture stomatitis | The patients were randomized to one of two therapy groups at random: Miconazole oral gel, 20 mg/g, was given to the control group (MIC) for 14 days. For 14 days, the PROP group was given a mucoadhesive formulation containing a standardized extract of 2% (20 mg/g) propolis (EPP-AF ®®). On days 1, 7 and 14, patients were assessed. | EPP-AF showed effect at par with miconazole. | [242] | |
| Cariogenic infections in a caries-active patient | Patients were randomly assigned to one of three experimental groups after their cavitated lesions were restored: (1) PROP-alcohol-free 2% propolis rinse (n = 20); (2) CHX- 0.12% chlorhexidine rinse; (3) PL-placebo mouth rinse. Patients were asked to rinse with 15 mL of respective rinses twice day for 60 s for 28 days. Salivary levels of Mutans Septococci (MS) and Lactobacilli (LACT) were evaluated at baseline, 7-day, 14-day and 28-day visits (experimental effects) and 45-day visits (residual effects). | Among all the treatments evaluated, propolis rinse was found to be the most efficient at suppressing cariogenic infections in caries-active participants. | [243] | |
| Dental Plaque and Gingivitis | A phase II clinical trial involved patients with a minimum of 20 healthy natural teeth, a mean plaque index of at least 1.5 P I, and a mean gingival index of at least 1.0 GI. Patients were advised to rinse with 10 mL of mouthwash test for one minute after brushing their teeth in the morning and at night. | Plaque and gingival index considerably reduced after 45 and 90 days of using mouthwash. | [244] | |
| Dentin hypersensitivity (DH) | 96 patients with DH in one or more teeth were studied in this study. The teeth were allocated in one of four treatment groups at random: Group 1—10% ethanolic extract of propolis. Group 2—30% ethanolic extract of propolis. Group 3—Single Bond Universal dentin bonding agent. Group 4—Distilled water as a placebo. The degree of DH was assessed using a visual analog scale based on the patients’ response to tactile and air blast stimuli. | Propolis extracts and dentin bonding agent were found to be equally effective in relieving DH. Propolis application was advised for patients experiencing mild to moderate discomfort whereas dentin bonding agent could be a preferable alternative for rapid relief. | [234] | |
| Oral malodor | Patients were allocated into three groups: I received a placebo (P), II received an ethanolic extract of propolis type-3 at a concentration of 3% (EEP), and III received chlorhexidine (CHX) at a concentration of 0.12%. Participants were instructed to rinse with respective rinses twice a day for 5 days. Each trial period was followed by a washout period of 21 days. Microbiological samples were obtained from the tongue dorsum at baseline and at the end of the rinse period to measure the concentration of volatile sulfur compounds (VSC) in the morning mouth breath. | Both EEP and CHX treatment resulted in a considerable reduction in the mean counts of bacterial pathogens, including certain VSC producers. The study suggested the use of mouth rinse containing 3% propolis type-3 prevents malodorous morning breath. | [245] | |
| Royal Jelly | Periodontal pathogens | Subgingival plaque samples were obtained and analyzed from 15 chronic periodontitis patients. RJ and chlorhexidine were tested in vitro for their ability to inhibit the growth of aerobic and anaerobic bacteria. | Chlorhexidine at low dose (50 μg/100 μL) was shown to be more sensitive in suppressing aerobic and anaerobic periodontopathic bacteria in subgingival plaque area whereas a larger dosage of RJ was required to have an inhibitory effect. | [150] |
| Radiotherapy-induced mucositis | Patients who needed chemoradiation for head and neck cancer were randomly allocated to one of two groups. During chemoradiation treatment, seven patients in the experimental group received RJ three times a day while six patients in the control group did not. The development of mucositis in both groups of patients was monitored twice a week. | The use of RJs reduced radiation-induced mucositis in patients with head and neck cancer. | [226] | |
| Radiotherapy and chemotherapy-induced mucositis | Patients were allocated into two categories. Both groups used benzydamine hydrochloride and nystatin mouthwashes. The experimental group also received 1 g RJ twice daily in addition to mouthwash. Both experimental groups administered mouthwash and/or RJ to patients until the mucositis resolved. | The RJ group resolved oral mucositis in a fraction of the time it took the control group. | [225] |
| Type of Product | Product | Patents | Bee Product Used | Intended Use | Reference |
|---|---|---|---|---|---|
| Toothpaste | Propolis toothpaste | CN102283795A | Propolis | To prevent and treat oral diseases | [255] |
| Propolis toothpaste | CN110755355A | Propolis | To improve bleeding gum and maintain oral health by inhibiting pathogenic bacteria | [256] | |
| Brazilian green propolis toothpaste | CN107412138B | Propolis | To prevent on gingivitis, periodontitis and halitosis | [269] | |
| Manuka honey toothpaste | CN105287328A | Manuka honey | To prevent prevents gingivitis, periodontitis, tooth decay and oral ulcer during pregnancy | [270] | |
| Mouthwash | Propolis mouthwash | CN104739738A | Propolis | Improved anti-bacterial and anti-inflammatory properties, as well as the prevention and treatment of oral disorders such oral cancer | [264] |
| Propolis mouthwash | KR20060041348A | Propolis | To prevent tooth decay and treat oral diseases | [271] | |
| Chewing gum | Hive honey chewing gum | CN107751533A | Honey | To improve health of oral cavity and remove bad breath | [272] |
| Chewable tablet | Tablet | CN107198189B | Propolis, royal jelly, honey, and beeswax | To improve immunity and highly suitable for patients suffering from pharyngitis | [266] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhary, P.; Tushir, S.; Bala, M.; Sharma, S.; Sangha, M.K.; Rani, H.; Yewle, N.R.; Kumar, P.; Singla, D.; Chandran, D.; et al. Exploring the Potential of Bee-Derived Antioxidants for Maintaining Oral Hygiene and Dental Health: A Comprehensive Review. Antioxidants 2023, 12, 1452. https://doi.org/10.3390/antiox12071452
Choudhary P, Tushir S, Bala M, Sharma S, Sangha MK, Rani H, Yewle NR, Kumar P, Singla D, Chandran D, et al. Exploring the Potential of Bee-Derived Antioxidants for Maintaining Oral Hygiene and Dental Health: A Comprehensive Review. Antioxidants. 2023; 12(7):1452. https://doi.org/10.3390/antiox12071452
Chicago/Turabian StyleChoudhary, Poonam, Surya Tushir, Manju Bala, Sanjula Sharma, Manjeet Kaur Sangha, Heena Rani, Nileshwari Raju Yewle, Parminder Kumar, Diksha Singla, Deepak Chandran, and et al. 2023. "Exploring the Potential of Bee-Derived Antioxidants for Maintaining Oral Hygiene and Dental Health: A Comprehensive Review" Antioxidants 12, no. 7: 1452. https://doi.org/10.3390/antiox12071452
APA StyleChoudhary, P., Tushir, S., Bala, M., Sharma, S., Sangha, M. K., Rani, H., Yewle, N. R., Kumar, P., Singla, D., Chandran, D., Kumar, M., & Mekhemar, M. (2023). Exploring the Potential of Bee-Derived Antioxidants for Maintaining Oral Hygiene and Dental Health: A Comprehensive Review. Antioxidants, 12(7), 1452. https://doi.org/10.3390/antiox12071452








